Abstract
Objectives
To describe the clinicopathological features, complement abnormalities, triggers, treatment and outcomes of patients with C3 glomerulopathy.
Patients and Methods
A total of 114 C3G patients seen at Mayo Clinic from January 1, 2007 to December 31, 2016.
Results
The mean age at diagnosis for the entire cohort was 40.4 (±22.3) years, with median serum creatinine and proteinuria of 1.6 mg/dl and 2605 mg/24h, respectively. Hematuria was present in 87.7% of patients. C3/C4 levels were low in 44.6%/11.8% of patients. A history of infection, positive autoimmune findings, or monoclonal gammopathy (MIg) was present in 28.9%, 24.6% and 37.9% of the cases, respectively. However, 65.1% of the patient’s ≥ 50 years had a MIg. A genetic variant in complement genes, C3 nephritic factor, and other autoantibodies were present in 37.1%, 43.5% and 13.4 % patients, respectively. Membranoproliferative and mesangial proliferative glomerulonephritis were the common patterns of injury. Patients without MIg were younger (mean age 32.3 (±20.6) years) with median serum creatinine and proteinuria of 1.4 mg/dL and 2450 mg/24 hours, with low C3/C4 in 49.4 %/12% of patients, respectively. Most patients received steroids and other immunosuppressive drugs. In patients without MIg, at a median follow-up of 22.3 months, median serum creatinine and proteinuria were 1.4 mg/dL and 825.5 mg/24 hours, with 7 patients (10.3%) progressing to ESRD.
Conclusion
C3 glomerulopathy is a heterogeneous disease entity with complex triggering events and abnormalities of the alternative pathway of complement. The disease tends to be progressive and shows a variable response to immunosuppressive therapy.
Keywords: C3 glomerulopathy, C3 glomerulonephritis, alternative pathway of complement, dense deposit disease, infection, monoclonal Ig
C3 glomerulopathy (C3G) is a rare disease entity that is characterized by accumulation of complement factors in the glomeruli due to over activation and abnormal regulation of the alternative pathway (AP) of complement.1–4 Abnormal control of the AP of complement may be due to acquired or genetic abnormalities of the complement regulatory proteins. The deposition of complement factors drives glomerular inflammation resulting in a proliferative glomerulonephritis.5, 6
The defining feature of C3G is the bright staining for C3 on immunofluorescence studies with minimal or no staining for immunoglobulins. C3G is further subdivided into C3 glomerulonephritis (C3GN) and dense deposit disease (DDD) based on the ultrastructural findings.1, 7 C3GN is characterized by mesangial and capillary wall electron dense deposits, whereas DDD is characterized by dense sausage shaped osmiophilic intramembranous and mesangial deposits.
A consensus on the defining characteristics of C3G was published only a few years ago.1 Most of the reports on the clinical characteristics, pathology, complement evaluation, treatment and outcomes of C3G are therefore based on individual cases or small series of C3GN or DDD patients, although 2 larger series were recently published.2, 8–10,11, 12In this manuscript, we describe the clinical and pathology findings, triggering factors, complement abnormalities, treatment and renal outcomes of a large series of 114 patients with C3G seen over a 10-year period (2007–2016) at the Mayo Clinic, Rochester, MN.
PATIENTS AND METHODS
We identified 114 patients evaluated at the Mayo Clinic from January 1, 2007 to December 31, 2016 with a diagnosis of C3 glomerulopathy (C3GN or DDD) in native kidney biopsies. As such, this represents patients actually seen at Mayo Clinic and not data gathered from patients whose biopsies were read at the Department of Laboratory Medicine and Pathology, Mayo Clinic, but not seen at the Mayo Clinic. Most are of the white race (92 of the 114 patients) and belong to an older (mean age 40.4(Std Dev: ± 22.3)) age group. C3G was defined as the presence of dominant C3 staining (2 orders or greater in magnitude compared to Ig) on immunofluorescence microscopy with minimal or no staining for Ig. SS reviewed all kidney biopsies and reports. Clinical information was obtained from the charts. The study was approved by the Mayo Clinic Institutional Review Board and conducted in accordance to the Declaration of Helsinki.
1. Data Analysis
Statistical analyses were performed using JMP Pro software version 13.0 (SAS Institute Inc., Cary, NC). Continuous variables are reported as median (range) and categorical variables as frequency (%). When the normality assumption was violated, we used nonparametric tests such as the Fischer exact test, Chi-square test or the Wilcoxon rank-sum/Mann Whitney as appropriate for test variables. Statistical significance was based on a two-sided significance level of .05. The main endpoints of the study were end stage renal disease (ESRD) or doubling of serum creatinine from the time of C3G diagnosis. The median follow-up was calculated as the median time on study for those event-free at the end of follow-up. Renal survival probabilities were determined using the Kaplan Meier method and group comparisons for survival were performed Wilcoxon and Log-Rank tests.13 Modeling for predictors of ESRD/doubling of serum creatinine was conducted using Cox proportional hazard methods.
For analyzing 24-hour proteinuria at diagnosis and at follow-up, the average predicted proteinuria values were considered for those patients where quantitative 24-hour urine protein was not performed. Further, treatment outcomes were defined based on a previous study by Rabasco et al14: i) Complete remission (CR) was defined as eGFR ≥ 60 mL/min/1.73 m2 (or return to ±15% of baseline if eGFR <60 mL/min/1.73 m2) and proteinuria <500 mg/24 hours; ii) Partial remission (PR) was defined as a reduction of >50%proteinuria (and a proteinuria value of <3500 mg/24 hours in patients with nephrotic-range proteinuria at baseline), plus stabilization (±25%) or improvement in renal function; iii) Responders were defined by > 50% reduction in proteinuria regardless of eGFR; iv) Stable disease (SD) was defined as eGFR within 10% of baseline and proteinuria of < 1500 mg/24 hours from onset to completion of treatment; and v) No response (NR) was defined by <50% reduction in proteinuria in patients with proteinuria ≥1500 mg/24 hours or 25% increase in serum creatinine from onset to completion of treatment.
2. Genetic Testing
Sample Preparation, Target Genomic Enrichment and Next Generation Sequencing (NGS)
For each sample, genomic DNA was extracted from peripheral blood using the Gentra Puregene Kit (Qiagen Inc., Valencia, CA); integrity was evaluated by 1% agarose gel electrophoresis. To ensure DNA samples met our minimal quality metrics of 1.8 for 260/280 and 260/230 ratios, absorbance was measured at 230:260:280 using a NanoDrop 1000 spectrophotometer (Thermo Scientific Inc., Wilmington, DE). DNA concentration was determined using the Qubit dsDNA HS Assay Kit (Life Technologies, Carlsbad, CA). Complement genes were analyzed by capturing the coding sequence and flanking splice sites using the Agilent SureSelect Target Enrichment System (Agilent Technologies Inc., Santa Clara, CA), as we have described.15 Library preparation was performed with SureSelect TGE baits and SureSelectXT Reagent Kits (Agilent Technologies Inc., Santa Clara, CA) following the manufacturer’s protocol. Preparation was automated using a Zephyr Workstation (PerkinElmer, Waltham, MI). Library quality and concentration were evaluated using a Bioanalyzer 2100 (Agilent Technologies Inc., Santa Clara, CA). Libraries passing quality control step were pooled and sequenced on a HiSeq 2500 (Illumina Inc., San Diego, CA) or a MiSeq Sequencer (Illumina Inc., San Diego, CA).
Next-Generation Sequencing (NGS) Data Analysis
NGS data storage and analysis were performed on dedicated computing resources maintained by the Iowa Institute of Human Genetics at the University of Iowa as described.15 Sequencing data were archived as fastq files on a secured storage server and then analyzed using locally implemented open-source Galaxy software on a high-performance computing cluster. The workflow for variant calling integrated publicly available tools: reads were mapped using Burrows–Wheeler Alignment against human reference genome GRCh37/hg19; duplicates were removed by Picard; realignment, calibration and variant calling were performed with GATK; variant annotation was performed with a CLCG Annotation and Reporting Tool developed by our bioinformatics team.
Variant Prioritization and Sanger Validation
Low quality variants (Depth<10 or QD<5) were filtered out by quality control. Common variants with MAF >1% in any population were excluded (based on the NHLBI GO Exome Sequencing Project [ESP, evs.gs.washington.edu], the 1000 Genomes Project [1000Gs, www.1000genomes.org] and the Exome Aggregation Consortium [ExAC, exac.broadinstitute.org]). Variants also were filtered based on predicted effect, retaining nonsynonymous single nucleotide variants (SNVs), canonical splicing changes and indels, which we prioritized based on MAF, nucleotide conservation, reported functional/expressional impact and phenotype correlation. Other reference databases routinely queried included the aHUS Mutation Database (www.fh-hus.org), Human Gene Mutation Database (HGMD), and our in-house Renal Variant Database (RVD). GERP++,16 PhyloP,17 MutationTaster,18 PolyPhen2,19 SIFT,20 and LRT,21 were used to calculate variant-specific pathogenicity scores. All reported variants were Sanger validated.
Copy Number Variation
CNVs across the CFHR3-CFHR1 region were identified using a MLPA set of 13 probes and six control probes, all designed following the MRC-Holland synthetic probe design protocol. Patients with deletion in CFHR3-CFHR1 were further tested for CNV of CFH, CFHR4, CFHR2, and CFHR5. MLPA was performed using the SALSA MLPA Reagent Kit (MRC-Holland, Amsterdam, Netherlands), resolved on a 3130xl Genetic Analyzer (Life Technologies, Carlsbad, CA), and analyzed with GeneMapper software (Life Technologies, Carlsbad, CA). Each MLPA run included eight control samples (five normal controls (three females, two males), two controls with a heterozygous deletion of CFHR3-CFHR1 (one female, one male), and one control with a homozygous deletion for CFHR3-CFHR1).
