Abstract
Objective:
Approximately 35–61% of individuals with posttraumatic stress disorder (PTSD) report insomnia. Further, upwards of 70% report clinically significant insomnia following PTSD treatment. There are converging lines of evidence suggesting that insomnia not only independently affects daytime functioning and worsens PTSD symptoms, but insomnia may also compromise response to PTSD treatment, such as prolonged exposure (PE). Taken together, integrated insomnia and PTSD treatment may increase client-centered care and treatment outcomes.
Method:
This paper reviews the theory and evidence for treating sleep prior to PTSD treatment, describes the key elements of integrated cognitive behavioral treatment for insomnia (CBT-I) and PE (2NITE protocol), and presents pilot data from a sample of 12 treatment-seeking veterans with PTSD and insomnia who completed the 2NITE protocol. Sleep data were collected with sleep diaries and actigraphy watches.
Results:
The Client Satisfaction Questionnaire indicated high satisfaction with the 2NITE protocol (mean score 29.66 out of 32 points). On average, there were statistical and clinically significant changes in all measures including a 20.16 point decrease in the PTSD Checklist 5 (PCL-5), a 11.75 point decrease in the insomnia severity index, a 18.58 point increase in the WHO-quality of life index, a 11% increase in sleep efficiency, and a 51 minute increase in total sleep time from the actigraphy data.
Conclusions:
Among individuals with insomnia and PTSD, integrating CBT-I and PE with the 2NITE protocol represents a logical, innovative, and empirically-informed method for augmenting existing treatments and optimizing outcomes that justifies further investigation.
Keywords: PTSD, Insomnia, Integrated Treatment, Psychotherapy
Introduction
Several studies found difficulty in sleep onset and maintenance to be the most frequently reported symptom of posttraumatic stress disorder (PTSD; e.g., Pruiksma et al., 2016). Upwards of 90% of individuals with PTSD report difficultly sleeping and 35–61% of individuals with PTSD meet diagnostic criteria for insomnia (Colvonen et al., in press). Insomnia severity is associated with greater severity of PTSD symptoms, poorer quality of life, and worse daily functioning (e.g., Martindale, Morissette, Rowland, & Dolan, 2017; Morgan et al., 2017). Reviews of studies that examine sleep during PTSD treatment suggest that while sleep does improve over the course of successful PTSD treatment, upwards of 80% still report clinically significant insomnia following treatment (Colvonen et al., in press). This is particularly concerning given that there are converging lines of evidence suggesting that insomnia may compromise underlying mechanisms used in trauma-focused PTSD treatment (e.g., Belleville, Guay, & Marchand, 2011; Gutner, Casement, Gilbert, & Resick, 2013). Taken together, addressing insomnia, prior to PTSD treatment, has the potential to increase PTSD treatment effectiveness and improve quality of life.
Although sleep disturbances are symptoms of PTSD, insomnia may be best considered a co-occurring and independent disorder (Germain, McKeon, & Campbell, 2017). For instance, insomnia may precede the trauma and predict the development of PTSD (Bryant, Creamer, O’Donnell, Silove, & McFarlane, 2010; Germain, Buysse, & Nofzinger, 2008; Wright et al., 2011). This is especially true in military populations where short sleep duration and irregular sleep patterns are common (Peterson, Goodie, Satterfield, & Brim, 2008). Additionally, when insomnia initially occurs as a symptom of PTSD, it can become an independent disorder when the behavioral and cognitive responses to acute insomnia lead to perpetuating factors (e.g., napping, sleeping pills) and conditioned arousal (e.g., hyperarousal symptoms lead to the pairing of the bed with wakefulness; Perlis et al., 2010). Thus, perpetuating factors and conditioned arousal are often responsible for the maintenance of insomnia even in the absence of PTSD (Bootzin, Epstein, & Wood, 1991). This suggests that insomnia may need to be assessed and treated separately from, or in conjunction with, PTSD.
In a review of PTSD treatment studies that tracked sleep, Colvonen et al. (in press) suggest that while sleep does improve over the course of treatment, it tends to remain above the clinical cut off for disturbed sleep. For example, in a study of active duty military personnel randomized to group Cognitive Processing Therapy (CPT) or group Present Centered Therapy (PCT; Pruiksma et al., 2016), sleep disturbance was the most frequently reported symptom of PTSD both before and after treatment. Of 108 participants, 92% reported sleep disturbance pre-treatment and 74% and 80% (CPT and PCT respectively) reported sleep disturbances following treatment. Among participants who no longer met criteria for PTSD post-treatment, 57% still reported sleep disturbances. Together, this suggests that PTSD treatment does not fully resolve insomnia, which may require direct intervention.
