Abstract
There is a paucity of recent data about the epidemiology and long‐term outcomes of nonalcoholic fatty liver disease (NAFLD) in the female population. Our aim was to assess the prevalence, risk factors, and mortality of NAFLD in female adults of the United States. Data from the National Health and Nutrition Examination Survey (NHANES) III and NHANES 1999‐2014 were used. NAFLD status was determined by the U.S. Fatty Liver Index (US‐FLI) in the absence of other liver diseases and excessive alcohol consumption. The prevalence rates, risk factors, and 5‐year all‐cause and cardiovascular mortality were determined in women with NAFLD. The most recent prevalence of NAFLD among female adults (2007‐2014) in the United States was 24.4% (95% confidence interval [CI], 22.48‐26.33). Prevalence was higher among women >44 years of age and those with body mass index ≥30 kg/m2. In addition, the average age of the female population with NAFLD has decreased over time. The fully adjusted odds ratios in women with NAFLD compared to those without NAFLD were 1.48 (95% CI, 1.20‐1.82) for cardiovascular disease (CVD), 1.89 (95% CI, 1.42‐2.52) for atherosclerotic cardiovascular disease (ASCVD) score ≥7.5%, and 1.76 (95% CI, 1.37‐2.25) for either CVD or ASCVD ≥7.5%. The 5‐year mortality for female adults with NAFLD was significantly higher than for those without NAFLD (adjusted hazard ratio, 1.48; 95% CI, 1.07‐2.05). Among women with NAFLD, those with ASCVD ≥7.5% had significantly higher 5‐year all‐cause mortality and CVD mortality. Conclusion: The prevalence of NAFLD in female NHANES participants from the United States has continued over recent years. In the female population with NAFLD, ASCVD ≥7.5% is an independent predictor of overall and cardiac‐specific mortality.
Short abstract
NAFLD is growing in the female population in the U.S. NAFLD is associated with metabolic risk factors and increases the risk of CVD and mortality. Detection of NAFLD in females consider a serious risk threat that needs to be addressed to potentially improve their long‐term outcomes.
Abbreviations
- ASCVD
atherosclerotic cardiovascular disease
- BMI
body mass index
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- CV
cardiovascular
- CVD
cardiovascular disease
- HL
hyperlipidemia
- HR
hazard ratio
- HTN
hypertension
- MS
metabolic syndrome
- NAFLD
nonalcoholic fatty liver disease
- NHANES
National Health and Nutrition Examination Survey
- PIR
poverty‐income ratio
- T2DM
type 2 diabetes mellitus
- US‐FLI
U.S. Fatty Liver Index
Nonalcoholic fatty liver disease (NAFLD) is a growing global health problem, affecting almost a quarter of the world’s population.1 NAFLD represents a spectrum of chronic liver diseases that range from simple steatosis to nonalcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma.2, 3
It has been shown that the global prevalence of NAFLD has been on the rise.1, 2, 3, 4 The prevalence of NAFLD has increased from 15% in 2005 to 25% in 2010 in the United States.4 A number of risk factors predispose individuals to develop NAFLD, including obesity, type 2 diabetes mellitus (T2DM), hypertension (HTN), and hyperlipidemia (HL).5, 6 In this context, NAFLD is considered the hepatic manifestation of the metabolic syndrome (MS), which is a multisystem disruption of energy homeostasis primarily driven by insulin resistance (IR).7, 8 In addition, some studies have demonstrated that the prevalence of NAFLD is higher among the male population, increases with age, and varies among different ethnicities.9, 10, 11 Moreover, it has been clearly demonstrated that NAFLD is associated with an increased risk of cardiovascular disease (CVD), independent of other risk factors of CVD, such as obesity and diabetes, and CVD is the most frequent cause of death among patients with NAFLD.12, 13
Although the spectrum of NAFLD is reported to be more common in men, NASH was initially described as a liver disease of elderly women.14 It is known that after menopause, the prevalence of NAFLD increases compared to the premenopausal state, with rates similar to the prevalence of NAFLD in men as well as a comparable risk of progression of liver disease.11 Given that most of these data come from tertiary care centers or review articles, there is a paucity of data using a population‐based data set about the burden and outcomes of NAFLD in the adult female population. Therefore, our aim was to assess the prevalence and mortality in female patients with NAFLD, using data from the National Health and Nutrition Examination Survey (NHANES)‐III, NHANES (1999‐2006), and NHANES (2007‐2014). We also assessed the association of CVD and cardiovascular (CV) mortality in patients with NAFLD.
Participants and Methods
Study Population
This study was based on the NHANES series conducted by the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention.15 The data were compiled through household interviews, physical examinations, and laboratory assays on collected blood and urine specimens to assess the prevalence of disease, disease risk factors, and nutritional status of the civilian noninstitutionalized U.S. population. NHANES III was carried out from 1988 to 1994, and starting in 1999, data were collected continuously and were released in 2‐year blocks. Therefore, we used data that included NHANES III (1988‐1994); combined NHANES 1999‐2000, 2001‐2002, 2003‐2004, and 2005‐2006 (NHANES 1999‐2006); and combined NHANES 2007‐2008, 2009‐2010, 2011‐2012, and 2013‐2014 (NHANES 2007‐2014), following NCHS recommendations. Participants were included if they were women, aged ≥20 years with available demographics (age and ethnicity), and seen in a mobile medical examination center where laboratory measurements were taken. Participants with insufficient data for the diagnosis of NAFLD were excluded from the study.
