Abstract
Hepatitis C has increasingly affected women of child‐bearing age over the past few years as a result of the opioid epidemic. In this review, we discuss the effect of hepatitis C on pregnancy outcomes, effect of pregnancy on hepatitis C, as well as implications on management of hepatitis C during pregnancy.
Abbreviations
- AASLD‐IDSA
American Association for the Study of Liver Diseases and the Infectious Diseases Society of America
- CI
confidence interval
- DAA
direct‐acting antiviral
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- ICP
intrahepatic cholestasis of pregnancy
- OR
odds ratio
- SMFM
Society for Maternal‐Fetal Medicine
- SVR
sustained virologic response
Hepatitis C virus (HCV) remains a leading cause of liver disease in the United States and worldwide. The World Health Organization goals of significantly reducing morbidity and mortality rates from HCV by 2030 require a comprehensive effort to identify and treat all those that are infected.1 Over the last 5‐10 years in the United States, there has been a shift in the populations infected with HCV, with the so‐called second wave of HCV infections fueled by the opioid crisis and increasing rates of new HCV infection among injection drug users 20‐40 years of age.2 Further, an increase in HCV infection among women of childbearing age presents the additional problem of HCV transmission from mother to child. In light of this pronounced increase in the infection rate among women of childbearing age, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America (AASLD‐IDSA) guidelines recently recommended screening of all pregnant women for HCV.3 As more women are diagnosed with HCV during pregnancy, liver specialists need to partner with obstetrics and pediatric providers to optimize care. Key among the management issues are the timing of antiviral therapy with direct‐acting antivirals (DAAs) in relation to pregnancy as well as postpartum care of infants exposed to HCV (Fig. 1). We review the epidemiology and natural history of HCV among women of childbearing age, impact of HCV on pregnancy outcomes, implications of pregnancy on HCV disease course, and potential management approaches in pregnant women with HCV.
Figure 1.
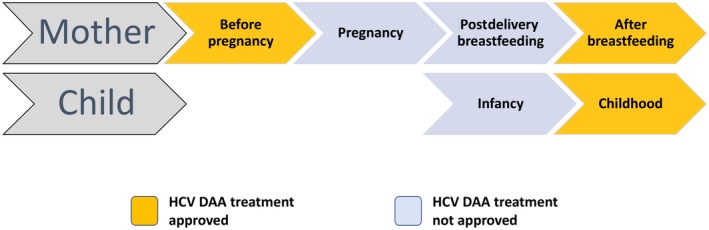
Potential times during the pregnancy care cascade for DAA therapy.
Epidemiology of Hepatitis C Virus Among Women of Childbearing Age
Of the approximately 3.5 million people estimated to have chronic HCV infection, only 50% are aware of their diagnosis, 16% have been prescribed treatment, and only 9% have achieved sustained virologic response (SVR).4 Men outnumber women in terms of prevalent HCV cases,5 but young persons who inject drugs include a substantial proportion of women of reproductive age.2 Women who inject drugs appear to have a higher risk of incident HCV, possibly due to higher risk‐injecting behaviors compared to men.6 Women are more likely to be injected by others, more frequently to be injected by sex partners, and more likely to engage in high‐risk sexual behaviors for drugs than their male counterparts.
There has also been an increase in HCV reported in women diagnosed specifically during pregnancy, with a 5‐fold increase from 1998 to 2011; this is more than reported for hepatitis B virus (HBV) or human immunodeficiency virus (HIV).7 Although this may reflect increased testing and reporting of HCV during pregnancy, the pronounced increase over time, especially when compared to other viral infections during pregnancy, suggests increased prevalence as well. According to data from the National Notifiable Diseases Surveillance System, of 425,322 women with confirmed HCV infection in the United States from 2006 to 2014, 171,801 (40.4%) were of reproductive age. The number of newly reported HCV cases among women of childbearing age has increased from around 15,500 in 2006 to 31,039 in 2014.2 Using data from a large commercial laboratory and birth certificate data in Kentucky (the state with the highest incidence of HCV during the period 2011‐2014) and nationwide, a significant increase in HCV detection was evident from 2011 to 2014, with the proportion of infants born to HCV‐infected mothers increasing by 68% nationally and by more than 200% in Kentucky.8 Similarly, in another report comparing rates of HCV among pregnant women in Tennessee and the United States from 2009 to 2014, the proportion of pregnant women with HCV giving birth increased by 89% nationally.9 Paralleling the rise in HCV infection among pregnant women is an increase in HCV detection among children, in particular those aged 2‐3 years,2 suggesting perinatal transmission of HCV.
