Abstract
Objective(s):
Black cumin (Nigella sativa) belonging to Ranunculaceae family has a long history of medicinal use in various folk and traditional systems of medicine, including Iranian traditional medicine (ITM). These valuable medicinal seeds have been used traditionally against a variety of diseases such as dyspepsia, diabetes, headache, influenza and asthma. In addition, several scientific investigations have reported the therapeutic properties of N. sativa and thymoquinone (TQ), one of the most important constituent of black cumin, for treatment of a large number of diseases, including ischemia. As there is no comprehensive review study about the anti-ischemic activity of black cumin and its mechanism of action, in the current study, we aimed to review the anti-ischemic activities of N. sativa and TQ in different organ-related disorders.
Materials and Methods:
We searched the words N. sativa or black cumin and ischemia in the combination of related organs through available databases including Scopus, Web of Science, and Google Scholar.
Results:
Several studies were found reporting the anti-ischemic activity of black cumin and its active constituent on different organs including brain, kidneys, heart, and liver. Black cumin exert its beneficial effects as an antioxidant, anti-inflammatory, anti-apoptosis, and anti-necrosis agent through inhibition of growth factors, biochemical and oxidative stress markers and regulating gene expression.
Conclusion:
Thus, N. sativa could be a potential candidate for treatment of ischemia related disorders in key organs such as brain, liver, digestive system, kidney, and heart. To figure out the exact mechanism of action, further investigations are proposed in this regard.
Key Words: Black cumin, Brain, Cardiovascular, Ischemia, Nigella sativa
Introduction
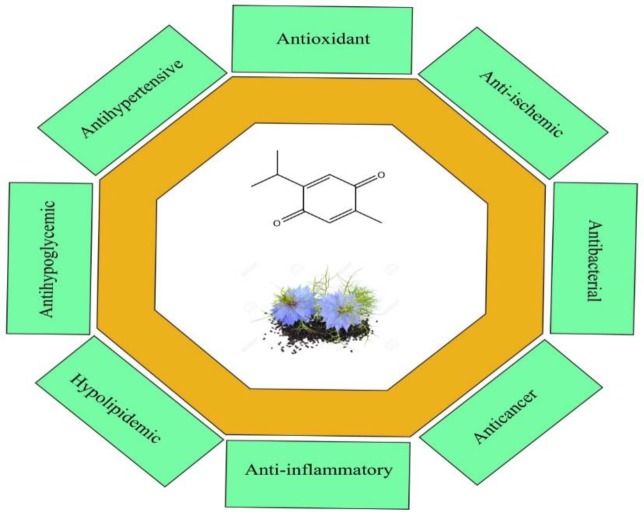
The date of using plants as medicines by humans is not certain but the historical findings show that at least it dates back nearly 4000 years ago. Interest in medicinal plants is due to newly observed valuable activities of natural drugs which lead to the growing interest in the field of natural product research. In addition, this interest has been increased because of significant adverse effects of synthetic drugs leading to substitution of natural products for the treatment of various diseases in the past decade (1, 2). Nigella sativa belonging to Ranunculaceae family is a dicotyledonous plant and is mostly distributed in Southern Europe, North Africa, and Asia Minor. This amazing plant has a rich religious and historical background (3). It is a bushy plant with white or pale to dark blue flowers. The seeds are the parts of the plant which is used in herbal medicines; when the fruit capsules open, the black seeds are dispersed in the air (4). N. sativa has been used for treatment of various diseases from ancient times. In the Holy Bible, it was identified as the curative black cumin and Hippocrates described it as the Melanthion and Dioscorides as the Gith of Pliny (5). This plant is also known as the black seed and black cumin. Historically, this plant has been used for the treatment of toothache, flatulence, as a choleretic, anti-spasmodic and uricosuric (6), nasal congestion, hypertension, obesity, back pain, amenorrhea (7), infections, and intestinal worms (3, 4, 8, 9). Studies have revealed that black cumin and its bioactive constituent, thymoquinone (TQ) have hypoglycemic (10, 11), hypolipidemic (12), antioxidant (13, 14), anti-inflammatory (15), anti-tumor, anti-convulsant, and anti-tussive activities (16-19) (Figure 1). In addition, it is a protective agent against natural or chemical toxins (20), an anti-aging agent (21) and a protective agent against ethanol induced toxicity (22).
Figure 1.
The chemical structure of thymoquinone (TQ) and a schematic diagram of the pharmacological activities of Nigella sativa and TQ
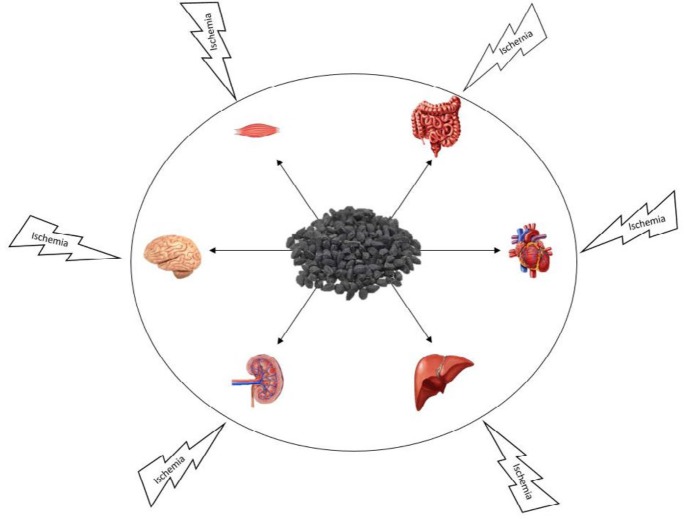
Ischemia-reperfusion injury (IRI) is a complex of intracellular chemical operation and inflammatory response. Reduction in cellular energy (ATP) content, as one of the mechanisms for IRI, leads to destroy cellular ion homeostasis with activation of hydrolases and damage of elective permanence of cell membranes and cause of decrease in organ function after transplantation and limit in survival of transplanted organs (23) (Figure 2). IRI damages O2-dependent cells of tissues and organs such as brain, liver, kidney, intestine and heart; mitochondrial oxidative phosphorylation can provide energy need of these cells which cannot be supplied by anaerobic glycolysis alone (24, 25). A reduction in mitochondrial energy leads to decrease in pH of the cell, increase in the anaerobic glycolytic level, and release of H+ from the lysosomal; Finally, the increasing flow rate of cytosolic Na+ and Ca2+ destroys cellular ion homeostasis (23).
Figure 2.
Protective effects of Nigella sativa against ischemia in different organs
In this study, the anti-ischemic activity of N. sativa and TQ on different organs including brain, liver, kidney, etc. is reviewed.
Methods
The data were collected by searching through scientific databases PubMed, Scopus, Google Scholar, and Web of Science. N. sativa, black cumin, and ischemia were the main keywords used as search terms. All kinds of relevant articles, abstracts, or books were included. Furthermore, the reference lists of key papers for further leads were searched. Both in vivo and in vitro studies were included to this investigation. No time limitation was considered in this review.
Phytochemicals
The phytochemicals found in black cumin can be divided in two categories; volatile compounds and non-volatile metabolites.
Volatile compounds
The biological activity of N. sativa is mostly attributed to its volatile oil compounds especially TQ (26). Black cumin contains 0.40–0.45 %w/w of a volatile oil including 18.4–24% TQ, and 46% other monoterpenes such as ρ-cymene, thymol, carvacrol, ρ-cymene and α- and β-pinene (27).
The essential oil of black cumin is usually obtained by hydrodistillation, however other methods such as microwave-assisted extraction and super critical fluid extraction is also used (28, 29). Botnick et al. has proposed a method for the isolation of black cumin essential oil. For this purpose, the seeds were ground with a mortar and pestle after they were frozen in liquid N2. The extraction of the volatile fraction was made by adding a 3 to 1 ratio (v/w) of tert-butyl methyl ether (MTBE). After a short vortex, the ground seeds were shaken for 2 h at room temperature (25-30 oC) for extracting volatile fraction (30).
Non-volatile compounds
The other category of secondary metabolites found in N. sativa is non-volatile compounds. Alkaloids are one of the most important bioactive constituents of N. sativa; the seeds have two different forms of alkaloids: isoquinoline alkaloids such as nigellicimine and pyrazol alkaloids including nigellidine and nigellicine. Moreover, saponins, fatty acids, carbohydrates, fixed oils, proteins, and phenolic compounds such as flavonoids, have been reported from this plant (31, 32). The plant is also a source of calcium, iron, and potassium (33).
