Abstract
Key points
One in two female athletes chronically take a combined, monophasic oral contraceptive pill (OCP). Previous thermoregulatory investigations proposed that an endogenous rhythm of the menstrual cycle still occurs with OCP usage.
Forthcoming large international sporting events will expose female athletes to hot environments differing in their thermal profile, yet few data exist on how trained women will respond from both a thermoregulatory and performance stand‐point.
In the present study, we have demonstrated that a small endogenous rhythm of the menstrual cycle still affects T core and also that chronic OCP use attenuates the sweating response, whereas behavioural thermoregulation is maintained.
Furthermore, humid heat affects both performance and thermoregulatory responses to a greater extent than OCP usage and the menstrual cycle does.
Abstract
We studied thermoregulatory responses of ten well‐trained (, 57 ± 7 mL min−1 kg−1) women taking a combined, monophasic oral contraceptive pill (OCP) (≥12 months) during exercise in dry and humid heat, across their active OCP cycle. They completed four trials, each of resting and cycling at fixed intensities (125 and 150 W), aiming to assess autonomic regulation, and then a self‐paced intensity (30‐min work trial) to assess behavioural regulation. Trials were conducted in quasi‐follicular (qF) and quasi‐luteal (qL) phases in dry (DRY) and humid (HUM) heat matched for wet bulb globe temperature (WBGT) (27°C). During rest and exercise at 125 W, rectal temperature was 0.15°C higher in qL than qF (P = 0.05) independent of environment (P = 0.17). The onset threshold and thermosensitivity of local sweat rate and forearm blood flow relative to mean body temperature was unaffected by the OCP cycle (both P > 0.30). Exercise performance did not differ between quasi‐phases (qF: 268 ± 31 kJ, qL: 263 ± 26 kJ, P = 0.31) but was 5 ± 7% higher during DRY than during HUM (273 ± 29 kJ, 258 ± 28 kJ; P = 0.03). Compared to matched eumenorrhoeic athletes, chronic OCP use impaired the sweating onset threshold and thermosensitivity (both P < 0.01). In well‐trained, OCP‐using women exercising in the heat: (i) a performance‐thermoregulatory trade‐off occurred that required behavioural adjustment; (ii) humidity impaired performance as a result of reduced evaporative power despite matched WBGT; and (iii) the sudomotor but not behavioural thermoregulatory responses were impaired compared to matched eumenorrhoeic athletes.
Keywords: Exercise physiology, Thermoregulation, Oral Contraception
Key points
One in two female athletes chronically take a combined, monophasic oral contraceptive pill (OCP). Previous thermoregulatory investigations proposed that an endogenous rhythm of the menstrual cycle still occurs with OCP usage.
Forthcoming large international sporting events will expose female athletes to hot environments differing in their thermal profile, yet few data exist on how trained women will respond from both a thermoregulatory and performance stand‐point.
In the present study, we have demonstrated that a small endogenous rhythm of the menstrual cycle still affects T core and also that chronic OCP use attenuates the sweating response, whereas behavioural thermoregulation is maintained.
Furthermore, humid heat affects both performance and thermoregulatory responses to a greater extent than OCP usage and the menstrual cycle does.
Introduction
The primary ovarian steroidal hormones influence several non‐reproductive organs and systems. Concerning thermoregulation, oestrogens promote heat dissipation and lower core body temperature (T core), whereas progestogens are thermogenic (Israel & Schneller, 1950; Charkoudian & Stachenfeld, 2014). Studies have investigated the impact of these hormones on thermoregulation during different phases of the menstrual cycle. In eumenorrhoeic women, the thermoregulatory balance‐point shows T core to be regulated ∼0.4°C higher during the post‐ovulatory (luteal) phase at rest and during passive and active heat stress, as the rise in progesterone exerts its dominant effect (Harvey & Crockett, 1932; Israel & Schneller, 1950). This is accompanied by an increased T core threshold for thermoregulatory effector responses such as sweating and cutaneous vasodilatation (Hessemer & Brück, 1985; Stephenson & Kolka, 1985; Stachenfeld et al. 2000). Therefore, several studies have suggested that, when performing exercise under environmental heat stress during their luteal phase, women should avoid competition or face a thermoregulatory and performance disadvantage (Stephenson & Kolka, 1993; Charkoudian & Joyner, 2004; Janse de Jonge et al. 2012). However, for a well‐trained and competitive female athlete, this may not be the case. First, trained females have a greater capacity to deal with a heat load as a result of their enhanced thermoeffector responses compared to less‐trained counterparts (Kuwahara et al. 2005 a,b). Second, trained women show smaller biphasic effects on T core and thermoeffector responses because of reduced ovarian hormone concentrations and fluctuation between their menstrual phases (Kuwahara et al. 2005 a,b). Third, most previous investigations have utilised a fixed‐intensity exercise protocol that is less ecologically‐valid (i.e. most athletes use pacing) and does not examine the fundamental premise of heat balance: that heat loss needs only to equal heat production (Gagnon et al. 2013). However, we recently demonstrated that, when well‐trained females can use behavioural thermoregulation (self‐pacing) during exercise in heat stressful environments, exercise intensity, and therefore, metabolic heat production is reduced (Lei et al. 2017). This eases the required evaporation and decreases thermoregulatory strain to the point where the menstrual phase‐related thermodynamic and autonomic differences become nullified (Lei et al. 2017). Furthermore, these menstrual phase‐related effects were relatively small in well‐trained women.
The prevalence of oral contraceptive (OC) use is high (42–83%) among athletically competitive and elite females, three‐quarters of whom reportedly use a combined monophasic OC pill (OCP) (Rechichi et al. 2009). The combined monophasic OCP provides a constant dose of synthetic oestrogen and progestogen for 21 days followed by 7 days of a placebo. Previous investigations on OCP users report that the phase‐related elevation in T core and concomitant increase in the T core threshold for effector responses is maintained during active and passive heating, and that this shift can be regarded as a strong and residual effect of the phase of the menstrual cycle (Grucza et al. 1993; Martin & Buono, 1997; Rogers & Baker, 1997; Charkoudian & Johnson, 1997 a; Tenaglia et al. 1999; Sunderland & Nevill, 2003). However, although these investigations generally describe their comparison between a quasi‐follicular and quasi‐luteal phase, the comparison always occurred when the females were taking active OC compared to their placebo week (withdrawal). This raises several important considerations. First, the variable tissue washout rates mean that the exogenous hormones, or their metabolites, probably remain elevated and able to exert an effect (Israel & Schneller, 1950; Rothchild & Barnes, 1952; Charkoudian & Stachenfeld, 2014). Second, towards the end of the placebo week, the concentration of endogenous oestrogens increases and, as such, this phase should be viewed as a transitory hormonal phase and not a controlled ‘low’ hormonal phase (i.e. compare OC use as a ‘high’ hormonal phase: Rechichi et al. 2008; Charkoudian & Stachenfeld, 2014). Third, the synthetic progestins found in OCPs differ in some of their basic actions to those of endogenous progesterone, probably influencing physiological systems differently (Charkoudian & Stachenfeld, 2014). Thus, the supposition that an endogenous rhythm of the menstrual cycle is maintained during OC use has not been investigated properly because, to our knowledge, no data exist on variation within the active OCP cycle. This was the principle objective of the present study. Moreover, data exist indicating that chronic OCP use may alter T core and thermoeffector responses to active and passive heating in women, as indicated by greater resting T core and attenuated changes in mean body temperature and gain for local sweat rate (Grucza et al. 1993; Sunderland & Nevill, 2003). Therefore, a secondary objective was to compare the results of the current OCP cohort matched in relevant physical and fitness characteristics with those of our previous eumenorrhoeic cohort (Lei et al. 2017).
Forthcoming large international events [e.g. 2019 International Association of Athletics Federations (IAAF) World Championships in Doha; 2020 Summer Olympic Games in Tokyo] will expose female athletes to high environmental heat stress, and the number of women participating at this elite level is ever increasing, now approximating that of males. However, these environments differ in their ambient thermal profile, with arid environments usually permitting almost full evaporation of sweat, whereas humid tropical environments do not. In our previous investigation, the performance of eumenorrhoeic athletes was impaired in humid compared to dry ambient heat matched for wet bulb globe temperature (WBGT) (27 °C) (Lei et al. 2017). To our knowledge, no previous study has compared thermoregulatory and performance responses in women taking OCP when exposed to humid vs. dry heat. This was our final objective.
In the present study, we aimed to characterise and compare the behavioural and autonomic thermoregulatory responses to exercise when exposed to equivalent dry and humid heat stress in well‐trained women who had been chronically taking a combined, monophasic OCP. Based on our previous study in eumenorrhoeic women, as well as the literature described above, we hypothesised that: (i) if an endogenous thermoregulatory rhythm persisted during their active OCP cycle, it would be small and nullified by behavioural adjustments; (ii) these differences in thermoregulatory control across the OCP cycle would interact with differences brought about by the thermal environment (i.e. dry vs. evaporative heat transfer); and (iii) compared to a matched cohort of eumenorrhoeic athletes, chronic OCP users would display an attenuated autonomic response.
Methods
Ethical approval
The present study was approved by the Massey University Human Ethics Committee: Southern A (14/99). The study conformed to the standards set by the latest revision of the Declaration of Helsinki, except for registration in a database, with each participant providing their informed, written consent.
Participants
Ten aerobically well‐trained and competitive women cyclists and triathletes volunteered for this study. Table 1 displays the participants’ mean ± SD physical characteristics alongside those of our matched eumenorrhoeic group (Lei et al. 2017); this was considered appropriate because all methods were identical between this group and the OCP group. The data of the eumenorrhoeic group have been reported previously with respect to testing unique aims and hypotheses (Lei et al. 2017). The training history for both OCP and eumenorrhoeic groups was similar at 5 ± 3 years (range 2–12 years). All OCP participants were taking a monophasic combination OCP (≥ 1 year) that provides a constant level of hormones for 21 days followed by a placebo pill for 7 days. Five were taking Ginet® (REX Medical Ltd, Auckland, New Zealand; containing 2 mg of cyproterone acetate and 35 μg of ethinylestradiol), four Ava 20 ED® (Teva Pharma Ltd, Auckland, New Zealand; containing 0.1 mg of levonorgestrel and 20 μg of ethinylestradiol) and one Norimin® (Pfizer Ltd, Auckland, New Zealand; containing 0.5 mg of norethisterone and 35 μg of ethinylestradiol).
