Abstract
Neuropsychiatric disorders have traditionally been difficult to study due to the complexity of the human brain and limited availability of human tissue. Induced pluripotent stem (iPS) cells provide a promising avenue to further our understanding of human disease mechanisms, but traditional 2D cell cultures can only provide a limited view of the neural circuits. To better model complex brain neurocircuitry, compartmentalized culturing systems and 3D organoids have been developed. Early compartmentalized devices demonstrated how neuronal cell bodies can be isolated both physically and chemically from neurites. Soft lithographic approaches have advanced this approach and offer the tools to construct novel model platforms, enabling circuit-level studies of disease, which can accelerate mechanistic studies and drug candidate screening. In this review, we describe some of the common technologies used to develop such systems and discuss how these lithographic techniques have been utilized to advance our understanding of neuropsychiatric disease. Finally, we address other in vitro model platforms such as 3D culture systems and organoids and compare these models with compartmentalized models and ask important questions regarding how we can further harness induced pluripotent stem cells in these engineered culture systems for the development of improved in vitro models.
Keywords: compartmentalization, microfluidics, stem cells, induced neurons, organoids, 3D culture, axonal transport, Huntington’s Disease, neuropsychiatric disorders, neurocircuitry
Introduction
Developmental and neuropsychiatric disorders are highly prevalent and generate a great socioeconomic burden for patients, families, and society. Data obtained through 2010 revealed that neuropsychiatric and mental health disorders are the third leading rank of disability-adjusted life years (DALYs) and the first of years lived with disability (YLDs) in Europe (World Health Organization, 2018). Despite an increase in disease incidence, efforts to study and treat such disorders have led to limited progress and many of the therapies available are often only partially effective or temporarily effective (Pankevich et al., 2014). Furthermore, many phase III clinical failures have caused many pharmaceutical companies to terminate trials or cut back on programs for the treatment of mental health disorders (Pankevich et al., 2014; Kesselheim et al., 2015; Hyman, 2016). Much of this lack of progress is due to a limited understanding of the complex function and circuitry of the human brain and, often, symptoms are only evident at later stages of disease. Animal models, which have offered much insight into connections between disease and behavior (Crawley, 2012; Shinoda et al., 2013), have practical advantages as model systems for neuropsychiatric disorder research. Results achieved from animal studies can be limited however, as evidenced by the lack of efficacy of many pharmaceutical candidates (Hyman, 2014; Pankevich et al., 2014). How can we generate improved models of neuropsychiatric disorders that generate greater predictive power? The answer may be found by utilizing other model systems which can, alongside animal models, shed additional light on the mechanism and onset of neuropsychiatric disorders.
Recent developments in stem cell biology, especially the invention of techniques to derive induced pluripotent stem cells (iPS) cells (Takahashi and Yamanaka, 2006; Takahashi et al., 2007) from patient subjects and subsequently differentiate them into functional human neurons, provide a model system highly complementary to animal studies, while faithfully maintaining the complex human neuronal context (Pang et al., 2011; Yang et al., 2017). One major advantage of human iPS cell-derived neurons is that they are human cells, expressing human proteins naturally, without ectopic expression. They also can be linked to a behavioral or disease phenotype in the donor. Furthermore, genome wide association studies have linked certain genetic loci with disease, suggesting a connection between the genome and disease onset and providing a starting point for mechanistic studies (Gratten et al., 2014; The Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium., 2015). While techniques for employing stem cells for such studies are still being developed, progress has been made to identify differences between diseased and normal cell populations (Farra et al., 2012; Oni et al., 2016; Yi et al., 2016). With the formation of stem cell repositories, larger numbers of patient-derived cell lines can be accessed and harnessed for such research, often with known genetic variations and without requiring review by an Institutional Review Board.
Many of the experiments using iPS cells have been limited to single or mixed neuronal population studies, e.g., evaluating the role of dopaminergic neurons in mediating Parkinson’s disease (Singh Dolt et al., 2017). The brain, however, is much more complex than 2D culture, with these populations of neurons forming a very specific neural network, wherein individual populations of neurons receive inputs from distinct brain nuclei. Recapitulation of defined neural circuits should provide greater insight into circuit dynamics and how the circuits are impacted under disordered conditions. This is important to consider, as many of these disorders target defined regions of the circuit, which naturally impacts the circuit as a whole. Understanding the dynamics of the neurocircuit and seeking treatments which can rescue circuit deficits will be of value in trying to aid therapeutic development directed toward neuropsychiatric disorders.
To circumvent the difficulty of modeling neuronal circuitry in culture, compartmentalized culturing systems have been developed and recent advances in soft lithographic techniques have made these model systems more appealing. These models take advantage of the unique morphology of the neuron: somata are separated from projecting axons and dendrites in different regions of a device, by defined microchannels of a limited height. The channels prevent cell bodies from freely moving between chambers but allow the outgrowth of projecting axons to extend between chambers and communicate with neurons on the opposite side. Such platforms can create mini neurocircuits analogous to the networks found between brain regions. Combining the research benefits of iPS cells and compartmentalized devices provides avenues for the study of “human” neurocircuit models in vitro. In the review, we begin by discussing the development of compartmentalized devices. We then discuss studies that have been performed using primary cultures to demonstrate proof-of-concept for compartmentalized devices. We then discuss the role of incorporation of iPS cell-derived neurons in these devices as well as 3D culture systems and organoids. Finally, we ask how these systems can best be used and further developed for the study of neuropsychiatric and developmental disorders.
