Abstract
Background/Purpose:
Studies conducted in Eastern Asia suggest that serum uric acid (SUA) level is highly related to nonalcoholic fatty liver disease (NAFLD). However, limited information is available in the USA. Our objective was to determine the association between NAFLD and SUA levels in the USA and to determine if this is independent of age, sex, and components of metabolic syndrome (MetS).
Methods:
We analyzed 5370 men and women aged 20–74 years from the Third National Health and Nutrition Examination Survey (NHANES III) (1988–1994) in the USA. We calculated the prevalence and odds ratio (OR) of NAFLD and elevated liver enzymes by SUA and sex-specific quin-tiles of SUA, adjusting for multiple factors.
Results:
The prevalence of NAFLD was higher in participants with higher SUA levels (10.9%,9.6%, 15.9%, 21.8% and 33.1%, respectively, from the second to the fifth sex-specific quintile of uric acid). After adjustment, individuals with hyperuricemia were more likely to have NAFLD (OR: 1.4, 95% CI: 1.1–1.9). Similarly, the adjusted odds of NAFLD were increasingly higher from the second to the fifth quintile of SUA (ORs: 0.8, 1.2, 1.5 and 1.7, respectively; p < 0.01) as compared to the lowest quintile. Finally, individuals with hyperuricemia were more likely to have elevated liver enzymes (aspartate aminotransferase or alanine aminotransferase) (adjusted OR: 1.8, 95% CI: 1.1–2.7).
Conclusion:
NAFLD and SUA levels were strongly and independently associated in this nationally representative sample of men and women after adjustment for multiple factors.
Keywords: hyperuricemia, nonalcoholic fatty liver disease, serum uric acid
Introduction
Hepatic steatosis is the accumulation of fat (usually triglyceride) within the hepatic parenchyma. It is usually asymptomatic, but can cause malaise, a sensation of fullness, or discomfort on the right side of the upper abdomen. In the absence of significant alcohol consumption and other causes of liver disease, hepatic steatosis is usually called nonalcoholic fatty liver disease (NAFLD).1 The prevalence of NAFLD exceeds 25% by ultrasound surveys of the general population in some countries.2
NAFLD contains a range of manifestations from simple steatosis, nonalcoholic steatohepatitis (NASH), fibrosis, and liver cirrhosis, which may lead to hepatocellular carcinoma (HCC) and liver failure.1,3 Nutritional, metabolic and genetic factors contribute to the occurrence of NAFLD.1,4,5 In addition, oxidative stress, insulin resistance and systemic inflammation are known as important risk factors for the development or progression of the liver diseases including NAFLD and NASH. Recently, several observational studies suggest that hyperuricemia (serum uric acid (SUA) level >7.0 mg/dL in men and >5.7 mg/dL in women) is a risk factor for NAFLD among eastern Asian populations independent of the components of metabolic syndrome (MetS).6–9 In fact, there is some evidence that insulin resistance can lead to reduced excretion of SUA and increased SUA level.10,11 However, data in the USA have been limited to liver enzymes and advanced liver disease.12 Therefore, we hypothesized that there also exists a relationship between hyperuricemia and NAFLD among the US population. In addition, there is a quantitative relationship between SUA level and NAFLD.
Our primary objective was: (1) to determine the association between ultrasound-defined NAFLD and SUA; and (2) to determine if hyperuricemia is associated with ultrasound-defined NAFLD independent of well-known risk factors. In secondary analyses we investigated the relationship between hyperuricemia and liver enzymes including aspartate aminotransferase (AST) and alanine aminotransferase (ALT), using data representative of the US population.