Pharmacological activities
A large number of activities have been reported for N. sativa and its bioactive constituents including anti-convulsant (34, 35), hypotensive, anti-nociceptive, uricosuric, choleretic, anti-fertility, anti-diabetic, anti-microbial and antibacterial (31, 36). N. sativa have also shown analgesic, anti-inflammatory (37), anti-cancer (38), anti-histaminic (39), and neuroprotective (40, 41) properties (Figure 1). Moreover, N. sativa and TQ had a potential role in the management of metabolic syndrome and cardiovascular diseases risk factors including high blood pressure, obesity, dyslipidemia and high blood glucose (42). Among these various activities, black cumin has strong effects against ischemia reperfusion (IR) injury on various organs. In the following paragraphs, the anti-ischemic properties of N. sativa and its bioactive compound namely TQ on various organs will be discussed.
Anti-ischemic activities
Brain
Ischemic brain damage as one of the most important causes of adult disability (43), stops the blood flow completely and produces inadequate delivery of oxygen to the brain tissue that lead to a decrease in glucose and adenosine triphosphate (ATP) levels through anaerobic glycolysis. Lactic acidosis produced by ATP discharge can reduce energy and operating cofactor redistribution of fast ions in neuronal and glial cell and depolarization occurs on cell membrane (44). Events that generally occur during ischemia including brain ischemia are: 1) The loss of membrane lipid by lipolysis and by radical-mediated peroxidation of poly-unsaturated fatty acids during reperfusion; 2) Protein synthesis in the brain is inhibited at the translation initiation and prolonged arrest of protein synthesis in elective vulnerable neurons during post-IR; 3) Apoptotic mechanisms, activation of proteolysis, and activation of endonucleases lead to injury of critical molecules and destroy cellular repair processes during reperfusion.
Antioxidants have essential roles in recovery of damaged brain cells (45). They regulate initiation of translation by various mechanisms including activation of initiation factors, inhibition of apoptosis, and improve the restoration of injured organelles at a fundamental level by signal transduction mechanisms involving growth factors (45). Studies show that different extracts of N. sativa and TQ have antioxidant and neuroprotective activities in cerebral ischemia (46, 47). In vitro and in vivo investigations have reported an increase in reduced glutathione (GSH), superoxide dismutase (SOD), and catalase levels which are indicative of cerebral ischemia. In addition, after administration of aqueous and hydroalcoholic extracts of N. sativa, a decrease in malondialdehyde (MDA) level and an inhibition in lipid peroxidation have also been reported in several studies (48). For example, Al-Majed et al. have reported that oral administration of TQ could reduce MDA level and elevate GSH and SOD levels in transient forebrain ischemia in the rat hippocampus (49). Hosseinzadeh et al. confirming the results of the previous study, reported the protective effects of N. sativa seeds oil and TQ on cerebral ischemia in a rat model. The results exhibited the beneficial effects of N. sativa on lipid peroxidation during global cerebral IRI (50). In some studies, the protective activity of TQ against hippocampal neurodegeneration has been investigated (51, 52). Remarkable changes were observed in biochemical factors after the treatment with TQ (5 mg/kg, PO); a significant decrease in MDA content, lipid peroxidation, and an increase in GSH level, SOD and catalase activities (52). Edema is one of the major consequences of both peripheral and intracellular global IRI which happens in pyramidal and interstitial cornu ammonis cells as well as supportive neuronal tissue such as cytoplasmic glial cells (53). In a rat model, different doses of N. sativa extracts (1, 10, and 50 mg/kg) were injected to the animals. The results showed that the extract has a significant ability to protect neuronal tissue of hippocampus and prevents edema in a dose-dependent manner (52). Soleimannejad et al. showed that hydroalcoholic extract of N. sativa (20 mg/kg) could increase the markers of cerebral angiogenesis after global ischemia of brain in rats (54). Accordingly, a significant decrease in brain edema and infarct volume, an increase in gene expression of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor (HIF), and a decrease in the expression level of matrix metallopeptidase-9 were observed. The inhibitory effect of black cumin against cerebral IRI is also confirmed by in vitro and in vivo studies (52, 55). Effects of various extracts of N. sativa such as aqueous, hydro-alcoholic, chloroform and petroleum ether refers to decline lipid peroxidation and increase glutathione and antioxidant enzymes, (superoxide dismutase and catalase) in middle cerebral artery occluded rats (56, 57). Black cumin extract also could reduce hippocampal neurons swelling and astrocytes loss after global cerebral IRI in rats. N. sativa extract (1 and 10 mg/kg) also could prevent the edema of pyramidal neurons (52). Moreover, pre- and co-administration of TQ (20 mg/kg) has also been reported to decrease brain ischemia induced by neurotoxic effects of lead in rats through the same mechanism of action (58).
Kidneys
Renal ischemia injury is one of the ordinary difficulty that occurs during transplantation, retail nephrectomy, cardiopulmonary bypass, or hydronephrosis that may lead to kidney dysfunction (59). This problem may also happen in the context of cardiac arrest during recovery vascular operation which is an important cause of renal cell death, renal failure, and delayed graft function (60, 61). There are substantial number of contributor to renal ischemia including anoxia, free radicals and inflammation responses (62, 63). ROS and stress oxidative lead to structural and functional damages (59) including detrimental effects in endothelial, glomerular, mesangial, and tubular epithelial cells (particularly S3 segment of proximal tubule) (64, 65), a reduction in the impact of ion pump activity and DNA damages such as DNA inhibiting transcription and repair (66, 67) causing acute kidney injury, acute rejection, acute tubular necrosis, chronic allograft failure, and acute renal failure (63, 68, 69). N. sativa has remarkable beneficial effects on renal ischemia mostly because of its immunomodulatory and antioxidant properties (70). Aqueous and ethanol extract of N. sativa (1.6 g/kg) as well as TQ are reported to have protective effects on renal ischemia by decreasing MDA content of renal tissue and increasing the renal thiol content. They can also diminish free radical-mediated lipid peroxidation, DNA damage and reduce thiobarbituric acid reactive substances (TBARS) levels (71-75). It is also reported that pre- and post-treatment of N. sativa seed oil can diminish kidney oxidative stress signs and tubular necrosis score after IRI (76). Several other studies confirm the protective effects of N. sativa on renal ischemia via decreasing oxidative stress markers. In 2008, Omer Bayraket et al. investigated the protective effect of N. sativa against IRI in rat kidneys. They demonstrated that pre- and post-treatment with N. sativa oil has protective effects on IRI through improving renal failure including a decrease in MDA levels, nitric oxide (NO) concentration and protein carbonyl content (PCC) in serum and tissue, as compared to the control group (77).
Pretreatment with N. sativa significantly can decrease the levels of creatinine, blood urea nitrogen, and uric acid as well as the water intake and urinary excretion. In addition, the ratio of renal to body weight (the renal-body weight degree) is diminished and the index of histopathological damages such as cellular vacuolation, interstitial edema, hyperemia, hemorrhage, tubular necrosis, and glomerular changes are improved (78). Studies show that N. sativa not only affect biochemical parameters, but also it has influences on gene and protein expression. TQ decreased cytochrome P450 3A1 (CYP3A1) and spermidine/spermine N-1-acetyl-transferase (SSAT) gene expression that were up-regulated in ischemia in the kidneys (79). In another study, confirming the protective effects of TQ, pre- and post- administration of TQ could result in a significant improvement in the tubular renal cells and hemodynamic functional parameters and in debilitation of the gene expression of some of the pro-fibrotic cytokines and pro-inflammatory, namely tumor transforming growth factor beta 1 (TGF-β1), the type-1 inhibitor of plasminogen activator (PAI) and necrosis factor alpha (TNF-α). In addition, TQ reduced the expression of some of the markers of acute renal injury such as neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecular 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) (80). Moreover, N. sativa could decrease the degenerative changes in the glomerulus and tubules of kidney cortex and the positivity of NF-kappa-B p65 subunit (NFkB p65) in the renal cortical tissues (81).
Table 1.
