Summary
Stem cells are unique cell populations able to copy themselves exactly as well as specialize into new cell types. Stem cells isolated from early stages of embryo development are pluripotent, i.e., can be differentiated into multiple different cell types. In addition, scientists have found a way of reverting specialized cells from an adult into an embryonic‐like state. These cells, that are as effective as cells isolated from early embryos, are termed induced pluripotent stem cells (iPSCs). The potency of iPSC technology is recently being employed by researchers aimed at helping wildlife and environmental conservation efforts. Ambitious attempts using iPSCs are being made to preserve endangered animals as well as reanimate extinct species, merging science fiction with reality. Other research to sustain natural resources and promote animal welfare are exploring iPSCs for laboratory grown animal products without harm to animals offering unorthodox options for creating meat, leather, and fur. There is great potential in iPSC technology and what can be achieved in consumerism, animal welfare, and environmental protection and conservation. Here, we discuss current research in the field of iPSCs and how these research groups are attempting to achieve their goals. Stem Cells Translational Medicine 2019;8:7–13
Significance Statement.
This article focuses on research utilizing a specific pluripotent stem cell type, known as induced pluripotent stem cells (iPSCs), in wildlife conservation and animal welfare, summarizing past and future avenues, and discussing its benefits and limitations.
Introduction
Reprogramming of adult somatic cells into induced pluripotent stem cells (iPSCs) has massive potential to revolutionize personalized medicine, drug discovery, and cell therapy, but this represents only a portion of iPSC technology's capabilities. iPSCs also have great promise to aid endangered animal species and native habitat preservation 1, revive several extinct species 2, and reduce consumer dependence on animal products 3. Similar to embryonic stem cells (ESCs), iPSCs can expand indefinitely and are capable of differentiating into all three germ layers 4, 5. However, unlike ESCs, iPSCs do not require embryonic tissues or oocytes for harvesting. For endangered species, the supply of embryos is often restricted and iPSCs from somatic tissue offer a more practical source of stem cells with less moral and ethical dilemmas. This also offers significant advantages for using iPSCs from domestic animals to reduce animal death for commercially produced animal products. Domestic farm animals are a large drain on natural recourses, requiring deforestation for pasture, significant water consumption, and produce considerable greenhouse gas emissions. Alternative animal products derived from iPSCs have the potential to reduce the environmental damage caused by large‐scale farming and present eco‐friendly commercial applications. Here, we discuss past and current research utilizing iPSCs and how they could benefit wildlife conservation and animal welfare as well as their limitations and future avenues for iPSC technology.
iPSC Lines for Revival of Endangered or Extinct Wild Animal Species
The ability to readily make iPSC lines from adult tissue raises the possibility of adding a critically important safety net in the preservation of endangered animal species. However, iPSCs must prove they can aid in animal assisted reproductive technologies (ART), where gametes can be developed in vitro. The first reported example of fully functional animals derived from iPSCs was in mice using the tetraploid complementation assay 6, 7, where iPSCs are injected into an in vitro cultured tetraploid blastocyst and transferred to a surrogate female for gestation 8. Since then, iPSC lines have been derived from an extremely diverse group of wild species including birds, primates, bovids, and large cats, as seen in greater detail in Table 1. The majority of the iPSC lines were created using Yamanaka reprogramming factors (OCT4, SOX2, KLF4, and c‐MYC) and almost all these cell lines have been reported to be derived from samples taken from adult tissue. Some of the samples used in this research were made possible through use of the San Diego Zoo Institute of Conservation Research's Frozen Zoo center, which currently has over 10,000 samples from approximately 1,000 species in a reference bank known as Frozen ZOO that began collecting samples in 1976 9. Another reference base, The Genome 10K project also provides vital data for animal genome sequences that will aid in using iPSCs for animal conservation 10. Even with this data, the mechanisms that control the generation of fully pluripotent iPSCs are still poorly understood. Difficulties in deriving iPSCs from large animals may be due to an inability to produce consistent transgene‐free iPSCs, without sustained expression of transcription factors for self‐renewal 11. More research into species specific reprogramming factors could provide missing biological data for creating iPSCs and generating viable offspring from endangered animals.
Table 1.