Methods
Study population
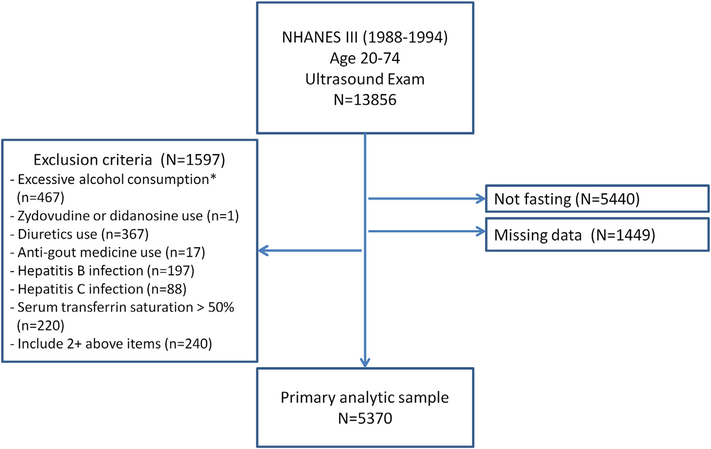
We used data from the Third National Health and Nutrition Examination Survey (NHANES III), which was conducted between 1988 and 1994, and contains a representative sample of the noninstitutionalized US civilian population.13 For our study, we initially included 6967 participants between the ages of 20 and 74 years who were eligible for ultrasound examination of the gallbladder14 and had a videotape reviewed for hepatic steatosis, had fasted at least 8 hours before the blood collection, had blood tested for SUA and liver enzymes, had complete information for definition of the MetS and had no other missing information. After excluding participants who reported only excessive alcohol consumption (n = 467) (>2 drinks for men; >1 drink for women; 1 drink = 14 g alcohol); who only had serum transferrin saturation >50% (n = 220), hepatitis B (n = 197) or C (n = 88) infection; who were only taking diuretics (n = 367), anti-gout medicine (n = 17), or zidovudine/didanosine (n = 1); those with two or more of the above conditions (n = 240), we included 5370 individuals as our analytic sample (Fig. 1).
Figure 1.
Flowchart of selection of study population. *Excessive alcohol consumption is defined as >1 drink/day for women and >2 drinks/day for men; 1 drink = 14 g alcohol.
Data collection
NHANES III collected data through interviewer questionnaires, physical examination, laboratory measurements, and gallbladder ultrasound examination. Detailed description of data collection can be found elsewhere.13 Questionnaires collected sociodemographic information, including age, sex and race/ethnicity. Race/ethnicity was categorized into non-Hispanic white, non-Hispanic black, Mexican American, and others. In addition, information on alcohol consumption (frequency and amount) and medical history (including medication use, and diagnosis of diabetes and hypertension) was also obtained. The physical examination included measurement of height, weight, waist circumference and systolic and diastolic blood pressure. Body mass index (BMI) was calculated. Gallbladder ultra-sound was performed using a Toshiba (Tustin, CA, USA) SSA-90A machine using 3.75 and 5.0 MHz transducers. Laboratory measurements included SUA, plasma glucose, serum total cholesterol, serum high-density lipoprotein cholesterol (HDL-C), serum triglyceride (TG) and liver enzymes, including serum AST and ALT.
All NHANES III participants aged 20–74 years who were part of the gallbladder ultrasound examination and had the archived ultrasound videotapes were eligible for review of liver echo-texture between 2009 and 2010. In total, 13,856 NHANES III participants had a successful hepatic steatosis ultrasound assessment (96.7% of all the participants with available gallbladder data). Five criteria including liver parenchymal brightness, liver-to-kidney contrast, deep beam attenuation, bright vessel walls, and gallbladder wall definition were used to determine hepatic steatosis. Detailed description can be found elsewhere.15 Briefly, the degree of steatosis was assessed by the following five criteria: (1) the degree of the liver parenchymal brightness (none/intermediate/moderate/severe); (2) the presence of liver-to-kidney contrast (yes/no/not assessed); (3) the presence of posterior deep bean attenuation (yes/no/not assessed); (4) the presence of bright vessel walls (yes/no/not assessed); (5) the definition of gallbladder (clear/intermediate/obliterated/not assessed). According to the five parameters, participants with steatosis were classified as normal, mild, moderate, or severe. In the current analysis, we defined NAFLD for participants with moderate or severe steatosis.
Hyperuricemia was defined as SUA level >7.0 mg/dL in men and >5.7 mg/dL (to convert to micromoles per liter, multiply by 59.48) in women according to the laboratory reference for NHANES.16 Overweight was defined as BMI ≥ 25 kg/m2. MetS was defined according to National Cholesterol Education Programe–Adult Treatment Panel III (NCEP-ATPIII) criteria: abdominal obesity (waist circumference >102 cm in men and >88 cm in women); hyper-triglyceridemia (≥150 mg/dL or 1.69 mmol/L); low HDL-C (<40 mg/dL or 1.04 mmol/L in men and <50 mg/dL or1.29 mmol/L in women); high blood pressure (≥130/85 mmHg); and high fasting glucose (≥100 mg/dL or ≥6.1 mmol/L).17 Hepatic steatosis was defined as the presence of moderate or severe steatosis by ultrasound regardless of the presence of other liver diseases. NAFLD was defined as the presence of steatosis without excessive alcohol consumption, or use of zydovudine or didanosine, which were found to be associated with the presence of steatosis. Finally, elevated liver enzymes were defined as an AST level >37 U/L in men or >31 U/L in women; and an ALT level >40 U/L in men or >31 U/L in women according to the laboratory reference for NHANES.16 We defined unexplained abnormal liver enzymes as elevations in AST and/or ALT without excessive alcohol consumption, hepatitis B infection, hepatitis C infection, or serum transferrin saturation >50%.