There are converging lines of evidence suggesting that insomnia may compromise underlying mechanisms used in trauma-focused PTSD treatment in general, and prolonged exposure (PE) specifically. Insomnia and rapid eye movement (REM) fragmentation, in particular, reduce the effectiveness of extinction learning (integrating new learning of decreased fear response through repetition; Spoormaker et al., 2012) and safety learning (the ability to distinguish safe from unsafe cues; Marshall, Acheson, Risbrough, Straus, & Drummond, 2014) that may be necessary for PE. This implies that insomnia could potentially interfere with both natural extinction (thus maintaining PTSD) and treatment-induced extinction (thus reducing the effects of PE) of PTSD-related fear (Marshall et al., 2014; Spoormaker et al., 2012). Additionally, insomnia may also interfere with the emotional functioning needed for successful PE treatment. For example, insomnia has been linked to worse emotional reactivity and emotional coping (Morin, Rodrigue, & Ivers, 2003), emotional processing (Walker & van Der Helm, 2009), and cognitive abilities (Harvey, 2002). Pace-Schott, Germain, and Milad (2015) suggest that improving sleep quality before PTSD treatment may strengthen the extinction process leading to better treatment outcomes.
Cognitive behavioral treatment for insomnia (CBT-I) is a behavioral treatment with strong efficacy (Morin et al., 2006). It is the first line treatment of chronic and severe insomnia (Wilson et al., 2010) and is recommended for veterans with PTSD (Veterans Affairs/Department of Defense, 2016). Additionally, CBT-I has been shown to be effective in treating insomnia in individuals with co-occurring PTSD, increasing sleep efficiency (SE) and decreasing daytime PTSD symptoms (Talbot et al., 2014; Taylor & Pruiksma, 2014). CBT-I is also associated with objective changes in sleep architecture including increased and consolidated REM (Cervena et al., 2004). Consolidated sleep may directly influence better safety learning and extinction learning/recall helpful in PE. Taken together, these studies support the use of CBT-I in patients with PTSD.
Targeting insomnia prior to PE may offer a unique and underutilized opportunity to advance clinical care and research. Despite CBT-I being an effective treatment, insomnia treatment is rarely a first line treatment with individuals who have PTSD. There is a single case study that uses CBT-I prior to “trauma-specific exposure therapy” (Baddeley & Gros, 2013), and the authors suggest that CBT-I was an acceptable “stepping stone” before starting PTSD treatment. Another study examined hypnosis for insomnia treatment prior to CPT treatment. Galovski et al. (2016) randomized patients to either hypnosis or symptom monitoring prior to CPT. They found that both conditions showed improvement in sleep and PTSD, but the hypnosis condition showed significantly greater improvement than the control condition in sleep and depression, but not PTSD. Unfortunately, this paper offers limited insight into the relationship between insomnia treatment and PE trajectories as hypnosis shows limited evidence in the reduction of insomnia severity and is not considered a first-line treatment (Schutte-Rodin, Broch, Buysse, Dorsey, & Sateia, 2008). Additionally, the mechanisms of CPT are different from the mechanisms of PE (Gallagher & Resick, 2012). Together, further examination of CBT-I prior to PE is required.
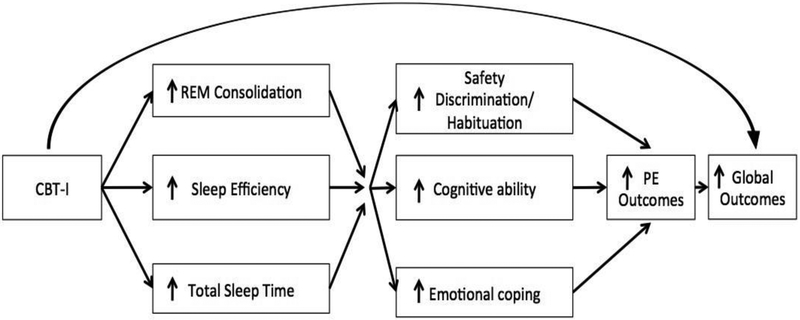
Based on the above rationale, we developed an integrated CBT-I and PE protocol (2NITE); a 15-session protocol designed to consolidate sleep, increase total sleep time (TST), and successfully complete PE (see Figure 1 for conceptual model). This intervention is based on evidence that untreated insomnia can persist for years, is independently associated with impaired health-related quality of life, can exacerbate daytime PTSD symptoms, and may interfere with the mechanisms of PE. The goals of this study were to describe the key elements of the integrated protocol for evidence-based CBT-I and PE, and to present pilot data from a sample of 12 treatment-seeking veterans with PTSD and insomnia who underwent the 2NITE protocol. In the pilot study, we hypothesized that veterans with PTSD and insomnia would successfully engage in the 2NITE protocol, report high satisfaction and acceptability of the intervention, and show reductions in insomnia and PTSD symptoms following the completion of the intervention.
Figure 1.
Conceptual Model of the 2NITE protocol.
