Abstract
In recent years, hopes for better treatment of traumatic brain injury (TBI) have focused on non-pharmacologic transcranial electrical brain stimulation; however, studies of perfusion changes after stimulation are few and contradictory. Therefore, the aim of this study was to assess cerebral perfusion after high-definition transcranial direct current stimulation (HD-tDCS) in patients with posttraumatic encephalopathy (PTE).
Methods.
Twenty patients with PTE (16 men and 4 women, aged 35.5 ± 14.8 years) underwent perfusion computed tomography (PCT), followed by anodal HD-tDCS and post-stimulation tomography at 21 days after TBI. The Westermark perfusion maps were constructed and quantitative per-fusion parameters calculated. Significance was preset to P < 0.05.
Results.
Qualitative analysis revealed that all patients had areas with reduced cerebral blood flow (CBF) and increased average mean transit time (MTT). HD-tDCS was accompanied by a significant decrease in the number of zones of both hypoperfusion and ischemia (p < 0.05). Quantitative analysis showed that, in all patients, HD-tDCS caused a significant increase in CBF (p < 0.001), cerebral blood volume (CBV) (p< 0.01) and MTT shortening (p < 0.05) in the frontotemporal region on the anode side. In the basal ganglia, a significant increase in CBF was found only in the 5 patients in whom this was initially reduced (p < 0.01) and only with an anode placed on the same side.
Conclusions.
In patients with complications due to PTE TBI, HD-tDCS causes a significant increase in CBV, CBF and a decrease in the average MTT, suggesting better oxygen delivery to tissue.
1. Introduction
Current achievements in neurology and neurosurgery have enabled to significantly reduce mortality in patients with severe traumatic brain injury (TBI). However, this progress is accompanied with a problem of an increasing number of surviving patients with severe posttraumatic neurological consequences, including posttraumatic encephalopathy (PTE). In recent years, hopes for the treatment of TBI have focused on non-pharmacologic electrical brain stimulation, particularly by transcranial application, in order to change neuronal activity. This technique is called transcranial direct current stimulation (tDCS) and is a non-invasive method for stimulation of the brain that modulates the spontaneous firing rate of neurons through direct current applied to the scalp surface [1]. However, studies of perfusion changes after tDCS in general, and in particular the high-definition direct current electrical stimulation (HD-tDCS) in patients with complications after TBI are few and contradictory, stressing the relevance of this investigation.
The aim of this study was to assess cerebral perfusion changes occurring after high-definition transcranial direct current stimulation (HD-tDCS) in patients with PTE after TBI.
2. Materials and Methods
The study was approved by the Ethics Committee of the Nizhny Novgorod State Medical Academy and conformed to the standards of the Declaration of Helsinki. An informed consent was obtained from each patient. Participants in this study were 20 patients (14 men, 6 women) with PTE after TBI: average age was 35.5 ± 14.8 (range 18–63 years), height 168.0 ± 8.8 cm and weight 61.6 ± 5.4 kg. All patients were free of any known cardiovascular or respiratory injury and were not taking any medications. Each patient received verbal and written explanation of the study objectives, measurement techniques, risks, and benefits associated with the investigation. The average Glasgow Coma Score was 12 ± 2. At 21 days after TBI, each patient underwent perfusion computed tomography (PCT), followed by anodal HD-tDCS. After HD-tDCS, the PCT study was repeated using the same parameters.
2.1. Anodal HD-tDCS
At 21 days after TBI, each patient underwent perfusion computed tomography (PCT), followed by anodal HD-tDCS. After HD-tDCS, the PCT study was repeated using the same parameters. The anodal and cathodal electrodes were placed over the left M1 and contralateral supraorbital region, respectively. HD-tDCS was delivered by a direct current stimulator (ActivaDose II, ActivaTek, USA) with a pair of gel-soaked surface sponge electrodes (Ambu, USA). The parameters were: 1 mA, 20 min, with current density ~ 0.15 mA/cm2. No complications caused by HD-tDCS were identified.