Statistical analysis
The distribution of hepatic steatosis, NAFLD, the MetS components, MetS, alcohol use, race/ethnicity, and abnormal liver enzymes were compared by the presence or absence of hyperuricemia using the x2 test. The means of age and BMI were compared by the presence or absence of hyperuricemia using unpaired t test. We calculated the unadjusted, age- and sex-adjusted, and multivariable-adjusted odds ratios of NAFLD by the presence or absence of hyperuricemia after adjusting for age, sex, race/ethnicity, alcohol consumption, BMI, fasting plasma glucose, systolic pressure, serum HDL-C, and serum TG. Likewise, the prevalence of NAFLD among the total study population was calculated according to five sex-specific percentiles (quintiles) of serum uric acid levels:0.8–3.6 mg/dL, 3.7–4.2 mg/dL, 4.3–4.7 mg/dL,4.8–5.5 mg/dL, and 5.6–10 mg/dL for women;1.7–5.1 mg/dL, 5.2–5.7 mg/dL, 5.8–6.3 mg/dL,6.4–7.0 mg/dL, and 7.1–11.4 mg/dL for men. We also performed logistic regression analyses to evaluate the association of NAFLD with SUA across quintile and calculated unadjusted odds ratio (OR), age-adjusted OR, and multivariable-adjusted OR (same adjustment as used for the prevalence estimates). Finally, we used logistic regression to evaluate the association between elevated SUA levels and abnormal liver enzymes and calculated unadjusted, age- and sex-adjusted, and multivariable-adjusted ORs after controlling for age, sex, race/ethnicity, alcohol consumption, BMI, fasting plasma glucose, systolic pressure, serum HDL-C, and serum TG.
All statistical analyses were computed using the survey commands of Stata statistical software to incorporate sample weights and adjust for clusters and strata of the complex sampling design (Version 11; Stata Corporation, College Station, TX, USA). Values were considered statistically significant if p values were less than 0.05.
Results
Clinical characteristics of study population
The characteristics of the study population are showed in Table 1 by the presence or absence of hyperuricemia. Overall 16.4% had hyperuricemia (unweighted). Those with hyperuricemia were significantly older (43.1 vs. 40.4 years, p = 0.001), more likely to be male (54.5% vs. 46.3%, p < 0.05), and overweight (82.3% vs. 49.2%, p < 0.001) than those without hyperuricemia. The prevalence of each individual MetS component was significantly higher among individuals with hyperuricemia as was the overall prevalence of the MetS (49.2% vs. 20.8%, p < 0.001).
Table 1.
Clinical characteristics of the study population with and without hyperuricemia.
| Weighted prevalence | Study cohort | Without hyperuricemiaa | With hyperuricemiaa | p |
|---|---|---|---|---|
| Unweighted, n | n = 5370 | n = 4489 | n = 881 | |
| Mean age (y) | 40.8 (40.3–41.4) | 40.4 (0.31) | 43.1 (0.62) | 0.001 |
| Sex | ||||
| Male | 47.6 | 46.3 | 54.5 | 0.004 |
| Race/ethnicity | ||||
| NH White | 75.7 | 75.7 | 75.8 | 0.38 |
| NH Black | 10.3 | 10.1 | 11.8 | |
| Mexican American | 5.9 | 6.0 | 5.1 | |
| Other races | 8.1 | 8.2 | 7.4 | |
| Alcohol consumptionb | ||||
| Never | 12.9 | 13.2 | 11.2 | 0.22 |
| Low | 87.1 | 86.8 | 88.9 | |
| Mean BMI (kg/m2) | 26.3 (26.1–26.5) | 25.6 (25.4–25.8) | 30.0 (29.4–30.6) | <0.001 |
| BMI ≥ 25 kg/m2 | 54.4 | 49.2 | 82.3 | <0.001 |
| Waist circumference (cm) >102 (M), >88 (W) | 34.2 | 29.8 | 57.6 | <0.001 |
| BP ≥ 130/85 mmHgc | 27.5 | 24.2 | 45.0 | <0.001 |
| Fasting glucose >100 mg/dLd | 24.9 | 23.3 | 33.4 | <0.001 |
| HDL-C (mg/dL) <40 (M), <50 (W) | 38.2 | 35.3 | 53.7 | <0.001 |
| TGs > 150 mg/dL | 27.2 | 23.0 | 49.4 | <0.001 |
| MetS components ≥3 | 25.3 | 20.8 | 49.2 | <0.001 |
Data are presented by incorporating sample weights and adjusted for clusters and strata of the complex sample design of the Third National Health and Nutrition Examination Survey; data are expressed as means (SD range) or as percentages.