Soft lithography as a tool for compartmentalization
Early versions of compartmentalization used Teflon dividers instead of the now-common polydimethylsiloxane (PDMS) polymer. The Campenot chamber, developed by Robert Campenot in 1977, was the first demonstration of separating different parts of the neurons into different device regions (Figure 1A) (Campenot, 1977; Campenot and Martin, 2001; Vikman et al., 2001; Campenot and MacInnis, 2004; Millet and Gillette, 2012). The devices are produced by scratching a series of channels into a collagen surface treatment and then affixing a Teflon divider with a silicon grease layer on top of the scratches. Axonal projections traversed the areas between the scratches while cell bodies were retained on their own side of the divider. Importantly, the chambers remained fluidically isolated, and could therefore be treated separately with reagents/media. This device was originally used to demonstrate the role of nerve growth factor (NGF) on neurite outgrowth (Campenot, 1977). Robert Campenot demonstrated compartmentalized treatment of soma and distal neurites within the device. Neurites entered chambers containing NGF, while no neurites entered chambers without NGF. Interestingly, when NGF was removed from the soma compartment, neurons whose neurites originally projected into NGF-containing compartment survived. This shows the utility for the study of local environmental effects. Since their inception, Campenot chambers have been used for a variety of studies, using the original setup (Manning et al., 1987; Atwal et al., 2000) or modified chambers, e.g., examining herpes simplex virus axonal transport in a two-chamber system (Penfold et al., 1994). While Campenot chambers have demonstrated proof-of-principle for compartmentalizing neuronal cultures, they require skill to produce and at times have had issues with leakage between compartments if the grease is not correctly applied (Taylor et al., 2005; Yang et al., 2009).
Figure 1.
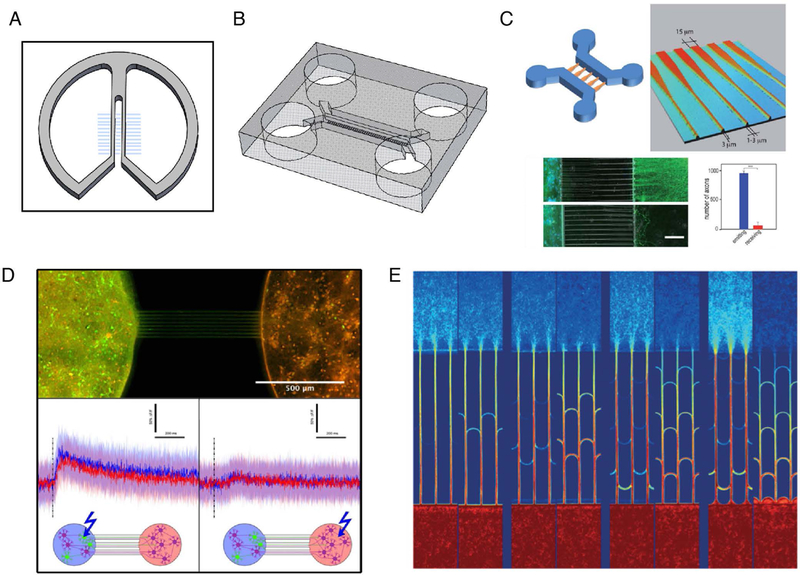
Tools for compartmentalized systems. A) Campenot chamber (re-drawn from Zweifel et al. (Zweifel et al., 2005) and Millet and Gillette (Millet and Gillette, 2012)). Scratches in collagen surface produce channels which direct axonal outgrowth. B) Compartmentalized device made from PDMS (re-drawn from Taylor et al. (Taylor et al., 2005)). Microchannels (3 μm tall, 10 μm wide) connect two opposing chambers. C) Axon diodes drive axonal outgrowth primarily in one direction. Wider channels (15 μm) provide greater opportunity for neurites to enter whereas thinner channels (3 μm) limit neurite entry. (Reproduced from Peyrin et al. (Peyrin et al., 2011) with slight modifications with permission.) D) Incorporation of optogenetics and calcium imaging in a culture system, coupled with axon diodes. Calcium imaging bursts in response to light seen in both compartments only when Channelrhodopsin-2-transduced neurons were stimulated. (Reproduced with permission under CCBY4.0(https://creativecommons.org/licenses/by/4.0/legalcode) from Renault et al. (Renault et al., 2015)with slight modifications.) E) “Return-to-sender” approach provides directionality between compartments. Axons entering from the “receiver” side encounter bifurcations (arches) which return them back to their original chamber. Axons from the “sender” side do not encounter bifurcations and pass through to the “receiver” side (Reproduced from Renault et al. (Renault et al., 2016) with permission.)
Ever since the early 2000s, microfluidic approaches have been developed to created compartmentalized devices using soft lithography. These devices investigate a wide range of CNS disorders and injuries and use a variety of techniques for study (Table 1).
Table 1.
