Abstract
Corticotropin-releasing factor (CRF) circuitry is a key component in plasticity underlying the transition to ethanol (EtOH) dependence. We have previously shown that chemogenetic silencing of CRF neurons stemming from the dorsolateral bed nucleus of the stria terminalis (dlBNST) and projecting to the ventral tegmental area (VTA) significantly blunts binge-like EtOH consumption. While CRF neurons in the BNST are thought to entail primarily a GABA phenotype, glutamatergic neurons within the BNST also innervate the VTA and influence consummatory behaviors. Here, we combined the well-validated Vgat-ires-Cre transgenic mice with chemogenetic tools to extend our previous findings and corroborate the contribution of the VTA-projecting dlBNST GABAergic circuitry in modulating binge-like EtOH consumption using “drinking-in-the-dark” procedures. Mice were given bilateral injection of Gi-coupled chemogenetic viral vector (or control virus) into the dlBNST and bilateral cannulae into the VTA. On test day, clozapine-N-oxide (CNO; or vehicle) was infused directly into the VTA to silence VTA-projecting dlBNST neurons and subsequent binge-like EtOH consumption was assessed. We then used immunohistochemistry (IHC) to determine the co-expression of CRF and viral vector. Our results showed that relative to vehicle treatment or CNO treatment in mice expressing the control virus, silencing VTA-projecting dlBNST GABAergic neurons by CNO treatment in mice expressing Gi-coupled chemogenetic virus significantly reduced binge-like EtOH intake. This effect was not seen with sucrose consumption. Our IHC results confirm a population of CRF-expressing GABAergic neurons within the dlBNST. This study directly establishes that VTA-projecting GABAergic neurons of the dlBNST modulate binge-like EtOH consumption.
Keywords: chemogenetics, corticotropin-releasing factor, drinking-in-the-dark, extended amygdala
1 |. INTRODUCTION
It has been established that the method and rate of alcohol consumption contribute to the susceptibility of transitioning from moderate alcohol (ethanol; EtOH) drinking to the development of alcohol dependence (Li, Hewitt, & Grant, 2007). A pattern of short bouts involving excessive alcohol consumption, referred to as binge drinking, is a major proponent of this transition. According to the National Institute on Alcohol Abuse and Alcoholism (NIAAA), binge drinking is consuming enough alcohol to achieve blood concentrations in excess of 80 mg/dl in a short period of time (NIAAA, 2004). The “Drinking-in-the-dark” (DID) model has been shown to induce binge-like levels of ethanol intake using a restricted access paradigm in C57BL/6J mice (Rhodes, Best, Belknap, Finn, & Crabbe, 2005; Thiele, Crabbe, & Boehm, 2014; Thiele & Navarro, 2014). Utilizing this approach to study system neurocircuitry has helped to provide insight into the underlying mechanisms involved with binge consumption and the transition from moderate alcohol use to dependence.
One neuropeptide system heavily involved in both binge-like EtOH intake and dependence-induced drinking is the corticotropin-releasing factor (CRF) system (Lowery & Thiele, 2010; Lowery-Gionta et al., 2012; Roberto et al., 2010). Previous work has shown that CRF-protein levels within the ventral tegmental area (VTA) become elevated after exposure to binge-like EtOH consumption (Lowery-Gionta et al., 2012). More recently, our laboratory has discovered that the inhibition of CRF axonal projections stemming from the dorsal lateral BNST (dlBNST) and innervating the VTA leads to a decrease in binge-like EtOH consumption in transgenic CRF-ires-Cre mice on a C57BL/6J background (Rinker et al., 2017). These CRF projection neurons have been shown to exert a GABAergic phenotype (Dabrowska, Hazra, Guo, DeWitt, & Rainnie, 2013). Consistently, GABA-A receptor inhibition in the posterior VTA has been shown to reduce binge-like EtOH consumption in C57BL/6J mice (Melón & Boehm, 2011). However, there are also other populations of neurons in the BNST that innervate the VTA, such as glutamatergic neurons, that have also been shown to be involved in consummatory behaviors (Jennings et al., 2013; Kudo et al., 2012; Stamatakis et al., 2014). The established co-expression of GABA and CRF in dlBNST neurons suggests that our previous results involved GABAergic neurons, though the direct assessment of VTA-projecting GABAergic neurons of the dlBNST in the modulating of binge-like EtOH has not been directly tested.