Method
2NITE Intervention
The integrated intervention was designed to maximize the effects of consolidated sleep and SE using CBT-I (Perlis et al., 2010) before starting in-vivo and imaginal exposures of PE (Foa, Hembree, & Rothbaum, 2007), while minimizing the total number of sessions needed to complete treatment. As such, the start of treatment focuses on sleep restriction and stimulus control of CBT-I while exposure components of PE (in-vivos and imaginals) do not start until session 6. Evidence for integrated PTSD treatments and comorbid disorders is still in its infancy. However, the PTSD and substance use literature show that both clinicians and patients have reported a preference for integrated treatment (Najavits, Norman, Kivlahan, & Kosten, 2010).
Cognitive Behavioral Therapy for Insomnia.
The standard CBT-I protocol consists of 5–6 weekly 60-minutes sessions and includes: 1) psychoeducation about the underlying causes of insomnia; 2) sleep restriction, or limiting patients’ time in bed to more closely correspond to total sleep time; 3) stimulus control, or instructing patients to use bed for sleep only in order to strengthen the association between bed and sleep; 4) sleep hygiene, or providing additional suggestions for behavioral modifications to improve sleep, and 5) modification of dysfunctional thoughts about sleep.
Prolonged Exposure.
The standard PE protocol consists of 10–12 weekly 90-minute sessions and includes psychoeducation about the underlying cause of PTSD, common symptoms and reactions to trauma, and uses repeated exposures (in-vivos and imaginals) to activate the feared response to stress-provoking situations to allow for a corrective experience. With repeated exposures to the situation, the anticipatory, initial, and maximum levels of distress will decrease, and the integration of new beliefs and responses can be learned. Specifically, PE includes: 1) an overview of treatment, review PTSD symptoms, and breathing retraining; 2) the rationale for exposure therapy (i.e., anxiety is reduced by habituation to feared stimuli); 3) in-vivo exposures (in real life) to avoided people, places, and activities; 4) imaginal exposures where patients review the trauma memory repeatedly; and 5) the processing of imaginal exposures.
Integrated Treatment (2NITE protocol; see Figure 1 for conceptual model).
We developed an integrated treatment that overlaps CBT-I (Perlis et al., 2010) and PE (Foa et al., 2007). Integrating CBT-I and PE allows patients to address both symptoms of insomnia and PTSD within a shortened timeframe (i.e., fewer than the 18–20 weeks required if the treatments were offered sequentially). Integrated treatment is delivered in 15 90-minute weekly sessions. The 2NITE protocol starts with CBT-I for the first 2 weeks with an added focus on the effects of PTSD on sleep and vice versa. PE session 1 (psychoeducation) begins on week 3 of the 2NITE protocol and both treatments overlap until week 6 when CBT-I effectively ends and imaginal and in-vivo exposures begin. Sleep diary review and sleep time adjustment continues until the end of treatment. The final session includes reviewing treatment goals, discussing progress and skills to be used post-treatment, and discussing relapse prevention and handling reemergence of insomnia and PTSD symptoms.
We chose to stagger the starts of CBT-I and PE for two reasons. First, this should minimize client burden (e.g., doing sleep restriction of CBT-I and in-vivos of PE at the same time). Second, the staggered treatment allows 6 weeks for sleep to consolidate (a proposed mechanism for trauma-focused treatment) before starting PE imaginal exposures.
Participants
Fourteen veterans consented to take part in the study; twelve of whom were included in the analyses. Of the 14 veterans consented, one veteran never initiated treatment, and one was removed from the study before the start of PE due to unmanaged obstructive sleep apnea (OSA; veteran was referred to the Pulmonary Sleep Clinic). Of the 12 veterans included (mean age = 38.50 years old; SD = 10.10; range = 24 to 55), 83.3% were male, 16.7% Hispanic, 66.7% self-identified as white, 25% were African American, and 8.3% were Asian. Eight of the veterans were taking sleep or PTSD medications, of which 3 of the veterans were taking multiple medications. Medications included prazosin (n = 4), trazodone (n = 2), hydroxyzine pamoate (n = 2), selective serotonin reuptake inhibitors (n = 2), lorazepam (n = 1), mirtazapine (n = 1), gabapentin (n = 1), and melatonin (n = 1). All medications remained unchanged over the course of treatment. Inclusion criteria were diagnoses of insomnia and PTSD. Exclusion criteria were acute safety concerns (i.e., suicidality, homicidality, substance use disorder requiring acute treatment), or an unmanaged bipolar or psychotic disorder.