3. Autoantibody Assays
IgGs were purified using a Melon gel column (Thermo Fisher Scientific, Inc, Waltham, MA) or protein G (SeraCare, Gaithersburg, MD).
C3 Nephritic factors (C3Nefs)
C3Nefs were measured by C3CSA (C3 Convertase Stabilizing Assay). This assay measures the ability of C3Nefs to stabilize surface-bound C3 convertase on sheep erythrocytes. The assay was performed as described.22
C4 Nephritic factors (C4Nefs)
C4Nefs were assayed to measure the ability of patient-derived IgG to stabilize preformed C4b2a, as we have described.23 Hemolysis of C4b2a-coated sheep erythrocytes (5×108/ml) was quantitated using increasing aliquots of patient-purified IgG, supplemented with GVB-EDTA buffer to a total volume of 250μl. Hemolysis was recorded as a measure of the optical density of the supernatant at 415nm.23
C5 Nephritic factors (C5Nefs)
C5Nefs were measured by C3CSAP (C3 Convertase Stabilizing Assay with Properdin. This assay is similar to C3CSA, although properdin is included to favor the generation of C5 convertases. To perform the assay, the convertase is allowed to decay after adding patient-purified IgG and activity is measured at 30 and 80 minutes. Results are reported as a function of hemolysis at 30 minutes.22
Factor H and Factor B Autoantibodies (FHAA and FBAA)
FHAA and FBAA were detected by ELISA. In brief, either FH or FB (both at 5μg/ml) (Complement Technology Inc, Tyler, TX) was coated on a 96-well micro-titer plate and allowed to sit overnight at 4°C. After washing three times with 1X PBST (1XPBS containing 0.5% Triton-X), free reactive sites were blocked with Ultrablock (AbD Serotec, Raleigh, NC) for 1 hour at RT. Patient serum (1:50 dilution) was added for 1 hour at RT. Plates were washed and incubated for 1 hour at RT with horseradish peroxidase-labeled goat anti-human IgG specific for the γ chain. After a final washing, enzymatic activity was measured using TMB (3,3´,5,5´-tetramethylbenzidine) and optical density (OD) was read at λ450. A positive result was an OD >0.15 for FHAA and >0.05 for FBAA, which exceed the mean + 2x standard deviations for 87 controls.
RESULTS
Clinical Features and Laboratory Findings
A total of 114 patients met the study criteria, of which 102 (89.5%) had a diagnosis of C3GN and 12 (10.5%) of DDD. Thus, C3GN was approximately 9-fold more common than DDD during the same period. The mean age at diagnosis was 40.4(Std Dev: ± 22.3) years (range: 3–85); 63 (55.3 %) were males and 51 (44.7%) females, respectively. C3GN patients were older than DDD patients with a mean age of 41.5 (Std Dev: ±22.1) versus 31.5 (Std Dev: ±23.2) respectively, p=.10. The median serum creatinine at diagnosis of C3G was 1.6 mg/dL (n=114, range: 0.3–14.7). No difference (p=.34) in median serum creatinine at onset between C3GN (median serum creatinine: 1.6 mg/dL, range: 0.3–14.7) and DDD (median serum creatinine: 1.4 mg/dL, range: 0.3–6.3) was observed. Urinalysis was positive for hematuria in 100 (87.7%) patients. The median proteinuria was 2605 mg/24 hours (range: 233–24,165). Patients with DDD presented with higher proteinuria (median proteinuria: 6478 mg/24 hours, range: 496–17,867) compared to patients with C3GN (median proteinuria: 2500 mg/24 hours, range: 233–24,165), p=.02. Forty-eight (42.1%) patients (39 C3GN and 9 DDD) had nephrotic range proteinuria (>3500 mg/24 hours) and 20 patients had nephrotic syndrome (16 C3GN and 4 DDD). Fifty (44.6%, n=112) patients had low/borderline low C3 (reference: 75–175 mg/dL) and 13 (11.8%, n=110) had low/borderline low C4 levels (reference: 14–40 mg/dL). The demographic, clinical and laboratory data of C3GN and DDD are given in Table 1A. Twenty-five (21.9%) patients, 22 with C3GN and 3 with DDD, had a family history of kidney disease. Within this group, only 2 C3GN patients had a family history of C3 glomerulopathy. Of the remaining 23 patients, 16 had kidney disease of unknown etiology, 1 monoclonal gammopathy of renal significance, 1 vasculitis, 1 pyelonephritis, 1 renal amyloidosis, 1 IgA nephropathy, 1 ESRD secondary to drug toxicity and 1 polycystic kidney disease.
Table 1A:
Clinical and Laboratory Findings at the time of diagnosis of C3 Glomerulopathy
| Data Variable n=114 |
C3GN (n=102) |
DDD (n=12) |
|---|---|---|
| Age at diagnosis (years) | Mean (Std Dev): 41.5 (±22.1) Range: 3–85 |
Mean (Std Dev): 31.5 (±23.2) Range: 3–65 |
| Sex | Male: 60 (58.8%) Female: 42 (41.2%) |
Male: 3 (25%) Female: 9 (75%) |
| Race | White: 82 (80.4%) Black: 2 (2.0%) Asian: 3 (2.9%) American Indian/Alaskan Native: 1 (1.0%) Other: 4 (3.9%) Unknown: 10 (9.8%) |
White: 10 (83.3%) Black: 1 (8.3%) Asian: 0 American Indian/Alaskan Native: 0 Other: 0 Unknown: 1 (8.3%) |
| Ethnicity | Hispanic or Latino: 2 (2.0%) Non-Hispanic or Latino: 86 (84.3%) Unknown: 14 (13.7%) |
Hispanic or Latino: 0 Non-Hispanic or Latino: 10 (83.3%) Unknown: 2 (16.7%) |
| Blood Urea Nitrogen (mg/dL) | Median: 30, n=62 Range: 5.5–95.8 |
Median: 21; n=6 Range: 7–68 |
| Serum Creatinine (mg/dL) at diagnosis | Median: 1.6 Range: 0.3–14.7 |
Median: 1.4 Range: 0.3–6.3 |
| Serum albumin at diagnosis (g/dL) | Median: 3.4; n=56 Range: 1.2–4.6 |
Median: 2.7; n=8 Range: 1.7–3.9 |
| Hematuria | Positive: 89 (87.3%) Negative: 13 (12.7%) |
Positive: 11 (91.7%) Negative: 1(8.3%) |
| Proteinuria (mg/24 hours) |
Median: 2500 Range: 233–24,165 |
Median: 6478 Range: 496–17,867 |
|
Immunology Anti-GBM antibody ANCA/PR-3/MPO Anti-streptolysin Anti-DNase B ANA Anti-ds DNA Cryoglobulins Cryofibrinogen |
Positive: 0; Negative: 36; NA: 66 Positive: 3; Negative: 73; NA: 26 Positive: 2; Negative: 24; NA: 76 Positive: 1; Negative: 11; NA: 90 Positive: 11, Negative: 79; NA: 12 Positive: 5; Negative: 63; NA: 34 Positive: 3; Negative: 52; NA: 47 Positive: 0; Negative: 54; NA: 48 |
Positive: 0; Negative: 3; NA: 9 Positive: 0; Negative: 6; NA: 6 Positive: 1; Negative: 2; NA: 9 Positive: 0, Negative: 3; NA: 9 Positive:1; Negative: 7; NA: 4 Positive: 1; Negative: 4; NA: 7 Positive:0; Negative: 3; NA:9 Positive: 0; Negative: 2; NA: 10 |
|
Complement
C3 C4 |
Low: 43; Normal: 57; NA: 2 Low: 12; Normal: 86; NA: 4 |
Low: 7; Normal: 5 Low: 1; Normal: 11 |
Abbreviations: ANA: antinuclear antibody, ANCA, anti-neutrophil cytoplasmic antibodies; GBM, glomerular basement membrane; NA, not available; PR-3, anti-proteinase antibodies; MPO, anti-myeloperoxidase antibodies; Std Dev, standard deviation
Patients with a monoclonal immunoglobulin (MIg, n=36) represented ~37.9% of the cohort. When C3G patients with positive MIg were excluded, the mean age at diagnosis was 32.3 (Std Dev: ±20.6) years (range: 3–84, n=78). The median serum creatinine at diagnosis was 1.4 mg/dL (range: 0.3–7.9). Urinalysis was positive for hematuria in 68 (87.2%) patients. The median proteinuria was 2450 mg/24 hours (range: 250–24,165). Thirty-two (41.0%) patients (26 C3GN and 6 DDD) had nephrotic range proteinuria (>3500 mg/24 hours) and 14 patients had nephrotic syndrome (11 C3GN and 3 DDD). Thirty-eight (49.4%, n=77) patients had low C3 and 9 (12.0%, n=75) had low C4 levels. The demographic, clinical and laboratory data of C3GN and DDD patients without MIg (n=78) are described in Table 1B.