Natural History of HCV Among Women
The natural history of HCV among women compared to men appears to differ. Studies reveal a higher rate of spontaneous clearance of HCV among women compared to men. In a cohort of injection drug users in San Francisco, CA, clearance of acute HCV was 34.6/100 person‐years of observation (PYO) (95% confidence interval [CI], 19.2‐62.5) among women compared to 12.1/100 PYO (95% CI, 6.3‐23.3) among men.10 More than 50% of women historically infected with HCV through contaminated anti‐D immune globulin spontaneously cleared. The mechanism behind the association of female sex with viral clearance may be related to immunologic differences between males and females due to sex steroid binding to specific receptors expressed in immune cells, although further study is necessary to confirm this.11 Furthermore, some data suggest that women chronically infected with HCV can also spontaneously clear their HCV infection in the postpartum period.12 One small study from Egypt demonstrated that 37/52 (71.2%) of pregnant women had a significant decline in their HCV viral load within the first 3 months after delivery, and 15 women had dropped their viral load to undetectable. Women with undetectable viral load by 12 months were most likely to spontaneously clear. In this study, the interleukin (IL)28B CT genotype had significantly lower odds of clearance then the CC allele. The potentially increased rate of spontaneous clearance postpartum in these women may be related to a surge of HCV‐specific T cells in the postdelivery period when “immune reconstitution” takes place. Further confirmation of this phenomenon can impact the timing and selection of treatment of HCV, especially surrounding pregnancy.
Progression of HCV disease in women may also relate to antifibrotic effects of estrogens, with pregnancy potentially having a beneficial impact on the long‐term progression of liver fibrosis and menopause (conversely, leading to more accelerated progression). Alternatively, the reduced prevalence of advanced fibrosis among women versus men may be due to a lower frequency of cofactors for liver disease progression, such as alcohol use.13, 14, 15
Influence of HCV on Maternal and Fetal Outcomes
Pregnancy has little to no substantial effect on the progression of active HCV infection. Maternal serum aminotransferase levels tend to fall during pregnancy, possibly reflecting the less immune‐reactive state of pregnancy.16 These changes may be related to the influence of immunosuppressive cytokines synthesized during pregnancy. Minor increases in HCV RNA can occur, especially during the second and third trimesters. Most pregnant women have minimal levels of fibrosis, although advanced fibrosis may be seen. Studies evaluating histologic changes prepartum versus postpartum present conflicting information, but most favor a benign effect. In one prospective study of 145 women in British Columbia who were infected with HCV and were monitored during pregnancy, the fibrosis‐4 (FIB‐4) score appeared to increase during pregnancy.17 However, the significance of FIB‐4 scores in pregnancy is not validated. Transient elastography is also not approved for use during pregnancy in the United States; therefore, serial fibrosis assessments in patients with HCV during pregnancy are not available.
In considering the associations between maternal HCV infection and pregnancy outcomes, the presence of concurrent risk factors of poor outcomes, such as poor perinatal care and concurrent drug or alcohol use, need to be considered. Women of childbearing age with HCV were found to have premature ovarian senescence leading to pregnancy complications, such as gestational diabetes, preeclampsia, and miscarriage.18 Successful treatment of HCV was found to reduce complications, such as miscarriage, in this cohort of women.18 Regarding fetal outcomes, a prospective study of 145 pregnant women from British Columbia who were HCV‐positive observed a 3.4% rate of intrauterine fetal death, 17.9% rate of preterm delivery, 11.3% rate of small for gestational age, and 12.5% rate of low birth weight infants, which were significantly higher than rates in the general British Columbia population (0.5%, 7%, and 5%, respectively). Interestingly, the authors did not find a significant association of adverse pregnancy outcomes with actual HCV viremia, which suggests that the risk factors for these adverse events may reflect other confounding factors, such as intravenous drug use and other background characteristics of the patient population.17 Nonetheless, given the relatively high rates of poor obstetric outcomes, these authors suggested population‐level screening for HCV in pregnancy. In a population‐based cohort in Washington state, mothers who were HCV‐positive were more likely to have infants that were low birth weight (odds ratio [OR], 2.17; 95% CI, 1.24‐3.80) and to be small for gestational age (OR, 1.46; 95% CI, 1.00‐2.13).19 Similarly, in a cohort of pregnant women in France who were HIV‐1‐positive, HCV coinfection appeared to be associated with cholestasis (adjusted OR, 4.1; 95% CI, 1.5‐10.8) and preterm delivery (OR, 3.0; 95% CI, 1.6‐5.7), whereas coinfection with HBV was not.20 The mechanism behind the association with preterm delivery was thought to be related to excessive local and/or systemic inflammation triggered by HCV infection. However, as highlighted above, HCV infection may co‐associate with high‐risk behaviors that are responsible for preterm birth and other pregnancy complications. It is difficult to adjust for potential confounders in HCV‐infected patients, such as educational level, socioeconomic status, and other psychosocial issues, which may be the true etiologic basis for the association of HCV with adverse pregnancy outcomes.
Recent studies have suggested that maternal HCV infection is a risk factor for intrahepatic cholestasis of pregnancy (ICP). In a meta‐analysis of three studies, the pooled odds of ICP in pregnant women who were infected with HCV compared to pregnant women who were not was 20.40 (95% CI, 9.39‐44.33), although significant heterogeneity was present among these studies.21 Although the mechanism behind this association is not entirely clear, the authors suggested several possible explanations, including the down‐regulation by HCV of adenosine triphosphate‐binding cassette transporter multidrug resistance protein 2 (MRP2), which would induce a failure in the transport of toxic substances and subsequent defect on bile transport; this effect on MRP2 would be further compounded by the high estrogen and progesterone levels during pregnancy. In addition, HCV viremia can cause direct cytopathic effects on biliary epithelial cells, creating an environment that facilitates the occurrence of ICP at an earlier gestational age. Given the increased association with ICP, women with HCV during pregnancy should be counseled and educated on the risk of developing ICP during pregnancy.