Comparison of some compartmentalized devices for both injury and circuit-based studies.
| Category | Reference | Publication Date |
Pathology Focus | Directionality | Neuron Cell Source |
Key Features/Assays |
|---|---|---|---|---|---|---|
| Mechanical Injury | Taylor et al. | 2005 | Axonal injury | None | Rat, Mouse | Axotomy through aspiration |
| Hosmane et al. | 2011 | Graded axonal injury |
None | Rat | Microvalves to actuate pressure injury |
|
| Dolle et al. | 2013 | Graded axonal injury |
None | Rat | Axon injury over different strain rates |
|
| Circuit studies |
Coquinco et al. | 2014 | Synaptic development |
None | Rat | Distinct compartment treatments |
| Renault et al. | 2015 | Undefined | Axon Diode | Rat | Optogenetics/calcium imaging, axon diodes |
|
| Fantuzzo et al. | 2017 | Undefined | None | Human (iNs) | Electrophysiology, 3-way circuit connections |
|
| Zhao et al. | 2016 | Huntington’s | None | Mouse: WT and BACHD |
Axonal trafficking, synaptic analysis |
|
| Virlogeux et al. | 2018 | Huntington’s | Laminin gradient |
Mouse: WT and HdhCAG140/+ KI |
Laminin gradient for axon directionality, axonal trafficking, synaptic analysis, calcium imaging |
|
| Sarkar et al. | 2018 | Schizophrenia | None | Human (ES and iPS cell derived neurons) |
Rabies virus tracing of pre- synaptic neurons |
Soft lithography is the molding of devices from a topographically defined relief structure on a substrate wafer, which is usually produced using photolithography. Ultraviolet light exposure to photoresists through a photomask can produce substrate topography with high resolution (Xia and Whitesides, 1998; Qin et al., 2010). Using this approach for compartmentalization takes its point of departure from the Jeon group, previously at University of California, Irvine (Figure 1B). Using two-layer soft lithography, the Jeon group demonstrated that a negative mold of microchannels can be produced using SU-8 photoresist on a silicon wafer, and which are traditionally arrayed at a height of 3 μm. The 3 μm height restricts transport of cell bodies but does not hinder the extension of neurites. The second layer of lithography defines compartment chambers, whose dividing wall sits on top of the microchannels of the first layer, and which can be arranged uniquely to serve the research question. The advantage of such an approach is precise definition of channels and compartments, high device reproducibility, and easy visualization of cultures (since devices were produced using transparent, cell-friendly PDMS) (Taylor et al., 2003; Park et al., 2006; Taylor and Jeon, 2011). Additionally, the Jeon group demonstrated that chambers can be fluidically separated by creating a back pressure to resist diffusion. The strategy, however, does have a distinct time limit for efficacy, but it is large enough to conduct experiments where chemical separation is desired. Importantly, they also showed that while dendrites can enter microchannels, they are restricted in their outgrowth beyond 450 μm. Therefore, to restrict the inter-chamber neurite access to axons only, microchannels must be 450 μm or greater in length. This device has since been commercialized by Xona Microfluidics (Temecula, CA). Overall, this approach can capture the benefits of the Campenot chamber while adding ease of fabrication and assembly, and greater customization to suit the research question.
Originally, the Jeon group designed their devices for axonal injury studies, where axons projecting to another compartment can be injured through media aspiration (Taylor et al., 2005). Since the development of this approach, a number of devices have been produced to demonstrate the use of the approach to query neuronal properties and injury dynamics. With respect to axonal injury models, Hosmane et al. show that a graded injury can be applied through the actuation of microfluidic injury-pads onto out-growing neurites (Hosmane et al., 2011). Dolle et al. also demonstrated through a similar approach that axonal injury can be induced by stretching microchannels containing axons (Dolle et al., 2013). This device uses a separate pressure chamber which can be actuated for injury, showing the customizability of microfluidic devices for neuronal studies. These studies among many others show how soft lithography can be expanded upon to investigate neuronal properties and circuit dynamics. With respect to developmental and psychiatric disorders in patients, however, aberrant circuit formation and morphology are often not due to injury but find their etiology in genetic (e.g., autism spectrum disorders), chemical (e.g., substance abuse), or psychological (e.g., post-traumatic stress disorder) trauma. To address issues related to circuit formation and communication between neuronal subtypes, devices must re-establish defined circuits in a way that enables the investigation of circuit communication and morphology.
Techniques for compartmentalization for neurocircuit studies
The human brain contains different nuclei which are largely composed of specific neuronal subtypes. These subtypes not only form synaptic connections within each nucleus, but between distinct nuclei, forming complex neural circuits. Understanding the function of neural circuit properties has been of great interest in trying to further our knowledge of the onset of psychiatric disease and therapeutic development. In vitro models, which study only single subtype populations of neurons, offer insight into the mechanism of disease, but connections formed within these non-compartmentalized models are believed to be randomly oriented. Mixed co-cultures between different subtypes can result in confounding synaptic connections making results difficult to interpret. Separation of cells into distinct compartments provides structure to the system and enables researchers to investigate the impact of one neuronal population on another (e.g., a disordered population versus wild type). Several proof-of-concept studies have shown that such connections can be established and monitored, and through lithographic design, can control circuit directionality.