Procedures
Study procedures were approved by the local VA Institutional Review Board. Data were collected between September 2014 and September 2017 due to limited clinical availability of the clinician (PC). Veterans who indicated that they were struggling with insomnia and PTSD during a mental health intake or other meetings with a mental health provider at the VA hospital were offered participation in the 2NITE pilot study if clinical openings with the study clinician (PC) were available. Interested veterans completed consent procedures and baseline assessments. They then proceeded to meet with the study therapist weekly for approximately 15 sessions to complete the 2NITE protocol. Participants did not take part in any other form of psychotherapy during the 2NITE protocol; however, no restrictions were placed on pharmacotherapy. Upon completion of treatment, participants completed post-treatment measures. Of the 12 veterans, compensation was given to 9 subjects for participation due to access to grant money starting in October of 2016. These 9 veterans also wore actigraphy watches to track sleep at pre- and post- treatment. No differences between the two groups were found in insomnia symptoms, PTSD symptoms, or quality of life. Self-report measures were delivered at pre-treatment, week 5 (before the start of PE), and post-treatment. Actigraphy and quality of life measures were only given at pre- and post-treatment.
Measures
PTSD:
The PTSD Checklist (PCL-5; Weathers et al., 2013) is a 20-item self-report measure of PTSD symptoms with good psychometric properties. The PCL-5 maps directly onto DSM-5 diagnostic criteria. All analyses also examined a modified PCL-5 with the 2 sleep items removed. This is typically used to limit overlap between sleep and PTSD measures. The full PCL-5 showed strong internal consistency (α = .80).
Depression:
Patient Health Questionnaire (PHQ-9; Kroenke & Spitzer, 2002) is a 9 item measure based directly on the diagnostic criteria for major depressive disorder as outlined by the DSM and assesses the severity and frequency of mood symptoms. PHQ-9 showed strong internal consistency (α = .77).
Insomnia:
Insomnia Severity Index (ISI; Morin & Barlow, 1993) was used to measure insomnia with well-established reliability and validity. The ISI consists of 7 items, three of which assess severity of insomnia (i.e., degree of difficulty falling asleep, staying asleep, and waking too early). The remaining items query satisfaction with sleep pattern, effect of sleep on daytime and social functioning, and concern about current sleep difficulties. Scores range from 0–28; scores of 0–7 are considered “no clinical significance insomnia”, 8–14 are subthreshold, 15– 21 are moderate, and 22–28 are considered severe clinical insomnia. ISI showed strong internal consistency (α = .77).
Sleep diaries:
Patients completed a daily sleep diary throughout the course of treatment. The sleep diaries were used to assess subjective measures (bed time, wake time, sleep latency, number and duration of awakenings, and total time in bed) and two calculated variables (TST and SE).
Actigraphy Watch:
A subgroup of participants (n = 9) wore the Actiwatch 2 (Philips Respironics, Bend, OR). Participants wore the watch for 7 days at baseline and for another 7 days at post-treatment. The Actiwatch is a wrist-worn device that measures body movements and light. Actigraphy-measured SE and TST were used as the primary variables.
Satisfaction:
The Client Satisfaction Questionnaire (CSQ; Larsen, Attkisson, Hargreaves, & Nguyen, 1979) is an 8-item self-report scale measuring satisfaction with treatment. It has excellent internal consistency (α = .79) and correlates with therapists’ estimates of client satisfaction. This instrument was used to measure participants’ satisfaction with the interventions at post-treatment where higher score indicates higher satisfaction.
Quality of Life:
World Health Organization Quality of Life-BREF (WHOQOL-BREF; Skevington, Lotfy, & O’Connell, 2004) is a 26-item measure, which has a total score and measures the following broad domains: physical health, psychological health, social relationships, and environment. Due to the small N, only the total score was used. The total score showed strong internal consistency (α = .84).
Feasibility:
Study feasibility and engagement were assessed through number of sessions veterans attended and drop-out rates.
Analyses
Mean differences between pre- and post-treatment in PTSD, depression, insomnia, and sleep diary indices were examined with correlations, paired t-tests, and Cohen’s d for effect sizes correcting for the dependence between means (Morris & DeShon, 2002). All analyses were also run using the modified PCL-5 with the two sleep items removed. All analyses between the full PCL-5 and modified PCL-5 were the same. As such, we present data on the fully validated PCL- 5. Of the 12 veterans, two stopped treatment before the final session (one at session 6 and one at session 10); their last data was used as post-treatment data in intent-to-treat analyses (Brown et al., 2008).
Results
Feasibility, Engagement, and Satisfaction
Of the 12 participants who started treatment, two did not complete the entire protocol. One veteran stopped treatment before completing session 6 (prior to the start of PE) due to a new job with increased responsibilities and increased hours working. The other veteran stopped treatment at session 10. This veteran had a moderate traumatic brain injury (TBI) with cognitive difficulties that may have impacted her emotional processing, memory for assignments, and filling out her sleep diaries; she had a noticeably challenging time with their PTSD in-vivos, sleep diaries, and imaginals. On average, participants attended 12.83 sessions (SD = 3.01). Following treatment, satisfaction for the protocol, as indicated by the CSQ, was high with a mean score of 29.66 out of a possible 32 points (SD = 2.08).