Perinatal Transmission of HCV
Several studies have evaluated the rate of perinatal transmission of HCV. In a large meta‐analysis including 109 studies, the pooled estimated rate of transmission was 5.8% (95% CI, 4.2%‐7.8%).22 Maternal coinfection with HIV was the most important predictor of risk, with an estimated rate of perinatal transmission of 10.8% (95% CI, 7.6%‐15.2%). Only viremic mothers are at risk of transmission; those with anti‐HCV who have spontaneously cleared HCV RNA or have been successfully treated are not at risk of transmission. Higher maternal HCV viral load may increase the risk of HCV transmission,23 although no threshold has been identified. In addition, maternal injection drug use has been suggested as an independent risk factor for HCV perinatal transmission.24 One potential explanation for injection drug use as an independent risk factor for perinatal transmission is that injection drug use may be associated with a higher likelihood of acute hepatitis during pregnancy (with higher titer viremia and absence of antibodies early in infection), leading to increased risk of transmission.25
The timing of mother‐to‐child transmission of HCV during pregnancy has also been evaluated. In a study of 54 children infected with HCV and tested within 3 days of birth,26 17 (31%) were positive in the first 3 days of life and therefore acquired infection in utero. However, 37 (68%) were negative during the first 3 days of life and 27 were positive when tested again at 3 months, suggesting that late intrauterine or intrapartum transmission occurred. Similarly, in a study conducted by the Tuscany Study Group of Hepatitis C Virus Infection in children, approximately half of the 5% of children born with HCV were HCV RNA‐positive at birth, suggesting intrauterine transmission (and not necessarily intrapartum transmission).27 Postpartum transmission of the virus through breastfeeding is rare.28 Thus, the majority of transmission appears to occur in the late intrauterine or in the peripartum period. However, more studies are needed to determine the exact mechanisms and timing of transmission.
Interventions to reduce perinatal transmission of HCV to the newborn are limited (Table 1). Although studies indicate that the majority of mother‐to‐child transmission occurs at or around the time of delivery, a clear benefit of caesarian section has not been established.29 Furthermore, avoidance of breastfeeding is not associated with a decreased risk of mother‐to‐child transmission. Two studies demonstrated a potential association with prolonged duration of ruptured membranes,30, 31 leading experts to recommend caesarean section if rupture of membranes is prolonged or invasive monitoring is required. The Society for Maternal‐Fetal Medicine (SMFM) has developed guidelines regarding pregnancy management in patients with HCV to reflect these data (Table 1).32
Table 1.
Pregnancy Features That May Impact Perinatal Transmission of HCV During Pregnancy and Postdeliverya
| Pregnancy Considerations | Studies; # of Women | Number of Women | Strength of Evidence | Summary of Findings | SMFM Recommendation |
|---|---|---|---|---|---|
| Elective C‐section versus vaginal delivery | 4 cohort studies30, 54, 55, 56 | 2,080 | Low | No differences, but trends in opposite directions in highest‐quality studies | Do not recommend C‐section solely for indication of HCV |
| All C‐section versus vaginal delivery | 11 cohort studies24, 27, 31, 57, 58, 59, 60, 61, 62, 63, 64 | 2,308 | Moderate | No association | |
| Amniocentesis and CVS | 3 cohort studies30, 54, 56 | 928 | Insufficient | Inconsistent, but one good quality study (OR, 6.7; 95% CI, 1.1‐36.0) | Counsel patients on potential risks of amniocentesis and CVS |
| Prolonged ROM | 2 cohort studies30, 31 | 245 | Low | Yes with >6 hours (OR, 9.3; 95% CI, 1.5‐1.8) | Active labor management if prolonged ROM to expedite delivery |
| Breastfeeding | 15 cohort studies24, 27, 30, 31, 54, 55, 59, 62, 63, 64, 65, 66, 67, 68, 69 | 2,971 | Moderate | No association between breastfeeding and risk for transmission | Do not discourage breastfeeding based on positive HCV status |
Adapted from Cottrell et al.29
Abbreviations: C‐section, cesarean section; CVS, chorionic villus sampling; ROM, rupture of membranes.
Anti‐HCV antibodies are present in the newborn’s blood as a result of passive placental transfer from infected mothers and can persist for the first 12‐15 months of life.33 Thus, perinatal transmission defined by antibody status requires the persistence of anti‐HCV antibodies to beyond 18 months of age. Alternatively, an early diagnosis can be made using HCV RNA testing, with presence of HCV RNA in an infant older than 2 months of age and confirmed on two different sampling occasions being consistent with chronic HCV infection in the infant.34
HCV Infection in Children
Perinatal transmission is the primary transmission route among children and is responsible for 60%‐90% of cases of HCV in children. In the United States, an estimated 23,000 to 46,000 children live with chronic HCV.35 However, a recent study found that among 1,026 infants exposed to HCV, only 96 (9%) were actually screened for HCV postpartum.36 Similarly, in a study conducted at the University of Pittsburgh, only 96 of 323 (30%) children of mothers with HCV who were seen in a pediatric clinic were ever tested for HCV,36 and in a prospective cohort of 879 women with opioid use disorder being seen in a specialized obstetrics clinic, only 180 (45%) had the appropriate diagnostic follow‐up.37 Thus, among women known to have HCV during or prior to pregnancy, many children often remained untested and may have had undiagnosed HCV infection.