Earliest studies of microfluidic compartmentalized systems used primary cultures seeded in either one compartment to measure axon outgrowth or induce injury (Taylor et al., 2005; Hellman et al., 2010; Hosmane et al., 2011; Dolle et al., 2013) or both compartments to investigate synaptic connections. Regarding compartmentalization for circuit studies, Coquinco et al. produced a model of synaptic competition, where synaptic pairings were analyzed between distinct side-chambers (Coquinco et al., 2014). This device used a three-chambered structure where rat cortical neurons seeded in the outer two chambers compete for synaptic contacts in the center chamber. They demonstrated that the chemical treatment of one outer population by the addition of GABAA receptor agonist muscimol increased the level of central chamber innervation by projecting axons from the untreated side. Additionally, they investigated the role of NMDA receptor antagonist APV, CamKII inhibitor KN-93, and GluR23y on the system. APV and KN-93 were able to block the muscimol-treatment effect, but not GluR23y (Coquinco et al., 2014). Coquinco et al. were also able to visualize axonal outgrowth over a time course, which was aided by the microchannel structures. Taken together, these studies show the utility of compartmentalization to probe a system with small molecules and assay the circuit response through visualization of morphological changes.
In addition to morphological studies, functional studies can be performed to investigate neuronal communication between compartments. To this end, researchers have used clever lithographic approaches to control the formation of communicating neurons in a circuit. Symmetric microchannels offer the opportunity for outgrowing axons to traverse microchannels in either direction. Building directionality into the connections focuses communication of one neuronal population to another without returning (and potentially confounding) communication in the reverse direction. One such approach is the use of “axon diodes”, originally developed by the labs of Viovy and Brugg (Figure 1C) (Peyrin et al., 2011). The diode has a larger channel entry and narrow channel exit. Projecting axons have a reduced probability of entering the narrow microchannels as compared to the wider microchannels, and therefore are primarily contained within their own compartment. In a later study, the same group demonstrated that calcium imaging, combined with optogenetic stimulation, can produce a unidirectional neuronal output (Figure 1D) (Renault et al., 2015). This study demonstrates that axon diodes provide directionality within the circuit on a functional level. The use of optogenetics and calcium imaging also show how novel genetic tools can be employed in such systems. An additional design instituted by the same authors uses the clever “return-to-sender” design (Figure 1E) (Renault et al., 2016). Here, axons entering the microchannels from one side are presented with a straight access channel to the other side. Axons from the opposing side (“receiving side”) are presented with bifurcations down the microchannels that return axons back to the receiving side. An advantage with this design is that microchannel thickness can remain the same, resulting in easier fabrication of devices. Additional approaches have used “barbed” channels, which provide obstructions to one direction and no obstructions in the reverse direction (le Feber et al., 2015) or use a series of tapering channels to restrict outgrowth to one direction (Gladkov et al., 2017) These approaches remove a layer a complexity from the system introduced through reciprocal communication, allowing the researcher to focus on a defined, unidirectional connection.
Examples of compartmentalized devices for psychiatric studies: Huntington’s disease (HD)
Most studies to date have served to demonstrate the concept of compartmentalization, although there have been some which have demonstrated directly the utility of compartmentalized devices for the study of neuropsychiatric disorders. The challenge of circuit studies for psychiatric disease research is experimental design. Questions regarding experimental setup, device design, and cell source must be carefully considered, so that unique mechanisms of disease, both at the cellular and circuit levels, can be extracted from the model. This requires basic understanding of disease endophenotypes and relies on mechanistic data from single cell in vitro models and neural circuit connections from animal models. The experimenter must also understand which endophenotypes the device is capable of modeling. Recently, two compartmentalized systems have been developed to model the pathology in Huntington’s disease.
Zhao et al. re-established the corticostriatal network, which is known to be affected in HD, in a two-chamber microfluidic device from Xona Microfluidics (Figure 2A) (Zhao et al., 2016). In this study, the authors investigated the role of mutant huntingtin gene (mHTT) and the rescuing effects of TriC (T-complex 1 ring complex). Cortical and striatal neurons were isolated from E17.5 embryos of wild type and BACHD mice (FVB/N-Tg(HTT*97Q)IXwy/J), which express human mHTT). Since mHTT impacts axonal trafficking between cortical and striatal neurons, it is beneficial to separate the two populations to see how molecular trafficking is altered. Zhao et al. demonstrated firstly that cortical and striatal neurons were maintained in their respective chambers. This was visualized through staining for both Tau (pan-neuronal marker) and DARPP32, a medium spiny striatal neuronal marker. Through immunostaining for synaptic puncta in the striatal chamber, a significant reduction in synapse number was seen in cases where BACHD cortical neurons were paired with wild type or BACHD striatal neurons. Next, they investigated the role of brain-derived neurotrophic factor (BDNF) and its support of the corticostriatal circuit, by modulating the relative BDNF concentration in the striatal chamber compartment through either direct addition of BDNF to the chamber (emulating cortical neuronal transport of BDNF to the striatal chamber) or siRNA knockdown of BDNF in the cortical chamber. Direct treatment with BDNF after starvation for 48 hours restored altered soma size of both WT and BACHD striatal neurons. siRNA knockdown demonstrated the reverse: that WT neurons, unable to traffic BDNF, produced the phenotypic smaller soma sizes found in BACHD circuits. Additionally, Zhao et al. examined reasons for reduced BDNF signaling and demonstrated that anterograde axonal transport of BDNF is impaired in BACHD cortical neurons. Trafficking, visualized through the connection of the molecule of interest to quantum dots, was observed in the microchannels over time (Zhao et al., 2014). Interestingly, treatment with either CCT3, a subunit of TriC, or ApiCCT1 restored normal anterograde transport in BACHD cortical neurons.
