Abstract
Nonalcoholic fatty liver disease (NAFLD) is an emerging entity, becoming the most prevalent pediatric chronic liver disease. Its broad spectrum of histological findings, comorbidities, and complications, including cirrhosis and liver failure, can occur in childhood, emphasizing the severity of pediatric NAFLD. Current lifestyle and diet modifications have been linked to the increasing prevalence of NAFLD, including the rise of fructose consumption, a monosaccharide present in foods that contain added sugar, such as sugar‐sweetened beverages. Excessive fructose consumption is believed to cause addiction like alcohol and other drugs. As such, the new term “fructoholism” refers to the consumption of a substance (fructose) that can cause psychological and physical damage and become a major public health concern, highlighting the seriousness of the excessive consumption of fructose in the pediatric age. Hepatic fructose metabolization leads to hepatic steatosis and progression to fibrosis through mechanisms comparable to alcoholic liver disease, hence the term “fructoholic liver disease.” Conclusion: The importance of implementing reliable global strategies, such as education campaigns to promote healthy diet, increasing taxes on foods that contain added sugars, subsidies to promote accessibility to fruit and vegetables, and strict food industry regulation to reduce sugar intake in children and adolescents, cannot be overemphasized.
Abbreviations
- DA
dopamine
- DNL
de novo lipogenesis
- FA
fatty acid
- HCC
hepatocellular carcinoma
- NAFLD
nonalcoholic fatty liver disease
- and NASH
nonalcoholic steatohepatitis
During the past century, modifications in lifestyle and nutrition have contributed to an increase in noncommunicable diseases. Nonalcoholic fatty liver disease (NAFLD) has emerged as the most prevalent chronic liver disease, paralleling the worldwide increase of obesity, in the pediatric age.1, 2 NAFLD includes a spectrum of hepatic histological alterations, which are diagnosed based on the presence of at least 5% of hepatocytes with fatty infiltration with no evidence of infection, metabolic or autoimmune disorder, or steatogenic drug or alcohol consumption.3, 4 NAFLD can progress to nonalcoholic steatohepatitis (NASH), which is histologically characterized by the presence of steatosis and lobular inflammation with hepatocyte damage.3, 4, 5 NAFLD can potentially evolve to fibrosis, end‐stage liver disease, and hepatocellular carcinoma (HCC).3, 4, 5 Today, it is recognized as the hepatic manifestation of metabolic syndrome, being linked to obesity, insulin resistance, dyslipidemia, and cardiovascular disease.6
Fructose is a monosaccharide that is present in foods that contain added sugar components and has been linked to the rising incidence of obesity, insulin resistance, and NAFLD in the pediatric population.7, 8 Its metabolism may parallel that of alcohol, with the production of similar subproducts and liver damage.9, 10 Fructose consumption has increased 300% in the past 20 years, and it may have an addictive effect similar to other drugs.11 Therefore, banning added sugars from children’s diets should be considered a public health priority.
The aim of this review is to provide evidence of the negative effect of high fructose consumption in childhood on future liver health, with many similarities to alcohol, and to propose strategies for its reduction and to support global health improvement in the pediatric age.
Natural History of Pediatric NAFLD
NAFLD, initially thought to be a disease of adulthood, is increasingly recognized as an emerging and harmful entity in the pediatric age.12 Biopsy is still the gold standard for diagnosis and accurate evaluation of fibrosis progression, which limits precise follow‐up due to its invasiveness and potential risk conjugated with the absence of impact in therapy management.13 Although NAFLD is the most common cause of elevated liver enzymes, alanine aminotransferase and aspartate aminotransferase cannot be considered a reliable method for diagnosis and follow‐up,4, 6 with several studies confirming all ranges of hepatic fibrosis in the presence of normal liver enzymes.1, 14, 15, 16
Some studies revealed that NAFLD might begin in utero. Neonates born to obese mothers have a higher prevalence of liver steatosis compared with newborns from normal weight mothers, which can be a sign of early metabolic programming for a future with the trend to metabolic syndrome.17, 18 Despite the difficulty of ascertaining the mean age of disease onset, most children are diagnosed in the second decade of life, with a slight male predominance.14, 19, 20 A retrospective study of autopsied children 2 to 19 years of age from 1993 to 2003 showed the presence of fatty liver in 13%, with an increased prevalence with age (0.7% for 2‐4 years old and 17.3% for 15‐19 years old).21 With the exponential increase in fructose consumption and pediatric obesity since this period, it is expected that this number might be higher today. Obesity is the main risk factor for pediatric NAFLD, with prevalence rising up to 80% in some countries.3, 12, 13, 15 Despite being more frequent in overweight children, NAFLD also occurs in children of normal weight.14
As described in adult cohorts, pediatric NAFLD has been associated with comorbidities affecting other organs, enhancing its multisystemic metabolic dysfunction.20 Comorbidities detected in childhood include cardiovascular disease (hypertension and left ventricular disfunction), metabolic impairment (low mineral bone density, insulin resistance with progression to type 2 diabetes mellitus, hypercholesterolemia, and hypertriglyceridemia), and pulmonary (obstructive sleep apnea) and psychosocial affection.19, 20 The latter should not be neglected, as it has a negative effect on a child’s quality of life and adherence to treatment.22 Feldestein et al. demonstrated that 83% of children with NAFLD have at least one characteristic of metabolic syndrome, and 29% have established metabolic syndrome (three or more features).19 Due to the broad spectrum of comorbidities associated with NAFLD, patients should be closely followed.