The effect of HCV infection on children may begin in utero. One study evaluated HCV‐exposed neonates and found them to have lower levels of regulatory T cells and rates of clusters of differentiation (CD)4+ and CD8+ T‐cell activation as well as lower plasma levels of proinflammatory markers than controls, suggesting a relative suppression of immune activation in neonates exposed to HCV.38 Another study suggested that HCV can be transmitted to the neonate as early as the first trimester, especially in women coinfected with HIV, which would potentially have implications on early organ development.39 Early transmission would potentially lead to long‐term tolerance to HCV antigens and loss of pathogen‐specific immune competence, although there are limited clinical data to support this.
Although 12%‐64% of vertically infected children spontaneously clear HCV, long‐term infection can lead to cirrhosis, hepatocellular carcinoma, decompensated liver disease, and need for liver transplant in later childhood or young adulthood.40, 41 Studies have demonstrated potential extrahepatic effects of HCV infection in childhood, with significant reduction in both physical and psychosocial health as well as cognitive function in children with HCV compared to children without HCV.42, 43 Health‐related quality of life in children with HCV has also been found to be significantly lower in children with HCV, with improvements after treatment.44 Finally, the presence of autoimmune disease has also been associated with HCV in children.45 Collectively, these studies highlight the need to consider treatment in all eligible children. Treatment with DAAs is currently available and approved for children over age 12 or over 35 kg in weight, and ongoing trials with DAAs are under way for younger children, with a recent trial demonstrating safety and efficacy of ledipasvir/sofosbuvir for children as young as 6 years of age.3, 46 These data emphasize the importance of taking all measures to decrease the risk of HCV transmission to the child and make a strong case for increasing treatment of HCV in women of childbearing age before pregnancy in order to prevent childhood infections occurring by perinatal transmission. Women of childbearing age have therefore been identified by AASLD‐IDSA as a high‐priority HCV treatment group before they become pregnant.3
Pregnancy Is a Unique Opportunity to Engage Women in HCV Care
Pregnancy represents a time of engagement in medical care and a unique opportunity to identify HCV infection. Moreover, with the expansion of Medicaid to cover women in pregnancy and for perinatal care,47 this may be the opportunity to engage vulnerable populations, including women who inject drugs. Recognizing the gap in diagnosis that plagues elimination efforts in the United States, the new AASLD‐IDSA recommendation to screen all women during pregnancy for HCV is to be applauded.3
Additionally, because pregnancy and the perinatal period may be the only time that women have health insurance, this may be the perfect opportunity to confirm viremia, evaluate stage of disease, and discuss future treatment. In high‐risk patient populations, such as injection drug users, treatment as a prevention strategy as well as a cure of HCV would potentially decrease further transmission to others and eliminate the risk of HCV transmission in future pregnancies. Indeed, this unique opportunity to engage women in HCV care has prompted consideration of HCV treatment during pregnancy.3
HCV Treatment During Pregnancy
Given the rise in HCV infection among women of childbearing age as well as the availability of highly effective DAA agents for HCV, the possibility of using DAAs during pregnancy has begun to be explored. Many of the DAA agents have been labeled as pregnancy category B (or equivalent), indicating that the DAAs are not associated with toxicity to the fetus in animal data but that adequate human studies are lacking.48 As a result, a phase 1 trial evaluating ledipasvir/sofosbuvir during the third trimester of pregnancy is under way, with results anticipated in 2019.49 In addition, another study is evaluating the use of sofosbuvir/velpatasvir in the postpartum period after breastfeeding is complete.50 Investigators from regions of the world where HCV is prevalent among pregnant women have already begun to develop protocols for treatment during pregnancy. For example, clinicians in India reported on 15 pregnant women treated with ledipasvir/sofosbuvir, with all patients achieving SVR at 12 weeks (SVR12) and no reports of adverse pregnancy outcomes.51 Furthermore, a recent survey of women with HCV or a history of HCV in the United States found that 60% of women would be interested in undergoing HCV therapy during pregnancy if it would reduce the risk of mother‐to‐child transmission, despite the limited current safety and efficacy data on HCV treatment during pregnancy.52
The optimal timing of treatment in relation to pregnancy and prevention of perinatal transmission is yet to be determined. Given the lack of current safety data on DAAs during pregnancy, AASLD‐IDSA guidelines recommend treatment of HCV prior to pregnancy or delayed until after pregnancy. Women of childbearing age should be prioritized for treatment to prevent future potential transmission to their children. However, there may be potential benefits to treatment during pregnancy. Timely antiviral therapy may be able to potentially decrease perinatal transmission, similar to what is achieved by third‐trimester treatment of high viremia mothers with chronic hepatitis B.47 In addition, as previously emphasized, pregnancy may be the only time when uninsured women have health coverage and can obtain access to DAA medications. Finally, most young women who become pregnant do not have advanced fibrosis and would therefore be eligible for an 8‐week course of DAA therapy timed during the third trimester of pregnancy when any potential adverse effect on the fetus would be minimized. On the other hand, safety of DAAs during pregnancy has not yet been established, and treatment during pregnancy cannot be recommended until adequate safety data become available.
