Abstract
Objectives:
To describe the relationship between metabolic factors and lower urinary tract symptoms (LUTS), overactive bladder syndrome (OAB) and urinary incontinence (UI).
Methods:
Adult male and female patients who presented to a clinician from the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN) were recruited. Urinary symptoms (presence of OAB, any UI, stress UI (SUI), urgency UI (UUI), urgency, frequency, and nocturia) were assessed with the LUTS Tool. Metabolic factors assessed included central obesity (waist circumference, using the Adult Treatment Panel III, the International Diabetes Federation thresholds, and waist circumference as a continuous variable), general obesity (body mass index [BMI] as dichotomous or continuous variables), diabetes mellitus, hypertension, and dyslipidemia. Multivariable logistic regression was used to test for associations.
Results:
920 participants were studied. In multivariable analyses, central obesity (per 10cm larger waist) was associated with higher odds of UI in both sexes (OR=1.16, p=0.008), SUI in females (OR=1.27, p=0.008), UUI in both sexes (OR=1.24, p=0.001), OAB in females (OR=1.248, p=0.003), as well as frequency and nocturia. General obesity (5-unit increase in BMI) was associated with UI, UUI, urgency and frequency in both sexes, and with SUI and OAB in females. We did not find associations between central or general obesity and OAB in males. Dyslipidemia was associated with nocturia ≥2.
Conclusions:
In patients, central and general obesity were key metabolic factors associated with UI in both males and females, and with OAB in females but not in males. The association between dyslipidemia and nocturia ≥2 needs further research.
Keywords: body mass index, central obesity, lower urinary tract symptoms, overactive bladder, urinary incontinence
Introduction:
The prevalence of metabolic factors such as obesity and diabetes in the United States has been increasing. The morbidity and mortality associated with metabolic factors are costly and pose a serious burden to society.1 Recent studies suggest that metabolic factors may play a role in the development of overactive bladder syndrome (OAB), urinary incontinence (UI) and other lower urinary tract symptoms (LUTS). However, the literature has major knowledge gaps. For females, studies have shown high body mass index (BMI) to be a risk factor for UI.2,3 However, the relationships between obesity and OAB are less clear.4 The results are conflicting; some studies5–7 showed that high BMI was associated with OAB while other studies8,9 showed no association. For males, studies have reported associations between metabolic factors and higher American Urological Association Symptom Index (AUA-SI) scores.10–13 However, the relationships between metabolic factors and UI and OAB in males have not been well studied. In addition, most studies were community-based; thus it is unclear whether the findings would apply to patients seeking care for their LUTS.2,3,5–8,10–12,14–19 Among studies that have examined obesity, most have only reported on BMI.5,7,8,15–18 Few studies have specifically evaluated central obesity for its association with urinary symptoms.6,9
The Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN)20,21 sought to address these knowledge gaps by recruiting a large cohort of male and female patients who were seeking care for their LUTS. We assessed multiple metabolic factors (including three measures of central obesity, three measures of BMI, diabetes, hypertension, and dyslipidemia), and their relationships to OAB, UI, and other LUTS. We expect to gain insights into the relevant and modifiable metabolic factor(s) associated with these LUTS that may inform future primary and secondary management and prevention strategies.
Materials and Methods:
Study Design and Population
The LURN Observational Cohort Study20,21 recruited adult male and female patients over the age of 18 who presented to a urologist or urogynecologist for treatment of their LUTS between June 2015 and January 2017 at one of six LURN clinical centers in the United States. Participants were followed prospectively for one year, although data reported herein only include baseline data. The inclusion and exclusion criteria and details of the study design have been previously reported.21 Participants with neurogenic conditions or neurogenic bladder were excluded. The following data were collected at the baseline visit: urinary symptoms, medical history, medication use, systolic and diastolic blood pressure, BMI, and waist circumference measurements. All participants provided informed consent. The protocol was approved by the institutional review boards of all participating research sites.
Measures
Central obesity was assessed based on waist circumference measurements. Three measures of central obesity were used: 1) the Adult Treatment Panel III (ATP III) Guidelines of ≥102 cm for males, ≥88 cm for females,1 2) the 2004 Consensus Statement from the International Diabetes Federation (IDF) of ≥94 cm for white males, ≥80 cm for white females.22 and 3) waist circumference as a continuous variable. Body weight and height were also measured, and BMI was calculated as the weight in kilograms divided by the square of the height in meters. General obesity was defined as BMI ≥30; overweight was defined as BMI ≥25; and BMI was also used as a continuous variable.
The majority of studies in the literature have only assessed BMI. Since central obesity is a key component of metabolic syndrome, we have also included measures of central obesity in addition to BMI. We have included the two most commonly used definitions of central obesity (ATP III and IDF) to be comprehensive. The ATP III definition used the same waist circumference cutoffs regardless of race, while the newer IDF definition used race-specific waist circumference cutoffs. Also, the IDF used lower cutoffs to include subjects who are overweight but not yet considered obese.
Hypertension was defined by a measured systolic pressure ≥130 mmHg, diastolic pressure ≥85 mmHg, or self-reported medication(s) to treat hypertension. Dyslipidemia was defined by self-reported medication(s) taken to treat elevated serum cholesterol or triglycerides. Diabetes was defined by a self-reported medical history of diabetes mellitus.
Urinary symptoms were assessed using the LUTS Tool23 with a one-week recall period. Seven LUTS Tool questions addressed urinary incontinence (UI); participants with responses of “rarely” or “never” to all seven UI questions were classified as “without UI” and responses of “sometimes” or greater to at least one symptom of UI were classified as “with UI”. Two LUTS Tool questions addressed stress urinary incontinence (SUI), leakage while exercising or during a laugh, cough, or sneeze. Participants were classified as having SUI if they had responses of “sometimes” or more to at least one of two leakage questions. Participants who answered “sometimes” or more to a sudden need to rush to urinate were classified as having urgency. A follow-up question asked about urinary leakage due to a sudden feeling of needing to rush to urinate; those who responded “sometimes” or more were classified as having urgency urinary incontinence (UUI).