Recent data provide evidence of the heterogeneity of NAFLD progression in the pediatric age.19 The whole spectrum of NAFLD can be seen in childhood, from liver steatosis to cirrhosis, to liver failure and HCC.16 Although rare, rapid progression to cirrhosis and end‐stage liver disease can disclose within a few years, and cirrhosis at diagnosis has also been described in childhood.19, 20, 23, 24 Surprisingly, NAFLD has revealed to be more deleterious than hepatitis C virus (HCV) infection. Pediatric HCV progresses to cirrhosis in 1% to 4% of children during a period of 2 to 9 years, which contrasts with the rise of cirrhosis due to NAFLD.25, 26
In the United States, NAFLD is the second indication for liver transplantation in the adult population and is expected to become the leading cause in the upcoming years.27, 28 The recent increase in transplantation before the age of 40 may express the natural evolution of a childhood NAFLD onset.29 Moreover, taking into account the ongoing rise of fructose consumption and childhood obesity and its role in NAFLD, a significative number of children might need liver transplantation and at earlier ages if no effective measures are assumed.30, 31 NASH recurrence in allograft with requirement of retransplantation is well recognized and can occur within a few months after transplantation if no solid lifestyle modification is attempted.19, 20, 32, 33
In adults, NAFDL is clearly associated with HCC even without the presence of significant fibrosis.34 Despite its rarity in the pediatric age range, HCC has been described in a 7‐year‐old boy with NAFLD, and childhood obesity raises the risk of HCC in adulthood.20, 35 The increase in pediatric severe NAFLD could raise the incidence of HCC in the young adult in the near future, strongly affecting NAFLD outcome due to the low survival rate of HCC.36
Pediatric NAFLD is associated with a lower long‐term survival as compared with the general population of the same age and sex, with a 13.8‐fold higher mortality risk.19 In the pediatric age, it is not clear which patients have an augmented risk of progression and shorter survival, but the evidence of a more aggressive disease in childhood than previously thought emphasizes the importance of systematic follow‐up. Pediatric NAFLD is a serious condition that should earn the attention of all clinicians to implement a reliable strategy to reduce morbidity and mortality seen in this population.
Fructose as the Main Cause of NAFLD
Fructose is the major constituent of common sweeteners, such as sucrose (table sugar), high fructose corn syrup, and agave syrup, which consist of a mixture of fructose and glucose monosaccharides.30 Fructose consumption has risen uncontrollably over the past century, especially by ingestion of sugar‐sweetened beverages.30 It can also be found in fruits and honey, although with less noxious outcome than fructose in added sugar compounds due to its lower concentration and the presence of other constituents (primarily antioxidants and fibers) that alter its metabolic effect.30, 31 Added sugars account for 15% of overall energy intake in the Western diet and are even higher among adolescents.30 Despite the World Health Organization’s recommendation of reduction of free sugar intake to less than 10% of total calorie intake, US adolescents ingest an average of 94 g of added sugar per day (17.9% of energy intake).37, 38
Fructose and glucose have considerably different metabolic pathways. Fructose enters in portal circulation and is totally metabolized in the liver, whereas glucose is partially metabolized in the liver and tends to enter into systemic circulation.31 Fructose hepatic metabolization to fructose‐1‐phosphate by fructokinase in glycolysis induces a fast depletion of adenosine triphosphate and increase of adenosine monophosphate, which is converted to inosine monophosphate with acid uric production.31, 39, 40 This transient decrease of adenosine triphosphate does not occur with glucose metabolization and is the key to fructose‐mediated negative metabolic effects. One of those effects is intrahepatic lipid accumulation (steatosis) due to an increase in hepatic fatty acid (FA) synthesis from de novo lipogenesis (DNL), an increase in hepatic delivery of FAs, and a decrease in lipid clearance.30, 31 Dietary sugars have been shown to enhance hepatic DNL, a metabolic pathway that affects lipid and glucose regulation and induces insulin resistance, cardiovascular disease, and hepatic steatosis.8, 41 Hepatic fructose metabolism results in available substrate to DNL and enhancement of regulatory signals to promote hepatic lipid synthesis, leading to serum triglycerides rising after acute fructose ingestion.42 Additionally, chronic fructose intake leads to up‐regulation of the DNL path with continuing overexpression of key transcription factors.43 In patients with NAFLD, DNL was shown to be 3‐fold greater than in healthy individuals.44 Furthermore, high fructose intake with consequent adenosine triphosphate