Anti-ischemic activity of Nigella sativa and its active constitute TQ on different organs
| Ischemic condition | Study design | Result | Ref. | |
|---|---|---|---|---|
| Transient global cerebral ischemia | Five N.G Wister albino rats i.p administration | Reduction in MDA and elevation in thiol (SH) | (137) | |
| Focal hippocampus ischemia | Wister rats (N=30), (1, 10, and 50 mg/kg, i.p) | Prevent intracellular edema of interneurons in 50 mg/kg group significantly compared to the sham group | (49) | |
| Transient global cerebral ischemia | TQ (2.5, 5 and 10 mg/kg) and N. sativa oil (0.048, 0.192 and 0.384 mg/kg, i.p), Rats | Dose-dependent reduction in the free radical-mediated lipid peroxidation as indicated by a decrease in the MDA levels | (138) | |
| Renal ischemia | Wistar albino rats, 0.3 ml gastric tube | Significant reduction in serum and tissue MDA, NO and PCC and subsequent elevation in anti-oxidant power | (69) | |
| Rats, 150-300 mg/kg, i.p | Dose-dependent reduction in MDA level and an elevation in total Thiol content and glutathione peroxidase Decreases in oxidative DNA damage |
(70) | ||
| Hepatic ischemia | Wister rats, Aqueous extract (0.7, 1 and 1.6 g/kg) ethanol extract (0.7, 1 and 1.6 g/kg) and TQ (2.5, 5 and 10 mg/kg, i.p.) | No significant effect on the radical-mediated lipid peroxidation (aqueous extract) A significant reduction in the MDA levels (ethanol extract, 1.6 g/kg) TQ in doses 5 and 10 mg/kg reduced free radical-mediated lipid peroxidation significantly, by a decrease in the MDA levels TQ pretreated groups, a reduction in TBARS levels, ethanolic extract only in upper dose increased anti-oxidant power |
(68) | |
| Wister-albino rats, TQ (5, 20, and 50 mg/kg) | A decrease in plasma ALT, AST, and LDH levels A significant decrease in pathological changes and histological tissue damages |
(89) | ||
| Heart ischemia | Wistar albino rats (n=44), TQ (10 mg/kg, i.p) | A significant decrease in infarct size Protective effect against reperfusion-induced arrhythmias |
(133) | |
| Wistar rats (20 mg/kg orally) | No significant change in biochemical and histological parameters A significant decrease in the heart weight/body weight ratio Improvement in myocardial SOD, GSH activity, A decrease in the level of MDA, AST, ALT, LDH, and CK (the diagnostic markers of myocardial) A significant decline in the myocardial levels of IL-1, IL-6, and TNF-α |
(139) | ||
| Intestinal ischemia | Rats, TQ (50 mg/kg i.p.) | Dose-dependent reduction in MDA An elevation in SOD activity, GSH-Px activity Reduction in the number of apoptotic cells |
(112) | |
| Skeletal ischemia | Rats, aqueous extract (1, 1.5 and 2 mg/kg), ethanol extracts (1.6, 2.4 and 3.2 g/kg, i.p.) | Decrease in MDA level, Increase SH level Increased anti-oxidant power (FRAP value) |
(45) | |
Liver
Another organ that may be influenced by ischemia is liver. Hepatic IRI is an important factor associated with a high morbidity and mortality that lead to destructive effect on metabolic and structural functions (82). Generally, it happens in conditions with low blood flow to the liver resulting in insufficient perfusion such as liver resections and transplantation (83), cardiogenic and hemorrhagic shock, surgical cutting, cardiovascular or laparoscopic surgery and abdominal compartment syndromes (84, 85). IRI of the liver is complex and multifactorial pathophysiological process involving the generation of numerous ROS and reactive nitrogen species (RNS) (86-88). In the early stages of reperfusion in the liver, cellular edema, vacuolization, endothelial cell disruption, neutrophil penetration, and hepatocellular necrosis may occur (89). To decrease these mechanisms and phenomena in hepatic IRI, several investigations have been established. In this regard, natural products play important roles. The activity of N. sativa as a potential medicinal plant against liver ischemia has been studied by different research groups (91, 92). For instance, it is reported that TQ has a strong positive effect against liver IRI, and can down-regulate the expression of CYP3A1 and SSAT gene in the ischemic liver (79). The efficacy of N. sativa to postpone progression in chronic liver diseases should be considered as preventive medicine in patients with hepatic disorders (91).
TQ could also decrease the adverse effects of ROS in IRI condition by increasing catalase activity which shows a potent protective effect on liver tissue. In the liver ischemia, apoptotic cell death is activated and as a consequence, the levels of caspases 8, 9, and 3 are increased. This study has shown that TQ (50 mg/kg) with anti-apoptotic effects could reduce the activities of caspases 8 and 3 via direct and indirect mechanisms. TQ could deactivate caspase 8 and inhibit cytochrome c release from mitochondria and finally inactivate caspase 3. Decrease in cytosolic cytochrome c rate lead to inhibition of lipid peroxidation measured as MDA liver tissue content. In addition, TQ could decrease pro-apoptotic bax protein expression and increase significantly the anti-apoptotic Bcl-2 protein. NF-κB signaling pathway is one of the multiplex apoptotic pathways activated by TNF-α; one of the anti-ischemic activity for TQ is reduction the expression of TNF-α and NF-κB in hepatic I/R models (92, 93). Moreover, administration of TQ (5, 20, and 50 mg/kg) could decrease both aspartate transaminase (AST) and alanine transaminase (ALT) activities as compared to the untreated group.
Other organs
Although most of the studies have focused on the protective activities of N. sativa against renal, hepatic, and brain ischemia, there are several reports about the anti-ischemic properties of this plant extract and its major component TQ on other important systems and organs. In the following paragraphs, you can find some investigations on the anti-ischemic activity of black cumin in digestive, and cardiovascular systems, as well as skeletal muscles.
Digestive system (Intestine)
Several conditions lead to intestinal IRI and numerous damages to the intestine, for instance, shock, incarcerated hernia, cardiopulmonary bypass, midgut volvulus, necrotizing enterocolitis, multiple traumas, and sepsis (94-96). Moreover, mesenteric ischemia caused by different kind of factors such as endogenous vasocontractile substances may lead to develop intestinal IRI (97). Interestingly, mesenteric IR lead to many damages such as hepatic and renal injuries (98). Iglesias et al. demonstrated that mesenteric IRI causes acute pulmonary edema and increase the pulmonary microvascular permeability to fluids and proteins (99). Intestinal IR damages lead to detrimental events such as systemic inflammatory response, increasing NO and poly morphonuclear lymphocytes (PMNL), and production of ROS and RNS (100-102). For example, Horton and White reported that production of ROS and lipid peroxidation in cardiac cell membranes play a significant role in cardiac dysfunction after intestinal IRI in rats (103). This multiple organ dysfunction syndrome is related to an interference in energy metabolism, oxygen radicals, overload of intracellular calcium, endothelial cell damage, and leukocyte adhesion (104-106). TQ as a potent antioxidant can suppress intestinal IR damage and decrease oxidative stress. TQ can decrease MDA, SOD, and erythrocyte glutathione peroxidase (GSH-Px)-GSH-Px levels in IR-damaged intestinal tissue (107). El-Abhar et al. have shown that black cumin can improve the antioxidant conditions due to an increase in mucin content of the gastric mucosa (90). Black cumin oil has also a protective effect against intestinal ischemia through inhibition the release of leukotrienes and histamine from mast cells (102, 105). Anti-apoptotic effect is reported to be an important protective mechanism of N. sativa against intestinal ischemia (108-113). Al Mofleh et al. showed pathologically that N. sativa can prohibit gastric ulcer formation induced by necrotizing factors (114). Treatment with TQ improved pathology and significantly decreased the number of TUNEL-positive cells (115, 116). Studies show that N. sativa have protective effect against ischemia-induced gastric mucosal ulcer (107).
Skeletal muscle
IRI in skeletal muscle, as an important cause of morbidity and mortality in populations, motivate an inflammatory response in the affected muscles (117). Blaisdell et al. reported that there are substantial numbers of mortality resulting from several system organ failures in severe cases of limb ischemia (118). It is characterized by a number of detrimental phenomena in tissue, such as cell elevation penetrance in the microcirculation, edema, mitochondrial electrolytic change, increased cytosolic free calcium concentration, decrease of membrane phospholipids in ischemic cells and releasing cytotoxic ROS and simulate an inflammatory response (119, 120).