Figure 2.

Examples of compartmentalization for modeling Huntington’s Disease. A) Zhao et al. used twocompartment devices for investigating the role of mHTT and rescuing effects of TriC. Tau and DARPP32 staining verify the presence of neurons on both sides but DARPP32-positive neurons restricted to one compartment. (Reproduced from Zhao et al. (Zhao et al., 2016) with permission). B) Three-compartment device developed by Virlogeux et al. A specific cortical-to-striatal setup was achieved through extending microchannels greater than 450 μm and creating a laminin gradient in the striatal chamber. (Reproduced from Virlogeux et al. (Virlogeux et al., 2018) with permission).
This study highlights key experimental approaches that would be difficult or impossible in non-compartmentalized systems. First, distinct cell sources and cell types can be co-cultured in a circuit. Here, both cortical and striatal neurons were used, both from wild type and BACHD mice. Four distinct neuronal pairings were investigated (BACHD/BACHD, WT/BACHD, BACHD/WT, and WT/WT), and phenotypes can be visualized in distinct compartments. This enables researchers to see whether one synaptic partner or the other may be responsible for circuit phenotypes. Second, chemical treatments (here, quantum dots used for visualization of retrograde trafficking) can be isolated to a given chamber specifically. Unbalancing the liquid levels between the two chambers, which creates a convective force to counter and therefore limit diffusion, enables researchers to screen drug compounds to investigate their impact on a circuit. Third, focusing axons into microchannels facilitates the investigation of axonal transport in real time. These properties of the compartmentalized system broaden the number of model parameters that can be investigated in neuroscience and psychiatric disease research.
More recently, Virlogeux et al. created a unique compartmentalized device for the study of Huntington’s disease (Virlogeux et al., 2018). Their microdevice featured two cellular compartments and one synaptic compartment (Figure 2B). The synaptic compartment was cleverly designed to receive only cortical axons and striatal dendrites. Cortical dendrites from one chamber were restricted from entering the synaptic chamber due to the length of the microchannels. Microchannels greater than 450 μm in length exceed dendritic outgrowth (Taylor et al., 2005), allowing only axons to enter the synaptic compartment. To limit the number of striatal axons from entering the synaptic compartment, a laminin gradient from the cortical chamber to the striatal chamber was generated. Axons have shown preference for high laminin concentrations and tend to project in that direction (Dertinger et al., 2002). Microchannels from the striatal side were only 75 μm in length to allow dendritic growth to the synaptic chamber. Therefore, by combining both the length microchannels from the cortical side and the laminin gradient from the striatal side, precise connections for a defined circuit pathway, e.g., cortical to striatal, were generated. This approach improves upon the directionality techniques described above in that synapses are specifically axodendritic from upstream (cortical) axons to downstream (striatal) dendrites. Previous directional approaches provide some level of control, but synapses may be axoaxonic or axosomatic.
Using fluorescent-tagged tropomyosin-related kinase receptor B (TrkB), Virlogeux et al. similarly demonstrated impaired anterograde and retrograde axonal trafficking, as well as altered number of synapses between conditions. Additionally, they used recently developed fluorescent indicators (iGluSnFR (Marvin et al., 2013) and GCaMP6f (Chen et al., 2013)) to probe functional activity of the corticostriatal circuit. Through cortical neuron treatment with glycine/strychnine, glutamate release was visualized in the striatal chamber through fluorescent imaging. Importantly, this approach revealed a deficit in glutamatergic transmission in HD networks. Further functional analysis also revealed global network differences between the two conditions. Striatal neurons were infected with GCaMP6f adeno-associated virus and their global activity was tracked over basal conditions. The HD network resulted in highly synchronized but overall less active cultures. The authors suggest that HD networks may be overactive, possibly due to a reduced synapse number. They next looked at glutamate transmission under different conditional pairings. HD cortical neurons paired with WT striatal neurons showed impaired glutamate release, analogous to the full HD-HD network. Conversely, WT cortical neurons paired with HD striatal neurons had similar glutamate transmission to the fully WT network. Virlogeux et al. added tetrodotoxin (a sodium channel blocker) to further probe the network dynamics. Interestingly, the demonstrated that large, synchronous network bursts in the HD-HD network were reduced, suggesting aberrant cortical activity as responsible for the HD network functional phenotype. Lastly, they compared levels of phospho-ERK staining in striatal dendrites both in the device and in vivo. Under stimulation conditions, in vivo results showed an analogous deficit in phospho-ERK levels as in the reconstructed HD-cortical-paired networks.
These two studies highlight the potential of compartmentalized microfluidic devices to serve as a tool for neuropsychiatric research. They were designed to limit the experimental parameters to defined metrics related to the disease pathology. In both cases, morphology (soma size or synapse number) could be investigated in distinct neuronal populations. Separation of cell bodies into compartments facilitates these studies, so that the researcher knows which population is revealing a phenotype. PDMS structures are transparent and can be bonded to very thin glass substrates (0.17 mm), enabling high resolution and high magnification microscopy to aid in these analyses. Time-lapse video enables researchers to perform axonal trafficking studies. Since axonal outgrowth is captured in the microchannel structures, both retrograde and anterograde trafficking data can be captured. Importantly, these devices are also amenable to functional analyses, and with the advent of genetically-encoded calcium indicators and other genetic tools (Bernstein and Boyden, 2011; Deisseroth, 2011; Pastrana, 2011; Chen et al., 2013), the global network activity can be investigated. These studies have, however, been limited to primary mouse cultures and rely on mouse disease model development and ectopic expression of human mutant proteins. To capture disease phenotypes in a more “humanized” context, human-derived neurons must be used.