depletion and uric acid formation inhibits enoyl coenzyme A (CoA) hydratase activity with consequent decrease of FA beta‐oxidation, which leads to hepatic lipid accumulation and gluconeogenesis.30, 31 Oxidative stress, an important factor for liver steatosis progression to NASH, is induced by uric acid produced in fructose metabolism.31 Another potential mechanism of fatty liver induction is related to the interaction of fructose with gut microbiome. Fructose metabolism in the gut by intestinal fructokinase leads to tight‐junction disruption, resulting in increased gut permeability and endotoxins translocation into portal vein and, consequently, increased oxidative stress.45, 46 Children with NAFDL have been shown to have elevated serum endotoxins levels and different microbiome profile when compared with controls, with more significant endogenous alcohol production and generation of hepatic oxidative stress.47, 48
Fructose intake has a dose‐dependent correlation with NAFLD development and its progression to fibrosis in children and adolescents.30 A study including 271 obese adolescents with biopsy‐proven NAFLD showed that fructose consumption was significantly higher in patients with NASH compared with NAFLD, and patients from the first group had significantly higher uric acid levels.49 Short‐term fructose‐restricted diet in obese children has demonstrated to decrease liver fat, visceral fat and DNL, and improve insulin kinetics—regardless of the calories consumed.50 Furthermore, long‐term (6‐month) fructose restriction was associated with improvement of serum markers of liver dysfunction and cardiometabolic risk, with significant reduction of systolic blood pressure, alanine aminotransferase levels, Apolipoprotein‐B100, and homeostasis model assessment of insulin resistance.51 Adherence to the Mediterranean diet, characterized by high monounsaturated FAs and low fructose, was associated with a decrease in insulin resistance and liver inflammation in pediatric patients with biopsy‐proven NAFLD.52
NAFLD in childhood has been demonstrated to have different characteristics from adults, primarily concerning histological findings with a predominant periportal inflammation in the pediatric age.4 Dietary fructose consumption with consequent hyperuricemia has been shown to induce greater damage in the periportal zone than the perivenous zone.39
Sugar as a Cause of Addiction
Traditionally, the term “addiction” is reserved for drugs of abuse, such as cocaine, morphine, heroin, nicotine, and alcohol. Nevertheless, non–drug‐related addictions have been investigated recently, including gambling, sex, and food.53 Sugar addiction is a specific type of food addiction, probably occurring due to its palatability combined with its postingestive effects.54
Sugar addiction from the viewpoint of behavior and brain neurochemistry has been shown in animal models, in which feeding comportment during intermittent access to sugar solutions was studied, demonstrating similar addiction‐related behaviors caused by drugs of abuse, including bingeing, withdrawal syndrome, cravings, and cross‐sensitization.55 Rats’ preference for sugar reward over an addictive drug such as cocaine has been evidenced, even for rats that are cocaine‐dependent.56 These effects may occur due to the same neural circuits involved in these consummatory behaviors for drugs of abuse and sugar, causing dopaminergic, cholinergic, and opioid effects.55
Two neural pathways have been implicated in energy intake and reward in the brain: the homeostatic system, which regulates feeding based on energy need; and the hedonic system, which is involved in “reward” and pleasure effects.55 The ventral tegmental area and nucleus accumbens are the structures of the hedonic system implicated in the “reward” of food intake.57 The dopaminergic effect is caused by a dopamine (DA) increase in the nucleus accumbens; DA receptors may change its expression or availability for the substance inducing release of DA or decrease of DA reuptake, the effect of most substances of abuse.11, 58, 59 Sugar addiction also appears to be opioid‐mediated. After repeated exposure to sugar, rats exposed to naloxone, an opioid antagonist that chemically blocks the synaptic receptor, showed withdrawal symptoms similar to that observed in mice that are chronically exposed to opioids drugs, leading to anxiety and behavioral depression similar to nicotine or morphine.60, 61 This effect appears to be due to opioids’ modifications and concerns two neurochemical manifestations: extracellular DA decrease in the nucleus accumbens and acetylcholine release from the nucleus accumbens interneurons.62 The cerebral cholinergic system is related to DA and the opioids system, and is implicated in food and drug intake, decreasing satiety when extracellular acetylcholine concentration is high and DA is low.63, 64 All of these neurochemical findings, comparing the effects of sugar and drugs of abuse in the brain and behavior, may explain sugar dependency.