Studies have revealed that hindlimb ischemia in a Wistar rat model is produced by clamping the common femoral artery and vein mitochondrial electrolytic changes, cell edema, increased permeability in the microcirculation, cytosolic calcium overload, ROS generation, decrease in membrane phospholipids (118, 121). Hosseinzadeh et al. have investigated the effects of N. sativa and its constituent TQ in decreasing skeletal muscle during ischemia. The results of this study show that after treatment with TQ, MDA content is decreased and GSH level is increased significantly (122). In a rat model, pre-administration of N. sativa seed aqueous (1, 1.5 and 2 g/kg) and ethanol (1.6, 2.4 and 3.2 g/kg) extracts intraperitoneally lead to a decrease in MDA levels, an increase in antioxidant capacity (FRAP value) of muscle homogenate samples, and a significant elevation in thiol (SH) concentration, as compared with control-ischemic group (48). It is reported that the administration of TQ plus alpha-tocopherol may strongly protect muscle and nerve tissues against IRI due to their synergistic effects (123). These compounds together could have protective effects on the sciatic nerve and femoral muscle as a result of lower limb IRI. They could significantly decrease the levels of MDA, interleukin-6 (IL-6), and neuronal nitric oxide synthase activity of nerve tissues and increase the level of GSH. By recording intramuscular electro myograph (EMG) signals, it is reported that TQ could increase the muscles activities after IRI in comparison to IR control group (119).
Heart
Myocardium ischemic can trigger a series of deleterious phenomena such as myocardial damage and life-threatening ventricular arrhythmias. Following the reperfusion of the ischemic myocardium, ROS products lead to decreases in antioxidant activity, generation of lethal ventricular arrhythmias and tissue injury. Studies show that TQ plays an important role on myocardial IRI and reduce the infarct size and suppresses arrhythmia scores, ventricular tachycardia and the incidence of ventricular fibrillation. Intraperitoneal administration of TQ (10 mg/kg) seems to be effective in preserving myocardial IRI induced lethal ventricular arrhythmias in anesthetized rats (124-126). A great number of research projects have shown that antioxidants with suppressive effects could delimit infarction rate and decrease myocardial dysfunction and decelerate development of myocardial infarction (MI) (127, 128). ROS play an important role in the pathophysiology of MI (129, 130). Moens et al. in 2005 noted that production of ROS after ischemia and reduction in antioxidant task lead to tissue dysfunction and produce ventricular arrhythmias (131). Various herbal antioxidants have a beneficial effect against myocardial IRI (132-135). TQ could reduce MI by affecting the antioxidant condition and decreasing the ROS levels. For instance, TQ (10 and 20 mg/kg) showed a protective effect in myocardial IR damage in a rat model (136). In another study, it is reported that chronic treatment (3 months) of TQ lead to reduction of oxidative stress in MI and help to maintain the activity of antioxidant enzymes in isoproterenol-induced MI rat model (137). TQ could also reduce heart rate and arterial blood pressure (137). In vitro studies have shown that TQ has cardiovascular activity, and regulates arterial force and rate of constriction intercede by blockade of voltage gated Ca+2 channels (138).
Ovary
Atasever et al. have studied the N. sativa oil on ovarian oxidative damage following IRI, using a rat model (139). They did not observe a significant improvement in N. sativa oil-treated group in comparison to control groups. Thus, they proposed further studies to confirm or reject the data.
Conclusion
All findings discussed above indicate that N. sativa and its active constituent TQ have strong effects against IRI on various organs, including brain, liver, digestive system, kidney, skeletal-muscle system, and heart. Black cumin has a long reputation in traditional medicine and in recent years it has been used for treatment of several disorders without any reported side effects. Therefore, this plant can be a valuable agent for ischemia problems. Thus, further complementary studies are proposed in this regard.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- 1.Dattner AM. From medical herbalism to phytotherapy in dermatology: back to the future. Dermatol Ther. 2003;16:106–113. doi: 10.1046/j.1529-8019.2003.01618.x. [DOI] [PubMed] [Google Scholar]
- 2.Fong HH. Integration of herbal medicine into modern medical practices: issues and prospects. Integr Cancer Ther. 2002;1:287–293. doi: 10.1177/153473540200100313. [DOI] [PubMed] [Google Scholar]
- 3.Goreja W. Black seed: nature’s miracle remedy. Karger Publishers; 2003. [Google Scholar]
- 4.Schleicher P, Saleh M. Black seed cumin: the magical Egyptian herb for allergies, asthma, and immune disorders. Rochester, Vermont: Healing Arts Press; 1998. [Google Scholar]
- 5.Junemann M, Luetjohann S. Three great healing herbs. 1st ed. Lotus Press (WI); 1998. [Google Scholar]
- 6.Pourbakhsh H, Taghiabadi E, Abnous K, Hariri AT, Hosseini SM, Hosseinzadeh H. Effect of Nigella sativa fixed oil on ethanol toxicity in rats. Iran J Basic Med sci. 2014;17:1020–1031. [PMC free article] [PubMed] [Google Scholar]
- 7.Ziaee T, Moharreri N, Hosseinzadeh H. Review of pharmacological and toxicological effects of Nigella sativa and its active constituents. J Med Plant. 2012;2:16–42. [Google Scholar]
- 8.El-Dakhakhny M. Studies on the Egyptian Nigella sativa L IV Some pharmacological properties of the seeds’ active principle in comparison to its dihydro compound and its polymer. Arzneim-Forsch. 1965;15:1227–1229. [PubMed] [Google Scholar]
- 9.Al-Rowais NA. Herbal medicine in the treatment of diabetes mellitus. Saudi Med J. 2002;23:1327–1331. [PubMed] [Google Scholar]
- 10.Kaleem M, Kirmani D, Asif M, Ahmed Q, Bano B. Biochemical effects of Nigella sativa seeds in diabetic rats. Indian J Exp Biol. 2006;44:745–748. [PubMed] [Google Scholar]
- 11.Meddah B, Ducroc R, Faouzi MEA, Eto B, Mahraoui L, Benhaddou-Andaloussi A, et al. Nigella sativa inhibits intestinal glucose absorption and improves glucose tolerance in rats. J Ethnopharmacol. 2009;121:419–424. doi: 10.1016/j.jep.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 12.Bamosa AO, Ali BA, al-Hawsawi ZA. The effect of thymoquinone on blood lipids in rats. Indian J Physiolo Pharmacol. 2002;46:195–201. [PubMed] [Google Scholar]
- 13.Uz E, Bayrak O, Kaya A, Bayrak R, Uz B, Turgut F, et al. Nigella sativa oil for prevention of chronic cyclosporine nephrotoxicity: an experimental model. Am J nephrol. 2008;28:517–522. doi: 10.1159/000114004. [DOI] [PubMed] [Google Scholar]
- 14.Kanter M, Coskun O, Uysal H. The antioxidative and anti-histaminic effect of Nigella sativa and its major constituent, thymoquinone on ethanol-induced gastric mucosal damage. Arch Toxicol. 2006;80:217–224. doi: 10.1007/s00204-005-0037-1. [DOI] [PubMed] [Google Scholar]
- 15.Al-Ghamdi M. The anti-inflammatory, analgesic and anti-pyretic activity of Nigella sativa. J Ethnopharmacol. 2001;76:45–48. doi: 10.1016/s0378-8741(01)00216-1. [DOI] [PubMed] [Google Scholar]
- 16.Ait Mbarek L, Ait Mouse H, Elabbadi N, Bensalah M, Gamouh A, Aboufatima R, et al. Anti-tumor properties of blackseed (Nigella sativa L) extracts. Braz J Med Biol Res. 2007;40:839–847. doi: 10.1590/s0100-879x2006005000108. [DOI] [PubMed] [Google Scholar]