BMI = body mass index; BP = blood pressure; HDL-C = high-density lipoprotein cholesterol; M = men; MetS = metabolic syndromes; NH = non-Hispanic; TGs = triglycerides; W = women.
Hyperuricemia: >7 mg/dL in men; >5.7 mg/dL in women.
Alcohol consumption: low (≤1 drink/day in women, ≤2 drinks/day in men); 1 drink = 14 g alcohol.
Systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥85 mmHg, or patients who reported currently using antihypertensive medications.
Containing patients who self-reported physician diagnosis of diabetes mellitis.
Associations between hepatic steatosis, NAFLD, and SUA levels
As shown in Table 2, the overall prevalence of NAFLD was17.7% [95% confidence interval (CI) 16.1–19.4%], and was significantly higher in those with hyperuricemia compared to those without (33.8% vs. 14.7%, p < 0.001). Individuals with hyperuricemia were 40% more likely to have NAFLD even after adjusting for age, sex, race/ethnicity, alcohol consumption, BMI, fasting plasma glucose, systolic pressure, serum HDL-C, and serum TG (OR 1.4, 95% CI 1.1–1.9). Overall, the likelihood of NAFLD increased with increasing SUA levels among total study population. As shown in Table 3, the overall prevalence of NAFLD was 10.9%, 9.6%, 15.9%, 21.8% and 33.1%, respectively from the first sex-specific quintile to the fifth. Compared to the lowest quintile, the odds of NAFLD was 0.8, 1.2, 1.5 and 1.7 across the second to the fifth quintile of uric acid even after adjustment for age, race/ethnicity, alcohol consumption, and components of the MetS (p < 0.001). Moreover, we further explored whether there was a dose–response relationship between the severity of NAFLD (none, intermediate, moderate, and severe) and quintiles of SUA levels and found no evidence supporting such a relationship.
Table 2.
Association between nonalcoholic fatty liver disease (NAFLD) and serum uric acid (SUA) levels.
| Weighted prevalence or odds ratio (OR) (95% confidence interval) | Study cohort | Without hyperuricemiaa | With hyperuricemiaa | p |
|---|---|---|---|---|
| Unweighted, n | n = 5370 | n = 4489 | n = 881 | |
| NAFLDb | 17.7% | 14.7% | 33.8% | <0.001 |
| NAFLD: | ||||
| Unadjusted OR | 1 | 3.0 (2.3–3.9) | <0.001 | |
| Age- and sex-adjusted OR | 1 | 2.8 (2.2–3.7) | <0.001 | |
| Multivariable-adjusted ORc | 1 | 1.4 (1.1–1.9) | <0.01 | |
Data are presented by incorporating sample weights and adjusted for clusters and strata of the complex sample design of the Third National Health and Nutrition Examination Survey.
Hyperuricemia: >7 mg/dL in men; >5.7 mg/dL in women.
NAFLD: hepatic steatosis without excessive alcohol consumption, or use of zydovudine or didanosine.
Adjusted for age, sex, race/ethnicity, alcohol consumption, body mass index, (systolic) blood pressure, fasting glucose, high-density lipoprotein cholesterol and triglycerides.
Table 3.