The goal of the present study was to confirm that our previous study, using CRF-ires-Cre mice, likely involved a GABAergic neuronal population housing this CRF peptide. To this end, we used well-established transgenic mice in which Cre was linked to the vesticular GABA transporter, VGAT (the Vgat-ires-Cre line; Vong et al., 2011) in combination with Cre-dependent adeno-associated viral vectors (AAVs) that were infused into the dlBNST of Vgat-ires-Cre mice. This chemogenetic technology that we employed has been referred to as Designer Receptors Exclusively Activated by Designer Drugs (DREADD) and acts by producing G-coupled protein receptors, reactive to naturally inert ligands, such as Clozapine-N-oxide (CNO) (Rogan & Roth, 2011; Roth, 2016). In addition to bilateral infusion of Cre-dependent Gi/o DREADD (or control virus lacking the DREADD construct) into the dlBNST, Vgat-ires-Cre mice were simultaneously given bilateral cannulae aimed at the VTA. By infusing CNO specifically into the VTA, we were able to specifically inhibit VTA-projecting GABAergic neurons arising from the dlBNST area where the virus was injected. We then applied immunohistochemistry (IHC) to assess co-expression of CRF with our DREADD virus in dlBNST neurons.
2 |. MATERIALS AND METHODS
Experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the University of North Carolina at Chapel Hill.
2.1 |. Animals
This study used both male and female vGat-ires-Cre mice (determined Cre+ by standard PCR genotyping) backcrossed with the C57BL/6J (Jackson Laboratories, Bar Harbor, MA, USA), which have previously been shown to consume high binge-like levels of EtOH in a restricted consumption model (Crabbe, Harris, & Koob, 2011). Mice were at least 60 days of age before the beginning of this study. Animals were individually housed in a ventilated cage system on a 12-hr reversed light/dark cycle with ad libitum access to Prolab® RMH 3000 (Purina labDiet®; St. Louis, MO) and water during the duration of the experiment; except for during EtOH access periods (Marshall et al., 2015). A total of 44 mice were used in this study with individual group n’s listed in figure captions. All mice within the sucrose portion of the experiment overlap with a portion from the EtOH trials. A separate group of five animals, three male and two female, were used in order to quantify overlap of CRF+ and DREADD-expressing neurons within the dlBNST region of viral injection.
2.2 |. Drug
Ethanol (20% v/v) solutions were prepared from 95% ethyl alcohol stock (Deacon Laboratories Inc, Prussia, PA, USA) and diluted with tap water. Sucrose (3% w/v) solutions were prepared with D-sucrose (Thermo Fisher Scientific, Waltham, MA, USA) dissolved in tap water. Clozapine-N-oxide (supplied by the NIDA Drug Supply Program) was dissolved in DMSO (1% v/v final concentration) and then diluted with 0.9% saline with a final concentration of 3 mM. Vehicle consisted of the same solution used to dissolve the CNO compound. Microinjections of CNO and vehicle were infused over the course of 1 min (0.3 μl/min) and injectors remained in the same place for at least 1-m in post-infusion for diffusion of the drug and to help minimize back flow as the injectors are being removed. This injection volume and rate have been consistently used in site-directed injection methods within similar experiments (Rinker et al., 2017).
2.3 |. Surgery procedures
Surgery methods were similar to those in our previous study with CRF+ neurons within the BNST (Rinker et al., 2017). Briefly, mice were anesthetized with a 1.5 g/kg dose of ketamine/xylazine cocktail (100 and 10 mg/kg, respectively) before beginning surgery procedures. Mice were then randomly assigned to receive bilateral 0.5 μl/side injections of either a Cre-dependent Gi/o-coupled DREADD (AAV8-hSyn-DIO-hM4d-mCherry) or a Cre-dependent control virus (AAV8-hSyn-DIO-mCherry) (Addgene.org, Cambridge, MA, USA) in the dlBNST (with respect to bregma; AP: +0.30 mm, ML: ± 1.10 mm, DV: −4.35 mm) at a rate of 0.1 μl/min The injectors remained in place for at least 10–15 min following injections in an effort to minimize backflow of the virus through the syringe tract. Simultaneously, bilateral cannulae (Plastics One, Anaheim, CA, USA) were implanted into the VTA (with respect to bregma; AP: −3.1 mm, ML: ± 0.5 mm, DV: −4.5 mm) of all animals with the assistance of Lecia Angle Two Stereotax (Lecia Biosystems, Buffalo Grove, IL, USA). Mice then remained in their homecage for at least 5–6 weeks in order to let the virus incorporate into the GABAergic neurons and terminals before beginning the behavioral drinking portions of the experiment.
2.4 |. Drinking-in-the-dark procedures
A 4-day ON/3-day OFF DID protocol was used to model binge-like EtOH consumption in mice. Water bottles were removed and replaced with bottles containing 20% EtOH (v/v) for 2-hr beginning approximately 3-hr into the dark cycle. Day 4 was considered a test day where animals were microinjected with either 900 pmol/side CNO or vehicle, using a Latin Square design, 30 min before a 2-hr EtOH drinking session where drinking was recorded every hour. Animals then went through a 3-day abstinence with only water ad libitum. The full 7 days are considered a cycle of the DID exposure.