Table 1.
Participant characteristics for the matched eummenorrhoeic (EUM) (Lei et al. 2017) and OCP groups
| Characteristic | OCP | EUM | P value |
|---|---|---|---|
| Age (years) | 25 ± 5 | 34 ± 9 | 0.02 |
| Height (cm) | 167 ± 5 | 165 ± 5 | 0.30 |
| Mass (kg) | 68 ± 10 | 62 ± 4 | 0.10 |
| A D (m2) | 1.76 ± 0.13 | 1.67 ± 0.06 | 0.10 |
| A D: mass | 0.026 ± 0.002 | 0.027 ± 0.001 | 0.14 |
| % fat | 24 ± 5 | 24 ± 5 | 0.85 |
| (L min−1) | 3.7 ± 0.5 | 3.5 ± 0.5 | 0.42 |
| (mL min−1 kg−1) | 55 ± 9 | 57 ± 7 | 0.69 |
| W max (W) | 278 ± 25 | 261 ± 30 | 0.19 |
| W max (W kg−1) | 4.2 ± 0.7 | 4.2 ± 0.4 | 0.82 |
Data are the mean ± SD. A D, Dubois body surface area. Both EUM and OCP cohorts: n = 10.
Experimental overview
The present study replicated all of the conditions used for our matched eumenorrhoeic group (Lei et al. 2017). All trials were conducted during autumn to spring in Palmerston North, New Zealand, when temperatures rarely exceed 22°C. No participant had spent time in warmer climates or training environments within 1 month preceding testing. All participants attended the laboratory on six occasions: (i) preliminary submaximal and maximal tests; (ii) experimental familiarisation; and (iii) to (vi) experimental trials. The four experimental trials were a full cross‐over of OCP phase (quasi‐follicular: qF; quasi‐luteal: qL) and environment (dry and humid, at matched WBGT). All trials were counterbalanced except that the same order of dry or humid environment was retained for each OCP phase within participants. Experimental trials were conducted at the same time of day (±1 h) and following >24 h of dietary and exercise control. Each trial consisted of 12 min of fixed‐intensity cycling followed immediately by 30 min of a self‐paced cycling performance trial. All exercise was performed on an electromagnetically‐braked cycle ergometer (Lode Excalibur, Groningen, The Netherlands) with a participant‐specific set up for the seat, handle bars and pedals, which was maintained constant for each trial with respect to a participant.
Preliminary testing and familiarisation
Submaximal and maximal capacity tests were undertaken in the qF phase to minimise the potential physiological effects of the menstrual/OCP cycle on performance. Following body mass and height measurement, preliminary testing was conducted in a temperate laboratory environment (18–22°C) with a fan‐generated airflow of 19 km h−1 facing participants. The submaximal test consisted of four consecutive 6‐min power outputs: 100, 125, 150 and 175 W, at comfortable but constant cadence. The rate of oxygen consumption () was measured during the last 2 min of each stage. Following a 10‐min rest, a maximal capacity test was undertaken to measure . Work rate began at 100 W and consisted of increments at 25 W min−1 until volitional fatigue. The linear relationship between power output and was subsequently used to calculate workload for experimental trials, as 75% (Jeukendrup et al. 1996).
At least 24 h following preliminary testing, the familiarisation trial was undertaken to ensure that participants were accustomed to the experimental procedures and to minimise learning effects. These trials replicated entirely the experimental trials outlined below.
Dietary and exercise control
The day of and prior to any experimental trial was marked by abstinence from alcohol, exercise and only habitual caffeine use (i.e. because abstinence would in itself confound from withdrawal effects). Participants were provided with a standardised dinner (2 × Watties Snack Meals; Heinz Watties, Hastings, New Zealand: 1363 ± 247 kJ providing 53 ± 6 g of carbohydrate, 12 ± 4 g of protein and 8 ± 0.3 g of fat) the night preceding the trial and were asked to consume the same light meal (consisting of toast or cereal) between 2 and 4 h prior to visiting the laboratory for the trial. Fluid was encouraged and a euhydrated state was further ensured by instructing the participants to drink 500 mL of water 2 h prior to each trial.
OCP cycle phase and type of heat stress
Participants were tested during the qF and qL phases to permit comparison with our eumenorrhoeic group. Testing occurred on days 3–5 and 18–20 following the start of OCP use, whereas testing of our eumenorrhoeic group occurred on days 3–6 (early follicular) and 18–21 (mid‐luteal) following the start of menses. This was unavoidable as a result of the study hypothesis and therefore design and, as such, this meant that the current OCP users were being tested on days 10–12 (quasi mid‐late follicular) and 25–27 (quasi mid‐late luteal) following the start of menses.
In accordance with previous studies investigating the influence of humid (HUM) vs. dry (DRY) environmental heat in women (Morimoto et al. 1967; Shapiro et al. 1980; Frye & Kamon, 1983), heat stress was indexed using WBGT because it is the most widely used empirical index (Brotherhood, 2008; Budd, 2008). Our decision‐making was guided by the typical (or possible) extreme conditions athletes would encounter at the 2018 Commonwealth Games (humid) compared to the 2019 IAAF World Championships (dry). Therefore, a WBGT equivalent to 27°C was chosen to elicit our HUM (29 ± 0.3°C, 83 ± 2% relative humidity) and DRY (34 ± 0.2°C, 42 ± 3% relative humidity) environments. Absolute humidity in these two environments was 3.4 ± 0.1 kPa and 2.2 ± 0.2 kPa, respectively. Within each OCP phase, exposure to DRY and HUM environments was separated by 3 days.
Experimental procedures
The four experimental sessions were conducted in the same environmental chamber with a 19 km h−1 airflow. However, the fan was turned off for each ∼2 min data collection period (of each 6 min stage or interval) to minimise the interference of airflow on measurement. On arrival at the laboratory, participants voided to produce a urine sample to confirm a urine specific gravity <1.010 and hence euhydration, nude mass was recorded and they then self‐inserted a rectal thermistor. A blood sample was obtained from the antecubital vein, following which participants entered the environmental chamber wearing only cycling shorts and top, shoes and socks. Participants rested seated on the ergometer for 20 min during which they were instrumented and baseline measurements were recorded. Participants then completed 6 min of cycling at each of 125 and 150 W to allow sufficient warm‐up and fixed‐intensity responses to be recorded. Physiological measurements taken during the final 2 min of each intensity included expired gas, heart rate (HR), blood pressure (BP), forearm blood flow (FBF) and cardiac output () responses, whereas rectal (T rec) and skin ( sk) temperatures, as well as local sweat rate (LSR), were measured continuously. Immediately on completion of the 150 W bout, the ergometer was set to linear mode based on the formula of Jeukendrup et al. (1996), and participants were instructed to perform as much work as possible over 30 min. During this 30 min of self‐paced period, work completed (kJ), HR and expired gas responses were recorded every 6 min, whereas T rec, sk and LSR were measured continuously. Total work completed was used as the performance criterion, whereas the time profile of power output was used as the behavioural criterion (Lei et al. 2017). Immediately following the 30 min self‐paced exercise, FBF was measured when participants began their 5 min cool‐down (100 W) before the nude mass of participants (towelled dry) was recorded to allow estimation of whole‐body sweat rate (WBSR). Tap water at 20°C and in aliquots of 3 mL kg−1 bodyweight was provided to drink ad libitum either at 15‐min intervals or when requested throughout each trial to minimise dehydration.
Measurements
Anthropometric
Participant height and mass were measured using a stadiometer (Seca, Hamburg, Germany; accurate to 0.1 cm) and scale (Jadever, Taipei, Taiwan; accurate to 0.01 kg), from which surface area was estimated (Dubois & Dubois, 1916). Body composition was measured using multifrequency bioelectrical impedance analysis (InBody 230; InBody, Seoul, Korea) in accordance with a standard procedure (Kyle et al. 2004).
Respiratory
Expired respiratory gases were collected and analysed to then calculate and carbon dioxide elimination (), ventilation () and respiratory exchange ratio (RER), using an online, breath‐by‐breath system (Vista Turbofit; VacuMed, Ventura, CA, USA) using a 30‐s average. The system was calibrated before each trial using β‐standard gas concentrations and a 3 L syringe (VacuMed).
Cardiovascular
The HR was recorded from detection of R‐R intervals (Polar Vantage XL; Polar Electro, Kempele, Finland), whereas BP was measured using a stethoscope and a sphygmomanometer over the right brachial artery, in duplicate and by the same experienced operator. Mean arterial pressure (MAP) was calculated as diastolic blood pressure + 1/3 pulse pressure. The FBF was measured in triplicate (mean values reported) using venous occlusion plethysmography (Whitney, 1953) with a mercury‐in‐silastic strain‐gauge on the widest part of the forearm supported at heart level. The voltage output was acquired (PowerLab; ADInstruments, Dunedin, New Zealand) and displayed (Labchart Pro; ADInstruments) in real time, as well as for offline analysis. The venous occlusion pressure was 50 mm Hg, cycle duration ≤10 s. Forearm vascular resistance (FVR) was calculated as MAP/FBF. The was measured using CO2 rebreathing (Defares, 1958), as described previously (Schlader et al. 2010). Pressure of end‐tidal CO2 () during the rebreathing procedure was measured (O2/CO2 gas analyser; ADInstruments), with data acquisition and display as noted above (AD Instruments). Differences between and venous and arterial were corrected in accordance with Paterson & Cunningham (1976) and Jones et al. (1979). The CO2 content difference was calculated as described by McHardy (1967). Stroke volume (SV) was calculated from the Fick equation.
Body temperatures
The T core was indexed from T rec, measured using a calibrated rectal thermistor (Mon‐a‐Therm; Covidien, Medtronic, Minneapolis, MN, USA; accurate to 0.1˚C) inserted 10 cm beyond the anal sphincter. The sk was measured at four sites using calibrated skin thermistors (Grant Instrument Ltd, Cambridgeshire, UK; accurate to 0.2°C) fastened on the calf, thigh, chest and forearm using surgical tape (3M Healthcare, St Paul, MN, USA). Area‐weighted mean sk was calculated in accordance with the equation of Ramanathan (1964). Core and skin temperatures were recorded using TracerDAQ® software (Measurement Computing Corporation, Norton, MA, USA). To account for the relative influence of T core and sk on the activation of heat loss responses (Hertzman et al. 1952), mean body temperature ( b) was calculated as: 0.8 × T rec + 0.2 × sk (Stolwijk & Hardy, 1966).
