Abstract
Pancreatic ductal adenocarcinoma is a lethal disease for which radical surgery and chemotherapy represent the only curative options for a small proportion of patients. Recently, FOLFIRINOX and nab-paclitaxel plus gemcitabine have improved the survival of metastatic patients but prognosis remains poor. A pancreatic tumor microenvironment is a dynamic milieu of cellular and acellular elements, and it represents one of the major limitations to chemotherapy efficacy. The continued crosstalk between cancer cells and the surrounding microenvironment causes immunosuppression within pancreatic immune infiltrate increasing tumor aggressiveness. Several potential targets have been identified among tumor microenvironment components, and different therapeutic approaches are under investigation. In this article, we provide a qualitative literature review about the crosstalk between the tumor microenvironment components and immune system in pancreatic cancer. Finally, we discuss potential therapeutic strategies targeting the tumor microenvironment and we show the ongoing trials.
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive disease accounting as the fourth leading cause of cancer-related deaths worldwide, and it is estimated to become the second within 2030 [1]. PDAC incidence and mortality are similar, and the five-year survival rate for all stages is around 8% [2]. The primary therapeutic strategies include surgery and chemotherapy. Unfortunately, majority of patients have unresectable, locally advanced, or metastatic disease at the time of diagnosis and treatment is only palliative in this setting [3]. Chemotherapy is the cornerstone of advanced PDAC treatment even if patients' outcome has been disappointing with this approach because of the occurrence of chemoresistance [4]. In addition, target agents have failed to improve survival both alone and in combination with standard chemotherapy [5]. Single-agent gemcitabine has been the mainstay of advanced PDAC treatment since 1997, despite of a small survival benefit [6]. In the last decade, drug portfolio has been enriched by novel combinations like FOLFIRINOX and nab-paclitaxel (nab-P) plus gemcitabine (GEM) that represent the standards of care in metastatic disease management [7, 8]. Nevertheless, treatment effectiveness is limited and patients' prognosis remains very poor. Several factors could explain the reduced efficacy of chemo- and targeted therapies: signalling redundancy, the role of stem cells, the tumor microenvironment (TME), and desmoplastic stroma [9–11]. PDAC is a “milieu” of distinct elements that compose the so-called TME, including fibroinflammatory stroma, extracellular matrix, infiltrating immune cells, and cancer cell population [12, 13]. A growing knowledge of the PDAC pathogenesis has led to better understanding of the immune components' role within the TME. Stimulation and mobilization of the human immune system as well as the enhancement of TME antitumor capacity have become a research focus in PDAC treatment [14, 15]. In this article, we will provide a qualitative literature review about the crosstalk between the TME components and immune system in PDAC. Finally, we will discuss potential therapeutic strategies targeting the TME and we will show the ongoing trials in this field.
2. Literature Research Methods
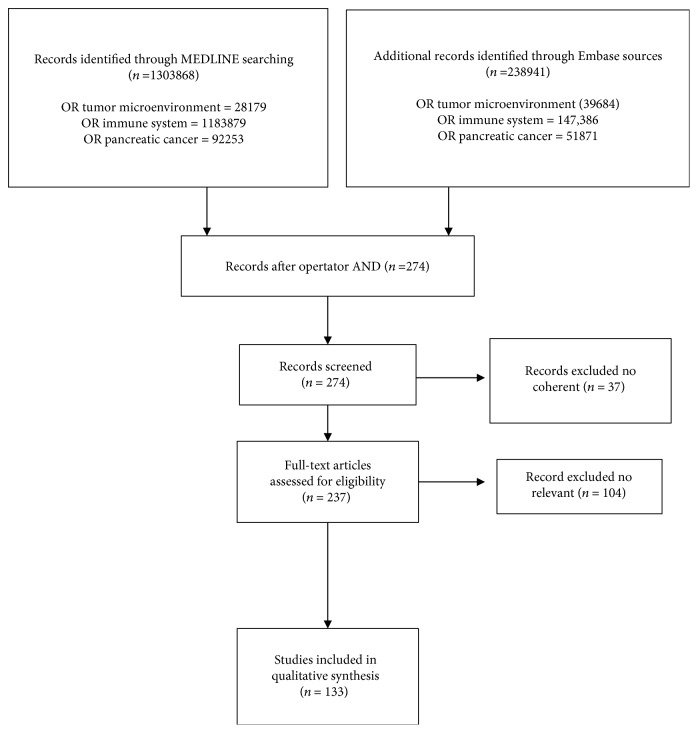
A systematic review of the literature was performed in compliance with the PRISMA guidelines [16]. Article titles or full text up to May 2018 using electronic databases MEDLINE and Embase was screened. The primary search terms included “tumor microenvironment,” “immune system,” and “pancreatic cancer” in the article titles using operator “OR.” Later, to narrow the scope of the review, operator “AND” was applied on the extracted records by using the abovementioned terms. Two hundred seventy-four articles met eligibility criteria for our qualitative systematic review. 37 papers were excluded because they were not coherent as well as 104 because they were not relevant, resulting in 133 full texts being included (Figure 1). In addition, ASCO, ASCO GI, and ESMO abstracts published during the last three years were evaluated in order to detect the most recent clinical data about drugs targeting the TME. Trials with negative or not clinically relevant results were excluded from this article. Finally, ClinicalTrials.gov website was interrogated and “recruiting,” “active, not recruiting,” and “not yet recruiting” trials in PDAC were selected. The National Cancer Institute Drug Dictionary was consulted to verify that the mechanism of action of screened drugs was clearly directed against the TME and immune system.
Figure 1.
Preferred reporting items for systematic reviews and meta-analysis (PRISMA) protocol used for the systematic review.
3. Pancreatic Cancer and the TME
A TME is an intricate system with peculiar physical and biochemical features, in which interactions between tumor and stromal cells promote carcinogenesis, progression, metastasis, and therapeutic resistance [17, 18]. Consistently, extracellular matrix (ECM) elements, vascular networks, and lymphatic networks show an abnormal behaviour within the TME [19]. In the normal pancreas, connective tissue, resident fibroblasts (PFs), pancreatic stellate cells (PSCs), immune cells, and vascular cells play a critical role in tissue repair and wound healing (Figure 2(a)). In response to pancreatic tissue damage, injured acinar cells secrete proinflammatory, proangiogenic growth factors and cytokines activating immune cells, PSCs/PFs, and vascular cells to restore normal pancreatic function (Figure 2(b)) [20]. However, in the presence of oncogenic mutations like KRAS, TP53, SMAD4, and CDKN2A, genetically altered epithelial cells transform into cancer cells and disrupt normal communications between PSCs and immune and vascular cells, creating a favorable microenvironment for cancer progression (Figure 2(c)) [21]. PDAC is characterized by a profuse fibrotic stromal reaction called “desmoplasia,” composed of cellular elements such as PSCs, PFs, vascular elements, immune cells, and acellular components such as collagens, fibronectin, cytokines, and growth factors stored in the extracellular matrix (Figure 2(c)). Abundance of stroma is a unique characteristic of PDAC, and it is well demonstrated that the microenvironment influences both responses to treatment and survival of PDAC patients [22]. Notably, during disease progression, tumor stroma exerts pressure on blood vessels, causing their constriction and hypoxic niche formation [23]. Consequently, low-oxygen content in the tumor induces the hypoxia-inducible factor 1 (HIF1A) stabilization. HIF1A mediates activation of different signals that alter metabolic pathways, induce invasiveness, promote chemoresistance, and lead to a poor prognosis of the patient. Upon hypoxic stress, HIF1A accumulates and compensates for low oxygen by increasing glycolysis and glucose uptake in the cells. The consequent metabolic switch from oxidative phosphorylation to aerobic glycolysis results in the production of lactate and acidification of the extracellular environment [24, 25]. Hypoxic conditions, acidic extracellular pH, and high interstitial fluid pressure in the TME are additional drivers of tumorigenesis and tumor progression [17]. The TME also develops an adapted metabolism, in which malignant epithelial cells consume proteins and lipids as a source of energy. Finally, an invasive epithelial to mesenchymal transition (ETM) and a metastatic phenotype complete the PDAC microenvironment [18, 26]. Although desmoplasia represents more than 80% of the tumor mass, the PDAC microenvironment is also replete with immune cells [27]. In particular, PDAC infiltrate is rich of T-cells, also known as tumor-infiltrating lymphocytes (TILs) [28]. Consistently, even if innate and adaptive immune responses are active against the tumor, PDAC by itself induces local and systemic immune dysfunction or immunosuppression to prevent eradication by effector immune cells [29]. Recent studies have showed that PDAC immune cells interact with TME components, resulting in the inactivation of the cytotoxic antitumoral response [29]. In this scenario, the TME could influence treatment efficacy through different mechanisms, including drug delivery modulation, immunosuppression, vascular remodelling, metabolic activities, and signalling pathways involved in DNA repair and apoptosis [30].
Figure 2.
