Abstract
Black patients have higher mortality and are less likely to receive liver transplantation for hepatocellular carcinoma (HCC) than white patients. Reasons for these disparities have not been fully elucidated. Comorbid disease, liver disease severity, cirrhosis etiologies, and tumor characteristics were compared between black and white patients with HCC seen at the Indiana University Academic Medical Center from January 2000 to June 2014. Logistic regression was used to investigate the primary outcome, which was liver transplantation. Log‐rank testing was used to compare survival between the two groups. Subgroup analysis explored reasons for failure to undergo liver transplantation in patients within Milan criteria. The cohort included 1,032 (86%) white and 164 (14%) black patients. Black and white patients had similar Model for End‐Stage Liver Disease (MELD) and Child‐Pugh scores (CPSs). There was a trend toward larger tumor size (5.3 cm versus 4.7 cm; P = 0.05) in black patients; however, Barcelona Clinic Liver Cancer (BCLC) staging and Milan criteria were similar. Black patients were less likely to undergo liver transplantation than white patients; this was a disparity that was not attenuated (odds ratio [OR], 0.43; 95% confidence interval [CI], 0.21‐0.90) on multivariable analysis. Substance abuse was more frequently cited as the reason black patients within Milan criteria failed to undergo transplantation compared to white patients. Survival was similar between the two groups. Conclusion: Racial differences in patient and tumor characteristics were small and did not explain the disparity in liver transplantation. Higher rates of substance abuse in black patients within Milan criteria who failed to undergo transplantation suggest social factors contribute to this disparity in this cohort.
Abbreviations
- AFP
alpha‐fetoprotein
- BCLC
Barcelona Clinic Liver Cancer
- BMI
body mass index
- CAD
coronary artery disease
- CDT
catheter‐directed therapy
- CI
confidence interval
- CPS
Child‐Pugh score
- DM
diabetes mellitus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- MELD
Model for End‐Stage Liver Disease
- NAFLD
nonalcoholic fatty liver disease
- OLT
orthotopic liver transplantation
- OR
odds ratio
- PVD
peripheral vascular disease
- SBRT
stereotactic body radiation therapy
- RFA
radio frequency ablation
- TNM
tumor‐node‐metastasis
The incidence of hepatocellular carcinoma (HCC) has more than doubled in recent decades, making it one of the fastest‐rising causes of cancer‐related mortality in the United States.1 Increases in both incidence and mortality have disproportionately impacted black patients,2 who have incidence rates twice that of their white counterparts.3 In a recent Surveillance, Epidemiology, and End Results (SEER) database study, the overall 5‐year survival rate for patients with HCC was lower among black patients than any other racial group.4
Analyses exploring racial disparities in HCC outcomes have failed to fully explain this disparity on multivariable analysis.5, 6, 7 Contributing factors that have been explored include late presentation, as black patients have been shown in some cohorts to be more likely to present with advanced tumor burden than white patients.4 Other analyses have suggested disparities in curative therapies contribute to the racial disparity in HCC mortality.8 Curative treatments for HCC include resection, ablation, and liver transplantation. However, only 20% of patients with HCC have sufficiently preserved liver function to undergo resection.9 Therefore, liver transplantation is often the treatment of choice to cure both HCC and the underlying liver disease. Despite this, Wong et al.8 found that from 2009 to 2010, of those potentially eligible for liver transplantation, only 11% of black patients underwent transplantation compared to 20% of white patients. Furthermore, even after adjusting for factors such as tumor stage, age, sex, treatment type, and income,5, 6, 7 black patients remained significantly more likely than white patients to die from HCC.
More than 80% of patients with HCC have underlying liver cirrhosis10; however, large cancer registry studies are limited in their ability to analyze liver disease etiology, severity, or comorbidities. The disparity seen in liver transplantation for HCC may be related to these patient‐specific factors. Therefore, in an effort to better understand racial disparities in liver transplantation in patients with HCC, we sought to conduct a detailed study to compare these factors between black and white patients seen over a 14.5‐year period at a tertiary care transplant center.
Patients and Methods
All adult patients with HCC seen at the Indiana University Academic Medical Center from January 2000 to June 2014 were identified using the Indiana State Cancer Registry. The study was reviewed and approved by the institutional review board at our institution and is compliant with ethical conduct of research. White and black patients with confirmed HCC by histologic and/or radiographic evidence were included in this study. Patients with fibrolamellar HCC or pure cholangiocarcinoma without combined HCC or belonging to other racial groups (n = 54) were excluded.