Sweat rates
The LSR was measured using a ventilated capsule (Graichen et al. 1982). The capsule (3.5 cm2) was attached to the neck dorsally and ventilated with dry air at 0.4 L min−1. The effluent gas was sensed for humidity (Honeywell Ltd, Auckland, New Zealand) and temperature (National Semiconductor, Santa Clara, CA, USA). The neck was used because all limbs were used for other measurements and it was not exposed directly to the fan. The WBSR was estimated from body mass loss, corrected for fluid consumed.
Thermodynamics
Heat stress compensability was estimated using the heat strain index (HSI), with >1.0 indicating uncompensable heat stress (Cheung et al. 2000). The HSI was calculated as the ratio of the required evaporative cooling for heat balance (E req; W m−2) and the maximal evaporative capacity of the environment (E max; W m−2) (Belding & Hatch, 1955). E req was calculated as E req = M‐W ± (C + R) ± (C res − E res). M − W represents metabolic heat production, where M is the metabolic rate (W m−2), calculated as (Kenney, 1998): M = [352 × (0.23 × RER + 0.77) × ]/body surface area, and W is the rate of energy lost as external work (W m−2). C + R is the rate of heat transfer from convection (C; W m−2) and radiation (R; W m−2), calculated as the sum of: (Kerslake, 1972) and R = 4.7 × (T Sk − T A) (Kenney, 1998), where h c is the convective heat transfer coefficient (W m−2 °C) (Kerslake, 1972) and T A is the ambient temperature (°C). C res + E res is the rate of respiratory conductive (C res) and evaporative (E res) heat transfer, and was calculated as (Kenney, 1998): ], where P A is ambient vapour pressure (kPa). E max was calculated as , where LR is the Lewis relationship (16.5°C kPa) and P Sk is the saturated vapour pressure at the skin (kPa).
Hormonal
Blood was collected by venipuncture into a vacutainer (Becton‐Dickinson, Wokingham, UK) containing clot activator. Following inversion and clotting, the whole blood was centrifuged at 4 °C and 805 g for 12 min and aliquots of serum were transferred into Eppendorf tubes (Genuine Axygen Quality; Corning Inc., Union City, CA, USA) and stored at −80 °C until further analysis. Serum samples were analysed using enzyme‐linked immunoassays for 17β‐oestradiol (Demeditec Diagnostics, Kiel, Germany) and progesterone (IBL International, Hamburg, Germany), with a sensitivity of 22.7 pmol L−1 and 0.14 nmol L−1, respectively, and an intra‐assay variation of 4% and 6%, respectively.
Statistical analysis
All statistical analyses were performed with SPSS, version 20 (IBM Corp., Armonk, NY, USA). Descriptive values were obtained and reported as the means ± SD, unless stated otherwise. Levene's test was used to ensure the data did not differ substantially from a normal distribution. Data were analysed using a three‐way (OCP phase × environment × time) ANOVA for repeated measures. Resting and fixed‐intensity exercise data were analysed separately from self‐paced exercise data. Sphericity was assessed and, where the assumption of sphericity could not be assumed, adjustments to the degrees of freedom were made (ε > 0.75 = Huynh–Feldt; ε < 0.75 = Greenhouse–Geisser). Where main or interaction effects occurred, post hoc pairwise analyses were performed using a paired samples t test (Bonferroni correction where relevant), with statistical significance set at P ≤ 0.05. Partial eta‐squared (ηp 2) is reported as a measure of effect size, with demarcations of small (<0.09), medium (>0.09 and <0.25) and large (>0.25) effects, respectively (Cohen, 1988). This combination of statistical significance and effect size provides an indication of the likelihood of committing a Type I (i.e. P ≤ 0.05 but ηp 2 < 0.09) or II (i.e. P < 0.10 but ηp 2 > 0.25) error. To examine how OCP phase and type of heat stress affected the thermal control of the effector responses (LSR and FBF), the visually determined linear portion of each response against b was analysed by the same experienced researcher using simple linear regression (y = y 0 + a * x) and compared using two‐way (menstrual phase × heat stress) ANOVA. We used linear regression, as opposed to segmental regression, to allow direct comparison with our previous cohort (Lei et al. 2017). The onset threshold was defined as the y‐intercept (y 0) of the regression line with values at baseline once instrumented in the environmental chamber, whereas the thermosensitivity was defined as the slope (a) of the regression line. Notably, although the absolute values determined for the onset threshold may not be physiologically plausible using linear regression, shifts or differences in the thresholds between groups are interpreted as physiologically meaningful. To allow comparison between our OCP and previous eumenorrhoeic group (Lei et al. 2017), a fourth (between‐group) factor was introduced using a mixed‐model (group × OCP phase × environment × time) ANOVA, with the group effect reported.
Results
Ovarian hormone concentrations (Table 2)
Table 2.
Individual and group progesterone and 17β‐oestradiol concentrations during the qF and qL phase for the matched eummenorrheic (EUM) (Lei et al. 2017) and OCP groups
| Progesterone (nmol L−1)* | 17β‐Oestradiol (pmol L−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| F | L | F | L | |||||
| Participant | EUM | OCP | EUM | OCP | EUM | OCP | EUM | OCP |
| 1 | 0.3, 0.3 | 0.4, 0.4 | 30, 42 | 0.5, 0.5 | 195, 202 | 79, 75 | 290, 264 | 89, 102 |
| 2 | 0.6, 3.8 | − | 49, 43 | − | 33, 7 | − | 330, 301 | − |
| 3 | 2.2, 2.9 | 0.0, 0.1 | 219, 168 | 0.0, 0.1 | 503, 602 | 8, 4 | 1057, 833 | 3, 0 |
| 4 | 0.3, 0.3 | 0.2, 0.3 | 4.1, 11 | 0.4, 0.5 | 88, 132 | 96, 95 | 176, 723 | 90, 77 |
| 5 | 2.9, 1.0 | 0.2, 0.1 | 39, 21 | 0.5, 0.2 | 92, 103 | 56, 57 | 162, 165 | 71, 62 |
| 6 | 2.9, 1.9 | 0.3, 0.2 | 18, 39 | 0.2, 0.3 | 117, 44 | 24, 67 | 198, 198 | 36, 29 |
| 7 | 1.3, 0.6 | 0.5, 0.4 | 61, 60 | 0.5, 0.8 | 40, 62 | 60, 66 | 139, 261 | 64, 72 |
| 8 | 1.3, 2.2 | 0.9, 1.4 | 19, 61 | 0.5, 1.1 | 147, 136 | 28, 64 | 242, 169 | 76, 46 |
| 9 | 3.5, 3.2 | 0.1, 1.0 | 35, 59 | 0.2, 0.2 | 396, 430 | 1, 9 | 426, 716 | 0, 12 |
| 10 | 1.0, 1.3 | 0.2, 0.1 | 27, 65 | 0.2, 0.2 | 176, 195 | 10, 2 | 272, 363 | 4, 7 |
| Mean ± SD | 1.7 ± 1.2 | 0.4 ± 0.4 | 54 ± 52 | 0.4 ± 0.3 | 185 ± 166 | 44 ± 33 | 364 ± 259 | 47 ± 36 |
–, Blood sample not obtained. *Significant difference between EUM and OCP. Both EUM and OCP cohorts: n = 10.
OCP use maintained constant endogenous concentrations of both progesterone and 17β‐oestradiol between qF and qL (P = 0.93, ηp 2 < 0.01 and P = 0.62, ηp 2 = 0.02, respectively) and concentrations were also not different between days of testing within a quasi‐phase (P = 0.24, ηp 2 = 0.17 and P = 0.22, ηp 2 = 0.19, respectively). Between‐group analysis revealed that endogenous concentrations of progesterone (P < 0.01, ηp 2 = 0.41) but not 17β‐oestradiol (P = 0.07, ηp 2 = 0.09, possible type I error) were significantly lower in the current OCP than in the previous eumenorrhoeic group.
Exercise performance and behaviour (Figure 1)
Figure 1. Mean (SD) power output (n = 10) and individual and mean ± SD work capacity (n = 10) during exercise in dry (DRY) and humid (HUM) heat during the qF and qL phase.
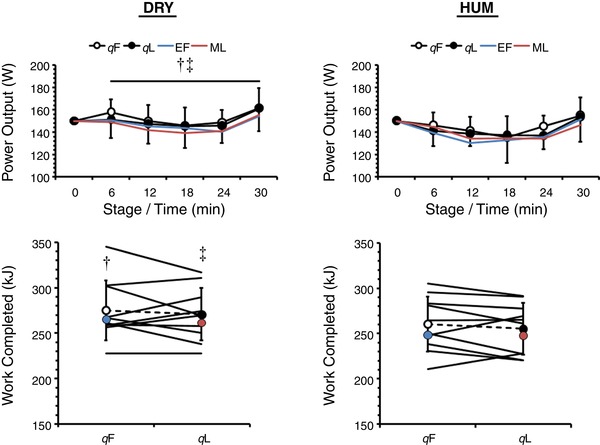
*Significant difference between qF − qL within environment. †Significant difference between corresponding qF − HUM value. ‡Significant difference between corresponding qL − HUM value. Mean early follicular (EF) and mid‐luteal (ML) values are provided for our previous eumenorrhoeic cohort (Lei et al. 2017).
Work capacity was similar between OCP phases (qF: 268 ± 31 kJ vs. qL: 263 ± 26 kJ, P = 0.31, ηp 2 = 0.12) but was 5 ± 7% higher during DRY than during HUM (273 ± 29 kJ vs. 258 ± 28 kJ; P = 0.03, ηp 2 = 0.45). Accordingly, mean power output was unaffected by OCP phase (P = 0.44, ηp 2 = 0.07) but was 5 ± 7% higher during DRY than during HUM (152 ± 16 W vs. 143 ± 16 W; P = 0.03, ηp 2 = 0.43). When viewing behaviour as the self‐paced exercise profile, behaviour was similar between OCP phases (P = 0.44, ηp 2 = 0.07) but was 8 ± 10 W higher during DRY than during HUM (P = 0.03, ηp 2 = 0.43) and changed over time (P < 0.01, ηp 2 = 0.61). Between‐group analysis revealed no differences between the OCP and eumenorrhoeic groups for work capacity (P = 0.50, ηp 2 = 0.03) or power output profile (P = 0.52, ηp 2 = 0.02).