NAFLD
Because abdominal ultrasounds were only performed in NHANES III, we used the U.S. Fatty Liver Index (US‐FLI), which has been validated to establish NAFLD in the U.S. population.16 The US‐FLI includes age, race, waist circumference, gamma‐glutamyltransferase, fasting insulin, and fasting glucose. Using ultrasound as standard, hepatic steatosis can be predicted by the US‐FLI with an area under the curve value of 0.80 (95% CI, 0.77‐0.83).16 For our study, participants were presumed to have NAFLD if they had a US‐FLI score of ≥30 in the absence of other causes of liver disease, such as extensive alcohol intake (>10 g/day), chronic hepatitis B (CHB) (hepatitis B surface antigen‐positive), chronic hepatitis C (hepatitis C virus RNA‐positive), or a history of autoimmune liver diseases or other chronic liver diseases.17
Demographics and Comorbidities
Sociodemographic characteristic of participants, including age (20‐44, 45‐64, and >65 years), race/ethnicity (non‐Hispanic white, non‐Hispanic black, Hispanic, or “other,” which included other Hispanics, Aleut, Eskimo, Asians, Native Americans, or Pacific Islander), sex, smoking status, and poverty‐income ratio (PIR) (PIR <1.3 as low, PIR 1.3‐3.5 as middle, and PIR >3.5 as high),18 were obtained as self‐reported data during the interview.
Obesity was defined as a body mass index (BMI) of ≥30 kg/m2. T2DM was defined as self‐reported use of oral hypoglycemic medications or insulin or a fasting glucose measure of ≥126 mg/dL.19 HTN was defined as a systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg, or self‐reported use of oral antihypertensive medications.20 HL was defined as a serum cholesterol level of >200 mg/dL, low‐density lipoprotein level of ≥130 mg/dL, or high‐density lipoprotein level of <40 mg/dL for men and <50 mg/dL for women. MS was defined as having at least three of the following: obesity, T2DM, HTN, or HL. IR was defined as having a homeostasis model assessment score of >3.21 Excessive alcohol consumption was defined as ≥20 g/day for men and ≥10 g/day for women. Elevated liver enzyme was defined as increased alanine aminotransferase of ≥40 U/L in men and ≥31 U/L in women or aspartate aminotransferase level of ≥37 U/L in men and ≥31 U/L in women. Alcoholic liver disease was defined by excessive alcohol use and elevated liver enzymes. Chronic hepatitis C was defined as positive hepatitis C virus RNA, and CHB was defined as positive hepatitis B surface antigen. CVD was defined by a self‐reported medical history of congestive heart failure, coronary artery disease, heart attack, or stroke. Chronic obstructive pulmonary disease (COPD) was defined by a self‐reported medical history of chronic bronchitis or emphysema. Cancer was defined as a self‐reported history of any cancer. The atherosclerotic cardiovascular disease (ASCVD) risk score was calculated from each participant’s age, race, sex, smoking status, presence of diabetes, systolic blood pressure, antihypertensive medication, serum cholesterol levels, and high‐density lipoprotein levels.22 ASCVD score ≥7.5% corresponded to a high‐risk group for a first ASCVD event.
Statistical Methods
Examination sample weights, accounting for nonresponse, noncoverage, and unequal selection probabilities for certain categories of the population, were incorporated in order to produce national estimates. The standard errors of estimates were estimated using Taylor series linearization, a design‐based method.23 For combining NHANES study cycles, appropriate selection of sampling weights and adjustment coefficients was implemented in compliance with the NHANES Analytic and Reporting Guidelines.18 Various sociodemographic and clinical parameters among U.S. female adults with NAFLD were compared among the three study cycles, i.e., NHANES III, NHANES (1999‐2006), and NHANES (2007‐2014), by using Rao‐Scott chi‐square for categorical variables or Wald test for continuous variables.24, 25 Female adults with NAFLD and no NAFLD were compared using the combined NHANES (1984‐2014). Logistic regressions were used to assess the association of CVD and ASCVD ≥7.5% with NAFLD.
To assess the effects of NAFLD on the 5‐year all‐cause mortality and CVD mortality using the Cox proportional hazards models, we restricted our analysis to the combined NHANES cohort (1984‐2006) to have at least 5 years of follow‐up. Mortality data were obtained by using the public‐use linked mortality file.26 For our study, CVD mortality was defined as death due to CV and cerebrovascular diseases (International Classification of Diseases, Tenth Revision, codes I00‐I90, I11, I13, I20‐I51, and I60‐I69).27 The proportional hazards assumption of the Cox models was examined by testing time‐dependent covariates,28 which showed no significant departure from proportionality over time. We used two models with a hierarchical fashion: model 1 adjusted for age and ethnicity and model 2 further adjusted for smoking status, MS, and important comorbidities. Due to collinearity between MS and metabolic components (obesity, T2DM, HTN, and HL), the latter components were not included in the fully adjusted model. All analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC), using the “SURVEY” procedure, which incorporates the sample design. Statistical tests were considered significant at P < 0.05 (2‐tailed). “SURVEY” functions were used to account for the complex survey design.
Results
The study cohort included 13,559 female subjects. Of these, 3,811 (28.1%) had NAFLD and 9,748 did not. Female patients with NAFLD were older, more commonly Hispanic, and had higher comorbidity, including metabolic conditions and CV disease and associated risks (Table 1).
Table 1.
