Abstract
A cascade reaction of thioamides with 6β-bromoandrostenedione in hexafluoroisopropanol formed substituted thiazolo-androstenones. This is a simple and mild protocol to synthesize novel molecules by using readily available reagents and substrates. Feasibility of the reaction has been rationalized by density functional theory calculations. Moreover, these compounds are potent growth inhibitors of colon, central nervous system, melanoma, ovarian, and renal cancer cell lines with 50% growth inhibition values as low as 1.04 μM.
Introduction
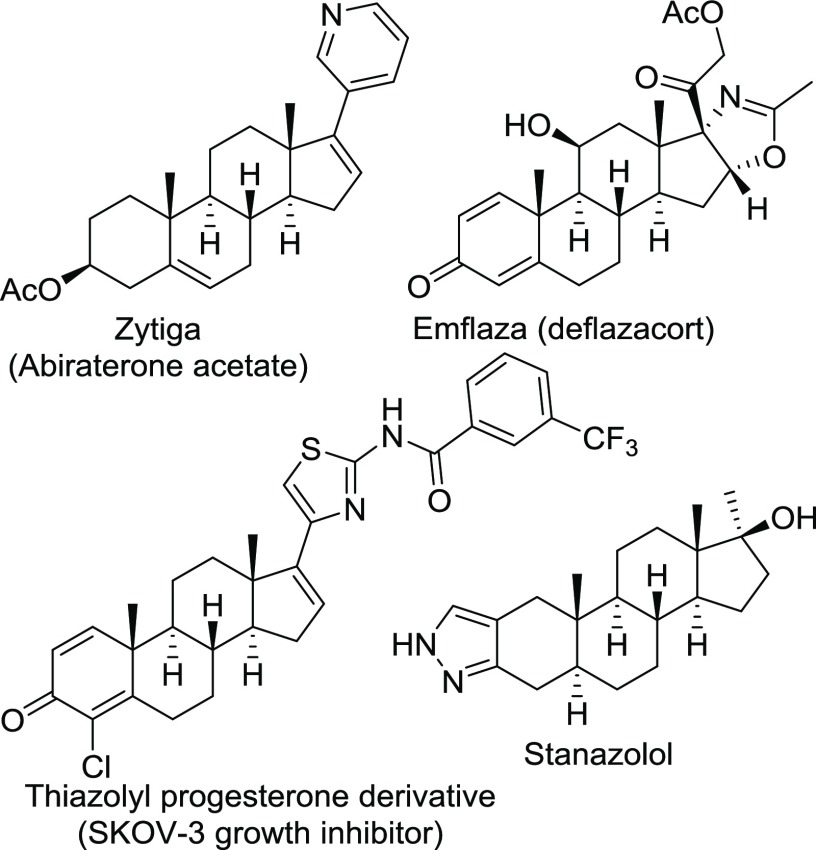
Several hormones of the steroidal skeleton are found in biological signaling in mammals.1,2 A large number of bioactive steroidal natural products have been isolated from various plants and microorganisms.3,4 Numerous synthetic derivatives have also been reported in literature in a quest of drugs, drug candidates, and other valuable materials including herbicides.5−11 Natural and synthetic steroidal derivatives are known to show a number of useful pharmacological properties such as agonists of cell-surface G-protein-coupled bile acid receptor,12 neuroprotective,13 anticancer,14 and anti-Alzheimer15 properties.7,16 Unnatural steroidal derivatives are one of the broadest spectra of therapeutic classes of compounds, which are used to treat different diseases including cancer.8,17 Thiazole derivatives are another class of important compounds with several approved drugs such as dasatinib, fanetizole, and nizatidine.18,19 Several steroidal drugs contain heterocyclic moieties: oxazole in Emflaza (deflazacort) and pyridine in Zytiga (abiraterone acetate).20−22 Thiazole-attached progesterone derivatives have been reported as potent SKOV-3 (ovarian cancer) growth inhibitor.23 Pyrazole-fused sterone, stanazolol, derivative is known for potent anabolic activities (Figure 1).24 Not surprisingly, syntheses of heterocycle-incorporated steroidal derivatives have been reported in a large number of literature.25−31 Novel molecules based on the steroidal core structure are synthesized in a multistep synthesis7,12,32−34 and using catalyst.29
Figure 1.
Representative examples of heterocycle containing steroidal drugs and pharmacologically active molecules.
Results and Discussion
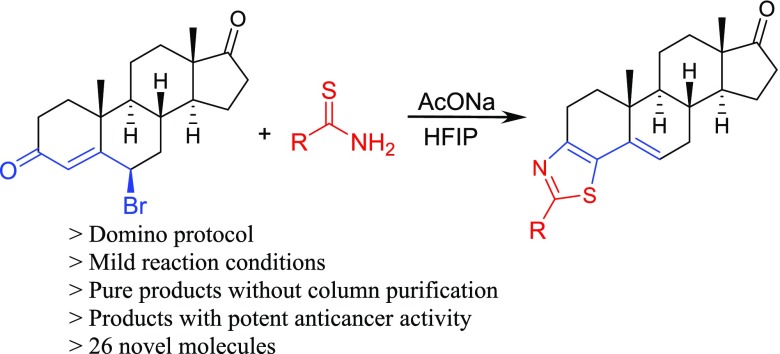
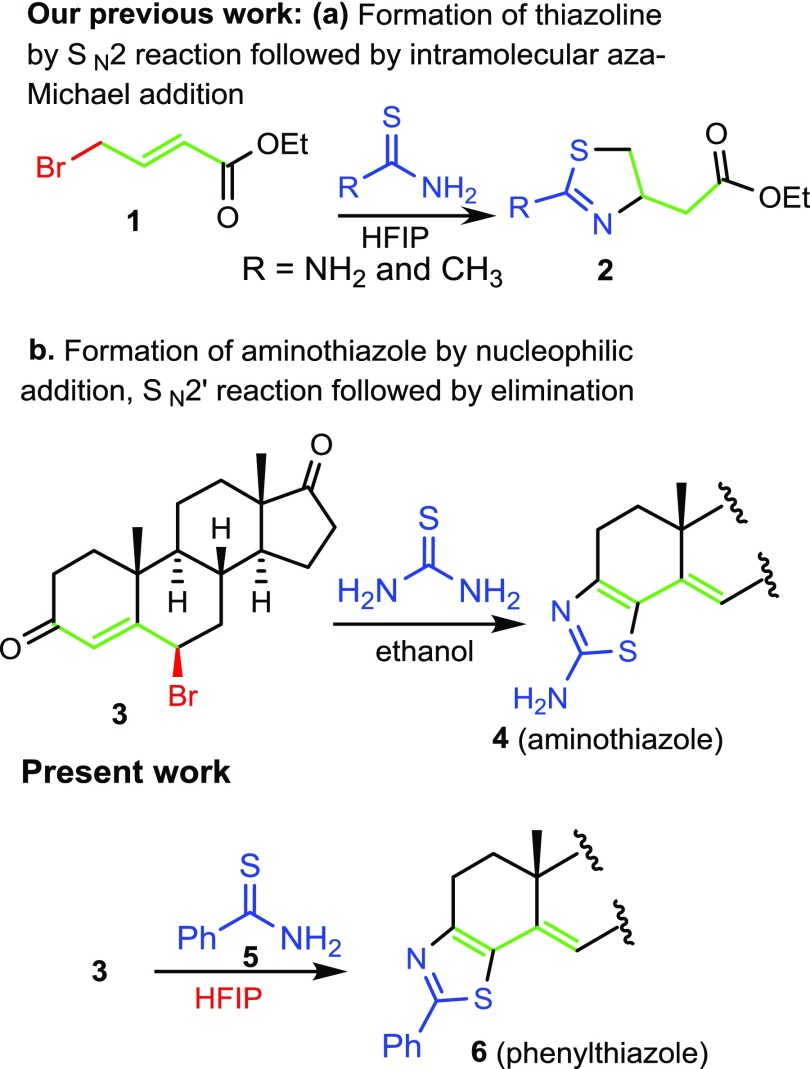
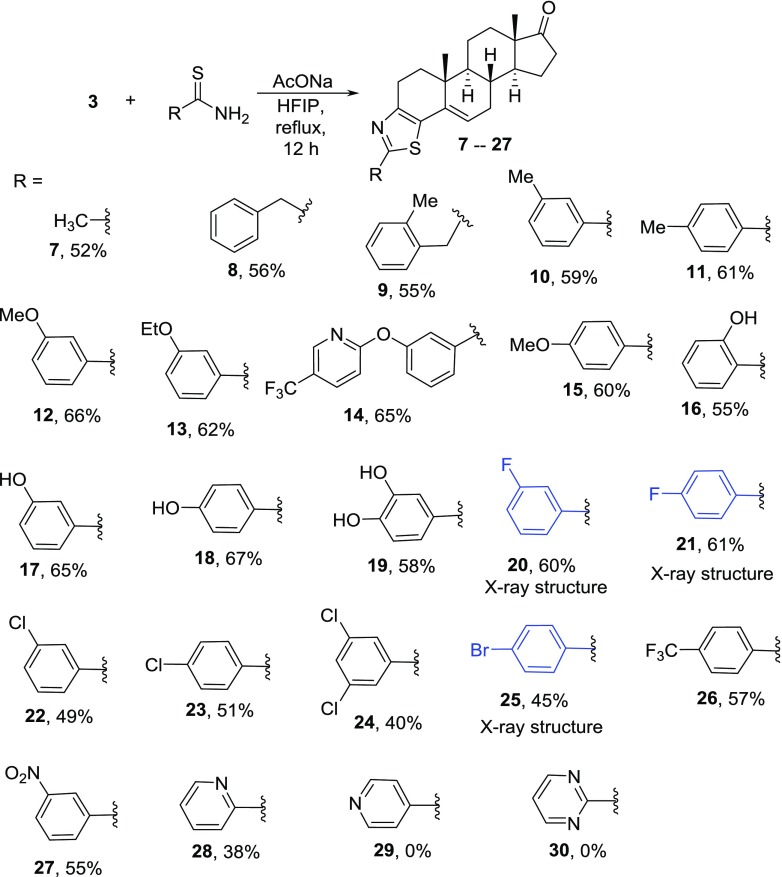
In our quest to synthesize bioactive molecules35−37 and to develop new domino reactions to synthesize heterocycles,38,39 we planned the synthesis of thiazolino-androstanedione derivatives by using our recently reported methodology, the synthesis of thiazoline derivatives (2) by reacting thioamides with γ-bromoenones (1).40 Reaction of 6β-bromoandrostenedione (3) with thiourea derivatives formed aminothiazoloandrostenone derivative (4) by an unexpected mechanism.41 Surprisingly, reaction of thioamide derivative (5) with the electrophile (3) did not form the product in refluxing ethanol, as we expected from our previous report (Scheme 1).41
Scheme 1. Synthesis of Thiazolo-androstenone Derivative (6).