Concluding Remarks and Future Directions
The prevalence of HCV among pregnant women is rising in the United States, and recent recommendations by AASLD‐IDSA guidelines are to screen all pregnant women for HCV, regardless of risk profile. On the other hand, the SMFM still endorses risk‐based screening for HCV.32 Efforts should be directed at unifying these recommendations across expert societies so that a clear recommendation is provided to obstetrics providers who will be screening pregnant women. If the universal HCV screening recommendation is endorsed by the SMFM, higher numbers of women with HCV will be identified during pregnancy.
The concept of HCV treatment during pregnancy requires thoughtful investigation of the risks and benefits to mother and fetus. More safety data on DAA use in pregnancy are urgently needed and will be hopefully available in the near future once results from clinical trials of DAAs during pregnancy are obtained. In the interim, all women exposed to DAAs during pregnancy, especially in HIV coinfected patients, can potentially be included in the Antiretroviral Pregnancy Registry, which prospectively tracks exposure to HIV and HBV medications during pregnancy.53 Once safety data become available, potential treatment during pregnancy, especially in high‐risk individuals, can be evaluated on a case‐by‐case basis.
Whether treatment during pregnancy will impact perinatal transmission remains to be determined. Therefore, all efforts should be made to treat women of childbearing age prior to pregnancy. Preconception counseling should be performed, and delaying pregnancy until DAA therapy is completed should be recommended if possible. The efficacy of the DAAs is not expected to be diminished during pregnancy, but this too remains an open question. Current trials of DAAs for children as young as 3 years of age, if successful, will potentially reduce the concern for perinatal transmission, with early treatment of infected children possible. Given the changing epidemiology of HCV infection in the United States and the goal to eradicate the virus, identifying more women with HCV prior to as well as during pregnancy and children who acquire HCV through perinatal transmission will provide an opportunity to treat HCV in an expanding patient population. In doing so, this will be an ideal setting to impact the HCV cascade of care and assist in the elimination of HCV.
Potential conflict of interest
Dr. Terrault has received grants from Gilead, Bristol‐Myers Squibb, AbbVie, and Merck. Dr. Kushner advises Gilead.
Contributor Information
Tatyana Kushner, Email: Tatyana.kushner@mssm.edu.
Norah A. Terrault, Email: Norah.terrault@ucsf.edu
References
- 1. Buckley GJ, Strom BL. A national strategy for the elimination of viral hepatitis emphasizes prevention, screening, and universal treatment of hepatitis C. Ann Intern Med 2017;166:895‐896. [DOI] [PubMed] [Google Scholar]
- 2. Ly KN, Jiles RB, Teshale EH, Foster MA, Pesano RL, Holmberg SD. Hepatitis C virus infection among reproductive‐aged women and children in the United States, 2006 to 2014. Ann Intern Med 2017;166:775‐782. [DOI] [PubMed] [Google Scholar]
- 3. AASLD‐IDSA . HCV guidance: recommendations for testing, managing, and treating hepatitis C. https://www.hcvguidelines.org. Accessed July 2018.
- 4. Zucker J, Aaron JG, Feller DJ, Slowikowski J, Evans H, Scherer ML, et al. Development and validation of an electronic medical record‐based algorithm to identify patient milestones in the hepatitis C virus care cascade. Open Forum Infect Dis 2018;5:ofy153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Edlin BR, Eckhardt BJ, Shu MA, Holmberg SD, Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology 2015;62:1353‐1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tracy D, Hahn JA, Fuller Lewis C, Evans J, Briceño A, Morris MD, et al. Higher risk of incident hepatitis C virus among young women who inject drugs compared with young men in association with sexual relationships: a prospective analysis from the UFO Study cohort. BMJ Open 2014;4:e004988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salemi JL, Spooner KK, Mejia de Grubb MC, Aggarwal A, Matas JL, Salihu HM, et al. National trends of hepatitis B and C during pregnancy across sociodemographic, behavioral, and clinical factors, United States, 1998‐2011. J Med Virol 2017;89:1025‐1032. [DOI] [PubMed] [Google Scholar]
- 8. Koneru A, Nelson N, Hariri S, Canary L, Sanders KJ, Maxwell JF, et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission—United States and Kentucky, 2011‐2014. MMWR Morb Mortal Wkly Rep 2016;65:705‐710. [DOI] [PubMed] [Google Scholar]