Clinical characteristics (including demographics and race), comorbidities, tumor characteristics, underlying liver disease etiologies, and treatment modalities received throughout the disease course were manually extracted from medical records. Chart documentation and laboratory data were used to judge the presence of medical comorbidities, including diabetes mellitus (DM), hypertension, hyperlipidemia, coronary artery disease (CAD), and peripheral vascular disease (PVD). Underlying liver disease etiologies were also collected, including hepatitis C virus (HCV), hepatitis B virus, alcoholic liver disease, nonalcoholic fatty liver disease (NAFLD), autoimmune liver diseases (autoimmune hepatitis, primary biliary cholangitis, and primary sclerosing cholangitis), and metabolic liver diseases (hemochromatosis and alpha‐1 antitrypsin deficiency). We defined NAFLD as a diagnosis assigned by the patient’s hepatologist based on metabolic risk factors or, in the absence of this documentation, evidence of hepatic steatosis, either by imaging or histology without an alternative liver disease etiology.11 The remaining patients with cryptogenic cirrhosis without evidence of NAFLD were classified as other.
History of alcohol abuse was defined as “a history of more than 3 drinks a day, documentation of alcoholism/alcohol abuse in a physician’s note, enrollment in a substance abuse treatment program, or history of alcoholic hepatitis.”12
Insurance information was obtained for each patient at the time of HCC diagnosis through the Regenstrief Institute.13 The Regenstrief Institute manages the Indiana Network for Patient Care, a large regional health information exchange that contains information on 17 million unique patients over a 30‐year period.13 Types of insurance were grouped into four categories: Medicare, Medicaid, private, and self‐pay.
The presence or absence of cirrhosis was assessed using criteria established by Mittal et al.12 Patients were unclassified if there were insufficient data in the medical records. Child‐Pugh score (CPS) and Model for End‐Stage Liver Disease (MELD) score were calculated only for patients with cirrhosis.
Tumor stage was defined using the tumor‐node‐metastasis (TNM) stage; histologic grade, presence/absence of Milan criteria,14 and the original Barcelona Clinic Liver Cancer (BCLC) classification were captured at the time of diagnosis.15 The size of the largest mass in centimeters was captured for each patient at the time of diagnosis.
The primary outcome was liver transplantation. The secondary outcome was combined liver transplantation, resection, or ablation. Other noncurative therapies, including catheter‐directed therapies (CDTs), stereotactic body radiation therapy (SBRT), sorafenib treatment, and palliative care referral, were compared between the two groups.
Survival data were ascertained from the Indiana Cancer Registry and the Regenstrief Institute at Indiana.13 The Regenstrief Institute links death data to patients in the Indiana Network for Patient Care, using a global match program. The overall survival period was computed using the date of HCC diagnosis and the date of death. For patients who were still alive or who died and their date of death was unknown, the date of last contact was used.
Statistical Analysis
Continuous variables are presented as mean ± SD or median with interquartile range and categorical variables as numbers and percentages. Chi‐square testing was used for comparison between different groups for discrete variables and independent samples, and Mann‐Whitney testing was used for continuous variables. A 2‐tailed test with P < 0.05 was considered statistically significant. For categorical variables with cells that had expected counts less than 5, Fisher’s exact test was used. The trends of HCC over a 14.5‐year period were studied in three intervals (2000‐2004, 2005‐2009, and 2010‐June 2014). The linear‐by‐linear progression P value was used for the time‐trend comparison between tested groups. Overall survival was calculated using the Kaplan‐Meier method and log‐rank test.
Logistic regression models were used to calculate odds ratio (OR) and 95% confidence interval (CI) for the primary outcome of liver transplantation and secondary combined curative outcome (liver transplantation, resection, or ablation), by race (black versus white). To attempt to attenuate any disparity, we adjusted for demographics, liver disease severity, and other covariates in a stepwise approach. An interaction was explored with insurance status and race and was not significant. A similar analysis was performed with matched propensity scores (4:1 match), using generalized linear mixed models to account for the matched groups, based on clinically relevant variables. Matched propensity scores were derived using the methods as outlined by Murphy and Fraeman.16
A subgroup analysis using the medical record and our liver transplant database to identify causes for failure to receive liver transplantation was then performed on patients who were within Milan criteria (potential liver transplant candidates) but did not undergo transplantation. Black potential candidates were matched by age (±3 years) and sex with white potential candidates in a 1:1 ratio. Chi‐square testing was then performed to compare the different causes of failure to receive a transplant between white and black patients.
