Abstract
Neither an animal model nor a cell culture system has been established for the genotype 5 hepatitis E virus (G5 HEV), and the pathogenicity, epidemiology, and replication mechanism of the virus remain unclear. In this study, we used a reverse genetics system to generate G5 HEV and examined the possibility of zoonotic infection. Capped and uncapped genomic G5 HEV RNAs generated by in vitro transcription were transfected into PLC/PRF/5 cells. Infectious G5 HEV was recovered from the capped G5 HEV RNA–transfected PLC/PRF/5 cells and the subsequently passaged cells. G5 HEV was also recovered from uncapped G5 HEV–transfected PLC/PRF/5 cells after a longer lag phase, suggesting that the 5′‐cap structure is not essential but affected the efficiency of G5 HEV replication. G5 HEV infection was neutralized not only by anti‐G5 HEV‐like particles (HEV‐LPs) antibody, but also by anti‐G1, anti‐G3, anti‐G4, and anti‐G7 HEV‐LPs antibodies. G5 HEV was capable of infecting cynomolgus monkeys negative for anti‐HEV antibody but not animals positive for anti‐G7 HEV immunoglobulin G (IgG), indicating that cynomolgus monkeys were susceptible to G5 HEV, and the serotype of G5 HEV was identical to that of G7 HEV and human HEVs. Moreover, G5 HEV replication was efficiently inhibited by ribavirin and partially inhibited by sofosbuvir. Conclusion: Infectious G5 HEV was produced using a reverse genetics system, and the antigenicity was identical to that of human HEVs and G7 HEV. Transmission of G5 HEV to primates was confirmed by an experimental infection, providing evidence of the possibility of zoonotic infection by G5 HEV.
Abbreviations
- AA
amino acid
- ALT
alanine aminotransferase
- ELISA
enzyme‐linked immunosorbent assay
- G
genotype
- HEV
hepatitis E virus
- HEV‐LPs
HEV‐like particles
- IFA
immunofluorescence assay
- IgG
immunoglobulin G
- IgM
immunoglobulin M
- OD
optical density
- ORF
open reading frame
- P
passage
- PBS
phosphate‐buffered saline
- p.i.
post inoculation
- p.t.
post transfection
- RBV
ribavirin
- RT‐qPCR
real‐time quantitative reverse‐transcription polymerase chain reaction
- WB
western blot
Hepatitis E virus (HEV) is a causative agent of acute hepatitis E and has been classified into the Hepeviridae family, which includes two genera, Orthohepevirus and Piscihepevirus. 1, 2 The genus Piscihepevirus includes only one member, the cutthroat trout virus, whereas the genus Orthohepevirus includes four species, Orthohepevirus A to D.2 The species A includes eight genotypes of HEV (G1 to G8 HEV) and is detected in humans, monkeys, pigs, wild boars, deer, mongooses, rabbits, and camels.3 G1 and G2 HEV are exclusively isolated from humans and do not infect pigs.4 G3 and G4 HEV are isolated not only from humans but also from many animal species, including monkeys, pigs, wild boars, deer, mongooses, and rabbits, and thus have the potential for zoonotic infection.5, 6, 7, 8, 9, 10, 11 G5 and G6 HEV have been detected in wild boars in Japan.12, 13, 14 G7 HEV and G8 HEV are detected in dromedary camels and Bactrian camels, respectively.15, 16 Because G7 HEV can infect cynomolgus monkeys and a G7 HEV strain has been isolated from a patient with hepatitis E, G7 HEV is likely to be a causative agent for human type E hepatitis.17, 18 However, whether G5, G6, and G8 HEV are transmissible to humans is unclear.
To date, only one G5 HEV strain, JBOAR135‐SHiz09(AB573435), has been detected, which was found in a wild boar in Japan.12 The genome is 7.2 kb in length and contains three major open reading frames (ORF1‐3). ORF1 encodes a nonstructural protein of 1,708 amino acids (AAs); ORF2 encodes a capsid protein of 660 AAs; and ORF3 encodes a phosphoprotein of 112 AAs.12 The full genome sequences of G5 HEV share 70% to 80% nucleotide identities with known isolates of Orthohepevirus A and less than 60% nucleotide identities with those of Orthohepevirus B, C, and D.
G5 HEV‐like particles (HEV‐LPs) have been produced by expressing N‐terminus‐deleted ORF2 proteins, which exhibited antigenic cross‐reactivity with rats, ferrets, G1 HEV, and G3 to G6 HEV, and neutralization tests confirmed that the anti‐G5 HEV‐LPs antibody neutralizes G1 and G3 HEV infections, suggesting that the serotype of G5 HEV is similar to that of G1 and G3 HEV.19 Because there is no cell culture system to grow G5 HEV, it is unknown whether G5 HEV is neutralized by antibodies against other genotype HEVs. Thus, it is necessary to establish a cell culture system for G5 HEV and examine the possibility of zoonotic infection.
In the present study, we generated an infectious G5 HEV by using a reverse genetics system and found that G5 HEV thus produced grows not only in PLC/PRF/5 cells but also in HepG2/C3A and A549 cells. Animal experiments using cynomolgus monkeys demonstrated that the monkeys are susceptible to G5 HEV, suggesting that G5 HEV has the potential for zoonotic infection.
Materials and Methods
In Vitro Transcription of G5 HEV RNA
The complete genome of G5 HEV was synthesized based on the nucleotide sequence of G5 HEV deposited in GenBank (accession No. AB573435), and a T7 RNA polymerase promoter sequence was added to the 5′ end and a unique XbaI‐site to the 3′ end. The genome was cloned into the pUC57 vector to generate the plasmid pUC57‐T7G5HEV (GeneScript, Piscataway, NJ). The plasmid was linearized with XbaI and purified by phenol/chloroform extraction. Capped G5 HEV RNA was synthesized using an mMESSAGE mMACHINE T7 kit (Ambion, Austin, TX), and uncapped G7 HEV RNA was synthesized using a MEGAscript kit (Ambion). All of the RNA purifications were carried out by lithium chloride precipitation.
Cell Culture
A human hepatocarcinoma cell line, PLC/PRF/5 (JCRB0406), and a human lung carcinoma cell line, A549 (IF050153), were obtained from the Health Science Research Resources Bank (Osaka, Japan). A human hepatocellular carcinoma cell line, HepG2/C3A (ATCC HB‐8065), was obtained from the American Type Culture Collection (ATCC). The cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% (vol/vol) heat‐inactivated fetal bovine serum (FBS; Nichirei, Biosciences Inc., Tokyo, Japan), 100 U/mL penicillin, and 100 µg/mL streptomycin (Gibco, Grand Island, NY) at 37°C in a humidified 5% CO2 atmosphere and passaged every 3 days.