Animals went through two cycles of the DID procedure with EtOH, and after a week of additional abstinence, two additional cycles of DID were conducted with a 3% sucrose (w/v) solution replacing the EtOH solution. Tail blood samples were taken at the end of the test day during both cycles of EtOH exposure in order to analyze the blood ethanol concentration (BEC) for each animal using the Analox Analyzer (Analox Instruments, Lunenburg, MA, USA). Approximately 30 μl of tail blood was collected from lateral tail vein of each animal. BECs were used to determine whether or not the animal was consuming EtOH in a binge-like pattern (at least 80 mg/dl) during the test day.
2.5 |. Tissue preparation and IHC procedures
Animals were sacrificed by a ketamine/xylazine cocktail, at a 10:1 ratio before transcardial perfusion with PBS at a rate of 2.5 ml/min for 4 min followed by a 4% paraformaldehyde solution at a rate of 2.5 ml/min for 7 min; sufficient to flush and fix brain tissue. Brains were extracted and allowed to set in the 4% paraformaldehyde solution in order to further fix the tissue for 48-hr before being transferred to PBS until tissue processing. Fixed brain tissue was cut into 40-μm slices using a Leica VT1000 S Vibratome (Lecia Biosystems, Buffalo Grove, IL, USA) and stored in a cryopreserve solution. Using every fourth slice of tissue, IHC procedures were conducted in order to label CRF protein within the dlBNST.
Tissue slices were exposed to the primary Anti-CRF Rabbit antibody (Abcam Inc., Cambridge, MA, USA; ab8901) at a 1:250 concentration in the blocking solution for 48–72 hr after an antigen retrieval at 65°C for 30 min in Antigen Retrieval Citra 1× Buffer to facilitate a more substantial binding potential (Cuevas Guaman et al., 2014; Garcia-Moreno et al., 2010). This concentration was determined by running a pilot concentration dilution experiment with the tissue used in a previous study to determine the proper primary concentration and antigen retrieval protocol. We then used a solution containing the secondary fluorescent antibody Donkey Anti-Rabbit Alexa Flour 488 (Abcam Inc., Cambridge, MA, USA) at a 1:1,000 concentration in order to attach a green label to the primary antibody for visualization. Three rinses made sure that all excess antibodies are rinsed away for maximum qualification of labeled neurons.
Tissue was then mounted onto glass slides and Vectashield HardSet Medium containing DAPI (Vector Laboratories, Burlingame, CA, USA) was used to mount the coverslips over the tissue. This allowed for easy visualization of cell bodies that expresses both the mCherry virus tag and the green-fluorescing CRF antibody tag. A Zeiss LSM 800 confocal microscope (Zeiss, USA) was used to image the tissue and allow 3D and z-stack imaging in order to fully determine whether co-localization is present within the dlBNST GABAergic projection neuron population. Images were then analyzed in order to quantify the overall percentage of CRF+ cells overlapped with the overall DREADD+ neurons within the dlBNST. This was done using the cell counting with 2–4 z-stacks per animal.
2.6 |. Statistical analysis
Ethanol consumption and BECs achieved during DID procedures at the 2-hr time-point data were analyzed using a repeated-measures MANOVA with factors of treatment and treatment order to ensure there were no drug carryover or order effects. Separate hour time points used Bonferroni corrected paired and unpaired t tests in order to uncover significant effects.
3 |. RESULTS
3.1 |. Anterograde tracing of fibers reveals VTA-projecting GABAergic pathway stemming from the dlBNST
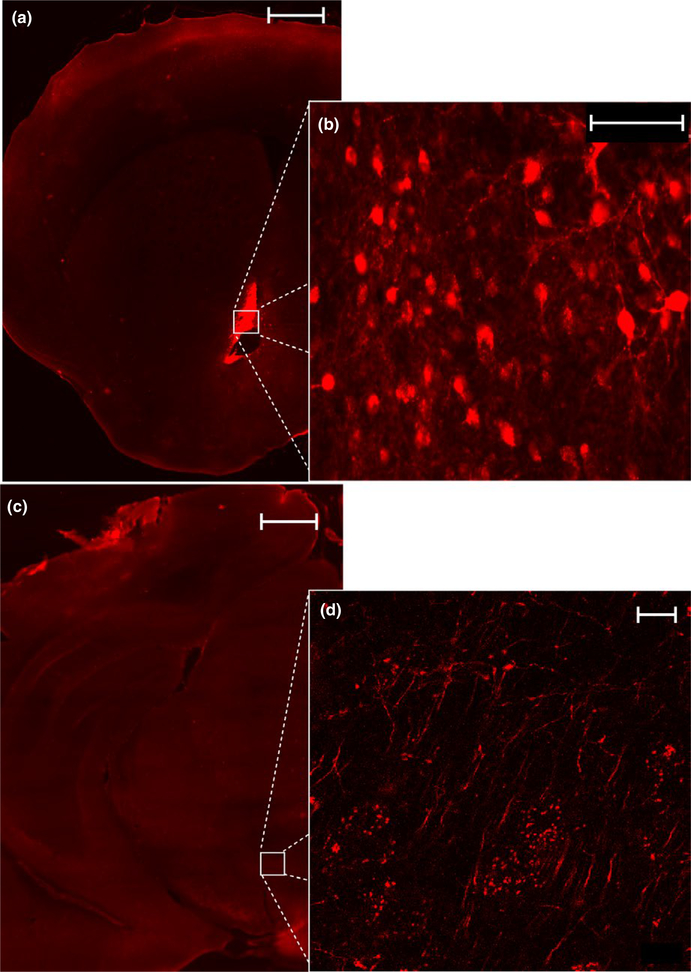
Visualization of AAV8-hSyn-DIO-hM4d-mCherry terminals within the VTA of VGat-ires-Cre mouse line confirmed an anterograde, GABAergic projection from the original injection site of the dlBNST after 6 weeks of incubation (Figure 1a, mCherry+ visualization of dlBNST; Figure 1b, visualization of mCherry+ cell bodies, Figure 1c, Section of VTA used to visualize terminals, Figure 1d, mCherry+ terminals is the VTA).