Timeline of advancements for iPSCs derived from domestic and wild animal species
| Year | Event | Citation |
|---|---|---|
| 1976 | Frozen ZOO | 9 |
| 2006 | Derived first iPSC line | 4 |
| 2008 | Derived iPSC from rhesus macaque (Macaca mulatta, 0235#) | 17 |
| 2009 | Initiation of the Genome 10K Project | 10 |
| 2009 | Adult mice generated from iPSCs | 6, 7 |
| 2011, 2012 | Functional mouse gametes, in vitro | 13, 14 |
| 2011 | Derived iPSCs from drill (Mandrillus leucophaeus) | 1 |
| 2011 | Derived iPSCs from bovine | 18 |
| 2011 | Derived iPSC from equine fibroblasts | 19 |
| 2012 | Derived iPSC from water buffalo (Bubalus bubalis) | 20 |
| 2012 | Derived iPSC from snow leopard (Panthera uncia) | 21 |
| 2012 | Derived iPSC from quail | 22 |
| 2013 | Piglets generated from iPSCs | 12 |
| 2013 | Derived iPSC from Bengal tiger (Panthera tigris), serval (Leptailurus serval), and jaguar (Panthera onca) | 23 |
| 2015 | Derived iPSC from orangutan (Pongo abelii) | 24 |
| 2015 | “Conservation by Cellular Technologies” meeting to discuss the potential for iPSCs to preserve the nearly extinct northern white rhinoceros (Ceratotherium simum cottoni) | 25 |
| 2015 | Report of editing Asian elephant cells to contain gene sequences of the Woolly mammoth (Mammuthus primigenius) | 26 |
| 2016 | Functional mouse oocytes from iPSCs, ex vivo. | 15 |
| 2018 | Development of hybrid northern white rhinoceros (Ceratotherium simum cottoni) and southern white rhinoceros (Ceratotherium simum simum) embryos in vitro with potential for implantation in surrogate | 16 |
The next successful attempt at creating live animals from iPSCs was achieved in 2013 with viable piglets 12. These piglets were achieved using iPSCs as nuclei donors for nuclear transfer into an enucleated donor egg (somatic cell nuclear transfer). It was reported that viable offspring were created from both the tetraploid complementation and intracellular nuclear injection methods. However, these methods have not yet transferred to any endangered species even though they have successfully derived iPSCs (Table 1). It should be noted that donor embryos or eggs are likely to be unavailable when working with endangered, extinct, or nearly extinct species. Therefore, the only way forward would be creation of embryos from iPSC‐derived gametes. Fully functional spermatozoids and oocytes derived from iPSCs have been reported in mice but required injection of these immature gametes into an adult mouse testis or ovary to become fully functional 13, 14. Generation of fully functional oocytes from mouse iPSCs was reported again in 2016, but this was achieved through ex vivo coculture with female gonadal somatic cells 15.
In a more recent development, ESCs and embryos have been created from the critically endangered northern white rhinoceros (NWR, Ceratotherium simum cottoni) 16. This represents a meaningful step in bridging ESCs and iPSCs research in domestic and laboratory animals to assist with endangered species preservation. In March 2018, the last male NWR died in captivity, leaving only two remaining females for the species and they are both infertile. Hildebrandt et al. recovered oocytes from the southern white rhinoceros (SWR, Ceratotherium simum simum), a related subspecies of the NWR and not currently endangered. The oocytes were matured in vitro, fertilized with intracytoplasmic sperm injection using NWR sperm, and allowed to develop into a blastocyst stage. The resulting SWR–NWR hybrid embryos have the potential to be implanted into a female SWR and carried to term, preserving NWR genes through ART. The next step is to attempt to develop artificially generated NWR oocytes from cryopreserved NWR somatic tissue using iPSCs. With such a limited supply of NWR genetic material, iPSCs offer the ability to create genetic diversity and increase the population size of a critically endangered species 1.