Descriptive model representing pancreatic microenvironment changes during PDAC carcinogenesis. (a) In the normal pancreas, connective tissue, resident fibroblasts (PFs), pancreatic stellate cells (PSCs), immune cells, and vascular cells play a critical role in tissue repair and wound healing. (b) Pancreatic tissue damages and oncogenic mutations lead to carcinogenesis and disrupt normal communications between PSCs/PFs and immune and vascular cells, determining a favorable microenvironment for cancer progression. Soluble and growth factors produced by cancer cells activate PSCs and PFs that play a key role in the development and maintenance of stromal cancer compartment increasing extracellular matrix synthesis. (c) The intense fibrotic stromal reaction of PDAC is characterized by PSCs, PFs, vascular elements, immune cells, and acellular components such as collagens and hyaluronan, fibronectin, cytokines, and growth factors stored in the extracellular matrix. In this stage of disease MDSCs, M2, N2, and Tregs induce a protumor phenotype.
4. Cellular Component of the TME
The cellular component includes pancreatic fibroblasts (PFs), pancreatic stellate cells (PSCs), vascular cells, and inflammatory/immune cells (Figure 2(c)). All these components interact with each other and with cancer cells in a complex fashion (Figure 3) [31]. In normal condition, PFs are inert and spindle-shaped cells in the connective tissue, embedded in physiological ECM. Differently, PDAC cells recruit PFs to the tumor mass and convert them in cancer-associated fibroblasts (CAFs) through genetic and epigenetic changes [32]. CAFs are a characteristic type of myofibroblastic cells expressing alpha-smooth muscle actin (α-SMA) that contribute to PDAC progression [32]. In the normal pancreas, quiescent PSCs are located in the periacinar space representing only a small proportion of all pancreatic cells (Figure 2(a)) [33]. Quiescent PSCs have a low mitotic index and synthesize matrix proteins [34]. Following activation by toxins, oxidant stress, smoking, cytokines, and growth factors, quiescent PSCs acquire a myofibroblast-like phenotype and are called “activated PSCs” (Figure 2(c)) [31]. Notably, activated PSCs express α-SMA and play a key role in the development and maintenance of the stromal cancer compartment, mediating an extracellular matrix synthesis increase [35, 36]. Microvessels contribute to normal pancreatic microenvironment regulation. Differently, in PDAC, a dysregulated vascular network is demonstrated. In particular, pericytes normally recruited by endothelial cells (ECs) could migrate from vessels and potentially undergo a pericyte-myofibroblast transition within the PDAC microenvironment [37, 38]. Furthermore, ECs could be indirectly activated by CAFs or tumor cells through secretion of proteases in the ECM [39]. Inflammatory and immune cells are crucial elements in the pancreatic TME, and their involvement in generating chemoresistance has become a matter of intense research. Bone marrow-derived cells (BMDCs) are recruited to the pancreatic stroma, leading to early carcinogenesis and metastases together with PSCs, CAFs, and inflammatory cells [40]. BMDCs differentiate into several cell types and contribute to both neovascularization and fibrosis in PDAC stroma by activating PSCs, myeloid-derived suppressor cells (MDSCs), and mast cells (Figure 2(c)) [41, 42]. High levels of MDSCs lead to premetastatic niche formation, tumor invasiveness, angiogenesis stimulation, and worse prognosis [43]. PDAC cells recruit also monocytes from bone marrow within the TME, transforming them into macrophages. Tumor-associated macrophages (TAMs) have been described as promoters of cancer initiation, progression, and metastasization and protect tumors from cytotoxic agents. In particular, TAMs can be converted into M1-like inflammatory macrophages that could activate an immune response against the tumor or into M2-like immunosuppressive macrophages that promote tumor immunity and tumor progression (Figures 2(b) and 2(c)) [44]. M2 TAMs have effect on tumor survival by inhibiting T-cell response and recruiting regulatory T-cells (Treg cells) that negatively influence cytotoxic T-cells [45]. Elevated CD4+ in the TME can promote tumor growth blocking CD8+-related antitumoral response [46]. Recently, several studies showed that B lymphocytes support PDAC carcinogenesis and progression stimulating cancer cell proliferation, suppressing CD8+ cells through the Bruton tyrosine kinase (BTK) pathway [47, 48]. Finally, depending on the stimuli, neutrophils may differentiate into two subtypes in PDAC. N1 neutrophils may potentially kill tumor cells under negative regulation of IFN-β. On the other hand, under TGF-β and G-CSF stimulation, neutrophils activate into a protumor phenotype called N2 (Figures 2(b) and 2(c)) [49].
Figure 3.
Crosstalk between cancer cells, the TME, the immune system, and potential therapeutic targets. TME components interplay with cancer cells (black arrows) through cytokines and growth factors becoming active and causing tumor proliferation, invasiveness, and metastasization. Communication between PDAC cells and activated PSCs/PFs induces soluble factor secretion increasing ECM production. This continued crosstalk determines immunosuppressive effects on TME immune infiltrate (red lines). Tumor-infiltrating lymphocytes produce high levels of PD-1 and interact with PDL-1 overexpressed by PDAC cells, resulting in T lymphocyte depletion. Several molecules (target agents or immunotherapies) with different mechanisms of action (green boxes) may interfere in the crosstalk between cancer cells and the TME restoring immune response, directly killing tumor cells, or destroying ECM components (green lines).
5. Acellular Component of the TME
The acellular component of the TME is made of collagens I, III, and IV; periostin; fibronectin; and hyaluronic acid (Figure 2(c)) [50, 51]. In many solid tumors as PDAC, elevated collagen deposition contributes to form the stromal barrier influencing both drug resistance and poor prognosis. ECM remodelling is made by lysyl oxidases (LOX), a family of amine oxidases that catalyze the posttranslational crosslinking of collagen molecules, thus favoring biogenesis and maturation. Tumor stroma is characterized by abnormal LOX expression; consequently, high collagen deposition is possible [52]. Hyaluronic acid (HA) is a glycosaminoglycan composed of repeated N-acetyl glucosamine and glucuronic acid units, alternating in β-1,3 and β-1,4 linkages. HA synthesis is regulated by HA synthases (HAS 1–3) and α-SMA-positive myofibroblasts, and its degradation is carried by six hyaluronidases [53, 54]. An elevated HA level has been found in PDAC where it binds and traps water molecules in the ECM, causing high pressure on neighboring structures as well as elevated interstitial fluid pressure within the tumor [53]. Furthermore, it is known that HA binds several receptors as CD44, receptor for hyaluronan-mediated motility (RHAMM), lymphatic vessel endothelial HA receptor-1 (LYVE-1), hyaluronan receptor for endocytosis (HARE), layilin, and Toll-like receptor 4, implicated in tumor migration, invasion, adhesion, and proliferation [55]. Periostin is an osteoblast-specific factor, preferentially expressed in the periosteum functioning as a cell adhesion molecule, and its expression is 42-fold higher in PDAC compared to that in the normal pancreas [31]. Notably, periostin promotes PDAC cell invasiveness, resistance to hypoxia-induced death, and EMT. Fibronectin (Fn) is one of the most abundant ECM proteins and binds to collagen, periostin, fibrillin, and tenascin-C facilitating their assembly and organization [56]. In PDAC, high levels of Fn are secreted by CAFs together with type I and II collagens causing an anisotropic fiber orientation that drives cancer cell migration [57].