FIGURE 1.
Tracing of GABAergic neuron terminals from the dlBNST to the VTA within the VGat-ires-Cre mouse line. (a) Visualization of mCherry-tagged DREADD viral transfection within the dlBNST at 5× magnification (Scale bar = 500 μm) and (b) visualization of DREADD-expressing cell bodies at 40× magnification (Scale bar = 75 μm). (c) Innervation of the VTA by dlBNST GABAergic fibers at 5× magnification (Scale bar = 500 μm) and (d) visualization of terminal fiber at × magnification (Scale bar = 10 μm)
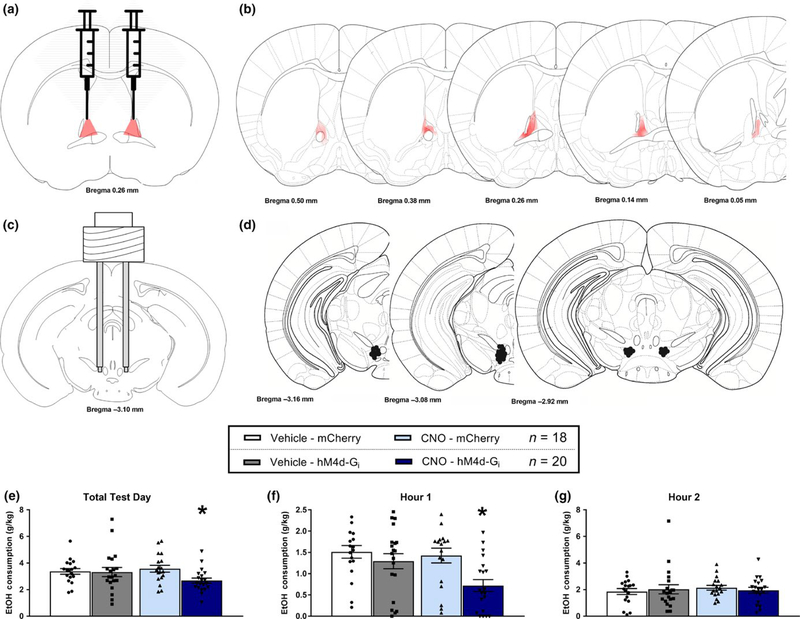
3.2 |. Inhibition of GABAergic VTA-projecting dlBNST neurons reduces binge-like ethanol consumption
Figure 2 shows data from the dlBNST → VTA silencing study during binge-like ethanol consumption. Figure 2a shows a schematic representation of the viral-vector injection sites in the dlBNST, Figure 2b depicts the average gradated spread of the DREADD construct in the dlBNST in VGat-ires-Cre mice given dlBNST injection of Cre-dependent viral vector, verifying the expression of viral vector that was confined to the dlBNST. Critically, previous work has functionally validated the hM4D-Gi DREADD when traduced into the dlBNST by showing that CNO application significantly blunted neuronal activity in vGat-ires-Cre mice (Mazzone et al., 2018). Figure 2c shows a schematic representation of the cannula placement in the VTA while Figure 2d shows a schematic depicting the termination sites of cannulae implanted into the VTA of individual subjects. There were six animals removed from analysis due to incorrect placement of cannula. A repeated-measures (RM) MANOVA with within groups factors (hour and drug) and between groups factors (virus and sex) performed on total ethanol consumption indicated a main effect of hour [F(1, 34) = 23.982, p < 0.001] and interaction of drug × virus [F(1, 34) = 5.883, p = 0.021] and drug × sex [F(1, 34) = 13.866, p = 0.001; male CNO = 1.72 ± 0.10, male vehicle = 1.53 ± 0.13, female CNO = 1.36 ± 0.11, female vehicle = 1.86 ± 0.15]. The drug × sex interaction reflected the tendency for male mice to drink more ethanol when treated with CNO, while the female mice tended to drink more ethanol when treated with vehicle. A further analysis, drug × virus interaction using the planned-comparison Bonferroni’s correction t test (with significance set at p < 0.008) revealed a significant difference between the AAV8-hSyn-DIO-hM4d-mCherry virus and the AAV8-hSyn-DIO-mCherry virus groups that were treated with CNO [p = 0.0079]. There were no differences between virus conditions in mice treated with vehicle (Figure 2e). Similarly, a significant difference emerged between the AAV8-hSyn-DIO-hM4d-mCherry virus and the AAV8-hSyn-DIO-mCherry virus groups that were treated with CNO at the 1-hr consumption measure [p = 0.003], with no differences between virus conditions in mice treated with vehicle (Figure 2f). There were no group differences when the second hour of consumption was analyzed (Figure 2g). Finally, analysis of BEC data failed to reveal significant effects (control virus-vehicle, 110 ± 13.84 mg/dl; control virus-CNO, 131.5 ± 13.66 dl; hM4d virus-vehicle, 109.4 ± 15.61 dl; hM4d virus-CNO, 112.4 ± 17.51 dl).