Prevalence and odds ratio (OR) of NAFLD according to sex-specific quintiles of serum uric acid.
| Weighted prevalence and OR | Serum uric acid levels (mg/dL) by quintilea | p for trend | ||||
|---|---|---|---|---|---|---|
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | ||
| Unweighted, n (total n = 5370) | n = 1166 | n = 1136 | n = 994 | n = 1083 | n = 991 | |
| NAFLDb | 10.9% | 9.6% | 15.9% | 21.8% | 33.1% | |
| Unadjusted OR (95% confidence interval, CI) | 1 | 0.9 (0.6–1.4) | 1.5 (1.0–2.3) | 2.3 (1.6–3.3) | 4.0 (2.8–5.8) | <0.001 |
| Age-adjusted OR (95% CI) | 1 | 0.9 (0.6–1.4) | 1.5 (1.0–2.3) | 2.3 (1.6–3.3) | 3.8 (2.7–5.5) | <0.001 |
| Multivariable-adjusted OR (95% CI)c | 1 | 0.8 (0.5–1.4) | 1.2 (0.8–1.9) | 1.5 (1.0–2.2) | 1.7 (1.1–2.5) | <0.001 |
Data are presented by incorporating sample weights and adjusted for clusters and strata of the complex sample design of the Third National Health and Nutrition Examination Survey.
Serum uric acid level (mg/dL) quintiles: for women 0.8–3.6, 3.7–4.2, 4.3–4.7, 4.8–5.5 and 5.6–10; for men 1.7–5.1, 5.2–5.7,5.8–6.3, 6.4–7.0 and 7.1–11.4.
NAFLD: hepatic steatosis without excessive alcohol consumption, or use of zydovudine or didanosine.
Adjusted for age, sex, race/ethnicity, alcohol consumption, body mass index, (systolic) blood pressure, fasting glucose, high-density lipoprotein cholesterol and triglycerides.
Association between liver enzymes and serum uric acid
As shown in Table 4, individuals with hyperuricemia had a higher prevalence of elevated liver enzymes compared to those without (AST 8.9% vs. 3.0%, p < 0.001; ALT 9.6% vs.4.7%, p < 0.001). In addition, individuals with hyperuricemia were more likely to have elevated liver enzymes (AST or ALT), even after adjusting for age, sex, race/ethnicity, alcohol consumption, BMI, fasting plasma glucose, systolic pressure, serum HDL-C, and serum TG (OR 1.8, 95% CI1.1–2.7).
Table 4.
Association between liver enzymes and serum uric acid.
| Weighted prevalence or odds ratio (OR) (95% confidence interval) | Study cohort | Without hyperuricemiaa | With hyperuricemiaa | |
|---|---|---|---|---|
| Unweighted, n | n = 5370 | n = 4489 | n = 881 | |
| AST (U/L) > 37 (M), >31 (W) | 3.9% | 3.0% | 8.9% | <0.001 |
| ALT (U/L) > 40 (M), >31 (W) | 5.5% | 4.7% | 9.6% | <0.001 |
| Abnormal liver enzymesb | 6.6% | 5.4% | 12.6% | <0.001 |
| Abnormal liver enzymes: | ||||
| Unadjusted OR | 1 | 2.5 (1.7–3.8) | <0.001 | |
| Age- and sex-adjusted OR | 1 | 2.5 (1.7–3.8) | <0.001 | |
| Multivariable-adjusted ORc | 1 | 1.8 (1.1–2.7) | 0.01 | |
Data are presented by incorporating sample weights and adjusted for clusters and strata of the complex sample design of the Third National Health and Nutrition Examination Survey.
ALT = alanine aminotransferase; AST = aspartate aminotransferase; M = men; W = women.
Hyperuricemia: >7 mg/dL in men; >5.7 mg/dL in women.
Abnormal liver enzymes were defined as elevated AST and/or ALT without excessive alcohol consumption, hepatitis B or C virus infections, or serum transferrin saturation >50%.
Adjusted for age, sex, race/ethnicity, alcohol consumption, body mass index, (systolic) blood pressure, fasting glucose, high-density lipoprotein cholesterol and triglycerides.
Discussion
In this nationally representative sample of men and women, we found that individuals with hyperuricemia had a higher prevalence of NAFLD and, furthermore, that there was a dose–response relationship between SUA level and NAFLD. Hyperuricemia was associated with NAFLD independent of age, sex, race/ethnicity, alcohol, BMI, and all components of the MetS. In addition, individuals with hyperuricemia are more likely to have elevated liver enzymes (AST or ALT) even after adjustment.