6. Crosstalk between Cancer Cells, the TME, and the Immune-System in PDAC
The continuous interaction between the glandular neoplastic component and TME has been widely investigated so far. Several authors demonstrated the reciprocal influence of PDAC cells on PSCs via intercellular signalling (Figure 3) [22]. In particular, PDAC cells stimulate PSC activation, proliferation, and migration through cytokines and growth factors such as pigment epithelium-derived factor (PEDF), platelet-derived growth factor (PDGF), PDGF-1, insulin-like growth factor (IGF), and ECM synthesis via TGF-β and fibroblast growth factor 2 (FGF2) [20]. On the other hand, PSCs stimulate cancer cell proliferation by production of paracrine factors as TGF-β, FGF2, PDGF, and epidermal growth factor (EGF) and inhibit apoptosis [20]. Moreover, metalloproteinase (MMPs) synthesis is mainly correlated to TGFβ-1 and tumor necrosis factor- (TNF-) α [58]. Secretion of MMPs, stroma cell-derived factor-1 (SDF-1), acidic secreted protein and rich in cysteine (SPARC), PDGF, and EGF by PSCs induces invasion and migration (Figure 3). Furthermore, PSCs promote invasion and metastasis by inducing the EMT phenotype in PDAC cells via loss of adhesion intercellular proteins such as E-cadherin and enhance tumor angiogenesis by secretion of vascular endothelial growth factor (VEGF) [59, 60]. Another candidate factor that has received some attention in recent years is the hepatocyte growth factor (HGF), which is secreted by activated PSCs and has a pivotal role in cancer cell proliferation and migration binding its transmembrane cell surface receptor c-MET, which is expressed on cancer cells. Furthermore, c-MET is present on the surface of ECs, enhancing PSC-EC interaction with a potential role in angiogenesis and metastatic spread [61]. Activation of fibroblasts into CAFs is induced by numerous cytokines and growth factors like TGF-β, EGF, PDGF, and FGF2 secreted in the TME (Figure 3) [62]. Tumor cells influencing PSCs and CAFs drive ECM remodelling through assembly, alignment, unfolding, and crosslinking of collagen type I and the fibronectin-rich matrix. Interestingly, CAFs produce both signalling factors and exosomes that reinforce the crosstalk with tumor cells [63]. In this context, PDAC cells recruit pericytes via PDGF secretion inducing both chemotaxis from microvessels and pericyte-myofibroblast transition [37]. Furthermore, ECs can be directly induced by cancer cells through soluble factors (FGF-1, FGF-2, VEGFA, and PDGF-B), activation of adhesion receptor (OPG and JAGGED1), gap junctions (CX43), and vesicles (or exosomes) [38]. Contemporarily, BMDCs are attracted in PDAC stroma by growth factors as fibroblast activation protein (FAP), PDGF, TGF-β1, VEGF, and EGF produced by tumor cells and participate to PSC activation [40]. In the PDAC microenvironment, cytokines including G-CSF, GM-CSF, IL-1β, IL-4, IL-6, prostaglandin E2 (PGE2), IFN-γ, and VEGF induce MDSCs to infiltrate the tumor (Figure 3) [64]. MDSCs are myeloid cells that suppress T-cell activation through TGF-β secretion, nitric oxide and reactive oxygen species (ROS) production, and arginase-1 depletion. Consistently, cancer cells upregulate a soluble protein named pancreatic adenocarcinoma upregulated factor (PAUF), increasing the accumulation of MDSCs and enhancing their immunosuppressive function (Figure 3) [43]. The intricate crosstalk between PDAC cells and the microenvironment involves also immune elements. Macrophage colony-stimulating factor receptor (M-CSF/M-CSFR) and C-C motif chemokine ligand 2-C-C motif chemokine receptor-2 (CCL2/CCR2) pathways are involved in the recruitment of TAMs. Once within the tumor, TAMs switch towards a M2 phenotype via colony-stimulating factor-1 (CSF-1). M2 are activated by cancer cells through IL-4, IL-10, and IL-13 production and secrete macrophage-derived EGF causing tumor cell migration around blood vessels [65]. Furthermore, M2 release nitric oxide synthase (NOS) and arginase I (ARGI) damaging T lymphocytes through L-arginine depletion in the TME (Figure 3) [66]. Interestingly, neutrophils contribute to tumor growth and invasiveness, producing neutrophil-derived proteases as elastase, PR3, cathepsin G, MMP-8, and MMP-9 that destroy the surrounding ECM [67]. In the dense fibrotic TME, cancer cells activate a wide variety of signalling pathways and suppress both innate and adaptive immune systems by decreasing cytotoxic CD8 T-cells and increasing the presence of immunosuppressive macrophages (M2), neutrophils (N2), and Treg cells (Figure 2(c)) [27]. Otherwise, tumor-infiltrating lymphocytes (TILs) produce high levels of programmed cell death protein 1 (PD-1) and interact with its specific ligand, known as programmed cell death ligand 1 (PDL-1) overexpressed by PDAC cells, resulting in T lymphocyte depletion (Figure 3) [68, 69].
7. Clinical Impact of TME and Immune System Components in PDAC
Recently, a wide genome-sequencing programme has been developed in order to better understand PDAC heterogeneity and get information that could have a clinical significance. In particular, whole genome sequencing and copy number variation analyses performed on 100 tumor samples classified four PDAC subtypes depending on chromosomal structure variation: stable, locally rearranged, scattered, and unstable. Each subtype could predict a different therapeutic responsiveness [21]. Subsequently, integrated genomic analysis of 456 PDACs identified 32 mutated genes that aggregate into 10 pathways (K-Ras, WNT, NOTCH, ROBO/SLIT signalling, G1/S transition, TGF-β, SWI-SNF, chromatin modification, DNA repair, and RNA processing). Notably, the TGF-β pathway is mainly involved in TME modelling, regulation, and crosstalk with the immune system. A further analysis of those pathways defined four PDAC subtypes that correlate with histopathological characteristics and have different prognoses: (a) squamous, (b) pancreatic progenitor, (c) immunogenic, and (d) aberrantly differentiated endocrine exocrine (ADEX). Interestingly, the immunogenic subtype is characterized by a predominant B- and T-cell (CD8+, Treg) infiltrate as well as cytotoxic T lymphocyte antigen-4 (CTLA4) and PD-1 upregulation [70]. Consistently, PDAC stromal features, immune elements, and their correlation with patients' outcome have been investigated in several research programmes. Knudsen et al. showed that PDAC stroma could be differentiated into three categories called “mature” with dense collagenous stroma and low number of CAFs, “immature” that is highly cellular and collagen poor, and an “intermediate form.” Among those phenotypes, the immature form strongly correlated with worse prognosis. Additionally, poor overall survival was observed in patients with lower stromal volume, high peritumoral T lymphocytes, monocytes/macrophages, CTLA4, and PDL-1 in TME [71]. Immunohistochemistry analysis performed on 88 PDAC samples demonstrated that patients with high-density M2 macrophage infiltration in the stroma had shorter overall survival than those with low M2 infiltration [72]. Furthermore, neutrophil infiltrates have been observed both in the neighborhood of tumor cells and in the stroma and correlated with undifferentiated tumor growth and poor prognosis in 363 pancreatic tumor samples [73]. Coherently, this pathological evidence could partially explain the prognostic significance of the neutrophil to lymphocyte ratio (NLR) value in the peripheral blood of PDAC patients. Several studies both on resected and metastatic PDACs showed that high NLR were related to significantly shorter OS [74, 75]. The impact of TILs on PDAC patients' prognosis is not yet clarified and the available data are not conclusive. The evaluation of TILs on tumor samples in the cohort enrolled in the PDAC adjuvant CONKO 001 study showed a significant correlation between high TIL levels and longer disease-free survival (DFS) and OS [76]. Those results had no confirmation in the Knudsen et al. data in which no correlation between TILs and survival was found [71]. In contrast, in many studies, the high presence of Treg in TME has shown to unfavorably impact the prognosis [77]. The D-1/PDL-1 axis has a well-established role in different neoplasms including PDAC. This pathway regulates the interaction between tumor cell and lymphocytes and their crosstalk with TME [68]. In the last years, several authors attempted to redefine the clinical relevance of PD-1/PDL-1 expression in PDAC, but also, in this field, the road will be long to run. A retrospective analysis of PDL-1 mRNA expression in 453 PDAC samples showed that PDL-1 upregulation was associated with worse DFS and OS. In the same study, PDL-1 upregulation was correlated with biological parameters, showing some degree of T-cell infiltration, signs of antitumor response, and profiles of lymphocyte exhaustion [78]. The PD-1/PDL-1 prognostic value was also evaluated in a group of 145 PDAC surgical samples. Patients with CD8+ and PD-1+, lymphocytes in the stroma had better outcomes compared to patients with low expression, independently from clinic-pathologic parameters like age, tumor site, TNM staging, resection margins, and previous chemotherapy. In this study, a correlation between the PDL-1 status and Bailey's molecular PDAC classification was found. In particular, PDL-1 mRNA was upregulated in the squamous subtype versus each other subtype [79]. The acellular component of the TME has been investigated in order to understand the clinical significance. A recent meta-analysis examined the clinical status and OS of PDAC patients with high HIF-1α expression compared to those with low expression. HIF-1α was associated with a higher rate of lymph node metastasis and advanced tumor stage. Notably, HIF-1α overexpression was significantly correlated with poor OS [24]. Interestingly, another study found negative correlation between survival and extracellular matrix deposition in primary PDACs. Median survival was significantly higher in low-collagen patients compared to high-level ones. Furthermore, low-HA level patients had longer OS than high-HA level patients. This analysis also indicated that extracellular matrix components, such as collagen and HA, are found in high levels in both primary tumors and metastatic lesions [80].
8. Potential Targets for Therapeutic Approaches: Insights into Clinical Data
The TME is involved in the lack of responsiveness to chemo- and target therapies favoring a hypoxic environment, causing difficulty in drug access and limiting the immune infiltration. The crosstalk between TME cellular elements and the immune system promotes a clearly immunosuppressive phenotype (Figure 3) [81, 82]. There is an intense research focused on the TME and immune system as therapeutic targets, and potentially, active agents are under investigation (Figure 3 and Tables 1 and 2).
Table 1.