FIGURE 2.
Silencing of VTA-projecting dlBNST GABAergic neurons reduces binge drinking during the first hour. (a) Diagram of bilateral viral placements within the dlBNST. (b) Representative figure of AAV8-hSyn-DIO-hM4d-mCherry expression and gradated spread in VGat-ires-Cre neurons of the dlBNST. (c) Diagram of bilateral cannula placements within the VTA. (d) Cannulae placements within the VTA for individual subjects. Due to the cannula being placed within a pedestal, only one hemisphere is shown for placements as each subject was consistent on both hemispheres. Missed placements were excluded from analysis. (e) Following site-directed microinjections of vehicle (1% DMSO v/v final concentration diluted in 0.9% saline) there were no differences in 20% ethanol consumption (mean g/kg ± SEM) between the control (mCherry) viral vector and hM4D-Gi viral groups; however, following site-directed microinfusions of CNO (same as vehicle with final concentration of 3 mM CNO) mice treated with dlBNST hM4D-Gi virus drank significantly less ethanol than mice treated with the control (mCherry) virus (*p = 0.0079). (f) There was a similar CNO-induced reduction of binge-like ethanol intake in hM4D-Gi treated mice during the first hour of ethanol consumption (*p = 0.003), but not during the second hour of ethanol consumption (g)
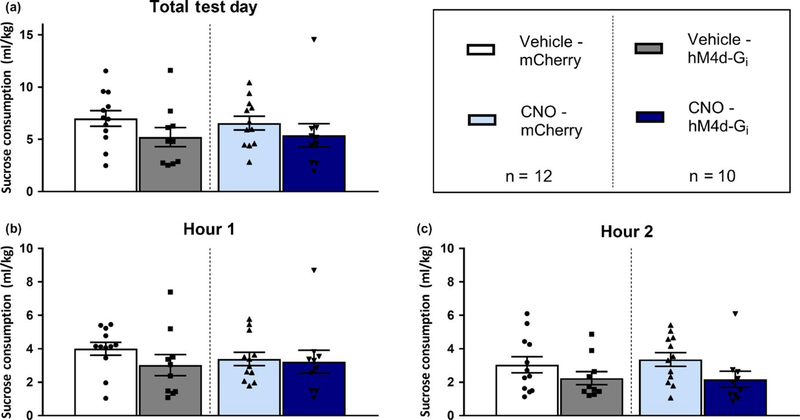
3.3 |. Inhibition of GABAergic VTA-projecting dlBNST neurons does not influence sucrose consumption
Figure 3 shows data from the dlBNST → VTA silencing study during binge-like sucrose consumption. A repeated-measures (RM) MANOVA with within groups factors (hour and drug) and between groups factors (virus and sex) performed on 2-hr sucrose consumption data failed to produce any main effects or interaction effects (Figure 3a). Further, there were no significant differences during the first (Figure 3b) or second (Figure 3c) hours of sucrose consumption.
FIGURE 3.