Fructose and Ethanol Similarities
Excessive alcohol intake is considered a drug of abuse, and its consumption entails a high‐risk factor for chronic liver disease pathogenesis, such as steatosis, cirrhosis, and HCC.65 In addition to their similar addictive effect, fructose and ethanol share analogies in liver metabolism (Fig. 1).9 They are both substrates for the DNL pathway, leading to FA synthesis, dyslipidemia, and hepatic steatosis.9 In the hepatocyte, ethanol is metabolized to acetaldehyde, which is transformed in acetic acid and then metabolized in acetyl‐CoA, which participates in FA synthesis through DNL.66 Ethanol also causes microsomal triglyceride transfer protein suppression, increasing very low‐density lipoprotein production and exportation, which contributes to hypertriglyceridemia and insulin resistance, as does fructose.9 In a comparable way as fructose metabolism, ethanol leads to reactive oxygen species production due to its hepatic metabolization to acetaldehyde.66, 67, 68 Consequently, this can promote hepatocellular damage as lipid peroxidation, fibrogenesis, and cirrhosis.67, 68, 69 Thus, metabolites from alcohol metabolism are comparable to those resulting of fructose metabolism, which leads to identical toxic cellular response and hepatocyte damage.
Figure 1.
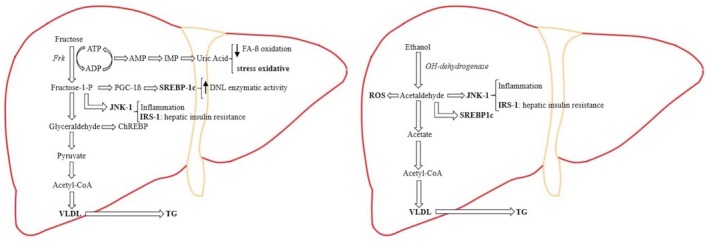
In hepatic fructose metabolism, the major part of fructose is metabolized directly to pyruvate. Due to the liver mitochondria cannot metabolizing the substrate excess, there is an overproduction of DNL and VLDL (very‐low‐density lipoprotein), which is exported out of the liver to contribute to fructose‐induced hypertriglyceridemia. Fructose induces substrate‐dependent phosphate depletion, which increases uric acid contributing to the decrease of FA ß‐oxidation, leading to intrahepatic lipid accumulation, and it is also primarily responsible for the oxidative stress. Fructose also stimulates the overexpression of key transcription factors, which lead to up‐regulation of DNL as ChREBP (carbohydrate response element binding protein) and PGC‐1ß (peroxisomal proliferator‐activated receptor‐γ coactivator‐1ß). PGC‐1ß is a transcriptional coactivator for SREBP‐1c (sterol regulatory element binding protein‐1c), which accentuates DNL enzymatic activity, and JNK‐1 (c‐jun N‐terminal kinase), which, once is induced, begins the inflammation cascade. As part of its inflammatory action, JNK‐1 activation induces IRS‐1 (insulin receptor substrate‐1), which promotes hepatic insulin resistance. In hepatic alcohol metabolism, ethanol is metabolized to acetaldehyde, which, because of its free aldehyde, can generate ROS (reactive oxygen species) formation and toxic damage. Furthermore, acetaldehyde stimulates SREBP‐1c, activating the enzymes of DNL and JNK‐1, promoting the inflammation process and hepatic insulin resistance by inducing IRS‐1. In the ethanol metabolization process there is an excess of the product intermediaries, which stimulates an overproduction of DNL, resulting in further intrahepatic lipid build‐up. By suppressing the activity of the protein that mediates the VLDL production, ethanol intake contributes to hypertriglyceridemia.
In conclusion, several data showed that fructose consumption is the primary cause of NAFLD, with a dose‐dependent effect leading to a pattern of liver injury comparable to alcohol ingestion. Additionally, it has been shown that excessive sugar intake may cause similar addiction behavior. Because of the negative effects of fructose described in children’s health, the authors suggest that excessive fructose consumption can be compared with alcohol intake in adults, leading to the term “fructoholism”—the consumption of a substance (fructose) that can cause psychological and physical damage, and fructoholic liver disease. Using these terms, the authors highlight the importance of taking seriously the fructose intake in the pediatric age and developing an active response to this problem.