Characteristics of U.S. Female Adults by NAFLD Status, NHANES (1988‐2014)
| Characteristics | No NAFLD (n = 9,748) | NAFLD (n = 3,811) | ALL (n = 13,559) | |||
|---|---|---|---|---|---|---|
| n | Weighted % ± SEM | n | Weighted % ± SEM* | n | Weighted % ± SEM | |
| Age, years † | 44.35 ± 0.32 | 53.16 ± 0.40 | 46.38 ± 0.29 | |||
| Age, % | ||||||
| 20‐44 | 5,482 | 57.62 ± 0.86 | 1,223 | 32.59 ± 1.18 | 6,705 | 51.86 ± 0.71 |
| 45‐64 | 2,466 | 27.98 ± 0.68 | 1,406 | 40.55 ± 1.25 | 3,872 | 30.87 ± 0.55 |
| ≥65 | 1,800 | 14.40 ± 0.63 | 1,182 | 26.86 ± 1.09 | 2,982 | 17.27 ± 0.63 |
| RACE, % | ||||||
| non‐Hispanic white | 4,550 | 72.77 ± 1.24 | 1,508 | 68.87 ± 1.52 | 6,058 | 71.87 ± 1.20 |
| non‐Hispanic black | 2,562 | 12.24 ± 0.70 | 618 | 9.02 ± 0.67 | 3,180 | 11.50 ± 0.65 |
| Hispanic | 1,967 | 7.29 ± 0.50 | 1,503 | 16.28 ± 1.16 | 3,470 | 9.36 ± 0.60 |
| Other | 669 | 7.70 ± 0.59 | 182 | 5.83 ± 0.77 | 851 | 7.27 ± 0.52 |
| Income (PIR), % | ||||||
| Low (PIR <1.3) | 2,722 | 19.76 ± 0.92 | 1,346 | 26.96 ± 1.09 | 4,068 | 21.41 ± 0.82 |
| Middle (1.3<PIR<3.5) | 3,558 | 38.79 ± 0.84 | 1,381 | 42.02 ± 1.38 | 4,939 | 39.53 ± 0.76 |
| High (PIR >3.5) | 2,677 | 41.45 ± 1.07 | 723 | 31.01 ± 1.59 | 3,400 | 39.07 ± 1.04 |
| Current smoker, % | 1,787 | 23.00 ± 0.68 | 543 | 18.88 ± 1.02 | 2,330 | 22.08 ± 0.64 |
| Metabolic components, % | ||||||
| Obesity | 2,083 | 18.13 ± 0.55 | 2,583 | 70.54 ± 1.08 | 4,666 | 30.15 ± 0.68 |
| Type 2 diabetes mellitus | 475 | 3.16 ± 0.19 | 1,135 | 26.24 ± 0.85 | 1,610 | 8.45 ± 0.31 |
| Hypertension | 2,598 | 21.85 ± 0.63 | 2,081 | 53.79 ± 1.06 | 4,679 | 29.20 ± 0.61 |
| Hyperlipidemia | 6,548 | 64.85 ± 0.78 | 3,320 | 88.49 ± 0.67 | 9,868 | 70.29 ± 0.64 |
| Metabolic syndrome | 848 | 6.32 ± 0.31 | 1,752 | 45.20 ± 1.03 | 2,600 | 15.27 ± 0.44 |
| Comorbidities, % | ||||||
| Cancer | 721 | 8.31 ± 0.36 | 380 | 12.26 ± 0.72 | 1,101 | 9.22 ± 0.33 |
| COPD | 654 | 7.44 ± 0.44 | 367 | 11.90 ± 0.86 | 1,021 | 8.46 ± 0.39 |
| CVD | 490 | 4.04 ± 0.25 | 457 | 10.30 ± 0.61 | 947 | 5.48 ± 0.27 |
| ASCVD ≥7.5% | 2,076 | 16.89 ± 0.58 | 1,565 | 37.16 ± 1.21 | 3,641 | 21.51 ± 0.60 |
| CVD or ASCVD ≥7.5% | 2,237 | 17.99 ± 0.60 | 1,687 | 38.66 ± 1.22 | 3,924 | 22.75 ± 0.61 |
Significantly different from no NAFLD.
Expressed as weighted mean ± SEM.
Characteristics of U.S. Female Adults With NAFLD
The unadjusted prevalence of NAFLD among female adults in the United States has increased: 18.54% (95% confidence interval [CI], 16.41‐20.66) in 1988‐1994; 21.36% (95% CI, 19.63‐23.09) in 1999‐2006; and 24.86% (95% CI, 22.9‐26.82) in 2007‐2014 (Fig. 1). The prevalence of NAFLD was lower in those aged 20 to 44 years than in those aged ≥45 years across NHANES cycles. Nevertheless, the prevalence rates for female adults aged 20 to 44 years also increased from 10.8% in 1988‐1994 to 17.3% in 2007‐2014. Finally, the prevalence of NAFLD in female adults with BMI ≥30 kg/m2 was significantly higher than in those with BMI <30 kg/m2 (Supporting Table S1).
Figure 1.