Participants who reported that they “sometimes”, “often” or “almost always” urinate too frequently were classified as having frequency. Those who reported that they wake up because of the need to urinate one or two times a night or more were classified as having nocturia ≥1 or nocturia ≥2 respectively. Participants who answered affirmatively to the urgency question, and who also reported frequency and/or nocturia were classified as having overactive bladder syndrome (OAB), in accordance with the definition of the International Continence Society (ICS).24 Among those with OAB, they were further classified into two groups: with UUI (also referred as “OAB-wet”) versus without UUI (“OAB-dry”).
Statistics
Demographics and other participant characteristics are shown as means and standard deviations, or medians and interquartile ranges, as appropriate, for continuous variables and as frequencies and percentages for categorical variables.
Multivariable logistic regression models were fitted to test associations between metabolic factors and the LUTS groups described above. For each outcome (e.g. UI, OAB), regression models were developed using the following metabolic predictors: central obesity cutoffs defined by ATP III or by IDF, waist circumference as a continuous variable, general obesity (BMI ≥30), overweight status (BMI ≥25), BMI as a continuous variable, hypertension, diabetes, and dyslipidemia. Additional candidate covariates included sex, age, race, ethnicity, and smoking status. Menopausal status, hormonal use, parity, and pelvic organ prolapse quantification classification were also included for females using interactions with sex. Model selection was guided by the method of best subsets. Interactions between sex and waist circumference/BMI (both continuous and dichotomous versions) were tested in all models and are presented when statistically significant (p<0.05). All p-values were adjusted for multiple comparisons using the false discovery rate (FDR) correction. Models are shown with the adjusted odds ratios and 95% confidence levels. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results:
Among the 1064 participants recruited into the LURN Observational Cohort Study, 920 had complete data on metabolic factors and had complete responses to at least 15 out of 22 questions on the LUTS Tool.23 Demographics and characteristics of these 920 participants (456 males and 464 females) are included in the Supplemental Table. The mean age was 59.1±13.9 years; 81.5% were white, most had central obesity per ATP III definition (60.4%) or IDF definition (70.2%), 43.4% had general obesity (BMI ≥30), 76.5% were over-weight (BMI ≥25), 65.2% had hypertension, 31.5% had dyslipidemia, and 17.1% had diabetes mellitus. The lower percentage with central obesity using the ATP III versus the IDF definition was due to higher cutoff values with ATP III. Regarding incontinence, 33.1% had no UI, 7.4% had SUI, 20.4% had UUI, 26.1% had mixed UI, and 13.0% had other UI; 63.4% of participants had OAB (urgency plus either frequency or nocturia). Among those with OAB, 66.4% had OAB with UUI (“OAB-wet”), and 33.6% had OAB without UUI (“OAB-dry”).
Table 1 shows the relationships between central obesity and LUTS (multivariable logistic regression). We include the results from all three obesity measures in the table (central obesity cutoffs defined by ATP III, defined by IDF, and waist circumference as a continuous variable), but will describe the results of the continuous predictors in more detail below, keeping in mind that the dichotomous predictors also identify several significant predictors.
Table 1:
Relationships between central obesity (waist circumference) and LUTS (multivariable logistic regression)
| Models using Central Obesity ATP III definition | Models using Central Obesity IDF definition | Models using Waist Circumference | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Yes vs. No) | (Yes vs. No) | (continuous measures) | |||||||||||||
| Dependent Variable |
Variable | Odds Ratio (OR) |
Lower 95% CI |
Upper 95% CI |
FDR adjusted p-value |
Variable | Odds Ratio (OR) |
Lower 95% CI |
Upper 95% CI |
FDR adjusted p-value |
Variable | Odds Ratio (OR) |
Lower 95% CI |
Upper 95% CI |
FDR adjusted p-value |
|
UI (odds of having any UI) |
Central Obesity |
1.411 | 1.009 | 1.973 | 0.058 | Central Obesity |
0.872 | 0.585 | 1.300 | 0.637 | Waist Circumference (per 10 cm) |
1.155 | 1.048 | 1.273 | 0.008 |
| Female vs. Male |
4.572 | 3.223 | 6.487 | <0.001 | Female vs. Male |
0.207 | 0.146 | 0.293 | <0.001 | Female vs. Male |