- 9. Patrick SW, Bauer AM, Warren MD, Jones TF, Wester C. Hepatitis C virus infection among women giving birth—Tennessee and United States, 2009‐2014. MMWR Morb Mortal Wkly Rep 2017;66:470‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Page K, Hahn JA, Evans J, Shiboski S, Lum P, Delwart E, et al. Acute hepatitis C virus infection in young adult injection drug users: a prospective study of incident infection, resolution, and reinfection. J Infect Dis 2009;200:1216‐1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grebely J, Page K, Sacks‐Davis R, van derLoeff MS, Rice TM, Bruneau J, et al. InC3 Study Group. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology 2014;59:109‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hashem M, Jhaveri R, Saleh DA, Sharaf SA, El‐Mougy F, Abdelsalam L, et al. Spontaneous viral load decline and subsequent clearance of chronic hepatitis C virus in postpartum women correlates with favorable interleukin‐28B gene allele. Clin Infect Dis 2017;65:999‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Di Martino V, Lebray P, Myers RP, Pannier E, Paradis V, Charlotte F, et al. Progression of liver fibrosis in women infected with hepatitis C: long‐term benefit of estrogen exposure. Hepatology 2004;40:1426‐1433. [DOI] [PubMed] [Google Scholar]
- 14. Yi SW, Choi JS, Yi JJ, Lee YH, Han KJ. Risk factors for hepatocellular carcinoma by age, sex, and liver disorder status: a prospective cohort study in Korea. Cancer 2018;124:2748‐2757. [DOI] [PubMed] [Google Scholar]
- 15. Yu MW, Chang HC, Chang SC, Liaw YF, Lin SM, Liu CJ, et al. Role of reproductive factors in hepatocellular carcinoma: impact on hepatitis B‐ and C‐related risk. Hepatology 2003;38:1393‐1400. [DOI] [PubMed] [Google Scholar]
- 16. Dibba P, Cholankeril R, Li AA, Patel M, Fayek M, Dibble C, et al. Hepatitis C in pregnancy. Diseases 2018;6:pii:E31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Money D, Boucoiran I, Wagner E, Dobson S, Kennedy A, Lohn Z, et al. Obstetrical and neonatal outcomes among women infected with hepatitis C and their infants. J Obstet Gynaecol Can 2014;36:785‐794. [DOI] [PubMed] [Google Scholar]
- 18. Karampatou A, Han X, Kondili LA, Taliani G, Ciancio A, Morisco F, et al. PITER Framework Investigators; PITER Investigators. Premature ovarian senescence and a high miscarriage rate impair fertility in women with HCV. J Hepatol 2018;68:33‐41. Erratum. In: J Hepatol 2018; 10.1016/j.jhep.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 19. Pergam SA, Wang CC, Gardella CM, Sandison TG, Phipps WT, Hawes SE. Pregnancy complications associated with hepatitis C: data from a 2003‐2005 Washington state birth cohort. Am J Obstet Gynecol 2008;199:38.e1‐e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Benhammou JN, Dong TS, May FP, Kawamoto J, Dixit R, Jackson S, et al. Race affects SVR12 in a large and ethnically diverse hepatitis C‐infected patient population following treatment with direct‐acting antivirals: analysis of a single‐center Department of Veterans Affairs cohort. Pharmacol Res Perspect 2018;6:e00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wijarnpreecha K, Thongprayoon C, Sanguankeo A, Upala S, Ungprasert P, Cheungpasitporn W. Hepatitis C infection and intrahepatic cholestasis of pregnancy: a systematic review and meta‐analysis. Clin Res Hepatol Gastroenterol 2017;41:39‐45. [DOI] [PubMed] [Google Scholar]
- 22. Benova L, Mohamoud YA, Calvert C, Abu‐Raddad LJ. Vertical transmission of hepatitis C virus: systematic review and meta‐analysis. Clin Infect Dis 2014;59:765‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yeung CY, Lee HC, Chan WT, Jiang CB, Chang SW, Chuang CK. Vertical transmission of hepatitis C virus: current knowledge and perspectives. World J Hepatol 2014;6:643‐651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Syriopoulou V, Nikolopoulou G, Daikos GL, Theodoridou M, Pavlopoulou I, Nicolaidou P, et al. Mother to child transmission of hepatitis C virus: rate of infection and risk factors. Scand J Infect Dis 2005;37:350‐353. [DOI] [PubMed] [Google Scholar]
- 25. Indolfi G, Azzari C, Resti M. Perinatal transmission of hepatitis C virus. J Pediatr 2013;163:1549‐1552.e1. [DOI] [PubMed] [Google Scholar]
- 26. Mok J, Pembrey L, Tovo PA, Newell ML. European Paediatric Hepatitis C Virus Network. When does mother to child transmission of hepatitis C virus occur? Arch Dis Child Fetal Neonatal Ed 2005;90:F156‐F160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Resti M, Azzari C, Mannelli F, Moriondo M, Novembre E, de Martino M, et al. Mother to child transmission of hepatitis C virus: prospective study of risk factors and timing of infection in children born to women seronegative for HIV‐1. Tuscany Study Group on Hepatitis C Virus Infection. BMJ 1998;317:437‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Polywka S, Schröter M, Feucht HH, Zöllner B, Laufs R. Low risk of vertical transmission of hepatitis C virus by breast milk. Clin Infect Dis 1999;29:1327‐1329. [DOI] [PubMed] [Google Scholar]
- 29. Cottrell EB, Chou R, Wasson N, Rahman B, Guise JM. Reducing risk for mother‐to‐infant transmission of hepatitis C virus: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2013;158:109‐113. [DOI] [PubMed] [Google Scholar]