Silencing of VTA-projecting dlBNST GABAergic neurons had no significant effect on binge drinking of 3% sucrose. (a) There was no significant reduction of 3% sucrose drinking within any of the four groups during the total 2-hr binge test. (b) There was no significant effect of virus or drug treatment on sucrose drinking during the first hour of sucrose drinking. (c) There was also no reduction of sucrose drinking during the second hour of sucrose consumption
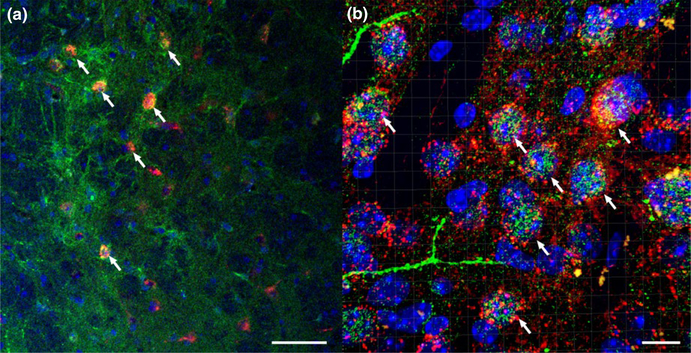
3.4 |. Co-localization of CRF immunoreactivity with Cre-dependent DREADD in the dlBNST of VGat-ires-Cre mice
Figure 4 shows confocal imaging of AAV8-hSyn-DIO-hM4d-mCherry (red) with anti-CRF in Alexaflour-488 (green) and cell nuclei DAPI staining (blue) at 20× (Figure 4a) and 63× (Figure 4b) magnification. When cell counting clearly labeled DREADD+ neurons, about 87% or 187 out of 209 cells, co-expressed CRF-protein staining within the dlBNST. A qualitative assessment of the co-expression suggests that most, if not all, of the AAV8-hSyn-DIO-hM4d-mCherry+neurons also expressed CRF immunoreactivity, consistent with the observation that 98% of GABAergic neurons in the dlBNST entail a CRF phenotype (Nguyen, Dela Cruz, Sun, Holmes, & Xu, 2016). These sets of data together provide strong evidence of a heavily populated CRF region within the dlBNST.
FIGURE 4.
CRF IHC co-localization with Cre-dependent AAV8-hSyn-DIO-hM4d-mCherry in VGat-ires-Cre mice. (a) Confocal imaging of AAV8-hSyn-DIO-hM4d-mCherry viral vector (red) with anti-CRF in Alexaflour-488 (green) and cell nuclei DAPI staining (blue) at × magnification. White arrows emphasize co-localized neurons (scale bar = 50 μm). (b) Confocal, z-stacked 3D reconstructed of co-localization of AAV8-hSyn-DIO-hM4d-mCherry virus (red) with CRF (green) and DAPI (blue) at 63× magnification. White arrows emphasize co-localized neurons (scale bar = 10 μm). When cell counting clearly labeled DREADD+ neurons, about 87% co-expressed CRF-protein staining within the dlBNST
4 |. DISCUSSION
Here, we show that silencing VTA-projecting GABAergic dlBNST neurons significantly reduced binge-like EtOH intake, particularly during the first hour of the 2-hr EtOH consumption test. Importantly, CNO did not alter binge-like ethanol intake relative to vehicle treatment in mice expressing the control viral vector, ruling out potential off-target effects of CNO with respect to ethanol intake. There was also no effect of virus or drug on sucrose drinking using the DID paradigm, suggesting that the effect of silencing VTA-projecting GABAergic dlBNST neurons was specific to EtOH consumption and did not influence the consumption of another salient, natural reinforcer. The IHC and confocal microscopy revealed GABAergic neurons in the dlBNST that also co-localized with the CRF peptide, reaffirming our previous findings showing blunted binge-like ethanol intake with silencing of VTA-projection dlBNST neurons expressing CRF (Rinker et al., 2017). When taken together, our results demonstrate that dlBNST GABAergic circuitry projecting to the VTA modulates binge-like EtOH drinking, and that many GABAergic neurons in this region co-express CRF.
We have previously shown that silencing VTA-projecting CRF+ neurons within the dlBNST significantly blunted binge-like EtOH intake (Rinker et al., 2017). A recent study found that while CRF+ neurons in the BNST co-express both glutamate and GABA, within the dlBNST, about 98% of CRF+ neurons are GABAergic (Nguyen et al., 2016). Together, these findings suggest that our previous study using CRF-ires-Cre mice may have stemmed from the silencing of a GABAergic population of neurons within the dlBNST. The observation by Nguyen et al. (2016) that a population of BNST glutamatergic neurons also co-express a CRF+phenotype, and research showing that a population of BNST glutamatergic neurons innervates the VTA and have effects on consummatory behavior (Jennings et al., 2013) together highlight the importance of future research to investigate the role of VTA-projecting glutamatergic BNST neurons in the modulating of binge-like ethanol intake. However, given that 98% of CRF+ neurons in the dlBNST are co-expressed with a GABAergic phenotype, and in light of our co-expression results, the contributions of a VTA-projecting glutamatergic dlBNST circuit in modulating binge-like ethanol intake seems minimal. One caveat is that we cannot be certain if the rate of GABAergic terminal staining co-expressing CRF in the VTA matches the rate of co-expression observed in the dlBNST.