All analyses were performed using Stata version 14.0 (StataCorp LLC, College Station, TX) and SPSS version 24.0 (IBM Corp., Armonk, NY).
Results
There were 1,032 (86%) white and 164 (14%) black patients in the cohort (Table 1). Over the three time intervals of the study (2000‐2004, 2005‐2009, 2010‐2014), the numbers of HCC cases did not differ between black (14%, 14%, 13%, respectively) and white (86%, 86%, 87%) patients (P = 0.68).
Table 1.
Comparison of Demographics and Clinical and Tumor Characteristics Between Black and White Patients With HCC.
| Black Patients (n = 164) | White Patients (n = 1,032) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age (years), mean (SD) | 59.7 (9.8) | 62.0 (10.3) | 0.005 |
| Male sex (%) | 70 | 74 | 0.33 |
| BMI (kg/m2), mean (SD) | 27.4 (6.3) | 29.0 (6.2) | 0.001 |
| Comorbidities (%) | |||
| Hypertension | 64 | 54 | 0.02 |
| DM | 37 | 38 | 0.78 |
| Dyslipidemia | 17 | 23 | 0.13 |
| CAD | 17 | 20 | 0.34 |
| PVD | 9 | 11 | 0.39 |
| Alcohol abuse | 59 | 42 | <0.001 |
| Liver disease characteristics | |||
| ALT (U/L), mean (SD) | 69 (54) | 73 (135) | 0.03 |
| AST (U/L), mean (SD) | 124 (110) | 111 (199) | <0.001 |
| Platelets (k/mm3), mean (SD) | 172 (111) | 142 (97) | <0.001 |
| Albumin (g/dL), mean (SD) | 3.1 (0.6) | 3.2 (0.7) | 0.23 |
| Total bilirubin (mg/dL), mean (SD) | 2.4 (3.4) | 2.3 (3.7) | 0.95 |
| MELD score, median (IQR) | 11 (7) | 11 (6) | 0.16 |
| Proportion Child‐Pugh class C (%)* | 21 | 20 | 0.79 |
| Liver cirrhosis (%) † | 92 | 88 | 0.22 |
| Tumor severity | |||
| Tumor size (cm), mean (SD) | 5.3 (3.8) | 4.7 (3.8) | 0.05 |
| AFP (ng/mL) (%) | 0.03 | ||
| <20 | 38 | 49 | |
| 20‐200 | 32 | 23 | |
| >200 | 30 | 29 | |
| Tumors within Milan criteria (%) | 42 | 48 | 0.19 |
| BCLC stage (%) | 0.60 | ||
| A | 25 | 24 | |
| B | 7 | 11 | |
| C | 47 | 44 | |
| D | 21 | 22 | |
| Tumor grade (%) | 0.09 | ||
| Well | 34 | 29 | |
| Moderate | 39 | 54 | |
| Poor | 27 | 17 | |
| Anaplastic | 0 | 1 | |
| Type of insurance (%) | 0.03 | ||
| Medicare | 38 | 46 | |
| Medicaid | 16 | 9 | |
| Private | 38 | 38 | |
| Self‐pay | 8 | 7 | |
| Treatment modalities received (%) | |||
| OLT | 14 | 26 | 0.001 |
| Palliative/hospice care | 31 | 20 | 0.001 |
| Surgical resection | 10 | 16 | 0.06 |
| CDT | 38 | 39 | 0.74 |
| SBRT | 15 | 12 | 0.26 |
| RFA | 2 | 3 | 0.61 |
| Sorafenib | 13 | 10 | 0.16 |
Abbreviations: AFP, alpha‐fetoprotein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; IQR, interquartile range; OLT, orthotopic liver transplantation; RFA, radio frequency ablation.
*CPS was only assessed for patients with cirrhosis.
†Unclassified cases excluded.
Demographics and Underlying Comorbidities
Black patients with HCC were significantly younger (59.7 years versus 62.0 years; P = 0.005) with lower body mass index (BMI) (27.4 kg/m2 versus 29.0 kg/m2; P = 0.001) than white patients. Black patients had more hypertension (64% versus 54%; P = 0.02) than white patients. Otherwise, there was no difference in the distribution of other comorbidities, including DM, dyslipidemia, CAD, and PVD, between the two groups (Table 1).