In Vivo Transfection
The confluent PLC/PRF/5 cells were trypsinized, and 5 × 105 cells were cultured in a 25‐cm2 tissue culture flask for 24 hours and then washed with phosphate‐buffered saline (PBS) and supplemented with 6.6 mL of new medium. The transfection was performed using a TransIT‐mRNA Transfection Kit (Mirus Bio, Madison, WI). Briefly, 6.6 µg of the capped or uncapped G5 HEV RNA was combined with 650 µL of Opti‐MEM (Gibco), and then 13.2 µL of mRNA Boost Reagent and 13.2 µL of TransIT‐mRNA reagent were added to the mixture. After 5 minutes of incubation at room temperature, the mixture was added to the PLC/PRF/5 cells. After a 12‐hour incubation at 37°C, the medium was replaced with 10 mL of maintenance medium: medium 199 (Invitrogen, Carlsbad, CA) containing 2% (vol/vol) heat‐inactivated FBS and 10 mM MgCl2. Further incubation was done at 36°C. The medium was replaced with new medium every 4 days, and the culture supernatant was used for the detection of the G5 HEV RNA and capsid proteins. The cells were observed daily by a light microscopy for the occurrence of the cytopathic effect.
Virus Inoculation
The confluent PLC/PRF/5, A549, and HepG2/C3A cells were trypsinized, and 5 × 105 cells were cultured in a 25‐cm2 tissue culture flask. After incubation for 24 hours, the cells were washed two times with PBS, and a total of 1 mL of the cell culture supernatant was inoculated onto the cells. After adsorption at 37°C for 1 hour, the cells were washed two times with PBS, and 10 mL of maintenance medium was added to the cells as described previously.
Detection of Anti‐G5 HEV Immunoglobulin G and Immunoglobulin M Antibodies and G5 HEV Capsid Protein
Anti‐G5 HEV immunoglobulin G (IgG) and immunoglobulin M (IgM) antibodies in monkey sera were detected by an enzyme‐linked immunosorbent assay (ELISA) using G5 HEV‐LPs as the antigen as described.19 The horseradish peroxidase (HRP)–conjugated goat anti‐monkey IgG‐heavy and light‐chain antibody (Bethyl Laboratories, Montgomery, TX) and HRP‐conjugated goat anti‐monkey IgM (KPL, Gaithersburg, MD) were used to detect the monkey IgG and IgM antibodies, respectively. Both antibodies were diluted 1:10,000 with PBS containing 0.05% Tween 20 (PBS‐T) and 1% skim milk.
The G5 HEV capsid protein was detected by an antigen‐capture ELISA as described with a slight modification17: A 96‐well microplate was coated with 1:2,000 diluted rabbit anti‐G5 HEV‐LPs serum, and 100 µL of the supernatant was added. The detection of the capsid protein was performed using guinea pig anti‐G5 HEV‐LPs hyperimmune serum (1:2,000). The normal cell culture supernatant (three wells per plate) served as the negative control. When the ratio of the optical density (OD) values between the sample and negative control was higher than 3.0, the sample was judged to be positive.
Western Blot Analysis and Immunofluorescence Assay
The capsid protein of G5 HEV was detected by western blot (WB) analysis. The culture supernatant of the G5 HEV–infected PLC/PRF/5 cells was separated by centrifugation at 10,000g for 30 minutes, and the supernatants were further concentrated by ultracentrifugation at 100,000g for 2 hours in a Beckman SW 55 Ti rotor. The pellet was resuspended in PBS. The viral proteins were separated by 5% to 20% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and electrophoretically transferred onto a nitrocellulose membrane. The membrane was then blocked with 5% skim milk in 50 mM Tris–HCl (pH 7.4) containing 150 mM NaCl and incubated with rabbit anti‐G5 HEV‐LPs polyclonal antibody (1:1,000 dilution with PBS‐T containing 1% skim milk). Detection of the rabbit IgG antibody was achieved using alkaline phosphatase–conjugated goat anti‐rabbit immunoglobulin (1:1,000 dilution) (Chemicon International, Temecula, CA). Nitroblue tetrazolium chloride and 5‐bromo‐4‐chloro‐3‐indolyl phosphate P‐toluidine were used as the coloring agents (Bio‐Rad Laboratories, Hercules, CA).
Immunofluorescence assay (IFA) with rabbit anti‐G5 HEV‐LPs hyperimmune serum (1:1,000 dilution) was used to detect the G5 HEV capsid protein in the cells as described.17, 20
Real‐Time Quantitative Reverse‐Transcription Polymerase Chain Reaction for Detection of G5 HEV
The RNA extraction was carried out by a MagNA Pure LC System with a MagNA Pure LC Total Nucleic Acid isolation kit (Roche Applied Science, Mannheim, Germany). A TaqMan assay for determination of the G5 HEV RNA copy number was performed using a 7500 FAST Real‐Time PCR System (Applied Biosystems, Foster City, CA) with TaqMan Fast Virus 1‐Step Master Mix (Applied Biosystems) and the forward primer 5′‐CCATGGAGGCCCACCAGTT‐3′ (nt 24‐42), a reverse primer 5′‐TCAGGGCGAAAGACCAGCTG‐3′ (nt 185‐204), and probe 5′‐FAM‐CCAACTCCGCCTTGGCGAATGC‐TAMRA‐3′ (nt 96‐117). One‐step real‐time quantitative reverse‐transcription polymerase chain reaction (RT‐qPCR) was performed for 5 minutes at 50°C and for 20 seconds at 95°C, followed by 50 cycles of 3 seconds at 95°C and 30 seconds at 60°C. A 10‐fold serial dilution of the capped G5 HEV RNA, 10 to 107 copies, was used as the standard. Amplification data were collected and analyzed with Sequence Detector software version 1.3 (Applied Biosystems).
Viral Genome Sequencing
The complete genome sequence of G5 HEV was determined by next‐generation sequencing (NGS) as described.21
Purification of G5 HEV
The culture supernatant of G5 HEV‐infected PLC/PRF/5 cells was collected at day 50 post inoculation (p.i.), and the cell debris were removed by centrifugation at 10,000g for 60 minutes, then concentrated by ultracentrifugation at 100,000g for 3 hours in a Beckman SW 55 Ti rotor. The resulting pellet was suspended in PBS at 4°C overnight, loaded onto a 10%‐50% (wt/vol) sucrose gradient, and centrifuged at 35,000g for 2 hours at 4°C in a Beckman SW 55 Ti rotor. The gradient was fractionated into 250 μL aliquots, and the G5 HEV capsid protein and HEV RNA in each fraction were detected by WB and RT‐qPCR, respectively.