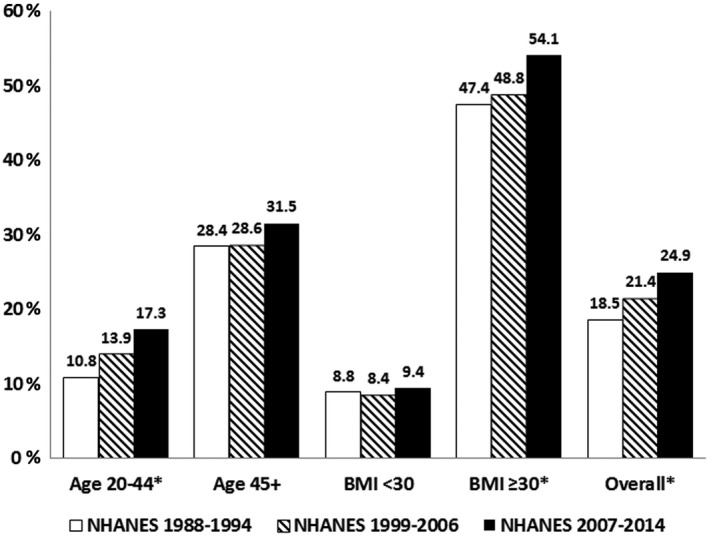
Prevalence (%) of NAFLD among U.S. female adults, NHANES (1988‐2014). *Indicates the linear trend over time with P < 0.05.
Among U.S. female adults with NAFLD, the prevalence of metabolic components, including obesity, T2DM, and HTN, increased from 1988‐1994 to 2007‐2014 (Table 2). Consistent with these changes, the prevalence of MS also increased from 40.1% in 1988‐1994 to 49.1% in 2007‐2014 (Table 2).
Table 2.
Characteristics of U.S. Female Adults with NAFLD, NHANES 1988‐2014
| Characteristics | 1988‐1994 (n = 1,563) | 1999‐2006 (n = 993) | 2007‐2014 (n = 1,256) | |||
|---|---|---|---|---|---|---|
| n | Weighted % ± SEM | n | Weighted % ± SEM | n | Weighted % ± SEM | |
| Age, years† | 54.64 ± 0.69 | 52.73 ± 0.64 | 51.85 ± 0.46* | |||
| Age, % | ||||||
| 20‐44 | 525 | 32.89 ± 2.49 | 315 | 32.05 ± 1.72 | 383 | 32.68 ± 1.66 |
| 45‐64 | 504 | 36.94 ± 2.11 | 358 | 40.48 ± 2.04 | 544 | 44.67 ± 2.14* |
| ≥65 | 534 | 30.17 ± 2.13 | 320 | 27.46 ± 1.90 | 328 | 22.65 ± 1.40* |
| RACE, % | ||||||
| non‐Hispanic white | 598 | 72.13 ± 2.72 | 418 | 67.54 ± 2.83 | 492 | 66.29 ± 2.62 |
| non‐Hispanic black | 309 | 9.76 ± 1.14 | 135 | 8.65 ± 1.17 | 174 | 8.49 ± 0.98 |
| Hispanic | 575 | 8.88 ± 1.25 | 415 | 20.73 ± 2.97* | 513 | 20.98 ± 2.08* |
| Other | 81 | 9.23 ± 1.94 | 25 | 3.08 ± 0.74* | 76 | 4.24 ± 0.75* |
| Income, % | ||||||
| Low | 596 | 27.22 ± 2.19 | 307 | 26.15 ± 2.05 | 443 | 27.34 ± 1.43 |
| Middle | 595 | 49.03 ± 2.28 | 365 | 37.18 ± 2.31* | 421 | 38.17 ± 1.83* |
| High | 210 | 23.74 ± 2.86 | 246 | 36.67 ± 2.30* | 267 | 34.49 ± 2.44* |
| Current smoker, % | 217 | 17.45 ± 1.47 | 142 | 22.80 ± 1.95* | 184 | 17.86 ± 1.65 |
| BMI | 33.22 ± 0.41 | 34.79 ± 0.36* | 35.56 ± 0.22* | |||
| HOMA | 7.91 ± 0.24 | 6.46 ± 0.25* | 6.52 ± 0.16* | |||
| Metabolic components,% | ||||||
| Obesity | 981 | 64.51 ± 2.01 | 684 | 72.99 ± 1.97* | 918 | 75.38 ± 1.16* |
| Type 2 diabetes mellitus | 472 | 26.76 ± 1.71 | 275 | 22.80 ± 1.37 | 388 | 28.48 ± 1.41* |
| Hypertension | 761 | 48.16 ± 2.08 | 588 | 58.95 ± 1.99* | 732 | 55.94 ± 2.05* |
| Hyperlipidemia | 1,382 | 89.54 ± 1.25 | 860 | 87.98 ± 1.33 | 1,078 | 87.73 ± 0.96 |
| Metabolic syndrome | 646 | 40.05 ± 1.84 | 476 | 47.52 ± 2.53* | 630 | 49.12 ± 1.64* |
| Comorbidities, % | ||||||
| Cancer | 132 | 11.45 ± 1.14 | 105 | 12.07 ± 1.04 | 143 | 13.31 ± 1.54 |
| COPD | 146 | 12.30 ± 1.22 | 93 | 12.80 ± 1.43 | 128 | 10.72 ± 1.46 |
| CVD | 208 | 11.44 ± 1.21 | 112 | 10.53 ± 1.17 | 137 | 8.82 ± 0.99 |
| ASCVD ≥7.5% | 703 | 42.69 ± 2.27 | 409 | 36.21 ± 1.74* | 453 | 31.61 ± 1.89* |
| CVD or ASCVD ≥7.5% | 746 | 43.97 ± 2.45 | 447 | 38.11 ± 1.86 | 494 | 33.12 ± 1.79* |
Abbreviation: HOMA, homeostasis model assessment.
Significantly different from NHANES III (1988‐1994).
Expressed as weighted mean ± SEM.
