Abstract
Patients with cirrhosis are growing older, which could have an impact on brain dysfunction beyond hepatic encephalopathy. Our aim was to study the effect of concomitant aging and cirrhosis on brain inflammation and degeneration using human and animal experiments. For the human study, age‐matched patients with cirrhosis and controls between 65 and 85 years underwent cognitive testing, quality of life (QOL) assessment, and brain magnetic resonance (MR) spectroscopy and resting state functional MR imaging (rs‐fMRI) analysis. Data were compared between groups. For the animal study, young (10‐12 weeks) and old (1.5 years) C57BL/6 mice were given either CCl4 gavage to develop cirrhosis or a vehicle control and were followed for 12 weeks. Cortical messenger RNA (mRNA) expression of inflammatory mediators (interleukin [IL]‐6, IL‐1β, transforming growth factor β [TGF‐β], and monocyte chemoattractant protein 1), sirtuin‐1, and gamma‐aminobutyric acid (GABA)‐ergic synaptic plasticity (neuroligin‐2 [NLG2], discs large homolog 4 [DLG4], GABA receptor, subunit gamma 1/subunit B1 [GABRG1/B1]) were analyzed and compared between younger/older control and cirrhotic mice. The human study included 46 subjects (23/group). Patients with cirrhosis had worse QOL and cognition. On MR spectroscopy, patients with cirrhosis had worse changes related to ammonia and lower N‐acetyl aspartate, whereas rs‐fMRI analysis revealed that these patients demonstrated functional connectivity changes in the frontoparietal cortical region compared to controls. Results of the animal study showed that older mice required lower CCl4 to reach cirrhosis. Older mice, especially with cirrhosis, demonstrated higher cortical inflammatory mRNA expression of IL‐6, IL‐1β, and TGF‐β; higher glial and microglial activation; and lower sirtuin‐1 expression compared to younger mice. Older mice also had lower expression of DLG4, an excitatory synaptic organizer, and higher NLG2 and GABRG1/B1 receptor expression, indicating a predominantly inhibitory synaptic organization. Conclusion: Aging modulates brain changes in cirrhosis; this can affect QOL, cognition, and brain connectivity. Cortical inflammation, microglial activation, and altered GABA‐ergic synaptic plasticity could be contributory.
Abbreviations
- CI
confidence interval
- DAN
dorsal attention network
- DLG4
discs large homolog 4
- ELISA
enzyme‐linked immunosorbent assay
- GABA
gamma‐aminobutyric acid
- GABRG1/B1
gamma‐aminobutyric acid receptor, subunit gamma 1/subunit B1
- GFAP
glial fibrillary acidic protein
- HE
hepatic encephalopathy
- IBA1
ionized calcium binding adaptor molecule 1
- IL
interleukin
- MCI
mild cognitive impairment
- MCP1
monocyte chemoattractant protein 1
- MRI
magnetic resonance imaging
- mRNA
messenger RNA
- NAA
N‐acetyl aspartate
- NLGN2
neuroligin‐2
- PHES
psychometric hepatic encephalopathy score
- QOL
quality of life
- rs‐fMRI
resting state functional magnetic resonance imaging
- RSN
resting state network
- SIP
Sickness Impact Profile
- TGF‐β
transforming growth factor β
- VAN
ventral attention network
- VCU
Virginia Commonwealth University
Patients with cirrhosis are growing older, and this changing disease demographic now includes patients with greater comorbid conditions.1, 2 Most changes of altered cognition in patients with cirrhosis are due to the sequelae of hepatic encephalopathy (HE). However, with increasing age, factors independent of liver disease that can affect cognition need to be evaluated.3 A prior study has evaluated the role of an altered gut–liver–brain axis in patients with early stages of the disease, which could be related to inflamm‐aging.4, 5 There are several processes in the brain related to neuroinflammation, aging, gamma‐aminobutyric acid (GABA) physiology, and synaptic plasticity and neuronal differentiation that could synergize in the pathogenesis of the consequences of cirrhosis in the aging brain.5, 6, 7, 8 Specifically, the role of sirtuins, especially sirtuin‐1, which are nicotinamide adenine dinucleotide‐dependent enzymes linked with protection against dementia, are being increasingly recognized as modulators of brain dysfunction with aging.9 However, their role in cirrhosis‐associated aging is unclear. These multiple concomitant processes need a translational approach with human experiences studied in the context of specific brain‐related changes in animal models of cirrhosis to improve insight. Our aim was to study the effect of concomitant aging and cirrhosis on brain inflammation and degeneration using human and animal experiments.
Materials and Methods
Human Study
Subjects
We enrolled outpatients with cirrhosis between 65 and 85 years of age and age‐matched patients without cirrhosis or chronic diseases to serve as their controls after obtaining written informed consent. Cirrhosis was diagnosed using liver biopsy, radiologic evidence of a nodular liver or portal hypertension, or endoscopic evidence of varices in the setting of chronic liver disease. We excluded patients who were not able to consent; those on psychoactive drugs or with recent alcohol/illicit drug use (within 3 months); those with prior or current history of or treatment for overt HE, dementia, or other neurologic diseases; those with end‐stage organ failure or recent hospitalization (<1 month); and those who had contraindications for magnetic resonance imaging (MRI). Controls were age‐matched subjects without chronic diseases, not on any psychoactive medications, without organ failures, without chronic liver disease or alcohol/illicit drug abuse, and able to give consent and tolerate the MRI. Controls were recruited by word of mouth or through community advertisements.