5.262 | 3.733 | 7.418 | <0.001 | |
|
SUI (in Females only) # |
Central Obesity |
2.026 | 1.102 | 3.725 | 0.036 | Central Obesity |
1.340 | 0.541 | 3.318 | 0.642 | Waist Circumference (per 10 cm) |
1.271 | 1.08 | 1.495 | 0.008 |
| UUI | Central Obesity |
1.716 | 1.175 | 2.507 | 0.011 | Central Obesity |
1.087 | 0.689 | 1.713 | 0.807 | Waist Circumference (per 10 cm) |
1.236 | 1.105 | 1.382 | 0.001 |
| Female vs. Male |
6.207 | 4.247 | 9.073 | <0.001 | Female vs. Male |
7.174 | 4.868 | 10.572 | <0.001 | Female vs. Male |
7.761 | 5.302 | 11.359 | <0.001 | |
| Black vs. Non-Black |
2.157 | 1.174 | 3.963 | 0.026 | Black vs. non- Black |
2.144 | 1.166 | 3.94 | 0.022 | ||||||
| Hypertension (Yes vs. No) |
1.548 | 1.039 | 2.305 | 0.059 | |||||||||||
| OAB | Central Obesity – Males |
1.023 | 0.684 | 1.530 | 0.911 | Central Obesity |
1.232 | 0.837 | 1.814 | 0.407 | Waist Circumference (per 10 cm) – Males |
1.022 | 0.902 | 1.159 | 0.731 |
| Central Obesity – Females |
2.493 | 1.530 | 4.063 | 0.001 | Waist Circumference (per 10 cm)– Females |
1.248 | 1.093 | 1.426 | 0.003 | ||||||
| Female vs. Male |
2.123 | 1.546 | 2.914 | <0.001 | |||||||||||
| Age (per 10 years) |
1.174 | 1.050 | 1.312 | 0.011 | Age (per 10 years) |
1.182 | 1.057 | 1.321 | 0.011 | Age (per 10 years) |
1.162 | 1.042 | 1.295 | 0.013 | |
| Black vs. Non-Black |
1.919 | 1.117 | 3.298 | 0.031 | Black vs. Non-Black |
1.947 | 1.137 | 3.333 | 0.033 | Black vs. non Black |
1.881 | 1.098 | 3.221 | 0.031 | |
|
OAB with UUI vs. without UUI (among those with OAB only) ^ |
Central Obesity |
1.422 | 0.931 | 2.172 | 0.123 | Central Obesity |
1.159 | 0.685 | 1.96 | 0.681 | Waist Circumference (per 10 cm) |
1.225 | 1.076 | 1.395 | 0.006 |
| Female vs. Male |
4.063 | 2.681 | 6.159 | <0.001 | Female vs. Male |
4.378 | 2.908 | 6.591 | <0.001 | Female vs. Male |
5.016 | 3.345 | 7.521 | <0.001 | |
|
Urgency Symptoms |
Central Obesity |
1.327 | 0.96 | 1.834 | 0.108 | Central Obesity |
1.036 | 0.698 | 1.536 | 0.862 | Waist Circumference (per 10 cm) |
1.086 | 0.986 | 1.195 | 0.096 |
| Female vs. Male |
2.791 | 2.008 | 3.88 | <0.001 | Female vs. Male |
3.122 | 2.237 | 4.356 | <0.001 | Female vs. Male |
3.144 | 2.273 | 4.347 | <0.001 | |
| Black vs. Non-Black |
1.784 | 1.018 | 3.126 | 0.058 | Black vs. Non-Black |
1.714 | 0.978 | 3.003 | 0.098 | Black vs. non- Black |
1.695 | 0.968 | 2.968 | 0.073 | |
| Hypertension (Yes vs. No) |
1.464 | 1.049 | 2.043 | 0.050 | Hypertension (Yes vs. No) |
1.417 | 1.01 | 1.986 | 0.051 | ||||||
|
Frequency Symptoms |
Central Obesity |
1.223 | 0.862 | 1.736 | 0.282 | Central Obesity |
1.042 | 0.678 | 1.602 | 0.862 | Waist Circumference (per 10 cm) |
1.157 | 1.046 | 1.28 | 0.009 |
| Black vs. Non-Black |
2.111 | 1.063 | 4.191 | 0.048 | Black vs. non- Black |
2.103 | 1.059 | 4.177 | 0.059 | Black vs. non- Black |
2.142 | 1.08 | 4.247 | 0.036 | |
| Hypertension (Yes vs. No) |
1.548 | 1.089 | 2.201 | 0.027 | Hypertension (Yes vs. No) |
1.593 | 1.122 | 2.261 | 0.026 | ||||||
| Nocturia ≥1 | Central Obesity – Males |
1.138 | 0.533 | 2.430 | 0.769 | Central Obesity |
1.094 | 0.607 | 1.970 | 0.825 | Waist Circumference (per 10 cm) |
1.161 | 1.018 | 1.322 | 0.035 |
| Central Obesity – Females |
2.787 | 1.563 | 4.969 | 0.001 | |||||||||||
| Female vs. Male |
0.528 | 0.322 | 0.865 | 0.026 | Female vs. Male |
0.558 | 0.351 | 0.889 | 0.022 | ||||||
| Age (per 10 years) |
1.367 | 1.171 | 1.597 | <0.001 | Age (per 10 years) |
1.334 | 1.136 | 1.567 | 0.002 | Age (per 10 years) |
1.377 | 1.184 | 1.601 | <0.001 | |
| Dyslipidemia (Yes vs. No) |
1.714 | 0.927 | 3.17 | 0.127 | |||||||||||
| Nocturia ≥2 | Central Obesity |
1.201 | 0.890 | 1.620 | 0.263 | Central Obesity |
1.203 | 0.813 | 1.781 | 0.473 | Waist Circumference (per 10 cm) |
1.122 | 1.029 | 1.225 | 0.016 |
| Female vs. Male |
0.762 | 0.560 | 1.038 | 0.127 | Female vs. Male |
0.775 | 0.578 | 1.04 | 0.096 | ||||||
| Age (per 10 years) |
1.374 | 1.226 | 1.54 | <0.001 | Age (per 10 years) |
1.352 | 1.204 | 1.517 | <0.001 | Age (per 10 years) |
1.336 | 1.194 | 1.495 | <0.001 | |
| Black vs. Non-Black |
2.883 | 1.703 | 4.882 | <0.001 | Black vs. non- Black |
2.873 | 1.695 | 4.87 | <0.001 | Black vs. non- Black |
2.739 | 1.616 | 4.643 | 0.001 | |
| Dyslipidemia (Yes vs. No) |
1.605 | 1.153 | 2.232 | 0.011 | Dyslipidemia (Yes vs. No) |
1.545 | 1.105 | 2.159 | 0.026 | Dyslipidemia (Yes vs. No) |
1.455 | 1.044 | 2.026 | 0.035 | |
Males with a history of prostate cancer were excluded from the study. Since male SUI was rare, only female SUI was analyzed.
Comparing OAB with UUI versus OAB without UUI (“OAB-wet” versus “OAB-dry”).