Human induced pluripotent stem cells in compartmentalized devices
The studies mentioned above have provided validation for the approach as well as disease-specific examples of how compartmentalization through microfluidics can be used to investigate circuit properties. These studies, though, have been limited to primary cultures, where disease conditions are produced through ectopic expression of a protein or gene or taken from a mouse disease model. For neuropsychiatric disorders, these models have value, but they may not always translate all disease-relevant genes, regulatory elements, receptors, or phenotypes into the network model. Species differences and brain complexity are important to consider when creating in vitro models of human disease (Nestler and Hyman, 2010; Hyman, 2014). The advent of induced pluripotent stem cells has provided human cell sources for disease modeling, with the additional advantage of possessing a known, fully human genome. The benefits of induced pluripotent stem (iPS) cell techniques goes hand in hand with genome wide association studies, which identify genetic loci linked with various psychiatric disorders such as autism spectrum disorders (ASDs) (Bailey et al., 2009; Etherton et al., 2011; Liu et al., 2017), schizophrenia (Rees et al., 2015), or addiction (Saccone et al., 2007; Tammimaki et al., 2012; Chen et al., 2015; Jensen et al., 2015; Oni et al., 2016). For the sake of mechanistic studies, iPS cells can be generated from patients with known genetic variants and can be used to produce a variety of neuronal subtypes (Vierbuchen et al., 2010; Pang et al., 2011; Yang et al., 2017; Sarkar et al., 2018). Single populations of stem cells for the study of psychiatric disorders have been widely utilized and extensively reviewed (Brennand and Gage, 2012; Panchision, 2016; Wen et al., 2016; Li et al., 2017; Soliman et al., 2017). With the advent of more differentiation protocols, we can begin to reconstruct commonly-targeted neural circuits such as the corticostriatal circuit or the mesolimbic dopamine circuit. Seeding different neuronal subtypes into distinct compartments serves to recapitulate network effects using human (and patient-specific) neurons. There are only a few studies to date using iPS cells in compartmentalized devices.
Fantuzzo et al. in 2017 developed a five-chambered microfluidic device capable of housing multiple neuronal subtypes (Figure 3A) (Fantuzzo et al., 2017). Four outer chambers were connected to a center chamber through a circular array of microchannels, fabricated in an analogous manner to previous studies (Taylor et al., 2003; Park et al., 2006). This device used simple, 10 μm-wide microchannels, without built-in directionality as described above. They demonstrated that three different neuronal populations could be generated through infection of iPS cells with lentivirus containing the required transcription factors for each desired subtype. Dopaminergic, glutamatergic, and GABA-ergic neurons were created and cultured in individual wells within the microdevice. This device was uniquely designed to be open to patch clamp apparatus in the center well, enabling the precise recording of neuronal activity in this compartment. Neurons within the center compartment can receive inputs from multiple side chambers, which adds a dimension of complexity to the neurocircuit model. This device, therefore, has the capacity to model a single brain nucleus receiving inputs from two other nuclei. Depending on the circuit modeled, neuronal subtypes can be re-ordered in the device, so that the center compartment neurons serve as the key population under investigation. The authors also demonstrated that excitatory transmission could be both perturbed through the addition of CNQX and stimulated through optogenetics. Excitatory neurons expressed viral-encoded channel-rhodopsin-2 (ChR2) and seeded in outer chambers and inhibitory neurons were seeded and recorded in the center chamber. Light pulses were time-coupled to post-synaptic currents seen in the central chamber, indicating that channel-rhodopsin2 positive excitatory neurons in the outer compartments were able to transmit action potentials through the microchannels to inhibitory neurons in the center (Figure 3A lower panel). This study further demonstrates how optogenetics serves as an important tool in compartmentalized devices, both to alter basal activity and provide a controlled stimulus that may be otherwise present endogenously in a human receiving sensory input.
Figure 3.