Underlying Liver Disease Etiology and Severity
Overall, viral hepatitis and alcoholic liver disease were the most common etiologies of liver disease in the cohort. HCV and/or alcohol were underlying liver disease etiology in 77% of the black patients compared to 49% of the white patients (P < 0.001). NAFLD was very rare in black patients; only 1% of black patients had NAFLD compared to 19% of white patients (P < 0.001) (Fig. 1). There were no significant differences in the occurrence of noncirrhotic HCC between black and white patients (8% versus 12%; P = 0.22) (Table 1).
Figure 1.
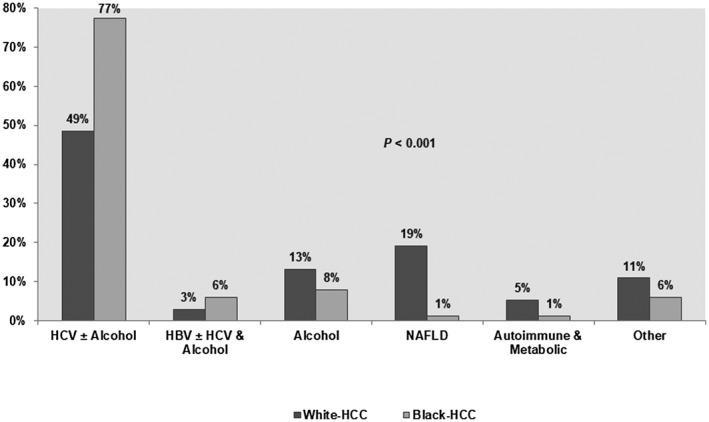
Distribution of liver disease etiologies among black and white patients. Abbreviation: HBV, hepatitis B virus.
Platelet count was significantly higher in black patients than white patients (172 k/mm3 versus 142 k/mm3; P < 0.001). Otherwise, black and white patients had similar MELD scores (median, 11 for both groups; P = 0.16) and CPS (21% versus 20% Child‐Pugh class C patients; P = 0.79) (Table 1).
Tumor Characteristics
There was a trend toward larger tumor size in black patients compared to white patients (5.3 cm versus 4.7 cm; P = 0.05); black patients also had higher alpha‐fetoprotein (AFP) levels (62% versus 52% had AFP >20 ng/mL; P = 0.02) than white patients. Although black patients presented with more advanced TNM stage, there was no difference in the distribution of BCLC stages and histologic tumor differentiation between the groups. The numbers of patients within Milan criteria were similar between black (42%) and white (48%) patients (P = 0.19) (Table 1).
History of Alcohol Abuse and Insurance Status
Of the 1,145 patients with known insurance status, 45% had Medicare, 38% had private insurance, 10% had Medicaid, and 8% were self‐pay. White patients were more likely to have Medicare (46% versus 38%) than black patients, but the opposite was true for Medicaid (16% black versus 9% white; P = 0.03). Forty‐four percent of our study population had a history of alcohol abuse; however, alcohol abuse history was more prevalent in black patients than white patients (59% versus 42%; P < 0.001). Medicaid patients (63%) were significantly more likely than Medicare (35%), privately insured (48%), or self‐pay (17%) patients to have a history of alcohol abuse (P < 0.001).
Treatments and Outcomes
Black patients were significantly less likely to undergo transplantation compared to white patients (14% versus 26%; P = 0.001) and more likely to receive palliative and/or hospice care (31% versus 20%; P = 0.001). The proportion of patients who underwent resection or received CDT, SBRT, radio frequency ablation (RFA), or sorafenib at any point throughout their disease course was similar in both groups (Table 1).
On univariable analysis, black patients were less likely to undergo transplantation than white patients (OR, 0.46; 95% CI, 0.29‐0.73) (Table 2). The disparity was not attenuated when the following variables were added to the model in a stepwise approach: age, BMI, underlying comorbidities (OR, 0.35; 95% CI, 0.21‐0.60), liver disease etiology and severity (OR, 0.42; 95% CI, 0.23‐0.75), tumor characteristics (OR, 0.43; 95% CI, 0.22‐0.85), insurance status, and history of alcohol use (OR, 0.43; 95% CI, 0.21‐0.90). This analysis was repeated in a cohort limited to diagnosis from 2002 to 2014 (after introduction of the MELD score), and the disparity remained on univariable and multivariable analysis. On univariable analysis, black patients were also less likely to achieve the secondary combined curative outcome of liver transplantation, resection, or ablation (OR, 0.44; 95% CI, 0.30‐0.64). This disparity was also not attenuated on multivariable analysis (OR, 0.43; 95% CI, 0.23‐0.83) (Table 2). Results of a propensity score match were similar to the standard unadjusted and adjusted logistic regression models, with similar P values and point estimates for the OR and with only a slight increase in CI width (OR, 0.49; 95% CI, 0.27‐0.89).