Abbreviations: CI = confidence limit; FDR = false discovery rate; OAB = overactive bladder syndrome; SUI = stress urinary incontinence; UI = urinary incontinence; UUI = urgency urinary incontinence
Higher waist circumference was associated with higher odds of having any UI (OR=1.16 per 10 cm increase, p=0.008) and UUI (OR=1.24 per 10 cm increase, p=0.001) in both sexes, and SUI in females (OR=1.27 per 10 cm increase, p=0.008). In females, there was a relationship between waist circumference and OAB (OR=1.25/10 cm increase, p=0.003), however the relationship was not detected in males (OR=1.02/10 cm increase, p=0.73). Among participants with OAB, higher waist circumference was associated with UUI versus without UUI (“OAB-wet” vs. “OAB-dry”, OR=1.23/10 cm increase, p=0.006). Higher waist circumference was additionally associated with higher odds of frequency and nocturia ≥1 and ≥2 (OR=1.16/10cm, p=0.009; OR=1.16, p=0.035; OR=1.12, p=0.016, respectively). Dyslipidemia was also associated with nocturia ≥2 in this model (OR=1.46, p=0.035)
Table 2 shows the relationships between general obesity, overweight status, continuous BMI, and LUTS (multivariable logistic regression). As above with waist circumference, we include the results from all three obesity measures in the table (BMI ≥30, BMI ≥25, BMI as a continuous variable), but will describe the results of the continuous predictors in more detail below, keeping in mind that the dichotomous predictors also identify several significant predictors.
Table 2:
Relationships between general obesity (BMI), overweight status and LUTS (multivariable logistic regression)
| Models using General Obesity | Models using Overweight Status | Models using BMI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (BMI ≥30 vs. <30) | (BMI ≥25 vs. <25) | (continuous measures) | |||||||||||||
| Dependent Variable |
Variable | Odds Ratio (OR) |
Lower 95% CI |
Upper 95% CI |
FDR adjusted p-value |
Variable | Odds Ratio (OR) |
Lower 95% CI |
Upper 95% CI |
FDR adjusted p-value |
Variable | Odds Ratio (OR) |
Lower 95% CI |
Upper 95% CI |
FDR adjusted p-value |
|
UI (odds of having any UI) |
General Obesity |
1.443 | 1.058 | 1.968 | 0.028 | Overweight | 1.323 | 0.922 | 1.898 | 0.160 | BMI (per 5 units) |
1.202 | 1.063 | 1.359 | 0.006 |
| Female vs. Male |
4.644 | 3.386 | 6.369 | <0.001 | Female vs. Male |
4.832 | 3.518 | 6.636 | <0.001 | Female vs. Male |
4.641 | 3.382 | 6.369 | <0.001 | |
|
SUI (in Females only) # |
General Obesity |
1.931 | 1.132 | 3.296 | 0.023 | Overweight | 1.395 | 0.792 | 2.457 | 0.283 | BMI (per 5 units) |
1.318 | 1.087 | 1.599 | 0.009 |
| UUI | General Obesity | 1.857 | 1.316 | 2.619 | 0.001 | Overweight | 1.280 | 0.840 | 1.949 | 0.283 | BMI (per 5 units) |
1.305 | 1.142 | 1.492 | <0.001 |
| Female vs. Male |
6.387 | 4.526 | 9.012 | <0.001 | Female vs. Male |
7.081 | 4.973 | 10.084 | <0.001 | Female vs. Male |
6.377 | 4.514 | 9.009 | <0.001 | |
| Hypertension (Yes vs. No) |
1.487 | 1.015 | 2.178 | 0.059 | |||||||||||
| OAB | General Obesity – Males |
1.157 | 0.787 | 1.699 | 0.502 | Overweight | 1.778 | 1.285 | 2.461 | 0.001 | BMI (per 5 units) Male |
1.047 | 0.886 | 1.238 | 0.588 |
| General Obesity – Females |
2.432 | 1.576 | 3.753 | <0.001 | BMI (per 5 units) Female |
1.385 | 1.182 | 1.623 | <0.001 | ||||||
| Female vs. Male |
2.220 | 1.665 | 2.960 | <0.001 | |||||||||||
| Age (per 10 years) |
1.141 | 1.030 | 1.263 | 0.019 | Age (per 10 years) |
1.129 | 1.019 | 1.251 | 0.037 | Age (per 10 years) |
1.135 | 1.025 | 1.257 | 0.021 | |
|
OAB with UUI vs. without UUI (among those with OAB only) ^ |
General Obesity |
1.789 | 1.22 | 2.624 | 0.007 | Overweight | 1.132 | 0.708 | 1.811 | 0.628 | BMI (per 5 units) |
1.26 | 1.079 | 1.472 | 0.006 |
| Female vs. Male |
4.498 | 3.073 | 6.585 | <0.001 | Female vs. Male |
4.716 | 3.230 | 6.885 | <0.001 | Female vs. Male |
4.449 | 3.039 | 6.512 | <0.001 | |
|
Urgency Symptoms |
General Obesity – Males |
1.217 | 0.828 | 1.789 | 0.366 | Overweight | 1.671 | 1.190 | 2.346 | 0.008 | BMI (per 5 units) |
1.22 | 1.083 | 1.375 | 0.003 |
| General Obesity – Females |
2.573 | 1.566 | 4.228 | 0.001 | |||||||||||
| Female vs. Male |
3.009 | 2.226 | 4.066 | <0.001 | Female vs. Male |
2.812 | 2.086 | 3.792 | <0.001 | ||||||
| Black vs. Non-Black |
1.742 | 1.022 | 2.969 | 0.050 | Black vs. Non-Black |
1.790 | 1.052 | 3.046 | 0.052 | ||||||
|
Frequency Symptoms |
General Obesity |
1.597 | 1.149 | 2.22 | 0.010 | Overweight | 1.229 | 0.848 | 1.782 | 0.300 | BMI (per 5 units) |
1.227 | 1.077 | 1.397 | 0.005 |
| Black vs. Non-Black |
2.248 | 1.172 | 4.313 | 0.023 | Black vs. Non-Black |
2.266 | 1.181 | 4.350 | 0.028 | Black vs. non- Black |
2.19 | 1.14 | 4.207 | 0.026 | |
| Hypertension (Yes vs. No) |
1.405 | 1.004 | 1.968 | 0.062 | |||||||||||
| Nocturia ≥1 | General Obesity |
1.132 | 0.737 | 1.74 | 0.598 | Overweight | 1.600 | 1.015 | 2.523 | 0.059 | BMI (per 5 units) Males |
0.792 | 0.598 | 1.049 | 0.116 |
| BMI (per 5 units) Female |
1.185 | 0.986 | 1.425 | 0.082 | |||||||||||
| Female vs. Male |
0.525 | 0.335 | 0.823 | 0.010 | Female vs. Male |
0.544 | 0.347 | 0.853 | 0.019 | ||||||