In addition to increasing populations of endangered species, there is current research to revive extinct species in the field of deextinction or resurrection biology. The southern gastric brooding frog (Rheobatrachus), the dodo (Raphus cucullatus), the Tasmanian tiger (Thylacinus cynocephalus), and the passenger pigeon (Ectopistes migratorius) are just a few of the candidates being discussed among the Australian scientists from the Lazarus project 27. However, since a well‐preserved woolly mammoth calf (Mammuthus primigenius) was discovered in Siberia in 2007 26, 28, 29, the majority of media attention and notoriety has focused on mammoth research. George Church and colleagues have stated they are editing Asian elephant genes to include gene sequences from the woolly mammoth 30. Utilizing Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)‐Cas9 genome‐editing technology, Church's team replaced 14 loci in the elephant genome with the mammoth version of those sequences with the goal of creating an elephant–mammoth hybrid that would be able to survive to colder climates. Genome editing has numerous applications in editing living organisms with extinct animal sequences, but extensive knowledge of the genetic differences between the two species is needed. Comparisons between Asian elephants and woolly mammoths found 1.5 million nucleotide differences 28, making it difficult to gauge if an elephant–mammoth hybrid would be more similar to one animal or the other in physicality or behavioral traits. Analogous to the NWR, to create a viable mammoth embryo, the potency of iPSCs could create gametes from woolly mammoth tissue to be implanted into a female Asian elephant surrogate. Combined with genetic engineering with similar subspecies, iPSCs have great potential for resurrecting extinct animals and allow scientists to create this diversity through other targeted insertion or deletions events 31, 32.
Future Prospects and Challenges of iPSCS for Wildlife Conservation
With the rapid acescent of ESCs and iPSC technology, it can be hypothesized that the iPSCs research will develop fully functioning, mature gametes without the use of extraneous tissue for other endangered or extinct species to form viable embryos. Nevertheless, despite the optimism for iPSC technology, there are practical and ethical obstacles to overcome before endangered or extinct animals can be generated in lab. The majority of animal reproductive research using stem cells has only a 5%–13% efficacy rate for viable animals 8, and the mechanisms that control the generation of fully pluripotent iPSCs have not been sufficiently investigated. More research into understanding iPSC reprogramming factors needs to be explored in order to aid in wildlife preservation and restoration. The main goal of conservation is to prevent an extinction event for any animal, and genetic engineering and iPSCs have the potential to reverse what was once thought to be irreparable damage. However, animals developed through these processes, (endangered, extinct, or hybrids) will be raised in captivity which may make these species ineligible for release into the wild. It is also unclear once a species is resurrected if its behavior will help or harm its previous native habitat or help reestablish previous ecosystem balance. Reintroduction of endangered animals into their original habitats has had success stories for ecosystem recovery, including the reintroduction of gray wolves (Canis lupus) to Yellowstone National Park through the U.S. Endangered Species Act of 1973. Bringing back wolves into the park reduced the excess elk population and consequently increased the populations of various trees and plants that the elk fed upon. Reintroduction of wolves also brought surges in population growth of other endangered species in the park including the beaver (Caster canadensis) and bison (Bison bison) 33. However, some efforts to restore animal populations hurt the species more than help. In July 2018, wildlife workers in Kenya tried relocating 11 black rhinoceroses (Diceros bicornis) to Tsavo East National Park to help restore their population, but 10 died due to contaminated drinking water at their new location 34. These events indicate ongoing work is needed, to ensure after species resurrection, that the newly revived species will be able to exist and live a healthy life outside of captivity and survive in a new habitat.
Cellular Agriculture and iPSC—Laboratory Research to Commercial Aspects
Due to human expansion, mass extinctions of other species have become common; current research suggests that the planet is entering a sixth “mass extinction” event with a dozen of animal and plant species lost daily 35, 36. In spite of conservation efforts, large‐scale land clearing for livestock, over‐fishing, and environmentally harmful farming practices continue to reduce and irreparably damage native habitats and species. Animal welfare is a priority but equally as important is reducing the need for livestock animals and the land resources they require. Reports from the United Nations Food and Agriculture Organization (FAO) have shown that livestock is the world's largest user of land resources, with 80% of all agricultural land dedicated to their development and feeding 37, leading to subsequent increased deforestation and greenhouse gas emissions 38. Additionally, to sustain large quantities of farm animals in enclosed environments, livestock are often given significant antibiotics and growth hormones generating antibiotic resistance in consumers 39. The knowledge of detrimental effects of industrial farming on the environment and consumer health has created a desire for more environmentally sustainable animal products. To reduce the impact of industrial farming and deforestation, the scientific community has established a new field of stem cell research deemed, cellular agriculture. Cellular agriculture seeks to create animal‐based products in the lab, such as meat, eggs, leather, or fur without harming or killing live animals while simultaneously reducing land resources for farming. Stem cells from the animals are collected using a small biopsy, multiplied in the lab, and then engineered to imitate the desired animal products. Large numbers of domestic animals have already had protocols for their respective iPSC lineage derived (Table 1), making it an advantageous moment to explore laboratory grown animal products. Cellular agriculture offers a more environmentally friendly alternative to farming, and iPSC technology presents an exciting opportunity to create animal products at industrial scale 40.