Target agents directed against TME or immunotherapies under investigation in PDAC.
| Name | Type/structure | Mechanism of action | Effect |
|---|---|---|---|
| ALT-803 | Fusion protein | Binds IL-2/IL-15 receptor beta common gamma chain (IL-2R beta gamma) receptor on natural killer (NK) and CD8+ | Activation and increase of NK cell memory CD8+ levels |
| AM0010 | Covalent conjugate of recombinant human interleukin-10 (IL-10) and polyethylene glycol (PEG) | Activates cell-mediated immunity against cancer cells by stimulating the CD8+ differentiation and expansion | Potential antifibrotic, anti-inflammatory, immunomodulating, and antineoplastic activities |
| AMG 820 | Fully human monoclonal antibody (IgG2) | Against the colony-stimulating factor-1 (CSF-1 or M-CSF) receptor c-fms (or CSFR1) | Suppresses recruitment and activation of TAMs |
| Anakinra | Recombinant human nonglycosylated IL-1 receptor antagonist | Blocks IL-1 activity | Inhibition of VEGF, TNF-α, and IL-6 cascade resulting in inhibition of tumor angiogenesis |
| Atezolizumab | Humanized, Fc optimized | Binds to PD-L1, blocking its binding to and activation of PD-1 on activated T-cells | Enhancement of T-cell-mediated immune response and reversal of T-cell inactivation |
| Avelumab | Human monoclonal antibody (IgG1) | Binds to PD-L1 preventing interaction with PD-1 | May restore immune function activation of cytotoxic T lymphocytes |
| BL-8040 | Short peptide | Binds to the chemokine receptor CXCR4, preventing the binding of stromal-derived factor-1 to the CXCR4 receptor | Decreases tumor cell proliferation and migration |
| CCX872-B | Small molecule | Human C-C chemokine receptor type 2 (CCR2) antagonist | Inhibition of both CCR2 activation and CCR2-mediated signal transduction |
| CD8 + NKG2D + AKT cell | Cells | Human CD8+ tumor specific engineered to express the natural killer cell-activating receptor group 2D (NKG2D) and the serine/threonine kinase AKT | Potential immunomodulating and antineoplastic activities |
| CRS-207 | Recombinant Listeria-based cancer vaccine expressing human mesothelin | Listeria invades professional phagocytes within the immune system and expresses mesothelin, activating a cytotoxic T lymphocyte response against mesothelin-expressing tumor cells | Potential immunostimulatory and antineoplastic activities |
| Durvalumab | Fc-optimized monoclonal antibody | Binds to PD-L1 blocking its binding to and activation of PD-1 expressed on activated T-cells | Reverses T-cell inactivation and activates the immune system to exert a cytotoxic T lymphocyte response against PD-L1-expressing tumor cells |
| Galunisertib | Small molecule | Antagonist of TGF-β receptor type 1 (TGFBR1) | Prevents the activation of the TGF-β-mediated signalling pathways inhibiting tumor proliferation |
| GVAX | Allogeneic cancer vaccine composed of lethally irradiated whole melanoma cancer cells that are genetically modified to secrete the immunostimulatory cytokine granulocyte-macrophage colony-stimulating factor | Stimulates the body's immune system against tumor cells | Enhances the activation of dendritic cells, promotes antigen presentation to both B- and T-cells, and increases IL-2-mediated lymphokine-activated killer cell function |
| iAPA-DC/CTL | A cell-based product composed of dendritic cells (DCs) pulsed with tumor-associated antigens and devoid of the inhibitory effect of antigen presentation attenuators (iAPA) combined with cytotoxic T lymphocytes | Prevents the expression of APA genes and inhibits attenuation of antigen presentation | Potential immunostimulating and antineoplastic activities |
| Ibrutinib | Small molecule | Binds to and irreversibly inhibits BTK activity | Prevents both B-cell activation and B-cell-mediated signalling leading to growth inhibition of the malignant B-cells overexpressing BTK |
| IDO-1 inhibitor | Small molecule | Targets and binds to indoleamine 2,3-dioxygenase 1, a cytosolic enzyme responsible for the oxidation of tryptophan into the immunosuppressive metabolite kynurenine | Restores and promotes proliferation and activation of various immune cells and causes a reduction in Tregs |
| Ipilimumab | Recombinant human monoclonal antibody (IgG1) | Binds to CTLA4 expressed on T-cells | Inhibits the CTLA4-mediated downregulation of T-cell activation leading to a cytotoxic T lymphocyte-mediated immune response |
| M7824 | Bifunctional fusion protein composed of a monoclonal antibody against PD-L1 fused to the extracellular domain of human TGF-β receptor II | “Trap” for all three TGF-β isoforms | Suppressed tumor growth and metastasis |
| MCS110 (Lacnotuzumab) | Humanized monoclonal antibody | Binds to M-CSF and blocks M-CSF-mediated signalling through the M-CSF receptor CD116 | Antineoplastic activities |
| Nivolumab | Fully human monoclonal antibody (IgG4) | Binds PD-1 and blocks its activation by PD-L1 | Activation of T-cell immune responses against tumor |
| Pamrevlumab | Humanized monoclonal antibody | Binds to connective tissue growth factor (CTGF) preventing the binding to the receptor and its subsequent activation | May prevent and reverse fibrosis; prevents tumor cell proliferation in CTGF-expressing tumor cells |
| PDR 001 (Spartalizumab) | Humanized monoclonal antibody | Directed against the negative immunoregulatory human cell surface receptor programmed death-1 | Prevents PD-1-mediated signalling and results in both T-cell activation and the induction of T-cell-mediated immune responses against tumor cells |
| PEGPH20 | Recombinant form of human hyaluronidase | Degrades hyaluronic acid- (HA-) coating tumor cells | Inhibition of tumor cell growth, lowering of the interstitial fluid pressure and allowing better penetration of chemotherapeutic agents into the tumor bed |
| Pembrolizumab | Humanized monoclonal immunoglobulin antibody (IgG4) | Directed against PD-1 | Restores T-cell activation and immune response |
| Pexidartinib | Small molecule | Binds to and inhibits phosphorylation of stem cell factor receptor (KIT), colony-stimulating factor-1 receptor (CSF1R), and FMS-like tyrosine kinase 3 (FLT3) | Inhibition of tumor cell proliferation and downmodulation of macrophages, osteoclasts, and mast cells |
| RO7009789 | Recombinant monoclonal antibody | Binds to CD40 on a variety of immune cell types | Activation of antigen-presenting cells (APCs), B-cells, and T-cells, resulting in an enhanced immune response |
| Tremelimumab | Human immunoglobulin monoclonal antibody (IgG2) | Directed CTLA4 | A cytotoxic T lymphocyte immune response against cancer cells |
| Vactosertib | Small molecule | Inhibitor of the serine/threonine kinase TGFBR1 also known as activin receptor-like kinase 5 (ALK5) | Inhibits the activity of TGFBR1 and prevents TGF-β/TGFBR1-mediated signalling and suppresses tumor growth |
| VCN-01 | Adenovirus | Replication-competent adenovirus encoding the human glycosylphosphatidylinositol-anchored enzyme PH20 hyaluronidase | Potential antitumor activity |
| γδ T-cell | Cells | Secrete interferon-gamma | Direct killing of tumor cells, activation of cytotoxic T lymphocyte response against tumor cells |
Table 2.
Current clinical trials investigating strategies directed against TME in PDAC.