| Age (per 10 years) |
1.311 | 1.131 | 1.521 | 0.001 | Age (per 10 years) |
1.305 | 1.124 | 1.515 | 0.001 | Age (per 10 years) |
1.316 | 1.132 | 1.529 | 0.001 | |
| Dyslipidemia (Yes vs. No) |
1.894 | 1.057 | 3.394 | 0.041 | Dyslipidemia (Yes vs. No) |
1.832 | 1.020 | 3.289 | 0.059 | Dyslipidemia (Yes vs. No |
1.854 | 1.031 | 3.335 | 0.047 | |
| Nocturia ≥2 | General Obesity |
1.028 | 0.779 | 1.357 | 0.845 | Overweight | 0.993 | 0.716 | 1.377 | 0.966 | BMI (per 5 units) |
1.029 | 0.93 | 1.139 | 0.588 |
| Female vs. Male |
0.728 | 0.551 | 0.961 | 0.044 | Female vs. Male |
0.723 | 0.547 | 0.955 | 0.03 | ||||||
| Age (per 10 years) |
1.374 | 1.236 | 1.528 | <0.001 | Age (per 10 years) |
1.355 | 1.218 | 1.507 | <0.001 | Age (per 10 years) |
1.355 | 1.218 | 1.507 | <0.001 | |
| Black vs. Non-Black |
2.833 | 1.728 | 4.643 | <0.001 | Black vs. Non-Black |
2.862 | 1.741 | 4.703 | <0.001 | Black vs. non- Black |
2.815 | 1.713 | 4.626 | <0.001 | |
| Dyslipidemia (Yes vs. No) |
1.547 | 1.135 | 2.109 | 0.010 | Dyslipidemia (Yes vs. No) |
1.493 | 1.092 | 2.039 | 0.026 | Dyslipidemia (Yes vs. No) |
1.478 | 1.081 | 2.021 | 0.021 | |
Males with a history of prostate cancer were excluded from the study. Since male SUI was rare, only female SUI was analyzed.
Comparing OAB with UUI versus OAB without UUI (“OAB-wet” versus “OAB-dry”).
Abbreviations: CI = confidence limit; FDR = false discovery rate; OAB = overactive bladder syndrome; SUI = stress urinary incontinence; UI = urinary incontinence; UUI = urgency urinary incontinence
Higher BMI was associated with any UI (OR=1.20/5 unit increase, p=0.006) and UUI (OR=1.31/5 unit increase, p<0.001) in both sexes, and SUI in females (OR=1.32/5 unit increase, p=0.009). In females, there was a relationship between BMI and OAB (OR=1.38/5 unit increase, p<0.001), however again no association was detected in males (OR=1.05/5 unit increase, p=0.59). Among participants with OAB, higher BMI was associated with UUI versus without UUI (“OAB-wet” vs. “OAB-dry”, OR=1.26/5 unit increase, p=0.006). Dyslipidemia was also associated with nocturia ≥2 in this model (OR=1.48, p=0.021). Of the other covariates, older age, female sex, and African American race were also associated with multiple urinary symptoms (Tables 1 and 2).
Discussion:
Previous community-based studies have demonstrated relationships between high BMI and UI in females,2,3 and between metabolic factors and higher AUA Symptom Index in males.10–13 However, the relationships between metabolic factors and OAB in females were less clear.4 Also the relationships between metabolic factors and UI and OAB in males have not been well studied. In this study we found associations between both central and general obesity and OAB in females, and UI and its subtypes in males and females. We did not find association between central or general obesity and OAB in males. We also found an association between dyslipidemia and nocturia ≥2 in the clinical population.
Our observations in the clinical cohort were consistent to those reported in the Boston Area Community Health (BACH) population-based cohort.6 In the BACH Study, the odds of having OAB increased with waist circumference and with BMI in females. However, in males, there were negative associations up to 100 cm waist circumference or a BMI of 27.5, and then positive associations beyond those thresholds.6 In longitudinal studies, Dallosso et al also showed that obesity at baseline was associated with new onset of OAB one year later in women;18 however obesity at baseline was not associated with new onset of OAB one year later in men.17 Taken together, the data suggested that there might be important sex difference in the relationships between obesity and OAB.
This sex difference raises some interesting research possibilities. For example, hormonal factors may play a role. Anatomic factors may account for some of the sex differences. Increased abdominal pressure from central (visceral) obesity could increase the pressure on the bladder, stretch the pelvic floor, or trigger urine entry into the proximal urethra, which could in turn cause prolapse, urethral hypermobility, and OAB symptoms in females. Males however are less susceptible to these anatomic forces since males do not develop prolapse, urethral hypermobility, or an open bladder neck. Besides local mechanical forces, systemic factors such as neuroendocrine, vascular or inflammatory processes can provide a link between obesity and OAB. Adipose tissue produces leptin, which can stimulate the sympathetic nervous system activity, and exacerbate urinary frequency.25,26 The generation of inflammatory factors (e.g. cytokines, C-reactive protein) from visceral adipose tissue may also influence bladder sensory function. Obesity is also associated with vascular dysfunction and ischemia. Vascular ischemia in pelvic structures including the bladder has been demonstrated to cause detrusor overactivity and OAB in animal models.27 In summary, the pathophysiology that links obesity and OAB is poorly understood, and the mechanisms behind the sex differences need to be further examined.