The first major cellular agriculture development occurred with Mark Post's group in 2013, where he and his team showed the world that lab grown meat was feasible 41. After thousands of muscle fibers were grown over three months, Post and his colleagues cooked and ate the cells in front of an audience in London, U.K., showcasing the world's first lab grown burger. It was a pivotal moment in laboratory food development and received heavy media attention. However, a major caveat included the large $325,000 sum to grow the burger. The single 85 g burger was composed of cultured bovine muscle strips that required significant maintenance and biological resources, therefore increasing the fabrication cost. Even with the financial burden, the design of a laboratory burger spurred multiple commercial companies to try growing meat products in the laboratory (Table 2).
Table 2.
Companies working on meat production based on cellular agriculture
| Company | Country | Founded | Website | Technology |
|---|---|---|---|---|
| Aleph Farms | Israel | 2017 | www.aleph-farms.com | iPSC? |
| Appleton Meats | BC, Canada | 2018? | www.appletonmeats.com | Bovine cell culture? |
| Balletic Foods | CA, US | 2017 | www.balleticfoods.com | Stem cells from muscle tissue |
| BlueNalu | CA, US | 2018 | www.bluenalu.com | Marine animal cells |
| Finless Foods | CA, US | 2017 | https://finlessfoods.com | Marine animal cells |
| Future Meat | Israel | 2018 | www.future‐meat.com | Stem cell culture |
| HigherSteaks | UK | 2017 | www.highersteaks.com | iPSC |
| Integriculture/Shojinmeat | Japan | 2014 |
http://integriculture.jp/?lang=en
www.shojinmeat.com |
Meat from cultured muscle cells |
| Just | CA, US | 2011 | https://justforall.com | Animal cell culture |
| Kiran Meats | CA, US | 2018 | www.kiranmeats.com | Animal cell culture |
| Mission Barn | CA, US | 2018 | Not available | Animal cell culture |
| Modern Meadow* | NJ, US | 2011 | www.modernmeadow.com | Meat from cultured muscle cells |
| Mosa Meat | The Netherlands | 2013 | www.mosameat.com | Stem cells from muscle tissue |
| Memphis Meats | CA, US | 2015 | www.memphismeats.com | Animal cell culture |
| New Age Meats | CA, US | 2018 | http://newagemeats.com/ | Pork from cultured cells |
| Seafuture | AL, Canada | 2017 | http://seafuturebio.com/ | Stem cells from fish muscles |
| Supermeat | Israel | 2015 | www.supermeat.com | Chicken stem cells |
| Wild Type | CA, US | 2016 | www.thewildtype.com | Animal cell culture |
In period 2011–2016, only seven companies worked in the field. In the last 2 years, however, trend becomes more obvious. Five new companies have entered the field in 2017. In 2018, only in the first 7 months, another six were established. Among 17 companies (in meantime, 1 “*” abandoned meat production program), 9 are in the United States and all of them in California. From those nine, eight are in San Francisco Bay Area.