In female adults, the age‐standardized prevalence of NAFLD increased from 20.96% (95% CI, 18.77‐23.15) in 1988‐1994 to 26.19% (95% CI, 24.32‐28.06) in 2007‐2014. In each survey cycle, the age‐standardized prevalence of NAFLD was substantially higher among Mexican American women, followed by non‐Hispanic whites and non‐Hispanic blacks. However, non‐Hispanic whites was the only group in which the prevalence of NAFLD increased from 19.28% (95% CI, 17.59‐20.98) in 1988‐1994 to 25.47% (95% CI, 23.24‐27.71) in 2007‐2014. In contrast, the prevalence of NAFLD among Mexican American women remained high but did not show a significant change over time.
Except for non‐Hispanic blacks, the age‐standardized prevalence of NAFLD was lower in female adults than male adults across the study cycles (Supporting Table S2).
CVD and High Risk for CVD in Female Subjects According to NAFLD
Age‐adjusted prevalence of CVD was 7.70% (95% CI, 6.83‐8.57) among women with NAFLD compared to 4.65% (95% CI, 4.16‐5.14) among those without NAFLD (P < 0.0001). Age‐adjusted prevalence of ASCVD ≥7.5% was 28.05% (95% CI, 26‐30.11) among women with NAFLD compared with 19.94% (95% CI, 19.42‐20.46) among those without NAFLD (P < 0.0001) (Table 3). The fully adjusted odds ratios (ORs) in female participants with NAFLD compared to those without NAFLD were 1.5 (95% CI, 1.21‐1.84) for CVD, 1.96 (95% CI, 1.47‐2.6) for ASCVD ≥7.5%, and 1.81 (95% CI, 1.41‐2.32) for either CVD or ASCVD ≥7.5% (Table 3).
Table 3.
Odds Ratios for CVD and ASCVD (≥7.5%) by NAFLD Status Among U.S. Female Adults, NHANES (1988‐2014)
| Age‐Adjusted Prevalence % (95% CI)* | Adjusted Odds Ratio (95% CI) | ||
|---|---|---|---|
| Model 1 † | Model 2 ‡ | ||
| CVD | |||
| No NAFLD | 4.65 (4.16‐5.14) | Reference | Reference |
| NAFLD | 7.70 (6.83‐8.57) § | 1.82 (1.54‐2.15) | 1.5 (1.21‐1.84) |
| ASCVD ≥7.5% | |||
| No NAFLD | 19.94 (19.42‐20.46) | Reference | Reference |
| NAFLD | 28.05 (26‐30.11) § | 3.08 (2.46‐3.86) | 1.96 (1.47‐2.6) |
| CVD or ASCVD ≥7.5% | |||
| No NAFLD | 21.04 (20.41‐21.67) | Reference | Reference |
| NAFLD | 29.66 (27.55‐31.77) § | 2.73 (2.23‐3.34) | 1.81 (1.41‐2.32) |
Age adjustment is based on the direct method to the census 2000 population, using the age groups 20‐44, 45‐64, and ≥65 years.
Model 1, adjusted for age and race.
Model 2, adjusted for Model 1 plus current smoker, MS, cancer, and COPD; due to multicollinearity between MS and metabolic components, the letters were excluded.
Significantly different from no NAFLD.
The 5‐Year Mortality From All Causes and CVDs According to NAFLD
The 5‐year all‐cause mortality, adjusted for age, was 3.49% (95% CI, 2.77‐4.2) among the female population with NAFLD compared to 2.51% (95% CI, 2.05‐2.98) among those without NAFLD. Age‐adjusted 5‐year CV mortality was 1.13% (95% CI, 0.68‐1.58) among female adults with NAFLD compared to 0.77% (95% CI, 0.53‐1.01) among those without NAFLD. The fully adjusted hazard ratios (HRs) in female adults with NAFLD compared to those without NAFLD were 1.5 (95% CI, 1.09‐2.07) for all‐cause mortality and 1.45 (95% CI, 0.78‐2.68) for CV mortality (Table 4).
Table 4.
Hazard Ratios of 5‐year Mortality by NAFLD Status Among U.S. Female Adults, NHANES (1988‐2014)
| Age‐Adjusted Mortality, % (95% CI)* | Hazard Ratio (95% CI) | |||
|---|---|---|---|---|
| Unadjusted | Model 1† | Model 2‡ | ||
| All‐cause mortality | ||||
| No NAFLD | 2.51 (2.05‐2.98) | Reference | Reference | Reference |
| NAFLD | 3.49 (2.77‐4.2) § | 2.57 (2.01‐3.29) | 1.43 (1.12‐1.81) | 1.5 (1.09‐2.07) |
| Cardiovascular mortality | ||||
| No NAFLD | 0.77 (0.53‐1.01) | Reference | Reference | Reference |
| NAFLD | 1.13 (0.68‐1.58) | 2.83 (1.72‐4.65) | 1.54 (0.96‐2.46) | 1.45 (0.78‐2.68) |
Age adjustment is based on the direct method to the census 2000 population, using the age groups 20‐44, 45‐64, and ≥65 years.
Model 1, adjusted for age and race.
Model 2, adjusted for Model 2 plus active smoker, income, metabolic syndrome, cancer, COPD, and ASCVD ≥7.5%; due to multicollinearity between MS and metabolic components, the letters were excluded.
Significantly different from no NAFLD.
Among female participants with NAFLD, those with higher ASCVD score (≥7.5%) had an increased risk of all‐cause mortality (HR, 514; 95% CI, 1.79‐14.72) and CV mortality (HR, 142.62; 95% CI, 17.36‐1171.5) (Table 5).
Table 5.