Among participants with OAB, increases in continuous BMI and waist circumference were associated with UUI versus without UUI (“OAB-wet” vs. “OAB-dry”). Our results were in contrast to a Korean community study which showed that high BMIs were associated with “OAB-dry” but not “OAB-wet”.16 Our clinical cohort consisted of patients who presented to tertiary urology and urogynecology clinics seeking care for their LUTS; thus, it was possible that the community sample may have less severe UUI, and therefore did not permit the discovery of an association.
Although a number of measures of adiposity are available that account for different aspects of obesity, they have rarely been evaluated together for their associations with urinary symptoms.6, 9 Among studies that have examined obesity, most have only reported on BMI.5,7,8,15–18 Since the distribution of fat in the abdominal viscera – central obesity – is a key component of metabolic syndrome,22 and central obesity is more predictive of cardiovascular risk than high BMI,4 we have specifically examined three measures of central obesity (the ATP III definition, the IDF definition, and waist circumference as a continuous measure) and three measures of BMI (general obesity [BMI≥30], overweight status [BMI≥25], and BMI as a continuous measure), to assess differences among these measures of obesity with respect to their relationships to UI and OAB. Overall the continuous measures of waist circumference or BMI showed the strongest relationships with incontinence measures, followed by central obesity (ATP III) and general obesity (BMI ≥30). As shown in Figure 1, there was high correlation between waist circumference and BMI in our care seeking cohort. No associations were found with central obesity defined by IDF, which has lower cutoffs than ATP III. This is likely due to the distribution of waist circumference in the LURN sample resulting in similar odds of LUTS when participants are grouped using the IDF definition despite a positive association with waist circumference (as a continuous variable). This is illustrated in Figure 2 using UUI in males as an example. It is also possible that the positive association is less strong at lower values of waist circumference, however we did not have adequate sample size to detect this difference. Despite this limitation, the use of continuous predictor variables for waist circumference and BMI revealed the substantial gain in power over use of dichotomized variables, and may explain some of the conflicting results from prior studies that used dichotomous predictors. For similar reasons, overweight status was not associated with many LUTS due to lower BMI cutoffs, even though there were positive associations with higher BMI (as a continuous variable).
Figure 1: Scatterplots of body mass index (BMI) by waist circumference for males and females.
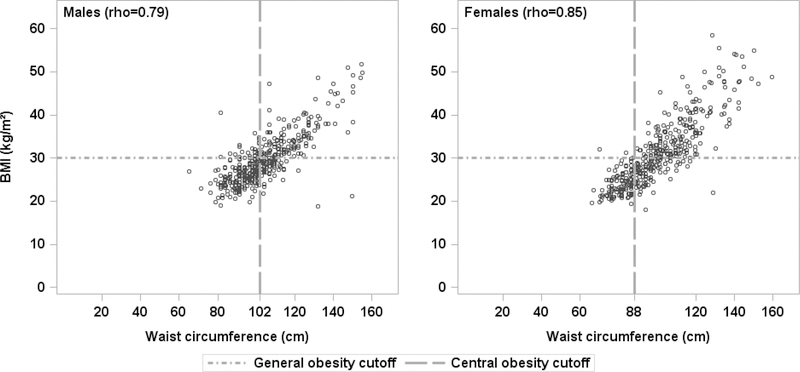
with Pearson correlation coefficients (both p<0.001). Dashed lines show the ATP III criteria for central obesity (waist circumference ≥102 cm for males, ≥88 cm for females) and the general obesity criterion of BMI ≥30.
Figure 2: Odds ratio of UUI in males for various measures of central obesity.
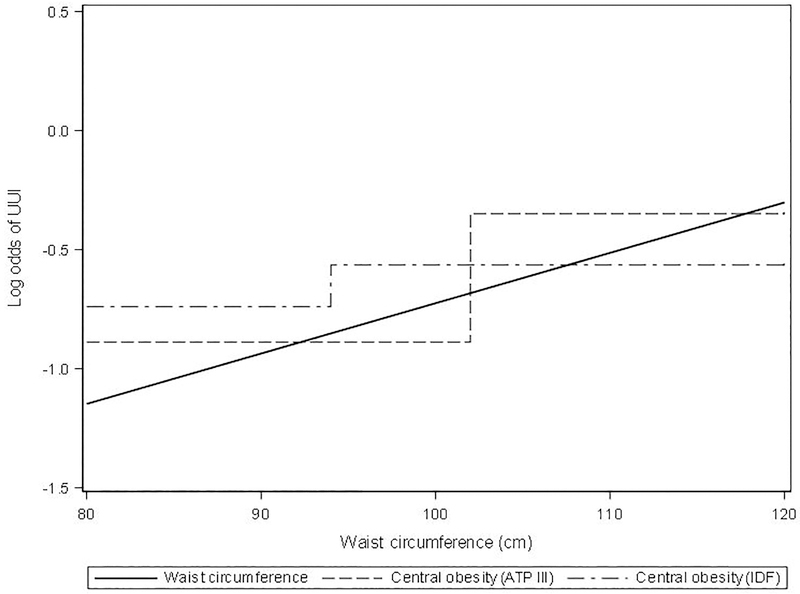
When waist circumference is included as a continuous variable, the log odds of UUI increases as waist circumference increases (solid line). When waist circumference is dichotomized, the averaged log odds is different enough that the difference is significant with the ATP III cutoffs (dashed line), but not with the IDF cutoffs (dashed-dotted line).
In this study we were not able to demonstrate any relationships to other metabolic factors including diabetes and hypertension. A novel association between dyslipidemia and nocturia ≥2 was observed, that to our knowledge has never been reported in the literature. One study from Taiwan (n=1827) found an increased odds of OAB in individuals with dyslipidemia.28 In the BACH Study, the use of statins was associated with lower prevalence of LUTS among older men but not among women or younger men.29 Unfortunately neither of these studies had looked specifically at nocturia. The significance of the potential link between nocturia and dyslipidemia is unclear at this time and should be further investigated.