Cognitive and Quality of Life Testing
Both groups underwent cognitive testing using psychometric hepatic encephalopathy score (PHES) and EncephalApp Stroop, along with quality of life (QOL) assessment using the Sickness Impact Profile (SIP).10, 11, 12 PHES consists of five tests; the SDs are compared against healthy controls, and the total sum is added. A low total score indicates poor performance. EncephalApp Stroop has two sections, an easier Off state, where the subject has to recognize the color of the pound or hashtag (#) signs appropriately and touch the screen at the corresponding color, and a more difficult On state, where the words meaning specific colors are presented in discordant colors. The time to complete five correct runs in each state is added with the total time, OffTime, OnTime, and the number of runs required to complete five states. A higher time required indicates poor performance. SIP is a 136‐question survey that inquires about health‐related changes over the last 24 hours. It has 12 dimensions and two overall domains (physical and psychosocial) along with a total score. A higher score indicates a poor QOL.
Brain MRI
Subsequently, all subjects underwent brain MRI for analysis of MR spectroscopy and resting state functional MRI (rs‐fMRI).13 MR spectroscopy focused on three voxels of interest in the brain: the right parietal white matter, posterior gray matter, and anterior cingulate cortex. Ammonia‐associated consequences in astrocytes manifest as higher creatine ratios of glutamate + glutamine and lower myoinositol and choline.13, 14, 15 On the other hand, N‐acetyl aspartate (NAA) is associated with neuronal integrity without the involvement of ammonia.16
Resting state networks (RSNs) were computed from rs‐fMRI data to determine which specific areas of the brain are functionally connected during rest when no activity is being performed.17, 18 Details of the MRI testing and analysis are in the Supporting Methods. The protocol was approved by the institutional review board at the Virginia Commonwealth University (VCU).
Animal Study
We performed a further analysis of specific brain‐related processes in animal studies that are not possible using human experiments in the MRI scanner or cognitive testing. Given the predominant changes in the frontal cortex in humans with cirrhosis and HE, we focused our analysis on this part of the mouse brain.18
In the animal study, we used two groups of C57BL/6 mice. The first group was between 10 and 12 weeks (young), and the second group was 1.5 years of age (old). Both groups were sourced from the Jackson Laboratory and underwent similar chow feeding, monitoring, and acclimatization at the VCU animal laboratories. Half of the young and old mice then underwent the development of cirrhosis using CCl4 gavage (1.0 mL/kg) twice a week for 12 weeks under careful monitoring per our prior protocols.19 The CCl4 model was used as a potential model for our human population with cirrhosis that was predominantly due to toxic‐inflammatory etiologies and did not use the bile duct ligation model, which reflects cholestatic cirrhosis etiologies. At week 12, the mice were killed and liver histology performed. The frontal cortices were extracted and evaluated for messenger RNA (mRNA) using real‐time quantitative polymerase chain reaction (Supporting Table S1) for (1) neuroinflammation (interleukin [IL]‐6, IL‐1β, monocyte chemoattractant protein 1 [MCP1], and transforming growth factor β [TGF‐β]); (2) glial/microglial activation (ionized calcium binding adaptor molecule 1 [IBA1] and glial fibrillary acidic protein [GFAP]); (3) synaptic plasticity (discs large homolog 4 [DLG4] and neuroligin‐2 [Nlgn2]); (4) aging (sirtuin‐1); and (5) GABA physiology (GABA receptor, subunit gamma 1 and subunit B1 [GABRG1, GABRB1]). Results were compared between older and younger mice with and without cirrhosis by using Kruskal‐Wallis and Mann‐Whitney tests with correction for multiple comparisons. Enzyme‐linked immunosorbent assay (ELISA) for cortical IL‐6 and MCP1 and western blot for IBA1 and sirtuin‐1 was also performed (details in supporting data). The protocol was approved by the Institutional Animal Care and Use Committee at VCU. All authors had access to the study data and reviewed and approved the final manuscript.
Results
Human Study
We included 46 subjects, 23 with cirrhosis and 23 without cirrhosis. There were no significant differences in demographics of the patients (Table 1). Patients with cirrhosis had a worse performance on the cognitive tests and QOL measures compared to their age‐matched controls. The mean Model for End‐Stage Liver Disease score in patients with cirrhosis was 9.3 ± 4.6, and none of the patients had a history of prior decompensation.
Table 1.