Table 1B:
Clinical and Laboratory Findings at the time of diagnosis of C3 Glomerulopathy among patients without a MIg
| Data Variable n=78 |
C3GN (n=70) |
DDD (n=8) |
|---|---|---|
| Age at diagnosis (years) | Mean (Std Dev): 33.9 (±20.6) Range: (3–84) |
Mean (Std Dev): 18.5 (15.4) Range: 3–51 |
| Sex | Male: 36 (51.4%) Female: 34 (48.6%) |
Male: 2 (25%) Female: 6 (75%) |
| Race | White: 54 (77.1%) Black: 1 (1.4%) Asian: 3 (4.3%) American Indian/Alaskan Native: 0 Other: 3 (4.3%) Unknown: 9 (12.9%) |
White: 8 (100%) Black: 0 Asian: 0 American Indian/Alaskan Native: 0 Other: 0 Unknown: 0 |
| Ethnicity | Hispanic or Latino: 1 (1.4%) Non-Hispanic or Latino: 58 (82.9%) Unknown: 11 (15.7%) |
Hispanic or Latino: 0 Non-Hispanic or Latino: 8 (100%) Unknown: 0 |
| Blood Urea Nitrogen (mg/dL) | Median: 23.5, n=44 Range: 5.5–85 |
Median: 21, n=6 Range: 7–68 |
| Serum Creatinine (mg/dL) at diagnosis | Median: 1.4 Range: 0.3–7.9 |
Median: 1.2 Range: 0.3–6.3 |
| Serum albumin at diagnosis (g/dL) | Median: 3.5, n=41 Range: 1.2–4.5 |
Median: 2.4. n=7 Range: 1.7–3.9 |
| Hematuria | Positive: 61 (87.1%) Negative: 9 (12.9%) |
Positive: 7 (87.5%) Negative: 1 (12.5%) |
| Proteinuria (mg/24 hours) |
Median: 2044.5 Range: 250–24,165 |
Median: 6792 Range: 1200–17,867 |
|
Immunology Anti-GBM antibody ANCA/PR-3/MPO Anti-streptolysin Anti-DNase B ANA Anti-ds DNA Cryoglobulins Cryofibrinogen |
Positive: 0; Negative: 21; NA: 49 Positive: 2; Negative: 51; NA: 17 Positive: 2; Negative: 18; NA: 50 Positive: 1; Negative: 7; NA: 62 Positive: 7; Negative: 57; NA: 6 Positive: 4; Negative: 46; NA: 20 Positive: 1a; Negative: 34; NA: 35 Positive: 0; Negative: 34; NA: 36 |
Positive: 1; Negative: 1; NA: 6 Positive: 0; Negative: 5; NA: 3 Positive: 1; Negative: 2; NA: 5 Positive: 0; Negative: 3; NA: 5 Positive: 1; Negative: 5; NA: 2 Positive: 1; Negative: 4; NA: 3 Positive: 0; Negative: 2; NA: 6 Positive: 0; Negative: 1; NA: 7 |
|
Complement
C3 C4 |
Low: 32; Normal: 37; NA: 1 Low: 9; Normal: 58; NA: 3 |
Low: 6; Normal: 2 Low: 0; Normal: 8 |
Abbreviations: ANA: antinuclear antibody, ANCA, anti-neutrophil cytoplasmic antibodies; GBM, glomerular basement membrane; NA, not available; PR-3, anti-proteinase antibodies; MPO, anti-myeloperoxidase antibodies; Std Dev, standard deviation
Type 3 cryoglobulinemia
C3G associated triggers/conditions
1. Monoclonal gammopathy
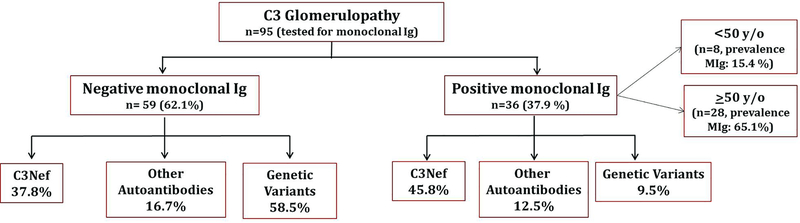
Ninety five (83.3%) of the 114 patients were evaluated for a monoclonal gammopathy by serum/urine electrophoresis and immunofixation studies. Overall, 36 (37.9%) had a MIg in serum and/or urine. Among patients ≥50 years (n=43), 28 (65.1%) were positive for a MIg (Figure 1).
Figure 1:
Breakdown of C3G by presence of monoclonal gammopathy and complement abnormalities.
C3 glomerulonephritis:
Eighty-eight (86.1%) of 102 patients with C3GN patients were tested for a MIg; 39 of these patients were ≥50 years of age. Overall, 32 (36.4%) were positive for a MIg (24 males; 8 females). Of C3GN patients ≥50 years (n=39), 25 (64.1%) were positive for a MIg.
Dense deposit disease:
Seven (58.3%) of 12 patients with DDD were tested for a monoclonal protein (MIg) and four (57.1%) were positive for a MIg (1 male; 3 female). Of DDD patients ≥50 years of age (n=4), three (75%) were positive for a MIg.
2. Infections
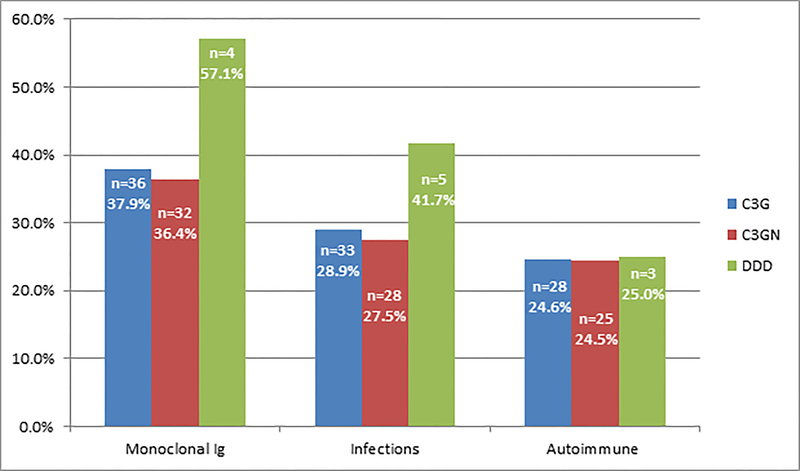
Of the 114, 33 (28.9%) patients had a history of infection at the time of diagnosis of which 3 had positive anti-streptolysin titers and one borderline positive anti-DNase B titer. All patients had persistent hematuria and were diagnosed as C3G on review of kidney biopsy.
C3 glomerulonephritis:
Twenty-eight (27.5%) patients had a history of recent infection at the time of C3GN diagnosis of which two had positive anti-Streptolysin O titers and one borderline positive anti-DNase B titer. Among these, 16 patients had a history of upper respiratory infection, 9 had urinary tract infection, 1 had pneumonia, 1 had human immunodeficiency virus infection and 1 had endocarditis. Twenty patients received antibiotics, 2 were managed conservatively and for the remaining patients treatment history was not available.
Dense deposit disease:
Five (41.7%) patients had a history of infection at diagnosis of DDD (3 had upper respiratory infection, 1 had pneumonia, and 1 had septic arthritis), of which one had positive anti-Streptolysin O titers but negative anti-DNase-B titer. Among these 5 patients, 2 received antibiotics, 1 was managed conservatively and for the remaining 2 patients treatment history was not available.
3. Autoimmune diseases
Twenty- eight (24.6%, n=114) patients had a positive autoimmune finding. The most common findings of autoimmunity was a positive ANA and anti-dsDNA in 12 (12.2%, n=98) and 6 (8.2%, n=73) patients, respectively. Other autoantibodies are listed in table 1. Other associated autoimmune conditions were antiphospholipid antibody syndrome and systemic lupus erythematosus in 1 patient, Grave’s disease in 1, celiac disease in 1, ulcerative colitis in 1, Sjogren’s syndrome in 2, and Henoch-Schönlein purpura in 1 patient. Two patients with no history of antiphospholipid antibody syndrome were positive for lupus anticoagulant. Autoantibodies to complement regulatory proteins are discussed in the section on complement evaluation.
The triggers and associated conditions are shown in Figure 2.
Figure 2:
C3G triggers/associated disorders.
The percentage of patients with a monoclonal gammopathy, infection or an autoimmune laboratory finding at the time of development of renal disease is given for the total group of C3G, as well as for the C3GN and DDD subgroups.
Evaluation of the AP of complement
A total of 77 (67.5%) patients were evaluated for complement abnormalities.22 Twenty-six (37.1%, n=70) were positive for rare or novel genetic variants in complement genes (Table 2) and 47 (97.9%, n=48) carried known C3G-associated genetic polymorphisms in the CFH and/or C3 genes. Although a genetic variant was found in 37.1% patients, the significance of many of these variants in causing C3G is not completely known. Thirty patients (43.5%, n=69) were positive for C3 nephritic factor (C3Nef) and nine (13.4%, n=67) patients were positive for other auto-antibodies including C4Nef, C5Nef and autoantibodies to complement factor B (CFB) and complement factor H (CFH).
Table 2.