Inoculation of Cynomolgus Monkeys and Sample Collection
Four cynomolgus monkeys (Tsukuba Primate Research Center, Ibaragi, Japan) were individually housed in Biosafety Level‐2 facilities. Two 10‐year‐old male cynomolgus monkeys (M4769 and M4770) were negative for HEV RNA by a nested broad‐spectrum RT‐PCR22 and negative for anti‐G1, anti‐G3, anti‐G4, anti‐G5, anti‐G6, anti‐G7, anti‐ferret, and anti‐rat HEV antibodies by respective HEV‐specific ELISAs.19, 23, 24 Two 15‐year‐old female cynomolgus monkeys (M4693 and M4694), which had been experimentally infected with G7 HEV and further challenged with G3 HEV,17 were positive for the anti‐G5 HEV IgG but negative for HEV RNA.
The cell culture supernatant collected at day 40 post transfection (p.t.), which was positive for both G5 HEV RNA and the capsid protein, was clarified by centrifugation at 10,000g for 30 minutes and then passed through a 0.45‐µm membrane filter (Millipore, Bedford, MA). Four cynomolgus monkeys were intravenously inoculated with 1 mL of the supernatant through the femoral vein. The serum samples were collected 2 times per week until 5 weeks p.i. and then collected weekly until 10 weeks p.i. and used for the detection of G5 HEV RNA, anti‐G5 HEV IgG and IgM antibodies, and alanine aminotransferase (ALT) values. The fecal samples were collected daily until day 35 p.i. and then collected weekly and used for the detection of the G5 HEV RNA. To observe whether G5 HEV is excreted into the urine and saliva, the urine (daily) and saliva (weekly) were collected from monkeys M4769 and M4770 until 7 weeks p.i. The experiments were reviewed by the institutional ethics committee, and all of the animal experiments were carried out according to the “Guides for Animal Experiments Performed at NIID” under code 516001.
Liver Enzyme Level
ALT values in the sera from cynomolgus monkeys were monitored weekly using a Fuji Dri‐Chem Slide GPT/ALT‐PIII kit (Fujifilm, Saitama, Japan). The geometric mean titers of the ALT values over the pre inoculation period were defined as the normal ALT value, and a 2‐fold or greater increase at the peak was considered a sign of hepatitis.
Neutralizing Activity Test
The neutralizing activity of various anti‐HEV antibodies against G5 HEV was evaluated. Antibodies against G1, G3, G4, G5, G7, ferret, and rat HEVs were prepared by inoculating rabbits with G1, G3, G4, G5, G7, ferret, and rat HEV‐LPs as described.19, 23, 24, 25, 26 The preimmunized and postimmunized rabbit sera were heated at 56°C for 30 minutes and diluted 1:20 with medium 199. Then, 1 mL of the solution containing 5 × 106 copies of G5 HEV was mixed with 1 mL antiserum and incubated at 37°C for 1 hour and then at 4°C for 3 hours. The PLC/PRF/5 cells cultured in 6‐well cell culture plates (2 × 105 cells/well) were inoculated with 1 mL of the virus/serum mixture. The adsorption was performed at 37°C for 1 hour. The cells were then washed three times with PBS, and 4 mL of maintenance medium was added. The culture medium was replaced with new medium every 3 days. The test was carried out in duplicate. We monitored the neutralizing activity by the antigen‐capture ELISA that detected the G5 HEV capsid protein in the supernatant at 3 weeks p.i.
Viral Infection Inhibition Experiments
Ribavirin (RBV) (Sigma Aldrich, St Louis, MO) and sofosbuvir (PSI‐7977; ChemScene, LLC, Monmouth Junction, NJ) were used to examine the inhibition of virus replication. The G5 HEV–infected PLC/PRF/5 cells were trypsinized, placed in cell culture bottles (25 cm3), and incubated at 36°C. The cells were maintained with 0 µM/mL, 50 µM/mL, 100 µM/mL, 200 µM/mL, and 400 µM/mL RBV or 0 µM/mL, 25 µM/mL, 50 µM/mL, 100 µM/mL, and 200 µM/mL PSI‐7977. The cell culture supernatants were collected every 4 days to detect G5 HEV RNA to evaluate the inhibition.
Results
Replication of G5 HEV in PLC/PRF/5 Cells
A total of 6.6 µg of the capped G5 HEV RNA was transfected into 5 × 105 PLC/PRF/5 cells. The supernatant was collected every 4 days and used for the detection of the G5 HEV RNA and the capsid protein. The G5 HEV RNA was detected in the cell culture supernatant at day 4 p.t. with a titer of 2.79 × 106 copies/mL, and the increase was observed from day 12 p.t. and reached a plateau at day 24 p.t., when the amount was 2.97 × 107 copies/mL (Fig. 1). From day 24 p.t. onward, the G5 HEV RNA was constantly detected with titers higher than 107 copies/mL until day 88 p.t. (Fig. 1A). The capsid protein was detected in the culture supernatant from day 4 p.t., quickly reached a high OD value of 1.365 at day 12 p.t. (Fig. 1B), and then was constantly detected with an OD value ranging from 1.289 to 1.712. G5 HEV recovered from the supernatant was designated as G5 HEV passage 1 (P1).
Figure 1.
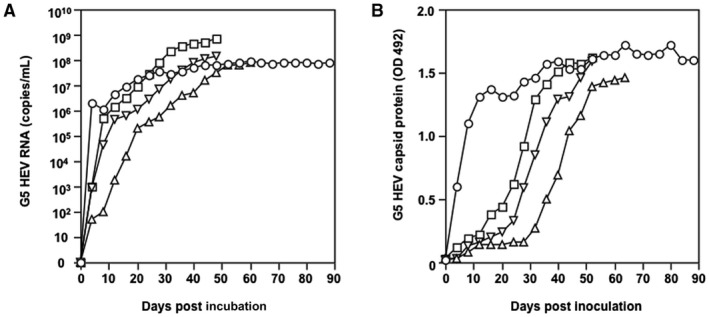
Replication and infection of G5 HEV in PLC/PRF/5 cells. (A) Detection of G5 HEV RNA. (B) Detection of G5 HEV capsid protein. PLC/PRF/5 cells were transfected with capped G5 HEV RNA, and P1 G5 HEV (○) was recovered from the cell culture supernatant. Inoculation of the P1 G5 HEV onto PLC/PRF/5 cells and generation of P2 G5 HEV (△), inoculation of the P2 G5 HEV onto PLC/PRF/5 cells and generation of P3 G5 HEV (▽), and inoculation of the P3 G5 HEV onto PLC/PRF/5 cells and generation of P4 G5 HEV (□) are shown. The culture supernatant was collected every 4 days and used for detection of the G5 HEV RNA by RT‐qPCR and the capsid protein by an antigen‐capture ELISA.