Characteristics Of Human Subjects
| No Cirrhosis (n = 23) | Cirrhosis (n = 23) | |
|---|---|---|
| Age (years) | 73.6 ± 5 | 70.1 ± 5 |
| Sex (% men) | 65% | 70% |
| Education (years) | 15.1 ± 2.9 | 14.8 ± 3.1 |
| Diabetes | 10 | 13 |
| Mini‐mental status (maximum 30) | 28.8 ± 1.2 | 28.4 ± 1.9 |
| EncephalApp Stroop (high time is worse) | ||
| Off time (seconds) | 85.4 ± 29.1 | 101.2 ± 26.7* |
| On time (seconds) | 105.6 ± 24.7 | 142.8 ± 29.5* |
| Median runs on | 5.0 | 6.0 |
| Median runs off | 6.0 | 6.0 |
| OffTime + OnTime (seconds) | 171.3 ± 32 | 228.6 ± 69* |
| PHES (low score is worse) | ||
| Number connection‐A (seconds) | 45.3 ± 25.0 | 54.8 ± 28.9 |
| Number connection‐B (seconds) | 102.7 ± 76.8 | 152.3 ± 69.0* |
| Digit symbol (raw score) | 54.7 ± 18.4 | 42.3 ± 14.5* |
| Line tracing test (seconds) | 107.4 ± 57.3 | 104.5 ± 45.4 |
| Line tracing errors | 32.4 ± 32.7 | 42.3 ± 29.9 |
| Serial dotting (seconds) | 56.5 ± 20.4 | 83.7 ± 28.3* |
| Median PHES | −1 | −5* |
| QOL SIP assessment (high is worse) | ||
| Total score | 1.4 ± 1.8 | 10.4 ± 16* |
| Psychosocial domain | 1.2 ± 3.5 | 9.3 ± 11.5* |
| Physical domain | 2.6 ± 5.5 | 10.4 ± 12.0* |
Data presented as mean ± SD unless mentioned otherwise. *P < 0.05 between groups on unpaired t tests or Mann‐Whitney tests as appropriate.
MRI Analysis
MR Spectroscopy
Patients with cirrhosis had a higher average glutamate + glutamine creatine ratio and a lower creatine ratio of choline and myoinositol compared to controls in most voxels of interest (Fig. 1A‐C). There was also a reduction in NAA concentrations in patients with cirrhosis compared to patients without cirrhosis (P = 0.01, all voxels).
Figure 1.
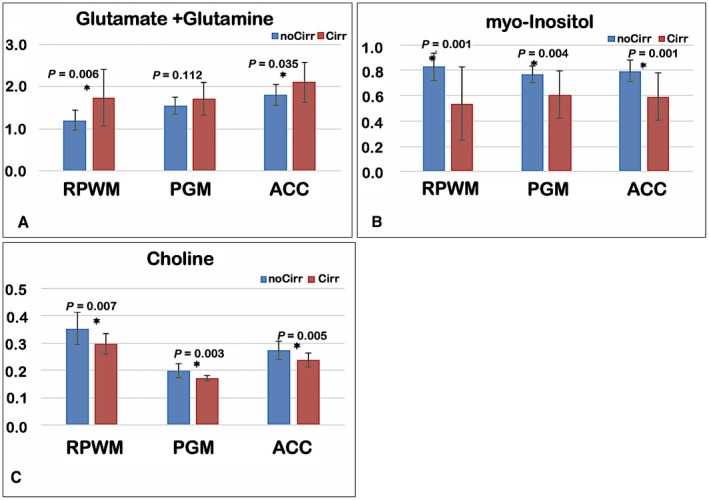
MR spectroscopic results. Creatine ratios of the three metabolites in patients with cirrhosis (red bars) and patients without cirrhosis (blue bars) are shown as mean ± SDs. P values of comparisons are displayed, with * indicating significant difference. (A) Glutamate + glutamine, (B) myo‐inositol, and (C) choline. There was a significantly higher glutamate + glutamine and lower myo‐inositol and choline in patients with cirrhosis compared to patients without cirrhosis (controls) at all sites except PGM glutamate + glutamine. Abbreviations: ACC, anterior cingulate cortex; Cirr, cirrhosis; NoCirr, without cirrhosis; PGM, posterior gray matter; RPWM, right parietal white matter.
RSN Analysis
We found lower functional connectivity in patients with cirrhosis compared to controls in two important networks detected by group‐independent component analysis (Fig. 2): visual RSN (right lingual gyrus, P = 0.016) and dorsal attention network (DAN)/ventral attention network (VAN) (left ventrolateral prefrontal cortex, P = 0.004; right ventrolateral prefrontal cortex, P = 0.04; and left frontoparietal operculum, P = 0.03).
Figure 2.
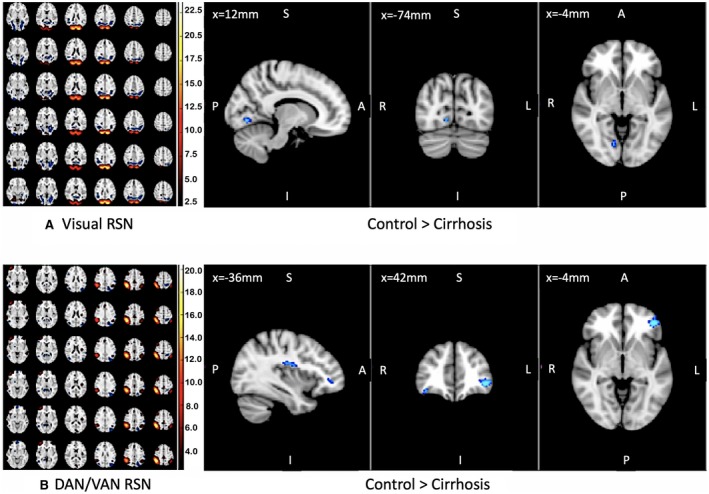
Resting state network analysis. Significant differences in visual and attentional RSNs in control compared to patients with cirrhosis are shown. On the left, we show group‐level RSNs corresponding to visual and attentional networks. Blue areas on the right correspond to areas of the brain that have greater functional connectivity to visual RSN (top) and attentional RSN (bottom) in controls compared to elderly patients with cirrhosis. None of the RSNs were higher in patients with cirrhosis compared to controls. Scale is Z‐transformed independent component coefficients.