C3 glomerulopathy – Genetic Analysis
| C3G | Age/Sex | Gene | Variant | Highest MAFa | Populationb | PPc | CADDd | Interpretatione | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | C3GN | 64/F | CFH | c.349G>A, p.Gly117Arg | No data | No data | 4/5 | 31 | Likely pathogenic |
| c.497G>A, p.Arg166Gln | 0.05% | NFE | 1/5 | 0.36 | VUS | ||||
| 2 | C3GN | 27/F | C3 | c.2390A>T, p.Asp797Val | No data | No data | 6/6 | 31 | Likely pathogenic |
| 3 | C3GN | 29/M | C8G | c.276–3C>T | 0.52% | AFR | N/A | N/A | VUS |
| 4 | C3GN | 42/F | CFI | c.782G>A, p.Gly261Asp | 0.20% | NFE | 1/6 | 8.26 | VUS |
| 5 | C3GN | 53/F | CFHR5 | c.646–647delinsTT, p.Asn216Phe | 0.01% | NFE | N/A | N/A | Pathogenic |
| 6 | C3GN | 73/F | CFH | c.2171delC, p.Thr724fs*725 | No data | No data | N/A | N/A | Pathogenic |
| 7 | C3GN | 47/F | C3 | c.2390A>T, p.Asp797Val | No data | No data | 6/6 | 31 | Likely pathogenic |
| 8 | C3GN | 23/M | CFH | c.3350A>G, p.Asn1117Ser | No data | No data | 22.8 | VUS | |
| 9 | C3GN | 69/M | CFH | c.2557T>C p.Cys853Arg | No data | No data | 5/5 | 24.1 | Pathogenic |
| 10 | C3GN | 4/M | CFH | c.497G>A, p.Arg166Gln | 0.05% | NFE | 1/5 | 0.36 | VUS |
| 11 | C3GN | 21/M | C8A | c.465G>T, p.Gly155Gly | No data | No data | N/A | N/A | VUS |
| 12f | C3GN | 20/M | C3 | c.463A>C p.Lys155GIn | 0.059% | NFE | 1/6 | 5.73 | VUS |
| 13 | C3GN | 15/F | CFHR2 | c.212C>T, p.Thr71Met | No data | No data | 0/6 | 18.04 | VUS |
| 14 | C3GN | 12/F | C5 | c.734A>G, p.Asn245Ser | 0.42% | EAS | 2/6 | 12.76 | VUS |
| 15 | C3GN | 19/M | C3 | c.2488T>G, p.Phe830Val | No data | No data | 6/6 | 25.2 | VUS |
| C8B | c.1144G>T, p.Asp382Tyr | 0.62% | NFE | 4/6 | 28.2 | VUS | |||
| 16 | C3GN | 34/F | C3 | c.2770G>A, p.Gly924Ser | No data | No data | 5/6 | 31 | VUS |
| 17 | C3GN | 42/F | C3 | c.463A>C, p.Lys155Gln | 0.59% | NFE | 1/6 | 5.73 | VUS |
| 18 | C3GN | 45/F | CFH | c.739delG, p.Gly247Glufs*34 | No data | No data | N/A | N/A | Pathogenic |
| 19 | C3GN | 28/M | CFHR5 | c.254–5C>T | 0.65% | FIN | N/A | N/A | VUS |
| 20 | C3GN | 22/M | C5 | c.3706G>C, p.Asp1236His | 0.01% | NFE | 5/6 | 23.9 | VUS |
| C8B | C.1625C>T, p.Thr542Ile | 0.76% | NFE | 1/6 | 0.033 | VUS | |||
| CFH | c.2867C>T, p.Thr956Met | 0.30% | SAS | 2/5 | 15.09 | VUS | |||
| 21 | C3GN | 14/M | C9 | c.499C>T, p.Pro167Ser | 0.66% | NFE | 5/6 | 25.3 | VUS |
| 22f | C3GN | 50/M | CFHR5 | c.254–5C>T | 0.65% | FIN | N/A | N/A | VUS |
| 23 | C3GN | 43/M | CFHR5 | c.1343A>T,p.Tyr448Phe | No data | No data | 2/5 | 12.72 | VUS |
| 24 | C3GN | 55/M | CFH | c.705T>A, p.Tyr235Stop | No data | No data | 2/4 | 35 | Pathogenic |
| CFI | c.1657C>T, p.Pro553Ser | 0.21% | NFE | 2/6 | 15.35 | VUS | |||
| 25 | DDD | 22/M | CFH | c.2850G>T, p.Gln950His | 0.66% | NFE | 2/5 | 21.4 | VUS |
| 26 | DDD | 51/F | CFH | c.3628C>T, p.Arg1210Cys | 0.03% | NFE | 1/5 | 11.77 | Pathogenic |
Abbreviations:
MAF minor allele frequency
Populations= NFE: Non-Finnish European; SAS: South Asian; AFR: African/African American; EAS: East Asian; FIN: Finnish
PP= Pathogenicity prediction: Pathogenicity prediction score using a maximum of 6 computational methods (PhyloP, SIFT, LRT, Mutation Taster, PolyPhen HDIV, and GERP) to assess functional significance and conservation of missense variants. A score of ≥4 (out of 4, 5, or 6) is generally considered to indicate a variant is more likely to be pathogenic than benign, but pathogenicity predictions are used only as a guideline to aid interpretation and are not used solely to determine pathogenicity.
CADD= Combined Annotation Dependent Depletion: http:wintervar.wglab.org; indication of the deleteriousness of a genetic variant
Interpretation= Variants are interpreted as VUS (variant of uncertain significance), likely pathogenic, pathogenic based on criteria developed by the American College of Medical Genetics and Genomics
Patient 12 and 22 had a positive MIg
C3 glomerulonephritis:
Twenty-four (39.3%) of 61 patients who underwent genetic testing carried rare or novel genetic variants in complement genes. Of these, 8 patients had a heterozygous variant in CFH gene, 2 patients had a heterozygous variant in CFI gene, 6 patients had a heterozygous variant in C3 gene, 2 patients had a heterozygous variant in C5 gene, 4 patients had a heterozygous variant in CFHR5 gene, 1 patient had a heterozygous variant in C9, 2 patients had a heterozygous variant in C8B gene, 1 patient had a heterozygous variant in C8A gene , 1 patient had a heterozygous variant in C8G gene, and 1 patient had heterozygous variant in CFHR2 gene. Acquired drivers of disease like C3Nefs, C5Nef, FH and FB autoantibodies were identified in 27 (45.8%), one (1.7%), two (3.4%) and five (8.5%) patients, respectively. One patient had a C4Nef in addition to Factor B autoantibody.
Dense deposit disease:
Two (22.2%) of the nine patients who underwent comprehensive genetic testing carried a single genetic variants in CFH (Table 2). Seven (87.5%) patients carried common polymorphisms in CFH and C3. C3Nef was detected in three (30%) of 10 patients and autoantibodies to FH was present in one (12.5%) of eight patients tested. None of the DDD patients had multiple abnormalities.
Single versus multiple AP abnormalities: Of 26 patients with genetic variants, 23 patients had a genetic variant(s) in a single gene, 2 patients had genetic variant(s) in 2 genes, and 1 patient had genetic variant(s) in 3 genes (table 2). Of the patients with acquired drivers, 18 patients had C3Nef only; 6 patients had a C3Nef and a genetic variant(s); 4 patients had C3Nef, other autoantibodies (3 had FB autoantibody, 1 had FH autoantibody) and a genetic variant (s); 1 patient had C3Nef and C5Nef; 1 patient had C3NeF and FH autoantibody; 1 patient had FB autoantibody and genetic variant (s), 1 patient had FH autoantibody only and 1 patient had FB autoantibody, C4Nef and a genetic variant.
Evaluation of the AP of complement in MIg-positive versus MIg-negative C3G: Genetic variants were more common in the MIg-negative group as compared to the MIg-positive group (58.5% vs 9.5%, p = .02) (Figure 1). On the other hand, C3Nef and other autoantibodies (CFH, CFB, C4Nef, C5Nef) were present in both the MIg negative group and MIg positive group (C3Nef37.8% vs 45.8%, other autoantibodies 16.7% vs 12.5% respectively).
Kidney Biopsy findings
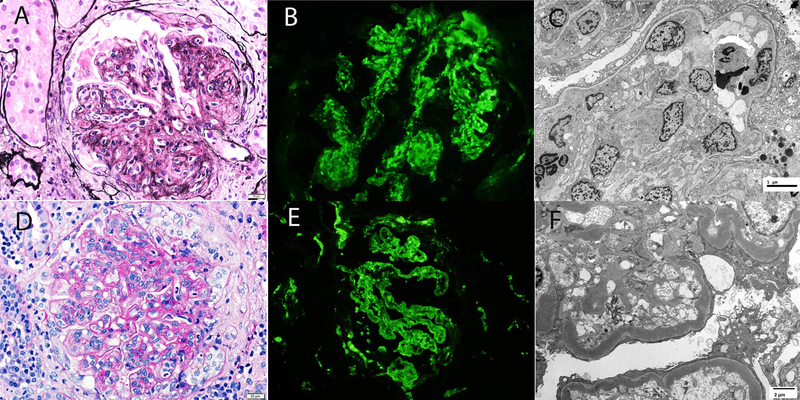
Kidney biopsy findings are summarized in Table 3 and representative biopsy findings are shown in Figure 3. Membranoproliferative glomerulonephritis (n=58, 51.3%) was the most common pattern of injury followed by mesangial proliferative glomerulonephritis (n=38, 33.6%). Nine (8.0%) biopsies showed glomeruli with greater than 40% crescents/fibrinoid necrosis. Of these, 1 also showed a diffuse proliferative glomerulonephritis and 2 showed a membranoproliferative pattern of injury in addition to the crescentic lesions. Interstitial fibrosis and tubular atrophy (IFTA) was minimal (<10%) in 29 (25.7%), mild (10–25%) in 56 (49.6%), moderate (26–50%) in 22 (19.5%) and severe (>50%) in 6 (5.3%) cases, respectively.24 Sixty-four (56.6%) biopsies showed arteriosclerosis. Immunofluorescence studies showed bright (2–3+/3) staining for C3 in 100% biopsies. In addition, trace-1+ immunoglobulins were present in 51 (45.1%) biopsies. Electron microscopy showed mesangial deposits in 101 (99%) of the C3GN biopsies. Capillary wall deposits (subepithelial, subendothelial, or intramembranous) were present in 99 (97.1%) of the C3GN biopsies. Subepithelial deposits including subepithelial humps were present in 48.5% of C3GN biopsies. An additional finding was the presence of subendothelial fluff in 5 (4.9%) of C3GN biopsies, indicating a component of thrombotic microangiopathy. Light microscopy features of thrombotic microangiopathy, including thrombi in glomeruli or arterioles were not present in these biopsies. Electron microscopy showed mesangial and capillary wall osmiophilic intramembranous electron dense deposits in all 12 cases of DDD. Subepithelial deposits including subepithelial humps were present in 25% of DDD biopsies.