The P1 cell culture supernatant was collected at day 36 p.t. and inoculated onto PLC/PRF/5 cells to examine the infectivity. The G5 HEV RNA was detected on day 4 p.i., the capsid protein was detected on day 32 p.i., and both molecules reached a peak on day 52 p.i., indicating that the G5 HEV P2 was generated. Similarly, G5 HEV P3 was obtained by inoculation with P2 collected at day 44 p.i., and G5 HEV P4 was obtained by inoculation with P3 collected at day 32 p.i. The growth patterns of P2, P3, and P4 HEVs were similar (Fig. 1), indicating that infectious G5 HEV was produced and the infectivity was maintained during the passages in PLC/PRF/5 cells.
G5 HEV was recovered from the G5 HEV P1–infected cells and the supernatants of A549 and HepG2/C3A cells, indicating that these cells are also susceptible to G5 HEV.
Detection of the G5 HEV Capsid Protein by WB and IFA
To further characterize the capsid protein of G5 HEV, the P1 G5 HEV–infected PLC/PRF/5 cells and the supernatant were collected at day 56 p.i. One protein band with a molecular mass of approximately 72 kDa corresponding to G5 HEV ORF2 was specifically detected in the cells and the supernatant by WB analysis (Fig. 2A), indicating that the capsid protein was released into the cell culture supernatant. The G5 HEV capsid protein was also examined by an IFA using rabbit anti‐G5 HEV‐LPs antiserum. The G5 HEV capsid protein was located in the cytoplasm of the infected cells as observed in other HEVs (Fig. 2C), and the protein did not react with preimmunized rabbit serum (Fig. 2B).
Figure 2.
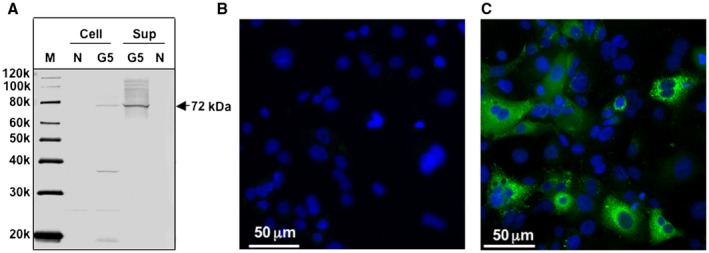
Detection of G5 HEV capsid protein. (A) The G5 HEV capsid protein in the PLC/PRF/5 cells and the cell culture supernatant (sup) was detected by a WB assay using a rabbit anti‐G5 HEV‐LPs serum. P1 G5 HEV–infected PLC/PRF/5 cells were incubated with preimmunized serum (1:1,000 dilution) (B) or rabbit anti‐G5 HEV‐LPs serum (1:1,000 dilution) (C), and then stained with an Alexa Fluor 488‐labeled anti‐rabbit IgG antibody. Nuclei were stained with 4′,6‐diamidino‐2‐phenylindole. Abbreviations: G5, G5 HEV–infected cells; M, molecular marker; and N, negative control (uninfected cells).
Generation of G5 HEV Virion
The generation of the G5 HEV virion was examined by sucrose gradient ultracentrifugation as described in the Materials and Methods section. The capsid protein with a molecular mass of approximately 72 kDa was detected primarily in fractions 8 and 9 by WB with band densities of 1.173 and 1.168 g/cm3, respectively (Fig. 3A). An HEV RNA peak appeared in the fractions 8 and 9 with copy numbers of 4.83 × 105 and 5.22 × 105 copies/µL, respectively (Fig. 3B). Both the RNA and capsid protein appeared in the same fractions, and the density of the virion was similar to that of G7 HEV,17 indicating that these fractions contained complete G5 HEV particles. Although we were unable to observe the virus particles by electron microscopy, fractions 8 and 9 could induce G5 HEV infection in PLC/PRF/5 cells (data not shown), demonstrating that the G5 HEV purified from the cell culture supernatants is infectious.
Figure 3.
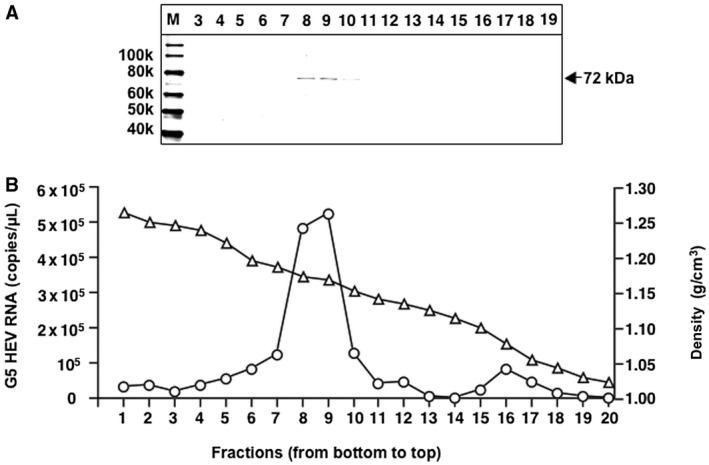
Purification of G5 HEV. The supernatant of G5 HEV (P1)‐infected PLC/PRF/5 cells was concentrated and the G5 HEV virions were purified by sucrose density gradient ultracentrifugation. (A) Aliquots from each fraction were analyzed by 5% to 20% polyacrylamide gel electrophoresis followed by WB with rabbit anti‐G5 HEV‐LPs serum. (B) The copy numbers of G5 HEV RNA (○) determined by RT‐qPCR and the density (△) of each fraction are depicted. Abbreviation: M, molecular weight marker.
Uncapped G5 HEV RNA Was Infectious
To examine whether the uncapped G5 HEV is capable of producing an infectious virus in PLC/PRF/5 cells, we compared the entire capped and entire uncapped G5 HEV genome RNAs. The replication of G5 HEV was examined by detection of G5 HEV RNA and the capsid protein in the cell culture supernatant (Fig. 4). Following transfection of the capped G5 RNA, both the viral RNA and capsid protein were detected on day 4 p.t. and then steadily increased (Fig. 4A,B). Interestingly, when the uncapped G5 HEV RNA was used, the RNA was first detected on day 4 p.t. with 6.89 × 105 copies/mL, and then the level gradually declined until day 24 p.t., when it began to increase, and it peaked at day 68 p.t. (Fig. 4A). The capsid protein was detected from day 36 p.t. and rapidly increased and reached the same level as the capped G5 HEV RNA on day 56 p.t. (Fig. 4B). These results demonstrated that the uncapped G5 HEV RNA is also infectious and capable of generating infectious G5 HEV.
Figure 4.
Comparison of capped and uncapped G5 HEV replication in PLC/PRF/5 cells. (A) Detection of G5 HEV RNA. (B) Detection of G5 HEV capsid protein. PLC/PRF/5 cells were transfected with capped (○) or uncapped (△) G5 HEV RNA. The G5 HEV RNA and the capsid protein in the cell culture supernatants were detected by RT‐qPCR and ELISA, respectively.