Physiological measures
Body temperatures (Figure 2)
Figure 2. Mean ± SD rectal temperature (T rec, n = 10) and weighted mean skin temperature ( sk, n = 10) during exercise in dry (DRY) and humid (HUM) heat during the qF and qL phase.
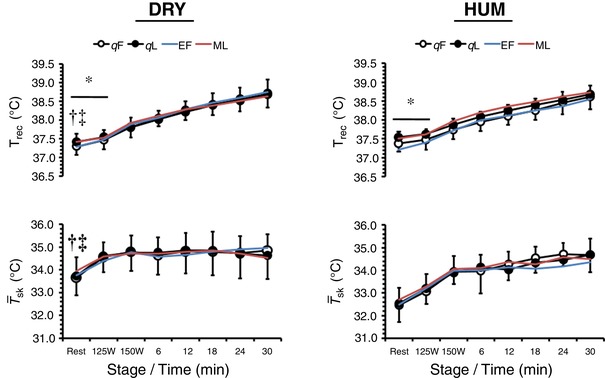
*Significant difference between qF − qL within environment. †Significant difference between corresponding qF − HUM value. ‡Significant difference between corresponding qL − HUM value. Mean early follicular (EF) and mid‐luteal (ML) values are provided for our previous eumenorrhoeic cohort (Lei et al. 2017).
The T rec when resting was 0.15 ± 0.21°C higher in qL than in qF (P = 0.05, ηp 2 = 0.35) and was 0.12 ± 0.12°C higher during HUM than during DRY (P = 0.01, ηp 2 = 0.52). The rise in T rec during fixed‐intensity exercise differed between OCP phases as a function of work‐rate (OCP phase × time: P = 0.05, ηp 2 = 0.35) but was not dependent on environment (interaction: P = 0.17, ηp 2 = 0.20), with the between‐phase difference seen at rest still being evident at 125 W (0.12 ± 0.19°C) but not at 150 W (0.06 ± 0.21°C). During self‐paced exercise, T rec was similar between OCP phases (P = 0.74, ηp 2 = 0.05) and environments (P = 0.54, ηp 2 = 0.08) but continued to increase with time (P < 0.01, ηp 2 = 0.94) until the end of exercise.
Resting sk was similar between OCP phases (P = 0.78, ηp 2 = 0.01) but was 1.2 ± 0.7°C higher during DRY than during HUM (P < 0.01, ηp 2 = 0.76). During fixed‐intensity exercise, sk was similar between OCP phases (P = 0.85, ηp 2 < 0.01) but differed between environments as a function of work‐rate (environment × work‐rate: P < 0.01, ηp 2 = 0.61) such that the difference between work rates was greater during HUM (0.79 ± 0.42°C) than during DRY (0.19 ± 0.52°C). During self‐paced exercise, sk differed between environments as a function of time (environment × time: P = 0.01, ηp 2 = 0.31). Specifically, sk values were maintained constant during DRY but continued to increase by ∼0.7°C during HUM.
Between‐group analysis revealed no differences between the OCP and eumenorrhoeic groups for T rec (all P > 0.47, ηp 2 < 0.03) or sk (all P > 0.58, ηp 2 < 0.03) during any stage of the protocol.
Cardiovascular and thermoeffectors (Table 3 and Figures 3–5)
Table 3.
MAP (n = 8), (n = 6), FVR (n = 8) and SV (n = 6) at rest and during fixed‐intensity exercise in dry (DRY) and humid (HUM) heat during the qF and qL phase
| DRY | HUM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| qF | qL | qF | qL | |||||||||
| Rest | 125 W | 150 W | Rest | 125 W | 150 W | Rest | 125 W | 150 W | Rest | 125 W | 150 W | |
| MAP (mmHg) | 90 ± 6 | 98 ± 5 | 103 ± 7 | 84 ± 5 | 95 ± 7 | 100 ± 7 | 87 ± 7 | 98 ± 6 | 103 ± 6 | 84 ± 6 | 95 ± 8 | 100 ± 9 |
| (L min−1) | 8 ± 2 | 20 ± 3 | 21 ± 4 | 7 ± 2 | 22 ± 7 | 20 ± 3 | 7 ± 2 | 20 ± 2 | 21 ± 3 | 8 ± 4 | 20 ± 3 | 23 ± 3a |
| FVR (mm Hg min dL−1) | 11.9 ± 5.8 | 7.4 ± 2.3 | 5.6 ± 1.9 | 7.7 ± 1.5b | 5.3 ± 1.7 | 4.9 ± 1.5 | 7.2 ± 1.4b | 5.2 ± 0.9 | 3.8 ± 0.5 | 6.6 ± 1.6 | 4.2 ± 1.8 | 4.0 ± 1.6 |
| SV (mL) | 109 ± 33 | 138 ± 24 | 133 ± 25 | 94 ± 33 | 160 ± 59 | 128 ± 28 | 91 ± 20 | 140 ± 18 | 132 ± 21 | 122 ± 72 | 149 ± 31 | 154 ± 23c, d |
Data are the mean ± SD.
Significant difference from the preceding time‐point.
Significant difference from the corresponding qF − DRY time‐point.
Significant difference from the corresponding qL − DRY time‐point.
Significant difference from the corresponding qF − HUM time‐point.
Resting SV, and MAP were similar between OCP phases and environments (all P > 0.22, ηp 2 < 0.20), whereas resting HR was 2 ± 2 beats min−1 higher in DRY, with this being more evident in qF than in qL (environment × OCP phase: P = 0.08, ηp 2 = 0.30, possible type II error), and resting FVR was 2.9 ± 2.6 mm Hg min dL−1 higher during DRY than during HUM (P = 0.02, ηp 2 = 0.59), although this may have been more evident in qF than in qL (environment × OCP phase: P = 0.08, ηp 2 = 0.37, possible type II error). During fixed‐intensity exercise, and possibly also SV differed between environments as a function of OCP phase and work‐rate (environment × phase × time: P = 0.01, ηp 2 = 0.75 and P = 0.09, ηp 2 = 0.47, possible type II error, respectively), such that values were highest at 150 W during qL‐HUM. MAP and HR were similar between OCP phases and environments (all P > 0.16, ηp 2 < 0.20) but increased with work rate (both P < 0.01, ηp 2 > 0.86). Thus, during fixed‐intensity exercise, FVR was 1.5 ± 1.0 mm Hg min dL−1 higher during DRY than during HUM (P < 0.01, ηp 2 = 0.74) and differed between OCP phase as a function of work‐rate (OCP phase × time: P < 0.01, ηp 2 = 0.82) such that FVR was lower during qL than qF at 125 W (by 1.6 ± 2.2 mL dL min mm Hg−1) but not at 150 W (0.2 ± 1.8 mL dL min mm Hg−1). During self‐paced exercise, HR changed over time (P < 0.01, ηp 2 = 0.71) independent of OCP phase and environment (both P > 0.24, ηp 2 < 0.14), with a characteristic end‐spurt higher than all previous time‐points by >10 beats min−1.
Figure 5. Individual traces, and group onset threshold and thermosensitivity for FBF.
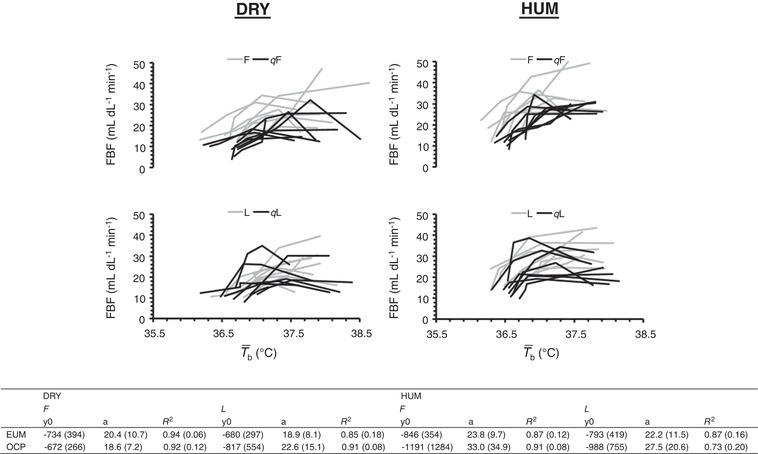
Upper: individual traces for FBF (n = 8) against mean body temperature ( b) during exercise in dry (DRY) and humid (HUM) heat during the qF and qL phase. Early follicular (EF) and mid‐luteal (ML) traces are provided for our previous eumenorrhoeic cohort (Lei et al. 2017). Lower: mean ± SD values for onset threshold (y 0, i.e. b in °C) and thermosensitivity (a) of FBF (in mL dL−1 min−1 °C−1) responses using simple linear regression (y = y 0 + a * x) during the qF and qL phase for the matched eummenorrhoeic (EUM) (Lei et al. 2017) and OCP groups. The values give an indication of the central modification of the thermoeffector (skin blood flow), thus demonstrating that chronic consumption of an OCP, (quasi‐) phase and environment, causes no meaningful shift in vasodilatatory control.
Between‐group analysis revealed no effect of OCP usage for SV, HR, and MAP (all P > 0.23, ηp 2 < 0.10); however, FVR was 2.7 ± 2.3 mm Hg min dL−1 (P < 0.01, ηp 2 = 0.45) and 1.1 ± 1.1 mm Hg min dL−1 (P = 0.01, ηp 2 = 0.37) higher at rest and during fixed‐intensity exercise, respectively, in the current OCP than in the previous eumenorrhoeic group.