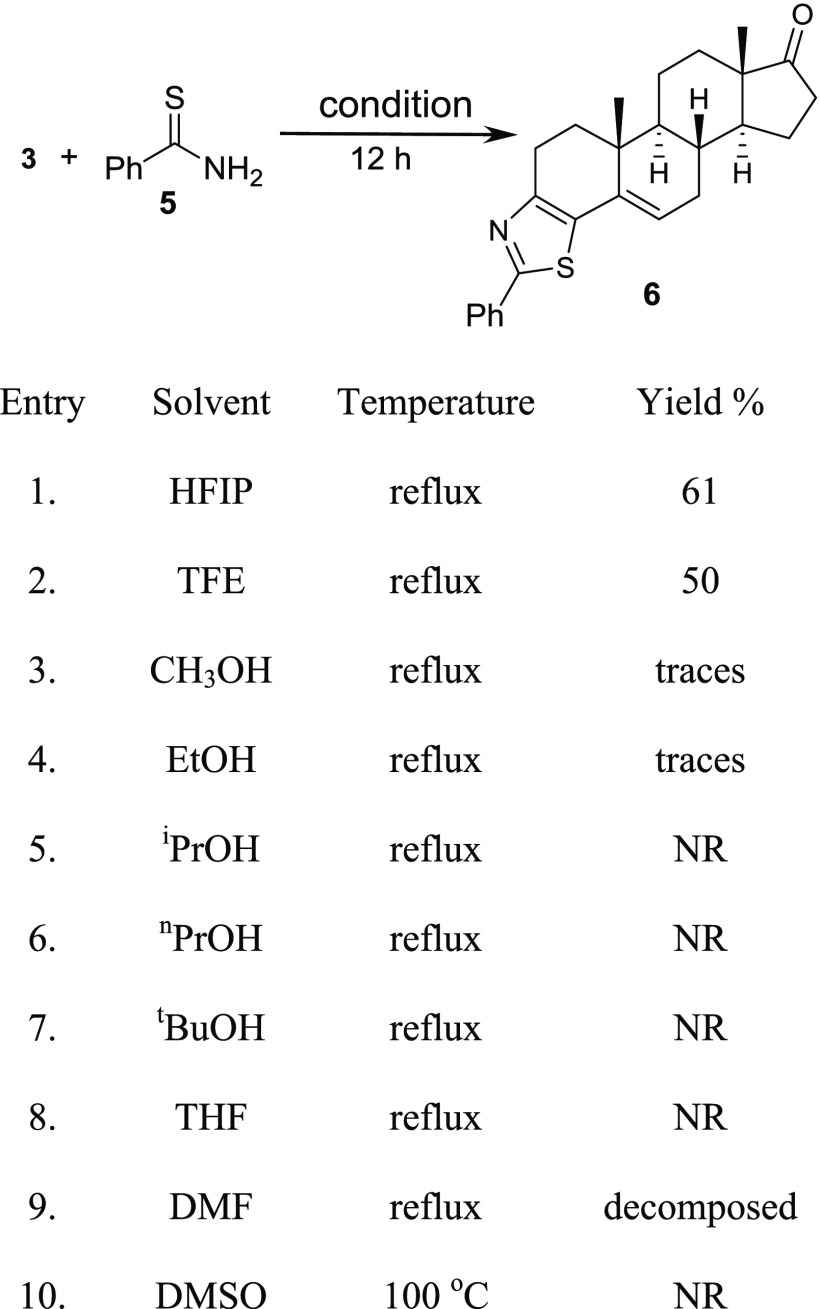
To our delight, the reaction happened in hexafluoroisopropanol (HFIP) in 61% yield, and the reaction did not require an anhydrous solvent and inert atmosphere. The products formed cleanly, and the pure material was isolated simply by distilling out HFIP followed by recrystallizing with methanol (Scheme 2). Column chromatography was not required to obtain the pure product (6). After identifying the product as thiazolo-androstenone in HFIP, we carried out the reaction in different solvents including different alcohols and polar aprotic solvents: tetrahydrofuran, dimethyl sulfoxide (DMSO), and N,N-dimethylformamide (DMF); however, the reaction was not successful in any solvent except trifuoroethanol in moderate yield. Refluxing the reaction mixture in DMF gave the unidentifiable decomposed products.42,43 On the basis of these observations, we can conclude that a very polar protic solvent is required for the product formation of this domino methodology, and HFIP has optimum properties for the success of this protocol.
Scheme 2. Reaction of Thiobenzamide with 6β-Bromoandrostenedione (3).
After establishing the optimum conditions, we reacted different thioamides with the electrophile (3), as shown in Scheme 3. Reaction of thioacetamide and 2-phenylthioacetamide with 6β-bromoandrostenone (3) formed the products (7 and 8) in 52 and 56% yields, respectively. 2-(2-Methylphenyl)thioacetamide also reacted with the electrophile (3) to give the benzyl derivative (9) in 55% yield. Substituted thiobenzamide derivatives were isolated under the established reaction conditions. m-Methyl- and p-methyl-substituted aryl products formed (10 and 11) in 59 and 61% yield, respectively. Similarly, 3-alkoxy-substituted products (12 and 13) formed in an average of 64% yield. A complex thioamide, 4-[5-(trifluoromethyl)pyrid-2-yloxy]thiobenzamide, did not hamper the reaction, and the product (14) was obtained in good yield. 4-Methoxy-substituted product (15) was obtained in 60% yield. Hydroxy-substituted products (16, 17, and 18) were obtained expectantly.
Scheme 3. Reaction of Thioamides with 6β-Bromoandrostenedione (3).
Furthermore, dihydroxy thiobenzamide also reacted smoothly to give the expected product (19) in 58% yield. Thus, the number and position of the electron-donating groups did not alter the outcome of the corresponding products. Products containing electron-withdrawing substituents were obtained under the established reaction conditions. 3-Fluoro-substituted thiazolo-androstenone derivative (20) formed in 60% yield, and 4-fluorophenyl-substituted compound (21) was obtained in 61% yield. 3-Chloro- and 4-chloro-substituted products (22 and 23) formed in an average of 50% yield. 3,5-Bischlorophenyl-substituted product (24) also formed accordingly. 4-Bromophenyl product (25) was obtained in 45% yield. 4-(Trifluoromethyl)thiobenzamide reacted with the electrophile to give the corresponding product (26). Very strong electron-withdrawing group containing aryl ring, such as 3-nitrothiobenzamide, also reacted with the electrophile (3) to give the corresponding product (27). Last but not least, pyridine-2-carbothioamide formed the product (28), albeit low yield (38%) was obtained. Thus, the nature and position of the electron-withdrawing group do effect the product formation of this methodology. Finally, the limitation we found in this methodology is that pyridine-4-carbothioamide and pyrimidine-2-carbotioamide failed to give the corresponding products (29 and 30). Thus, this methodology has the potential to generate a new class of novel molecules based on the fused thiazolo-androstane scaffold.
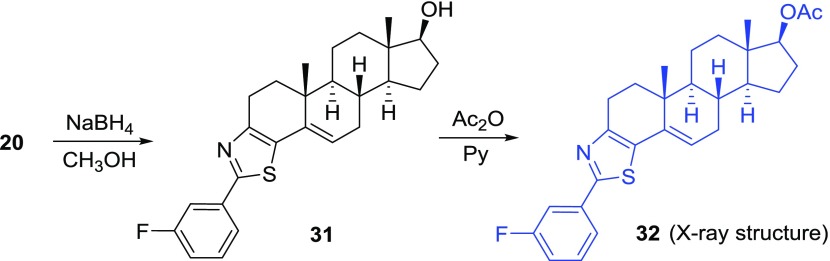
These molecules can be further transformed into new entities by simple reactions (Scheme 4). 17-Hydroxy and 17-aceloxy derivatives of androstane skeleton are integral parts of drugs, hormones, natural products, and synthetic bioactive molecules.44−46 Ketone derivative (20) was reduced with NaBH4 and led stereoselectively to the corresponding hydroxy product (31) in an excellent yield. Further acetylation with Ac2O/pyridine afforded the acetylated product (32) in quantitative yield.
Scheme 4. Reduction and Acetylation of Ketone Derivative (18) to Generate a Library of Molecules.
The structures of compounds (20, 21, 25, and 32) were confirmed by single-crystal X-ray diffraction analysis, which has helped to establish the regiochemistry and stereochemistry of the reactions. Crystal structures (20, 21, and 25) have helped to confirm the regiospecificity of this methodology. The final product (32) confirmed the formation of substrate-controlled β-hydroxy product exclusively in NaBH4 reduction (Figure 2).