Table 2.
MULTIVARIABLE ANALYSIS: COMPARISON OF PRIMARY OUTCOME TRANSPLANT AND SECONDARY OUTCOME TRANSPLANT, RESECTION, OR ABLATION BETWEEN BLACK AND WHITE PATIENTS
| Univariable Analysis | Multivariable Analysis † | |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Liver transplantation* | 0.46 (0.29‐0.73) | 0.43 (0.21‐0.90) |
| Liver transplantation, resection, or ablation* | 0.44 (0.30‐0.64) | 0.43 (0.23‐0.83) |
White race used as reference.
Multivariable analysis included age, BMI, comorbidities (hypertension, CAD, and PVD), liver disease severity (CPS and liver cirrhosis), liver disease etiologies, tumor characteristics (tumor size, Milan status, and BCLC), insurance, and history of alcohol abuse.
To further explore this disparity, a subgroup analysis was performed on patients with HCC within Milan criteria who did not undergo liver transplantation (potential liver transplant candidates). Sixteen out of 68 (24%) black patients and 208 out of 474 (44%) white patients within Milan criteria underwent transplantation (P = 0.001) (Fig. 2). Alcohol or drug abuse was the most common reason for not undergoing liver transplantation in both races (25 out of 88 patients; 28%), followed by medical comorbidities and older age (19%) (Table 3). Nearly 15% of patients within Milan criteria were never referred for liver transplantation, and 10% after referral reported they were not interested. Black potential liver transplant candidates were significantly more likely than white potential liver transplant candidates to be declined transplantation because of alcohol and drug abuse (39% versus 18%; P = 0.03). Although insurance status did not explain the disparity seen in liver transplantation on multivariable analysis, 42% of white potential liver transplant candidates with Medicaid underwent liver transplantation compared to 17% of black potential liver transplant candidates with Medicaid.
Figure 2.
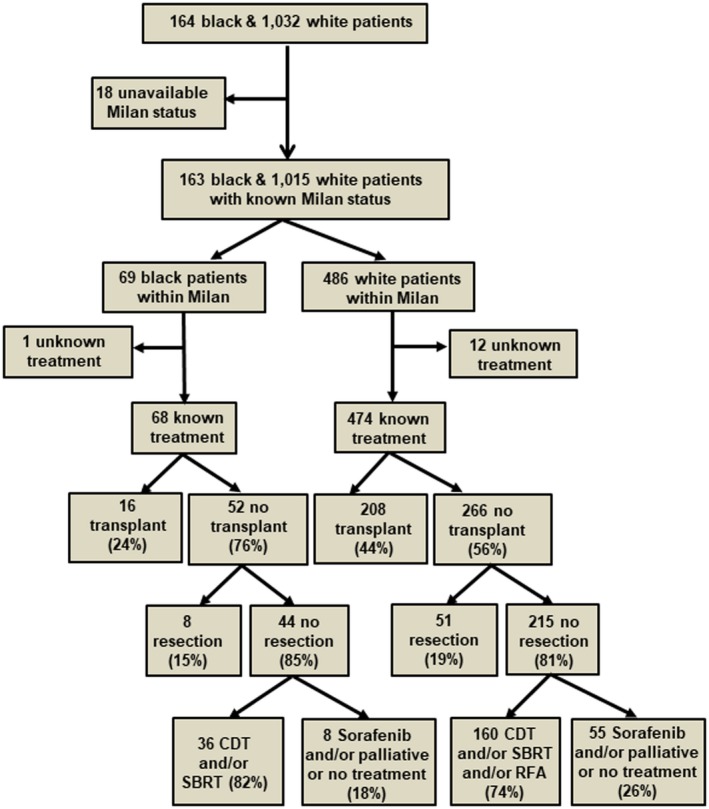
Flow diagram comparing the proportion of patients within Milan criteria who did or did not receive surgical resection/liver transplantation in both races.
Table 3.