Table 3:
Kidney Biopsy Findings
| Pathological findings | C3GN (n=102) |
DDD (n=12)a |
|---|---|---|
| Total Glomeruli | Mean: 19.8 Range: 4–83 |
Mean: 15.6 Range: 4–30 |
| Number of globally sclerosed glomeruli | Mean: 4.2 Range: 0–36 |
Mean: 2.5 Range: 0–7 |
| Glomerular pattern of injury | MPGN: 53 (51.9%) MES: 35 (34.3%) CRES: 7 (6.9%) DPGN: 6 (5.9%) SCL: 1 (1.0%) |
MPGN: 5 (45.4%) MES: 3 (27.3%) CRES: 2 (18.2%) DPGN: 0 (0%) SCL: 1 (9.1%) |
| Crescents/Fibrinoid necrosis | ≥40% crescents: 7 (6.9%) <40% crescents: 12 (11.8%) |
≥40% crescents: 2 (16.4%) <40% crescents: 1 (8.3%) |
| FSGS | Present: 33 (32.4%) Absent: 69 (67.6%) |
Present: 6 (54.5%) Absent: 5 (45.4%) |
| IFTA | Minimal: 27 (26.5%) Mild: 50 (49.0%) Moderate: 20 (19.6%) Severe: 5 (4.9%) |
Minimal: 2 (18.2%) Mild: 6 (54.5%) Moderate: 2 (18.2%) Severe: 1 (9.1%) |
| Arteriosclerosis | None: 43 (42.1%) Mild: 37 (36.3%) Moderate: 21 (20.6%) Severe: 1 (1.0%) |
None: 6 (54.5%) Mild: 2 (18.2%) Moderate: 2 (18.2%) Severe: 1 (9.1%) |
|
Immunofluorescence microscopy |
C3: 2–3+/3 102 (100%) Immunoglobulins: 47 (46.1%) IgA: Trace: 3, 1+: 1 IgM: Trace: 11, 1+: 17 IgG: Trace: 5, 1+: 18 |
C3: 11 (100%) Immunoglobulins (n=4, 36.4%) IgA: 1+: 1 IgM: Trace :1, 1+:2 |
| Electron microscopy | Mesangial deposits: 101 (99.0%) Capillary wall deposits: 99 (97.1%) With humps: 48 (48.5%) Subendothelial fluff: 5 (4.9%) |
Intramembranous dense deposits (100%) With humps: 3 (25%) |
Abbreviations: CRES, crescentic; DPGN, diffuse proliferative glomerular nephritis; FSGS, focal segmental glomerular sclerosis; MPGN, membranoproliferative glomerular nephritis; MES, mesangial; SCL, sclerotic; IFTA- interstitial fibrosis and tubular atrophy: grading: Minimal (<10%); Mild (10–25%); Moderate (26–50%); Severe (>50%), IF, Immunofluorescence; EM, electron microscopy.
Of the 12 patients with DDD-light microscopy details was not available for 1 patient and the diagnosis was based on electron microscopy finding.
Figure 3:
Kidney biopsy findings in C3GN and DDD.
Top panel shows a biopsy of C3GN and bottom panel shows a biopsy of DDD. A. Light microscopy demonstrating an MPGN pattern of injury (silver methenamine 40x); B. Immunofluorescence microscopy confirming bright C3 staining in the mesangium and along capillary walls (40x); C. Electron microscopy revealing mesangial and capillary wall deposits (2500x); D. Light microscopy showing an MPGN pattern of injury (periodic acid Schiff 40x); E. Immunofluorescence microscopy highlighting bright C3 staining in the mesangium and along capillary walls (40x); F. Electron microscopy displaying intramembranous dense deposits (4800x).
Treatment and follow-up of C3G
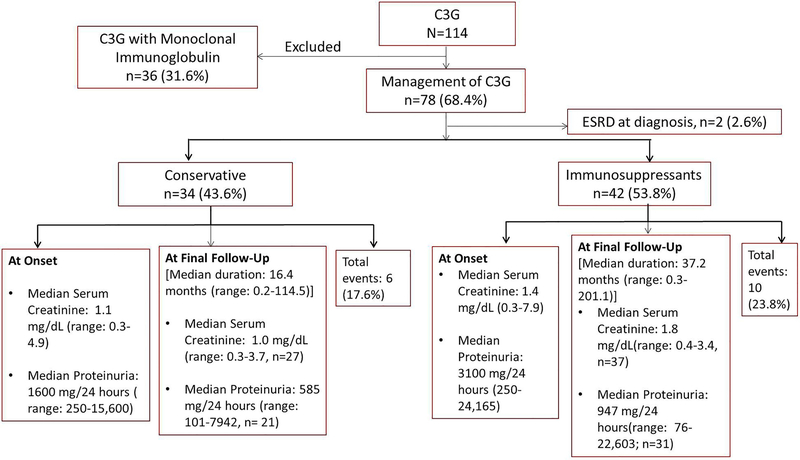
For treatment outcomes, we have excluded the 36 patients with a MIg (table 4a) since this group of patients represents a unique subset of C3G patients and was the subject of recent study that included treatment and outcomes.25 Of the remaining 78 patients, 34 (43.6%) patients were treated conservatively, 42 (53.8%) patients were treated with immunosuppressive drugs, and two (2.6%) patients presented with ESRD at diagnosis and subsequently received kidney transplant. Four patients (5.1%) received therapeutic plasma exchange. The median serum creatinine was 1.4 mg/dL (range: 0.3–3.7, n=64) and median serum proteinuria was 825.5 mg/24 hours (range: 76–22,603, n=52) after a median follow-up of 22.3 months (range: 0.1–201.1). Thirty-one patients (58.5%, n=53) had persistent hematuria. The summary of clinical course of the patients that were managed conservatively versus with immunosuppressants is shown in Figure 4.
Table 4a):
Treatment of follow-up patients with C3 Glomerulopathy, with no MIg
| Treatment and follow-up labs | C3GN (n=70) |
DDD (n=8) |
|---|---|---|
| Duration of follow-up | Median: 22.3 months Range: 0.1–201.1 |
Median: 21.1 months Range: 0.2– 125.2 |
| Conservative management only | 31 (44.3%) | 3 (37.5%) |
| Therapeutic plasma exchange | 3 (4.3%) | 1 (12.5%) |
| Immunosuppressive therapy | 38 (54.3%) -Steroids only: 12 -Other immunosuppressive drugs with/without steroids: 26a(21 Mycophenolate Mofetil, 6 Eculizumab, 7Calcineurin inhibitors, 2 Rituximab, 3 Azathioprine, 4 Cyclophosphamide) |
4 (50%) -Steroid only: 0 -Other Immunosuppressive with/without steroids: 4a(3 Mycophenolate Mofetil, 1 Cyclophosphamide, 1 Eculizumab) |
| ACE-I/ARB | 52 (74.3%) | 8 (100%) |
| ESRD | 7 (10%)b | 2 (25%)b |
| Kidney transplant | 5 (7.1%) | 1 (12.5%) |
| Recurrent C3G post-transplant | 1 | 1 |
| Serum Creatinine (mg/dL) | Median: 1.5 (n=58) Range: 0.4–3.7 | Median: 0.7, (n=6) Range: 0.3–1.7 |
| Proteinuria (mg/24 hours) | Median: 904 (n=47) Range: 76–22,603 |
Median: 472 (n=5) Range: 101–1500 |
Abbreviations: ACE-I, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; MIg, monoclonal Ig; NA; not available,
More than one immunosuppressive drug was given,
One patient presented with ESRD at diagnosis
Figure 4:
Flow Chart depicting the summary of clinical parameters among patients managed conservatively versus immunosuppressive treatment.
Of the 42 patients treated with immunosuppressants, 12 received steroid monotherapy and 30 received other immunosuppressants with/without steroids. The most common immunosuppressants included mycophenolate mofetil (MMF) in 24 (30.8%) patients, eculizumab in 7 (9.0%), and calcineurin inhibitors in 7 (9.0%) patients, respectively. The renal outcomes (ESRD or doubling of creatinine) was worse among patients managed conservatively versus those treated with immunosuppressants (17.6% vs 23.8%, p=.12, Hazard ratio: 2.41, 95% CI: 0.77–7.05).