- 17.Machmudah S, Shiramizu Y, Goto M, Sasaki M, Hirose T. Extraction of Nigella sativa L using supercritical CO2: a study of antioxidant activity of the extract. Sep Sci Technol. 2005;40:1267–1275. [Google Scholar]
- 18.Parvardeh S, Nassiri-Asl M, Mansouri M, Hosseinzadeh H. Study on the anti-convulsant activity of thymoquinone, the major constituent of Nigella sativa L seeds through intracerebroventricular injection. J Med Plant. 2005;2:45–52. [Google Scholar]
- 19.Hosseinzadeh H, Eskandari M, Ziaee T. Anti-tussive effect of thymoquinone, a constituent of Nigella sativa seeds, in guinea pigs. Pharmacologyonline. 2008;2:480–484. [Google Scholar]
- 20.Tavakkoli A, Ahmadi A, Razavi BM, Hosseinzadeh H. Black seed (Nigella sativa) and its constituent thymoquinone as an anti-dote or a protective agent against natural or chemical toxicities. Iran J Pharm Res. 2017;16:2–23. [PMC free article] [PubMed] [Google Scholar]
- 21.Shahroudi MJ, Mehri S, Hosseinzadeh H. Anti-aging effect of Nigella sativa fixed oil on D-galactose-induced aging in mice. J pharmacopuncture. 2017;20:29–35. doi: 10.3831/KPI.2017.20.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosseini SM, Taghiabadi E, Abnous K, Hariri AT, Pourbakhsh H, Hosseinzadeh H. Protective effect of thymoquinone, the active constituent of Nigella sativa fixed oil, against ethanol toxicity in rats. Iran J Basic Med Sci. 2017;20:927–939. doi: 10.22038/IJBMS.2017.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Groot H, Rauen U. Ischemia-reperfusion injury: processes in pathogenetic networks: a review. Transplant Proc: Elsevier; 2007. [DOI] [PubMed] [Google Scholar]
- 24.Buja LM. Myocardial ischemia and reperfusion injury. Cardiovasc Pathol. 2005;14:170–175. doi: 10.1016/j.carpath.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Zweier JL, Talukder MH. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Padhye S, Banerjee S, Ahmad A, Mohammad R, Sarkar FH. From here to eternity-the secret of Pharaohs: therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008;6:495–510. [PMC free article] [PubMed] [Google Scholar]
- 27.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–328. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 28.Liu X, Abd El-Aty AM, Shim J-H. Various extraction and analytical techniques for isolation and identification of secondary metabolites from Nigella sativa seeds. Mini Rev Med Chem. 2011;11:947–955. doi: 10.2174/138955711797068472. [DOI] [PubMed] [Google Scholar]
- 29.Kalidasu G, Reddy GS, Kumari SS, Kumari AL, Sivasankar A. Secondary volatiles and metabolites from Nigella sativa L seed. Indian J Nat Prod Resour. 2017;8:151–158. [Google Scholar]
- 30.Botnick I, Xue W, Bar E, Ibdah M, Schwartz A, Joel DM, Lev E, Fait A, Lewinsohn E. Distribution of primary and specialized metabolites in Nigella sativa seeds, a spice with vast traditional and historical uses. Molecules. 2012;17:10159–10177. doi: 10.3390/molecules170910159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forouzanfar F, Bazzaz BSF, Hosseinzadeh H. Black cumin (Nigella sativa) and its constituent (thymoquinone): a review on antimicrobial effects. Iran J Basic Med Sci. 2014;17:929–938. [PMC free article] [PubMed] [Google Scholar]
- 32.Tavakkoli A, Mahdian V, Razavi BM, Hosseinzadeh H. Review on clinical trials of black seed (Nigella sativa) and its active constituent, thymoquinone. J Pharmacopuncture. 2017;20:107–111. doi: 10.3831/KPI.2017.20.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Gaby A. Amino acid composition and biological effects of supplementing broad bean and corn proteins with Nigella sativa (black cumin) cake protein. Food/Nahrung. 1998;42:290–294. doi: 10.1002/(sici)1521-3803(199810)42:05<290::aid-food290>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 34.Hosseinzadeh H, Parvardeh S. Anti-convulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine. 2004;11:56–64. doi: 10.1078/0944-7113-00376. [DOI] [PubMed] [Google Scholar]
- 35.Hosseinzadeh H, Parvardeh S, Nassiri-Asl M, Mansouri M-T. Intracerebroventricular administration of thymoquinone, the major constituent of Nigella sativa seeds, suppresses epileptic seizures in rats. Med Sci Monit. 2005;11:BR106–BR110. [PubMed] [Google Scholar]
- 36.Hosseinzadeh H, Fazly Bazzaz BS, Haghi MM. Antibacterial activity of total extracts and essential oil of Nigella sativa L seeds in mice. Pharmacologyonline. 2007;2:429–435. [Google Scholar]
- 37.Amin B, Hosseinzadeh H. Black cumin (Nigella sativa) and its active constituent, thymoquinone: an overview on the analgesic and anti-inflammatory effects. Planta Med. 2016;82:8–16. doi: 10.1055/s-0035-1557838. [DOI] [PubMed] [Google Scholar]
- 38.Mollazadeh H, Afshari AR, Hosseinzadeh H. Review on the potential therapeutic roles of Nigella sativa in the treatment of patients with cancer: involvement of apoptosis-black cumin and cancer. J Pharmacopuncture. 2017;20:158–172. doi: 10.3831/KPI.2017.20.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L seed. Int Immunopharmacol. 2005;5:1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 40.Amin B, Taheri MMH, Hosseinzadeh H. Effects of intraperitoneal thymoquinone on chronic neuropathic pain in rats. Planta Med. 2014;80:1269–1277. doi: 10.1055/s-0034-1383062. [DOI] [PubMed] [Google Scholar]
- 41.Mehri S, Shahi M, Razavi BM, Hassani FV, Hosseinzadeh H. Neuroprotective effect of thymoquinone in acrylamide-induced neurotoxicity in Wistar rats. Iran J Basic Med Sci. 2014;17:1007–1011. [PMC free article] [PubMed] [Google Scholar]
- 42.Razavi B, Hosseinzadeh H. A review of the effects of Nigella sativa L and its constituent, thymoquinone, in metabolic syndrome. J Endocrinol Invest. 2014;37:1031–1040. doi: 10.1007/s40618-014-0150-1. [DOI] [PubMed] [Google Scholar]
- 43.Levy DE, Caronna JJ, Singer BH, Lapinski RH, Frydman H, Plum F. Predicting outcome from hypoxic-ischemic coma. Jama. 1985;253:1420–1426. [PubMed] [Google Scholar]
- 44.Katsura KI, Kristián T, Smith ML, Siesjö BK. Acidosis induced by hypercapnia exaggerates ischemic brain damage. J Cerebral Blood Flow & Metabolism. 1994;14:243–250. doi: 10.1038/jcbfm.1994.31. [DOI] [PubMed] [Google Scholar]
- 45.White BC, Sullivan JM, DeGracia DJ, O’Neil BJ, Neumar RW, Grossman LI, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- 46.Javidi S, Razavi BM, Hosseinzadeh H. A review of neuropharmacology effects of Nigella sativa and its main component, thymoquinone. Phytother Res. 2016:1219–1229. doi: 10.1002/ptr.5634. [DOI] [PubMed] [Google Scholar]
- 47.Houghton PJ, Zarka R, de las Heras B, Hoult J. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995;61:33–36. doi: 10.1055/s-2006-957994. [DOI] [PubMed] [Google Scholar]
- 48.Hosseinzadeh H, Moghim FF, Mansouri SMT. Effect of Nigella sativa seed extracts on ischemia-reperfusion in rat skeletal muscle. Pharmacologyonline. 2007;2:326–335. [Google Scholar]