Hazard Ratios of 5‐year Mortality by ASCVD (≥7.5%) Among U.S. Female Adults With NAFLD, NHANES (1988‐2014)
| Age‐Adjusted Mortality, % (95% CI)* | Hazard Ratios (95% CI) | |||
|---|---|---|---|---|
| Unadjusted | Model 1† | Model 2‡ | ||
| All‐cause mortality | ||||
| NAFLD with ASCVD <7.5% | 1.18 (−0.08‐2.44) | Reference | Reference | Reference |
| NAFLD with ASCVD ≥7.5% | 4.95 (3.44‐6.47)§ | 15.92 (7.33‐34.59) | 5.68 (2.21‐14.58) | 5.14 (1.79‐14.72) |
| Cardiovascular mortality | ||||
| NAFLD with ASCVD <7.5% | 0.02 (0‐0.04) | Reference | Reference | Reference |
| NAFLD with ASCVD ≥7.5% | 1.83 (0.81‐2.85) | 151.56 (40.48‐567.45) | 55.42 (12.07‐254.5) | 142.62 (17.36‐1171.5) |
Age adjustment is based on the direct method to the census 2000 population, using the age groups 20‐44, 45‐64, and ≥65 years.
Model 1, adjusted for age and race.
Model 2, adjusted for Model 2 plus active smoker, income, metabolic syndrome, cancer, and COPD; due to multicollinearity between MS and metabolic components, the letters were excluded.
Significantly different from no NAFLD.
Discussion
This study presents the most in‐depth analysis of the prevalence, risks, and outcomes of female patients with NAFLD in the United States. Based on these data, there is clear evidence that the prevalence of NAFLD among the female population is rising and is fueled by the epidemic of metabolic diseases, such as T2DM and obesity.1 Furthermore, the relationship between older age and increased prevalence of NAFLD is confirmed in female subjects from the NHANES.29, 30 However, among the female population, various other factors, including alterations in body composition, fat distribution, hormonal and metabolic changes after menopause, as well as polycystic ovary disease, have been associated with NAFLD.31, 32, 33, 34 In fact, data suggest that estrogen appears to have a protective role. In this context, females with NAFLD seem to have lower levels of estradiol,34, 35 and receiving hormone replacement therapy may result in lower rates of NAFLD.36 However, given significantly higher rates of metabolic derangement and the increasing prevalence of obesity, it can be suggested that a change in the metabolic trends is a more important factor contributing to higher rates of NAFLD than other sex‐specific mechanisms. Moreover, the mean age of women with NAFLD has decreased over time from 54.6 years to 52 years, and the prevalence of NAFLD among younger patients (20‐44 years) has increased from 11% to 17%. This can be explained by the rising prevalence of obesity among young women in the United States.37 Again, our findings are in agreement with previous studies demonstrating higher rates of components of MS in patients with NAFLD.6, 38, 39, 40
Racial differences in the prevalence of NAFLD led to some interesting observations. Our data suggest that Mexican American female adults have a higher prevalence of NAFLD than non‐Hispanic black and non‐Hispanic white female adults. The cause for this has not been well established but may be related to genetic predisposition and a higher prevalence of components of MS. Recent data from the Centers for Disease Control and Prevention showed that in Hispanic women, the rate of obesity is 50.6%, hyperlipidemia is 41.2%, and diabetes is 11.7%, which places Hispanic women at significant risk for NAFLD.41, 42, 43 Additionally, there may be genetic predisposition for development and progression of NAFLD. In fact, Chincilla‐Lopez et al.44 have recently suggested that polymorphism of the patatin‐like phospholipase domain containing 3 (PNPLA3) gene may be more common in native Mexicans, and this association places these individuals at higher risk for NAFLD (OR, 1.711; 95% CI, 1.014‐2.886; P = 0.044). Despite these data, additional investigation is needed to elucidate the driving factors for this increased prevalence of NAFLD in the Mexican American female population.
Another important finding of our study is the assessment of long‐term mortality outcomes. Our data show that women with NAFLD have a significantly higher rate of 5‐year all‐cause mortality. Similarly, patients with NAFLD and an ASCVD score of >7.5% had an even higher risk for all‐cause and CV mortality. It is well known that, due to the shared pathophysiologic pathways and comorbidities, NAFLD has been strongly associated with CVD,45, 46, 47 and CVD is the leading cause of mortality among patients with NAFLD.13, 48, 49 Furthermore, it has been recently demonstrated that having an ASCVD score of >7.5 is an independent predictor of both overall and cardiac‐specific mortality in patients with NAFLD.50 Moreover, among patients with NAFLD, the presence of diabetes and existing heart disease was proven to increase CVD mortality.5 These data that have been shown for all patients with NAFLD or using data from tertiary care centers were confirmed for the female population using a population database.
A key limitation of our study is that we used a noninvasive test (US‐FLI) to identify NAFLD because hepatic ultrasound data were not always available in population‐based studies. Although US‐FLI correlates well with NAFLD, the gold standard remains liver biopsy or other sensitive radiologic tests. The recent public‐use mortality file provides follow‐up data on vital status by December 31, 2011; therefore, only six cycles from NHANES III (1988‐1994) to NHANES (2005‐2006) have at least 5 years of follow‐up. A longer follow‐up period is needed to establish an independent association of CVD mortality with NAFLD. Despite these limitations, this study includes a large cohort of female adults with NAFLD where in‐depth clinical and laboratory data are available and generalizes the results to the U.S. general population.