| Study ID | Setting | Study drugs | Phase | Status |
|---|---|---|---|---|
| NCT02715804 | Metastatic PDAC (I line—HA high pts) |
Nab-P + GEM ± PEGPH20 | III rand | Recruiting |
| NCT02923921 | Metastatic PDAC (II line) | FOLFOX ± AM0010 | III rand | Recruiting |
| NCT02436668 | Metastatic (I line) | Nab-P + GEM ± ibrutinib | II-III rand | Active, not recruiting |
| NCT02030860 | Resectable | Nab-P + GEM ± Paricalcitol | II rand | Active, not recruiting |
| NCT02243371 | Advanced | GVAX + CY + CRS-207 ± Nivolumab | II rand | Active, not recruiting |
| NCT03006302 | Metastatic | Epacadostat + Pembrolizumab + CRS-207 ± CY/GVAX | II rand | Recruiting |
| NCT02648282 | Locally advanced | CY, pembrolizumab, GVAX, and SBRT | II | Recruiting |
| NCT01088789 | Resected | Boost vaccinations∗ of pancreatic tumor cell vaccine | II | Recruiting |
| NCT02826486 | Metastatic | BL-8040 + Pembrolizumab | II | Active, not recruiting |
| NCT03432676 | Advanced | IDO-1 inhibitor + Epacadostat + Pembrolizumab in PDAC with CIS/HRD | II | Not yet recruiting |
| NCT02910882 | Localized, unresectable | PEGPH20 + GEM + radiotherapy | II | Active, not recruiting |
| NCT02451982 | Resectable | GVAX + CY ± Nivolumab | I-II rand | Recruiting |
| NCT03193190 | Metastatic | Atezolizumab + Cobimetinib or Atezolizumab + PEGPH20 or Atezolizumab + BL-8040 | I-II rand | Recruiting |
| NCT02210559 | Locally advanced | GEM + Nab-P ± FG-3019 | I-II rand | Active, not recruiting |
| NCT02311361 | Metastatic | Tremelimumab and/or Durvalumab + radiation therapy | I-II | Recruiting |
| NCT02583477 | Metastatic | Durvalumab | I-II | Active, not recruiting |
| NCT02305186 | Resectable | Pembrolizumab | I-II | Recruiting |
| NCT02077881 | Metastatic | IDO Inhibitor + Nab-P + GEM | I-II | Recruiting |
| NCT02562898 | Metastatic | Ibrutinib + Nab-P + GEM | I-II | Active, not recruiting |
| NCT02529579 | Advanced | iAPA-DC/CTL + GEM | I-II | Recruiting |
| NCT03180437 | Resectable/advanced/metastatic | γδ T-cell | I-II | Recruiting |
| NCT02311361 | Unresectable | Tremelimumab and/or MEDI4736 + radiation therapy+ | I-II | Recruiting |
| NCT03451773 | Advanced | M7824 + GEM | I-II | Recruiting |
| NCT02713529 | Advanced | AMG 820 + Pembrolizumab | I-II | Active, not recruiting |
| NCT02807844 | Metastatic | MCS110 + Spartalizumab | I-II | Recruiting |
| NCT02929797 | Locally advanced | GEM ± CD8 + NKG2D + AKT cell | I rand | Recruiting |
| NCT03519308 | Resectable | Nivolumab + Paricalcitol | I | Recruiting |
| NCT02559674 | Metastatic | ALT-803 + Nab-P + GEM | I | Active, not recruiting |
| NCT02588443 | Resectable | RO7009789 alone or RO7009789 + Nab-P + GEM ➔ RO7009789 + Nab-P + GEM | I | Recruiting |
| NCT02345408 | Advanced | CCX872-B | I | Active, not recruiting |
| NCT02550327 | Advanced | Nab-P + GEM + Cisplatin + Anakinra | I | Recruiting |
| NCT02930902 | Resectable | Pembrolizumab + Paricalcitol ± Nab-P + GEM | I | Recruiting |
| NCT02868632 | Locally advanced | MEDI4736 + SBRT or Tremelimumab + SBRT or MEDI4736 + Tremelimumab + SBRT | I | Recruiting |
| NCT01473940 | Metastatic | Ipilimumab + GEM | I | Active, not recruiting |
| NCT02777710 | Metastatic | Durvalumab + Pexidartinib | I | Recruiting |
| NCT02345408 | Unresectable | CCX872-B | I | Active, not recruiting |
| NCT02045589 | Advanced | VCN-01 + Nab-P + GEM | I | Active, not recruiting |
| NCT03481920 | Advanced or locally advanced | PEGPH20 + Avelumab | I | Recruiting |
| NCT02734160 | Metastatic | Galunisertib + Durvalumab | I | Recruiting |
rand: randomized; pts: patients; GEM: gemcitabine; Nab-P: nab-paclitaxel; CY: Cyclophosphamide; SBRT: stereotactic body radiation therapy; CIS: chromosomal instability; HRD: homologous recombination repair deficiency; ➔: followed by. ∗PANC 10.05 pcDNA-1/GM-Neo and PANC 6.03 pcDNA-1 neo vaccine.
8.1. Targeting Tumor Stroma and the Extracellular Matrix
To date, the only drug approved for metastatic PDAC treatment that works against the TME is Nab-P [83]. Nab-P is an innovative molecule obtained by the combination of traditional paclitaxel with nanoparticles of albumin that binds tumor and stromal SPARC enhancing paclitaxel-selective delivery in PDAC cells [84]. The randomized phase III MPACT study showed that combination of Nab-P and GEM significantly increased median OS, progression-free survival (PFS), and response rates versus GEM alone in metastatic PDAC patients [8]. Unfortunately, a post hoc analysis on PDAC samples of the MPACT study failed to show the prognostic and predictive roles of SPARC [85]. Nab-P plus GEM is actually under investigation as the backbone of chemotherapy for novel combinations with immunotherapies or target agents directed against TME (Table 2). In particular, hyaluronidase treatment has been suggested to enhance degradation of HA [86]. Hyaluronidase synergizes with chemotherapy reducing HA levels and intratumoral pressure and increasing drug penetration [31, 46]. Pegvorhyaluronidase alfa (PEGPH20) was made with polyethylene glycol molecules linked to hyaluronidase, prolonging its half-life to >10 h. An open-label randomized phase 2 trial of PEGPH20 + Nab-P/GEM (PAG) versus Nab-P/GEM (AG) in 279 untreated metastatic PDAC patients showed a superior median PFS for the PAG versus AG, only in patients with high intratumoral HA content. Conversely, a modest trend towards better OS was found only in a small subgroup of high-HA tumor patients [87]. Actually, a global randomized phase III study in metastatic PDAC patients with high HA levels detected by immunohistochemistry is evaluating PAG (Table 2). Connective tissue growth factor (CTGF) is a profibrotic mediator that results as abundant in the stroma of PDAC. A human monoclonal antibody against CTGF (Pamrevlumab, FG-3019) was tested with GEM and erlotinib in stage III or IV PDAC [81]. Moreover, the combination of Nab-P + GEM with or without Pamrevlumab has been investigated in a phase I/II randomized study in locally advanced PDAC patients showing an increased resection rate and subsequent longer survival in the triplet arm [88].
8.2. Targeting the Immune Microenvironment
In PDAC, the TFG-β signalling pathway is involved in tumor progression and it is associated with poor prognosis. TGF-β has been related to tumor aggressiveness and invasiveness and to the activation of PSCs, leading to pancreatic desmoplasia. TGF-β is also associated to immune cell regulation, migration, and proliferation [89]. Therefore, targeting the TGF-β signalling pathway could be a rational therapeutic approach in PDAC [90]. A randomized phase II study assigned 156 patients to receive Galunisertib (anti-TGF-β) plus GEM or placebo plus GEM in stage II to stage IV unresectable PDAC. The combination of Galunisertib/GEM resulted in improvement of OS and PFS and a manageable toxicity profile compared to that of placebo/GEM. A major OS benefit was observed for the subgroup of patients with baseline TGF-β1 levels ≤ 4224 pg/mL [91]. Another mechanism that target indirectly the TGF-β pathway is the inhibition of the renin-angiotensin system with losartan. Fifty locally advanced PDAC patients were enrolled in a phase II study receiving FOLFIRINOX and losartan for a median of 8 cycles. This combination met the criteria for feasibility without severe toxicities, showing 61% of the R0 resection rate [92]. Vactosertib is a potent, highly selective, oral TGFBR1 inhibitor. Twenty-nine PDAC patients were enrolled in a phase I study, and vactosertib was safe and well tolerated [93]. Anti TGF-β agents are currently under investigation in clinical trials both in combination with chemotherapy and immunotherapy in PDAC treatment (Tables 1 and 2). Preclinical data showed that vitamin D analog therapy decreased MDSCs and Tregs, turning PDAC into a more “immune friendly” microenvironment. Preliminary results of a phase II pilot trial of Nivolumab + nab-P + Cisplatin + Paricalcitol + GEM in previously untreated metastatic PDAC patients showed 80% of the objective response rate and median PFS of 8.2 months. This regimen was related to 100% grade 3-4 thrombocytopenia, 50% grade 3-4 anemia, and 20% grade 3 colitis. This trial is still on going and data presented so far regarded only 10 patients (Table 2) [94]. CCR2 inhibition decreases TAMs and Tregs, increasing CD8+ and CD4+ cells in pancreatic tumors. A clinical trial evaluating CCR2 oral selective inhibitor CCX872-B in combination with FOLFIRINOX in locally advanced or metastatic PDAC showed 29% of OS at 18 months with no safety issues ascribed to CCX872-B use. Better OS was associated with lower peripheral blood monocyte counts at baseline [95]. The BTK pathway has a role in TME modulation. Ibrutinib demonstrated antitumor activity in preclinical PDAC models inhibiting mast cell degranulation, decreasing tumor-associated inflammation and desmoplasia, and enhancing cytotoxic T-cells [48]. A phase II-III trial is evaluating ibrutinib, in combination with Nab-P/GEM versus Nab-P/GEM alone, in 320 metastatic PDAC patients (Table 2). AM0010 is a covalent conjugate of recombinant IL-10 and polyethylene glycol (PEG), with potential antifibrotic, anti-inflammatory, immunomodulating, and antineoplastic activities. Upon subcutaneous administration, AM0010 may activate cell-mediated immunity against cancer cells stimulating CD8+ T-cell differentiation and expansion (Table 1). In a recent phase II trial, PDAC patients progressing on a median of 2 prior therapy were enrolled to AM0010 + FOLFOX resulting in a 15.8% response rate, 78.9% disease control rate, and 10.2-month median OS with good tolerability [96]. A phase III study of AM0010 with FOLFOX compared to FOLFOX alone as second-line therapy in metastatic PDAC patients is ongoing (Table 2). Recently, immune checkpoint inhibitors have been investigated in metastatic PDAC treatment (Table 1). To date, few data from early clinical trials are available. In particular, anti-PD-1 inhibitors have showed a safe toxicity profile but limited activity in combination with standard chemotherapy in “unselected” PDAC patients [94, 97]. Inhibiting the CSF-1/receptor pathway can reduce the intrinsic or acquired resistance to PD-1 inhibitors. Lacnotuzumab, a humanized antibody directed against CSF-1, in combination with Spartalizumab, anti-PD-1 humanized antibody, is under evaluation in a phase Ib/II study, showing good safety results [98].