Possible Strategies for Preventing Excessive Sugar Intake
It is imperative to adopt measures at different levels that could support the reduction of sugar intake in the population, especially in the youngest and most vulnerable community, such as children. Despite the existence of several ongoing clinical trials, there is no effective pharmacological treatment for pediatric NAFLD, with lifestyle intervention remaining the first line of treatment.70 Avoidance of sugar‐sweetened beverages, increase in daily physical activity, a healthy and well‐balanced diet, and limitations on screen time activities to fewer than 2 hours per day are among the most recommended ones.4 Moreover, to achieve successful lifestyle change, it is important to take into consideration family involvement and a solid interaction with primary care. The Mediterranean diet, consisting of vegetables, fruits, primarily unrefined cereals, olive oil and fish, has been studied as an appropriate therapeutic option for patients with NASH, because of its increase in monosaturated FAs and decrease in saturated fat and cholesterol, along with a high content of complex carbohydrates and fibers.52, 71
Finally, it is imperative to contemplate public policy measures and to raise awareness of public institutions about the importance of limiting fructose intake, as this is a real public health problem. The authors propose the following potential strategies, some of which have already been implemented successfully:
Food education at school to promote knowledge about nutrients and the proper combination of them to achieve a healthy diet, along with the importance of physical exercise.
Distribution of fruits and vegetables at schools to promote the benefits of a healthy diet and to encourage their consumption, which is already done in some countries of the European Union through the “EU school fruit, vegetable, and milk scheme” program.72
Promotion of a healthy diet through education campaigns and favoring its consumption using, for instance, vending machines in strategical places, such as train stations or airports.
Increasing taxes on foods that contain any form of added sugar (processed foods, juices, and soft drinks), like some countries have already done with foods high in saturated fat.73, 74 The World Health Organization, through the Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013‐2020, considers adding taxes and subsidies to promote accessibility to healthier food (with lower prices) and discourage the consumption of less healthy products.75
Regulation of the amount of sugars added by food industries as well as the regulation of the type of sugar they are allowed to use and discrimination of sugar quantity in all products.
In conclusion, NAFLD is an emerging entity in the pediatric age, becoming the most prevalent pediatric chronic liver disease. The increasing prevalence of NAFLD is linked to the rise of fructose consumption, which causes a comparable pattern of liver injury to alcohol consumption and is believed to have a similar addiction effect, named by the authors as “fructoholism,” potentially leading to “fructoholic liver diseases.” It is imperative to consider the importance of different approaches to limit sugar consumption and its health consequences and raise society’s awareness of this public health problem, especially in the youngest and most vulnerable population.
Potential conflict of interest
Dr. Sokal consults for, received grants from, owns intellectual property rights, is a board member for, is CMO for, and is employed by Promethera Biosciences.
References
- 1. Nasr P, Ignatova S, Kechagias S, Ekstedt M. Natural history of nonalcoholic fatty liver disease: a prospective follow‐up study with serial biopsies. Hepatol Commun 2018;2:199‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berardis S, Sokal E. Pediatric non‐alcoholic fatty liver disease: an increasing public health issue. Eur J Pediatr 2014;173:131‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vajro P, Lenta S, Socha P, Dhawan A, McKiernan P, Baumann U, et al. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN hepatology committee. J Pediatr Gastroenterol Nutr 2012;54:700‐713. [DOI] [PubMed] [Google Scholar]
- 4. Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohliet R, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2017;64:319‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005‐2023. [DOI] [PubMed] [Google Scholar]