Subgroup Analysis: Main Reasons for Not Receiving Liver Transplantation in Patients With Tumors Within Milan Criteria in Both Race Groups
| Cause | Black Patients (n = 44) | White Patients (n = 44) | P Value |
|---|---|---|---|
| Ongoing alcohol/drug abuse (n = 25) (%) | 39 | 18 | 0.03 |
| Comorbidities/malignancy/older age (n = 17) (%) | 21 | 18 | NS |
| Never referred (n = 13) (%) | 16 | 14 | NS |
| Not interested in OLT (n = 9) (%) | 5 | 16 | NS |
| Insurance/financial problems (n = 6) (%) | 7 | 7 | NS |
| Lost to follow‐up (n = 6) (%) | 5 | 9 | NS |
| Too sick/died before transplantation (n = 5) (%) | 0 | 11 | ‐ |
| Unknown/insufficient information (n = 5) (%) | 7 | 5 | NS |
| Noncompliance (n = 1) (%) | 2 | 0 | ‐ |
| Lack of social support (n = 1) (%) | 0 | 2 | ‐ |
Abbreviation: NS, not significant.
The 1‐year (54% versus 56%; P = 0.41), 3‐year (27% versus 32%; P = 0.20), and 5‐year (14% versus 20%; P = 0.07) survival rates were similar in black and white patients, respectively. When explored by transplant status, the 1‐year, 3‐year, and 5‐year survival rates between the two groups also did not differ (Table 4; Fig. 3).
Table 4.
Comparison of Survival Rates Between Black and White Patients With HCC
| Black Patients (n = 164) | White Patients (n = 1,032) | P Value | |
|---|---|---|---|
| Overall survival rates (%) | |||
| 1 year | 54 | 56 | 0.41 |
| 3 years | 27 | 32 | 0.20 |
| 5 years | 14 | 20 | 0.07 |
| Survival rates of patients who received OLT (%) | |||
| 1 year | 78 | 81 | 0.61 |
| 3 years | 68 | 61 | 0.48 |
| 5 years | 43 | 44 | 0.97 |
| Survival rates of patients who did not receive OLT (%) | |||
| 1 year | 48 | 39 | 0.21 |
| 3 years | 16 | 11 | 0.18 |
| 5 years | 5 | 4 | 0.19 |
Figure 3.
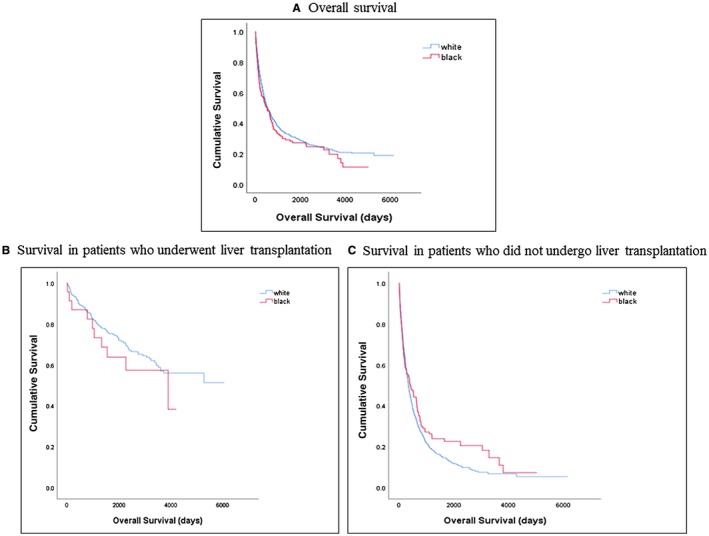
Kaplan‐Meier survival curves. (A) Overall survival. (B) Survival in patients who underwent liver transplantation. (C) Survival in patients who did not undergo liver transplantation.
Discussion
Black patients are disproportionately impacted by increasing incidence of and mortality from HCC. Reasons for this disparity are not clear, and a long‐term in‐depth review of a large cohort of black patients has been lacking in the literature. In this study, we sought to compare demographics, underlying comorbidities, liver disease etiologies, tumor characteristics, treatment modalities, and survival between white and black patients with HCC seen at a tertiary care transplant center, as these factors may explain the disparities that continue to be reported. Ultimately, our study found that black patients were significantly less likely to undergo transplantation than white patients and more likely to receive palliative care or hospice. They also had more hypertension and viral hepatitis, trended toward larger tumors, and were more likely to have Medicaid. These factors, however, did not explain the disparity in liver transplantation. Interestingly, despite being less likely to undergo transplantation, black patients seen at a tertiary care center had similar 1‐year, 3‐year, and 5‐year survival to white patients.