Figure 2.
Oak ridge thermal ellipsoid plot diagrams of 20 (CCDC 1859241), 21 (CCDC 1859222), 25 (CCDC 1861085), and 32 (CCDC 1859223).
Computational Analysis
All density functional theory calculations were carried out using Gaussian 09 suite of programs.46 The hybrid density functional method (M06-2X)/6-311++G(d,p) + PCM (solvent = HFIP) has been used to compute the feasibility of all four pathways. We have used polarizable continuum model (PCM) using the integral equation formalism variant as the self consistent reaction field method. This method creates solute cavity via a set of overlapping spheres. The free energy of all of the species was calculated using the PCM solvent model i.e., HFIP. The calculations were done at 1 atm pressure and 298 K.
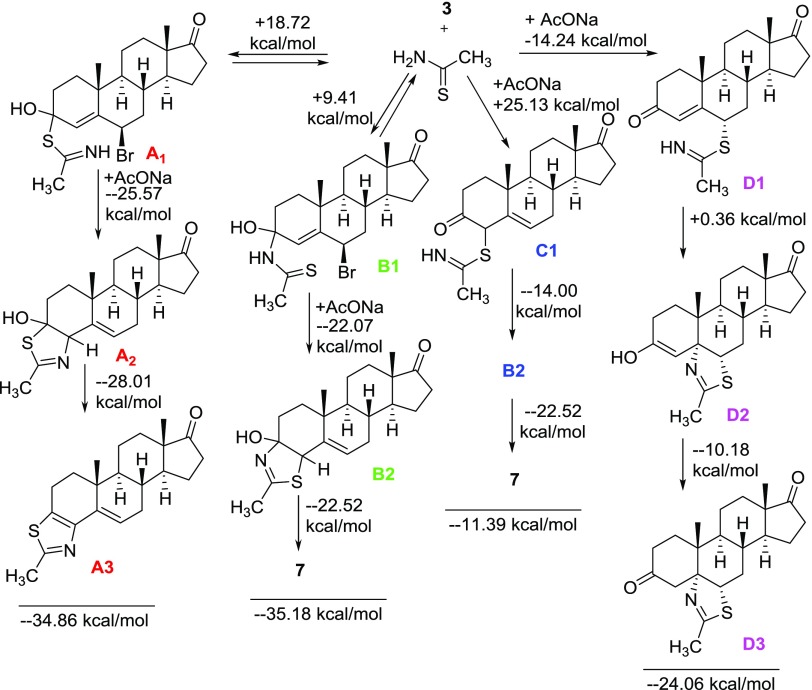
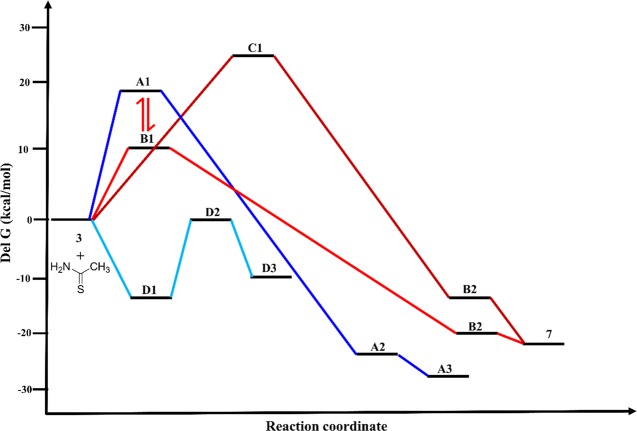
HFIP is a very strong hydrogen bond donor, which makes hydrogen bonding with the carbonyl oxygen of the enone of β-bromoandrostenedione (3).39 This hydrogen bonding makes the enone carbonyl group a better electrophile for the nucleophilic addition of thioacetamide, which is the key for the success of this methodology. Sulfur or nitrogen atom of thioacetamide can undergo nucleophilic addition to the carbonyl group of β-bromoandrostenedione (3) to form two possible intermediates, hemithioacetal (A1) or hemiaminal (B1), respectively. Both of these two reactions are endergonic, and the Gibb’s free energy for the formation of hemithioacetal (A1, +18.72 kcal/mol) and hemiaminal (B1, +9.41 kcal/mol) is achieved by refluxing the reaction mixture in HFIP. These reactions for the formation of hemithioacetal (A1) and hemiaminal (B1) are reversible under the reaction condition. Hemithioacetal (A1) and hemiaminal (B1) undergo intramolecular SN2′ reaction to form the thiazoline regioisomers (A2 and B2). This SN2′ reaction of hemithioacetal (A1) is more favorable than that of hemiaminal (B1) (−25.57 vs −22.07 kcal/mol). Dehydration, the final step, is more favorable for the hemithioacetal than the hemiaminal derivative (−28.01 vs −22.52 kcal/mol) to form the final products A3 and 7, respectively. Among the three steps for the formation of possible products, the last two steps are irreversible and exergonic (Scheme 5). Hence, the less-energy barrier for the formation of hemiaminal (B1) in the first step is the deciding factor for the formation of the actual product (7). The other possible pathway (C1 → B2) for the formation of expected product (7) is the least favorable. The expected product (D3) based on our previous report40 is also thermodynamically not favorable (Figure 2). Probable potential energy surface for all of the possible pathways is shown in Figure 3.
Scheme 5. Plausible Mechanism for the Formation of Product (7) Using M06-2X/6-311++G(d,p) + PCM (Solvent = HFIP) Level of Theory.
Figure 3.
Probable potential energy surface of formation of actual and expected products calculated at M06-2X/6-311++G(d,p) + PCM (solvent = HFIP).
In Vitro Anticancer Studies
After the successful synthesis of these novel molecules, some of them have been tested against NCI-60 cancer cell lines.47 Many of these compounds have shown promising activity against several cancer cell lines at 10 μM concentration. Selected growth inhibition data for compound 20 are shown in Table 1. This compound has inhibited the growth of 31 of 60 cell lines with 50% growth inhibition (GI50) value <2.80 μM concentration. The total growth inhibition (TGI) value is also in the low micromolar range. The compound (20) has also shown good lethal concentration (LC50) values against nine cancer cell lines. HCT-116 and SF-539 were inhibited with LC50 values of 7.30 and 5.90 μM concentrations, respectively.
Table 1. Cytotoxic Data of Compound 20 against NCI-60 Cell Lines.
|
20 |
||||
|---|---|---|---|---|
| cancer panel | cell line | GI50 | TGI | LC50 |
| leukemia | HL-60(TB) | 2.00 | 5.35 | >10 |
| K-562 | 1.82 | 9.08 | >10 | |
| MOLT-4 | 1.69 | 7.89 | >10 | |
| RPMI-8226 | 1.91 | 5.65 | >10 | |
| NSCLC | HOP-92 | 2.44 | 5.63 | >10 |
| NCI-H226 | 1.94 | 4.39 | >10 | |
| NCI-H460 | 2.79 | 8.27 | >10 | |
| NCI-H522 | 2.40 | 6.35 | >10 | |
| colon cancer | COLO 205 | 2.42 | 5.46 | >10 |
| HCT-116 | 1.73 | 3.56 | 7.33 | |
| HCT-15 | 1.56 | 4.17 | >10 | |
| SW-620 | 2.14 | 4.50 | >10 | |
| CNS cancer | SF-295 | 1.59 | 4.91 | >10 |
| SF-539 | 1.04 | 2.48 | 5.90 | |
| melanoma | LOX IMVI | 1.74 | 3.36 | 6.47 |
| MALME-3M | 1.80 | 4.13 | 9.48 | |
| SK-MEL-28 | 1.53 | 4.07 | >10 | |
| UACC-62 | 1.90 | 5.70 | >10 | |
| ovarian cancer | IGROV1 | 2.09 | 4.88 | >10 |
| OVCAR-3 | 1.92 | 3.79 | 7.47 | |
| SKOV-3 | 2.91 | 9.13 | >10 | |
| renal cancer | 786-0 | 1.30 | 2.80 | >10 |
| ACHN | 1.64 | 3.01 | 5.53 | |
| CAKI-1 | 1.87 | 3.76 | 7.57 | |
| RXF 393 | 1.41 | 2.94 | 6.14 | |
| TK-10 | 2.77 | 8.29 | >10 | |
| UO-31 | 1.61 | 2.98 | 5.54 | |
| prostate cancer | DU-145 | 2.01 | 4.13 | >10 |
| breast cancer | MCF7 | 1.78 | 4.26 | >10 |
| MDA-MB-231/ATCC | 2.60 | 7.34 | >10 | |
| BT-549 | 2.20 | 8.06 | >10 | |
| T-47D | 2.00 | 5.24 | >10 | |
| MDA-MB-468 | 1.95 | 4.27 | >10 | |
Colorectal cancer is the third most common cancer in men and women in the US, nevertheless, this cancer is second leading cause of cancer-related deaths in this country.47 Central nervous system (CNS) cancer is one of the most lethal forms of cancer with very limited treatment options.48 Our tested molecule (20) has shown significant activity against four colon cancer cell lines with GI50 values as low as 1.56 μM concentration. This molecule inhibited the growth of HCT-116 cell line with TGI and LC50 values 3.56 and 7.33 μM, respectively. Growths of two of the six CNS cancer cell lines were also inhibited significantly with GI50 values ∼1 μM concentration. In vitro growth inhibition of SF-539 cancer cell line of the CNS panel is very significant with TGI and LC50 values of 2.48 and 5.90 μM, respectively.