Animal Study
We included at least six mice in each group. All younger mice assigned to the CCl4 group tolerated the gavage without any issues and were killed with the development of cirrhosis at week 12 (Supporting Fig. S1). On the other hand, the older mice showed symptoms of sickness with the dose of CCl4 used in the younger mice, and instead of gavaging them twice per week, we gavaged them once per week for 12 weeks. The exploratory movements of older mice on CCl4 were 60% less than those of the younger mice on CCl4. In addition, the older mice had a 50% reduction in grooming behavior and a 40% reduction in tactile feedback. After the spacing out of the gavages, the symptoms above resolved, and the mice were then killed at week 12 with the development of cirrhosis (Supporting Fig. S1). As expected, no behavioral changes were seen as is typical of the CCl4 model. The control mice in both younger and older mice were fed the same chow as their counterparts and administered CCl4 and were killed at week 12 without evidence of cirrhosis or steatosis in the studied livers (Supporting Fig. S1).
Neuroinflammation
Neuroinflammation was significantly higher in the older mice regardless of cirrhosis but was higher in older cirrhotic mice with respect to IL‐1β, TGF‐β, and IL‐6 mRNA expression. MCP1 expression was equally higher in both cirrhosis groups and was higher than controls (Fig. 3).
Figure 3.
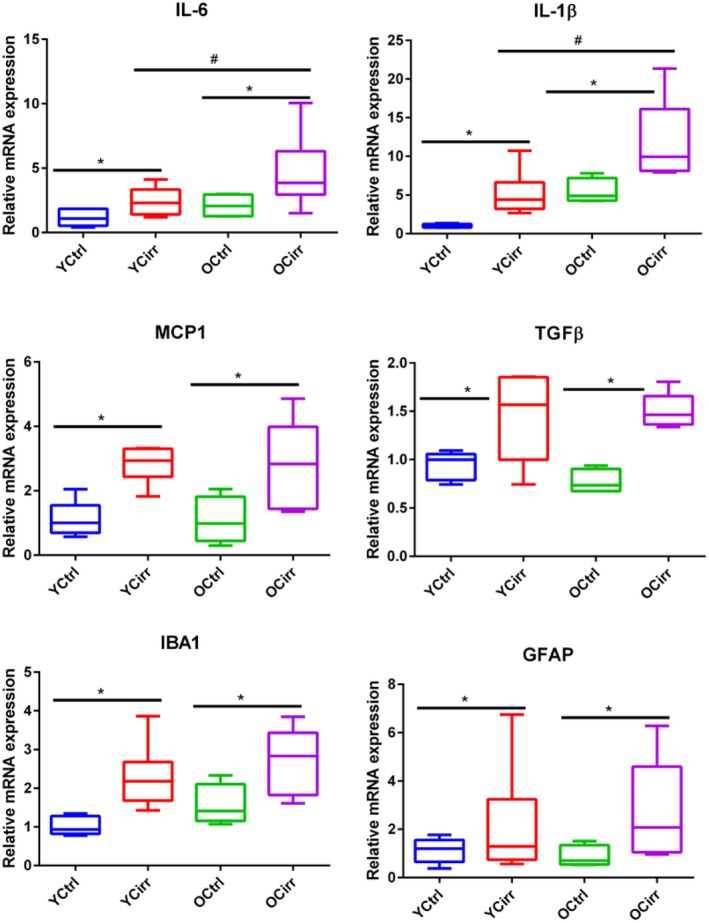
Cortical inflammation and glial/microglial activation. Comparisons among the four mouse groups. All data are presented as median and 95% confidence interval (CI). * indicates significant differences by the Mann‐Whitney test between cirrhotic and noncirrhotic mice in the same age group; # indicates significant differences by the Mann‐Whitney test between cirrhotic mice in different age groups. Abbreviations: OCirr, old cirrhosis; OCtrl, old control; YCirr, young cirrhosis; YCtrl, young control.
Glial and Microglial Activation
There was significantly higher GFAP and IBA expression in both cirrhotic groups compared to their noncirrhotic counterparts. In addition, IBA expression was higher in the older cirrhotic mice compared to the younger cirrhotic mice (Fig. 3). These changes were also reflected in ELISA for IL‐1β and MCP1 as well as the western blot for IBA1 (Fig. 4).
Figure 4.