Resting LSR was similar between OCP phases and environments (both P > 0.21, ηp 2 < 0.17), whereas resting FBF differed between environments as a function of OCP phase (environment × OCP phase: P = 0.05, ηp 2 = 0.46), such that values were higher during HUM than during DRY at both quasi‐phases and higher in qL than in qF during DRY. During fixed‐intensity exercise, LSR was 0.07 ± 0.06 mg cm−2 min−1 lower in qL than in qF (P < 0.01, ηp 2 = 0.60), 0.08 ± 0.10 mg cm−2 min−1 lower during DRY than during HUM (P = 0.04, ηp 2 = 0.40) and increased with work‐rate (P < 0.01, ηp 2 = 0.86). Whereas FBF differed between OCP phases as a function of environment (environment × OCP phase: P = 0.05, ηp 2 = 0.45) and work‐rate (time × OCP phase: P < 0.01, ηp 2 = 0.53), such that FBF was higher during DRY in qF (by 7 ± 3 mL dL−1 min−1) but not in qL (by 4 ± 6 mL dL min−1) and increased from 125 to 150 W (by 6 ± 4 mL dL−1 min−1) in qF but not in qL. During self‐paced exercise, LSR was 0.13 ± 0.10 mg cm−2 min−1 higher during HUM than during DRY (P < 0.01, ηp 2 = 0.64) and continued to increase with time (P < 0.01, ηp 2 = 0.79) until the end of exercise, although this tended to be more pronounced during HUM (environment × time: P = 0.06, ηp 2 = 0.22, possible type II error), regardless of OCP phase (interaction: P = 0.43, ηp 2 = 0.10). Neither onset thresholds, nor thermosensitivities of the effector responses were affected by OCP phase or environment (all P > 0.19, ηp 2 < 0.23). Water consumption was similar between OCP phases and environments (597 ± 193 mL; both P > 0.46, ηp 2 < 0.06), as was WBSR (843 ± 218 g h−1; both P > 0.28, ηp 2 < 0.13), resulting in a 1.3 ± 0.6% loss of body mass that was similar between OCP phases and environments (both P > 0.47, ηp 2 < 0.06).
Between‐group analysis revealed no differences between the OCP and eumenorrhoeic groups for LSR at rest or during fixed‐intensity exercise (both P > 0.19, ηp 2 < 0.11); however, LSR was 0.23 ± 0.21 mg cm−2 min−1 lower (P < 0.01, ηp 2 = 0.43) during the self‐paced time trial in the current OCP than in the previous eumenorrhoeic group. FBF was 4 ± 3 mL dL−1 min−1 (P < 0.01, ηp 2 = 0.54) and 6 ± 6 mL dL−1 min−1 (P = 0.01, ηp 2 = 0.38) lower at rest and during fixed‐intensity exercise, respectively, in the current OCP than in the previous eumenorrhoeic group. The onset threshold and thermosensitivity for LSR (both P < 0.01, ηp 2 = 0.54) but not FBF (both P > 0.54, ηp 2 < 0.03) revealed differences between the OCP and eumenorrhoeic groups, such that LSR occurred at a higher b and its sensitivity was lower in the current OCP users. Water consumption and percentage loss of body mass were similar between groups (both P > 0.11, ηp 2 < 0.04); however, WBSR was 200 ± 265 g (P = 0.03, ηp 2 = 0.24) lower in the current OCP than in the previous eumenorrhoeic group.
Respiratory (data not shown)
Hyperventilation was evident at rest (14–16 L min−1), although similar between OCP phases and environments (both P > 0.27, ηp 2 < 0.13) and therefore data derived from this (e.g. thermodynamic) were not analysed further or displayed. During fixed‐intensity exercise, ventilation was 2.5 ± 3.1 L min−1 higher in qL than in qF (P = 0.03, ηp 2 = 0.41) and increased with work‐rate (P < 0.01, ηp 2 = 0.95). was similar between OCP phases and environments (both P > 0.56, ηp 2 < 0.06) during fixed‐intensity exercise. Participants were exercising at 61 ± 11%, 72 ± 12% and 75 ± 8% of their at 125 W, 150 W and during the self‐paced time‐trial, respectively, and similar between OCP phases and environments (both P > 0.57, ηp 2 < 0.04) but increased with work‐rate (P < 0.01, ηp 2 = 0.78) from 125 W to 150 W only. Between‐group analysis revealed no differences between the OCP and eumenorrhoeic groups for ventilation, or percentage during fixed‐intensity or self‐paced exercise (all P > 0.47, ηp 2 < 0.05).
Thermodynamics (Figure 6)
Figure 6. Mean ± SD rate of metabolic heat production (M − W, n = 10), required evaporative cooling for heat balance (E req, n = 10), maximal evaporative capacity of the environment (E max, n = 10) and HSI (n = 10) during exercise in dry (DRY) and humid (HUM) heat during the qF and qL phase.
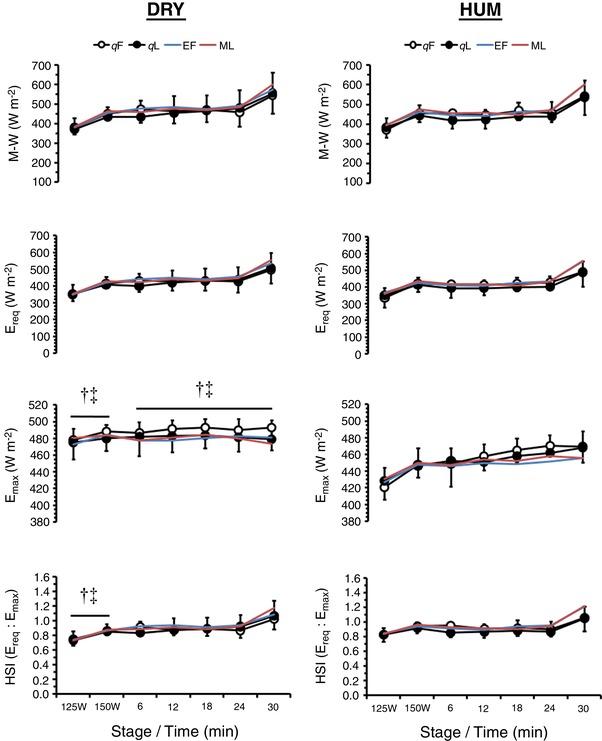
†Significant difference between corresponding qF − HUM value. ‡Significant difference between corresponding qL − HUM value. Mean early follicular (EF) and mid‐luteal (ML) values are provided for our previous eumenorrhoeic cohort (Lei et al. 2017).
During fixed‐intensity exercise M − W was similar between OCP phases and environments (both P > 0.50, ηp 2 < 0.06) but increased with work‐rate (P < 0.01, ηp 2 = 0.94). E max was 44 ± 14 W m−2 higher during DRY than during HUM (P < 0.01, ηp 2 = 0.92) and increased with work‐rate (P < 0.01, ηp 2 = 0.80), whereas E req was similar between OCP phases and environments (both P > 0.57, ηp 2 < 0.04) but increased with work‐rate (P < 0.01, ηp 2 = 0.94). Consequently, the HSI was similar between OCP phases (P = 0.71, ηp 2 < 0.02) but was 0.09 ± 0.06 a.u. lower during DRY than during HUM (P < 0.01, ηp 2 = 0.71) and increased with work‐rate (P < 0.01, ηp 2 = 0.82). During self‐paced exercise, M − W was similar between OCP phases (P = 0.48, ηp 2 = 0.07) but increased with time (P < 0.01, ηp 2 = 0.75). E max was 26 ± 15 W m−2 higher during DRY than during HUM (P < 0.01, ηp 2 = 0.75) and increased with time (P < 0.01, ηp 2 = 0.54). E req was similar between OCP phases and environments (both P > 0.18, ηp 2 < 0.19) but increased with time (P < 0.01, ηp 2 = 0.70). Consequently, the HSI was similar between OCP phases and environments (both P > 0.47, ηp 2 < 0.06) but increased with time (P < 0.01, ηp 2 = 0.66).
Between‐group analysis revealed no differences between the OCP and eumenorrhoeic groups for M − W during fixed‐intensity or self‐paced exercise (both P > 0.25, ηp 2 < 0.08). E max, E req and HSI were similar between groups at all time‐points (all P > 0.12, ηp 2 < 0.15).
Discussion
We tested the hypotheses that, in female athletes who are chronic users of the combined monophasic OCP: (i) a small endogenous thermoregulatory rhythm would persist during their active OCP cycle, and would be nullified by behavioural adjustments when faced with the heat stress of exercise in warm environments; (ii) there would be an interplay with the different thermal environments; and (iii) autonomic responses would be attenuated compared to our matched cohort of eumenorrhoeic athletes. In support of our hypotheses, (i) a small increase in T core occurs during qL at rest and fixed‐intensity exercise, although this disappears before behavioural adjustments are utilised; (ii) autonomic heat loss mechanisms were activated to a greater extent during HUM, whereas behavioural thermoregulation was effective in minimising further strain and did not differ between environments; and (iii) chronic OCP use impairs the sweating response, although this impairment was not sufficient to affect T core during exercise. These results indicate that, under the conditions of this investigation, the evaporative capacity of the environment determines endurance performance and, although the sudomotor response is attenuated by chronic OCP use, behavioural adjustments by female athletes are influenced by the environmental conditions but not chronic OCP use.
A quasi‐phase related shift in T core and, to a lesser extent, heat loss mechanisms occurs despite OCP use
We observed a consistent and significant but small quasi‐phase increase in resting T core, by 0.15°C from qF to qL that persisted into the fixed‐intensity exercise (Fig. 2). To our knowledge, this finding during active OC use is unique. Previous investigations suggested that this shift be regarded as a strong and residual effect of the endogenous menstrual cycle (Grucza et al. 1993; Martin & Buono, 1997; Rogers & Baker, 1997; Charkoudian & Johnson, 1997 a; Tenaglia et al. 1999; Sunderland & Nevill, 2003), yet, in these studies, the comparison always occurred when the females were actively taking OC compared to their placebo week. However, this finding is difficult to explain because, in the present study, both endogenous (Table 2) and exogenous (by design, not measured) concentrations of progestogens and oestrogens remained unchanged between qF and qL. This is probably not an estrogenic effect because it is known that, when progestogens and oestrogens are naturally elevated or administered, a progestogen‐dominant thermogenic response ensues (Israel & Schneller, 1950; Rothchild & Barnes, 1952). However, the current design of maintained OC use is sub‐optimal for determining causal mechanisms for the apparent ‘lack of an OC effect in resetting the thermoregulatory balance point’ (Tenaglia et al. 1999). For this, other study designs (i.e. gonadotrophin‐releasing hormone suppression) would be required (Stachenfeld & Taylor, 2014), although we are unaware of any such published data on measures of regulated T core. Furthermore, hormone exposure does not necessarily determine its effect, and both central and peripheral thermoregulatory receptors respond differently to synthetic progestin compared to progesterone (Charkoudian & Stachenfeld, 2014).
The quasi‐phase related difference in T core had disappeared by 12 min of fixed‐intensity exercise. This could be the result of a sufficient reserve in the capacity of the thermoregulatory system. This ‘disappearance’ has been observed by some (Tenaglia et al. 1999; Sunderland & Nevill, 2003) but not all investigators (Grucza et al. 1993; Rogers & Baker, 1997), whereas the reverse has also been reported (Martin & Buono, 1997). Notably, this quasi‐phase related difference in T core is similar in magnitude to that observed in our matched eumenorrhoeic cohort (Lei et al. 2017) and supports previous observations at rest and during heat stress of a smaller difference in the biphasic T core in trained women (Kuwahara et al. 2005 a,b; Lei et al. 2017).