Cross Neutralization of G5 HEV With Various Anti–HEV‐LPs Antibodies
The antigenicity of G5 HEV was examined by neutralizing activity tests. The solution containing 5 × 106 copies of G5 HEV was mixed with rabbit anti‐G1, anti‐G3, anti‐G4, anti‐G5, anti‐G7, anti‐ferret, or anti‐rat HEV‐LPs serum and inoculated onto PLC/PRF/5 cells. The cell culture supernatant was collected at 3 weeks p.t., and the G5 HEV capsid protein was detected by ELISA. The G5 HEV capsid protein was detected in the samples incubated with anti‐rat and anti‐ferret HEV‐LPs and preimmunized rabbit serum with the OD values ranging from 0.657 to 0.938 at 3 weeks p.i. (Fig. 5). In contrast, the G5 HEV capsid protein was not detected in samples incubated with sera against G1, G3, G4, G5, and G7 HEV‐LPs. These results indicated that the serotype of G5 HEV is identical to that of G1, G3, G4, and G7 HEV but different from that of ferret and rat HEV.
Figure 5.
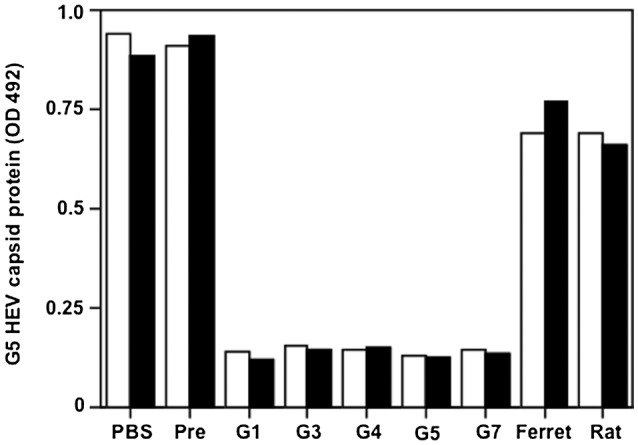
Antigenicity of G5 HEV. The antigenicity of G5 HEV was analyzed by a cell culture–based neutralization test with anti‐G1, anti‐G3, anti‐G4, anti‐G5, anti‐G7, anti‐ferret, and anti‐rat HEV‐LPs sera. The mixture of G5 HEV and 1:20 diluted antiserum was incubated and used to inoculate the PLC/PRF/5 cells. The capsid protein of G5 HEV in the supernatant was measured by an antigen‐capture ELISA. The test was carried out in duplicate (white bar and black bar). Abbreviations: Ferret, rabbit anti‐ferret HEV‐LPs serum; G1, rabbit anti‐G1 HEV‐LPs serum; G3, rabbit anti‐G3 HEV‐LPs serum; G4, rabbit anti‐G4 HEV‐LPs serum; G5, rabbit anti‐G5 HEV‐LPs serum; G7, rabbit anti‐G7 HEV‐LPs serum; Pre, pre immunized rabbit serum; and Rat, rabbit anti‐rat HEV‐LPs serum.
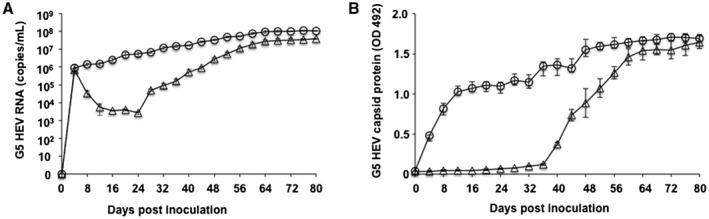
G5 HEV Is Capable of Infecting Monkeys
To examine whether G5 HEV infects primates, we challenged four cynomolgus monkeys, including two naive animals, M4769 and M4770, and two antibody‐positive animals, M4693 and M4694, with P1 G5 HEV (3.49 × 107 copies/mL) collected at day 40 p.i. (Fig. 6). After inoculation, 5.23 × 104 copies/g of the G5 HEV RNA was detected in the feces of M4769 on day 10 p.i., and the titer reached a peak of 2.73 × 105 copies/g on day 15 p.i. The copy number of the RNA decreased and became undetectable after day 24 p.i. The G5 HEV RNA in the serum was detected only on day 11 p.i., when it was present at 262.5 copies/mL (Fig. 6, M4769, top). Infection of another naive monkey, M4770, yielded a similar pattern. The viral RNA was detected in the feces on day 9 p.i., showing 2.66 × 104 copies/g, and reached a peak of 1.54 × 106 copies/g on day 20 p.i.; after day 30 p.i., it was undetectable. The RNA titers in the sera at days 14 and 19 p.i. were 186 copies/mL and 288 copies/mL, respectively (Fig. 6, M4770, top).
Figure 6.
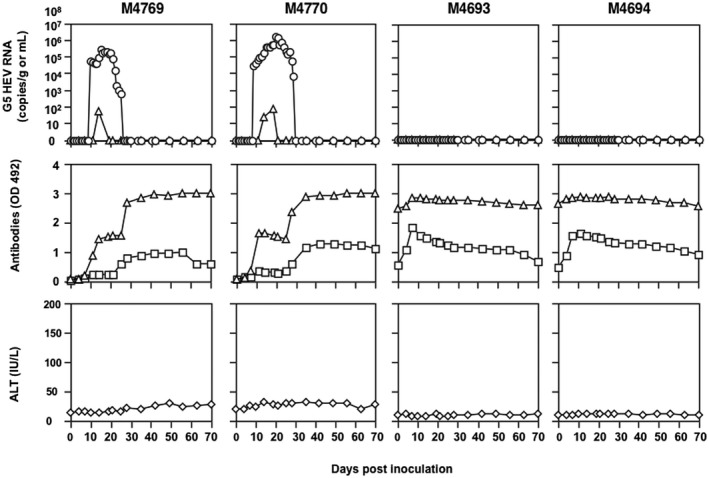
G5 HEV infection in cynomolgus monkeys. Two naive monkeys, M4769 and M4770, and two monkeys positive for anti‐HEV IgG, M4693 and M4694, were intravenously inoculated with G5 HEV, and the kinetics of the RNA, antibodies, and ALT were measured. The G5 HEV RNA copy numbers in the feces (○) and sera (△) were determined by RT‐qPCR. Anti‐HEV IgG (△) and anti‐HEV‐IgM (□) in sera were determined by ELISAs. ALT (◊) in monkey sera were determined by a commercial kit.
The IgM and IgG antibodies elicited in the two naive monkeys followed a similar pattern. The IgM antibodies were first detected at day 6 p.i. and reached a plateau at day 24 p.i. The IgG antibodies were first detected at day 6 p.i., and the OD values increased to approximately 1.50 and then remained constant until day 24 p.i. (Fig. 6, M4769, middle) or even decreased (Fig. 6, M4770, middle); they then increased again and reached a plateau with OD values of 3.0. In addition, the G5 HEV RNA was not detectable in the urine or saliva from either monkey, and there was no significant elevation of ALT during the period of the infection experiment (Fig. 6, M4769 and M4770, bottom), indicating that the G5 HEV was infectious to the cynomolgus monkeys but did not induce serious liver damage.