Melanoma is the most serious type of skin cancer. It is the 5–7th most common cancer in the United States, and the incidence of this cancer is increasing rapidly. Other than the skin, this cancer can also develop in the eyes and in internal organs such as the intestines.49 Development of new therapeutic options is urgently needed to treat this rapidly rising malignancy.50 Our lead compound (20) has shown promising growth inhibition activity against four melanoma cell lines with GI50 less than 2 μM concentration. TGI values are also in low micromolar concentration for these four melanoma cell lines. This compound also inhibited the two melanoma cell lines: LOX IMVI and MALME-3M cell lines with LC50 values of 6.47 and 9.48 μM, respectively. We have found several lead molecules such as 20 to generate a library of molecules to develop potent antimelanoma agents.
In addition, compound 20 has shown promising activity against four ovarian cancer cell lines with GI50 values at low micromolar concentration. The LC50 value for ovarian cancer cell line, OVCAR-3, is less than 10 μM. Renal cancer is the most common type of kidney cancer.51 The fluorophenyl derivative (20) has shown potent activity against six cancer cell lines with GI50 and TGI values as low as 1.30 and 2.80 μM, respectively. Four renal cancer cell lines were inhibited with LC50 values less than 10 μM. Significant growth inhibition of prostate and breast cancer cell lines was also observed by this lead molecule (20). Complete data are shown in the Supporting Information. Detailed findings and mode of action of potent molecules will be reported soon.
Conclusions
In summary, we have discovered a one-pot protocol to synthesize novel thiazolo-androstenones by using readily available starting materials and benign reaction conditions. On the basis of the availability of a number of thioamide starting materials and ease of reaction conditions, a large number of novel molecules as potent anticancer agents can be synthesized. Furthermore, these new scaffolds can be easily transformed into a variety of potential bioactive molecules. Further derivatization, structure–activity relationship, anticancer, and toxicity studies of these novel compounds are in progress and will be reported soon.
Experimental Section
General Consideration
All of the reactions were carried out under air atmosphere in round-bottom flasks. Solvents, reagents, and the substrate were bought from Fisher Scientific and Oakwood chemical.
Characterization
1H NMR and 13C NMR spectra were recorded with a Varian Mercury-300 MHz and Varian Mercury-75 MHz, respectively, with tetramethylsilane (TMS) as internal standard. CDCl3 (>99.9%), DMSO-d6 (>99.8%), or mixture of both were used to record NMR spectra. In some spectra, trifluoroacetic acid-d was also used to increase the solubility of the samples. The electrospray ionization-Fourier transform mass spectra (ESI-FTMS) were recorded using Bruker ApexII-FTMS system.
Crystals were grown in chloroform–methanol mixture for single-crystal diffraction.
General Procedure for the Synthesis of Thiazole-androstenones (6–28)
A mixture of β-bromoandrostenedione (1 mmol), thioamide derivative (1.1 mmol), and sodium acetate (82 mg, 1.0 mmol) in 10 mL of hexafluoroisopropanol was refluxed for 12 h to complete the reaction. Progress of the reaction was monitored by thin-layer chromatography. After the completion of the reaction, HFIP was distilled out and methanol (10 mL) was added. The solid precipitate was filtered followed by washing with ∼10 mL of methanol and ∼20 mL of water under vacuum to afford the pure product.
Characterization Data
(1S,2R,13R,14S,18S)-2,18-Dimethyl-7-phenyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (6)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.92–7.90 (m, 2H), 7.46–7.40 (m, 3H), 5.86–5.84 (m, 1H), 3.05–2.86 (m, 2H), 2.56–2.34 (m, 2H), 2.20–1.80 (m, 6H), 1.68–1.16 (m, 7H), 1.09 (s, 3H), 0.95 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.8, 164.2, 150.4, 136.6, 133.8, 131.6, 129.7, 128.8, 126.3, 121.4, 51.7, 48.1, 47.6, 36.7, 35.8, 34.3, 31.3, 31.1, 30.8, 24.1, 21.8, 20.7, 18.7, 13.6. HRMS (ESI-FTMS, m/z): calcd for C26H30NOS [M + H]+ 404.2042, found 404.2046. Yield (245 mg, 61%).
(1S,2R,13R,14S,18S)-2,7,18-Trimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (7)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 5.70–5.69 (m, 1H), 2.91–2.71 (m, 2H), 2.62 (s, 3H), 2.54–2.21 (m, 2H), 2.18–1.66 (m, 7H), 1.63–1.14 (m, 6H), 1.03 (s, 3H), 0.94 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.8, 162.7, 148.7, 136.5, 130.9, 120.5, 51.7, 48.1, 47.6, 36.7, 35.8, 34.3, 31.3, 31.1, 30.6, 23.9, 21.8, 20.7, 19.4, 18.6, 13.6. HRMS (ESI-FTMS, m/z): calcd for C21H28NOS [M + H]+ 342.1886, found 342.1890. Yield (177 mg, 52%).
(1S,2R,13R,14S,18S)-2,18-Dimethyl-7-phenyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (8)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.40–7.21 (m, 5H), 5.67–5.65 (m, 1H), 4.25 (s, 2H), 2.95–2.74 (m, 2H), 2.50 (dd, J = 8.7, 19.1 Hz, 1H), 2.34–2.25 (m, 1H), 2.18–1.29 (m, 12H), 1.22–1.10 (m, 1H), 1.03 (s, 3H), 0.93 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.8, 166.9, 148.9, 137.9, 136.5, 131.6, 129.0, 128.7, 127.0, 120.7, 51.7, 48.1, 47.6, 40.7, 36.7, 35.8, 34.3, 31.3, 31.1, 30.6, 24.0, 21.8, 20.7, 18.6, 13.6. HRMS (ESI-FTMS, m/z): calcd for C27H32NOS [M + H]+ 418.2199, found 418.2192. Yield (233 mg, 56%).
(1S,2R,13R,14S,18S)-2,18-Dimethyl-7-(o-tolylmethyl)-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),6,10-trien-17-one (9)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.27–7.22 (m, 4H), 5.63–5.62 (m, 1H), 4.25 (s, 2H), 2.95–2.74 (m, 2H), 2.49 (dd, J = 8.7, 19.2 Hz, 1H), 2.34 (s, 3H), 2.34–2.28 (m, 1H), 2.17–1.78 (m, 7H), 1.58–1.14 (m, 6H), 1.03 (s, 3H), 0.93 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.8, 167.3, 148.8, 136.7, 136.5, 136.3, 131.3, 130.5, 130.0, 127.5, 126.3, 120.6, 51.7, 48.1, 47.6, 37.8, 36.6, 35.8, 34.3, 31.3, 31.1, 30.6, 24.0, 21.8, 20.7, 19.6, 18.6, 13.6. HRMS (ESI-FTMS, m/z): calcd for C27H32NOS [M + H]+ 432.2356, found 432.2349. Yield (237 mg, 55%).
(1S,2R,13R,14S,18S)-2,18-Dimethyl-7-(m-tolyl)-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (10)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.76 (s, 1H), 7.69 (d, J = 7.6 Hz, 1H), 7.33–7.28 (m, 1H), 7.21 (d, J = 7.4 Hz, 1H), 5.85–5.84 (m, 1H), 3.05–2.82 (m, 2H), 2.55–2.34 (m, 5H), 2.19–1.80 (m, 7H), 1.68–1.25 (m, 5H), 1.22–1.16 (m, 1H), 1.09 (s, 3H), 0.95 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.7, 164.4, 150.4, 138.6, 136.6, 133.7, 131.4, 130.5, 128.7, 126.8, 123.6, 121.3, 51.7, 48.2, 47.6, 36.7, 35.8, 34.3, 31.4, 31.1, 30.8, 24.1, 21.8, 21.3, 20.7, 18.7, 13.6. HRMS (ESI-FTMS, m/z): calcd for C27H32NOS [M + H]+ 418.2199, found 418.2204. Yield (246 mg, 59%).
(1S,2R,13R,14S,18S)-2,18-Dimethyl-7-(p-tolyl)-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (11)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.80 (d, J = 8.0 Hz, 2H), 7.23 (d, J = 8.2 Hz, 2H), 5.83–5.52 (m, 1H), 3.03–2.87 (m, 2H), 2.55–2.33 (m, 2H), 2.39 (s, 3H), 2.19–1.80 (m, 7H), 1.68–1.15 (m, 6H), 1.08 (s, 3H), 0.94 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.8, 164.5, 150.2, 140.0, 136.6, 131.1, 129.5, 126.3, 121.2, 51.7, 48.1, 47.6, 36.7, 35.8, 34.3, 31.3, 31.1, 30.8, 24.1, 21.8, 21.4, 20.7, 18.7, 13.6. HRMS (ESI-FTMS, m/z): calcd for C27H32NOS [M + H]+ 418.2199, found 418.2204. Yield (254 mg, 61%).
(1S,2R,13R,14S,18S)-7-(3-Methoxyphenyl)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (12)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.47 (m, 2H), 7.32 (d, J = 8.2 Hz, 1H), 6.95–6.92 (m, 1H), 5.83 (s, 1H), 3.87 (s, 3H), 3.02–2.80 (m, 2H), 2.53–2.33 (m, 2H), 2.17–1.78 (m, 6H), 1.65–1.13 (m, 7H), 1.06 (s, 3H), 0.92 (m, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.7, 164.0, 159.9, 150.3, 136.5, 135.1, 131.7, 129.8, 121.5, 119.0, 116.0, 110.9, 55.4, 51.6, 48.1, 47.5, 36.7, 35.8, 34.3, 31.3, 31.1, 30.7, 24.1, 21.8, 20.7, 18.7, 13.6. HRMS (ESI-FTMS, m/z): calcd for C27H32NO2S [M + H]+ 434.2148, found 434.2154. Yield (286 mg, 66%).