- 30. Mast EE, Hwang LY, Seto DS, Nolte FS, Nainan OV, Wurtzel H, et al. Risk factors for perinatal transmission of hepatitis C virus (HCV) and the natural history of HCV infection acquired in infancy. J Infect Dis 2005;192:1880‐1889. [DOI] [PubMed] [Google Scholar]
- 31. Spencer JD, Latt N, Beeby PJ, Collins E, Saunders JB, McCaughan GW, et al. Transmission of hepatitis C virus to infants of human immunodeficiency virus‐negative intravenous drug‐using mothers: rate of infection and assessment of risk factors for transmission. J Viral Hepat 1997;4:395‐409. [DOI] [PubMed] [Google Scholar]
- 32. Society for Maternal‐Fetal Medicine ; Hughes BL, Page CM, Kuller JA. Hepatitis C in pregnancy: screening, treatment, and management. Am J Obstet Gynecol 2017;217:B2‐B12. [DOI] [PubMed] [Google Scholar]
- 33. Roberts EA, Yeung L. Maternal‐infant transmission of hepatitis C virus infection. Hepatology 2002;36(Suppl. 1):S106‐S113. [DOI] [PubMed] [Google Scholar]
- 34. Espinosa C, Jhaveri R, Barritt AS 4th. Unique challenges of hepatitis C in infants, children, and adolescents. Clin Ther 2018;40:1229‐1307. [DOI] [PubMed] [Google Scholar]
- 35. Pawlowska M, Sobolewska‐Pilarczyk M, Domagalski K. Hepatitis C virus infection in children in the era of direct‐acting antiviral. World J Gastroenterol 2018;24:2555‐2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chappell CA, Hillier SL, Crowe D, Meyn LA, Bogen DL, Krans EE. Hepatitis C virus screening among children exposed during pregnancy. Pediatrics 2018;141:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Epstein RL, Sabharwal V, Wachman EM, Saia KA, Vellozzi C, Hariri S, et al. Perinatal transmission of hepatitis C virus: defining the cascade of care. J Pediatr 2018; 10.1016/j.jpeds.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Babik JM, Cohan D, Monto A, Hartigan‐O'Connor DJ, McCune JM. The human fetal immune response to hepatitis C virus exposure in utero. J Infect Dis 2011;203:196‐206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fauteux‐Daniel S, Larouche A, Calderon V, Boulais J, Béland C, Ransy DG, et al. Vertical transmission of hepatitis C virus: variable transmission bottleneck and evidence of midgestation in utero infection. J Virol 2017;91:e01372‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cervino L, Hynicka LM. Direct‐acting antivirals to prevent vertical transmission of viral hepatitis C: when is the optimal time to treat? Ann Pharmacother 2018;52:1152‐1157. [DOI] [PubMed] [Google Scholar]
- 41. Bortolotti F, Vajro P, Cadrobbi P, Lepore L, Zancan L, Barbera C, et al. Cryptogenic chronic liver disease and hepatitis C virus infection in children. J Hepatol 1992;15:73‐76. [DOI] [PubMed] [Google Scholar]
- 42. Nydegger A, Srivastava A, Wake M, Smith AL, Hardikar W. Health‐related quality of life in children with hepatitis C acquired in the first year of life. J Gastroenterol Hepatol 2008;23:226‐230. [DOI] [PubMed] [Google Scholar]
- 43. Rodrigue JR, Balistreri W, Haber B, Jonas MM, Mohan P, Molleston JP, et al. Impact of hepatitis C virus infection on children and their caregivers: quality of life, cognitive, and emotional outcomes. J Pediatr Gastroenterol Nutr 2009;48:341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Younossi ZM, Stepanova M, Balistreri W, Schwarz K, Murray KF, Rosenthal P, et al. Health‐related quality of life in adolescent patients with hepatitis C genotype 1 treated with sofosbuvir and ledipasvir. J Pediatr Gastroenterol Nutr 2018;66:112‐116. [DOI] [PubMed] [Google Scholar]
- 45. Indolfi G, Bartolini E, Olivito B, Azzari C, Resti M. Autoimmunity and extrahepatic manifestations in treatment‐naive children with chronic hepatitis C virus infection. Clin Dev Immunol 2012;2012:785627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murray KF, Balistreri WF, Bansal S, Whitworth S, Evans HM, Gonzalez‐Peralta RP, et al. Safety and efficacy of ledipasvir‐sofosbuvir with or without ribavirin for chronic hepatitis C in children ages 6‐11. Hepatology 2018; 10.1002/hep.30123. [DOI] [PubMed] [Google Scholar]
- 47. Wherry LR. State medicaid expansions for parents led to increased coverage and prenatal care utilization among pregnant mothers. Health Serv Res 2018;53:3569‐3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kanninen TT, Dieterich D, Asciutti S. HCV vertical transmission in pregnancy: new horizons in the era of DAAs. Hepatology 2015;62:1656‐1658. [DOI] [PubMed] [Google Scholar]
- 49. Clinicaltrials.gov . Study of hepatitis C treatment during pregnancy (HIP). https://www.clinicaltrials.gov/ct2/show/NCT02683005?recrs=ab&cond=hepatitis+c+pregnancy&rank=2. Accessed October 2018.
- 50. Clinicaltrials.gov . Transmission of chronic hepatitis C in pregnancy. https://www.clinicaltrials.gov/ct2/show/NCT03570112?recrs=ab&cond=hepatitis+c+pregnancy. Accessed October 2018.