Of the 24 patients with MMF, treatment responses were varied (table 4b) and at median duration of therapy at 9.6 months (range: 1–96.2), 1 achieved CR, 2 achieved PR, 4 had SD, 15 showed NR (of which 3 progressed to ESRD) and 2 were lost to follow-up. Similarly, of the 7 patients treated with eculizumab, at a median duration of therapy at 8.5 months (range: 1.6–26.7), 4 showed a response (2 CR, 1 PR, 1 responder) and 3 had NR (of which 2 progressed to ESRD). Of 7 patients treated with calcineurin inhibitors (tacrolimus/cyclosporine), at a median duration of 17.4 months (range: 3.1–50.9), 1 showed PR and 4 showed NR (of which 1 progressed to ESRD).
Table 4b).
Summary of response rates to various treatments in C3G
| Response | Steroid monotherapy (n=12) |
Mycophenolate mofetil (n=24) |
Eculizumab (n=7) |
Calcineurin inhibitors (n=7) |
Rituximab (n=2) |
|---|---|---|---|---|---|
| Median Duration (range) | 2.5months(0.2–80.1) | 9.6 months (Range; 1–96.2) | 8.5 months (range: 1.6–26.7) | 17.4months (range: 3.1–50.9) | 0.75 months (0.5–1.0) |
| CR | 2 | 1 | 2 | 0 | 0 |
| PR | 3 | 2 | 1 | 1 | 1 |
| Responder | 1 | 0 | 1 | 0 | 0 |
| NR | 4 | 15 (of which 3 reached ESRD) | 3 (of which 2 reached ESRD) | 6 (of which 1 reached ESRD) | 0 |
| SD | 1 | 4 | 0 | 0 | 1 |
| Lost to follow-up | 1 | 2 | 0 | 0 | 0 |
Abbreviations: CR, complete response; PR, partial response; NR, no response; SD, stable disease.
C3 glomerulonephritis:
Of 70 patients with C3GN, 31 (44.3%) patients were treated conservatively, 26 (37.1%) patients were treated with immunosuppressive drugs, and one (1.4%) patient presented with ESRD at diagnosis and subsequently received a kidney transplant with no evidence of recurrence at 41months post-transplant. Of 26 patients on immunosuppressants, three patients (4.3%) also underwent therapeutic plasma exchange during the course of C3G. The median follow-up from diagnosis was 22.3 months (range: 0.1–201.1). Six patients progressed to ESRD during the course of C3GN, of which four received kidney transplant and two continued on dialysis. At a median follow-up of 22.6 months (range: 6.7–42.3) post-transplant, 1 developed early recurrent C3GN and 3 showed no recurrence (based on both clinical findings and protocol biopsies). For the remaining patients with stable renal function at final follow-up, the median serum creatinine was 1.5 mg/dL (range: 0.4–3.7, n=58); the median proteinuria was 904 mg/24 hours (range: 76–22,603; n=47); urinalysis was positive for persistent hematuria in 29 (59.2%, n=49) patients. Four patients (5.7%) died during the course of follow-up, of which 1 had ESRD.
Dense deposit disease:
Of 8 patients with DDD, 3 (37.5%) patients were treated conservatively, 4 (50%) patients were treated with immunosuppressive drugs, and one (12.5%) patient presented with ESRD at diagnosis and subsequently received a kidney transplant but had recurrent DDD at 38.8 months post-transplant. Of 4 patients on immunosuppressants, one also underwent therapeutic plasma exchange during the course of DDD. The median duration of final follow-up from diagnosis was 21.1 months (range: 0.2–125.2).
One patient progressed to ESRD during the course of DDD and continued on dialysis at final follow-up. For the remaining patients with stable renal function at final follow-up, the median serum creatinine was 0.7 mg/dL (range: 0.3–1.7, n=6); the median proteinuria was 472 mg/24 hours (range: 101–1500; n=5); urinalysis was positive for persistent hematuria in 2 (50%, n=4) patients, respectively. One patient died of Pneumocystis pneumonia as a consequence of chronic immunosuppression post kidney transplant.
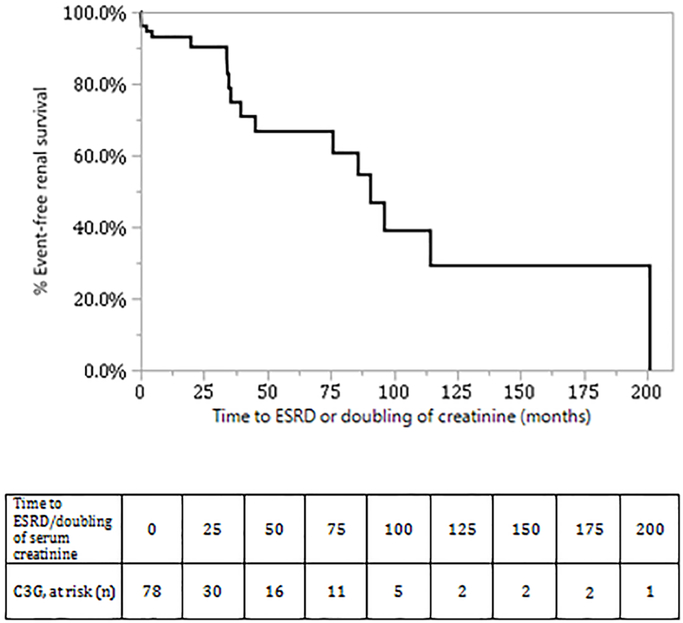
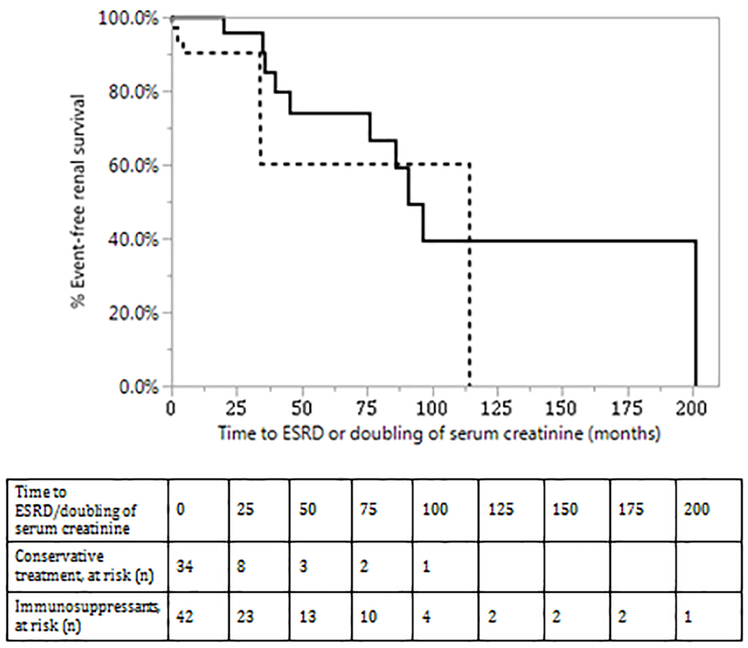
Predictors of ESRD/doubling of serum creatinine:
The predictors of ESRD on univariate analysis (Table 5) were serum creatinine, proteinuria >3 g/24 hours at diagnosis, severity of global glomerulosclerosis and the extent of tubular atrophy and interstitial fibrosis. Kaplan-Meier analysis showing renal survival during follow-up is shown in Figure 5A with an overall median survival of C3G patients of 90.8 months (95% CI: 45.3 to 201.1). Kaplan-Meier analysis showing renal survival of patients treated conservatively and patients treated with immunosuppressants is shown in Figure 5B (p-value by Wilcoxon: .05, Log Rank: .10). Kaplan-Meier analysis of renal survival showed no difference in renal survival between DDD and C3GN (Log -Rank p value=.74) (data not shown).
Table 5:
Predictors of ESRD or doubling of serum creatinine: Univariate Analysis by Cox Regression
| Factor | n | Hazard Ratio | 95% Confidence Interval | P Value |
|---|---|---|---|---|
| Age at diagnosis | 78 | 1.001 | 0.97–1.03 | .94 |
| Male Sex | 78 | 1.05 | 0.39–2.78 | .91 |
| S. Creatinine at diagnosis | 78 | 1.36 | 1.09–1.63 | .008 |
| Proteinuria >3 g/24 hours, at diagnosis | 78 | 3.07 | 1.13–9.68 | .03 |
| Global glomerulosclerosis (%) | 77a | 1.02 | 1.002–1.05 | .03 |
| Interstitial fibrosis and tubular atrophy (%) | 77a | 1.03 | 1.01–1.05 | .007 |
n<77 – For one patient, a diagnosis of DDD was based on characteristic electron microscopic findings and details of light microscopy findings were not available.
Figure 5:
Kaplan Meier analysis of renal survival of C3G (without MIg) patients 5(A): Overall renal survival of C3G patients. 5(B) Renal survival of C3G patients treated conservatively (dotted line) and C3G patients treated with immunosuppressants (solid line), p-value (Wilcoxon): .05, p-value (Log-Rank): .10.
DISCUSSION
In this study, the largest to date of C3G, we describe the clinicopathologic findings, triggering factors (monoclonal gammopathy, infections or autoimmune diseases), complement evaluation, treatment and follow-up of 114 patients seen at the Mayo Clinic, Minnesota. Although C3G is defined by the common denominator of glomerular C3 accumulation, our data reveal that the disease is heterogeneous and that there is a broad range of clinicopathologic findings, triggering events, complement abnormalities and renal outcomes.