(1S,2R,13R,14S,18S)-7-(3-Ethoxyphenyl)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (13)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.48–7.47 (m, 2H), 7.32 (t, J = 5.1 Hz, 1H), 6.94 (dd, J = 2.4, 8.3 Hz, 1H), 5.86–5.85 (m, 1H), 4.12 (q, J = 3.9 Hz, 2H), 3.04–2.82 (m, 2H), 2.56–2.34 (m, 2H), 2.20–1.81 (m, 7H), 1.71–1.20 (m, 9H), 1.09 (s, 3H), 0.95 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.8, 164.1, 159.3, 150.4, 136.6, 135.1, 131.6, 129.8, 121.4, 118.8, 116.5, 111.6, 63.6, 51.7, 48.1, 47.6, 36.7, 35.8, 34.3, 31.4, 31.1, 30.8, 24.1, 21.8, 20.7, 18.7, 14.8, 13.6. HRMS (ESI-FTMS, m/z): calcd for C28H34NO2S [M + H]+ 448.2305, found 448.2301. Yield (291 mg, 62%).
(1S,2R,13R,14S,18S)-2,18-Dimethyl-7-[3-[[5-(trifluoromethyl)-2-pyridyl]oxy]phenyl]-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),6,10-trien-17-one (14)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 8.47 (s, 1H), 7.99–7.92 (m, 3H), 7.21 (d, J = 8.4 Hz, 2H), 7.07 (d, J = 8.5 Hz, 1H), 5.86–5.85 (m, 1H), 3.04–3.02 (m, 2H), 2.56–2.35 (m, 2H), 2.20–1.19 (m, 13H), 1.09 (s, 3H), 0.95 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.9, 165.4, 163.2, 154.2, 150.5, 145.5 (J = 4.3 Hz), 136.8, 136.5, 131.7, 131.2, 127.9, 125.4, 122.1, 121.8, 121.7, 121.6, 111.6, 51.7, 48.1, 47.6, 36.7, 35.8, 34.3, 31.3, 31.1, 30.8, 24.1, 21.8, 20.7, 13.6. HRMS (ESI-FTMS, m/z): calcd for C32H32N2O2F3S [M + H]+ 565.2131, found 565.2125. Yield (366 mg, 65%).
(1S,2R,13R,14S,18S)-7-(4-Methoxyphenyl)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (15)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.86 (d, J = 8.6 Hz, 2H), 6.94 (d, J = 8.7 Hz, 2H), 5.82–5.81 (m, 1H), 3.86 (s, 3H), 3.02–2.86 (m, 2H), 2.55–2.33 (m, 2H), 2.19–1.81 (m, 6H), 1.68–1.16 (m, 7H), 1.09 (s, 3H), 0.95 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.7, 164.2, 160.9, 150.2, 136.7, 130.6, 127.8, 126.8, 120.9, 114.2, 55.3, 51.7, 48.2, 47.6, 36.7, 35.8, 34.3, 31.4, 31.1, 30.8, 24.1, 21.8, 20.7, 18.7, 13.6. HRMS (ESI-FTMS, m/z): calcd for C27H32NO2S [M + H]+ 434.2148, found 434.2153. Yield (259 mg, 60%).
(1S,2R,13R,14S,18S)-7-(3-Hydroxy)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (16)
Yellowish solid, 1H NMR (300 MHz, CDCl3 + DMSO-d6) δ ppm: 12.13 (br s, 1H), 7.49 (d, J = 7.7 Hz, 1H), 7.19 (t, J = 8.2 Hz, 1H), 6.88 (d, J = 8.2 Hz, 1H), 6.81 (t, J = 7.4 Hz, 1H), 5.78 (br s, 1H), 2.87–2.74 (m, 2H), 2.51–2.31 (m, 2H), 2.07–1.71 (m, 6H), 1.60–1.12 (m, 7H), 0.98 (s, 3H), 0.84 (s, 3H); 13C NMR (75 MHz, CDCl3 + DMSO-d6) δ ppm: 220.3, 164.9, 156.6, 147.6, 135.6, 130.9, 129.2, 126.7, 121.7, 118.8, 117.2, 116.7, 51.2, 47.6, 47.1, 36.3, 35.3, 33.6, 30.9, 30.7, 30.3, 23.2, 21.4, 20.3, 18.3, 13.2. HRMS (ESI-FTMS, m/z): calcd for C26H29NOS [M + H]+ 420.1992, found 420.1997. Yield (230 mg, 55%).
(1S,2R,13R,14S,18S)-7-(3-Hydroxy)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (17)
Yellowish solid, 1H NMR (300 MHz, CDCl3 + DMSO-d6) δ ppm: 9.41 (d, J = 5.2 Hz, 1H), 7.27–7.13 (m, 3H), 6.77 (d, J = 7.9 Hz, 1H), 5.77 (s, 1H), 2.89–2.70 (m, 2H), 2.43–2.30 (m, 2H), 2.04–1.72 (m, 6H), 1.60–1.11 (m, 7H), 0.99 (s, 3H), 0.84 (s, 3H); 13C NMR (75 MHz, CDCl3 + DMSO-d6) δ ppm: 219.7, 163.4, 158.0, 150.3, 136.3, 134.8, 131.4, 130.1, 121.9, 117.3, 117.1, 112.9, 51.4, 48.0, 47.3, 36.6, 35.7, 34.1, 31.4, 31.0, 30.7, 24.1, 21.8, 20.7, 18.8, 13.7. HRMS (ESI-FTMS, m/z): calcd for C26H30NO2S [M + H]+ 420.1992, found 420.1989. Yield (273 mg, 65%).
(1S,2R,13R,14S,18S)-7-(4-Hydroxy)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (18)
Yellowish solid, 1H NMR (300 MHz, CDCl3 + DMSO-d6) δ ppm: 9.90 (s, 1H), 7.67 (d, J = 8.3 Hz, 2H), 6.81 (d, J = 8.3 Hz, 2H), 5.74 (s, 1H), 2.85–2.69 (m, 2H), 2.50–2.27 (m, 2H), 2.06–1.71 (m, 6H), 1.62–1.15 (m, 7H), 1.00 (s, 3H), 0.84 (s, 3H); 1H NMR (300 MHz, CDCl3 + DMSO-d6) δ ppm: 219.7, 163.8, 159.7, 150.1, 136.5, 130.0, 127.9, 125.0, 121.5, 116.1, 51.3, 48.0, 47.3, 36.6, 35.7, 34.2, 31.5, 31.0, 30.6, 24.2, 21.8, 20.7, 18.9, 13.7. HRMS (ESI-FTMS, m/z): calcd for C26H29NO2S [M + H]+ 420.1992, found 420.1997. Yield (281 mg, 67%).
(1S,2R,13R,14S,18S)-7-(3,4-Dihydroxyphenyl)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),6,10-trien-17-one (19)
Yellowish solid, 1H NMR (300 MHz, CDCl3 + DMSO-d6) δ ppm: 8.95 (s, 1H), 8.78 (s, 1H), 7.30 (d, J = 2.0 Hz, 1H), 7.13 (dd, J = 2.0, 8.1 Hz, 1H), 6.74 (d, J = 8.2 Hz, 1H), 5.68 (br s, 1H), 2.83–2.71 (m, 2H), 2.50–2.29 (m, 3H), 2.07–1.73 (m, 6H), 1.56–1.12 (m, 6H), 0.98 (s, 3H), 0.84 (s, 3H); 1H NMR (75 MHz, CDCl3 + DMSO-d6) δ ppm: 220.1, 164.2, 149.8, 147.6, 145.5, 136.4, 130.0, 125.7, 120.9, 118.2, 115.9, 113.6, 51.4, 48.0, 47.4, 36.6, 35.7, 34.2, 31.4, 31.0, 30.6, 24.1, 21.7, 20.6, 18.7, 13.6. HRMS (ESI-FTMS, m/z): calcd for C26H30NO3S [M + H]+ 436.1941, found 436.1927. Yield (252 mg, 58%).
(1S,2R,13R,14S,18S)-7-(3-Fluorophenyl)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (20)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.69 (m, 2H), 7.42–7.35 (m, 1H), 7.12–7.06 (m, 1H), 5.88–5.85 (m, 1H), 3.04–2.88 (m, 2H), 2.53–2.11 (m, 2H), 2.08–1.81 (m, 7H), 1.69–1.20 (m, 6H), 1.09 (s, 3H), 0.95 (s, 3H); 1H NMR (75 MHz, CDCl3) δ ppm: 220.6, 163.0 (1JCF = 244.9 Hz), 162.5, 150.7, 136.5, 135.9 (3JCF = 8.0 Hz), 132.3, 130.4 (3JCF = 8.3 Hz), 122.0, 121.9, 116.5 (2JCF = 21.3 Hz), 113.0 (2JCF = 23.3 Hz), 51.7, 48.1, 47.6, 36.7, 35.8, 34.2, 31.4, 31.1, 30.8, 24.1, 21.8, 20.7, 18.7, 13.6. HRMS (ESI-FTMS, m/z): calcd for C26H29FNOS [M + H]+ 422.1948, found 422.1951. Yield (252 mg, 60%).
(1S,2R,13R,14S,18S)-7-(4-Fluorophenyl)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (21)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.91–7.87 (m, 2H), 7.11 (t, J = 8.5 Hz, 2H), 5.84–5.83 (m, 1H), 3.03–2.86 (m, 2H), 2.56–2.34 (m, 2H), 2.20–1.80 (m, 7H), 1.68–1.18 (m, 6H), 1.08 (s, 3H), 0.95 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.8, 163.6 (1JCF = 248.6 Hz), 162.9, 150.4, 136.5, 131.7, 130.2, 128.2 (3JCF = 8.3 Hz), 121.5, 116.0 (2JCF = 21.8 Hz), 51.7, 48.1, 47.6, 36.7, 35.8, 34.3, 31.3, 31.1, 30.8, 24.1, 21.8, 20.7, 18.7, 13.6. HRMS (ESI-FTMS, m/z): calcd for C26H29FNOS [M + H]+ 422.1948, found 422.1953. Yield (256 mg, 61%).