- 6. Clemente MG, Mandato C, Poeta M, Vajro P. Pediatric non‐alcoholic fatty liver disease: recent solutions, unresolved issues, and future research directions. World J Gastroenterol 2016;22:8078‐8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdelmalek MF, Suzuki A, Guy C, Unalp‐Arida A, Colvin R, Johnson RJ, et al. Increased fructose consumption is associated with fibrosis severity in patients with NAFLD. Hepatology 2010;51:1961‐1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Horst KW, Schene MR, Holman R, Romijn JA, Serlie MJ. Effect of fructose consumption on insulin sensitivity in nondiabetic subjects: a systematic review and meta‐analysis of diet‐intervention trials. Am J Clin Nutr 2016;104:1562‐1576. [DOI] [PubMed] [Google Scholar]
- 9. Lustig RH. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc 2010;110:1307‐1321. [DOI] [PubMed] [Google Scholar]
- 10. Harrington S. The role of sugar‐sweetened beverage consumption in adolescent obesity: a review of the literature. J Sch Nurs 2014;24:3‐12. [DOI] [PubMed] [Google Scholar]
- 11. Avena NM, Long KA, Hoebel BG. Sugar‐dependent rats show enhanced responding for sugar after abstinence: evidence of a sugar deprivation effect. Physiol Behav 2005;84:359‐362. [DOI] [PubMed] [Google Scholar]
- 12. Nobili V, Socha P. Pediatric non‐alcoholic fatty liver disease: current thinking. J Pediatr Gastroenterol Nutr 2017;66:188‐192. [DOI] [PubMed] [Google Scholar]
- 13. Mouzaki M, Ling SC, Schreiber RA, Kamath BM. Management of pediatric nonalcoholic fatty liver disease by academic hepatologists in Canada: a nationwide survey. J Pediatr Gastroenterol Nutr 2017;65:380‐383. [DOI] [PubMed] [Google Scholar]
- 14. Akader HH, Henderson J, Vanhoesen K, Ghishan F, Bhattacharyya A. Nonalcoholic fatty liver disease in children: a single center experience. Clin Gastroenterol Hepatol 2008;6:799‐802. [DOI] [PubMed] [Google Scholar]
- 15. Corte CD, Ferrari F, Villani A, Nobili V. Epidemiology and natural history of NAFLD. J Med Biochem 2014;34:13‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Doycheva I, Watt KD, Alkhouri N. Nonalcoholic fatty liver disease in adolescents and young adults: the next frontier in the epidemic. Hepatology 2017;65:2100‐2109. [DOI] [PubMed] [Google Scholar]
- 17. Brumbaugh DE, Tearse P, Cree‐Green M, Fenton LZ, Brown M, Scherzinger A, et al. Intrahepatic fat is increased in neonatal offspring of obese women with gestational diabetes. J Pediatr 2013;162:930‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Modi N, Murgasova D, Ruager‐Martin R, Thomas L, Hyde M, Gale C, et al. The influence of maternal body mass index on infant adiposity and hepatic lipid content 2695. Pediatr Res 2011;70:287‐291. [DOI] [PubMed] [Google Scholar]
- 19. Feldstein AE, Charatcharoenwitthaya P, Treeprasertuk S, Benson JT, Enders FB, et al. The natural history of nonalcoholic fatty liver disease in children: follow‐up study for up to 20 years. Gut 2009;58:1538‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goyal NP, Schwimmer JB. The progression and natural history of pediatric nonalcoholic fatty liver disease. Clin Liver Dis 2017;20:325‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics 2006;118:1388‐1393. [DOI] [PubMed] [Google Scholar]
- 22. Kerkar N, D’Urso C, Van Nostrand K, Kochin I, Gault A, Suchy F, et al. Psychosocial outcomes for children with nonalcoholic fatty liver disease over time and compared with obese controls. J Pediatr Gastroenterol Nutr 2013;56:77‐82. [DOI] [PubMed] [Google Scholar]
- 23. Molleston JP, White F, Teckman J, Fitzgerald JF. Obese children with steatohepatitis can develop cirrhosis in childhood. Am J Gastroenterol 2002;97:2460‐2462. [DOI] [PubMed] [Google Scholar]
- 24. Kohli R, Boyd T, Lake K, Dietrich K, Nicholas L, Balistreri WF, et al. Rapid progression of NASH in childhood. J Pediatr Gastroenterol Nutr 2010;50:453‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khaderi S, Shepherd R, Goss JA, Leung DH. Hepatitis C in the pediatric population: transmission, natural history, treatment and liver transplantation. World J Gastroenterol 2014;20:11281‐11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Indolfi G, Hierro L, Dezsofi A, Jahnel J, Debray D, Hadzic N, et al. Treatment of chronic hepatitis C virus infection in children. A position paper by the Hepatology Committee of European Society of Paediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr 2018;66:505‐515. [DOI] [PubMed] [Google Scholar]
- 27. Wong RJ, Aguilar M, Cheung R, Perumpail RB, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148:547‐555. [DOI] [PubMed] [Google Scholar]
- 28. Vreman RA, Goodell AJ, Rodriguez LA, Porco TC, Lustig RH, Kahn JG. Health and economic benefits of reducing sugar intake in the USA, including effects via non‐alcoholic fatty liver disease: a microsimulation model. BMJ Open 2017;7:1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alkhouri N, Hanouneh IA, Zein NN, Lopez R, Kelly D, Eghtesad B, et al. Liver transplantation for nonalcoholic steatohepatitis (NASH) in children and young adults: the true burden of pediatric nonalcoholic fatty liver disease. Transpl Int 2016;29:418‐424. [DOI] [PubMed] [Google Scholar]