Symptom Change
Table 1 presents means, standard deviations, t-tests, and effect sizes for pre- and post- treatment measure scores. Despite the small number of participants, there were statistical and clinically significant reductions in PTSD and insomnia symptoms from pre- to post-treatment. On average, there was a 20.17 point decrease in PCL-5 scores, where a 10 point decrease or more is considered clinically significant. There was an 11.75 point decrease in the ISI, where a 7 point decrease is considered clinically significant. Sleep diaries indicated a 14.97% increase in SE, and a 57.24 minute increase in TST. Actigraphy watches confirmed these findings with a 10.86% increase in SE and a 51.29 minute increase in TST. The strength of these findings are further supported by the large effect sizes. The PHQ-9 decreased by 6.5 points, but was not significant.
Table 1.
Means and standard deviations of key variables (N = 12)
| Variable | Pre M (SD) |
Week 6 M (SD) |
Post M (SD) |
Paired Sample t-test (Pre and Post) |
Effect Size |
|---|---|---|---|---|---|
| PCL-5 | 48.33 (10.99) | 41.33 (12.25) | 28.16 (17.16) | t(11) = 4.77, p = .001 | d = 1.48 *** |
| PCL-5 (no sleep) | 42.58 (10.29) | 37.75 (11.26) | 26.17 (15.85) | t(11) = 4.01, p = .002 | d = 1.26 ** |
| ISI | 19.03 (4.48) | 10.42 (5.62) | 7.33 (5.99) | t(11) = 8.01, p <.001 | d = 2.38*** |
| WHOQOL-BREF | 69.67 (9.18) | - - | 88.25 (13.43) | t(3) = −2.55, p = .08 | d = −1.35 |
| PHQ-9 | 13.33 (4.08) | - - | 6.80 (5.76) | t(4) = 1.87, p = .14 | d = 0.84 |
|
Sleep Diary (In Minutes) |
|||||
| Sleep Efficiency | 78.79% (7.02) | 88.35 (5.72) | 92.71% (4.23) | t(10) = −9.72, p < .001 | d = −3.15 *** |
| Total Sleep Time | 322.53 (52.89) | 329.02 (46.23) | 383.19 (53.17) | t(10) = −4.41, p = .001 | d = −1.34 *** |
| Sleep Latency | 36.78 (22.77) | 15.06 (7.44) | 14.34 (9.31) | t(10) = 3.04, p = .01 | d = 1.22 ** |
| Wake After Sleep Onset | 22.39 (13.52) | 16.63 (15.65) | 6.19 (5.42) | t(10) = 4.16, p = .002 | d = 1.48 ** |
| Morning Time in Bed | 23.41 (20.62) | 7.53 (8.39) | 8.14 (8.98) | t(10) = 3.87, p = .003 | d = 1.65 ** |
|
Actigraphy Watch (In Minutes; N = 9) |
|||||
| Sleep Efficiency | 74.67% (10.08) | - - | 84.57 (4.35) | t(6) = −3.05, p = .02 | d = −1.56 * |
| Total Sleep Time | 321.36 (62.36) | - - | 382.45 (33.71) | t(6) = −2.87, p = .03 | d = −1.40 * |
Note:
= p < 0.05;
= p < 0.01;
PCL-5 = The PTSD Checklist DSM-5; ISI = Insomnia Severity Index; PHQ-9 = Patient Health Questionnaire
We also examined the correlations between changes in PCL-5 and sleep indices pre- to post-treatment (see Table 2). Findings showed that correlations were moderate to high; larger decreases in PTSD symptoms from pre- to post-treatment were associated with larger changes in ISI scores, SE, and TST. Finally, we examined changes in PTSD and insomnia symptoms between baseline and week 6 (before the start of imaginal exposures). PCL-5 scores decreased by 7 points (SD = 7.42; t(11) = 3.27, p = 0.008) and ISI scores decreased by 8.67 points (SD = 6.05; t(11) = 4.96, p < 0.001). Sleep diaries showed SE increased by 9.57% (SD = 8.55; t(11) = 3.88, p = 0.003) while TST only increased by 6.47 minutes (SD = 40.62; t(11) = 3.27, p = 0.59).
Table 2.