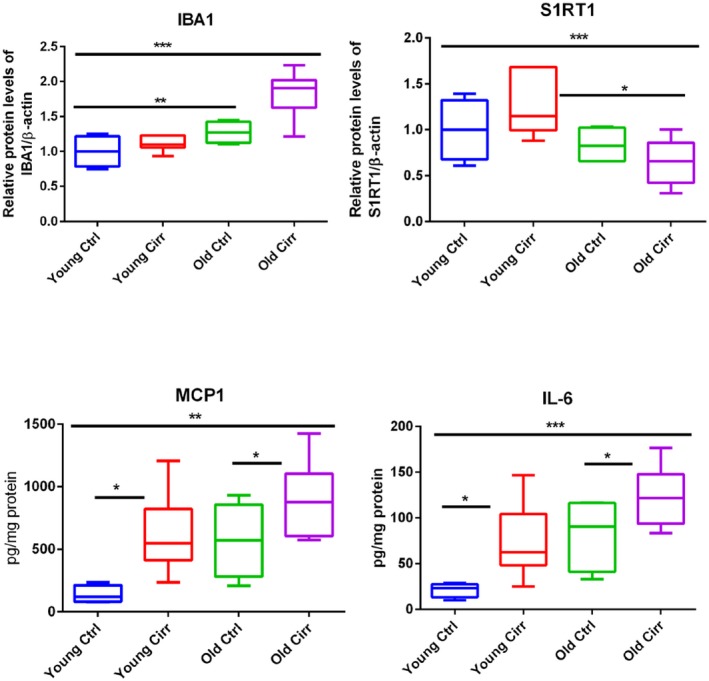
ELISA and western blot changes. Comparisons among the four mouse groups. Data presented in the graphs are median and 95% CI. *P < 0.05, **P < 0.01, ***P < 0.001 indicate significant differences by the Mann‐Whitney or Kruskal‐Wallis test. Abbreviations: Cirr, cirrhosis; Ctrl, control; S1RT1, sirtuin‐1.
Aging, Plasticity, and GABA
Sirtuin‐1 expression was lowest in older mice, similar to DLG4 expression. On the other hand, NLGN2 was higher in cirrhotic mice with higher expression of GABRG1 and GABRB1 (Fig. 5).
Figure 5.
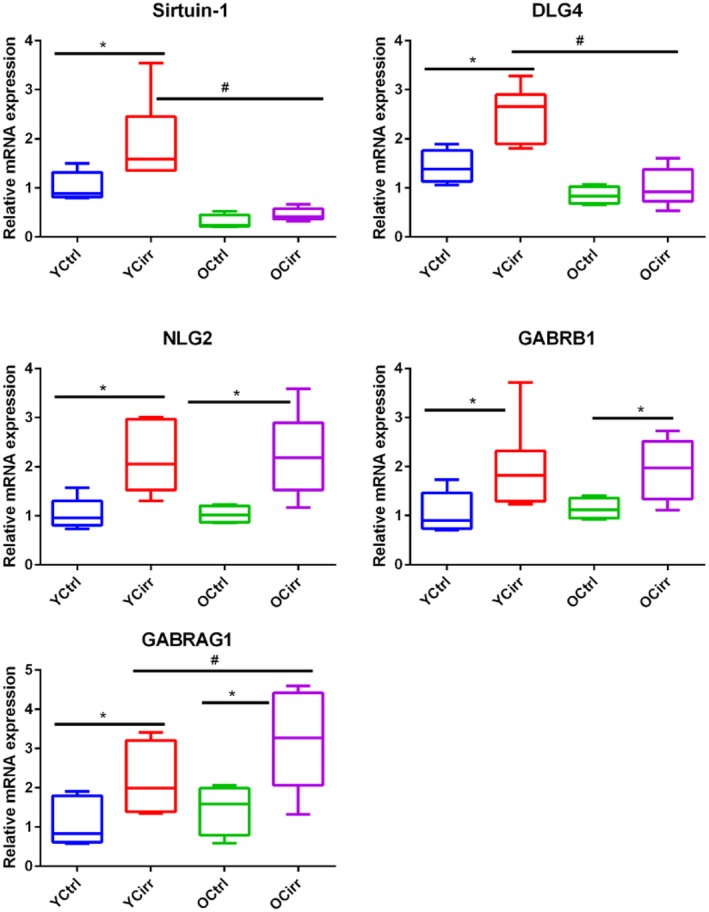
Cortical synaptic plasticity and GABA receptor expression. Comparisons among the four mouse groups. All data are presented as median and 95% CI. * indicates significant differences by the Mann‐Whitney test between cirrhotic and noncirrhotic mice in the same age group; # indicates significant differences by the Mann‐Whitney test between cirrhotic mice in different age groups. Abbreviations: OCirr, old cirrhosis; OCtrl, old control; YCirr, young cirrhosis; YCtrl, young control.
Discussion
The results of the current study demonstrate that older patients with cirrhosis have greater cognitive and QOL impairment compared to age‐matched patients without cirrhosis. This is linked with changes in brain MR focused on both connectivity and expression of astrocytic and neuronal changes. These changes were reflected in the animal data showing that advancing age is associated with higher neuroinflammation and modulation of synaptic plasticity and GABA physiology with the development of cirrhosis.
With the increasing population of older patients with cirrhosis, factors other than HE could contribute to the overall neurocognitive burden.4 These lead to an increased burden of older patients with cirrhosis on the caregivers and the health care system.20 With the potential comorbid conditions, including mild cognitive impairment (MCI) and dementia, potential listing for liver transplant also becomes difficult.21, 22 Therefore, the extent and potential pathogenesis of these changes are important to delineate.