9. Concluding Remarks
Pancreatic cancer management remains a challenge for oncologists despite that new therapeutic options have showed incremental survival advantage. TME and its components are main actors of tumor aggressiveness and treatment resistance. Stromal barrier, intense ECM production, high interstitial fluid pressure, hypoxia, and acidic extracellular pH contribute to make PDAC a chemorefractory tumor. Moreover, the crosstalk between TME and cancer cells causes immunosuppressive condition within PDAC immune infiltrate. Several signals deeply involved in early carcinogenesis, proliferation, invasiveness, and metastasization are activated by growth factors, chemokines, and cytokines released in this milieu. In the absence of predictive biomarkers for response and patient selection, an intriguing therapeutic approach should aim to normalize stroma, interfere in the crosstalk between TME and cancer cells, and restore the antitumoral activity of the immune system. Therefore, novel potential treatment strategies should include chemo/target/immunotherapy combinations or sequences in order to prevent or overcome resistances and improve outcomes.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
PaP, CC, NO, PiP, and GG performed the literature research and wrote the paper. PiP, NO, and GG assessed the figures and tables. TPL, AR, MP, PG, and EM made the text revision. PaP and GG supervised the project. All Authors approved the final manuscript. Paola Parente and Pietro Parcesepe contributed equally to this work.
References
- 1.Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.SEER stat fact sheets: pancreas cancer. 2015. April 2016 http://seer.cancer.gov/statfacts/html/pancreas.html.
- 3.Ferlay J., Partensky C., Bray F. More deaths from pancreatic cancer than breast cancer in the EU by 2017. Acta Oncologica. 2016;55(9-10):1158–1160. doi: 10.1080/0284186X.2016.1197419. [DOI] [PubMed] [Google Scholar]
- 4.Chin V., Nagrial A., Sjoquist K., et al. Chemotherapy and radiotherapy for advanced pancreatic cancer. Cochrane Database of Systematic Reviews. 2018;(3, article CD011044) doi: 10.1002/14651858.CD011044.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silvestris N., Gnoni A., Brunetti A. E., et al. Target therapies in pancreatic carcinoma. Current Medicinal Chemistry. 2014;21(8):948–965. doi: 10.2174/09298673113209990238. [DOI] [PubMed] [Google Scholar]
- 6.Burris H. A., 3rd, Moore M. J., Andersen J., et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. Journal of Clinical Oncology. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 7.Conroy T., Desseigne F., Ychou M., et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. New England Journal of Medicine. 2011;364(19):1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 8.Von Hoff D. D., Ervin T. J., Arena F. P., et al. Randomized phase III study of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic adenocarcinoma of the pancreas (MPACT) Journal of Clinical Oncology. 2013;31, article LBA148(4_Supplement) doi: 10.1200/jco.2013.31.4_suppl.lba148. [DOI] [Google Scholar]
- 9.Hidalgo M. Pancreatic cancer. The New England Journal of Medicine. 2010;362(17):1605–17. doi: 10.1056/NEJMra0901557. [DOI] [PubMed] [Google Scholar]
- 10.Zheng X., Carstens J. L., Kim J., et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–530. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dauer P., Nomura A., Saluja A., Banerjee S. Microenvironment in determining chemo-resistance in pancreatic cancer: Neighborhood matters. Pancreatology. 2017;17(1):7–12. doi: 10.1016/j.pan.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delitto D., Black B. S., Sorenson H. L., et al. The inflammatory milieu within the pancreatic cancer microenvironment correlates with clinicopathologic parameters, chemoresistance and survival. BMC Cancer. 2015;15(1):p. 783. doi: 10.1186/s12885-015-1820-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Apte M. V., Xu Z., Pothula S., Goldstein D., Pirola R. C., Wilson J. S. Pancreatic cancer: the microenvironment needs attention too! Pancreatology. 2015;15(4):S32–S38. doi: 10.1016/j.pan.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Delitto D., Wallet S. M., Hughes S. J. Targeting tumor tolerance: a new hope for pancreatic cancer therapy? Pharmacology & Therapeutics. 2016;166:9–29. doi: 10.1016/j.pharmthera.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Schreiber R. D., Old L. J., Smyth M. J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science. 2011;331(6024):1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- 16.Moher D., Liberati A., Tetzlaff J., Altman D. G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine. 2009;6(7, article e1000097) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turley S. J., Cremasco V., Astarita J. L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nature Reviews Immunology. 2015;15(11):669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 18.Balkwill F. R., Capasso M., Hagemann T. The tumor microenvironment at a glance. Journal of Cell Science. 2012;125(23):5591–5596. doi: 10.1242/jcs.116392. [DOI] [PubMed] [Google Scholar]
- 19.Kaur S., Kumar S., Momi N., Sasson A. R., Batra S. K. Mucins in pancreatic cancer and its microenvironment. Nature Reviews Gastroenterology & Hepatology. 2013;10(10):607–620. doi: 10.1038/nrgastro.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhan H. X., Zhou B., Cheng Y. G., et al. Crosstalk between stromal cells and cancer cells in pancreatic cancer: new insights into stromal biology. Cancer Letters. 2017;392:83–93. doi: 10.1016/j.canlet.2017.01.041. [DOI] [PubMed] [Google Scholar]
- 21.Australian Pancreatic Cancer Genome Initiative, Waddell N., Pajic M., et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518(7540):495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haqq J., Howells L. M., Garcea G., Metcalfe M. S., Steward W. P., Dennison A. R. Pancreatic stellate cells and pancreas cancer: current perspectives and future strategies. European Journal of Cancer. 2014;50(15):2570–2582. doi: 10.1016/j.ejca.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 23.Erkan M., Kurtoglu M., Kleeff J. The role of hypoxia in pancreatic cancer: a potential therapeutic target? Expert Review of Gastroenterology & Hepatology. 2016;10(3):301–316. doi: 10.1586/17474124.2016.1117386. [DOI] [PubMed] [Google Scholar]
- 24.Ye L.-Y., Zhang Q., Bai X.-L., Pankaj P., Hu Q.-D., Liang T.-B. Hypoxia-inducible factor 1α expression and its clinical significance in pancreatic cancer: a meta-analysis. Pancreatology. 2014;14(5):391–397. doi: 10.1016/j.pan.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Guillaumond F., Iovanna J. L., Vasseur S. Pancreatic tumor cell metabolism: focus on glycolysis and Its connected metabolic pathways. Archives of Biochemistry and Biophysics. 2014;545:69–73. doi: 10.1016/j.abb.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 26.Wang S., Huang S., Sun Y. L. Epithelial-mesenchymal transition in pancreatic cancer: a review. BioMed Research International. 2017;2017:10. doi: 10.1155/2017/2646148.2646148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang J. H., Jiang Y., Pillarisetty V. G. Role of immune cells in pancreatic cancer from bench to clinical application: an updated review. Medicine. 2016;95(49, article e5541) doi: 10.1097/MD.0000000000005541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lianyuan T., Dianrong X., Chunhui Y., Zhaolai M., Bin J. The predictive value and role of stromal tumor-infiltrating lymphocytes in pancreatic ductal adenocarcinoma (PDAC) Cancer Biology & Therapy. 2018;19(4):296–305. doi: 10.1080/15384047.2017.1416932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halbrook C. J., Pasca di Magliano M., Lyssiotis C. A. Tumor cross-talk networks promote growth and support immune evasion in pancreatic cancer. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2018;315(1):G27–G35. doi: 10.1152/ajpgi.00416.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klemm F., Joyce J. A. Microenvironmental regulation of therapeutic response in cancer. Trends in Cell Biology. 2015;25(4):198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erkan M., Hausmann S., Michalski C. W., et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nature Reviews Gastroenterology & Hepatology. 2012;9(8):454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 32.Tao L., Huang G., Song H., Chen Y., Chen L. Cancer associated fibroblasts: an essential role in the tumor microenvironment. Oncology Letters. 2017;14(3):2611–2620. doi: 10.3892/ol.2017.6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vonlaufen A., Joshi S., Qu C., et al. Pancreatic stellate cells: partners in crime with pancreatic cancer cells. Cancer Research. 2008;68(7):2085–2093. doi: 10.1158/0008-5472.CAN-07-2477. [DOI] [PubMed] [Google Scholar]