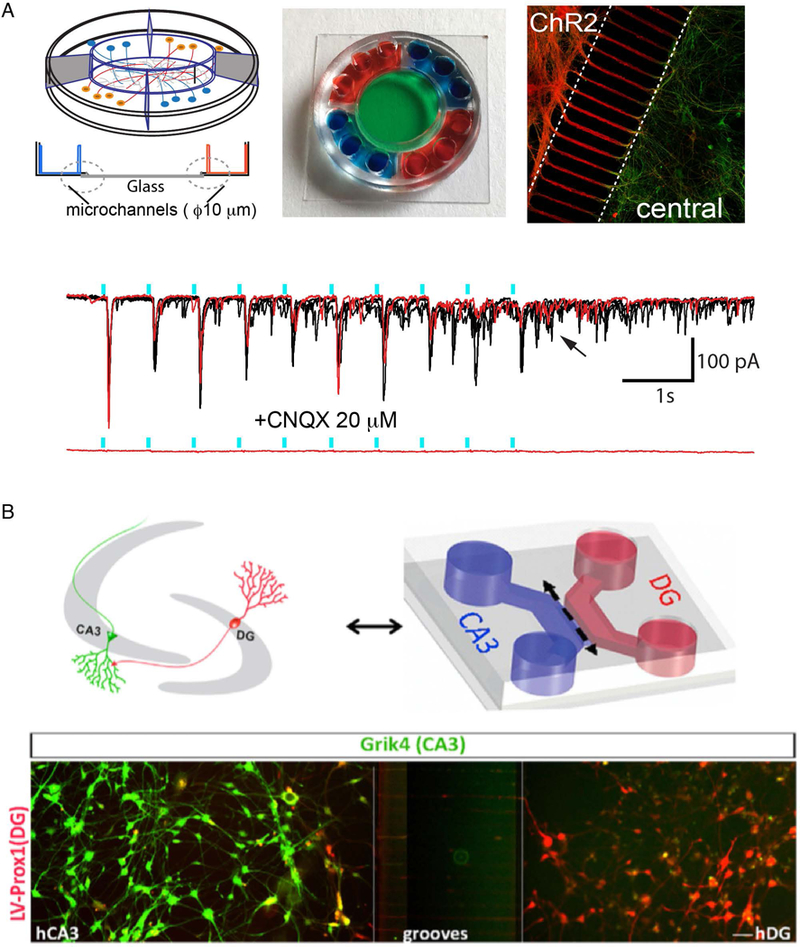
Devices using human induced neurons. A) Five-compartment device developed by Fantuzzo et al. (Fantuzzo et al., 2017). Center chamber enables access for electrophysiological analysis of circuit. Optogenetic stimulation (blue bar) is coupled to post-synaptic currents, indicating formation of a functional circuit. (Reproduced from Fantuzzo et al. (Fantuzzo et al., 2017) with permission). B) Hippocampal circuit (CA3-DG) modeled with human neurons. (Reproduced from Sarkar et al. (Sarkar et al., 2018) with permission).
Most recently, the Gage laboratory produced a model of the hippocampal Cornu Ammonis 3 (CA3) – Dentate Gyrus (DG) circuit using a compartmentalized device for the study of schizophrenia (Figure 3B) (Sarkar et al., 2018). The authors describe a newly developed protocol for differentiating human embryonic stem (hES) cells and iPS cells into both human CA3 (hCA3) neurons and human dentate gyrus (hDG) neurons. Extensive RNA-seq and immunocytochemistry was performed to profile these subtypes and to verify their match to known genetic markers of those brain regions, including the human secretagoggin (SCGN)-expressing CA3 neurons, which is not present in mice. The majority of their functional studies were performed in multi-electrode arrays (MEAs) or in simple 2D co-culture. The authors also demonstrated the formation of CA3-DG circuits within a two-compartment microfluidic device (Figure 3B lower panel). They show, using a rabies virus tracing protocol, that CA3 neurons receive inputs from opposing-chamber DG neurons. Co-culture studies were performed using cell lines from both healthy and schizophrenic patient lines. Electrophysiology and network activity measured by MEA revealed functional differences between the two cell populations. Here, the authors demonstrated the ability to perform compartmentalized co-culture, but did not further pursue this approach for functional analyses. Compartmentalized devices open to patch clamp apparatus (Fantuzzo et al., 2017) or constructed on an MEA (Berdichevsky et al., 2009) may provide an option for studies interested in electrophysiology within compartmentalized contexts.
Outside of establishing brain-specific circuits, researchers have also used iPS cells in microfluidic devices to look at CNS to PNS connections (Takayama and Kida, 2016) and connections from neuron to glia (Gao et al., 2016). There are a limited number of brain circuit studies using induced neurons to date, but with the generation of new and robust differentiation protocols (Gunhanlar et al., 2017; Yang et al., 2017), there remains robust opportunity for such studies.
Additional approaches using human induced neurons: 3D culture and organoids
Compartmentalized devices are advantageous in that they are easy to produce and manipulate, and they are well-studied. Most systems exist in a 2D format, which makes immunocytochemistry, calcium imaging, and electrophysiology easy to perform within the circuit context. The 2D cultures, however, remove an element from innate brain physiological architecture. Biomaterials have provided one way of creating constructs in 3D on which to seed human neurons. For example, Jakobsson et al. created a 3D electrospun scaffold on which to seed human neuronal cells (Jakobsson et al., 2017). Zhang et al. demonstrated the use of layered hydrogels made from methacrylate-modified hyaluronic acid for culture of iPS cell-derived neurons (Figure 4A) (Zhang et al., 2016). They were able to demonstrate morphological differences (reduced synapse number and neurite outgrowth) between neurons from a Rett patient (Q83X, which is a single nucleotide polymorphism resulting in a premature stop codon at position 83) (Tang et al., 2016), and a wild type parent. This study highlights one of the prime benefits of iPS cell-derived neurons: patient-specific cells can be used to connect genetic alterations to endophenotypes, which are then visualized in a model system. Another approach to generating 3D cultures were produced by Alessandri et al. Using 3D printing, they created a device which is capable of encapsulating neural progenitor cells in a Matrigel/alginate capsule (Figure 4B) (Alessandri et al., 2016). They demonstrated that progenitors can proliferate within the microcapsule and be differentiated into mature neurons, which extend neurites in different directions. Importantly, they were able to achieve high viability results (~98%) with human neurons within the capsules.