We found that the patients with cirrhosis had a higher cognitive burden than those without cirrhosis, despite being age matched and free of most comorbid and decompensating conditions and medications. This was expressed by worse performance on two validated testing strategies, the EncephalApp Stroop and PHES, which test psychomotor speed, cognitive flexibility, and visuospatial coordination.3, 12 These tests have prognostic value and, as was found in this case, are also associated with important patient‐reported outcomes, such as QOL.3, 12 The concurrence of aging, potential covert HE, as well as other comorbid illnesses would make this an important population for further study. Although these changes have been described in younger patients with cirrhosis, the impact of these changes could be higher in this aging population given the other potential comorbid conditions.
To study the potential pathophysiology of these impairments, we performed multimodal brain MRI. MR spectroscopy showed consequences of hyperammonemia and neuronal impairment. The consequence of ammonia‐related injury was found with expected higher creatine ratios of glutamate and glutamine and lower myoinositol and choline in most voxels of interest.13 The reduction in NAA in all three sites points toward a concomitant reduction in neuronal integrity that is not typical of ammonia‐associated impairment or HE but rather a neurodegenerating process. These findings were further bolstered by the rs‐fMRI analysis that studied the DAN, which is involved in goal‐driven attention processing (top‐down), and the VAN, which is involved in stimuli‐driven attention processing (bottom‐up). These two networks have been reported to be preferentially affected in MCI and Alzheimer’s disease.23 The largest cluster of voxels showing differences between patients with cirrhosis and the control group was found in the ventrolateral prefrontal cortex, which is part of the VAN and may indicate deficits in the ability to reorient to novel or unexpected stimulus‐relevant information (bottom‐up processing). In addition, there were altered connections with the frontoparietal opercular cortex, which is associated with verbal and spatial working memory.24 The visual cortex RSN that was impaired in cirrhosis is also important for visual processing, including visual memory, word processing, and selective attention. RSN changes have been described in younger patients with cirrhosis. In hepatitis B cirrhosis, there is widespread alteration in connectivity in the cortical and subcortical areas.25 Chen et al.26 also showed functional connectivity changes between patients with minimal HE and other patients with cirrhosis within the DAN (left superior/inferior parietal lobule and right inferior parietal lobule) and VAN (right superior parietal lobule). In this study, we did not include younger patients with cirrhosis, but prior studies in that group have already shown abnormalities in cerebral connectivity compared to younger controls.18 Although similar results were seen between older individuals with and without cirrhosis, we additionally found a reduction in NAA on MR spectroscopy, which is not found in younger patients with cirrhosis and HE compared to controls.13 This distinct change in brain MRI in older individuals is further supported by the changes seen in the same RSNs typically implicated in aging, MCI, or Alzheimer’s disease in older patients with cirrhosis. Our findings extend these to the elderly patients with cirrhosis within the same networks, albeit in different areas, especially those associated with verbal and spatial memory and learning.
The potential mechanisms of these changes in humans could be related to a combination of inflammation along with changes in synaptic plasticity, with an overlay of aging and altered neurotransmitter findings. Therefore, our animal model focused on changes in the cortex of these specific processes. In the CCl4 gavage model, we found that older mice were more sensitive to the same dose of CCl4 compared to the younger models and ultimately required lesser amounts of the toxin to develop cirrhosis. The main readout in mice with CCl4‐induced cirrhosis is neuroinflammation. We found evidence of higher cortical mRNA expression related to IL‐6, IL‐1β, TGF‐β, and MCP1 in the older mice compared to the younger mice. In addition, IL‐6, IL‐1β, and TGF‐β were significantly higher in the older cirrhotic mice compared to the older controls. This indicates that older mice may have a predisposition toward neuroinflammation even without cirrhosis, and addition of the CCl4 synergized to worsen this phenomenon. Neuroinflammation seems to be aided by microglial and glial activation through GFAP and IBA1 mRNA and IBA1 protein increase in older mice and follows the trend seen in younger mice, except with a higher relative magnitude.19, 27, 28, 29
In addition to neuroinflammation, changes related to aging and synaptic plasticity could play a role in the synergism between old age and cirrhosis‐associated brain dysfunction. The neuroprotective sirtuin‐1, which is altered in human and animal cerebral cortex samples in Alzheimer’s disease, was significantly lower in mRNA and protein expression in older mice, especially those with cirrhosis.30, 31, 32 This was associated with a lower expression of DLG4 and higher expression of NLG2. These findings are important because DLG4 expression is critical for the formation of synapses that interact with the excitatory N‐methyl‐d‐aspartic acid, whereas NLG2 is important to stabilize the GABA‐associated inhibitory synapses.33, 34 DLG4 receptor knockout animals suffer from defective synaptic plasticity and impaired spatial learning, whereas NLG2 overexpression is associated with attention deficit.33 Both these cognitive domain alterations are found in patients with cirrhosis, especially those with advancing age. In addition to the increase in NLG2 expression, there was also a higher expression of GABRG1 and GABRB1 mRNA in older cirrhotic mice, which could be potentiated by the NLG2‐associated stabilization and maturation of GABA synapses.35, 36 Ultimately, these changes paint a more complex picture of the multiple processes that can modulate the end product of brain dysfunction in elderly individuals with cirrhosis that goes beyond inflammation and could be associated with increased expression of inhibitory GABA‐ergic synapses.