Intercorrelations between Change in PTSD, Insomnia, and Sleep Diary Variables (N = 12)
| Variables | 1. | 2. | 3. | 4. |
|---|---|---|---|---|
| 1. PCL-5 Change | --- | |||
| 2. ISI Change | .71** | --- | ||
| 3. SE Change | −.74** | −.78** | --- | |
| 4. TST Change | −.62* | −.51 | .81** | --- |
| 5. WHOQOL Change | −.98* | −.84 | .94 | .35 |
Note: Changes scores = post-score minus pre-score;
= p < 0.05;
PCL-5 = The PTSD Checklist DSM-5; ISI = Insomnia Severity Index; SE = Sleep efficiency; TST = Total sleep time
Discussion
Results of this study showed that the 2NITE protocol was feasible, had high engagement, and showed promise for decreasing insomnia and PTSD symptoms while increasing quality of life. Satisfaction with the intervention was high, and participants showed statistically and clinically meaningful reductions in PTSD and insomnia symptoms. Additionally, both the sleep diary and actigraphy watch data showed increases in SE and TST. This finding is in contrast to PTSD treatment studies which generally only showed significant decreases in PTSD symptoms (Colvonen et al., in press). Interestingly, our study showed significant increases in TST from baseline to the end of treatment where many CBT-I studies only showed increases in SE, and not TST, in the 5–6 week protocols (Morin et al., 2006). This suggests that tracking sleep for another 8–10 weeks with the 2NITE protocol may allow further opportunity to reinforce positive sleep/wake habits and titrate wake and sleep times using the sleep diary. Integrated CBT-I and PE treatment with the 2NITE protocol represents a logical, innovative, and empirically-informed method for augmenting existing treatments for individuals with PTSD. However, due to the small sample size and lack of control group, modest interpretation of the results is warranted, and further research is needed.
Results showed that by week 6, before the start of PE, SE increased and PTSD symptom severity decreased. This suggests that the staggered start of the two treatments was effective and allowed for consolidated sleep before the start of PE. This also indicates that increasing SE may have a positive effect on daytime PTSD symptoms even prior to initiating core components of PE. While the literature suggests that for many individuals, addressing PTSD does not decrease insomnia (Belleville et al., 2011; Gutner et al., 2013), it is possible that addressing insomnia first may help alleviate symptoms of PTSD (Talbot et al., 2014; Taylor & Pruiksma, 2014). These findings should be interpreted cautiously as psychoeducation of PTSD and a therapeutic alliance may also account for decreases in PTSD.
Our study showed high correlations between changes in PTSD symptoms and changes in insomnia symptoms from pre- to post-treatment that could suggest an influential relationship between the sleep and PTSD treatments. It is possible that the decreases in sleep symptoms prior to the start of PTSD treatment helped make PE more effective. Again, this interpretation needs further study. Another possibility is that insomnia and PTSD symptoms decrease together with the administration of two effective treatments (CBT-I and PE) and the two treatments may not influence each other. However, having both insomnia and PTSD decrease together may translate to higher quality of life than addressing either single disorder. A final possibility, albeit unlikely, is that the two treatments interfere with each other, lowering the effectiveness of each treatment. Future studies can help clarify the directionality and relationship between changes in insomnia and PTSD symptoms.
We used an iterative process of manual development that allowed us to make necessary changes and suggestions for future studies. One major shift to our protocol had to do with screening for OSA. We recommend screening for and addressing OSA prior to the start of treatment as CBT-I and PE would be compromised with severe apnea and hypopnea events (Goldstein, Colvonen, & Sarmiento, 2017). This recommendation should be given heightened consideration in veteran samples with PTSD who have been shown to screen as high-risk for OSA at much higher rates than those seen in community studies and may not show all classic predictors of OSA (i.e., older age and higher BMI; Colvonen et al., 2015).
Limitations of this study include the small sample, the lack of a control condition, and lack of follow-up data. Given our small sample size, replication studies will be essential to further elucidate the potential efficacy of the 2NITE protocol as well as theoretical mechanisms of change (e.g., fear conditioning, extinction, concentration, memory). Additionally, we were unable to control for medications use or medication adherence that could potentially influence insomnia and PTSD. Our study used the self-report PCL-5 for PTSD symptoms; future research would benefit from using the Clinician Administered PTSD Scale (CAPS). Given these limitations, this study is best viewed as a theory-based manual development pilot study with positive initial results.
Based on the empirical relationship between insomnia and PTSD, as well as the promising pilot data, future research is warranted. Future directions could include, first, a randomized control trial to examine if and how CBT-I before PE compares to PE alone. Second, studies should examine global functioning to see if addressing sleep and PTSD is more beneficial than just addressing a single disorder. Third, studies should examine the mechanisms through which sleep treatment may affect PE outcomes (i.e., extinction/safety learning, emotional processing). Fourth, studies could include other sleep interventions that help decrease fragmented sleep (e.g., Imagery Rehearsal Therapy or Exposure, Rescripting, and Relaxation Therapy). Finally, studies should include prospective follow-up assessments beyond post- treatment to examine the stability of treatment gains.