Concurrent to the quasi‐phase difference observed for T core, LSR during fixed‐intensity exercise was lower during qL, whereas FBF was indistinguishable between environments and intensity during qL yet during qF was increased during DRY (vs. HUM) and at 150 W (vs. 125 W), indicating a differential response between quasi‐phases (Fig. 3). However, the activation of heat loss responses relative to b (onset threshold, thermosensitivity) was unaffected by OCP phase. Therefore, the physiological significance of a shift in resting T core < 0.2°C that effectively disappears during exercise should be considered closely.
Figure 3. Mean ± SD LSR (n = 9) and FBF (n = 8) against time and mean body temperature ( b) during exercise in dry (DRY) and humid (HUM) heat during the qF and qL phase.
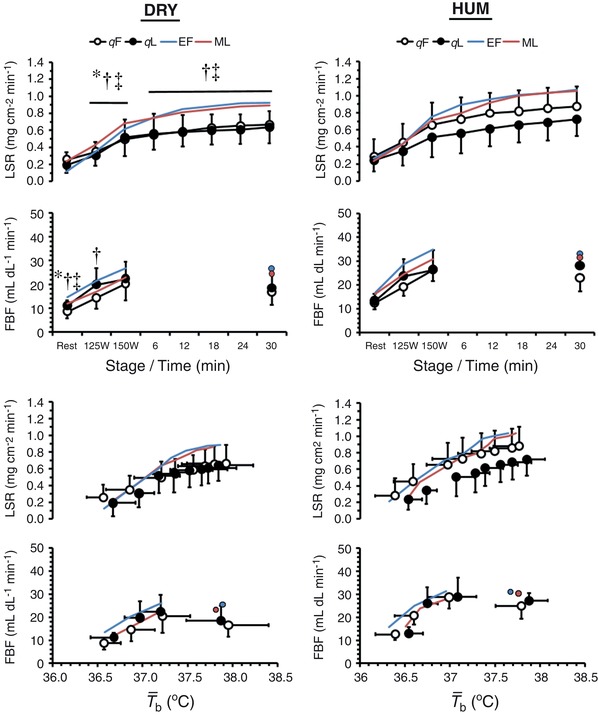
*Significant difference between qF − qL within environment. †Significant difference between corresponding qF − HUM value. ‡Significant difference between corresponding qL − HUM value. Mean early follicular (EF) and mid‐luteal (ML) values are provided for our previous eumenorrhoeic cohort (Lei et al. 2017).
The evaporative capacity of the environment infers greater performance and thermoregulatory strain than does an OCP cycle
Exercise performance was unaffected by the quasi‐phase of the OCP cycle but was impaired by the humid tropical environment despite it being WBGT‐matched to the dry heat (Fig. 1). This result confirms our observations in eumenorrhoeic athletes (Lei et al. 2017) and indicates that a reduction in the evaporative power of the environment is of greater performance consequence to a female athlete than is her menstrual/OCP cycle. Evaporative cooling rates decrease when the vapour pressure gradient between skin and environment is reduced (Morimoto et al. 1967; Shapiro et al. 1980; Frye & Kamon, 1983). Therefore, our females demonstrated a performance–thermoregulatory trade‐off, where their reduction in (self‐paced) work completed during HUM (Fig. 1) maintained a similar thermoregulatory strain (Fig. 2). These results are also in agreement with those of the study by Tenaglia et al. (1999) but are in contrast to those of Sunderland & Nevill (2003) who observed an improved running distance during qL in their OCP users. However, both studies compared their females in the active pill vs. placebo phases.
Chronic OCP use impairs sudomotor but not behavioural thermoregulatory responses
The careful matching of groups and experimental procedures of this and our previous study on eumenorrhoeic females (Lei et al. 2017) permits us to isolate the effects of chronic OCP use on autonomic and behavioural thermoregulation during heat stress. Namely, we used the same exercise protocols, ambient conditions and phases of the endogenous menstrual cycle, and matched our OCP and eumenorrhoeic cohorts for all appropriate physical and functional characteristics. This matching was so successful in as much as comparable self‐paced mean power output and work were completed between the groups, which lead to M − W being similar (Figs. 1 and 6). Thus, the between‐group differences evident for the onset threshold and thermosensitivity of the sweating response (Figs. 3 and 4) demonstrate that long‐term combined, monophasic OCP use in endurance athletes affects an autonomic thermoregulatory response. However, behavioural thermoregulation was maintained (in that self‐pacing was evident) with no differential effect on regulated body temperature(s), which indicates that overall thermoregulation and performance were not compromised. Thus, although both LSR and WBSR were higher in our previous cohort, this sweat was either not evaporated (dripped) or too small to affect evaporative heat loss.
Figure 4. Individual traces, and group onset threshold and thermosensitivity for LSR.
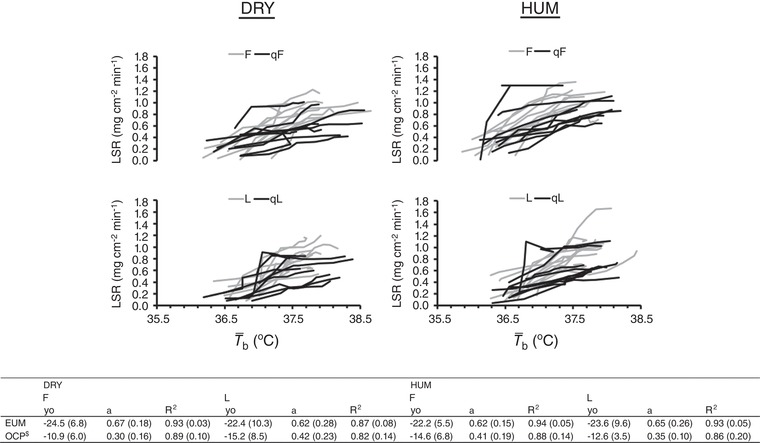
Upper: individual traces for LSR (n = 9) against mean body temperature ( b) during exercise in dry (DRY) and humid (HUM) heat during the qF and qL phase. Early follicular (EF) and mid‐luteal (ML) traces are provided for our previous eumenorrhoeic cohort (Lei et al. 2017). Lower: mean ± SD values for onset threshold (y 0, i.e. b in °C) and thermosensitivity (a) of LSR (in mg cm−2 min−1 °C−1) responses using simple linear regression (y = y 0 + a * x) during the qF and qL phase for the matched eummenorrhoeic (EUM) (Lei et al. 2017) and OCP groups. $Significantly different from EUM. The values give an indication of the central modification of the thermoeffector (sweating), thus demonstrating that chronic consumption of an OCP, but not (quasi‐) phase, nor environment, causes a meaningful shift in sweating control.
The potential mechanism(s) through which chronic OCP use affects an autonomic heat loss mechanism is not well understood, although this probably concerns a direct central action given the upward (i.e. rightward) shift in T core for the onset of sweating (Figs. 3 and 4). Oestrogens and progestogens both readily cross the blood–brain barrier and can inhibit temperature‐sensitive neurons in the preoptic/anterior hypothalamus (Lincoln, 1967; Nakayama, 1975). The direction of this shift implies that this constraint of heat dissipation is a result of progestin inhibiting warm‐sensitive neuronal activity (Nakayama, 1975). Mediation by a pyrogen or heat shock proteins (Rogers & Baker, 1997; Charkoudian & Johnson, 1997 b; Chang et al. 1998) is probably not possible. Equally, our results are probably not the result of an interaction with the system(s) that regulate volume of the extracellular fluid (Fortney et al. 1981; Fortney et al. 1983) because, although we did not quantify plasma volume, SV and were similar at rest and during exercise between our OCP and eumenorrhoeic cohorts. However, our findings could be a result of changes in osmotic pressure (Fortney et al. 1984) because some studies (Stachenfeld et al. 1999; Stachenfeld et al. 2000) but not others (Rogers & Baker, 1997) have reported a reduction in plasma osmolality with ≥1 month of OCP use. Interestingly, Rogers & Baker (1997) still observed a delay in the sweating onset despite no reduction in osmolality, whereas Stachenfeld et al. (2000) observed no delay with OCP use, indicating that the effect of plasma osmolality may be small.
Acute and chronic OCP use has different effects on thermoregulation (Charkoudian & Johnson, 1997 a; Stachenfeld et al. 2000). Previous investigations have compared women when actively taking OC compared to their placebo week, even when separate groups of chronic OCP‐ and non‐users were compared (Grucza et al. 1993; Tenaglia et al. 1999; Sunderland & Nevill, 2003). Nevertheless, data comparing our OCP and eumenorrhoeic females support the findings of several previous observations, such as a higher onset threshold for sweating (Rogers & Baker, 1997; Charkoudian & Johnson, 1997 a) and an attenuated gain for local sweat rate (Grucza et al. 1993). However, our result of resting (and exercising) T core being similar between groups is in contrast to the upward‐shift reported as a result of OCP use (Grucza et al. 1993; Sunderland & Nevill, 2003).
The OCP and quasi‐phase exert haemodynamic effects
A combined OCP affects both central (Walters & Lim, 1970; Lehtovirta, 1974 a) and peripheral (Lehtovirta, 1974 b) haemodynamics at rest and during exercise (Lehtovirta et al. 1977), which is probably caused by the oestrogen component (Lehtovirta, 1974 a,b) and requires longer than 1 month of OC consumption (Walters & Lim, 1970; Lehtovirta, 1974 a,b; Stachenfeld et al. 2000). We observed no differences in the central cardiovascular response (SV and ) between the OCP and eumenorrhoeic groups. However, FVR was higher in our current OCP compared to the eumenorrhoeic cohort, a finding that contrasts with the lower peripheral resistance found following 2 months of combined OCP use (Lehtovirta, 1974 a,b). Methodological differences probably account for these discrepant results because (i) vascular effects of the synthetic and endogenous hormones may be a confounding factor and our OCP had a lower concentration of 17β‐oestradiol (Table 2) and (ii) our OCP users had been taking the OCP ≥12 months, whereas Lehtovirta (1974 a,b) used a within‐subject design (pre‐post) following 2 months of OCP use.