Our findings confirm those of previous studies in eastern Asia (i.e., elevated SUA levels significantly contributed to the risk for NAFLD among patients who participated in health check-ups).6–9 In addition, our results extend previous findings from the NHANES which relied on elevated liver enzymes only.12 The associations are robust and occur in a nationally representative sample of the US population for two manifestations of NAFLD: (1) hepatic steatosis; and(2) elevated liver enzymes (AST or ALT), and among different subgroups (by sex, age, and race/ethnicity) as well as the entire population.
The development of NAFLD is complex and still not well understood. Many factors, including genetic, metabolic, and dietary risk factors, are postulated to contribute to the development of NAFLD.4 Mechanistically, NAFLD is thought to represent the results of two distinct steps of hepatic insults (the “two-hit” theory of NAFLD). The first “hit” is the development of hepatic steatosis caused by an imbalance of hepatic lipid metabolism; and the second “hit” is a concurrent inflammatory process potentially resulting from oxidative stress, peroxidation, and cytokines actions.4 Uric acid is produced as the end-product of purine breakdown. People generally acquire purine from increased intake of purine-rich foods (such as meats and seafood), monosaccharide fructose, and alcohol.18 As for fructose, once it enters into hepatocytes, it is rapidly phosphorylated and intracellular phosphate levels fall, which triggers the activity of adenosine mono-phosphate (AMP) deaminase. Finally, AMP deaminase results in substrate-dependent phosphate depletion [adenosine triphosphate (ATP) depletion], which increases uric acid production.18,19 Some studies have shown that hepatic ATP depletion also leads to arrest in protein synthesis and produces inflammatory and pro-oxidative changes.20,21 One recent review article by Lim et al. postulates that excessive dietary fructose consumption may contribute to the development of NAFLD.4 Additionally, increased SUA itself may also produce pro-inflammatory and pro-oxidative effects.22 Ruggiero et al. showed a positive and significant relationship between SUA and many inflammatory markers in Italian men.23 Thus ATP depletion and SUA itself may lead to hepatocellular injury (elevated liver enzymes) and progression of NAFLD. Indeed, some studies have showed that SUA levels are associated with the progression of chronic liver diseases such as NAFLD and NASH12 and hyperuricemia is independently associated with the severity of liver damage among NAFLD patients.24 However, in our study we did not find an association between NASH and SUA level among patients with NAFLD. Maybe this is because of the small number of NASH participants (n = 231). In addition, one prospective study of SUA and NAFLD has been reported. Xu et al in China followed 6890 men and women without NAFLD for 3 years and found a higher incidence of NAFLD in those with higher baseline SUA levels.25
Our study has several limitations. First, given the cross-sectional design, we are unable to determine causality. Second, the diagnosis of NAFLD by ultrasound is relatively insensitive as compared to that by biopsy26 although the recent study showed that ultrasound allows for reliable and accurate in the detection of moderate-to-severe fatty liver compared to histology.27 Thus, we are unable to determine the association between SUA and lesser degrees of steatosis. Third, we did not consider other potential confounding factors on SUA level such as diet patterns. Fourth, it should be a weakness to use in this study only around 40% of the cohort. Finally, individuals with more severe NAFLD, especially if hospitalized or in a nursing home, would have been unlikely to take part in NHANES, thus our associations are likely limited to moderate NAFLD, and not end-stage liver disease. Nevertheless, our study was performed using a nationally representative sample of US women and men, allowing for population-based estimates that can be generalized to the noninstitutionalized US population. In addition, given the large sample size and design of NHANES, we were able to control for multiple confounders, including all of the components of the MetS, establishing an association independent of these factors.
In conclusion, we found a significant association between SUA levels and NAFLD among the US population, independent of multiple metabolic risk factors. More prospective studies are needed to clarify the temporality of the association. If hyperuricemia proves to be in the causal pathway for NAFLD, then prevention or treatment of hyperuricemia may reduce the risk of development or progression of NAFLD. If hyperuricemia proves to be a consequence of NAFLD, then hyperuricemia could serve as a trigger for physicians to screen for NAFLD and liver disease, since it can identify someone at high risk for NAFLD independent of other currently acceptable factors.