In our study, black patients with HCC were 54% less likely to undergo transplantation than white patients. This finding has been consistently reported in the last decade.6, 17, 18 In a recent SEER analysis of patients within Milan criteria under the age of 65 years, 20% of white patients underwent liver transplantation compared to 11% of black patients.8
Many explanations have been proposed for these disparities, including concerns that black patients present with more advanced diseases, more comorbid illnesses, or more severe liver disease. In our study, black patients were more likely to have hypertension and just as likely to have CAD or PVD, diseases that often preclude liver transplantation. They also trended toward larger tumors and more advanced TNM staging. However, the staging used to determine liver transplantation eligibility (BCLC and Milan criteria) was similar between the white and black patients. An analysis of 63 black patients seen at Columbia University found they had more advanced HCC, higher CPS, more comorbid disease,17 and poorer 1‐year, 3‐year, and 5‐year survival than white patients. In our study, 42% of black patients were within Milan criteria compared to only 23% in the cohort at Columbia.17 These data suggest that patients seen at our tertiary care center may be healthier and pre‐sent earlier than black patients seen at other transplant institutions. This may also reflect a referral bias, with only healthier black patients being referred to our center.
Conventional explanations for racial disparities in liver transplantation did not explain the disparity in our cohort. Although black patients had slightly larger tumor size and there was a complex relationship between race, insurance status, and receipt of liver transplantation, these factors did not attenuate the disparity. Furthermore, when comorbidities, liver disease etiology, and tumor severity were added to multivariable models, the differences in liver transplantation were not attenuated. This prompted a subgroup analysis that revealed a number of social factors prohibited potential candidates from undergoing liver transplantation. Thirty‐nine percent of black patients compared to 18% of white patients within Milan criteria had ongoing alcohol or drug abuse. Throughout the study period, our institution’s alcohol policy required 6 months of sobriety as well as completion of an alcohol treatment program for patients sober less than 2 years. Lack of referral, lack of insurance, financial issues, and lack of interest in liver transplantation were other factors that prohibited potential candidates from undergoing transplantation. These social factors appear to be pivotal in these potential candidates’ failure to undergo liver transplantation. The social determinants of health are the structural determinants and conditions in which people are born, grow, live, work, and age.19 They include factors such as socioeconomic status, the physical environment, employment, and social support networks as well as access to health care.19 The distributions of economic and social conditions among the population are known to influence individual and group differences in health status.20 Deficiencies in these areas may disproportionately affect black patients.20
Although our study identified disparities in liver transplantation, survival in black and white patients without liver transplantation was similar. There were few differences in comorbidity and disease severity in our cohort to explain black patients’ similar survival to white patients despite the disparity in liver transplantation. Black patients in our cohort, however, were more likely to receive palliative care. Palliative care has been shown to lead to less aggressive care at the end of life but longer survival in non‐small cell lung cancer.21 This is a provocative hypothesis for the similar survival between black patients and white patients in our cohort that will require further study.
Our study strength is that it contains comorbidities, underlying liver disease severity, and tumor characteristics on the largest cohort of black and white patients with HCC to date. We also have a transplant database that allows us to perform a detailed subgroup analysis on potential liver transplant candidates. This analysis revealed interesting hypothesis‐generating findings about reasons for failure to undergo transplantation. Our study is limited because it contains data from one academic medical center. However, our center is the only transplant center in the state and is located in the heart of metropolitan Indianapolis where more than 75% of HCC occurs in black patients. Nevertheless, after comparison to the state cancer registry, we estimated that we captured only approximately 25% of black patients with HCC. This referral practice highlights potential disparities in HCC care for black patients and warrants further study. It is likely that black patients who are referred and seen at our tertiary care transplant center compose a select subgroup within a more widely disadvantaged population of black patients with cirrhosis with HCC. Even in this select group, significant disparities in liver transplantation are still seen. Although treatments for HCC vary by center and our institutional practice for treatment of HCC trends toward resection and SBRT and less toward ablation, there were no differences seen in these therapies by race. Furthermore, this should not impact the disparity seen in liver transplantation.