Recent evidence has suggested that CNO may back-metabolize to clozapine, which is a psychoactive drug that may act as a potential confound to chemogenetic studies (MacLaren et al., 2016). However, there are two observations within our study that placate this concern: First, CNO had no effect, relative to vehicle treatment, in mice that expressed the control viral vector. Second, there was no effect of CNO on sucrose consumption in either the AAV8-hSyn-DIO-hM4d-mCherry or control virus groups. Together, these observations rule out the likely possibility that off-target effects from the use of CNO influenced binge-like ethanol intake. There is also the concern that CNO may have diffused outside of the VTA injection region and possibly moved to other regions in which DREADD+ fibers were present. While we cannot completely rule of this possibility, this concern seems unlikely as we did not observe fiber staining in regions near the VTA. Further, we used a slow injection rate and volume also utilized in our previous work (Rinker et al., 2017).
Interestingly, Rinker et al. (2017) found that site-directed infusion of a CRF-1 receptor (CRF1R) antagonist into the VTA blunted binge-like ethanol consumption, but that while using simultaneous infusion of CRF1R and CRF2R antagonists, the paradigm failed to alter intake. Thus, intact CRF2R signaling in the VTA is necessary for CRF1R antagonism to blunt binge-like ethanol intake. Rinker et al. presented a theoretical model whereby GABAergic transmission from VTA-projecting dlBNST neurons promotes binge-like ethanol intake, and that GABAergic signaling from these neurons is facilitated or inhibited by CRF1R or CRF2R, respectively. Further, they postulate that blockade of CRF1R “unmasks” the ability of CRF2R to blunt GABAergic signaling, resulting in reduced binge intake. Blockade of both receptors simultaneously negates this effect, leaving GABAergic signaling unregulated which is consistent with the observed unaltered binge-like drinking when both CRF receptor antagonist are infused into the VTA. The present results provide additional support for this theoretical model by directly demonstrating the central role of GABAergic signaling in this circuit in the modulating of binge-like ethanol intake. However, additional studies are necessary to directly establish co-release of GABA and CRF as well as a functional link between CRF receptor signaling and GABA activity in the VTA with respect to effects on binge-like ethanol intake as this was not done in the present work.
In summary, we show that inhibition of a VTA-projecting GABAergic circuit stemming from the dlBNST reduces binge-like ethanol drinking, suggesting that this circuit plays a critical role in modulating binge-like EtOH consumption. This effect was specific to EtOH consumption, as silencing this circuit had no impact on the consumption of the natural reinforcer, sucrose. We also show that the GABAergic population under investigation within the dlBNST co-expresses CRF. Overall, this study and Rinker et al. (2017) emphasize the importance of VTA-projecting GABAergic and CRF+ neurons from the dlBNST in the early stages of binge-like EtOH intake. Neuroadaptations in these pathways may be an important component of increased voluntary consumption of ethanol stemming from repeated bouts of binge drinking (Cox et al., 2013), thus further characterization of this neurocircuitry may provide valuable insight into the mechanisms underlying the development of alcohol use disorders.
ACKNOWLEDGEMENTS
We gratefully acknowledge the support from the National Institute on Alcoholism and Alcohol Abuse (NIAAA; R01 AA022048, R01 AA013573, & R01 AA015148) and the National Institute on Drug Abuse (NIDA; T32 DA00724426). CNO was supplied by the NIDA Drug Supply Program.
Funding information National Institute on Alcoholism and Alcohol Abuse, Grant/Award Number: R01 AA022048, R01 AA013573 and R01 AA015148; National Institute on Drug Abuse, Grant/Award Number: T32 DA00724426; NIDA Drug Supply Program
Abbreviations:
- AAV
adeno-associated viral vectors
- BEC
blood ethanol concentration
- BNST
bed nucleus of the stria terminalis
- CNO
clozapine-N-oxide
- CRF1R
corticotropin-releasing factor 1 receptor
- CRF2R
corticotropin-releasing factor 2 receptor
- CRF
corticotropin-releasing factor
- dlBNST
dorsal lateral bed nucleus of the stria terminalis
- DREADD
designer receptors exclusively activated by designer drug
- eYFP
enhanced yellow fluorescent protein
- IHC
immunohistochemistry
- MANOVA
multivariate analysis of variance
- RM
repeated-measures
- VGAT
vesicular γ-aminobutyric acid transporter
- VTA
ventral tegmental area
Footnotes
CONFLICT OF INTEREST
The authors declare no competing financial interests.
DATA ACCESSIBILITY
The data from the current study will not be stored on a public repository. Upon request, access to the data will be provided by the corresponding author.