In contrast to the naive monkeys, no viral RNA was detected in the monkeys positive for anti‐HEV IgG (Fig. 6, M4693 and M4694, top). The existing IgG antibodies were slightly increased and the IgM antibodies were transiently increased at day 4 p.i. in both monkeys (Fig. 6, M4693 and M4694, middle). No elevation of ALT was observed in either monkey (Fig. 6, M4693 and M4694, bottom). These results indicated that anti‐HEV antibodies elicited 2 years earlier were capable of protecting monkeys from G5 HEV infection.
Comparison of G5 HEV Genome Sequences
The entire genome sequences of the P1 to P6 G5 HEVs recovered from the cell culture supernatants and the fecal samples of the two naive cynomolgus monkeys, M4769 and M4770, were determined by NGS and compared with that of the original G5 HEV (Table 1). Only two nucleotide changes, A372C and C5387T, were found in the genome after six passages. The nucleotide change A372C appeared in the P2 viruses accompanied by a nonsynonymous change in the ORF1 (Q116R), and C5387T appeared in the P4 viruses accompanied by two nonsynonymous changes of P66S (ORF2) and T69I (ORF3) in the overlapping region. In addition, the nucleotide sequences of the virus recovered from the fecal samples of both M4769 and M4770 were identical to those of the synthesized complementary DNA and P1 G5 HEV.
Table 1.
Nucleotide and Amino Acid Changes During Passages
| Nucleotide | Amino Acid | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Position* | ORFs | Position | C† | P1 | P2 | P3 | P4 | P5 | P6 | M‡ | Position | C | P1 | P2 | P3 | P4 | P5 | P6 | M |
| 372 | ORF1 | 347 | A | —§ | C | C | C | C | C | — | 116 | Q | — | R | R | R | R | R | — |
| 5,387 | ORF2 | 197 | C | — | — | — | T | T | T | — | 66 | P | — | — | — | S | S | S | — |
| 5,387 | ORF3 | 205 | C | — | — | — | T | T | T | — | 69 | T | — | — | — | I | I | I | — |
Nucleotide positions in the genomic RNA.
Sequence of the original G5 HEV.
Monkey.
No exchange.
RBV and PSI‐7977 Abrogated the Replication of G5 HEV
To examine whether G5 HEV replication is inhibited by RBV and PSI‐7977, we incubated the G5 HEV–infected PLC/PRF/5 cells with different concentrations of RBV or PSI‐7977 and determined the kinetics of the copy numbers of the G5 HEV RNA by RT‐qPCR. The copy numbers in the culture supernatants from RBV‐treated cells were drastically decreased in the presence of RBV (Fig. 7A). The viral RNA became undetectable at day 20 after treatment at the concentrations of 200 µM and 400 µM and at day 24 at the concentration of 100 µM, indicating that replication of G5 HEV was efficiently inhibited by RBV.
Figure 7.

Inhibition of G5 HEV replication by RBV and PSI‐7977. (A) Inhibitory effect of RBV. G5 HEV–infected PLC/PRF/5 cells were cultured in the presence of 0 μM (□), 50 μM (△), 100 μM (◊), 200 μM (×), or 400 μM (○) of RBV, respectively. (B) Inhibitory effect of PSI‐7977. G5 HEV–infected PLC/PRF/5 cells were cultured in the presence of 0 μM (□), 25 μM (△), 50 μM (◊), 100 μM (×), or 200 μM (○) of PSI‐7977, respectively. Triplicate samples were prepared for each dose. The culture supernatants were collected every 4 days and used for the detection of G5 HEV RNA.
In contrast, a moderate decrease of the copy number of the G5 HEV RNA was observed in the presence of 200 µM of PSI‐7977, indicating that the inhibitory effect of PSI‐7977 on the replication of G5 HEV was limited (Fig. 7B).
Discussion
The species Orthohepevirus A includes eight genotypes of HEV, and five of them—G1, G2, G3, G4, and G7 HEV—are known to be causative agents of human hepatitis E.3 Because G5 HEV also belongs to the same species, Orthohepevirus A, and shows greater genetic similarity to the other seven HEV genotypes in this species than the HEVs from other animals, such as rat, ferret, moose, and bat HEVs, we considered that it would be interesting to determine whether G5 HEV has the potential to infect humans. To answer this question, we used a reverse genetics system to produce infectious G5 HEV. Because PLC/PRF/5 cells have been shown to permit the growth of several HEV strains and infectious G7 HEV has been successfully recovered by a reverse genetics system,17, 20, 27, 28, 29, 30 we examined cell lines for the G5 HEV RNA transfection and confirmed that infectious G5 HEV could be produced not only in PLC/PRF/5 cells but also in A549 and HepG2/C3A cells.
Our previous study found that the G7 HEV RNA replication followed a long lag phase that extended to day 40 of the incubation, and the capsid protein of the virus was first detected in the culture supernatant at day 72 p.i.17 This replication pattern was different from that of G5 HEV, for which the viral RNA titers rapidly increased and the capsid protein was detected at day 3 p.i., clearly indicating that G5 HEV replicated much faster than G7 HEV. Although the mechanism is still unclear, this extensive replication will be of great benefit for the HEV research.
The HEV genome encodes a domain of methyltransferase that functions as a virus‐specific mRNA capping enzyme, and this domain is known to be a crucial requirement for the infectivity.31, 32 However, G5 HEV was unexpectedly recovered from the cell culture supernatants by transfection with uncapped G5 HEV, demonstrating that the cap structure is not absolutely required for the initiation of viral replication. Nonetheless, the longer lag phase after transfection of the uncapped RNA, which extended the incubation for 36 days, suggested that the 5′‐cap structure strongly affected the efficiency of the G5 HEV replication. In a future study, it would be interesting to examine whether the RNA recovered from uncapped RNA–transfected PLC/PRF/5 cells has the 5′‐cap structure.
Novel HEV strains have been discovered in various animal hosts around the world, and it is interesting to examine whether these HEVs cause cross‐species transmission. Our infection experiments clearly demonstrated that G5 HEV could infect and replicate in cynomolgus monkeys. This suggested that G5 HEV not only infects wild boars but also transmits to the primates, raising a potential public health concern for zoonosis. From the viewpoint of zoonotic infection, the development of a vaccine and the discovery of antiviral agents against G5 HEV are urgently needed. Our previous study demonstrated that G7 HEV–infected monkeys were protected from the challenge with G3 and G5 HEVs, and in the present study, we confirmed that the anti‐G1, anti‐G3, anti‐G4, and anti‐G7 HEV sera neutralized G5 HEV. These results indicated that the serotype of these HEVs is identical, suggesting that a single genotype of the HEV capsid protein would be sufficient to protect against infection by the other HEV genotypes.