- 30. Jensen T, Abdelmalek MF, Sullivan S, Nadeau KJ, Green M, Roncal C, et al. Fructose and sugar: a major mediator of nonalcoholic fatty liver disease. J Hepatol 2018;68:1063‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ter Horst KW, Serlie MJ. Fructose consumption, lipogenesis, and non‐alcoholic fatty liver disease. Nutrients 2017;9:1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jankowska I, Socha P, Pawlowska J, Teisseyre M, Gliwicz D, Czubkowski P, et al. Recurrence of non‐alcoholic steatohepatitis after liver transplantation in a 13‐yr‐old boy. Pediatr Transplant 2007;11:796‐798. [DOI] [PubMed] [Google Scholar]
- 33. Malik HM, Devera ME, Fontes P, Shaikh O, Sasatomi E, Ahmad J. Recurrent disease following liver transplantation for nonalcoholic steatohepatitis cirrhosis. Liver Transplant 2009;15:1843‐1851. [DOI] [PubMed] [Google Scholar]
- 34. Lindenmeyer CC, McCullough AJ. The natural history of nonalcoholic fatty liver disease—an evolving view. Clin Liver Dis 2018;22:11‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nobili V, Alisi A, Grimaldi C, Liccardo D, Francalanci P, Monti L, et al. Non‐alcoholic fatty liver disease and hepatocellular carcinoma in a 7‐year‐old obese boy: coincidence or comorbidity? Pediatr Obes 2014;9:e99‐e102. [DOI] [PubMed] [Google Scholar]
- 36. Jemal A, Bray F, Ferlay J. Global cancer statistics: 2011. CA Cancer J Clin 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 37. World Health Organization . Guideline: Sugars Intake for Adults and Children. Geneva, Switzerland: World Health Organization; 2015. [PubMed] [Google Scholar]
- 38. Rodríguez LA, Madsen KA, Cotterman C, Lustig R. Added sugar intake and metabolic syndrome in US adolescents: cross‐sectional analysis of the National Health and Nutrition Examination Survey 2005–2012. Public Health Nutr 2016;19:2424‐2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abdullah E, Idris A, Saparon A. Liver zonation in children with NAFLD: associations with dietary fructose and uric acid concentrations. Liver Int 2018;38:1102‐1109. [DOI] [PubMed] [Google Scholar]
- 40. Abdelmalek MF, Lazo M, Horska A, Bonekamp S, Liplin EW, Balasubramanyam A, et al. Higher dietary fructose is associated with impaired hepatic ATP homeostasis in obese individuals with type 2 diabetes. Hepatology 2012;56:952‐960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sekkarie A, Welsh JA, Vos MB. Carbohydrates and diet patterns in nonalcoholic fatty liver disease in children and adolescents. Curr Opin Clin Nutr Metab Care 2018;21:283‐288. [DOI] [PubMed] [Google Scholar]
- 42. Herman M, Samuel V. The sweet path to metabolic demise: fructose and lipid synthesis. Trends Endocrinol Metab 2016;27:719‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Arden C, Tudhope SJ, Petrie JL, Al‐Oanzi ZH, Cullen K, Lange AJ, et al. Fructose 2,6‐bisphosphate is essential for glucose‐regulated gene transcription of glucose‐6‐phosphatase and other ChREBP target genes in hepatocytes. Biochem J 2012;44:111‐123. [DOI] [PubMed] [Google Scholar]
- 44. Lambert JE, Ramos‐Roman MA, Browning JD, Parks EJ. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology 2014;146:726‐735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johnson RJ, Rivard C, Lanaspa MA, Otabachian‐Smith S, Ishimoto T, Cicerchi C, et al. Fructokinase, fructans, intestinal permeability, and metabolic syndrome: an equine connection? J Equine Vet Sci 2013;33:120‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Konigsrainer A, et al. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr 2008;138:1452‐1455. [DOI] [PubMed] [Google Scholar]
- 47. Jin R, Willment A, Patel SS, Sun X, Song M, Mannery Y, et al. Fructose induced endotoxemia in pediatric nonalcoholic fatty liver disease. Int J Hepatol 2014;2014:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Michail S, Lin M, Frey MR, Fanter R, Paliy O, Hilbush B, et al. Altered gut microbial energy and metabolism in children with non‐alcoholic fatty liver disease. FEMS Microbiol Ecol 2015;91:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mosca A, Nobili V, De Vito R, Crudele A, Scorletti E, Villani A, et al. Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J Hepatol 2017;66:1031‐1036. [DOI] [PubMed] [Google Scholar]