- 49.Al-Majed AA, Al-Omar FA, Nagi MN. Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur J Pharmacol. 2006;543:40–47. doi: 10.1016/j.ejphar.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 50.Hosseinzadeh H, Parvardeh S, Asl MN, Sadeghnia HR, Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine. 2007;14:621–627. doi: 10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 51.Yaman İ, Balikci E. Protective effects of Nigella sativa against gentamicin-induced nephrotoxicity in rats. Exp Toxicol Pathol. 2010;62:183–190. doi: 10.1016/j.etp.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 52.Hobbenaghi R, Javanbakht J, Sadeghzadeh S, Kheradmand D, Abdi F, Jaberi M, et al. Neuroprotective effects of Nigella sativa extract on cell death in hippocampal neurons following experimental global cerebral ischemia-reperfusion injury in rats. J Neurol Sci. 2014;337:74–79. doi: 10.1016/j.jns.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 53.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soleimannejad K, Rahmani A, Hatefi M, Khataminia M, Hafezi Ahmadi MR. Effects of Nigella sativa extract on markers of cerebral angiogenesis after global ischemia of brain in rats. J Stroke Cerebrovasc Dis. 2017;26:1514–1520. doi: 10.1016/j.jstrokecerebrovasdis.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 55.Mousavi S, Tayarani-Najaran Z, Asghari M, Sadeghnia H. Protective effect of Nigella sativa extract and thymoquinone on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol. 2010;30:591–598. doi: 10.1007/s10571-009-9484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akhtar M, Maikiyo AM, Najmi AK, Khanam R, Mujeeb M, Aqil M. Neuroprotective effects of chloroform and petroleum ether extracts of Nigella sativa seeds in stroke model of rat. J Pharm Bioallied Sci. 2013;5:119–125. doi: 10.4103/0975-7406.111825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akhtar M, Maikiyo AM, Khanam R, Mujeeb M, Aqil M, Najmi AK. Ameliorating effects of two extracts of Nigella sativa in middle cerebral artery occluded rat. J Pharm Bioallied Sci. 2012;4:70–75. doi: 10.4103/0975-7406.92740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radad K, Hassanein K, Al-Shraim M, Moldzio R. Thymoquinone ameliorates lead-induced brain damage in Sprague Dawley rats. Exp Toxicol Pathol. 2014;66:13–17. doi: 10.1016/j.etp.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Ahmadiasl N, Banaei S, Alihemmati A. Combination antioxidant effect of erythropoietin and melatonin on renal ischemia-reperfusion injury in rats. Iran J basic Med Sci. 2013;16:1209–1216. [PMC free article] [PubMed] [Google Scholar]
- 60.Almond P, Matas A, Gillingham K, Dunn D, Payne W, Gores P, et al. Predictors of chronic rejection in renal transplant recipients. Transplant Proc. 1993;25:936. [PubMed] [Google Scholar]
- 61.Pirsch JD, Ploeg RJ, Gange S, D’Alessandro AM, Knechtle SJ, Sollinger HW, et al. Determinations of graft survival after renal transplantation. Transplantation. 1996;61:1581–1586. doi: 10.1097/00007890-199606150-00006. [DOI] [PubMed] [Google Scholar]
- 62.Paller MS. The cell biology of reperfusion injury in the kidney. J Investig Med: the official publication of the American Federation for Clinical Research. 1994;42:632–639. [PubMed] [Google Scholar]
- 63.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Greene EL, Paller MS. Xanthine oxidase produces O2 in posthypoxic injury of renal epithelial cells. Am J Physiol-Renal Physiol. 1992;263:F251–F255. doi: 10.1152/ajprenal.1992.263.2.F251. [DOI] [PubMed] [Google Scholar]
- 65.Zager RA, Gmur D. Effects of xanthine oxidase inhibition on ischemic acute renal failure in the rat. Am J Physiol-Renal Physiol. 1989;257:F953–F958. doi: 10.1152/ajprenal.1989.257.6.F953. [DOI] [PubMed] [Google Scholar]
- 66.Chatterjee PK, Cuzzocrea S, Brown PA, Zacharowski K, Stewart KN, Mota-Filipe H, et al. Tempol, a membrane-permeable radical scavenger, reduces oxidant stress-mediated renal dysfunction and injury in the rat. Kidney Int. 2000;58:658–673. doi: 10.1046/j.1523-1755.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 67.Chatterjee PK, Cuzzocrea S, Thiemermann C. Inhibitors of poly (ADP-ribose) synthetase protect rat proximal tubular cells against oxidant stress. Kidney Int. 1999;56:973–984. doi: 10.1046/j.1523-1755.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- 68.Nafar M, Parvin M, Sadeghi P, Ghoraishian M, Soleimani M, Tabibi A, et al. Effects of stem cells and granulocyte colony stimulating factor on reperfusion injury. Iran J kidney Dis. 2010;4:207–213. [PubMed] [Google Scholar]
- 69.Radhakrishnan J, Kiryluk K. Acute renal failure outcomes in children and adults. Kidney Int. 2006;69:17–19. doi: 10.1038/sj.ki.5000094. [DOI] [PubMed] [Google Scholar]
- 70.Boozari M, Hosseinzadeh H. Natural medicines for acute renal failure: A review. Phytother Res. 2017;31:1824–1835. doi: 10.1002/ptr.5943. [DOI] [PubMed] [Google Scholar]
- 71.Hosseinzadeh H, Montahaei R. Protective effect of Nigella sativa L extracts and thymoquinone, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. Pharmacologyonline. 2007;1:176–189. [Google Scholar]
- 72.Bayrak O, Bavbek N, Karatas OF, Bayrak R, Catal F, Cimentepe E, et al. Nigella sativa protects against ischaemia/reperfusion injury in rat kidneys. Nephrol Dial Transplant. 2008;23:2206–2212. doi: 10.1093/ndt/gfm953. [DOI] [PubMed] [Google Scholar]
- 73.Havakhah S, Sadeghnia HR, Mosa-Al-Reza Hajzadeh NM, Roshan SS, Hosseinzadeh H, Mohareri N, et al. Effect of Nigella sativa on ischemia-reperfusion induced rat kidney damage. Iran J Basic Med Sci. 2014;17:986–992. [PMC free article] [PubMed] [Google Scholar]
- 74.Mungli P, Shetty MS, Tilak P, Anwar N. Total thiols: biomedical importance and their alteration in various disorders. Online J Health Allied Sci. 2009;8:1–9. [Google Scholar]
- 75.Mousavi G, Sadeghnia HR, Ziaee T, Danaee A. Study on the effect of black cumin (Nigella sativa Linn) on experimental renal ischemia-reperfusion injury in rats. Acta Cir Bras. 2015;30:542–550. doi: 10.1590/S0102-865020150080000005. [DOI] [PubMed] [Google Scholar]
- 76.Yildiz F, Coban S, Terzi A, Savas M, Bitiren M, Celik H, et al. Protective effects of Nigella sativa against ischemia-reperfusion injury of kidneys. Ren Fail. 2010;32:126–131. doi: 10.3109/08860220903367577. [DOI] [PubMed] [Google Scholar]
- 77.Bayrak O, Bavbek N, Karatas OF, Bayrak R, Catal F, Cimentepe E, et al. Nigella sativa protects against ischaemia/reperfusion injury in rat kidneys. Nephrol Dial Transplant. 2008;23:2206–2212. doi: 10.1093/ndt/gfm953. [DOI] [PubMed] [Google Scholar]
- 78.Mousavi G. Study on the effect of black cumin (Nigella sativa Linn) on experimental renal ischemia-reperfusion injury in rats. Acta Cir Bras. 2015;30:542–550. doi: 10.1590/S0102-865020150080000005. [DOI] [PubMed] [Google Scholar]
- 79.Awad AS, Kamel R, Sherief MAE. Effect of thymoquinone on hepatorenal dysfunction and alteration of CYP3A1 and spermidine/spermine N-1-acetyl-transferase gene expression induced by renal ischaemia–reperfusion in rats. J Pharm Pharmacol. 2011;63:1037–1042. doi: 10.1111/j.2042-7158.2011.01303.x. [DOI] [PubMed] [Google Scholar]
- 80.FT Hammad LL. The effect of thymoquinone on the renal functions following ischemia-reperfusion injury in the rat. Int J Physiol. 2016;8:152–159. [PMC free article] [PubMed] [Google Scholar]
- 81.Turhan Caskurlu MK, Mustafa Erboga, Zeynep Fidanol Erboga MO, Gokhan Atis. Protective effect of Nigella Sativa on renal reperfusion injury in rat. Iran J kidney. 2016;10:135–143. [PubMed] [Google Scholar]
- 82.Caldwell-Kenkel JC, Currin RT, Tanaka Y, Thurman RG, Lemasters JJ. Kupffer cell activation and endothelial cell damage after storage of rat livers: effects of reperfusion. Hepatology. 1991;13:83–95. [PubMed] [Google Scholar]