REFERENCES
- Cox BR, Olney JJ, Lowery-Gionta EG, Sprow GM, Rinker JA, Navarro M, … Thiele TE (2013). Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcoholism, Clinical and Experimental Research, 37, 1688–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Harris RA, & Koob GF (2011). Preclinical studies of alcohol binge drinking. Annals of the New York Academy of Sciences, 1216, 24–40. 10.1111/j.1749-6632.2010.05895.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas Guaman M, Sbrana E, Shope C, Showalter L, Hu M, Meloche S, & Aagaard K (2014). Administration of antenatal glucocorticoids and postnatal surfactant ameliorates respiratory distress syndrome-associated neonatal lethality in Erk3(−/−) mouse pups. Pediatric Research, 76, 24–32. 10.1038/pr.2014.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabrowska J, Hazra R, Guo J, DeWitt S, & Rainnie D (2013). Central CRF neurons are not created equal: Phenotypic differences in CRF-containing neurons of the rat paraventricular hypothalamus and the bed nucleus of the stria terminalis. Frontiers in Neuroscience, 7, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Moreno F, Pedraza M, Di Giovannantonio LG, Di Salvio M, Lopez-Mascaraque L, Simeone A, & De Carlos JA (2010). A neuronal migratory pathway crossing from diencephalon to telencephalon populates amygdala nuclei. Nature Neuroscience, 13, 680–689. 10.1038/nn.2556 [DOI] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, & Stuber GD (2013). Distinct extended amygdala circuits for divergent motivational states. Nature, 496, 224–228. 10.1038/nature12041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, … Watanabe M (2012). Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. Journal of Neuroscience, 32, 18035–18046. 10.1523/JNEUROSCI.4057-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li TK, Hewitt BG, & Grant BF (2007). The alcohol dependence syndrome, 30 years later: A commentary the 2006 H. David Archibald lecture. Addiction, 102, 1522–1530. 10.1111/j.1360-0443.2007.01911.x [DOI] [PubMed] [Google Scholar]
- Lowery EG, & Thiele TE (2010). Pre-clinical evidence that corticotropin-releasing factor (CRF) receptor antagonists are promising targets for pharmacological treatment of alcoholism. CNS & Neurological Disorders Drug Targets, 9, 77–86. 10.2174/187152710790966605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, … Thiele TE (2012). Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. Journal of Neuroscience, 32, 3405–3413. 10.1523/JNEUROSCI.6256-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLaren DAA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, España RA, & Clark SD (2016). Clozapine N-Oxide administration produces behavioral effects in long–evans rats: Implications for designing DREADD experiments. eNeuro, 3, ENEURO.0219–16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall SA, Rinker JA, Harrison LK, Fletcher CA, Herfel TM, & Thiele TE (2015). Assessment of the effects of 6 standard rodent diets on binge-like and voluntary ethanol consumption in male C57BL/6J mice. Alcoholism, Clinical and Experimental Research, 39, 1406–1416. 10.1111/acer.12773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzone CM, Pati D, Michaelides M, DiBerto J, Fox JH, Tipton G, … Kash TL (2018). Metabolic mapping of downstream network activity following CNO-induced activation of hM3Dq in BNST VGAT neurons. Molecular Psychiatry, 23, 1 10.1038/mp.2017.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melón LC, & Boehm SL (2011). GABAA receptors in the posterior, but not anterior, ventral tegmental area mediate Ro15–4513-induced attenuation of binge-like ethanol consumption in C57BL/6J female mice. Behavioral Brain Research, 220, 230–237. 10.1016/j.bbr.2011.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AQ, Dela Cruz JA, Sun Y, Holmes TC, & Xu X (2016). Genetic cell targeting uncovers specific neuronal types and distinct subregions in the bed nucleus of the stria terminalis. The Journal of Comparative Neurology, 524, 2379–2399. 10.1002/cne.23954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA. (2004). National Institute on Alcohol Abuse and Alcoholism Council approves definition of binge drinking. NIAAA Newsletter. [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, & Crabbe JC (2005). Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiology & Behavior, 84, 53–63. 10.1016/j.physbeh.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Rinker JA, Marshall SA, Mazzone CM, Lowery-Gionta EG, Gulati V, Pleil KE, … Thiele TE (2017). Extended amygdala to ventral tegmental area corticotropin-releasing factor circuit controls binge ethanol intake. Biological Psychiatry, 81, 930–940. 10.1016/j.biopsych.2016.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M, … Parsons LH (2010). Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biological Psychiatry, 67, 831–839. 10.1016/j.biopsych.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan SC, & Roth BL (2011). Remote control of neuronal signaling. Pharmacological Reviews, 63, 291–315. 10.1124/pr.110.003020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth BL (2016). DREADDs for neuroscientists. Neuron, 89, 683–694. 10.1016/j.neuron.2016.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, & Stuber GD (2014). Amygdala and bed nucleus of the stria terminalis circuitry: Implications for addiction-related behaviors. Neuropharmacology, 76(Pt B), 320–328. 10.1016/j.neuropharm.2013.1005.1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Crabbe JC, & Boehm SL II (2014). “Drinking in the Dark” (DID): A simple mouse model of binge-like alcohol intake. Current Protocols in Neuroscience, 68, 9.491–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, & Navarro M (2014). “Drinking in the dark” (DID) procedures: A model of binge-like ethanol drinking in non-dependent mice. Alcohol, 48, 235–241. 10.1016/j.alcohol.2013.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong L, Ye C, Yang Z, Choi B, Chua S, & Lowell BB (2011). Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron, 71, 142–154. 10.1016/j.neuron.2011.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]