- 34.Bynigeri R. R., Jakkampudi A., Jangala R., et al. Pancreatic stellate cell: Pandora’s box for pancreatic disease biology. World Journal of Gastroenterology. 2017;23(3):382–405. doi: 10.3748/wjg.v23.i3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apte M. V., Wilson J. S. Dangerous liaisons: pancreatic stellate cells and pancreatic cancer cells. Journal of Gastroenterology and Hepatology. 2012;27(Supplement 2):69–74. doi: 10.1111/j.1440-1746.2011.07000.x. [DOI] [PubMed] [Google Scholar]
- 36.Yen T. W. F., Aardal N. P., Bronner M. P., et al. Myofibroblasts are responsible for the desmoplastic reaction surrounding human pancreatic carcinomas. Surgery. 2002;131(2):129–134. doi: 10.1067/msy.2002.119192. [DOI] [PubMed] [Google Scholar]
- 37.Hosaka K., Yang Y., Seki T., et al. Pericyte–fibroblast transition promotes tumor growth and metastasis. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(38):E5618–E5627. doi: 10.1073/pnas.1608384113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hida K., Maishi N., Torii C., Hida Y. Tumor angiogenesis—characteristics of tumor endothelial cells. International Journal of Clinical Oncology. 2016;21(2):206–212. doi: 10.1007/s10147-016-0957-1. [DOI] [PubMed] [Google Scholar]
- 39.Lee E., Pandey N. B., Popel A. S. Crosstalk between cancer cells and blood endothelial and lymphatic endothelial cells in tumour and organ microenvironment. Expert Reviews in Molecular Medicine. 2015;17, article e3 doi: 10.1017/erm.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scarlett C. J. Contribution of bone marrow derived cells to the pancreatic tumor microenvironment. Frontiers in Physiology. 2013;4:p. 56. doi: 10.3389/fphys.2013.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scarlett C. J., Colvin E. K., Pinese M., et al. Recruitment and activation of pancreatic stellate cells from the bone marrow in pancreatic cancer: a model of tumor-host interaction. PLoS One. 2011;6(10, article e26088) doi: 10.1371/journal.pone.0026088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mizukami Y. Bone marrow-derived proangiogenic cells in pancreatic cancer. Journal of Gastroenterology and Hepatology. 2012;27:23–26. doi: 10.1111/j.1440-1746.2011.07012.x. [DOI] [PubMed] [Google Scholar]
- 43.Gabrilovich D. I., Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews Immunology. 2009;9(3):162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Habtezion A., Edderkaoui M., Pandol S. J. Macrophages and pancreatic ductal adenocarcinoma. Cancer Letters. 2016;381(1):211–216. doi: 10.1016/j.canlet.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kurahara H., Shinchi H., Mataki Y., et al. Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. The Journal of Surgical Research. 2011;167(2):e211–e219. doi: 10.1016/j.jss.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 46.Neesse A., Algül H., Tuveson D. A., Gress T. M. Stromal biology and therapy in pancreatic cancer: a changing paradigm. Gut. 2015;64(9):1476–1484. doi: 10.1136/gutjnl-2015-309304. [DOI] [PubMed] [Google Scholar]
- 47.Pylayeva-Gupta Y., Das S., Handler J. S., et al. IL35-producing B cells promote the development of pancreatic neoplasia. Cancer Discovery. 2016;6(3):247–255. doi: 10.1158/2159-8290.cd-15-0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gunderson A. J., Kaneda M. M., Tsujikawa T., et al. Bruton tyrosine kinase-dependent immune cell cross-talk drives pancreas cancer. Cancer Discovery. 2016;6(3):270–285. doi: 10.1158/2159-8290.CD-15-0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gaida M. M., Steffen T. G., Günther F., et al. Polymorphonuclear neutrophils promote dyshesion of tumor cells and elastase-mediated degradation of E-cadherin in pancreatic tumors. European Journal of Immunology. 2012;42(12):3369–3380. doi: 10.1002/eji.201242628. [DOI] [PubMed] [Google Scholar]
- 50.Feig C., Gopinathan A., Neesse A., Chan D. S., Cook N., Tuveson D. A. The pancreas cancer microenvironment. Clinical Cancer Research. 2012;18(16):4266–4276. doi: 10.1158/1078-0432.CCR-11-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y., Li F., Gao F., et al. Role of microenvironmental periostin in pancreatic cancer progression. Oncotarget. 2017;8(52):89552–89565. doi: 10.18632/oncotarget.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barker H. E., Cox T. R., Erler J. T. The rationale for targeting the LOX family in cancer. Nature Reviews Cancer. 2012;12(8):540–552. doi: 10.1038/nrc3319. [DOI] [PubMed] [Google Scholar]
- 53.Provenzano P. P., Hingorani S. R. Hyaluronan, fluid pressure, and stromal resistance in pancreas cancer. British Journal of Cancer. 2013;108(1):1–8. doi: 10.1038/bjc.2012.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Provenzano P. P., Cuevas C., Chang A. E., Goel V. K., von Hoff D., Hingorani S. R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(3):418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bourguignon L. Y. W., Singleton P. A., Zhu H., Diedrich F. Hyaluronan-mediated CD44 interaction with RhoGEF and Rho kinase promotes Grb2-associated binder-1 phosphorylation and phosphatidylinositol 3-kinase signaling leading to cytokine (macrophage-colony stimulating factor) production and breast tumor progression. Journal of Biological Chemistry. 2003;278(32):29420–29434. doi: 10.1074/jbc.M301885200. [DOI] [PubMed] [Google Scholar]
- 56.Kii I., Nishiyama T., Li M., et al. Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. The Journal of Biological Chemistry. 2010;285(3):2028–2039. doi: 10.1074/jbc.M109.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J. P., Hielscher A. Fibronectin: how its aberrant expression in tumors may improve therapeutic targeting. Journal of Cancer. 2017;8(4):674–682. doi: 10.7150/jca.16901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Knapinska A. M., Estrada C. A., Fields G. B. The roles of matrix metalloproteinases in pancreatic cancer. Progress in Molecular Biology and Translational Science. 2017;148:339–354. doi: 10.1016/bs.pmbts.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Kikuta K., Masamune A., Watanabe T., et al. Pancreatic stellate cells promote epithelial-mesenchymal transition in pancreatic cancer cells. Biochemical and Biophysical Research Communications. 2010;403(3-4):380–384. doi: 10.1016/j.bbrc.2010.11.040. [DOI] [PubMed] [Google Scholar]
- 60.Froeling F. E. M., Mirza T. A., Feakins R. M., et al. Organotypic culture model of pancreatic cancer demonstrates that stromal cells modulate E-cadherin, β-catenin, and ezrin expression in tumor cells. The American Journal of Pathology. 2009;175(2):636–648. doi: 10.2353/ajpath.2009.090131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pothula S. P., Xu Z., Goldstein D., et al. Targeting the HGF/c-MET pathway: stromal remodelling in pancreatic cancer. Oncotarget. 2017;8(44):76722–76739. doi: 10.18632/oncotarget.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Räsänen K., Vaheri A. Activation of fibroblasts in cancer stroma. Experimental Cell Research. 2010;316(17):2713–2722. doi: 10.1016/j.yexcr.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 63.Kalluri R., Zeisberg M. Fibroblasts in cancer. Nature Reviews Cancer. 2006;6(5):392–401. doi: 10.1038/nrc1877. [DOI] [PubMed] [Google Scholar]
- 64.Song J., Lee J., Kim J., et al. Pancreatic adenocarcinoma up-regulated factor (PAUF) enhances the accumulation and functional activity of myeloid-derived suppressor cells (MDSCs) in pancreatic cancer. Oncotarget. 2016;7(32):51840–51853. doi: 10.18632/oncotarget.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanford D. E., Belt B. A., Panni R. Z., et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clinical Cancer Research. 2013;19(13):3404–3415. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Noy R., Pollard J. W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bausch D., Pausch T., Krauss T., et al. Neutrophil granulocyte derived MMP-9 is a VEGF independent functional component of the angiogenic switch in pancreatic ductal adenocarcinoma. Angiogenesis. 2011;14(3):235–243. doi: 10.1007/s10456-011-9207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feng M., Xiong G., Cao Z., et al. PD-1/PD-L1 and immunotherapy for pancreatic cancer. Cancer Letters. 2017;407:57–65. doi: 10.1016/j.canlet.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J., Wolfgang C., Zheng L. Precision immuno-oncology: prospects of individualized immunotherapy for pancreatic cancer. Cancers. 2018;10(2):p. 39. doi: 10.3390/cancers10020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Australian Pancreatic Cancer Genome Initiative, Bailey P., Chang D. K., et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531(7592):47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 71.Knudsen E. S., Vail P., Balaji U., et al. Stratification of pancreatic ductal adenocarcinoma: combinatorial genetic, stromal, and immunologic markers. Clinical Cancer Research. 2017;23(15):4429–4440. doi: 10.1158/1078-0432.CCR-17-0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hu H., Hang J. J., Han T., Zhuo M., Jiao F., Wang L. W. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumour Biology. 2016;37(7):8657–8664. doi: 10.1007/s13277-015-4741-z. [DOI] [PubMed] [Google Scholar]