There are few data comparing the incidence of C3GN as compared to DDD although Medjeral-Thomas et al., and Bomback et al., have reported that C3GN is approximately 3–4 times more common than DDD.26, 27 In our study, however, C3GN was ~9 times more common than DDD, a finding that could reflect selection bias, as more adult patients are seen at the Mayo Clinic. Both C3GN and DDD affected all age groups, although C3GN was more common in the older patients (mean ± Std Dev: 41.5±22.1) as compared to DDD (mean± Std Dev: 31.5±23.2) respectively, p=.10. C3GN and DDD patients alike presented with hematuria and proteinuria, the latter often in the nephrotic range. Interestingly, low C3 levels were present in only 43.0% of C3GN patients and 58.3% of DDD patients.
C3G is caused by dysregulation of the AP of complement from either acquired abnormalities or genetic factors.28 Our results confirmed the presence of both C3 nephritic factors and genetic variants in complement regulatory proteins in a significant proportion of patients. Although we detected a genetic variant in 37.1% patients, in only 9 (12.9 %) patients the variant was pathogenic or likely pathogenic and in the remainder of cases the pathogenicity was of uncertain significance. Consistent with other reports, we found the prevalence of the CFH p.Y402H polymorphism to be high and antibodies to other complement proteins such as CFH and CFB to be uncommon.5 These differences are quite distinct from atypical HUS, where antibodies to CFH are frequently detected.29
Three recent studies have shown that both C3GN and DDD are associated with a monoclonal gammopathy.30–33 However, while the association of C3G with monoclonal gammopathy is documented, the prevalence of monoclonal gammopathy in patients with C3G is not well known. We found monoclonal gammopathy to be very common: 37.9% patients with C3G had a monoclonal gammopathy, and in patients over 50 years of age, the prevalence was 65.1%. The latter subgroup also was more likely to be positive for C3 nephritic factors and autoantibodies to complement regulatory proteins. These findings suggest that the MIg may act as an autoantibody to C3 convertase or as an autoantibody to FH, adding an additional layer to the complex pathophysiology of C3G.34, 35 In these cases, targeting the MIg and the underlying hematologic disorder may be central to treating C3G. In support of this concept, a recent study has shown that treatment of the underlying B cell lymphoproliferative disorder with hematological response results in higher renal response rates in C3GN patients.25,36 In addition, in a recent case report of C3GN with advanced renal failure, chemotherapy and stem cell transplantation for multiple myeloma resulted in renal recovery.37
Our study underscores that an infection, most commonly an upper respiratory infection, often precedes or is present at the clinical presentation of C3G. Hematuria and proteinuria persist even after resolution of the infection. Furthermore, careful review of the history often revealed exacerbation of renal disease during episodes of re-infection. The AP of complement is activated during an infection. Under normal circumstances, the AP is quickly brought under control once the infection resolves. However, in the setting of genetic variation and/or the development of acquired drivers of complement deregulation, activation of the AP continues, with resultant deposition of complement proteins and their cleavage products in the glomeruli and ensuing inflammation. This process ultimately leads to C3G. Indeed, the term ‘atypical’ post-infectious glomerulonephritis has been used to describe C3G occurring in the setting of infections as an infection can ‘unmask’ a latent underlying complement abnormality.38, 39
Kidney biopsy finding for C3G showed that MPGN was the most common pattern of injury followed by mesangial proliferative glomerulonephritis. Crescentic and diffuse proliferative glomerulonephritis were also present, although less commonly. Only the extent of global glomerulosclerosis but not the pattern of injury (mesangial proliferative, membranoproliferative, and crescentic glomerulonephritis) was predictive of ESRD. Most biopsies featured only minimal or mild (≤ 25%) interstitial fibrosis and tubular atrophy. Immunofluorescence studies showed the hallmark bright glomerular C3 staining in all biopsies. Trace-1+ immunoglobulins were present in 46.1% C3GN and 36.4% DDD biopsies. This is in keeping with other studies that also found low intensity positive Ig staining in a significant number of C3GN and DDD kidney biopsies40,41 Electron microscopy revealed mesangial and capillary wall deposits in almost all C3GN biopsies. Subepithelial hump-like deposits were present in almost 50% of the C3GN biopsies, indicating that they are not restricted to infection-related glomerulonephritis. There was no difference in the C3G kidney biopsy findings of patients with or without MIg.
A major challenge is how to best treat these patients. Rabasco et al, reported a retrospective analysis of 60 patients with C3GN seen among multiples sites within the GLOSEN group.11 Of these, 20 patients were treated conservatively, 22 patients received corticosteroids and MMF, and 18 patients received corticosteroids either as monotherapy or in combination with cyclophosphamide. The number of patients developing ESRD was significantly lower among treated compared with untreated patients (3 versus 7 patients, respectively). No patient in the corticosteroids plus MMF had a doubling of serum creatinine or progressed to ESRD, as compared with 7 and 3 patients, respectively, treated with other immunosuppressive regimens. Clinical remission was significantly higher in patients treated with corticosteroids plus MMF as compared with patients treated with other immunosuppressive regimens or patients on conservative therapy, (86, 50, and 25%) respectively. Of the patients treated with MMF, 32 achieved complete remission and 68% partial remission.
Similar results were recently reported in by Avasare et al. on a retrospective study of 30 patients treated with MMF for at least 3 months and follow-up for at least 1 year.12 Patients were categorized as “responders” if they had either complete (stable or improved eGFR with decline in proteinuria to <0.5 g/g creatinine by urine protein/creatinine ratio) or partial remission (stable or improved eGFR with ≥50% decline in proteinuria to between 0.5 and 3.5 g/g creatinine by urine protein-to-creatinine ratio) concordant with MMF treatment. Stable eGFR was defined as within 15% of baseline. Patients were categorized as “nonresponders” if they did not achieve remission on MMF. Twenty (67%) patients were classified as responders. Of note, initial proteinuria was lower (median 2468 mg/g creatinine) in responders compared with nonresponders (median 5000 mg/g creatinine). Although patients studied by Avasare et al. were younger (median 25 years old) and had lower proteinuria (median 3200 mg/g creatinine) than the patients treated with MMF by Rabasco et al, (median age 35 years old, proteinuria 6500 mg/24h), there were more patients progressing to ESRD (10%) than patents included in the GLOSEN study (0%).
In our study, the renal outcome (doubling of serum creatinine or ESRD) was worse in patients managed conservatively versus patients treated with immunosuppressive therapy. This is even more impressive in that the group with immunosuppressive therapy had a much longer follow-up compared to patients treated conservatively (Figure 5B). However, when evaluating the group of patients treated with MMF, our results were less impressive than those reported in the two studies discussed above. This may due to the worse kidney function at baseline in our group, (median creatinine 1.4 mg/dl) versus Avasare et al. (median creatinine 1.0 mg/dl) but it does not explain the discrepancy between our study and the one by Rabasco et al. that included patients of similar age (35 years) and kidney function (median creatinine 1.3 mg/dl) to our study. In our study versus Rabasco et al study, there were a similar number of patients with biopsies showing moderate to severe interstitial fibrosis and tubular atrophy (25% vs 21%). However, our patient population of C3G without MIg had a significantly larger number of patients (58.5%) with genetic forms compared to the Rabasco study (13%). In addition, our study had a lower number of C3Nef patients (37.8%) compared to the Rabasco study (48%). Thus, it is likely that the lower response rate observed in our study to MMF was due to a population enriched with the genetic forms of C3G. Taken together, these studies suggest that patients with the autoimmune form of the disease are more likely to respond to immunosuppressive therapy including MMF compared to the genetic forms of C3G that are less likely to respond to immunosuppressive therapy.
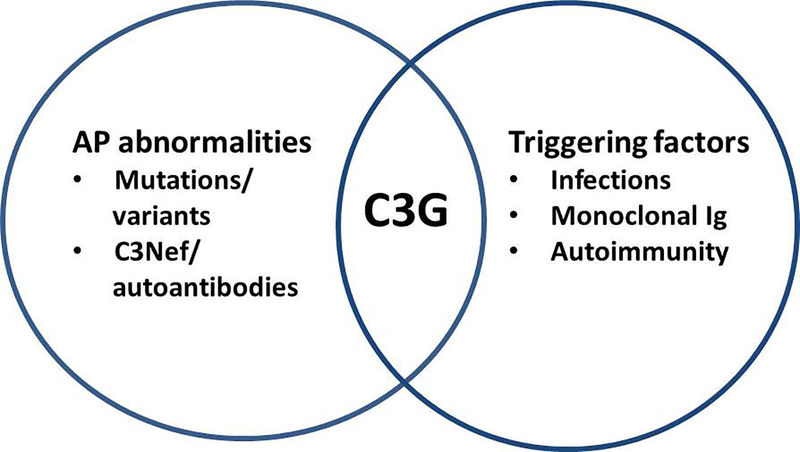
The present study also shows that C3G is a heterogeneous group of diseases that reflect the common underlying pathophysiology, which is deregulation of the AP of complement. The development of C3G likely depends on a complex interaction of triggering events and the underlying abnormalities of the AP of complement (figure 6). If the group of C3G patients is divided based on the presence or absence of monoclonal gammopathy, in the latter subgroup, patients are younger and have both genetic and acquired abnormalities of the AP of complement. In the monoclonal gammopathy subgroup, in contrast, patients are older and are less likely to carry genetic variants in complement genes.
Figure 6:
Schematic depicting that C3G often results due to interactions between the triggering events and abnormalities of the AP of complement.
One of the strengths of the study is that all patients were seen at a single institution and detailed clinical and laboratory findings kidney biopsy, treatment and follow-up were available. A limitation of the study is that complement evaluation varied, particularly as more sophisticated and detailed testing only became available for patients diagnosed during the latter part of the study. In addition, while many mutations/variants have been previously reported in C3G, the significance of some of the mutations/variants is not known and is the subject of an on-going study by our group.