(1S,2R,13R,14S,18S)-7-(3-Chlorophenyl)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (22)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.93 (s, 1H), 7.79–7.75 (m, 1H), 2.36–7.32 (m, 2H), 5.88–5.87 (m, 1H), 3.04–2.81 (m, 2H), 2.56–2.34 (m, 2H), 2.20–1.81 (m, 6H), 1.66–1.32 (m, 6H), 1.26–1.19 (m, 1H), 1.08 (s, 3H), 0.95 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.8, 162.3, 150.7, 136.4, 135.4, 134.9, 132.4, 130.1, 129.6, 126.2, 124.4, 122.0, 51.7, 48.1, 47.6, 36.7, 35.8, 34.2, 31.3, 31.1, 30.8, 24.1, 21.8, 20.7, 18.7, 13.6. HRMS (ESI-FTMS, m/z): calcd for C26H29ClNOS [M + H]+ 438.1653, found 438.1654. Yield (214 mg, 49%).
(1S,2R,13R,14S,18S)-7-(4-Chlorophenyl)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),6,10-trien-17-one (23)
Recrystallized from acetonitrile. Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.85 (d, J = 8.4 Hz, 2H), 7.40 (J = 8.5 Hz, 2H), 5.86–5.85 (m, 1H), 3.03–2.87 (m, 2H), 2.56–2.37 (m, 2H), 2.20–1.80 (m, 6H), 1.65–1.34 (m, 6H), 1.26–1.21 (m, 1H), 1.08 (s, 3H), 0.95 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.7, 162.7, 150.6, 136.5, 135.5, 132.3, 132.0, 129.0, 127.5, 121.8, 51.7, 48.1, 47.6, 36.7, 35.8, 34.2, 31.3, 31.1, 30.8, 24.1, 21.8, 20.7, 18.7, 13.6. HRMS (ESI-FTMS, m/z): calcd for C26H29ClNOS [M + H]+ 438.1653, found 438.1656. Yield (222 mg, 51%).
(1S,2R,13R,14S,18S)-7-(3,5-Dichlorophenyl)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),6,10-trien-17-one (24)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.80–7.73 (d, J = 1.8 Hz, 2H), 7.37 (t, J = 1.8 Hz, 1H), 5.89–5.88 (m, 1H), 3.02–2.87 (m, 2H), 2.56–2.35 (m, 2H), 2.20–1.81 (m, 7H), 1.69–1.20 (m, 6H), 1.08 (s, 3H), 0.95 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.7, 160.6, 150.9, 136.49, 136.40, 135.5, 133.1, 129.2, 124.5, 122.5, 51.7, 48.1, 47.6, 36.7, 35.8, 34.2, 31.3, 31.1, 30.8, 24.1, 21.8, 20.7, 18.7, 13.6. HRMS (ESI-FTMS, m/z): calcd for C26H28Cl2NOS [M + H]+ 442.1263, found 472.1264. Yield (188 mg, 40%).
(1S,2R,13R,14S,18S)-7-(4-Bromophenyl)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (25)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.78 (d, J = 8.3 Hz, 2H), 7.55 (d, J = 8.3 Hz, 2H), 5.86–5.85 (m, 1H), 3.03–2.87 (m, 2H), 2.56–2.33 (m 2H), 2.20–1.81 (m, 6H), 1.68–1.31 (m, 6H), 1.26–1.17 (m, 1H), 1.08 (s, 3H), 0.95 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 222.2, 164.2, 152.1, 138.0, 134.2, 133.6, 133.5, 129.2, 125.3, 123.3, 53.1, 49.6, 49.0, 38.2, 37.3, 35.7, 32.8, 32.6, 32.3, 25.6, 23.3, 22.2, 20.1, 15.1. HRMS (ESI-FTMS, m/z): calcd for C26H29BrNOS [M + H]+ and [M + 2 + H]+ 482.1148, 484.1128, found 482.1149, 484.1129 respectively. Yield (216 mg, 45%).
(1S,2R,13R,14S,18S)-2,18-Dimethyl-7-[4-(trifluoromethyl)phenyl]-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (26)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 8.02 (d, J = 8.1 Hz, 2H), 7.68 (d, J = 8.2 Hz, 2H), 5.91–5.89 (m, 1H), 3.07–2.84 (m, 2H), 2.57–2.39 (m, 2H), 2.21–1.82 (m, 7H), 1.69–1.19 (m, 6H), 1.09 (s, 3H), 0.96 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.8, 162.0, 151.0, 136.9, 136.4, 132.9, 131.3, 130.9, 126.4, 125.9–125.7 (m), 122.3, 51.7, 48.1, 47.6, 36.7, 35.8, 34.2, 31.3, 31.1, 30.8, 24.1, 21.8, 20.7, 18.7, 13.6. HRMS (ESI-FTMS, m/z): calcd for C27H29NOSF3 [M + H]+ 472.1916, found 472.1910. Yield (268 mg, 57%).
(1S,2R,13R,14S,18S)-2,18-Dimethyl-7-(3-nitrophenyl)-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),6,10-trien-17-one (27)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 8.74 (s, 1H), 8.24 (d, J = 7.7 Hz, 2H), 7.61 (t, J = 7.9 Hz, 1H), 5.93–5.91 (m, 1H), 305–2.84 (m, 2H), 2.57–2.37 (m, 2H), 2.21–1.82 (m, 7H), 1.70–1.25 (m, 6H), 1.10 (s, 3H), 0.96 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.2, 160.4, 150.6, 148.1, 135.9, 135.0, 132.8, 131.2, 129.4, 123.4, 122.2, 120.6, 51.2, 47.6, 47.1, 36.3, 35.3, 33.7, 30.9, 30.6, 30.4, 23.6, 21.3, 20.3, 18.2, 13.2. HRMS (ESI-FTMS, m/z): calcd for C26H29NOS [M + H]+ 449.1893, found 449.1891. Yields (246 mg, 0.55 mmol, 55%).
(1S,2R,13R,14S,18S)-2,18-Dimethyl-7-(2-pyridyl)-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),7,10-trien-17-one (28)
Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 8.59 (s, 1H), 8.13 (d, J = 7.6 Hz, 1H), 7.76 (t, J = 7.5 Hz, 1H), 7.27 (s, 1H), 5.94 (s, 1H), 3.02–2.84 (m, 2H), 2.54–2.38 (m, 2H), 2.18–1.79 (m, 7H), 1.67–1.20 (m, 6H), 1.07 (s, 3H), 0.93 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 220.7, 164.8, 151.5, 150.8, 149.4, 136.8, 136.6, 133.9, 124.0, 122.2, 119.3, 51.7, 48.1, 47.5, 36.6, 35.8, 34.3, 31.3, 31.1, 30.8, 24.1, 21.8, 20.7, 18.7, 13.6. HRMS (ESI-FTMS, m/z): calcd for C25H29N2OS [M + H]+ 405.1995, found 405.2000. Yield (153 mg, 38%).
(1S,2R,13R,14S,17S,18S)-7-(4-Fluorophenyl)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),6,10-trien-17-ol (31)
A solution of compound 20 (210.5 mg, 0.5 mmol) in methanol was cooled in ice and NaBH4 (189 mg, 5 mmol) was added portionwise, and the reaction mixture was stirred for 8 h. After completion of the reaction, aqueous 10% HCl was added, and the reaction was stirred for 2 h to precipitate the product. Filtration and washing with water gave the pure product (203 mg, 96%). Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.69–7.63 (m, 1H), 7.42–7.34 (m, 1H), 7.12–7.05 (m, 1H), 5.85–5.83 (m, 1H), 3.69 (t, J = 8.3 Hz, 1H), 3.04–2.81 (m, 2H), 2.31–2.22 (m, 1H), 2.18–2.05 (m, 2H), 1.93–1.28 (m, 10H), 1.22–1.11 (m, 3H), 1.07 (s, 3H), 0.82 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 163.5 (1J = 244.9 Hz), 162.4 (4J = 3.1 Hz), 150.5, 136.3, 135.9 (3J = 8.0 Hz), 132.5, 130.4 (3J = 8.2 Hz), 122.6, 122.1 (4J = 2.8 Hz), 116.5 (2J = 21.1 Hz), 113.1 (2J = 23.3 Hz), 81.7, 51.3, 48.1, 42.8, 36.7, 36.5, 34.3, 31.5, 31.5, 30.5, 24.1, 23.4, 21.0, 18.7, 11.0. HRMS (ESI-FTMS, m/z): calcd for C26H30NOS [M + H]+ 428.2105, found 424.2107. Yield (407 mg, 96%).
[(1S,2R,13R,14S,17S,18S)-7-(3-Fluorophenyl)-2,18-dimethyl-8-thia-6-azapentacyclo[11.7.0.02,10.05,9.014,18]icosa-5(9),6,10-trien-17-yl] acetate (32)
A solution of compound 31 (150 mg, 0.35 mmol) in dichloromethane (CH2Cl2, 4 mL) was cooled in ice and acetic anhydride (1 mL) and pyridine (0.2 mL) were added, and reaction mixture was stirred for 24 h at room temperature. After the completion of the reaction, CH2Cl2 was removed by evaporation and 5 mL of methanol was added to the reaction mixture followed by adding 10 mL of water. The precipitate was filtered and washed with water to get the pure product (158 mg, 0.34 mmol, 96%). Yellowish solid, 1H NMR (300 MHz, CDCl3) δ ppm: 7.68–7.62 (m, 2H), 7.42–7.34 (m, 1H), 7.00 (dd, J = 2.4, 9.0 Hz, 1H), 5.85–5.83 (m, 1H), 4.64 (t, J = 8.0 Hz, 1H), 3.03–2.81 (m, 2H), 2.32–2.11 (m, 3H), 2.07 (s, 3H), 1.87–1.68 (m, 6H), 1.61–1.09 (m, 6H), 1.06 (s, 3H), 0.86 (s, 3H); 13C NMR (75 MHz, CDCl3) δ ppm: 171.2, 163.0 (1J = 244.8 Hz), 162.4 (4J = 3.0 Hz), 150.6, 136.4, 136.0 (3J = 8.0 Hz), 132.5, 130.4 (3J = 8.2 Hz), 122.4, 122.0 (4J = 2.9 Hz), 116.4 (2J = 21.3 Hz), 113.1 (2J = 23.2 Hz), 82.6, 77.2, 51.0, 47.9, 42.4, 36.6, 34.3, 31.4, 31.3, 27.5, 24.1, 23.5, 21.2, 20.9, 18.7, 12.0. HRMS (ESI-FTMS, m/z): calcd for C28H33FNO2S [M + H]+ 466.2211, found 466.2214.