2.2. Perfusion computed tomography
Double perfusion computed tomography (PCT) was done using a 64-slice Philips Ingenuity CT® (Philips Medical systems, Cleveland, USA). The perfusion examination report included an initial native CT of the brain followed by 4 extended scanning of the ‘region of interest’, 32 mm in thickness, within 55 seconds, with a contrast agent administered (the Perfusion JOG mode). The scanning parameters were: 120 kVp, 70 mA, 70 mAs and 1000 msec. The contrast agent (Ultravist 370, Shering AG, Germany) was administered with an automatic syringe-injector (Stellant, One Medrad, Indianola, PA, USA) into a peripheral vein through a standard catheter (20 G) at a rate of 4–5 ml/sec in a dose of 30–50 ml per examination.
Acquired data were transferred to a Philips Ingenuity Core workstation (Philips Healthcare Nederland B.V., the Netherlands, 2013, v.3.5.5.25007). Artery and vein marks were automatically recorded followed by manual control of indices in a time-concentration diagram. Color-coded perfusion maps were produced to describe cerebral perfusion: cerebral blood volume (CBV), cerebral blood flow (CBF) and mean transit time (MTT), the ratio of cerebral blood volume to cerebral blood flow (CBV/CBF) which is a valuable indicator of the cerebral circulation. The qualitative analysis covered Westermark perfusion maps and the quantitative research involved the perfusion parameters in frontotemporal cortex regions (directly in areas of electrodes) as well as in the basal nuclei (“at a distance”). Pixels that characterized large vascular structures were excluded from the calculations at a moment when the peak concentration of the contrast in the vessel exceeded the concentration of contrast in the superior sagittal sinus (‘Remote Vessels’ mode), and perfusion maps were refined [2].
2.3. Statistical Analysis
Data are shown as a mean ± standard deviation. Statistical analysis of all results was performed using chi-square criterion and Student’s t-test. The level of significance was preset at p < 0.05.
3. Results
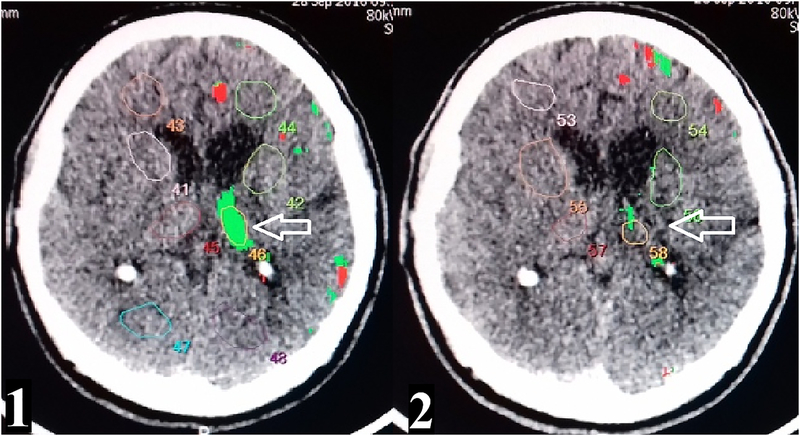
The qualitative analysis revealed that all patients had areas with reduced CBF rate (less than 25 ml/100 g per min) and increased average mean transit time (MTT) (more than 4 s), which were identified as zones of hypoperfusion suggesting reduced oxygen delivery to brain tissue (Fig. 1). In 18 (~90%) of the patients, ischemia-relevant areas with CBV less than 2 ml/100 g were noted, suggesting tissue hypoxia in these areas (Table 1). HD-tDCS was accompanied by a significant decrease in the number of both, hypoperfusion and ischemia zones (Figure 1).
Fig.1.
Comparison of CBF levels before 1) and after HD-tDCS 2). The white arrow shows a decrease of hypoperfusion zone in the left thalamus.
Table 1.