Other meats, such as pork have also been investigated. Using iPSC‐based technology, Genovese et al. published a method for efficient derivation of skeletal muscle from porcine iPSCs in culture that has the potential to be used for meat; however, the cells still required animal products for proliferation, so the final product was not entirely “animal‐free” 42. It is a significant challenge to culture iPSCs without the use of any animal products in the media or on the culture substrate. Most in vitro cell culture protocols require animal products like fetal bovine serum (FBS) or surface bound extracellular matrix meshwork to have healthy cell proliferation. Currently, the regulatory agencies such as the U.S. Food and Drug Administration or European Medicines Agency require that all cells cultured for cellular therapy are grown under animal product‐free conditions and these regulations could also apply to future iPSCs meats or products for consumers. Some recent cell culture methods have developed xeno‐free and feeder‐free methods of stem cell culture, reducing or completely eliminating animal products from their protocols to comply with future regulatory restrictions as well as have improved quality control processes 43, 44. An advantage of having highly standardized media will not only be the elimination of animal proteins but also the elimination of hormones and antibiotics that are given to farm animals that can be transferred to consumers. The final laboratory meat product would offer a competitive alternative to traditional meat producers. The U.S. Cattlemen's Association has taken notice and in February 2018 filed a petition with the U.S. Department of Agriculture to prevent laboratory meat product from being labeled with the words “beef” or “meat,” stating it might misinform consumers about their food 45, however research labs continue to work on building and marketing their products as comparable animal product alternatives. The petition will create disputes for when these products inevitably go to market and may initiate regulations on how iPSCs will be labeled for consumption.
Another challenge for cellular agriculture is to address the high cost of its fabricated products. At ~$40,000 per kg, laboratory meat is currently not financially available for most consumers. Improved methods of mass cell culture, in particular iPSCs, need to be engineered to reduce the cost. Bioreactors, where cells can be grown in suspension instead of on two‐dimensional (2D) plates, provide an environment to culture cells with higher efficiency producing billions of iPSCs and their derivatives in only a few days. Shafa et al. had successful proliferation of mouse iPSCs in stirred bioreactors 46, and Abecasis et al. demonstrated human iPSCs scale up in bioreactors using xeno‐free media 47. To imitate meat like ground beef or pork, animal iPSCs from a bioreactor could be collected and mixed together to mimic the real processed meat product substantially lowering the cost compared to traditional cell culture methods.
For the formation of highly ordered complex tissue, such as skin, fur, or meat fillets, the collected cells must also be integrated into a scaffold with defined porosity and vascularization system. Fabrication of dense tissue with micro vascularization for nutrient exchange is exceedingly difficult to build, but there has been significant progress in the field of three‐dimensional (3D) bioprinting to overcome this issue. Bioprinting uses living cells suspended in a hydrogel bioink that can be extruded or polymerized into a complex 3D structure with assistance of computer generated models 48. Ma et al. were successful at printing 3D microscale hexagonal gelatin constructs for human iPSCs with improved phenotypic and functional enhancements when compared to cells in 2D cultures 49. Artificial skin constructs, also created using human iPSCs, were designed by Abaci et al. using a printed vasculature within an alginate hydrogel 50. Skin and fur derived from animal iPSCs would offer a suitable leather and fur alternative, particularly for exotic animals such as crocodiles farmed for their pelts. Common leather types, such as bovine or porcine, are collected from domestic animals that are bred principally for meat, but iPSC‐derived sources of this animal leather offer a first‐step for consumers and industry to move away from industrial farming. As meat, leather, fur, and other animal derived products move closer to being released for market, public awareness to animal and environmental welfare becomes greater, creating more incentive for research in the cellular agriculture field and greater demand for more environmentally sustainable products.
Future Directions
Looking forward, iPSC technology will continue to play a major role not only in the advancement of medical sciences, but also improving animal conservation and environmental protection. iPSCs, gene editing, and “biobanks” with tissues of all living and some extinct animals will allow us to save current and future endangered species through increasing genetic diversities, or in worst case scenarios, de‐extinction events. Although this type of research awaits funding, we should keep in mind that the iPSC technology might have much broader potential. Cellular agriculture from iPSCs of pig, cow, and other domestic animals will create clean meat and reduce the impact on the environment caused by commercial‐scale animal husbandry 51. We can also imagine using iPSC technology to manufacture and commercialize exotic animal products without killing animals. For example, high‐quality ivory generated in vitro and made available commercially could compete with black market and would decrease poaching, indirectly protecting the species from extinction. There are boundless opportunities to use iPSCs to protect the environment. The future potentials of iPSC technology and what we can achieve in environmental protection and conversation are directed by our own creativity and ambition (Fig. 1).
Figure 1.