The current data are limited by the CCl4 model, which does not develop obvious changes in cognition and sickness behaviors and in which the readout is limited to brain‐associated changes.37 However, we did not perform in‐depth cognitive assessments, including the Y‐maze and rotorod assessment, in this group and restricted ourselves to daily evaluation of grooming, exploratory behavior, lethargy, and tactile feedback. Therefore, we could not link the mRNA expression profiles with overt cognitive changes. We specifically studied the animal models to perform analysis related to differential gene expressions and proteins that cannot be achieved in the human context. Although there is no ideal animal model for HE type C, we selected the one that would represent our human cirrhosis population as much as possible.37 We also were not able to perform immunohistochemistry; however, western blot analyses of IBA1 and sirtuin‐1 showed results that were reflective of the mRNA analyses. We also did not study newer pathways linked with bile acids and lactate given the specific results of the CCl4 model compared to bile duct‐ligated and azoxymethane models.38, 39 We also excluded patients with prior overt HE who were on medications for it to exclude the overwhelming effect of that condition on brain function and MR analysis.14 We also did not include younger patients with cirrhosis as mentioned above, but distinct MR differences between older individuals with and without cirrhosis were observed that drove these overall changes.
We conclude that older patients with cirrhosis have impaired cognition and QOL related to changes in brain connectivity and integrity of astrocytic and neuronal structures compared to age‐matched patients without cirrhosis. In animal models of cirrhosis, these changes are linked with increases in neuroinflammation and GABA‐ergic synaptic plasticity in the cerebral cortex with increasing age in cirrhosis. Further studies are needed in more advanced stages of cirrhosis to determine the synergism between advancing age and cirrhosis.
Potential conflict of interest
Nothing to report.
Supporting information
Supported in part by the McGuire Research Institute, Virginia Commonwealth University PeRQ award, VA Merit Review awards (I0CX001076 to J.S.B. and I0BX004033 to H.Z.), the National Center for Advancing Translational Sciences (award R21TR002024 to J.S.B.), and the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (awards R01‐DK104893 and R01‐DK115377 to H.Z. and P.B.H.).
References
- 1. Frith J, Jones D, Newton JL. Chronic liver disease in an ageing population. Age Ageing 2009;38:11‐18. [DOI] [PubMed] [Google Scholar]
- 2. Kim IH, Kisseleva T, Brenner DA. Aging and liver disease. Curr Opin Gastroenterol 2015;31:184‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014;60:715‐735. [DOI] [PubMed] [Google Scholar]
- 4. Bajaj JS, Ahluwalia V, Steinberg JL, Hobgood S, Boling PA, Godschalk M, et al. Elderly patients have an altered gut‐brain axis regardless of the presence of cirrhosis. Sci Rep 2016;6:38481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, et al. Inflamm‐aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci 2000;908:244‐254. [DOI] [PubMed] [Google Scholar]
- 6. Butterworth RF. Hepatic encephalopathy: A central neuroinflammatory disorder? Hepatology 2011;53:1372‐1376. [DOI] [PubMed] [Google Scholar]
- 7. Jones EA, Mullen KD. Theories of the pathogenesis of hepatic encephalopathy. Clin Liver Dis 2012;16:7‐26. [DOI] [PubMed] [Google Scholar]
- 8. Rodrigo R, Cauli O, Gomez‐Pinedo U, Agusti A, Hernandez‐Rabaza V, Garcia‐Verdugo JM, et al. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology 2010;139:675‐684. [DOI] [PubMed] [Google Scholar]
- 9. Bonda DJ, Lee HG, Camins A, Pallas M, Casadesus G, Smith MA, et al. The sirtuin pathway in ageing and Alzheimer disease: mechanistic and therapeutic considerations. Lancet Neurol 2011;10:275‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bergner M, Bobbitt RA, Carter WB, Gilson BS. The sickness impact profile: development and final revision of a health status measure. Med Care 1981;19:787‐805. [DOI] [PubMed] [Google Scholar]
- 11. Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol 2001;34:768‐773. [DOI] [PubMed] [Google Scholar]
- 12. Allampati S, Duarte‐Rojo A, Thacker LR, Patidar KR, White MB, Klair JS, et al. Diagnosis of minimal hepatic encephalopathy using Stroop EncephalApp: a multicenter US‐based, norm‐based study. Am J Gastroenterol 2016;111:78‐86. [DOI] [PubMed] [Google Scholar]
- 13. Sarma MK, Huda A, Nagarajan R, Hinkin CH, Wilson N, Gupta RK, et al. Multi‐dimensional MR spectroscopy: towards a better understanding of hepatic encephalopathy. Metab Brain Dis 2011;26:173‐184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, et al. Impaired gut‐liver‐brain axis in patients with cirrhosis. Sci Rep 2016;6:26800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ahluwalia V, Wade JB, Heuman DM, Hammeke TA, Sanyal AJ, Sterling RK, et al. Enhancement of functional connectivity, working memory and inhibitory control on multi‐modal brain MR imaging with rifaximin in cirrhosis: implications for the gut‐liver‐brain axis. Metab Brain Dis 2014;29:1017‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang H, Tan L, Wang HF, Liu Y, Yin RH, Wang WY, et al. Magnetic resonance spectroscopy in Alzheimer’s disease: systematic review and meta‐analysis. J Alzheimers Dis 2015;46:1049‐1070. [DOI] [PubMed] [Google Scholar]