- 51. Yattoo GN. Treatment of chronic hepatitis C with ledipasvir/sofosbuvir combination during pregnancy [Abstract]. Hepatol Int 2018;12(Suppl. 2):S292‐S293. [Google Scholar]
- 52. Kushner T, Cohen J, Tien PC, Terrault NA. Evaluating women's preferences for hepatitis C treatment during pregnancy. Hepatol Commun 2018; 10.1002/hep4.1264. Accessed October 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Antiretroviral Pregnancy Registry Steering Committee . The antiretroviral pregnancy registry interim report 1 January 1989 through 31 January 2018. Wilmington, NC: Registry Coordinating Center; 2017. https://www.apregistry.com/InterimReport.aspx. Accessed October 2018. [Google Scholar]
- 54. European Paediatric Hepatitis C Virus Network . A significant sex–but not elective cesarean section–effect on mother‐to‐child transmission of hepatitis C virus infection. J Infect Dis 2005;192:1872‐1879. [DOI] [PubMed] [Google Scholar]
- 55. Gibb DM, Goodall RL, Dunn DT, Healy M, Neave P, Cafferkey M, et al. Mother‐to‐child transmission of hepatitis C virus: evidence for preventable peripartum transmission. Lancet 2000;356:904‐907. [DOI] [PubMed] [Google Scholar]
- 56. McMenamin MB, Jackson AD, Lambert J, Hall W, Butler K, Coulter‐Smith S, et al. Obstetric management of hepatitis C‐positive mothers: analysis of vertical transmission in 559 mother‐infant pairs. Am J Obstet Gynecol 2008;199:315.e1‐e5. [DOI] [PubMed] [Google Scholar]
- 57. European Paediatric Hepatitis C Virus Network . Effects of mode of delivery and infant feeding on the risk of mother‐to‐child transmission of hepatitis C virus. European Paediatric Hepatitis C Virus Network. BJOG 2001;108:371‐377. [PubMed] [Google Scholar]
- 58. Ceci O, Margiotta M, Marello F, Francavilla R, Lerardi E, Loizzi P, et al. High rate of spontaneous viral clearance in a cohort of vertically infected hepatitis C virus infants: what lies behind? J Hepatol 2001;35:687‐688. [DOI] [PubMed] [Google Scholar]
- 59. Conte D, Fraquelli M, Prati D, Colucci A, Minola E. Prevalence and clinical course of chronic hepatitis C virus (HCV) infection and rate of HCV vertical transmission in a cohort of 15,250 pregnant women. Hepatology 2000;31:751‐755. [DOI] [PubMed] [Google Scholar]
- 60. Garland SM, Tabrizi S, Robinson P, Hughes C, Markman L, Devenish W, et al. Hepatitis C–role of perinatal transmission. Aust N Z J Obstet Gynaecol 1998;38:424‐427. [DOI] [PubMed] [Google Scholar]
- 61. Okamoto M, Nagata I, Murakami J, Hino S, Shiraki K. Shift in the buoyant density of hepatitis C virus particles in infants infected by mother‐to‐infant transmission. Pediatr Int 1999;41:369‐373. [DOI] [PubMed] [Google Scholar]
- 62. Tajiri H, Miyoshi Y, Funada S, Etani Y, Abe J, Onodera T, et al. Prospective study of mother‐to‐infant transmission of hepatitis C virus. Pediatr Infect Dis J 2001;20:10‐14. [DOI] [PubMed] [Google Scholar]
- 63. Zanetti AR, Tanzi E, Newell ML. Mother‐to‐infant transmission of hepatitis C virus. J Hepatol 1999;31(Suppl. 1):96‐100. [DOI] [PubMed] [Google Scholar]
- 64. La Torre A, Biadaioli R, Capobianco T, Colao MG, Monti M, Pulli F, et al. Vertical transmission of HCV. Acta Obstet Gynecol Scand 1998;77:889‐892. [PubMed] [Google Scholar]
- 65. Moriya T, Sasaki F, Mizui M, Ohno N, Mohri H, Mishiro S, et al. Transmission of hepatitis C virus from mothers to infants: its frequency and risk factors revisited. Biomed Pharmacother 1995;49:59‐64. [DOI] [PubMed] [Google Scholar]
- 66. Pipan C, Amici S, Astori G, Ceci GP, Botta GA. Vertical transmission of hepatitis C virus in low‐risk pregnant women. Eur J Clin Microbiol Infect Dis 1996;15:116‐120. [DOI] [PubMed] [Google Scholar]
- 67. Tanzi M, Bellelli E, Benaglia G, Cavatorta E, Merialdi A, Mordacci E, et al. The prevalence of HCV infection in a cohort of pregnant women, the related risk factors and the possibility of vertical transmission. Eur J Epidemiol 1997;13:517‐521. [DOI] [PubMed] [Google Scholar]
- 68. Zanetti AR, Tanzi E, Romano L, Zuin G, Minola E, Vecchi L, et al. A prospective study on mother‐to‐infant transmission of hepatitis C virus. Intervirology 1998;41:208‐212. [DOI] [PubMed] [Google Scholar]
- 69. Lin HH, Kao JH, Hsu HY, Ni YH, Chang MH, Huang SC, et al. Absence of infection in breast‐fed infants born to hepatitis C virus‐infected mothers. J Pediatr 1995;126:589‐591. [DOI] [PubMed] [Google Scholar]