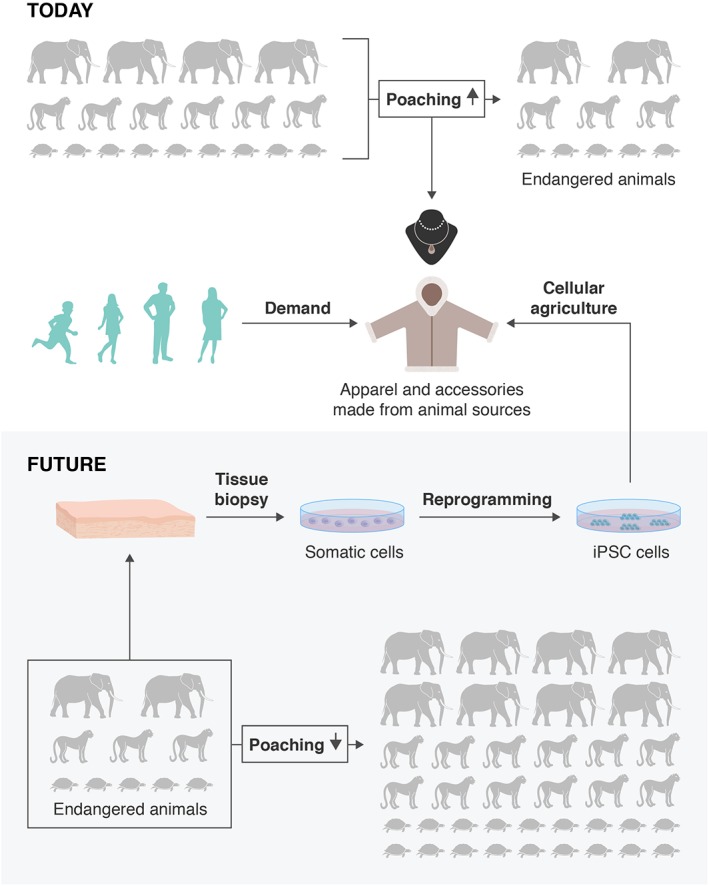
Schematic drawing showing how a novel use of iPSC technology for commercial purpose might be indirectly protecting the species of going toward extinction.
Author Contributions
M.M.S.: conception and design, manuscript writing, final approval of manuscript; E.T., M.D., and I.H.: manuscript writing, administrative support; N.K.: collection and/or assembly of the data.
Disclosure of Potential Conflict of Interest
M.M.S., E.T., M.D., I.H, and D.I. are associated with VitroLabs, Inc., a company built on the standards of the 3Rs and principles of animal welfare.
Acknowledgments
The authors thank Prof Caroline Ogilvie, King's College London, for useful comments and editing.
Contributor Information
Morgan M. Stanton, Email: morgan@vitrolabsinc.com
Dusko Ilic, Email: dusko.ilic@kcl.ac.uk.
References
- 1. Ben‐Nun IF, Montague SC, Houck ML et al. Induced pluripotent stem cells from highly endangered species. Nat Methods 2011;8:829–831. [DOI] [PubMed] [Google Scholar]
- 2. Shapiro B. Mammoth 2.0: Will genome engineering resurrect extinct species? Genome Biol 2015;16:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mattick CS. Cellular agriculture: The coming revolution in food production. Bull Atom Sci 2018;74:32–35. [Google Scholar]
- 4. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 5. Okita K, Ichisaka T, Yamanaka S. Generation of germline‐competent induced pluripotent stem cells. Nature 2007;448:313–317. [DOI] [PubMed] [Google Scholar]
- 6. Boland MJ, Hazen JL, Nazor KL et al. Adult mice generated from induced pluripotent stem cells. Nature 2009;461:91–94. [DOI] [PubMed] [Google Scholar]
- 7. Zhao XY, Li W, Lv Z et al. iPS cells produce viable mice through tetraploid complementation. Nature 2009;461:86–90. [DOI] [PubMed] [Google Scholar]
- 8. Boland MJ, Hazen JL, Nazor KL, Rodriguez A. R., Martin G., Kupriyanov S., Baldwin K. K. Generation of Mice Derived from Induced Pluripotent Stem Cells. J Vis Exp 2012;(69):e4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Benirschke K. The frozen ZOO concept. Zoo Biol 1984;3:325–328. [Google Scholar]
- 10. Koepfli KP, Paten B, Genome 10K Community of Scientists et al. The Genome 10K Project: A way forward. Annu Rev Anim Biosci 2015;3:57–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Du X, Feng T, Yu D et al. Barriers for deriving transgene‐free pig iPS cells with episomal vectors. Stem Cells 2015;33:3228–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan N, Chen J, Shang Z et al. Piglets cloned from induced pluripotent stem cells. Cell Res 2013;23:162–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hayashi K, Ogushi S, Kurimoto K et al. Offspring from oocytes derived from in vitro primordial germ cell–like cells in mice. Science 2012;338:971–975. [DOI] [PubMed] [Google Scholar]
- 14. Hayashi K, Ohta H, Kurimoto K et al. Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells. Cell 2011;146:519–532. [DOI] [PubMed] [Google Scholar]