- 50. Schwarz JM, Noworolski SM, Erkin‐Cakmak A, Korn NJ, Wen MJ, Tai VW, et al. Effects of dietary fructose restriction on liver fat, de novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology 2017;153:743‐752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mager DR, Iñiguez IR, Gilmour S, Yap J. The effect of a low fructose and low glycemic index/load (FRAGILE) dietary intervention on indices of liver function, cardiometabolic risk factors, and body composition in children and adolescents with nonalcoholic fatty liver disease (NAFLD). J Parenter Enteral Nutr 2015;39:73‐84. [DOI] [PubMed] [Google Scholar]
- 52. Della Corte C, Mosca A, Vania A, Alterio A, Iasevoli S, Nobili V. Good adherence to the Mediterranean diet reduces the risk for NASH and diabetes in pediatric patients with obesity: the results of an Italian study. Nutrition 2017;39‐40:8‐14. [DOI] [PubMed] [Google Scholar]
- 53. Meule A, Gearhardt AN. Food addiction in the light of DSM‐5. Nutrients 2014;6:3653‐3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Westwater ML, Fletcher PC, Ziaudddeen H. Sugar addiction: the state of the science. Eur J Nutr 2016;55:55‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 2008;32:20‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS ONE 2007;2:e698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron 2011;69:664‐679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Alonso‐Alonso M, Woods SC, Pelchat M, Grigson PS, Stice E, Farooqi S, et al. Food reward system: current perspectives and future research needs. Nutr Rev 2015;73:296‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Colantuoni C, Schwenker J, McCarthy J, Rada P, Ladenheim B, Cadet JL, et al. Excessive sugar intake alters binding to dopamine and mu‐opioid receptors in the brain. NeuroReport 2001;12:3549‐3552. [DOI] [PubMed] [Google Scholar]
- 60. Colantuoni C, Rada P, McCarthy J, Pattern C, Avena NM, Chadeayne A, et al. Evidence that intermittent, excessive sugar intake causes endogenous opioid dependence. Obes Res 2002;10:478‐488. [DOI] [PubMed] [Google Scholar]
- 61. Schulteis G, Yackey M, Risbrough V, Koob GF. Anxiogenic‐like effects of spontaneous and naloxone‐precipitated opiate withdrawal in the elevated plus‐maze. Pharmacol Biochem Behav 1998;60:727‐731. [DOI] [PubMed] [Google Scholar]
- 62. Rada P, Barson JR, Leibowitz SF, Hoebel BG. Opioids in the hypothalamus control dopamine and acetylcholine levels in the nucleus accumbens. Brain Res 2010;1312:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rada PV, Hoebel BG. Supraadditive effect of d‐fenfluramine plus phentermine on extracellular acetylcholine in the nucleus accumbens: possible mechanism for inhibition of excessive feeding and drug abuse. Pharmacol Biochem Behav 2000;65:369‐373. [DOI] [PubMed] [Google Scholar]
- 64. Avena NM, Rada P, Moise N, Hoebel BG. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience 2006;139:813‐820. [DOI] [PubMed] [Google Scholar]
- 65. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 66. Siler SQ, Neese RA, Hellerstein MK. De novo lipogenesis, lipid kinetics, and whole‐body lipid balances in humans after acute alcohol consumption. Am J Clin Nutr 1999;70:928‐936. [DOI] [PubMed] [Google Scholar]
- 67. Sozio M, Crabb DW. Alcohol and lipid metabolism. Am J Physiol Endocrinol Metab 2008;295:10‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ramirez T, Longato L, Dostalek M, Tong M, Wans JR, Monte SM. Insulin resistance, ceramide accumulation and endoplasmic reticulum stress in experimental chronic alcohol‐induced steatohepatitis. Alcohol Alcohol 2013;48:39‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Farfán Labonne BE, Gutiérrez M, Gómez‐Quiroz LE, Konigsberg Fainstein M, Bucio L, Souza V, et al. Acetaldehyde‐induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damage. Cell Biol Toxicol 2009;25:599‐609. [DOI] [PubMed] [Google Scholar]
- 70. Lassailly G, Caiazzo R, Pattou F, Mathurin P. Perspectives on treatment for nonalcoholic steatohepatitis. Gastroenterology 2016;150:1835‐1848. [DOI] [PubMed] [Google Scholar]
- 71. Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol 2018;24:2083‐2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. European Commission . School fruit, vegetables and milk scheme. https://ec.europa.eu/agriculture/school-scheme_en. Published May 31, 2018. Accessed August 4, 2018.
- 73. Blecher E. Taxes on tobacco, alcohol and sugar sweetened beverages: linkages and lessons learned. Soc Sci Med 2015;136‐137:175‐179. [DOI] [PubMed] [Google Scholar]
- 74. Smed S, Scarborough P, Rayner M, Jensen JD. The effects of the Danish saturated fat tax on food and nutrient intake and modelled health outcomes: an econometric and comparative risk assessment evaluation. Eur J Clin Nutr 2016;70:681‐686. [DOI] [PubMed] [Google Scholar]
- 75. World Health Organization . Global Action Plan for the Prevention and Control of Noncommunicable Diseases 2013‐2020. Geneva, Switzerland: World Health Organization; 2013:102. [Google Scholar]