- 83.Deschênes M, Belle SH, Krom RA, Zetterman RK, Lake JR. Early allograft dysfunction after liver transplantation: a definition and predictors of outcome1. Transplantation. 1998;66:302–310. doi: 10.1097/00007890-199808150-00005. [DOI] [PubMed] [Google Scholar]
- 84.Rezende-Neto JB, Moore EE, Masuno T, Moore PK, Johnson JL, Sheppard FR, et al. The abdominal compartment syndrome as a second insult during systemic neutrophil priming provokes multiple organ injury. Shock. 2003;20:303–308. doi: 10.1097/01.shk.0000082487.34705.d3. [DOI] [PubMed] [Google Scholar]
- 85.Selzner M, Clavien P-A, editors Fatty liver in liver transplantation and surgery. Semin liver Dis. 2001;21:105–113. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 86.Czaja MJ. Induction and regulation of hepatocyte apoptosis by oxidative stress. Anti-oxid Redox Signal. 2002;4:759–767. doi: 10.1089/152308602760598909. [DOI] [PubMed] [Google Scholar]
- 87.Tanaka T, Yamamoto J, Iwasaki S, Asaba H, Hamura H, Ikeda Y, et al. Activation of peroxisome proliferator-activated receptor δ induces fatty acid β-oxidation in skeletal muscle and attenuates metabolic syndrome. Proc Natl Acad Sci. 2003;100:15924–15929. doi: 10.1073/pnas.0306981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Selzner N, Rudiger H, Graf R, Clavien P-A. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–936. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 89.Serracino-Inglott F, Habib NA, Mathie RT. Hepatic ischemia-reperfusion injury. Am J Surg. 2001;181:160–166. doi: 10.1016/s0002-9610(00)00573-0. [DOI] [PubMed] [Google Scholar]
- 90.El-Abhar H, Abdallah D, Saleh S. Gastroprotective activity of Nigella sativa oil and its constituent, thymoquinone, against gastric mucosal injury induced by ischaemia/reperfusion in rats. J Ethnopharmacol. 2003;84:251–258. doi: 10.1016/s0378-8741(02)00324-0. [DOI] [PubMed] [Google Scholar]
- 91.Mollazadeh H, Hosseinzadeh H. The protective effect of Nigella sativa against liver injury: a review. Iran J Basic Med Sci. 2014;17:958–966. [PMC free article] [PubMed] [Google Scholar]
- 92.El-Ghany R, Sharaf N, Kassem L, Mahran L, Heikal O. Thymoquinone triggers anti-apoptotic signaling targeting death ligand and apoptotic regulators in a model of hepatic ischemia reperfusion injury. Drug Discov Ther. 2009;3:296–306. [PubMed] [Google Scholar]
- 93.Sethi G, Ahn KS, Aggarwal BB. Targeting nuclear factor-κB activation pathway by thymoquinone: role in suppression of anti-apoptotic gene products and enhancement of apoptosis. Mol Cancer Res. 2008;6:1059–1070. doi: 10.1158/1541-7786.MCR-07-2088. [DOI] [PubMed] [Google Scholar]
- 94.Mallick IH, Yang W, Winslet MC, Seifalian AM. Ischemia—reperfusion injury of the intestine and protective strategies against injury. Dig Dis Sci. 2004;49:1359–1377. doi: 10.1023/b:ddas.0000042232.98927.91. [DOI] [PubMed] [Google Scholar]
- 95.Sizlan A, Guven A, Uysal B, Yanarates O, Atim A, Oztas E, et al. Proanthocyanidin protects intestine and remote organs against mesenteric ischemia/reperfusion injury. World J Surg. 2009;33:1384–1391. doi: 10.1007/s00268-009-0011-9. [DOI] [PubMed] [Google Scholar]
- 96.Al Salamah SM, El Keyali AY. Ileo-caecal volvulus post-cesarean section: a case report. Saudi J Gastroenterol. 2000;6:163–164. [PubMed] [Google Scholar]
- 97.Gul H, Yildiz O, Simsek A, Balkan M, Ersoz N, Cetiner S, et al. Pharmacologic characterization of contractile serotonergic receptors in human isolated mesenteric artery. J Cardiovasc Pharmacol. 2003;41:307–315. doi: 10.1097/00005344-200302000-00021. [DOI] [PubMed] [Google Scholar]
- 98.Horie Y, Yamagishi Y, Kato S, Kajihara M, Kimura H, Ishii H. Low-dose ethanol attenuates gut ischemia/reperfusion-induced liver injury in rats via nitric oxide production. J Gastroenterol Hepatol. 2003;18:211–217. doi: 10.1046/j.1440-1746.2003.02929.x. [DOI] [PubMed] [Google Scholar]
- 99.Iglesias JL, LaNoue JL, Rogers TE, Inman L, Turnage RH. Physiologic basis of pulmonary edema during intestinal reperfusion. J Surg Res. 1998;80:156–163. doi: 10.1006/jsre.1998.5435. [DOI] [PubMed] [Google Scholar]
- 100.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol-cell Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 101.Daniel RAF, Cardoso VK, Góis Jr E, Parra RS, Garcia SB, Rocha JJRd, et al. Effect of hyperbaric oxygen therapy on the intestinal ischemia reperfusion injury. Acta Cir Bras. 2011;26:463–469. doi: 10.1590/s0102-86502011000600010. [DOI] [PubMed] [Google Scholar]
- 102.Takahashi A, Tomomasa T, Kaneko H, Watanabe T, Tabata M, Morikawa H, et al. Intestinal motility in an in vivo rat model of intestinal ischemia–reperfusion with special reference to the effects of nitric oxide on the motility changes. J Pediatr Gastroenterol Nutr. 2001;33:283–288. doi: 10.1097/00005176-200109000-00010. [DOI] [PubMed] [Google Scholar]
- 103.Horton JW, White DJ. Lipid peroxidation contributes to cardiac deficits after ischemia and reperfusion of the small bowel. Am J Physiol-Heart Circ Physiol. 1993;264:H1686–H1692. doi: 10.1152/ajpheart.1993.264.5.H1686. [DOI] [PubMed] [Google Scholar]
- 104.Shirasugi N, Wakabayashi G, Shimazu M, Oshima A, Shito M, Kawachi S, et al. Up-regulation of oxygen-derived free radicals by interleukin-1 in hepatic ischemia/reperfusion injury1. Transplantation. 1997;64:1398–1403. doi: 10.1097/00007890-199711270-00004. [DOI] [PubMed] [Google Scholar]
- 105.Murphy BA, Martin A-M, Furney P, Elliott JA. Absence of a serum melatonin rhythm under acutely extended darkness in the horse. J Circadian Rhythms. 2011;9:3. doi: 10.1186/1740-3391-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 107.Kojima M, Iwakiri R, Wu B, Fujise T, Watanabe K, Lin T, et al. Effects of anti-oxidative agents on apoptosis induced by ischaemia-reperfusion in rat intestinal mucosa. Aliment Pharmacol Ther. 2003;18:139–145. doi: 10.1046/j.1365-2036.18.s1.16.x. [DOI] [PubMed] [Google Scholar]
- 108.Noda T, Iwakiri R, Fujimoto K, Matsuo S, Aw TY. Programmed cell death induced by ischemia-reperfusion in rat intestinal mucosa. Am J Physiol-Gastrointest Liver Physiol. 1998;274:G270–G276. doi: 10.1152/ajpgi.1998.274.2.G270. [DOI] [PubMed] [Google Scholar]
- 109.Ikeda H, Suzuki Y, Suzuki M, Koike M, Tamura J, Tong J, et al. Apoptosis is a major mode of cell death caused by ischaemia and ischaemia/reperfusion injury to the rat intestinal epithelium. Gut. 1998;42:530–537. doi: 10.1136/gut.42.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Soliman MM. Effects of aminoguanidine, a potent nitric oxide synthase inhibitor, on myocardial and organ structure in a rat model of hemorrhagic shock. J Emerg Trauma Shock. 2014;7:190–195. doi: 10.4103/0974-2700.136864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guven A, Tunc T, Topal T, Kul M, Korkmaz A, Gundogdu G, et al. α-Lipoic acid and ebselen prevent ischemia/reperfusion injury in the rat intestine. Surg Today. 2008;38:1029–1035. doi: 10.1007/s00595-007-3752-9. [DOI] [PubMed] [Google Scholar]
- 112.Koltuksuz U, Özen S, Uz E, Aydinç M, Karaman A, Gültek A, et al. Caffeic acid phenethyl ester prevents intestinal reperfusion injury in rats. J Pediatr Surg. 1999;34:1458–1462. doi: 10.1016/s0022-3468(99)90103-3. [DOI] [PubMed] [Google Scholar]
- 113.Yildiz Y, Serter M, Ek RO, Ergin K, Cecen S, Demir EM, et al. Protective effects of caffeic acid phenethyl ester on intestinal ischemia-reperfusion injury. Dig Dis Sci. 2009;54:738–744. doi: 10.1007/s10620-008-0405-9. [DOI] [PubMed] [Google Scholar]