- 15. Hikabe O, Hamazaki N, Nagamatsu G et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature 2016;539:299–303. [DOI] [PubMed] [Google Scholar]
- 16. Hildebrandt TB, Hermes R, Colleoni S et al. Embryos and embryonic stem cells from the white rhinoceros. Nat Commun 2018;9:2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu H, Zhu F, Yong J et al. Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell 2008;3:587–590. [DOI] [PubMed] [Google Scholar]
- 18. Han X, Han J, Ding F et al. Generation of induced pluripotent stem cells from bovine embryonic fibroblast cells. Cell Res 2011;21:1509–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagy K, Sung HK, Zhang P et al. Induced pluripotent stem cell lines derived from equine fibroblasts. Stem Cell Rev 2011;7:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Deng Y, Liu Q, Luo C et al. Generation of induced pluripotent stem cells from buffalo (Bubalus bubalis) fetal fibroblasts with buffalo defined factors. Stem Cells Dev 2012;21:2485–2494. [DOI] [PubMed] [Google Scholar]
- 21. Verma R, Holland MK, Temple‐Smith P et al. Inducing pluripotency in somatic cells from the snow leopard (Panthera uncia), an endangered felid. Theriogenology 2012;77:220–228, 228.e1–2. [DOI] [PubMed] [Google Scholar]
- 22. Lu Y, West FD, Jordan BJ et al. Avian‐induced pluripotent stem cells derived using human reprogramming factors. Stem Cells Dev 2012;21:394–403. [DOI] [PubMed] [Google Scholar]
- 23. Verma R, Liu J, Holland MK et al. Nanog is an essential factor for induction of pluripotency in somatic cells from endangered felids. Biores Open Access 2013;2:72–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramaswamy K, Yik W, Wang XM et al. Derivation of induced pluripotent stem cells from orangutan skin fibroblasts. BMC Res Notes 2015;8:577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saragusty et al., Zoo Biol 2016;35:280–292, DOI: 10.1002/zoo.21284. [DOI] [PubMed] [Google Scholar]
- 26. Leake J. Science close to creating a mammoth. The Times 2015. Available at https://www.thetimes.co.uk/article/science‐close‐to‐creating‐a‐mammoth‐z8zlvbgr9fl. Accessed on July 23, 2018.
- 27. Thomson C. The Lazarus Project—To Bring Back Australia's Southern Gastric‐Brooding Frog. 2017. Available at https://awpc.org.au/the‐lazarus‐project‐to‐bring‐back‐australias‐southern‐gastric‐brooding‐frog/. Accessed July 23, 2018.
- 28. Lynch VJ, Bedoya‐Reina OC, Ratan A et al. Elephantid genomes reveal the molecular bases of woolly mammoth adaptations to the arctic. Cell Rep 2015;12:217–228. [DOI] [PubMed] [Google Scholar]
- 29. Fisher DC, Tikhonov AN, Kosintsev PA et al. Anatomy, death, and preservation of a woolly mammoth (Mammuthus primigenius) calf, Yamal Peninsula, northwest Siberia. Quat Int 2012;255:94–105. [Google Scholar]
- 30.Progress to Date. Available at http://reviverestore.org/projects/woolly-mammoth/progress-to-date/. Accessed July 23, 2018.