Black and white patients had different etiologies of their underlying liver disease, but their liver disease severity was comparable. Differences in comorbidities and tumor severity were minor in this cohort. Black patients were significantly less likely to undergo liver transplantation, a disparity that could not be accounted for by comorbid illness, liver disease severity, tumor characteristics, or insurance status. Nevertheless, mortality was similar between black and white patients seen at a tertiary care transplant center. Subgroup analysis suggests that racial disparities in liver transplantation in a cohort referred to a tertiary care transplant center are largely driven by social factors that warrant further study.
Potential conflict of interest
Nothing to report.
Acknowledgments
We thank the Indiana State Cancer Registry and Dr. Julia Wattacheril (Columbia University) and Dr. Kathleen Corey (Harvard Medical School) for their assistance with the study methodology.
Support for publication was in part provided by the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award (grant numbers KL2 TR002530 and UL1 TR002529 to A.S.)
SEE EDITORIAL ON PAGE 5.
Contributor Information
Naga Chalasani, Email: nchalasa@iu.edu.
Lauren Nephew, Email: lnephew@iu.edu.
References
- 1. El‐Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology 2014;60:1767‐1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rich NE, Hester C, Odewole M, Murphy CC, Parikh ND, Marrero JA, et al. Racial and ethnic differences in presentation and outcomes of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2018;pii:S1542‐S3565:30571‐30578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. White DL, Thrift AP, Kanwal F, Davila J, El‐Serag HB. Incidence of hepatocellular carcinoma in all 50 United States, from 2000 through 2012. Gastroenterology 2017;152:e815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ha J, Yan M, Aguilar M, Tana M, Liu B, Frenette CT, et al. Race/ethnicity‐specific disparities in hepatocellular carcinoma stage at diagnosis and its impact on receipt of curative therapies. J Clin Gastroenterol 2016;50:423‐430. [DOI] [PubMed] [Google Scholar]
- 5. Stewart SL, Kwong SL, Bowlus CL, Nguyen TT, Maxwell AE, Bastani R, et al. Racial/ethnic disparities in hepatocellular carcinoma treatment and survival in California, 1988‐2012. World J Gastroenterol 2016;22:8584‐8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Siegel AB, McBride RB, El‐Serag HB, Hershman DL, Brown RS Jr, Renz JF, et al. Racial disparities in utilization of liver transplantation for hepatocellular carcinoma in the United States, 1998‐2002. Am J Gastroenterol 2008;103:120‐127. [DOI] [PubMed] [Google Scholar]
- 7. Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015;61:191‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the Surveillance, Epidemiology, and End Results registry. Liver Transpl 2014;20:528‐535. [DOI] [PubMed] [Google Scholar]
- 9. Corey KE, Pratt DS. Current status of therapy for hepatocellular carcinoma. Therap Adv Gastroenterol 2009;2:45‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Crissien AM, Frenette C. Current management of hepatocellular carcinoma. Gastroenterology Hepatol (N Y) 2014;10:153‐161. [PMC free article] [PubMed] [Google Scholar]
- 11. Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328‐357. [DOI] [PubMed] [Google Scholar]
- 12. Mittal S, El‐Serag HB, Sada YH, Kanwal F, Duan Z, Temple S, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2016;14:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Regenstrief Institute . https://www.regenstrief.org/. Accessed February 26, 2018.
- 14. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693‐699. [DOI] [PubMed] [Google Scholar]
- 15. Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 1999;19:329‐338. [DOI] [PubMed] [Google Scholar]
- 16. Murphy B, Fraeman KH. A general SAS ® macro to implement optimal N: 1 propensity score matching within a maximum radius. https://docplayer.net/54384962-A-general-sas-macro-to-implement-optimal-n-1-propensity-score-matching-within-a-maximum-radius.html. Published 2016. Accessed August 2018.
- 17. Yu JC, Neugut AI, Wang S, Jacobson JS, Ferrante L, Khungar V, et al. Racial and insurance disparities in the receipt of transplant among patients with hepatocellular carcinoma. Cancer 2010;116:1801‐1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nguyen GC, Thuluvath PJ. Racial disparity in liver disease: biological, cultural, or socioeconomic factors. Hepatology 2008;47:1058‐1066. [DOI] [PubMed] [Google Scholar]
- 19. Braveman P, Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep 2014;129(Suppl. 2):19‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Walker RJ, Strom Williams J, Egede LE. Influence of race, ethnicity and social determinants of health on diabetes outcomes. Am J Med Sci 2016;351:366‐373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non‐small‐cell lung cancer. N Engl J Med 2010;363:733‐742. [DOI] [PubMed] [Google Scholar]