Among individuals with insomnia and PTSD, the 2NITE protocol represents a logical, innovative, and empirically-informed method for augmenting existing treatments and optimizing outcomes. The preliminary findings of the 2NITE protocol demonstrate feasibility and showed positive outcomes on insomnia symptoms, PTSD symptoms, and quality of life. Integrating CBT-I and PE justifies further investigation and may be useful among anyone presenting with PTSD and insomnia.
Clinical Impact:
Integrating insomnia and PTSD treatment may increase client-centered care and treatment outcomes beyond CBT-I or PE alone. Among individuals with insomnia and PTSD, integrating CBT-I treatment with PE represents a logical, innovative, and empirically-informed method for augmenting existing treatments and optimizing outcomes. The preliminary findings of the integrated protocol demonstrated feasibility and showed positive outcomes on insomnia symptoms, PTSD symptoms, and quality of life. Integrating CBT-I and PE justifies further investigation and may be useful among anyone presenting with PTSD and insomnia.
Acknowledgments:
This study was funded by VA RR&D CDA Grant #1lK2Rx002120–01 to Peter Colvonen. This project was also supported by a VA Center of Excellence for Stress and Mental Health (CESAMH) fellowship. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
References
- Baddeley JL, & Gros DF (2013). Cognitive behavioral therapy for insomnia as a preparatory treatment for exposure therapy for posttraumatic stress disorder. American journal of psychotherapy, 67(2), 199–210. [DOI] [PubMed] [Google Scholar]
- Belleville G, Guay S, & Marchand A (2011). Persistence of sleep disturbances following cognitive-behavior therapy for posttraumatic stress disorder. Journal of Psychosomatic Research, 70(4), 318–327. [DOI] [PubMed] [Google Scholar]
- Bootzin RR, Epstein D, & Wood JM (1991). Stimulus control instructions Case studies in insomnia (pp. 19–28): Springer. [Google Scholar]
- Brown CH, Wang W, Kellam SG, Muthén BO, Petras H, Toyinbo P, . . . Chamberlain P (2008). Methods for testing theory and evaluating impact in randomized field trials: Intent- to-treat analyses for integrating the perspectives of person, place, and time. Drug & Alcohol Dependence, 95, S74–S104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Creamer M, O’Donnell M, Silove D, & McFarlane AC (2010). Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep, 33(1), 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervena K, Dauvilliers Y, Espa F, Touchon J, Matousek M, Billiard M, & Besset A (2004). Effect of cognitive behavioural therapy for insomnia on sleep architecture and sleep EEG power spectra in psychophysiological insomnia. Journal of Sleep Research, 13(4), 385–393. [DOI] [PubMed] [Google Scholar]
- Colvonen PJ, Masino T, Drummond SP, Myers US, Angkaw AC, & Norman SB (2015). Obstructive Sleep Apnea and Posttraumatic Stress Disorder among OEF/OIF/OND Veterans. Journal of clinical sleep medicine, 11(5), 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvonen PJ, Straus LD, Stepnowsky C, Goldstein LA, McCarthy M, & Norman SB (in press). Recent Advancements in Treating Sleep Disorders in Co-Occurring PTSD Current psychiatry reports. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E, Hembree E, & Rothbaum BO (2007). Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences therapist guide: Oxford University Press. [Google Scholar]
- Gallagher MW, & Resick PA (2012). Mechanisms of change in cognitive processing therapy and prolonged exposure therapy for PTSD: Preliminary evidence for the differential effects of hopelessness and habituation. Cognitive therapy and research, 36(6), 750–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galovski TE, Harik JM, Blain LM, Elwood L, Gloth C, & Fletcher TD (2016). Augmenting cognitive processing therapy to improve sleep impairment in PTSD: A randomized controlled trial. Journal of consulting and clinical psychology, 84(2), 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, Buysse DJ, & Nofzinger E (2008). Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep medicine reviews, 12(3), 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, McKeon AB, & Campbell RL (2017). Sleep in PTSD: Conceptual model and novel directions in brain-based research and interventions. Current opinion in psychology, 14, 84–89. [DOI] [PubMed] [Google Scholar]
- Goldstein LA, Colvonen PJ, & Sarmiento KF (2017). Advancing treatment of comorbid PTSD and OSA. Journal of clinical sleep medicine, 13(6), 843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutner CA, Casement MD, Gilbert KS, & Resick PA (2013). Change in sleep symptoms across Cognitive Processing Therapy and Prolonged Exposure: A longitudinal perspective. Behaviour research and therapy, 51(12), 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG (2002). A cognitive model of insomnia. Behaviour research and therapy, 40(8), 869–893. [DOI] [PubMed] [Google Scholar]