Our data showed that obesity is a key modifiable metabolic factor associated with UI, SUI, UUI, and OAB. This finding may have important implications for primary and secondary preventive strategies to reduce the prevalence and burden of OAB and UI. Weight loss interventions targeting physical activity and healthy diet might be considered as an adjuvant therapy in OAB and UI patients who are obese. A 2015 Cochrane systematic review concluded that the therapeutic effect of weight loss on UI is building and should be a research priority.30
The strength of the study included: enrollment of a large cohort (>900) of patients who were seeking care for their LUTS at six clinical centers; inclusion of both males and females; and measurement of both waist circumference and BMI so we could examine various measures of obesity along with other metabolic factors. Our use of continuous predictor variables for waist circumference and BMI also revealed the substantial gain in power over use of dichotomized variables. There are several potential weaknesses of the study. We did not collect fasting glucose, hemoglobin A1c, HDL-cholesterol, or triglyceride serum levels to fully assess metabolic syndrome.22 For participants who took medications for hypertension, dyslipidemia or diabetes, we did not further sub-categorize them into those whose conditions were poorly controlled versus well controlled. Also, instead of using multiple instruments to assess different LUTS (e.g. OAB-q for OAB), we chose to use one instrument (LUTS Tool) as a comprehensive tool to assess a variety of LUTS. Only participants from specialty clinics (urology, urogynecology) in tertiary academic medical centers were enrolled, which may limit the generalizability of the findings. Finally our cross-sectional data does not permit one to draw inference on causality or temporal relationships.
Conclusions:
In care-seeking patients with LUTS, central obesity and general obesity were key metabolic factor associated with UI in both males and females, and with OAB in females. We did not demonstrate association between obesity and OAB in males. The association between dyslipidemia and nocturia ≥2 needs further research. Weight loss is a promising therapeutic intervention. Future research could focus on optimizing this intervention.
Supplementary Material
Acknowledgments:
The following individuals were instrumental in the planning and conduct of this study at each of the participating institutions:
Duke University, Durham, North Carolina (DK097780): PI: Cindy Amundsen, MD, Kevin Weinfurt, PhD; Co-Is: Kathryn Flynn, PhD, Matthew O. Fraser, PhD, Todd Harshbarger, PhD, Eric Jelovsek, MD, Aaron Lentz, MD, Drew Peterson, MD, Nazema Siddiqui, MD, Alison Weidner, MD; Study Coordinators: Carrie Dombeck, MA, Robin Gilliam, MSW, Akira Hayes, Shantae McLean, MPH
University of Iowa, Iowa City, IA (DK097772): PI: Karl Kreder, MD, MBA, Catherine S Bradley, MD, MSCE, Co-Is: Bradley A. Erickson, MD, MS, Susan K. Lutgendorf, PhD, Vince Magnotta, PhD, Michael A. O’Donnell, MD, Vivian Sung, MD; Study Coordinator: Ahmad Alzubaidi
Northwestern University, Chicago, IL (DK097779): PIs: David Cella, Brian Helfand, MD, PhD; Co-Is: James W Griffith, PhD, Kimberly Kenton, MD, MS, Christina Lewicky-Gaupp, MD, Todd Parrish, PhD, Jennie Yufen Chen, PhD, Margaret Mueller, MD; Study Coordinators: Sarah Buono, Maria Corona, Beatriz Menendez, Alexis Siurek, Meera Tavathia, Veronica Venezuela, Azra Muftic, Pooja Talaty, Jasmine Nero. Dr. Helfand, Ms. Talaty, and Ms. Nero are at NorthShore University HealthSystem.
University of Michigan Health System, Ann Arbor, MI (DK099932): PI: J Quentin Clemens, MD, FACS, MSCI; Co-Is: Mitch Berger, MD, PhD, John DeLancey, MD, Dee Fenner, MD, Rick Harris, MD, Steve Harte, PhD, Anne P. Cameron, MD, John Wei, MD; Study Coordinators: Morgen Barroso, Linda Drnek, Greg Mowatt, Julie Tumbarello
University of Washington, Seattle Washington (DK100011): PI: Claire Yang, MD; Co-I: John L. Gore, MD, MS; Study Coordinators: Alice Liu, MPH, Brenda Vicars, RN
Washington University in St. Louis, St. Louis Missouri (DK100017): PI: Gerald L. Andriole, MD, H. Henry Lai; Co-I: Joshua Shimony, MD, PhD; Study Coordinators: Susan Mueller, RN, BSN, Heather Wilson, LPN, Deborah Ksiazek, BS, Aleksandra Klim, RN, MHS, CCRC
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Kidney, Urology, and Hematology, Bethesda, MD: Project Scientist: Ziya Kirkali MD; Project Officer: John Kusek, PhD; NIH Personnel: Tamara Bavendam, MD, Robert Star, MD, Jenna Norton
Arbor Research Collaborative for Health, Data Coordinating Center (DK097776 and DK099879): PI: Robert Merion, MD, FACS; Co-Is: Victor Andreev, PhD, DSc, Brenda Gillespie, PhD, Gang Liu, PhD, Abigail Smith, PhD; Project Manager: Melissa Fava, MPA, PMP; Clinical Study Process Manager: Peg Hill-Callahan, BS, LSW; Clinical Monitor: Timothy Buck, BS, CCRP; Research Analysts: Margaret Helmuth, MA, Jon Wiseman, MS; Project Associate: Julieanne Lock, MLitt
Funding/Support:
This is publication number 10 of the Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN).
This study is supported by the National Institute of Diabetes & Digestive & Kidney Diseases through cooperative agreements (grants DK097780, DK097772, DK097779, DK099932, DK100011, DK100017, DK097776, DK099879).