In conclusion, our data show that NAFLD is growing in the female population in the United States. In this context, NAFLD is associated with metabolic risk factors and increases the risk of CVD and mortality. This issue is important because CV diseases and mortality in female adults have been underemphasized. Our data suggest that detection of NAFLD in women is considered a serious risk threat that needs to be addressed to potentially improve their long‐term outcomes.
Potential conflict of interest
Dr. Younossi has received research funds from or served as consultant to Gilead Sciences, Intercept, Novo Nordisk, Bristol‐Myers Squibb AbbVie, Terns, and Viking.
Supporting information
References
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease‐meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 2. Angulo P. Nonalcoholic fatty liver disease. N Engl J Med 2002;346:1221‐1231. [DOI] [PubMed] [Google Scholar]
- 3. Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non‐alcoholic fatty liver disease. Metabolism 2016;65:1017‐1025. [DOI] [PubMed] [Google Scholar]
- 4. Perumpail BJ, Khan MA, Yoo ER, Cholankeril G, Kim D, Ahmed A. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol 2017;23:8263‐8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Golabi P, Otgonsuren M, de Avila L, Sayiner M, Rafiq N, Younossi ZM. Components of metabolic syndrome increase the risk of mortality in nonalcoholic fatty liver disease (NAFLD). Medicine (Baltimore) 2018;97:e0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yki‐Järvinen H. Non‐alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol 2014;2:901‐910. [DOI] [PubMed] [Google Scholar]
- 7. Diehl AM, Day C. Cause, pathogenesis, and treatment of nonalcoholic steatohepatitis. N Engl J Med 2017;377:2063‐2072. [DOI] [PubMed] [Google Scholar]
- 8. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol 2013;10:330‐344. [DOI] [PubMed] [Google Scholar]
- 9. Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the United States and the rest of the world. Clin Liver Dis 2016;20:205‐214. [DOI] [PubMed] [Google Scholar]
- 10. Kalia HS, Gaglio PJ. The prevalence and pathobiology of nonalcoholic fatty liver disease in patients of different races or ethnicities. Clin Liver Dis 2016;20:215‐224. [DOI] [PubMed] [Google Scholar]
- 11. Ballestri S, Nascimbeni F, Baldelli E, Marrazzo A, Romagnoli D, Lonardo A. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther 2017;34:1291‐1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stepanova M, Younossi ZM. Independent association between nonalcoholic fatty liver disease and cardiovascular disease in the US population. Clin Gastroenterol Hepatol 2012;10:646‐650. [DOI] [PubMed] [Google Scholar]
- 13. Ballestri S, Lonardo A, Bonapace S, Byrne CD, Loria P, Targher G. Risk of cardiovascular, cardiac and arrhythmic complications in patients with non‐alcoholic fatty liver disease. World J Gastroenterol 2014;20:1724‐1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc 1980;55:434‐438. [PubMed] [Google Scholar]
- 15. Centers for Disease Control and Prevention ; National Center for Health Statistics . National Health and Nutrition Examination Survey . Documentation files 1988‐1994, 1999‐2000, 2001‐2002, 2003‐2004, 2005‐2006, 2007‐2008, 2009‐2010, 2011‐2012, and 2013‐2014. https://www.cdc.gov/nchs/nhanes.htm. Accessed March 1, 2018.
- 16. Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 2015;41:65‐76. [DOI] [PubMed] [Google Scholar]
- 17. Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319‐327. [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention, National Center for Health Statistics . Analytic and reporting guidelines: The Third National Health and Nutrition Examination Survey, NHANES III (1988‐94). https://wwwn.cdc.gov/nchs/data/nhanes3/manuals/nh3gui.pdf. Published October 1996. Accessed December 26, 2017.
- 19. AACE Task Force for Developing a Diabetes Comprehensive Care Plan Writing Committee . American Association of Clinical Endocrinologists and American College of Endocrinology‐Clinical Practice Guidelines for Developing a Diabetes Mellitus Comprehensive Care Plan‐2015. Endocr Pract 2015;21(Suppl. 1):1‐87. https://www.aace.com/files/dm-guidelines-ccp.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. American Heart Association . High blood pressure. https://www.heart.org/en/health-topics/high-blood-pressure. Accessed October 2018.
- 21. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285:2486‐2497. [DOI] [PubMed] [Google Scholar]
- 22. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(Suppl. 2):S49‐S73. [DOI] [PubMed] [Google Scholar]
- 23. Wolter KM. Introduction to Variance Estimation. 2nd ed New York, NY: Springer; 2007. [Google Scholar]
- 24. Rao JNK, Scott AJ. The analysis of categorical data from complex sample surveys: chi‐squared tests for goodness of fit and independence in two‐way tables. J Am Stat Assoc 1981;76:221‐230. [Google Scholar]
- 25. Rao JNK, Scott AJ. On chi‐squared tests for multiway contingency tables with cell proportions estimated from survey data. Ann Stat 1984;12:46‐60. [Google Scholar]
- 26. National Center for Health Statistics . Office of Analysis and Epidemiology, NCHS 2011 linked mortality files matching methodology. https://www.cdc.gov/nchs/data/datalinkage/2011_linked_mortality_file_matching_methodology.pdf. Published September 2013. Accessed February 14, 2018.
- 27. National Center for Health Statistics . NCHS Surveys: 2011 linked mortality files: public‐use data dictionary. https://www.cdc.gov/nchs/data/datalinkage/Public_use_Data_Dictionary_23_2015.pdf. Updated February 24, 2015. Accessed January 10, 2018.