In the present study, we examined the antiviral effectiveness of RBV and PSI‐7977 and found that G5 HEV replication was remarkably abrogated by RBV in a dose‐dependent manner, suggesting that RBV has the potential for treatment of hepatitis E caused by G5 HEV. In contrast, PSI‐7977 did not show significant inhibition for G5 HEV replication. PSI‐7977, an isopropyl ester of sofosbuvir, is known to inhibit HCV replication with broad genotype coverage.33 Because sofosbuvir inhibits G3 HEV replication in vitro and has shown antiviral activity in a patient with hepatitis E, it was recently reported as a candidate anti‐HEV drug.34, 35 However, other reports have suggested that the inhibition of HEV replication by sofosbuvir is limited.36, 37 In our present experiments, a high concentration of PSI‐7977 had only a limiting inhibitory effect against the virus replication, suggesting that sofosbuvir is not likely to be useful for the treatment of hepatitis E caused by G5 HEV infection.
HEV is generally transmitted by the fecal–oral route or by blood transfusion. In addition, recent studies have reported that G4 HEV was excreted into the urine collected from patients and monkeys in the acute phase of HEV infection.38 In a related finding, HEV RNA was detected in the breast milk collected from a patient with hepatitis E during acute infection, although the genotype was unknown.39 Thus, urine and milk could be another source of HEV infection. In our study, however, the G5 HEV RNA was not detected in any urine or saliva samples in the infected monkeys, indicating that such a transmission route does not apply in the case of this genotype.
When the entire genome sequences of P1 to P6 G5 HEVs were compared, we found two nucleotide changes accompanying three AA changes. Further study is needed to examine whether the mutations influence the infectivity, antigenicity, and pathogenesis of G5 HEV.
In conclusion, we succeeded in producing infectious G5 HEV using a reverse genetic system, and the resulting virus replicated in PLC/PRF/5 cells. This cell culture system would be useful for the characterization of G5 HEV as well as for studies of fundamental molecular biology, mechanisms of replication, cross‐species infection, and vaccine development. In addition, G5 HEV was experimentally transmitted to primates, demonstrating the possibility of zoonotic infection.
Potential conflict of interest
Nothing to report
Author contributions
T. L. contributed to the study concept and design, acquisition of data, analysis and interpretation of data, and drafting of the manuscript; H.B., S.Y., K.T., and HY.D. analyzed the data; Y.A. and Y. S. collected the specimens from monkeys; N.T. and S.M. contributed to revision of the manuscript; and T.W. supervised the study.
Acknowledgments
We thank Satoko Sato and Miyuki Oizumi for the technical assistance.
Supported by the Research Program on Hepatitis (JP18fk0210043 to T.L.) and the Program on Emerging and Reemerging Infectious Diseases (17fk0108218 to T.L.) from the Japan Agency for Medical Research and Development and by a Grant‐in‐Aid for Scientific Research (C) (17K08090 to T.L.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
References
Author names in bold designate shared co‐first authorship.
- 1. Meng XJ, Anderson DA, Arankalle VA, Emerson SU, Harrison TJ, Jameel S, et al. Hepeviridae In: King AMA, Michael J, Carstens EB, Lefkowitz EJ, eds. Virus Taxonomy: Ninth Report of the ICTV. London: Elsevier/Academic Press; 2012: 1021‐1028. [Google Scholar]
- 2. Smith DB, Simmonds P, Jameel S, Emerson SU, Harrison TJ, Meng XJ, et al. Consensus proposals for classification of the family Hepeviridae. J Gen Virol 2014;95:2223‐2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Purdy MA, Harrison TJ, Jameel S, Meng XJ, Okamoto H, Van der Poel WHM, et al. ICTV virus taxonomy profile: Hepeviridae . J Gen Virol 2017;98:2645‐2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meng XJ, Halbur PG, Haynes JS, Tsareva TS, Bruna JD, Royer RL, et al. Experimental infection of pigs with the newly identified swine hepatitis E virus (swine HEV), but not with human strains of HEV. Arch Virol 1998;143:1405‐1415. [DOI] [PubMed] [Google Scholar]
- 5. Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A 1997;94:9860‐9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamamoto H, Suzuki J, Matsuda A, Ishida T, Ami Y, Suzaki Y, et al. Hepatitis E virus outbreak in monkey facility, Japan. Emerg Infect Dis 2012;18:2032‐2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tei S, Kitajima N, Takahashi K, Mishiro S. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 2003;362:371‐373. [DOI] [PubMed] [Google Scholar]
- 8. Li TC, Chijiwa K, Sera N, Ishibashi T, Etoh Y, Shinohara Y, et al. Hepatitis E virus transmission from wild boar meat. Emerg Infect Dis 2005;11:1958‐1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nakamura M, Takahashi K, Taira K, Taira M, Ohno A, Sakugawa H, et al. Hepatitis E virus infection in wild mongooses of Okinawa, Japan: demonstration of anti‐HEV antibodies and a full‐genome nucleotide sequence. Hepatol Res 2006;34:137‐140. [DOI] [PubMed] [Google Scholar]
- 10. Zhao C, Ma Z, Harrison TJ, Feng R, Zhang C, Qiao Z, et al. A novel genotype of hepatitis E virus prevalent among farmed rabbits in China. J Med Virol 2009;81:1371‐1379. [DOI] [PubMed] [Google Scholar]
- 11. Abravanel F, Lhomme S, El Costa H, Schvartz B, Peron JM, Kamar N, et al. Rabbit hepatitis E virus infections in humans. France. Emerg Infect Dis 2017;23:1191‐1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takahashi K, Terada S, Kokuryu H, Arai M, Mishiro S. A wild boar‐derived hepatitis E virus isolate presumably representing so far unidentified “genotype 5”. Kanzo 2010;51:536‐538. [Google Scholar]
- 13. Takahashi M, Nishizawa T, Sato H, Sato Y, Jirintai Nagashima S, et al. Analysis of the full‐length genome of a hepatitis E virus isolate obtained from a wild boar in Japan that is classifiable into a novel genotype. J Gen Virol 2011;92:902‐908. [DOI] [PubMed] [Google Scholar]