Research reported in this publication was supported at Northwestern University, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences, Grant Number UL1TR001422. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of Interest: None
References:
- 1.Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285: 2486–97, 2001 [DOI] [PubMed] [Google Scholar]
- 2.Brown JS, Seeley DG, Fong J, et al. Urinary incontinence in older women: who is at risk? Study of Osteoporotic Fractures Research Group. Obstet Gynecol 87: 715–21, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Mommsen S, Foldspang A. Body mass index and adult female urinary incontinence. World J Urol 12: 319–22, 1994 [DOI] [PubMed] [Google Scholar]
- 4.Bunn F, Kirby M, Pinkney E, et al. Is there a link between overactive bladder and the metabolic syndrome in women? A systematic review of observational studies. Int J Clin Pract 69: 199–217, 2015 [DOI] [PubMed] [Google Scholar]
- 5.de Boer TA, Slieker-ten Hove MC, Burger CW, et al. The prevalence and risk factors of overactive bladder symptoms and its relation to pelvic organ prolapse symptoms in a general female population. Int Urogynecol J 22: 569–75, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Link CL, Steers WD, Kusek JW, et al. The association of adiposity and overactive bladder appears to differ by gender: results from the Boston Area Community Health survey. J Urol 185: 955–63, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teleman PM, Lidfeldt J, Nerbrand C, et al. Overactive bladder: prevalence, risk factors and relation to stress incontinence in middle-aged women. BJOG 111: 600–4, 2004 [DOI] [PubMed] [Google Scholar]
- 8.McGrother CW, Donaldson MM, Hayward T, et al. Urinary storage symptoms and comorbidities: a prospective population cohort study in middle-aged and older women. Age Ageing 35: 16–24, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Uzun H, Zorba OU. Metabolic syndrome in female patients with overactive bladder. Urology 79: 72–5, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Rohrmann S, Smit E, Giovannucci E, et al. Association between markers of the metabolic syndrome and lower urinary tract symptoms in the Third National Health and Nutrition Examination Survey (NHANES III). Int J Obes (Lond) 29: 310–16, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Kupelian V, McVary KT, Kaplan SA, et al. Association of lower urinary tract symptoms and the metabolic syndrome: results from the Boston Area Community Health Survey. J Urol 182: 616–24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mondul AM, Giovannucci E, Platz EA. A prospective study of obesity, and the incidence and progression of lower urinary tract symptoms. J Urol 191: 715–21, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee RK, Chung D, Chughtai B, et al. Central obesity as measured by waist circumference is predictive of severity of lower urinary tract symptoms. BJU Int 110: 540–5, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Hong GS, Shim BS, Chung WS, et al. Correlation between Metabolic Syndrome and Lower Urinary Tract Symptoms of Males and Females in the Aspect of Gender-Specific Medicine: A Single Institutional Study. Korean J Urol 51: 631–5, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawrence JM, Lukacz ES, Liu IL, et al. Pelvic floor disorders, diabetes, and obesity in women: findings from the Kaiser Permanente Continence Associated Risk Epidemiology Study. Diabetes Care 30: 2536–41, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Choo MS, Ku JH, Lee JB, et al. Cross-cultural differences for adapting overactive bladder symptoms: results of an epidemiologic survey in Korea. World J Urol 25: 505–11, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Dallosso HM, Matthews RJ, McGrother CW, et al. The association of diet and other lifestyle factors with the onset of overactive bladder: a longitudinal study in men. Public Health Nutr 7: 885–91, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Dallosso HM, McGrother CW, Matthews RJ, et al. The association of diet and other lifestyle factors with overactive bladder and stress incontinence: a longitudinal study in women. BJU Int 92: 69–77, 2003 [DOI] [PubMed] [Google Scholar]
- 19.Hammarsten J, Hogstedt B, Holthuis N, et al. Components of the metabolic syndrome-risk factors for the development of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis 1: 157–162, 1998 [DOI] [PubMed] [Google Scholar]
- 20.Yang CC, Weinfurt KP, Merion RM, et al. Symptoms of Lower Urinary Tract Dysfunction Research Network. J Urol 196: 146–52, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron AP, Lewicky-Gaupp C, Smith AR, et al. Baseline Lower Urinary Tract Symptoms in Patients Enrolled in the LURN: A Prospective, Observational Cohort Study. J Urol 199: 1023–31, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 23: 469–80, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Coyne KS, Barsdorf AI, Thompson C, et al. Moving towards a comprehensive assessment of lower urinary tract symptoms (LUTS). Neurourol Urodyn 31: 448–54, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Abrams P, Cardozo L, Fall M et al. The standardisation of terminology of lower urinary tract function: report from the Standardisation Sub-committee of the International Continence Society. Neurourol Urodyn 21: 167–78, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Shen J, Tanida M, Niijima A, et al. In vivo effects of leptin on autonomic nerve activity and lipolysis in rats. Neurosci Lett 416: 193–7, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Steers WD, Clemow DB, Persson K, et al. The spontaneously hypertensive rat: insight into the pathogenesis of irritative symptoms in benign prostatic hyperplasia and young anxious males. Exp Physiol 84: 137–47, 1999 [DOI] [PubMed] [Google Scholar]
- 27.Azadzoi KM, Tarcan T, Kozlowski R, et al. Overactivity and structural changes in the chronically ischemic bladder. J Urol 162: 1768–78, 1999 [PubMed] [Google Scholar]
- 28.Yu HJ, Liu CY, Lee KL, et al. Overactive bladder syndrome among community-dwelling adults in Taiwan: prevalence, correlates, perception, and treatment seeking. Urol Int 77: 327–33, 2006 [DOI] [PubMed] [Google Scholar]
- 29.Hall SA, Chiu GR, Link CL, et al. Are statin medications associated with lower urinary tract symptoms in men and women? Results from the Boston Area Community Health (BACH) Survey.” Ann Epidemiol 21: 149–55, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Imamura M, Williams K, Wells M, et al. Lifestyle interventions for the treatment of urinary incontinence in adults. Cochrane Database Syst Rev 12: CD003505, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