- 28. Allison PD. Survival Analysis Using SAS: A Practical Guide. Cary, NC: SAS Institute, Inc.; 1995. [Google Scholar]
- 29. Souza MR, Diniz Mde F, Medeiros‐Filho JE, Araújo MS. Metabolic syndrome and risk factors for non‐alcoholic fatty liver disease. Arq Gastroenterol 2012;49:89‐96. [DOI] [PubMed] [Google Scholar]
- 30. Bruno Ade S, Rodrigues MH, Alvares MC, Nahas‐Neto J, Nahas EA. Non‐alcoholic fatty liver disease and its associated risk factors in Brazilian postmenopausal women. Climacteric 2014;17:465‐471. [DOI] [PubMed] [Google Scholar]
- 31. Ryu S, Suh BS, Chang Y, Kwon MJ, Yun KE, Jung HS, et al. Menopausal stages and non‐alcoholic fatty liver disease in middle‐aged women. Eur J Obstet Gynecol Reprod Biol 2015;190:65‐70. [DOI] [PubMed] [Google Scholar]
- 32. Rodrigues MH, Bruno AS, Nahas‐Neto J, Sandrim VC, Muniz LG, Nahas EA. Evaluation of clinical and inflammatory markers of nonalcoholic fatty liver disease in postmenopausal women with metabolic syndrome. Metab Syndr Relat Disord 2014;12:330‐338. [DOI] [PubMed] [Google Scholar]
- 33. Romanowski MD, Parolin MB, Freitas AC, Piazza MJ, Basso J, Urbanetz AA. Prevalence of non‐alcoholic fatty liver disease in women with polycystic ovary syndrome and its correlation with metabolic syndrome. Arq Gastroenterol 2015;52:117‐123. [DOI] [PubMed] [Google Scholar]
- 34. Suzuki A, Abdelmalek MF. Nonalcoholic fatty liver disease in women. Womens Health (Lond) 2009;5:191‐203. [DOI] [PubMed] [Google Scholar]
- 35. Gutierrez‐Grobe Y, Ponciano‐Rodríguez G, Ramos MH, Uribe M, Méndez‐Sánchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, postmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol 2010;9:402‐409. [PubMed] [Google Scholar]
- 36. Chen KL, Madak‐Erdogan Z. Estrogens and female liver health. Steroids 2018;133:38‐43. [DOI] [PubMed] [Google Scholar]
- 37. National Center for Health Statistics . Health, United States, 2016: With Chartbook on Long‐Term Trends in Health. Hyattsville, MD: National Center for Health Statistics; 2017. [PubMed] [Google Scholar]
- 38. Dietrich P, Hellerbrand C. Non‐alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol 2014;28:637‐653. [DOI] [PubMed] [Google Scholar]
- 39. Tarantino G, Finelli C. What about non‐alcoholic fatty liver disease as a new criterion to define metabolic syndrome? World J Gastroenterol 2013;19:3375‐3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lim HW, Bernstein DE. Risk factors for the development of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis, including genetics. Clin Liver Dis 2018;22:39‐57. [DOI] [PubMed] [Google Scholar]
- 41. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015‐2016. NCHS Data Brief 2017;288. https://www.cdc.gov/nchs/data/databriefs/db288.pdf. [PubMed]
- 42. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation 2017;135:e146‐e603. Erratum in: Circulation 2017;135:e646 and Circulation 2017;136:e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Centers for Disease Control and Prevention . National diabetes statistics report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2017. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. [Google Scholar]
- 44. Chinchilla‐López P, Ramírez‐Pérez O, Cruz‐Ramón V, Canizales‐Quinteros S, Domínguez‐López A, Ponciano‐Rodríguez G, et al. More evidence for the genetic susceptibility of Mexican population to nonalcoholic fatty liver disease through PNPLA3. Ann Hepatol 2018;17:250‐255. [DOI] [PubMed] [Google Scholar]
- 45. Lonardo A, Sookoian S, Chonchol M, Loria P, Targher G. Cardiovascular and systemic risk in nonalcoholic fatty liver disease‐atherosclerosis as a major player in the natural course of NAFLD. Curr Pharm Des 2013;19:5177‐5192. [PubMed] [Google Scholar]
- 46. Bhatia LS, Curzen NP, Byrne CD. Nonalcoholic fatty liver disease and vascular risk. Curr Opin Cardiol 2012;27:420‐428. [DOI] [PubMed] [Google Scholar]
- 47. Mellinger JL, Pencina KM, Massaro JM, Hoffmann U, Seshadri S, Fox CS, et al. Hepatic steatosis and cardiovascular disease outcomes: an analysis of the Framingham Heart Study. J Hepatol 2015;63:470‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Younossi ZM, Otgonsuren M, Venkatesan C, Mishra A. In patients with non‐alcoholic fatty liver disease, metabolically abnormal individuals are at a higher risk for mortality while metabolically normal individuals are not. Metabolism 2013;62:352‐360. [DOI] [PubMed] [Google Scholar]
- 49. Stepanova M, Rafiq N, Makhlouf H, Agrawal R, Kaur I, Younoszai Z, et al. Predictors of all‐cause mortality and liver‐related mortality in patients with non‐alcoholic fatty liver disease (NAFLD). Dig Dis Sci 2013;58:3017‐3023. [DOI] [PubMed] [Google Scholar]
- 50. Golabi P, Fukui N, Otgonsuren M, deAvila L, Sayiner M, Younossi ZM. Can atherosclerotic cardiovascular risk score (ASCVD) predict mortality in patients with non‐alcoholic fatty liver disease (NAFLD)? J Hepatol 2017;66(Suppl):S598. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