- 14. Takahashi M, Nishizawa T, Nagashima S, Jirintai S, Kawakami M, Sonoda Y, et al. Molecular characterization of a novel hepatitis E virus (HEV) strain obtained from a wild boar in Japan that is highly divergent from the previously recognized HEV strains. Virus Res 2014;180:59‐69. [DOI] [PubMed] [Google Scholar]
- 15. Woo PC, Lau SK, Teng JL, Tsang AK, Joseph M, Wong EY, et al. New hepatitis E virus genotype in camels, the Middle East. Emerg Infect Dis 2014;20:1044‐1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Woo PC, Lau SK, Teng JL, Cao KY, Wernery U, Schountz T, et al. New hepatitis E virus genotype in Bactrian camels, Xinjiang, China, 2013. Emerg Infect Dis 2016;22:2219‐2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li TC, Zhou X, Yoshizaki S, Ami Y, Suzaki Y, Nakamura T, et al. Production of infectious dromedary camel hepatitis E virus by a reverse genetic system: potential for zoonotic infection. J Hepatol 2016;65:1104‐1111. [DOI] [PubMed] [Google Scholar]
- 18. Lee GH, Tan BH, Teo EC, Lim SG, Dan YY, Wee A, et al. Chronic infection with camelid hepatitis E virus in a liver transplant recipient who regularly consumes camel meat and milk. Gastroenterology 2016;150:355‐357, e353. [DOI] [PubMed] [Google Scholar]
- 19. Li TC, Kataoka M, Takahashi K, Yoshizaki S, Kato T, Ishii K, et al. Generation of hepatitis E virus‐like particles of two new genotypes G5 and G6 and comparison of antigenic properties with those of known genotypes. Vet Microbiol 2015;178:150‐157. [DOI] [PubMed] [Google Scholar]
- 20. Li TC, Yoshizaki S, Yang T, Kataoka M, Nakamura T, Ami Y, et al. Production of infectious ferret hepatitis E virus in a human hepatocarcinoma cell line PLC/PRF/5. Virus Res 2016;213:283‐288. [DOI] [PubMed] [Google Scholar]
- 21. Li TC, Yang T, Yoshizaki S, Ami Y, Suzaki Y, Ishii K, et al. Construction and characterization of an infectious cDNA clone of rat hepatitis E virus. J Gen Virol 2015;96:1320‐1327. [DOI] [PubMed] [Google Scholar]
- 22. Johne R, Plenge‐Bonig A, Hess M, Ulrich RG, Reetz J, Schielke A. Detection of a novel hepatitis E‐like virus in faeces of wild rats using a nested broad‐spectrum RT‐PCR. J Gen Virol 2010;91:750‐758. [DOI] [PubMed] [Google Scholar]
- 23. Li TC, Yamakawa Y, Suzuki K, Tatsumi M, Razak MA, Uchida T, et al. Expression and self‐assembly of empty virus‐like particles of hepatitis E virus. J Virol 1997;71:7207–7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou X, Kataoka M, Liu Z, Takeda N, Wakita T, Li TC. Characterization of self‐assembled virus‐like particles of dromedary camel hepatitis E virus generated by recombinant baculoviruses. Virus Res 2015;210:8‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang T, Kataoka M, Ami Y, Suzaki Y, Kishida N, Shirakura M, et al. Characterization of self‐assembled virus‐like particles of ferret hepatitis E virus generated by recombinant baculoviruses. J Gen Virol 2013;94(Pt 12):2647‐2656. [DOI] [PubMed] [Google Scholar]
- 26. Li TC, Yoshimatsu K, Yasuda SP, Arikawa J, Koma T, Kataoka M, et al. Characterization of self‐assembled virus‐like particles of rat hepatitis E virus generated by recombinant baculoviruses. J Gen Virol 2011;92:2830‐2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tanaka T, Takahashi M, Kusano E, Okamoto H. Development and evaluation of an efficient cell‐culture system for hepatitis E virus. J Gen Virol 2007;88:903‐911. [DOI] [PubMed] [Google Scholar]
- 28. Takahashi M, Tanaka T, Takahashi H, Hoshino Y, Nagashima S, Jirintai S, et al. Hepatitis E virus (HEV) strains in serum samples can replicate efficiently in cultured cells despite the coexistence of HEV antibodies: characterization of HEV virions in blood circulation. J Clin Microbiol 2010;48:1112‐1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jirintai S, Jinshan Tanggis, Manglai D, Mulyanto Takahashi M, et al. Molecular analysis of hepatitis E virus from farm rabbits in Inner Mongolia, China and its successful propagation in A549 and PLC/PRF/5 cells. Virus Res 2012;170:126‐137. [DOI] [PubMed] [Google Scholar]
- 30. Jirintai S, Tanggis Mulyanto, Suparyatmo JB, Takahashi M, Kobayashi T, et al. Rat hepatitis E virus derived from wild rats (Rattus rattus) propagates efficiently in human hepatoma cell lines. Virus Res 2014;185:92‐102. [DOI] [PubMed] [Google Scholar]
- 31. Kabrane‐Lazizi Y, Meng XJ, Purcell RH, Emerson SU. Evidence that the genomic RNA of hepatitis E virus is capped. J Virol 1999;73:8848–8850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Magden J, Takeda N, Li T, Auvinen P, Ahola T, Miyamura T, et al. Virus‐specific mRNA capping enzyme encoded by hepatitis E virus. J Virol 2001;75:6249‐6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lam AM, Espiritu C, Bansal S, Micolochick Steuer HM, Niu C, Zennou V, et al. Genotype and subtype profiling of PSI‐7977 as a nucleotide inhibitor of hepatitis C virus. Antimicrob Agents Chemother 2012;56:3359‐3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dao Thi VL, Debing Y, Wu X, Rice CM, Neyts J, Moradpour D, et al. Sofosbuvir inhibits hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology 2016;150: 82–85, e84. [DOI] [PubMed] [Google Scholar]
- 35. van de Garde MD, Pas SD, van der Net G, de Man RA, Osterhaus AD, Haagmans BL, et al. Hepatitis E virus (HEV) genotype 3 infection of human liver chimeric mice as a model for chronic HEV infection. J Virol 2016;90:4394‐4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang W, Peppelenbosch MP, Pan Q. Targeting viral polymerase for treating hepatitis E infection: How far are we? Gastroenterology 2016;150:1690. [DOI] [PubMed] [Google Scholar]
- 37. Wang W, Hakim MS, Nair VP, de Ruiter PE, Huang F, Sprengers D, et al. Distinct antiviral potency of sofosbuvir against hepatitis C and E viruses. Gastroenterology 2016;151:1251‐1253. [DOI] [PubMed] [Google Scholar]
- 38. Geng Y, Zhao C, Huang W, Harrison TJ, Zhang H, Geng K, et al. Detection and assessment of infectivity of hepatitis E virus in urine. J Hepatol 2016;64:37‐43. [DOI] [PubMed] [Google Scholar]
- 39. Rivero‐Juarez A, Frias M, Rodriguez‐Cano D, Cuenca‐Lopez F, Rivero A. Isolation of hepatitis E virus from breast milk during acute infection. Clin Infect Dis 2016;62:1464. [DOI] [PubMed] [Google Scholar]