- 31. Pimm SL, Alibhai S, Bergl R et al. Emerging technologies to conserve biodiversity. Trends Ecol Evol 2015;30:685–696. [DOI] [PubMed] [Google Scholar]
- 32. Johnson JA, Altwegg R, Evans DM et al. Is there a future for genome‐editing technologies in conservation? Anim Conserv 2016;19:97–101. [Google Scholar]
- 33. Mao JS, Boyce MS, Smith DW et al. Habitat selection by elk before and after wolf reintroduction in Yellowstone National Park. J Wildl Manage 2005;69:1691–1707. [Google Scholar]
- 34. Karimi F. CNN 2018. 11 endangered rhinos were moved to start a new population—10 died. Available at https://edition.cnn.com/2018/07/27/africa/black-rhinos-dead-kenya-relocation/index.html. Accessed July 27, 2018.
- 35. Ceballos G, Ehrlich PR, Barnosky AD et al. Accelerated modern human‐induced species losses: Entering the sixth mass extinction. Sci Adv 2015;1:e1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.The Extinction Crisis. Available at www.biologicaldiversity.org/programs/biodiversity/elements_of_biodiversity/extinction_crisis/. Accessed July 23, 2018.
- 37. Steinfeld H, Gerber P, Wasenaar T et al. Livestock's Long Shadow: Environmental Issues and Options. Food and Agriculture Organization of the United Nations. Rome, 2006. Available at http://www.fao.org/docrep/010/a0701e/a0701e.pdf. Accessed July 27, 2018.
- 38. Garnett T. Livestock‐related greenhouse gas emissions: Impacts and options for policy makers. Environ Sci Policy 2009;12:491–503. [Google Scholar]
- 39. Ronquillo MG, Angeles Hernandez JC. Antibiotic and synthetic growth promoters in animal diets: Review of impact and analytical methods. Food Cont 2017;72:255–267. [Google Scholar]
- 40. Specht EA, Welch DR, Rees Clayton EM et al. Opportunities for applying biomedical production and manufacturing methods to the development of the clean meat industry. Biochem Eng J 2018;132:161–168. [Google Scholar]
- 41. Post MJ. An alternative animal protein source: Cultured beef. Ann N Y Acad Sci 2014;1328:29–33. [DOI] [PubMed] [Google Scholar]
- 42. Genovese NJ, Domeier TL, Telugu BP et al. Enhanced development of skeletal myotubes from porcine induced pluripotent stem cells. Sci Rep 2017;7:41833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Devito L, Petrova A, Miere C et al. Cost‐effective master cell bank validation of multiple clinical‐grade human pluripotent stem cell lines from a single donor. Stem Cell Transl Med 2014;3:1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stephenson E, Jacquet L, Miere C et al. Derivation and propagation of human embryonic stem cell lines from frozen embryos in animal product‐free environment. Nat Prot 2012;7:1366–1381. [DOI] [PubMed] [Google Scholar]
- 45. Rowland MP. Labeling Wars: The U.S. Cattlemen's Association has beef with its competition. Forbes 2018. https://www.forbes.com/sites/michaelpellmanrowland/2018/02/14/usda-labeling-laws-meat-beef/ [Google Scholar]
- 46. Shafa M, Day B, Yamashita A et al. Derivation of iPSCs in stirred suspension bioreactors. Nat Methods 2012;9:465–466. [DOI] [PubMed] [Google Scholar]
- 47. Abecasis B, Aguiar T, Arnault É et al. Expansion of 3D human induced pluripotent stem cell aggregates in bioreactors: Bioprocess intensification and scaling‐up approaches. J Biotechnol 2017;246:81–93. [DOI] [PubMed] [Google Scholar]
- 48. Stanton MM, Samitier J, Sánchez S. Bioprinting of 3D hydrogels. Lab Chip 2015;15:3111–3115. [DOI] [PubMed] [Google Scholar]
- 49. Ma X, Qu X, Zhu W et al. Deterministically patterned biomimetic human iPSC‐derived hepatic model via rapid 3D bioprinting. Proc Natl Acad Sci U S A 2016;113:2206–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Abaci HE, Guo Z, Coffman A et al. Human skin constructs with spatially controlled vasculature using primary and iPSC‐derived endothelial cells. Adv Healthc Mater 2016;5:1800–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pimentel D, Pimentel M. Sustainability of meat‐based and plant‐based diets and the environment. Am J Clin Nutr 2003;78:660S–663S. [DOI] [PubMed] [Google Scholar]