Acknowledgments
We are thankful to the INBRE for a pilot grant (grant #224658) and ABI mini-grant 200138. This publication was made possible by the Research Technology Core of the Arkansas INBRE Program, supported by a grant from the National Institute of General Medical Sciences, (NIGMS), P20 GM103429-16 from the National Institutes of Health to record the Mass Spectrometry data. We are thankful to Dr Victor, Director/Senior Scientist X-ray Crystallography Laboratory University of Kansas, (NSF-MRI grant CHE-0923449) for recording crystal structure of compounds. M.A.A. thanks National Research Foundation of South Korea for the financial support.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02840.
The authors declare no competing financial interest.
Supplementary Material
References
- Terán-Pérez G.; Arana-Lechuga Y.; Esqueda-Leon E.; Santana-Miranda R.; Rojas-Zamorano J. A.; Moctezuma J. V. Steroid hormones and sleep regulation. Mini-Rev. Med. Chem. 2012, 12, 1040–1048. 10.2174/138955712802762167. [DOI] [PubMed] [Google Scholar]
- Wilkenfeld S. R.; Lin C.; Frigo D. E. Communication between genomic and non-genomic signaling events coordinate steroid hormone actions. Steroids 2018, 133, 2–7. 10.1016/j.steroids.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai J.; Yoshida W. Y.; Kelly M.; Williams P. Pregnane-10,2-carbolactones from a Hawaiian Marine Sponge in the Genus Myrmekioderma. J. Nat. Prod. 2016, 79, 1464–1467. 10.1021/acs.jnatprod.6b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoben C. V.; Ibezim A.; Ntie-Kang F.; Nwodo J. N.; Lifongo L. L. Exploring Cancer Therapeutics with Natural Products from African Medicinal Plants, Part I: Xanthones, Quinones, Steroids, Coumarins, Phenolics and other Classes of Compounds. Anti-Cancer Agents Med. Chem. 2015, 15, 1092–1111. 10.2174/1871520615666150113110241. [DOI] [PubMed] [Google Scholar]
- Liu J.; Zhang D.; Sun X.; Ding T.; Lei B.; Zhang C. Structure-activity relationship of brassinosteroids and their agricultural practical usages. Steroids 2017, 124, 1–17. 10.1016/j.steroids.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Calle J. M.; Pérez A. J.; Simonet A. M.; Guerra J. O.; Macías F. A. Steroidal Saponins from Furcraea hexapetala Leaves and Their Phytotoxic Activity. J. Nat. Prod. 2016, 79, 2903–2911. 10.1021/acs.jnatprod.6b00702. [DOI] [PubMed] [Google Scholar]
- Qian M.; Krishnan K.; Kudova E.; Li P.; Manion B. D.; Taylor A.; Elias G.; Akk G.; Evers A. S.; Zorumski C. F.; Mennerick S.; Covey D. F. Neurosteroid analogues. 18. Structure-activity studies of ent-steroid potentiators of gamma-aminobutyric acid type A receptors and comparison of their activities with those of alphaxalone and allopregnanolone. J. Med. Chem. 2014, 57, 171–190. 10.1021/jm401577c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal R.; Acharya P. C. Man-made cytotoxic steroids: exemplary agents for cancer therapy. Chem. Rev. 2014, 114, 6986–7005. 10.1021/cr4002935. [DOI] [PubMed] [Google Scholar]
- Le Bideau F.; Dagorne S. Synthesis of transition-metal steroid derivatives. Chem. Rev. 2013, 113, 7793–850. 10.1021/cr400269j. [DOI] [PubMed] [Google Scholar]
- El-Desoky E. S. I.; Reyad M.; Afsah E. M.; Dawidar A. A. Synthesis and chemical reactions of the steroidal hormone 17alpha-methyltestosterone. Steroids 2016, 105, 68–95. 10.1016/j.steroids.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Hamilton N. M.; Dawson M.; Fairweather E. E.; Hamilton N. S.; Hitchin J. R.; James D. I.; Jones S. D.; Jordan A. M.; Lyons A. J.; Small H. F.; Thomson G. J.; Waddell I. D.; Ogilvie D. J. Novel Steroid Inhibitors of Glucose 6-Phosphate Dehydrogenase. J. Med. Chem. 2012, 55, 4431–4445. 10.1021/jm300317k. [DOI] [PubMed] [Google Scholar]
- Sepe V.; Renga B.; Festa C.; D’Amore C.; Masullo D.; Cipriani S.; Di Leva F. S.; Monti M. C.; Novellino E.; Limongelli V.; Zampella A.; Fiorucci S. Modification on ursodeoxycholic acid (UDCA) scaffold. discovery of bile acid derivatives as selective agonists of cell-surface G-protein coupled bile acid receptor 1 (GP-BAR1). J. Med. Chem. 2014, 57, 7687–7701. 10.1021/jm500889f. [DOI] [PubMed] [Google Scholar]
- Mendell A. L.; Creighton C. E.; Kalisch B. E.; MacLusky N. J. 5α-Androstane-3α,17β-Diol Inhibits Neurotoxicity in SH-SY5Y Human Neuroblastoma Cells and Mouse Primary Cortical Neurons. Endocrinology 2016, 157, 4570–4578. 10.1210/en.2016-1508. [DOI] [PubMed] [Google Scholar]
- Ning X.; Yang Y.; Deng H.; Zhang Q.; Huang Y.; Su Z.; Fu Y.; Xiang Q.; Zhang S. Development of 17β-hydroxysteroid dehydrogenase type 3 as a target in hormone-dependent prostate cancer therapy. Steroids 2017, 121, 10–16. 10.1016/j.steroids.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Ji Z. H.; Xu Z. Q.; Zhao H.; Yu X. Y. Neuroprotective effect and mechanism of daucosterol palmitate in ameliorating learning and memory impairment in a rat model of Alzheimer’s disease. Steroids 2017, 119, 31–35. 10.1016/j.steroids.2017.01.003. [DOI] [PubMed] [Google Scholar]
- Larik F. A.; Saeed A.; Shahzad D.; Faisal M.; El-Seedi H.; Mehfooz H.; Channar P. A. Synthetic approaches towards the multi target drug spironolactone and its potent analogues/derivatives. Steroids 2017, 118, 76–92. 10.1016/j.steroids.2016.12.010. [DOI] [PubMed] [Google Scholar]
- Moreno Y. B. L.; Urban E.; Gelbcke M.; Dufrasne F.; Kopp B.; Kiss R.; Zehl M. Structure-activity relationship analysis of bufadienolide-induced in vitro growth inhibitory effects on mouse and human cancer cells. J. Nat. Prod. 2013, 76, 1078–1084. 10.1021/np400034d. [DOI] [PubMed] [Google Scholar]
- Rouf A.; Tanyeli C. Bioactive thiazole and benzothiazole derivatives. Eur. J. Med. Chem. 2015, 97, 911–927. 10.1016/j.ejmech.2014.10.058. [DOI] [PubMed] [Google Scholar]
- Ayati A.; Emami S.; Asadipour A.; Shafiee A.; Foroumadi A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015, 97, 699–718. 10.1016/j.ejmech.2015.04.015. [DOI] [PubMed] [Google Scholar]
- FDA Approves Drug To Treat Duchenne Muscular Dystrophy. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm540945.htm (Feb 9, 2017).