- 114.Al Mofleh IA, Alhaider AA, Mossa JS, Al-Sohaibani MO, Al-Yahya MA, Rafatullah S, et al. Gastroprotective effect of an aqueous suspension of black cumin Nigella sativa on necrotizing agents-induced gastric injury in experimental animals. Saudi J of Gastroenterology. 2008;14:128–134. doi: 10.4103/1319-3767.41731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tas U, Ayan M, Sogut E, Kuloglu T, Uysal M, Tanriverdi HI, et al. Protective effects of thymoquinone and melatonin on intestinal ischemia–reperfusion injury. Saudi J Gastroenterol: official J of the Saudi Gastroenterology Association. 2015;21:284–289. doi: 10.4103/1319-3767.166203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ikeda K, Negishi H, Yamori Y. Antioxidant nutrients and hypoxia/ischemia brain injury in rodents. Toxicol. 2003;189:55–61. doi: 10.1016/s0300-483x(03)00152-5. [DOI] [PubMed] [Google Scholar]
- 117.Ozyurt H, Ozyurt B, Koca K, Ozgocmen S. Caffeic acid phenethyl ester (CAPE) protects rat skeletal muscle against ischemia–reperfusion-induced oxidative stress. Vasc Pharmacol. 2007;47:108–112. doi: 10.1016/j.vph.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 118.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Vascular. 2002;10:620–630. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 119.Hosseinzadeh H, Taiari S, Nassiri-Asl M. Effect of thymoquinone, a constituent of Nigella sativa L on ischemia–reperfusion in rat skeletal muscle. Naunyn-Schmiedeberg’s Arch Pharmacol. 2012;385:503–508. doi: 10.1007/s00210-012-0726-2. [DOI] [PubMed] [Google Scholar]
- 120.Ascher E, Hanson JN, Cheng W, Hingorani A, Scheinman M. Glycine preserves function and decreases necrosis in skeletal muscle undergoing ischemia and reperfusion injury. Surgery. 2001;129:231–235. doi: 10.1067/msy.2001.112594. [DOI] [PubMed] [Google Scholar]
- 121.Woodruff TM, Arumugam TV, Shiels IA, Reid RC, Fairlie DP, Taylor SM. Protective effects of a potent C5a receptor antagonist on experimental acute limb ischemia-reperfusion in rats. J Surg Res. 2004;116:81–90. doi: 10.1016/j.jss.2003.04.001. [DOI] [PubMed] [Google Scholar]
- 122.Hosseinzadeh H, Modaghegh MH, Saffari Z. Crocus sativus (Saffron) extract and its active constituents (crocin and safranal) on ischemia-reperfusion in rat skeletal muscle. Evid-Based Complement Alternat Med. 2009;6:343–350. doi: 10.1093/ecam/nem125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Erkut A, Cure MC, Kalkan Y, Balik MS, Guvercin Y, Yaprak E, et al. Protective effects of thymoquinone and alpha-tocopherol on the sciatic nerve and femoral muscle due to lower limb ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci. 2016;20:1192–1202. [PubMed] [Google Scholar]
- 124.Gonca E, Kurt C. Cardioprotective effect of thymoquinone: a constituent of Nigella sativa L against myocardial ischemia/reperfusion injury and ventricular arrhythmias in anaesthetized rats. Pak J Pharm Sci. 2015;28:1267–1273. [PubMed] [Google Scholar]
- 125.Tullio F, Angotti C, Perrelli M-G, Penna C, Pagliaro P. Redox balance and cardioprotection. Basic Res Cardiol. 2013;108:1–26. doi: 10.1007/s00395-013-0392-7. [DOI] [PubMed] [Google Scholar]
- 126.Tappia PS, Hata T, Hozaima L, Sandhu MS, Panagia V, Dhalla NS. Role of oxidative stress in catecholamine-induced changes in cardiac sarcolemmal Ca2+ transport. Arch Biochem Biophys. 2001;387:85–92. doi: 10.1006/abbi.2000.2234. [DOI] [PubMed] [Google Scholar]
- 127.Agrawal YO, Sharma PK, Shrivastava B, Ojha S, Upadhya HM, Arya DS, et al. Hesperidin produces cardioprotective activity via PPAR-γ pathway in ischemic heart disease model in diabetic rats. PloS One. 2014;9:111–212. doi: 10.1371/journal.pone.0111212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Goyal S, Arora S, Bhatt TK, Das P, Sharma A, Kumari S, et al. Modulation of PPAR-γ by telmisartan protects the heart against myocardial infarction in experimental diabetes. Chem-Biol interact. 2010;185:271–280. doi: 10.1016/j.cbi.2010.03.030. [DOI] [PubMed] [Google Scholar]
- 129.Rona G. Catecholamine cardiotoxicity. J Mol Cell Cardiol. 1985;17:291–306. doi: 10.1016/s0022-2828(85)80130-9. [DOI] [PubMed] [Google Scholar]
- 130.Ojha S, Goyal S, Kumari S, Arya DS. Pyruvate attenuates cardiac dysfunction and oxidative stress in isoproterenol-induced cardiotoxicity. Exp Toxicol Pathol. 2012;64:393–399. doi: 10.1016/j.etp.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 131.Moens A, Claeys M, Timmermans J, Vrints C. Myocardial ischemia/reperfusion-injury, a clinical view on a complex pathophysiological process. Int J Cardiol. 2005;100:179–190. doi: 10.1016/j.ijcard.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 132.Zhang T, Yang S, Du J. Protective effects of berberine on isoproterenol-induced acute myocardial ischemia in rats through regulating HMGB1-TLR4 axis. Evid-Based Complement Alternat Med. 2014;2014 doi: 10.1155/2014/849783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Frank A, Bonney M, Bonney S, Weitzel L, Koeppen M, Eckle T, editors Myocardial ischemia reperfusion injury from basic science to clinical bedside. Semin Cardiothorac Vasc Anesth. 2012;16:123–132. doi: 10.1177/1089253211436350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ojha SK, Nandave M, Arora S, Narang R, Dinda AK, Arya DS. Chronic administration of Tribulus terrestris Linn extract improves cardiac function and attenuates myocardial infarction in rats. Int J Pharmacol. 2008;4:1–10. [Google Scholar]
- 135.Marczin N, El-Habashi N, Hoare GS, Bundy RE, Yacoub M. Antioxidants in myocardial ischemia–reperfusion injury: therapeutic potential and basic mechanisms. Arch Biochem Biophys. 2003;420:222–236. doi: 10.1016/j.abb.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 136.Gonca E, Kurt Ç. Cardioprotective effect of Thymoquinone: A constituent of Nigella sativa L against myocardial ischemia/reperfusion injury and ventricular arrhythmias in anaesthetized rats. Pak J Pharm Sci. 2015;28:1267–1273. [PubMed] [Google Scholar]
- 137.El Tahir KE, Ashour MM, Al-Harbi MM. The cardiovascular actions of the volatile oil of the black seed (Nigella sativa) in rats: elucidation of the mechanism of action. Gen Pharmacol. 1993;24:1123–1131. doi: 10.1016/0306-3623(93)90359-6. [DOI] [PubMed] [Google Scholar]
- 138.Ghayur MN, Gilani AH, Janssen LJ. Intestinal, airway, and cardiovascular relaxant activities of thymoquinone. Evid-Based Complement Alternat Med. 2012;2012:305–319. doi: 10.1155/2012/305319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Atasever M Z B. Nigella sativa oil protects the rat ovary from oxidative injury due to ischemia-reperfusion. Med Sci Monit. 2017;23:5027–5033. doi: 10.12659/MSM.905356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Abdulhakeem A, Al-Majed FAA-O, Mahmoud N. Nagi. Neuro protective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur J Pharmacol. 2006;543:40–47. doi: 10.1016/j.ejphar.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 141.Hosseinzadeh H PS, Nassiri Asl M, Sadeghnia HR, Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine. 2007;14:621–627. doi: 10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 142.Ojha S, Azimullah S, Mohanraj R, Sharma C, Yasin J, Arya DS, et al. Thymoquinone protects against myocardial ischemic injury by mitigating oxidative stress and inflammation. Evid-Based Complement Alternat Med. 2015;2015:1–12. doi: 10.1155/2015/143629. [DOI] [PMC free article] [PubMed] [Google Scholar]