Data on comparison of the analyzed parameters.
| CBV (ml/100 g) |
CBF (ml/100 g × min) |
MTT (sec) | |
|---|---|---|---|
| 1 Frontotemporal region on the anode side before tDCS | 3.01±0.15 | 30.51±7.29 | 5,93±1,24 |
| 2 Frontotemporal region on the anode side after tDCS | 4.12±0.35 | 50.33±18.5 | 4.94±1.16 |
| 3 Basal ganlia region on the anode side before tDCS | 1.71±0.08 | 26.87±8.44 | 3.84±0.61 |
| 4 Basal ganlia region on the anode side after tDCS | 2.33±0.13 | 34.28±9.25 | 4.08±0.86 |
| P (1–2) | <0.01* | <0.001* | 0.01* |
| P (3–4) | <0.001* | 0.06 | 0.315 |
Significant difference (р<0.05)
The quantitative analysis of perfusion parameters showed that, in all patients, HD-tDCS caused a significant increase in CBV (<0.01), CBF (p <0.001) and MTT shortening (p <0.05) in the frontotemporal region on the anode side (Table 1). In the basal ganglia, a significant increase in CBV (p <0.001) was identified. However, a significant increase in CBF rate in the basal ganglia was only identified in those 5 patients (41.6%) in whom it was initially reduced (p <0.05) and only with an anode placed on the same side (Table 1).
4. Discussion
Several explanations of the observed phenomena may be proposed. It has been recently shown that tDCS causes changes not only in the transmembrane potential of neurons but also in astrocytes [3]. Considering the fact that the number of glial cells exceeds the number of neurons in human brain [4] and that astrocytes are directly involved in the maintenance of vascular tone [5], the CBF increase is probably not only the consequence of activation of neurons but also glia. It was also shown that tDCS stimulates secretion of nitic oxide in a culture of vascular endothelial cells [6]. From that, we can assume that tDCS may directly cause vasodilation of resistive vessels leading to increased CBF which suggested to be effective at better oxygen delivery to tissue [7].
One of the possible concerns for tDCS-induced CFB increase is a potential dangerous consequences of hyperoxia that have long been raised related to oxygen toxicity from free radical reactions in conditions such as TBI or stroke sequelae described, for example, for hyperbaric oxygen exposure [8]. However, others have reported that normobaric hyperoxia has neuroprotective effects in TBI [9]. Nevertheless, since HD-tDCS treatment in this study was done at a late post-traumatic period (21 days after TBI), when acute pathophysiological cascades already terminated, oxygen toxicity from free radical reactions should already not be the case.
5. Conclusion
In patients with TBI complicated by posttraumatic encephalopathy, HD-tDCS causes a significant increase in the volumetric cerebral blood flow, cerebral blood volume and decrease in the average transit time, suggesting better oxygen delivery to tissue which could contribute to a better recovery.
Acknowledgments
DB was supported by NIH P20GM109089 and DOD DM160142 and RSF № 17-15-01263.
References
- 1.Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527(3):633–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miles KA, Eastwood JD, Konig M, eds. (2007) Multidetector computed tomography in cerebrovascular disease CT perfusion imaging. Oxon, United Kingdom: Informa Healthcare Ltd [Google Scholar]
- 3.Monai H, Ohkura M, Tanaka M et al. (2016) Calcium imaging reveals glial involvement in transcranial direct current stimulation-induced plasticity in mouse brain. Nat Commun 22;7:11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26:523–530 [DOI] [PubMed] [Google Scholar]
- 5.Filosa JA, Iddings J (2013) Astrocyte regulation of cerebral vascular tone. Am J Physiol Heart Circ Physiol 305(5):H609–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Long H, Yang G, Wang Z (2011). Galvanotactic migration of EA.Hy926 endothelial cells in a novel designed electric field bioreactor. Cell Biochem Biophys 61:481–491 [DOI] [PubMed] [Google Scholar]
- 7.Hlatky R, Valadka AB, Gopinath SP et al. (2008). Brain tissue oxygen tension response to induced hyperoxia reduced in hypoperfused brain. J Neurosurg 108:53–58 [DOI] [PubMed] [Google Scholar]
- 8.Kochanek PM, Bayır H (2017) Titrating the Dose of Oxygen after Severe Traumatic Brain Injury in the Era of Precision Medicine. J Neurotrauma 34(22):3067–3069 [DOI] [PubMed] [Google Scholar]
- 9.Palzur E, Vlodavsky E, Mulla H et al. (2004) Hyperbaric oxygen therapy for reduction of secondary brain damage in head injury: an animal model of brain contusion. J Neurotrauma 21:41–48 [DOI] [PubMed] [Google Scholar]