- Kroenke K, & Spitzer RL (2002). The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann, 32(9), 1–7. [Google Scholar]
- Larsen DL, Attkisson CC, Hargreaves WA, & Nguyen TD (1979). Assessment of client/patient satisfaction: development of a general scale. Evaluation and program planning, 2(3), 197–207. [DOI] [PubMed] [Google Scholar]
- Marshall AJ, Acheson DT, Risbrough VB, Straus LD, & Drummond SP (2014). Fear conditioning, safety learning, and sleep in humans. Journal of Neuroscience, 34(35), 11754–11760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martindale SL, Morissette SB, Rowland JA, & Dolan SL (2017). Sleep quality affects cognitive functioning in returning combat veterans beyond combat exposure, PTSD, and mild TBI history. Neuropsychology, 31(1), 93. [DOI] [PubMed] [Google Scholar]
- Morgan JK, Hourani L, Tueller S, Strange L, Lane ME, & Lewis GF (2017). Effects of Sleep Issues on Suicidal Ideation in a Military Sample: The Mediating Role of Mental Health. Military Behavioral Health, 1–9. [Google Scholar]
- Morin CM, & Barlow DH (1993). Insomnia: Psychological assessment and management: Guilford Press; New York. [Google Scholar]
- Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, & Lichstein KL (2006). Psychological and behavioral treatment of insomnia: update of the recent evidence (1998– 2004). Sleep, 29(11), 1398–1414. [DOI] [PubMed] [Google Scholar]
- Morin CM, Rodrigue S, & Ivers H (2003). Role of stress, arousal, and coping skills in primary insomnia. Psychosomatic medicine, 65(2), 259–267. [DOI] [PubMed] [Google Scholar]
- Morris SB, & DeShon RP (2002). Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychological methods, 7(1), 105. [DOI] [PubMed] [Google Scholar]
- Najavits LM, Norman SB, Kivlahan D, & Kosten TR (2010). Improving PTSD/substance abuse treatment in the VA: A survey of providers. The American Journal on Addictions, 19(3), 257–263. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Germain A, & Milad MR (2015). Effects of sleep on memory for conditioned fear and fear extinction. Psychological bulletin, 141(4), 835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Smith MT, Jungquist C, Nowakowski S, Orff H, & Soeffing J (2010). Cognitive-behavioral therapy for insomnia Clinical Handbook of Insomnia (pp. 281–296): Springer. [Google Scholar]
- Peterson AL, Goodie JL, Satterfield WA, & Brim WL (2008). Sleep disturbance during military deployment. Military medicine, 173(3), 230–235. [DOI] [PubMed] [Google Scholar]
- Pruiksma KE, Taylor DJ, Wachen JS, Mintz J, Young-McCaughan S, Peterson AL, . . . Litz BT. (2016). Residual sleep disturbances following PTSD treatment in active duty military personnel. Psychological Trauma: Theory, Research, Practice, and Policy, 8(6), 697. [DOI] [PubMed] [Google Scholar]
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, & Sateia M (2008). Clinical guideline for the evaluation and management of chronic insomnia in adults. Journal of clinical sleep medicine, 4(5), 487. [PMC free article] [PubMed] [Google Scholar]
- Skevington SM, Lotfy M, & O’Connell KA (2004). The World Health Organization’s WHOQOL-BREF quality of life assessment: psychometric properties and results of the international field trial. A report from the WHOQOL group. Quality of life Research, 13(2), 299–310. [DOI] [PubMed] [Google Scholar]
- Spoormaker VI, Schröter MS, Andrade KC, Dresler M, Kiem SA, Goya‐Maldonado R, . . . Czisch M (2012). Effects of rapid eye movement sleep deprivation on fear extinction recall and prediction error signaling. Human brain mapping, 33(10), 2362–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot LS, Maguen S, Metzler TJ, Schmitz M, McCaslin SE, Richards A, & Neylan TC (2014). Cognitive behavioral therapy for insomnia in posttraumatic stress disorder: a randomized controlled trial. Sleep, 37(2), 327–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DJ, & Pruiksma KE (2014). Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. International review of psychiatry, 26(2), 205–213. [DOI] [PubMed] [Google Scholar]
- Veterans Affairs/Department of Defense (2016). VA/DOD clinical practice guideline for management of post-traumatic stress. Washington (DC): Department of Veterans Affairs, Department of Defense. [Google Scholar]
- Walker MP, & van Der Helm E (2009). Overnight therapy? The role of sleep in emotional brain processing. Psychological bulletin, 135(5), 731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Blake DD, Schnurr PP, Kaloupek DG, Marx BP, & Keane TM (2013). The clinician-administered PTSD scale for DSM-5 (CAPS-5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Nutt D, Alford C, Argyropoulos S, Baldwin DS, Bateson A, . . . Espie C (2010). British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. Journal of Psychopharmacology, 24(11), 1577–1601. [DOI] [PubMed] [Google Scholar]
- Wright KM, Britt TW, Bliese PD, Adler AB, Picchioni D, & Moore D (2011). Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. Journal of clinical psychology, 67(12), 1240–1258. [DOI] [PubMed] [Google Scholar]