- 73.Reid M. D., Basturk O., Thirabanjasak D., et al. Tumor-infiltrating neutrophils in pancreatic neoplasia. Modern Pathology. 2011;24(12):1612–1619. doi: 10.1038/modpathol.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mowbray N. G., Griffith D., Hammoda M., Shingler G., Kambal A., al-Sarireh B. A meta-analysis of the utility of the neutrophil-to-lymphocyte ratio in predicting survival after pancreatic cancer resection. HPB. 2018;20(5):379–384. doi: 10.1016/j.hpb.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 75.Tabernero J., Chiorean E. G., Infante J. R., et al. Prognostic factors of survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. The Oncologist. 2015;20(2):143–150. doi: 10.1634/theoncologist.2014-0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Striefler J. K., Sinn M., Jöhrens K., et al. Influence of cytotoxic tumor-infiltrating T lymphocytes on outcome in resectable pancreatic cancer: results from the CONKO 001 trial. Journal of Clinical Oncology. 2017;35(4_Supplement):p. 281. doi: 10.1200/JCO.2017.35.4_suppl.281. [DOI] [Google Scholar]
- 77.Jang J. E., Hajdu C. H., Liot C., Miller G., Dustin M. L., Bar-Sagi D. Crosstalk between regulatory T cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Reports. 2017;20(3):558–571. doi: 10.1016/j.celrep.2017.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Birnbaum D. J., Finetti P., Lopresti A., et al. Prognostic value of PDL1 expression in pancreatic cancer. Oncotarget. 2016;7(44):71198–71210. doi: 10.18632/oncotarget.11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Diana A., Wang L. M., D’Costa Z., et al. Prognostic value, localization and correlation of PD-1/PD-L1, CD8 and FOXP3 with the desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget. 2016;7(27):40992–41004. doi: 10.18632/oncotarget.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Whatcott C. J., Diep C. H., Jiang P., et al. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clinical Cancer Research. 2015;21(15):3561–3568. doi: 10.1158/1078-0432.CCR-14-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Melstrom L. G., Salazar M. D., Diamond D. J. The pancreatic cancer microenvironment: a true double agent. Journal of Surgical Oncology. 2017;116(1):7–15. doi: 10.1002/jso.24643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sahin I. H., Askan G., Hu Z. I., O’Reilly E. M. Immunotherapy in pancreatic ductal adenocarcinoma: an emerging entity? Annals of Oncology. 2017;28(12):2950–2961. doi: 10.1093/annonc/mdx503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Giordano G., Pancione M., Olivieri N., et al. Nano albumin bound-paclitaxel in pancreatic cancer: current evidences and future directions. World Journal of Gastroenterology. 2017;23(32):5875–5886. doi: 10.3748/wjg.v23.i32.5875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Desai N., Trieu V., Yao Z., et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor-free, albumin-bound paclitaxel, ABI-007, compared with cremophor-based paclitaxel. Clinical Cancer Research. 2006;12(4):1317–1324. doi: 10.1158/1078-0432.CCR-05-1634. [DOI] [PubMed] [Google Scholar]
- 85.Hidalgo M., Plaza C., Musteanu M., et al. SPARC expression did not predict efficacy of nab-paclitaxel plus gemcitabine or gemcitabine alone for metastatic pancreatic cancer in an exploratory analysis of the phase III MPACT trial. Clinical Cancer Research. 2015;21(21):4811–4818. doi: 10.1158/1078-0432.CCR-14-3222. [DOI] [PubMed] [Google Scholar]
- 86.Wong K. M., Horton K. J., Coveler A. L., Hingorani S. R., Harris W. P. Targeting the tumor stroma: the biology and clinical development of pegylated recombinant human hyaluronidase (PEGPH20) Current Oncology Reports. 2017;19(7):p. 47. doi: 10.1007/s11912-017-0608-3. [DOI] [PubMed] [Google Scholar]
- 87.Hingorani S. R., Zheng L., Bullock A. J., et al. HALO 202: randomized phase II study of PEGPH20 plus nab-paclitaxel/gemcitabine versus nab-paclitaxel/gemcitabine in patients with untreated, metastatic pancreatic ductal adenocarcinoma. Journal of Clinical Oncology. 2018;36(4):359–366. doi: 10.1200/JCO.2017.74.9564. [DOI] [PubMed] [Google Scholar]
- 88.Picozzi V. J., Pishvaian M. J., Mody K., et al. Effect of anti-CTGF human recombinant monoclonal antibody pamrevlumab on resectability and resection rate when combined with gemcitabine/nab-paclitaxel in phase 1/2 clinical study for the treatment of locally advanced pancreatic cancer patients. Journal of Clinical Oncology. 2018;36, article 4016(15_Supplement) doi: 10.1200/JCO.2018.36.15_suppl.4016. [DOI] [Google Scholar]
- 89.Naber H., ten Dijke P., Pardali E. Role of TGF- β in the tumor stroma. Current Cancer Drug Targets. 2008;8(6):466–472. doi: 10.2174/156800908785699342. [DOI] [PubMed] [Google Scholar]
- 90.Sawyer J. S., Anderson B. D., Beight D. W., et al. Synthesis and activity of new aryl- and heteroaryl-substituted pyrazole inhibitors of the transforming growth factor-β type I receptor kinase domain. Journal of Medicinal Chemistry. 2003;46(19):3953–3956. doi: 10.1021/jm0205705. [DOI] [PubMed] [Google Scholar]
- 91.Melisi D., Garcia-Carbonero R., Macarulla T., et al. A phase II, double-blind study of galunisertib+gemcitabine (GG) vs gemcitabine+placebo (GP) in patients (pts) with unresectable pancreatic cancer (PC) Journal of Clinical Oncology. 2016;34, article 4019(15_Supplement) doi: 10.1200/JCO.2016.34.15_suppl.4019. [DOI] [Google Scholar]
- 92.Murphy J. E., Wo J. Y. L., Ryan D. P., et al. Potentially curative combination of TGF-b1 inhibitor losartan and FOLFIRINOX (FFX) for locally advanced pancreatic cancer (LAPC): R0 resection rates and preliminary survival data from a prospective phase II study. Journal of Clinical Oncology. 2018;36, article 4116(15_Supplement) doi: 10.1200/JCO.2018.36.15_suppl.4116. [DOI] [Google Scholar]
- 93.Keedy V. L., Bauer T. M., Clarke J. M., et al. Association of TGF-β responsive signature with anti-tumor effect of vactosertib, a potent, oral TGF-β receptor type I (TGFBRI) inhibitor in patients with advanced solid tumors. Journal of Clinical Oncology. 2018;36, article 3031(15_Supplement) doi: 10.1200/JCO.2018.36.15_suppl.3031. [DOI] [Google Scholar]
- 94.Borazanci E. H., Jameson G. S., Borad M. J., et al. A phase II pilot trial of nivolumab + albumin bound paclitaxel + paricalcitol + cisplatin + gemcitabine (NAPPCG) in patients (pts) with previously untreated metastatic pancreatic ductal adenocarcinoma. Journal of Clinical Oncology. 2017;35, article TPS511(4_Supplement) doi: 10.1200/JCO.2017.35.4_suppl.TPS511. [DOI] [Google Scholar]
- 95.Linehan D., Noel M. S., Hezel A. F., et al. Overall survival in a trial of orally administered CCR2 inhibitor CCX872 in locally advanced/metastatic pancreatic cancer: correlation with blood monocyte counts. Journal of Clinical Oncology. 2018;36(5_Supplement):p. 92. doi: 10.1200/JCO.2018.36.5_suppl.92. [DOI] [Google Scholar]
- 96.Hecht J. R., Naing A., Falchook G. S., et al. Overall survival of PEGylated pegilodecakin with 5-FU/LV and oxaliplatin (FOLFOX) in metastatic pancreatic adenocarcinoma (PDAC) Journal of Clinical Oncology. 2018;36, article 4119(15_Supplement) doi: 10.1200/JCO.2018.36.15_suppl.4119. [DOI] [Google Scholar]
- 97.Wainberg Z. A., Hochster H. S., George B., et al. Phase I study of nivolumab (nivo) + nab-paclitaxel (nab-P) ± gemcitabine (Gem) in solid tumors: interim results from the pancreatic cancer (PC) cohorts. Journal of Clinical Oncology. 35(4_Supplement):p. 412. doi: 10.1200/JCO.2017.35.4_suppl.412. [DOI] [Google Scholar]
- 98.Calvo A., Joensuu H., Sebastian M., et al. Phase Ib/II study of lacnotuzumab (MCS110) combined with spartalizumab (PDR001) in patients (pts) with advanced tumors. Journal of Clinical Oncology. 2018;36, article 3014(15_Supplement) doi: 10.1200/JCO.2018.36.15_suppl.3014. [DOI] [Google Scholar]