CONCLUSION
In summary, we describe the clinical and pathological findings, triggering events and complement abnormalities in the largest cohort of C3G patients reported to date, along with the treatment and outcomes data. It is of paramount importance that each patient of C3G be evaluated for complement abnormalities and triggering factors, to allow appropriate treatment to be recommended.
ACKNOWLEDGMENTS
Supported in part by grant RO1-DK110023 from the NIDDK (RJHS)
ABBREVIATIONS:
- C3G
C3 Glomerulopathy
- C3GN
C3 Glomerulonephritis
- CR
Complete Response
- CI
Confidence Interval
- DDD
Dense Deposit Disease
- ESRD
End Stage Renal Disease
- MIg
Monoclonal Immunoglobulin
- NR
No Response
- PR
Partial Response
- Std
Dev: standard deviation
Footnotes
Disclosure: None
Conflict of Interest: None
References
- 1.Pickering MC, D’Agati VD, Nester CM, et al. C3 glomerulopathy: consensus report. Kidney Int. 2013;84(6):1079–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sethi S, Fervenza FC, Zhang Y, et al. C3 glomerulonephritis: clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int. 2012;82(4):465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Vriese AS, Sethi S, Van Praet J, Nath KA, Fervenza FC. Kidney Disease Caused by Dysregulation of the Complement Alternative Pathway: An Etiologic Approach. Journal of the American Society of Nephrology. 2015;26(12):2917–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angioi A, Fervenza FC, Sethi S, et al. Diagnosis of complement alternative pathway disorders. Kidney International. 2016;89(2):278–288. [DOI] [PubMed] [Google Scholar]
- 5.Servais A, Noel L- H, Roumenina LT, et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82(4):454–464. [DOI] [PubMed] [Google Scholar]
- 6.Appel GB, Cook HT, Hageman G, et al. Membranoproliferative Glomerulonephritis Type II (Dense Deposit Disease): An Update. J Am Soc Nephrol. 2005;16(5):1392–1403. [DOI] [PubMed] [Google Scholar]
- 7.Sethi S, Nester CM, Smith RJH. Membranoproliferative glomerulonephritis and C3 glomerulopathy: resolving the confusion. Kidney Int. 2012;81(5):434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Servais A, Fremeaux-Bacchi V, Lequintrec M, et al. Primary glomerulonephritis with isolated C3 deposits: a new entity which shares common genetic risk factors with haemolytic uraemic syndrome. Journal of Medical Genetics. 2007;44(3):193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi S, Fervenza FC, Zhang Y, et al. Proliferative Glomerulonephritis Secondary to Dysfunction of the Alternative Pathway of Complement. Clinical Journal of the American Society of Nephrology. 2011;6(5):1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasr SH, Valeri AM, Appel GB, et al. Dense Deposit Disease: Clinicopathologic Study of 32 Pediatric and Adult Patients. Clinical Journal of the American Society of Nephrology. 2009;4(1):22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabasco C, Cavero T, Román E, et al. Effectiveness of mycophenolate mofetil in C3 glomerulonephritis. Kidney International. 2015;88(5):1153–1160. [DOI] [PubMed] [Google Scholar]
- 12.Avasare RS, Canetta PA, Bomback AS, et al. Mycophenolate Mofetil in Combination with Steroids for Treatment of C3 Glomerulopathy: A Case Series. Clinical Journal of the American Society of Nephrology. 2018;13(3):406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez Custodio, Maria Ruvie Lou, “Diagnostics for Choosing between Log-Rank and Wilcoxon Tests” (2007). Dissertations. 895 http://scholarworks.wmich.edu/dissertations/895 [Google Scholar]
- 14.Rabasco C, Cavero T, Roman E, et al. Effectiveness of mycophenolate mofetil in C3 glomerulonephritis. Kidney Int. 2015;88(5):1153–1160. [DOI] [PubMed] [Google Scholar]
- 15.Bu F, Borsa NG, Jones MB, et al. High-Throughput Genetic Testing for Thrombotic Microangiopathies and C3 Glomerulopathies. Journal of the American Society of Nephrology. 2016;27(4):1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davydov EV, Goode DL, Sirota M, Cooper GM, Sidow A, Batzoglou S. Identifying a high fraction of the human genome to be under selective constraint using GERP++. PLoS computational biology. 2010;6(12):e1001025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper GM, Stone EA, Asimenos G, et al. Distribution and intensity of constraint in mammalian genomic sequence. Genome research. 2005;15(7):901–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nature methods. 2014;11(4):361–362. [DOI] [PubMed] [Google Scholar]
- 19.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nature methods. 2010;7(4):248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar P, Henikoff S, Ng PC. Predicting the effects of coding non-synonymous variants on protein function using the SIFT algorithm. Nature protocols. 2009;4(7):1073–1081. [DOI] [PubMed] [Google Scholar]
- 21.Chun S, Fay JC. Identification of deleterious mutations within three human genomes. Genome research. 2009;19(9):1553–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Meyer NC, Wang K, et al. Causes of Alternative Pathway Dysregulation in Dense Deposit Disease. Clinical Journal of the American Society of Nephrology. 2012;7(2):265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Meyer NC, Fervenza FC, et al. C4 Nephritic Factors in C3 Glomerulopathy: A Case Series. American Journal of Kidney Diseases. 2017;70(6):834–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sethi S, D’Agati VD, Nast CC, et al. A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney International. 2017;91(4):787–789. [DOI] [PubMed] [Google Scholar]
- 25.Ravindran A, Fervenza FC, Smith RJ, Sethi S. C3 glomerulopathy associated with monoclonal Ig: a distinct subtype. Kid Int. 2018;in press( [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medjeral-Thomas NR, O’Shaughnessy MM, O’Regan JA, et al. C3 Glomerulopathy: Clinicopathologic Features and Predictors of Outcome. Clinical Journal of the American Society of Nephrology. 2014;9(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bomback AS, Santoriello D, Avasare RS, et al. C3 glomerulonephritis and dense deposit disease share a similar disease course in a large United States cohort of patients with C3 glomerulopathy. Kidney International.93(4):977–985. [DOI] [PubMed] [Google Scholar]
- 28.Sethi S, Fervenza FC. Membranoproliferative Glomerulonephritis: A New Look at an Old Entity. New England Journal of Medicine. 2012;366(12):1119–1131. [DOI] [PubMed] [Google Scholar]
- 29.Dragon-Durey M- A, Sethi SK, Bagga A, et al. Clinical Features of Anti-Factor H Autoantibody–Associated Hemolytic Uremic Syndrome. Journal of the American Society of Nephrology. 2010;21(12):2180–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zand L, Kattah A, Fervenza FC, et al. C3 Glomerulonephritis Associated With Monoclonal Gammopathy: A Case Series. American Journal of Kidney Diseases. 2013;62(3):506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sethi S, Sukov WR, Zhang Y, et al. Dense Deposit Disease Associated With Monoclonal Gammopathy of Undetermined Significance. American Journal of Kidney Diseases. 2010;56(5):977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bridoux F, Desport E, Frémeaux-Bacchi V, et al. Glomerulonephritis With Isolated C3 Deposits and Monoclonal Gammopathy: A Fortuitous Association? Clinical Journal of the American Society of Nephrology. 2011;6(9):2165–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lloyd IE, Gallan A, Huston HK, et al. C3 glomerulopathy in adults: a distinct patient subset showing frequent association with monoclonal gammopathy and poor renal outcome. Clinical Kidney Journal. 2016;9(6):794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meri S, Koistinen V, Miettinen A, Tornroth T, Seppala I. Activation of the alternative pathway of complement by monoclonal lambda light chains in membranoproliferative glomerulonephritis. . J Exp Med. 1992;175(4):939–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanc C, Togarsimalemath SK, Chauvet S, et al. Anti–Factor H Autoantibodies in C3 Glomerulopathies and in Atypical Hemolytic Uremic Syndrome: One Target, Two Diseases. The Journal of Immunology. 2015;194(11):5129–5138. [DOI] [PubMed] [Google Scholar]
- 36.Chauvet S, Frémeaux-Bacchi V, Petitprez F, et al. Treatment of B-cell disorder improves renal outcome of patients with monoclonal gammopathy–associated C3 glomerulopathy. Blood. 2017;129(11):1437. [DOI] [PubMed] [Google Scholar]
- 37.Hamzi M, Zniber A, Badaoui G, et al. C3 glomerulopathy associated to multiple myeloma successfully treated by autologous stem cell transplant. Indian J Nephrol. 2017;27(2):141–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sethi S, Fervenza FC, Zhang Y, et al. Atypical postinfectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int. 2013;83(2):293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Ghaithi B, Chanchlani R, Riedl M, Thorner P, Licht C. C3 Glomerulopathy and post-infectious glomerulonephritis define a disease spectrum. Pediatric Nephrology. 2016;31(11):2079–2086. [DOI] [PubMed] [Google Scholar]
- 40.Hou J, Markowitz GS, Bomback AS, et al. Toward a working definition of C3 glomerulopathy by immunofluorescence. Kidney Int. 2014;85(2):450–456. [DOI] [PubMed] [Google Scholar]
- 41.Larsen CP, Walker PD. Redefining C3 glomerulopathy: /`C3 only/’ is a bridge too far. Kidney Int. 2013;83(2):331–332. [DOI] [PubMed] [Google Scholar]