- 17. Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo‐planar MRI. Magn Reson Med 1995;34:537‐541. [DOI] [PubMed] [Google Scholar]
- 18. Zhang XD, Zhang LJ, Wu SY, Lu GM. Multimodality magnetic resonance imaging in hepatic encephalopathy: an update. World J Gastroenterol 2014;20:11262‐11272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kang DJ, Betrapally NS, Ghosh SA, Sartor RB, Hylemon PB, Gillevet PM, et al. Gut microbiota drive the development of neuroinflammatory response in cirrhosis in mice. Hepatology 2016;64:1232‐1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rakoski MO, McCammon RJ, Piette JD, Iwashyna TJ, Marrero JA, Lok AS, et al. Burden of cirrhosis on older Americans and their families: analysis of the health and retirement study. Hepatology 2012;55:184‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin P, DiMartini A, Feng S, Brown R Jr, Fallon M. Evaluation for liver transplantation in adults: 2013 Practice Guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014;59:1144‐1165. [DOI] [PubMed] [Google Scholar]
- 22. Wang CW, Covinsky KE, Feng S, Hayssen H, Segev DL, Lai JC. Functional impairment in older liver transplantation candidates: from the functional assessment in liver transplantation study. Liver Transpl 2015;21:1465‐1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vemuri P, Jones DT, Jack CR Jr. Resting state functional MRI in Alzheimer’s disease. Alzheimers Res Ther 2012;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reuter‐Lorenz PA, Jonides J, Smith EE, Hartley A, Miller A, Marshuetz C, et al. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J Cogn Neurosci 2000;12:174‐187. [DOI] [PubMed] [Google Scholar]
- 25. Zhang LJ, Zheng G, Zhang L, Zhong J, Wu S, Qi R, et al. Altered brain functional connectivity in patients with cirrhosis and minimal hepatic encephalopathy: a functional MR imaging study. Radiology 2012;265:528‐536. [DOI] [PubMed] [Google Scholar]
- 26. Chen HJ, Wang Y, Zhu XQ, Li PC, Teng GJ. Classification of cirrhotic patients with or without minimal hepatic encephalopathy and healthy subjects using resting‐state attention‐related network analysis. PLoS One 2014;9:e89684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shawcross DL, Davies NA, Williams R, Jalan R. Systemic inflammatory response exacerbates the neuropsychological effects of induced hyperammonemia in cirrhosis. J Hepatol 2004;40:247‐254. [DOI] [PubMed] [Google Scholar]
- 28. Shawcross DL, Sharifi Y, Canavan JB, Yeoman AD, Abeles RD, Taylor NJ, et al. Infection and systemic inflammation, not ammonia, are associated with grade 3/4 hepatic encephalopathy, but not mortality in cirrhosis. J Hepatol 2011;54:640‐649. [DOI] [PubMed] [Google Scholar]
- 29. Zemtsova I, Gorg B, Keitel V, Bidmon HJ, Schror K, Haussinger D. Microglia activation in hepatic encephalopathy in rats and humans. Hepatology 2011;54:204‐215. [DOI] [PubMed] [Google Scholar]
- 30. Lalla R, Donmez G. The role of sirtuins in Alzheimer’s disease. Front Aging Neurosci 2013;5:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Julien C, Tremblay C, Emond V, Lebbadi M, Salem N Jr, Bennett DA, et al. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol 2009;68:48‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim D, Nguyen MD, Dobbin MM, Fischer A, Sananbenesi F, Rodgers JT, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J 2007;26:3169‐3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tzanoulinou S, Garcia‐Mompo C, Riccio O, Grosse J, Zanoletti O, Dedousis P, et al. Neuroligin‐2 expression in the prefrontal cortex is involved in attention deficits induced by peripubertal stress. Neuropsychopharmacology 2016;41:751‐761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng MC, Lu CL, Luu SU, Tsai HM, Hsu SH, Chen TT, et al. Genetic and functional analysis of the DLG4 gene encoding the post‐synaptic density protein 95 in schizophrenia. PLoS One 2010;5:e15107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sigel E, Steinmann ME. Structure, function, and modulation of GABA(A) receptors. J Biol Chem 2012;287:40224‐40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci 2008;9:331‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Butterworth RF, Norenberg MD, Felipo V, Ferenci P, Albrecht J, Blei AT; M embers of the ISHEN Commission on Experimental Models of HE . Experimental models of hepatic encephalopathy: ISHEN guidelines. Liver Int 2009;29:783‐788. [DOI] [PubMed] [Google Scholar]
- 38. McMillin M, Grant S, Frampton G, Petrescu AD, Kain J, Williams E, et al. FXR‐mediated cortical cholesterol accumulation contributes to the pathogenesis of type A hepatic encephalopathy. Cell Mol Gastroenterol Hepatol 2018;6:47‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bosoi CR, Zwingmann C, Marin H, Parent‐Robitaille C, Huynh J, Tremblay M, et al. Increased brain lactate is central to the development of brain edema in rats with chronic liver disease. J Hepatol 2014;60:554‐560. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