References
- 1.Angulo P Nonalcoholic fatty liver disease. N Engl J Med 2002; 346:1221–31. [DOI] [PubMed] [Google Scholar]
- 2.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis 2010;28:155–61. [DOI] [PubMed] [Google Scholar]
- 3.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatol 2010;51:1820–32. [DOI] [PubMed] [Google Scholar]
- 4.Lim JS, Mietus-Snyder M, Valente A, Schwarz JM, Lustig RH. The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat Rev Gastroenterol Hepatol 2010;7: 251–64. [DOI] [PubMed] [Google Scholar]
- 5.Fan J-G, Saibara T, Chitturi S, Kim BI, Sung JJY, Chutaputti A. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J Gastroenterol Hepatol 2007;22: 794–800. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Xu C, Yu C, Xu L, Miao M. Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J Hepatol 2009;50:1029–34. [DOI] [PubMed] [Google Scholar]
- 7.Kuo CF, Yu KH, Luo SF, Chiu CT, Ko YS, Hwang JS, et al. Gout and risk of non-alcoholic fatty liver disease. Scand J Rheumatol 2010;39:466–71. [DOI] [PubMed] [Google Scholar]
- 8.Lee YJ, Lee HR, Lee JH, Shin YH, Shim JY. Association between serum uric acid and non-alcoholic fatty liver disease in Korean adults. Clin Chem Lab Med 2010;48:175–80. [DOI] [PubMed] [Google Scholar]
- 9.Yamada T, Suzuki S, Fukatsu M, Wada T, Yoshida T, Joh T. Elevated serum uric acid is an independent risk factor for nonalcoholic fatty liver disease in Japanese undergoing a health checkup. Acta Gastroenterol Belg 2010;73:12–7. [PubMed] [Google Scholar]
- 10.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 2008;359:1811–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinones Galvan A, Natali A, Baldi S, Frascerra S, Sanna G, Ciociaro D, et al. Effect of insulin on uric acid excretion in humans. Am J Physiol 1995;268:E1–5. [DOI] [PubMed] [Google Scholar]
- 12.Afzali A, Weiss NS, Boyko EJ, Ioannou GN. Association between serum uric acid level and chronic liver disease in the United States. Hepatol 2010;52:578–89. [DOI] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–1994 1-July-1994. Washington, DC: DHHS publication no. (PHS) 94–1308. Vital and health statistics. Series 1. No. 32. Ref Type: Serial (Book, Monograph). [Google Scholar]
- 14.Third National Health and Nutrition Examination Survey Gall-bladder ultrasonography procedure manual. Rockville, MD: Westat, Inc.; 1988. [Google Scholar]
- 15.Third National Health and Nutrition Examination Survey. Hepatic steatosis assessment procedure manual. Hyattsville, MD: National Center for Health Statistics; 2010. [Google Scholar]
- 16.Laboratory procedures used for the THIRD National Health and Nutrition Examination Survey (NHANES III) 1988–1994 U.S. Department of Health and Human Services; 1996.
- 17.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol 2006;21:1–6. [DOI] [PubMed] [Google Scholar]
- 18.Hediger MA, Johnson RJ, Miyazaki H, Endou H. Molecular physiology of urate transport. Physiol (Bethesda) 2005;20:125–33. [DOI] [PubMed] [Google Scholar]
- 19.Cortez-Pinto H, Chatham J, Chacko VP, Arnold C, Rashid A, Diehl AM. Alterations in liver ATP homeostasis in human nonalcoholic steatohepatitis: a pilot study. J Am Med Assoc 1999;282:1659–64. [DOI] [PubMed] [Google Scholar]
- 20.Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, et al. Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol 2009;20:545–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glushakova O, Kosugi T, Roncal C, Mu W, Heinig M, Cirillo P, et al. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J Am Soc Nephrol 2008;19:1712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, et al. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 2003;41:1287–93. [DOI] [PubMed] [Google Scholar]
- 23.Ruggiero C, Cherubini A, Ble A, Bos AJ, Maggio M, Dixit VD, et al. Uric acid and inflammatory markers. Eur Heart J 2006; 27:1174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petta S, Camma C, Cabibi D, Di Marco V, Craxi A. Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2011;34:757–66. [DOI] [PubMed] [Google Scholar]
- 25.Xu C, Yu C, Xu L, Miao M, Li Y. High serum uric acid increases the risk for nonalcoholic fatty liver disease: a prospective observational study. PLoS One 2010;5:e11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joseph AE, Saverymuttu SH, al-Sam S, Cook MG, Maxwell JD. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol 1991;43: 26–31. [DOI] [PubMed] [Google Scholar]
- 27.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultra-sonography for the detection of fatty liver: a meta-analysis. Hepatol 2011;54(3):1082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]