The central cardiovascular response was similar between quasi‐phases of the OCP cycle at rest but showed augmentation of SV (∼20‐30 mL) and (∼2‐4 L min−1) during qL compared to qF at matched workloads (∼70% ) and reduced sweating power (during HUM) (Table 3). The peripheral vascular response was correspondingly affected by the OCP cycle, such that FVR was lower at rest during qL during HUM and during exercise at ∼60% qL (Table 3). Most previous investigations (Walters & Lim, 1970; Lehtovirta, 1974 a,b; Stachenfeld et al. 2000) did not compare across an OCP cycle, such as in the current study. Moreover, the observed qL reduction in FVR at rest and during fixed‐intensity exercise is similar in magnitude to that of our eumenorrhoeic cohort (Lei et al. 2017), indicating that the menstrual cycle could be exerting a peripheral cardiovascular effect beyond the regulated T core in these OCP users. In support of this, the menstrual cycle phase has been shown to modulate vessel conductance and resistance that parallel changes in oestrogen (Williams et al. 2001), nitric oxide production (Kharitonov et al. 1994) and endothelial nitric oxide synthase expression (Taguchi et al. 2000), the most probable cause(s) of vascular smooth muscle relaxation (Charkoudian & Stachenfeld, 2016). Our a priori study design does not allow for further insight; this would require, for example, cutaneous microdialysis with concurrent pharmacological administration to determine endothelium‐dependent and independent factors.
Considerations
By design, the present study tested whether any quasi‐phase related endogenous thermoregulatory rhythms persist during the active pill phase in chronic OCP users, therefore capturing 75% of their OCP cycle and mimicking real‐world use in athletes (i.e. competition/performance occurring during active pill use). Therefore, it would simply be speculation and beyond the scope of the data obtained in the present study to determine how these responses compare to the 25% of the OCP cycle in which athletes consume a placebo pill; nevertheless, this warrants further investigation. Furthermore, we tested women taking the combined, monophasic OCP because this reflects most athlete use, yet there is evidence that these responses could differ in those taking a progestin‐only OCP (Stachenfeld et al. 2000).
The same de‐limitations are present in the present study as in our previous study on eumenorrhoeic females (Lei et al. 2017) and were unavoidable in the protocol and design as a result of a direct comparison between these cohorts; namely, the lack of an untrained cohort, periods of fixed‐intensity and a variable‐intensity exercise that were unequal (and limited) in duration, as well as a lack of other physiological measures such as leg blood flow and arterial oxygenation. Similarly, it is worth noting that the current data are derived from an index of T core known to exhibit a lag‐time (compared to oesophageal temperature; Mündel et al. 2016) and from limited data points for effector responses. Equally, and as noted above, several of the statistical differences between quasi‐phases probably have little physiological consequence and are within the biological variability or measurement error, such as the onset threshold for sweating (∼0.1 °C; Brengelmann et al. 1994) and local sweat rate (0.05–0.2 mg cm−2 min−1; Kenefick et al. 2012; Morriss et al. 2013), albeit these investigations yield data predominantly from male not female participants. Nevertheless, the above is probably not the case for the between‐group (OCP vs. eumenorrhoeic) results, which demonstrate clear differences in the onset threshold for sweating (Figure 4). However, verification of these results is warranted probably as a result of our less conventional method of determining onset thresholds. The sensitivity of our partitional calorimetry‐derived data could be improved by the use of a direct calorimeter, especially because we were unable to accurately estimate the rate of evaporative heat loss. However, such a facility has very limited access at much greater expense, exhibits a greater lag and the conditions used during HUM are probably beyond the reported operating range of such a calorimeter (Reardon et al. 2006; Kenny et al. 2008; Kenny & Jay, 2013). We also recognise that homeostatic systems interact, such that the regulation of body temperature is not separate and distinct from, for example, that of fluid, energy substrate and metabolite balance (Boulant & Silva, 1988). Therefore, representative measures, particularly of plasma osmolality and volume, would have further strengthened our conclusions. Despite careful matching of the groups, our current OCP cohort were an average of 9 years younger than our previous eumenorrhoeic females (Lei et al. 2017). Nevertheless, all other relevant physical and functional characteristics were similar (Table 1) and we are unaware of any research indicating that, in such pre‐menopausal women, this magnitude of an age difference should confound the results.
Finally, the results of the present study should be of interest to those (thermoregulation) researchers whose participants include OC users because there is now evidence to question the validity of treating the active OCP cycle as a way of controlling for menstrual cycle hormones that are known to affect thermoregulation.
Conclusions
The present study demonstrates that, when well‐trained women chronically using the combined, monophasic OCP exercise in heat‐stressful environments, a performance‐thermoregulatory trade‐off occurs to ensure overall thermoregulation is not impaired. The biggest determinant of this trade‐off is the evaporative capacity of the environment. Finally, an endogenous thermoregulatory rhythm persists despite chronic OCP use.
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
TM was responsible for the conception of the study. THL, JDC, ZJS, SRS, BGP, MJB and TM were responsible for the design of the work. THL, SRS, BGP, MJB and TM were responsible for data acquisition. THL and TM were responsible for data analysis. THL, JDC, ZJS and TM were responsible for data interpretation. THL and TM were responsible for drafting intellectual content. THL, JDC, ZJS, SRS, BGP, MJB and TM were responsible for critically revising intellectual content. All authors approved the final version of the manuscript submitted for publication. All experimental procedures were performed in the School of Sport, Exercise and Nutrition, Massey University, Palmerston North.
Funding
No funding was received for the present study.
Acknowledgements
We would like to specifically thank the very dedicated group of women that participated in this study.
Biographies
Toby Mündel is a tenured academic at Massey University in New Zealand, where he is teacher, supervisor and researcher of human exercise physiology, particularly temperature regulation during heat stress.

Tze‐Huan (Joe) Lei is a doctoral candidate under the supervision of Dr Toby Mündel at Massey University in New Zealand. His research has focussed on human exercise thermoregulation, particularly with environmental heat stress. His work has determined the physiological, behavioural and perceptual consequences of (i) the menstrual cycle and oral contraception and (ii) the thermal profile of the environment (i.e. ambient temperature and humidity). Having previously been educated and trained in New Zealand and his native Taiwan, Joe will be continuing his research apprenticeship via a prestigious JSPS International Fellowship for Research in Japan, aiming to study the induction and decay of dry and humid heat acclimation on sweat gland function with Dr Narihiko Kondo at Kobe University.
Edited by: Scott Powers & Bettina Mittendorfer
References
- Belding HS & Hatch TF (1955). Index for evaluating heat stress in terms of resulting physiological strain. Heat Piping Air Cond 27, 129–136. [Google Scholar]
- Brengelmann GL, Savage MV & Avery DH (1994). Reproducibility of core temperature threshold for sweating onset in humans. J Appl Physiol 77, 1671–1677. [DOI] [PubMed] [Google Scholar]
- Brotherhood JR (2008). Heat stress and strain in exercise and sport. J Sci Med Sport 11, 6–19. [DOI] [PubMed] [Google Scholar]
- Budd GM (2008). Wet‐bulb globe temperature (WBGT) – its history and its limitations. J Sci Med Sport 11, 20–32. [DOI] [PubMed] [Google Scholar]
- Chang RT, Lambert GP, Moseley PL, Chapler FK & Gisolfi CV (1998). Effect of estrogen supplementation on exercise thermoregulation in premenopausal women. J Appl Physiol 85, 2082–2088. [DOI] [PubMed] [Google Scholar]
- Charkoudian N & Johnson JM (1997. a). Modification of active cutaneous vasodilation by oral contraceptive hormones. J Appl Physiol 83, 2012–2018. [DOI] [PubMed] [Google Scholar]
- Charkoudian N & Johnson JM (1997. b). Altered reflex control of cutaneous circulation by female sex steroids is independent of prostaglandins. Am J Physiol Heart Circ Physiol 276, H1634–H1640. [DOI] [PubMed] [Google Scholar]
- Charkoudian N & Joyner MJ (2004). Physiologic considerations for exercise performance in women. Clin Chest Med 25, 247–255. [DOI] [PubMed] [Google Scholar]
- Charkoudian N & Stachenfeld NS (2014). Reproductive hormone influences on thermoregulation in women. Compr Physiol 4, 793–804. [DOI] [PubMed] [Google Scholar]
- Charkoudian N & Stachenfeld N (2016). Sex hormone effects on autonomic mechanisms of thermoregulation in humans. Auton Neurosci 196, 75–80. [DOI] [PubMed] [Google Scholar]
- Cheung SS, McLellan TM & Tenaglia S (2000). The thermophysiology of uncompensable heat stress. Physiological manipulations and individual characteristics. Sports Med 29, 329–359. [DOI] [PubMed] [Google Scholar]
- Defares J (1958). Determination of PvCOCO2 from the exponential CO2 rise during rebreathing. J Appl Physiol 13, 159–164. [DOI] [PubMed] [Google Scholar]
- Dubois D & Dubois EF (1916). A formula to estimate approximate surface area if height and weight be known. Arch Intern Med 17, 863–871. [Google Scholar]
- Fortney SM, Nadel ER, Wenger CB & Bove JR (1981). Effect of blood volume on sweating rate and body fluids in exercising humans. J Appl Physiol Respir Environ Exerc Physiol 51, 1594–1600. [DOI] [PubMed] [Google Scholar]
- Fortney SM, Wenger CB, Bove JR & Nadel ER (1983). Effect of blood volume on forearm venous and cardiac stroke volume during exercise. J Appl Physiol Respir Environ Exerc Physiol 55, 884–890. [DOI] [PubMed] [Google Scholar]