- Madhra M. K.; Sriram H. M.; Inamdar M.; Sharma M. K.; Prasad M.; Joseph S. Improved Procedure for Preparation of Abiraterone Acetate. Org. Process Res. Dev. 2014, 18, 555–558. 10.1021/op500044p. [DOI] [Google Scholar]
- Szychowski J.; Truchon J.-F.; Bennani Y. L. Natural Products in Medicine: Transformational Outcome of Synthetic Chemistry. J. Med. Chem. 2014, 57, 9292–9308. 10.1021/jm500941m. [DOI] [PubMed] [Google Scholar]
- Fan N.-J.; He Q.-R.; Duan M.; Bai Y.-B.; Tang J.-J. Synthesis and antiproliferative activity of D-ring substituted steroidal benzamidothiazoles. Steroids 2016, 112, 103–108. 10.1016/j.steroids.2016.04.009. [DOI] [PubMed] [Google Scholar]
- Žofková I.; Roejdmark S.; Kancheva R. L. Stanazolol - an anabolic steroid that does not influence parathyroid hormone response to hypercalcemia in postmenopausal women. Calcif. Tissue Int. 1994, 54, 521–522. 10.1007/BF00334336. [DOI] [PubMed] [Google Scholar]
- Vitellozzi L.; McAllister G. D.; Genski T.; Taylor R. J. K. Organometallic Routes to Novel Steroids Containing Heterocyclic C-17 Side-Chains. Synthesis 2015, 48, 48–56. 10.1055/s-0035-1560353. [DOI] [Google Scholar]
- Zhang B. L.; Song L. X.; Li Y. F.; Li Y. L.; Guo Y. Z.; Zhang E.; Liu H. M. Synthesis and biological evaluation of dehydroepiandrosterone-fused thiazole, imidazo[2,1-b]thiazole, pyridine steroidal analogues. Steroids 2014, 80, 92–101. 10.1016/j.steroids.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Martinez Botella G.; Salituro F. G.; Harrison B. L.; Beresis R. T.; Bai Z.; Shen K.; Belfort G. M.; Loya C. M.; Ackley M. A.; Grossman S. J.; Hoffmann E.; Jia S.; Wang J.; Doherty J. J.; Robichaud A. J. Neuroactive Steroids. 1. Positive Allosteric Modulators of the (gamma-Aminobutyric Acid)A Receptor: Structure-Activity Relationships of Heterocyclic Substitution at C-21. J. Med. Chem. 2015, 58, 3500–3511. 10.1021/acs.jmedchem.5b00032. [DOI] [PubMed] [Google Scholar]
- Li J.; Huo H.; Guo R.; Liu B.; Li L.; Dan W.; Xiao X.; Zhang J.; Shi B. Facile and efficient access to Androsten-17-(1′,3′,4′)-pyrazoles and Androst-17beta-(1′,3′,4′)-pyrazoles via Vilsmeier reagents, and their antiproliferative activity evaluation in vitro. Eur. J. Med. Chem. 2017, 130, 1–14. 10.1016/j.ejmech.2017.02.033. [DOI] [PubMed] [Google Scholar]
- Metz T. L.; Lutovsky G. A.; Stanley L. M. An Acid-Catalyzed Addition and Dehydration Sequence for the Synthesis of Heteroarylated Steroidal Dienes. J. Org. Chem. 2018, 83, 1643–1648. 10.1021/acs.joc.7b03045. [DOI] [PubMed] [Google Scholar]
- Ambrose A. J.; Santos E. A.; Jimenez P. C.; Rocha D. D.; Wilke D. V.; Beuzer P.; Axelrod J.; Kanduluru A. K.; Fuchs P. L.; Cang H.; Costa-Lotufo L. V.; Chapman E.; Clair J. J. L. Ritterostatin GN1N, a Cephalostatin–Ritterazine Bis-steroidal Pyrazine Hybrid, Selectively Targets GRP78. ChemBioChem 2017, 18, 506–510. 10.1002/cbic.201600669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolottsev V. A.; Tkachev Y. V.; Latysheva A. S.; Kostin V. A.; Novikov R. A.; Timofeev V. P.; Morozevich G. E.; Kuzikov A. V.; Shumyantseva V. V.; Misharin A. Y. Comparison of [17(20)E]-21-Norpregnene oxazolinyl and benzoxazolyl derivatives as inhibitors of CYP17A1 activity and prostate carcinoma cells growth. Steroids 2018, 129, 24–34. 10.1016/j.steroids.2017.11.009. [DOI] [PubMed] [Google Scholar]
- Festa C.; Renga B.; D’Amore C.; Sepe V.; Finamore C.; De Marino S.; Carino A.; Cipriani S.; Monti M. C.; Zampella A.; Fiorucci S. Exploitation of cholane scaffold for the discovery of potent and selective farnesoid X receptor (FXR) and G-protein coupled bile acid receptor 1 (GP-BAR1) ligands. J. Med. Chem. 2014, 57, 8477–8495. 10.1021/jm501273r. [DOI] [PubMed] [Google Scholar]
- Kudova E.; Chodounska H.; Slavikova B.; Budesinsky M.; Nekardova M.; Vyklicky V.; Krausova B.; Svehla P.; Vyklicky L. A New Class of Potent N-Methyl-D-Aspartate Receptor Inhibitors: Sulfated Neuroactive Steroids with Lipophilic D-Ring Modifications. J. Med. Chem. 2015, 58, 5950–5966. 10.1021/acs.jmedchem.5b00570. [DOI] [PubMed] [Google Scholar]
- Michalak K.; Morawiak M.; Wicha J. Synthetic Approach to the Core Structure of Oleandrin and Related Cardiac Glycosides with Highly Functionalized Ring D. Org. Lett. 2016, 18, 6148–6151. 10.1021/acs.orglett.6b03157. [DOI] [PubMed] [Google Scholar]
- Zakeyah A. A.; Whitt J.; Duke C.; Gilmore D. F.; Meeker D. G.; Smeltzer M. S.; Alam M. A. Synthesis and antimicrobial studies of hydrazone derivatives of 4-[3-(2,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid and 4-[3-(3,4-difluorophenyl)-4-formyl-1H-pyrazol-1-yl]benzoic acid. Bioorg. Med. Chem. Lett. 2018, 28, 2914–2919. 10.1016/j.bmcl.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison D.; Delancey E.; Ramey H.; Williams C.; Alsharif Z. A.; Al-khattabi H.; Ontko A.; Gilmore D.; Alam M. A. Synthesis and antimicrobial studies of novel derivatives of 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-Acinetobacter baumannii agents. Bioorg. Med. Chem. Lett. 2017, 27, 387–392. 10.1016/j.bmcl.2016.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brider J.; Rowe T.; Gibler D. J.; Gottsponer A.; Delancey E.; Branscum M. D.; Ontko A.; Gilmore D.; Alam M. A. Synthesis and antimicrobial studies of azomethine and N-arylamine derivatives of 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-methicillin-resistant Staphylococcus aureus agents. Med. Chem. Res. 2016, 25, 2691–2697. 10.1007/s00044-016-1678-8. [DOI] [Google Scholar]
- Alam M. A.; Alsharif Z.; Alkhattabi H.; Jones D.; Delancey E.; Gottsponer A.; Yang T. Hexafluoroisopropyl alcohol mediated synthesis of 2,3-dihydro-4H-pyrido[1,2-a]pyrimidin-4-ones. Sci. Rep. 2016, 6, 36316 10.1038/srep36316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharif Z.; Ali M. A.; Alkhattabi H.; Jones D.; Delancey E.; Ravikumar P. C.; Alam M. A. Hexafluoroisopropanol mediated benign synthesis of 2H-pyrido[1,2-a]pyrimidin-2-ones by using a domino protocol. New J. Chem. 2017, 41, 14862–14870. 10.1039/C7NJ03376A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharif Z. A.; Alam M. A. Modular synthesis of thiazoline and thiazole derivatives by using a cascade protocol. RSC Adv. 2017, 7, 32647–32651. 10.1039/C7RA05993K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M. A.; Okolo C.; Alsharif Z. A.; Whitt J.; Chambers S. A.; Varma R. S.; Alam M. A. Benign Synthesis of Thiazolo-androstenone Derivatives as Potent Anticancer Agents. Org. Lett. 2018, 20, 5927–5932. 10.1021/acs.orglett.8b02587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos A.; Girán L.; Mittendorfer H.; Schmidhammer H.; Berényi S. Synthesis of 1,4-thiazino- and benzo-1,4-thiazinomorphinans: their acid-catalyzed rearrangement and study of the formation of unexpected oxidation products. Tetrahedron 2008, 64, 1023–1028. 10.1016/j.tet.2007.08.075. [DOI] [Google Scholar]
- Toth M.; Zsuzsanna G.; Sandor B.; Attila S. Synthesis and Transformation of Thiazolomorphinanedienes. Lett. Org. Chem. 2007, 4, 539–543. 10.2174/157017807782795538. [DOI] [Google Scholar]
- Chen M.; Drury J. E.; Christianson D. W.; Penning T. M. Conversion of human steroid 5beta-reductase (AKR1D1) into 3beta-hydroxysteroid dehydrogenase by single point mutation E120H: example of perfect enzyme engineering. J. Biol. Chem. 2012, 287, 16609–16622. 10.1074/jbc.M111.338780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Świzdor A.; Panek A.; Milecka-Tronina N. Microbial Baeyer-Villiger oxidation of 5alpha-steroids using Beauveria bassiana. A stereochemical requirement for the 11alpha-hydroxylation and the lactonization pathway. Steroids 2014, 82, 44–52. 10.1016/j.steroids.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Krishnan K.; Manion B. D.; Taylor A.; Bracamontes J.; Steinbach J. H.; Reichert D. E.; Evers A. S.; Zorumski C. F.; Mennerick S.; Covey D. F. Neurosteroid Analogues. 17. Inverted Binding Orientations of Androsterone Enantiomers at the Steroid Potentiation Site on γ-Aminobutyric Acid Type A Receptors. J. Med. Chem. 2012, 55, 1334–1345. 10.1021/jm2014925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Colorectal (colon) Cancer. https://www.cdc.gov/cancer/colorectal/statistics/index.htm (April 10, 2018).
- Heffron T. P. Small Molecule Kinase Inhibitors for the Treatment of Brain Cancer. J. Med. Chem. 2016, 59, 10030–10066. 10.1021/acs.jmedchem.6b00618. [DOI] [PubMed] [Google Scholar]
- Mayo Clinic Melanoma. http://www.mayoclinic.org/diseases-conditions/melanoma/basics/definition/con-20026009 (July 11, 2016).
- Reedy J. L.; Hedlund D. K.; Gabr M. T.; Henning G. M.; Pigge F. C.; Schultz M. K. Synthesis and Evaluation of Tetraarylethylene-based Mono-, Bis-, and Tris(pyridinium) Derivatives for Image-Guided Mitochondria-Specific Targeting and Cytotoxicity of Metastatic Melanoma Cells. Bioconjug. Chem. 2016, 2424–2430. 10.1021/acs.bioconjchem.6b00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Kidney Cancer. https://www.cdc.gov/cancer/kidney/index.htm (April 10, 2018).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.