- Fortney SM, Wenger CB, Bove JR & Nadel ER (1984). Effect of hyperosmolality on control of blood flow and sweating. J Appl Physiol Respir Environ Exerc Physiol 57, 1688–1695. [DOI] [PubMed] [Google Scholar]
- Frye A & Kamon E (1983). Sweating efficiency in acclimated men and women exercising in humid and dry heat. J Appl Physiol 54, 972–977. [DOI] [PubMed] [Google Scholar]
- Gagnon D, Jay O & Kenny GP (2013). The evaporative requirement for heat balance determines whole‐body sweat rate during exercise under conditions permitting full evaporation. J Physiol 591, 2925–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graichen H, Rascati R & Gonzalez R (1982). Automatic dew‐point temperature sensor. J Appl Physiol 52, 1658–1660. [DOI] [PubMed] [Google Scholar]
- Grucza R, Pekkarinen H, Titov EK, Kononoff A & Hänninen O (1993). Influence of the menstrual cycle and oral contraceptives on thermoregulatory responses to exercise in young women. Eur J Appl Physiol Occup Physiol 67, 279–285. [DOI] [PubMed] [Google Scholar]
- Harvey O & Crockett HE (1932). Individual differences in temperature changes of women during the course of the menstrual cycle. Hum Biol 4, 453–468. [Google Scholar]
- Hertzman A, Randall W, Peiss C & Seckendorf R (1952). Regional rates of evaporation from the skin at various environmental temperatures. J Appl Physiol 5, 153–161. [DOI] [PubMed] [Google Scholar]
- Hessemer V & Brück K (1985). Influence of menstrual cycle on shivering, skin blood flow, and sweating responses measured at night. J Appl Physiol 59, 1902–1910. [DOI] [PubMed] [Google Scholar]
- Israel SL & Schneller O (1950). The thermogenic property of progesterone. Fertil Steril 1, 53–65. [Google Scholar]
- Janse de Jonge XA, Thompson MW, Chuter VH, Silk LN & Thom JM (2012). Exercise performance over the menstrual cycle in temperate and hot, humid conditions. Med Sci Sports Exerc 44, 2190–2198. [DOI] [PubMed] [Google Scholar]
- Jeukendrup A, Saris W, Brouns F & Kester AD (1996). A new validated endurance performance test. Med Sci Sports Exerc 28, 266–270. [DOI] [PubMed] [Google Scholar]
- Jones NL, Robertson DG & Kane JW (1979). Difference between end‐tidal and arterial PCO2 in exercise. J Appl Physiol 47, 954–960. [DOI] [PubMed] [Google Scholar]
- Kenefick RW, Cheuvront SN, Elliott LD, Ely BR, Sawka MN (2012). Biological and analytical variation of the human sweating response: implications for study design and analysis. Am J Physiol Regul Integr Comp Physiol 302, R252–R258. [DOI] [PubMed] [Google Scholar]
- Kenney WL (1998). Heat flux and storage in hot environments. Int J Sports Med 19 Suppl 2, S92–95. [DOI] [PubMed] [Google Scholar]
- Kenny GP, Webb P, Ducharme MB, Reardon FD & Jay O (2008). Calorimetric measurement of postexercise net heat loss and residual body heat storage. Med Sci Sports Exerc 40, 1629–1636. [DOI] [PubMed] [Google Scholar]
- Kenny GP & Jay O (2013). Thermometry, calorimetry, and mean body temperature during heat stress. Compr Physiol 3, 1689–1719. [DOI] [PubMed] [Google Scholar]
- Kerslake DM (1972). The Stress of Hot Environments. Cambridge University Press, London. [PubMed] [Google Scholar]
- Kharitonov SA, Logan‐Sinclair RB, Busset CM & Shinebourne EA (1994). Peak expiratory nitric oxide differences in men and women: relation to the menstrual cycle. Br Heart J 72, 243–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara T, Inoue Y, Abe M, Sato Y & Kondo N (2005. a). Effects of menstrual cycle and physical training on heat loss responses during dynamic exercise at moderate intensity in a temperate environment. Am J Physiol Regul Integr Comp Physiol 288, R1347–R1353. [DOI] [PubMed] [Google Scholar]
- Kuwahara T, Inoue Y, Taniguchi M, Ogura Y, Ueda H & Kondo N (2005. b). Effects of physical training on heat loss responses of young women to passive heating in relation to menstrual cycle. Eur J Appl Physiol 94, 376–385. [DOI] [PubMed] [Google Scholar]
- Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent‐Smith L, Melchior JC & Pirlich M (2004). Bioelectrical impedance analysis – part I: review of principles and methods. Clin Nutr 23, 1226–1243. [DOI] [PubMed] [Google Scholar]
- Lehtovirta P (1974. a). Haemodynamic effects of combined oestrogen‐progestogen oral contraceptives. J Obstet Gynaecol Br Commonw 81, 517–525. [PubMed] [Google Scholar]
- Lehtovirta P (1974. b). Peripheral haemodynamic effects of combined oestrogen‐progestogen oral contraceptives. J Obstet Gynaecol Br Commonw 81, 526–534. [DOI] [PubMed] [Google Scholar]
- Lehtovirta P, Kuikka J & Pyörälä T (1977). Hemodynamic effects of oral contraceptives during exercise. Int J Gynaecol Obstet 15, 35–37. [DOI] [PubMed] [Google Scholar]
- Lei TH, Stannard SR, Perry BG, Schlader ZJ, Cotter JD & Mündel T (2017). Influence of menstrual phase and arid vs. humid heat stress on autonomic and behavioural thermoregulation during exercise in trained but unacclimated women. J Physiol 595, 2823–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln DW (1967). Unit activity in the hypothalamus, septum and preoptic area of the rat: characteristics of spontaneous activity and the effect of oestrogen. J Endocrinol 37, 177–189. [DOI] [PubMed] [Google Scholar]
- Martin GJ & Buono MJ (1997). Oral contraceptives elevate core temperature and heart rate during exercise in the heat. Clin Physiol 17, 401–408. [DOI] [PubMed] [Google Scholar]
- Mchardy GJR (1967). Relationship between differences in pressure and content of carbon dioxide in arterial and venous blood. Clin Sci 32, 299–309. [PubMed] [Google Scholar]
- Morimoto T, Slabochova Z, Naman R & Sargent F (1967). Sex differences in physiological reactions to thermal stress. J Appl Physiol 22, 526–532. [DOI] [PubMed] [Google Scholar]
- Morris NB, Cramer MN, Hodder SG, Havenith G & Jay O (2013). A comparison between the technical absorbent and ventilated capsule methods for measuring local sweat rate. J Appl Physiol 114, 816–823. [DOI] [PubMed] [Google Scholar]
- Mündel T, Carter JM, Wilkinson DM & Jones DA (2016). A comparison of rectal, oesophageal and gastro‐intestinal tract temperatures during moderate‐intensity cycling in temperate and hot conditions. Clin Physiol Funct Imaging 36, 11–16. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Suzuki M & Ishizuka N (1975). Action of progesterone on preoptic thermosensitive neurones. Nature 258, 80. [DOI] [PubMed] [Google Scholar]
- Paterson DH & Cunningham DA (1976). Comparison of methods to calculate cardiac output using the CO2 rebreathing method. Eur J Appl Physiol Occup Physiol 35, 223–230. [DOI] [PubMed] [Google Scholar]
- Ramanathan N (1964). A new weighting system for mean surface temperature of the human body. J Appl Physiol 19, 531–533. [DOI] [PubMed] [Google Scholar]
- Reardon FD, Leppik KE, Wegmann R, Webb P, Ducharme MB & Kenny GP (2006). The Snellen human calorimeter revisited, re‐engineered and upgraded: design and performance characteristics. J Appl Physiol 94, 2350–2357. [DOI] [PubMed] [Google Scholar]
- Rechichi C, Dawson B & Goodman C (2008). Oral contraceptive phase has no effect on endurance test. Int J Sports Med 29, 277–281. [DOI] [PubMed] [Google Scholar]
- Rechichi C, Dawson B & Goodman C (2009). Athletic performance and the oral contraceptive. Int J Sports Physiol Perform 4, 151–162. [DOI] [PubMed] [Google Scholar]
- Rogers SM & Baker MA (1997). Thermoregulation during exercise in women who are taking oral contraceptives. Eur J Appl Physiol Occup Physiol 75, 34–38. [DOI] [PubMed] [Google Scholar]
- Rothchild I & Barnes AC (1952). The effects of dosage, and of estrogen, androgen or salicylate administration on the degree of body temperature elevation induced by progesterone. Endocrinology 50, 485–496. [DOI] [PubMed] [Google Scholar]
- Schlader ZJ, Mündel T, Barnes MJ & Hodges LD (2010). Peak cardiac power output in healthy, trained men. Clin Physiol Func Imag 30, 480–484. [DOI] [PubMed] [Google Scholar]
- Shapiro Y, Pandolf KB, Avellini BA, Pimental NA & Goldman RF (1980). Physiological responses of men and women to humid and dry heat. J Appl Physiol 49, 1–8. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Silva C, Keefe DL, Kokoszka CA & Nadel ER (1999). Effects of oral contraceptives on body fluid regulation. J Appl Physiol 87, 1016–1025. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, Silva C & Keefe DL (2000). Estrogen modifies the temperature effects of progesterone. J Appl Physiol 88, 1643–1649. [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS & Taylor HS (2014). Challenges and methodology for testing young healthy women in physiological studies. Am J Physiol Endocrinol Metab 306, E849–E853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson LA & Kolka MA (1985). Menstrual cycle phase and time of day alter reference signal controlling arm blood flow and sweating. Am J Physiol Regul Integr Comp Physiol 249, R186–R196. [DOI] [PubMed] [Google Scholar]
- Stephenson LA & Kolka MA (1993). Thermoregulation in women. Exerc Sport Sci Rev 21, 231–262. [PubMed] [Google Scholar]
- Stolwijk JAJ & Hardy JD (1966). Partitional calorimetric studies of man during exposures to thermal transients. J Appl Physiol 21, 967–977. [DOI] [PubMed] [Google Scholar]
- Sunderland C & Nevill M (2003). Effect of the menstrual cycle on performance of intermittent, high‐intensity shuttle running in a hot environment. Eur J Appl Physiol 88, 345–352. [DOI] [PubMed] [Google Scholar]
- Taguchi M, Alfer J, Chwalisz K, Beier HM & Classen‐Linke I (2000). Endothelial nitric oxide synthase is differently expressed in human endometrial vessels during the menstrual cycle. Mol Hum Reprod 6, 185–190. [DOI] [PubMed] [Google Scholar]
- Tenaglia SA, McLellan TM & Klentrou PP (1999). Influence of menstrual cycle and oral contraceptives on tolerance to uncompensable heat stress. Eur J Appl Physiol Occup Physiol 80, 76–83. [DOI] [PubMed] [Google Scholar]
- Walters WA & Lim YL (1970). Haemodynamic changes in women taking oral contraceptives. J Obstet Gynaecol Br Commonw 77, 1007–1012. [DOI] [PubMed] [Google Scholar]
- Whitney R (1953). The measurement of volume changes in human limbs. J Physiol 121, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K & Komesaroff PA (2001). J Clin Endocrinol Metab 86, 5389–5395. [DOI] [PubMed] [Google Scholar]