Figure 4.

Alternative approaches for modeling neuropsychiatric disorders with human cells. A) Layered hydrogel model used for the study of Rett syndrome. (Reproduced from Zhang et al. (Zhang et al., 2016) with permission). B) A 3D culture in Matrigel hydrogel produced through 3D-printed apparatus. (Reproduced from Alessandri et al. (Alessandri et al., 2016) with permission). C) The OrganoPlate®, which provides a high-throughput 3D culture model for human induced neurons. (Reproduced under CCBY4.0 (https://creativecommons.org/licenses/by/4.0/legalcode) from Wevers et al. (Wevers et al., 2016)). D) Generation of human organoids in Spin_ bioreactors by the Ming laboratory to study Zika virus. (Reproduced from Qian et al. (Qian et al., 2016) with permission).
High-throughput devices have also been generated. Wevers et al. describe the development of the OrganoPlate®, which is a compartmentalized device built into a 384-well plate (Figure 4C) (Wevers et al., 2016). The OrganoPlate® offers 96 different culture chambers, each made from four linearly arranged wells in the 384-well plate. An advantage of this setup is that media exchange can be performed without perturbing the cell culture. In a manner similar to that employed by Alessandri et al., a 3D matrix was generated through the gelation of Matrigel mixed in the cell suspension. Wevers et al. demonstrated the successful culture and differentiation of different types of human induced neurons (excitatory, inhibitory, and dopaminergic) co-cultured with glia. They also demonstrate two important features for drug screening. First, they show that they can use the device for calcium imaging studies, which provides a functional readout within a high-throughput system. Second, they show that they can introduce compounds into the OrganoPlate® to investigate their impact on the culture both functionally (calcium imaging) and morphologically (neurite outgrowth and cell viability). Overall, this study provides a useful tool and demonstrates an approach where microfluidics can be used to create a high-throughput drug screening platform using human neurons.
These approaches successfully demonstrate the formation and culture of 3D neuronal networks. 3D approaches have the advantage of bringing the models closer to their physiological, 3D state. However, it is more difficult to achieve compartmentalization in such models, as stem cells or progenitors are generally seeded throughout the entire matrix. Compartmentalization requires some form of physical barrier (or preservation of developmental layers) which can restrain certain populations of neurons. Achieving network activity between distinct subpopulations of neurons within a 3D device may be challenging. Organoid cultures have been developed, however, which are able to overcome some of these limitations, since they form spontaneously in three dimensions and maintain physiological layers similar to those found in the human brain (McCauley and Wells, 2017). While organoids are not generally used in compartmentalized devices, we briefly discuss their role in disease modelling below for comparison with other in vitro models.
Organoids can be generated using induced pluripotent stem cells grown in suspension. This approach and its application to the study of disease has been extensively reviewed (Fatehullah et al., 2016; Quadrato et al., 2016; Di Lullo and Kriegstein, 2017; Lee et al., 2017). Here, we highlight one report focused toward the study of Zika virus and microcephaly. This model is particularly important given the recent global concern with the connection of Zika virus and microcephaly. The Ming group demonstrated the successful generation of iPS cell-based organoids created to model certain regions of the brain (Figure 4D). To achieve greater reproducibility, they developed a bioreactor platform, SpinΩ, which parallelizes the development and growth of organoids (Qian et al., 2016). Using these reactors, they developed protocols for the generation of forebrain, midbrain, and hypothalamic organoids. The authors demonstrated the production of an outer subventricular zone-like region within the organoids as well as an outer radial glial cell layer, a feature specific to humans. Formation and identification of these distinct regions shed light on the role of pathogens in targeting specific cell types or cortical layers. They used their cortical organoids to test varying levels of exposure to Zika virus (ZIKV) in culture. Interestingly, they show that ZIKV targets neural progenitor cells and outer radial glial cells, resulting in reduced progenitor proliferation, neuronal layer thickness, and overall organoid size. The Ming group also demonstrated the capacity of the organoid system for drug screening. Using both 2D cultures and 3D organoid cultures, they screened approximately 6,000 compounds and identified Emricasan, a pan-caspase inhibitor, which provided a protective effect on neural progenitor cells, as well as other small molecules that impaired ZIKV replication (Xu et al., 2016).
Organoid models have not been limited to Zika virus studies. Among other models (Stachowiak et al., 2017; Forsberg et al., 2018), Birey et al. demonstrated the assembly of two distinct organoids for neuronal migration studies (Birey et al., 2017). The authors generated human pallial and subpallial spheroids and placed them adjacent to one another in a conical tube. This facilitated the merging of the spheroids into a pallial-subpallial bi-spheroid model, wherein migration of GABAergic interneurons from subpallium to pallium can be observed. Birey et al. demonstrate the recapitulation of this developmental phenomenon, and further apply it to a model of Timothy syndrome (TS), a neurodevelopmental disorder which impacts multiple body systems (Splawski et al., 2004). Through the use of their hybrid organoid model, the authors demonstrate that subpallial spheroids generated from patient-derived TS cell lines exhibit impaired neuronal migration into the pallial spheroid region of the model (Birey et al., 2017). Additionally, Mansour et al. demonstrated the successful transplantation of human organoids into a mouse host. They found that the organoid integrates into host tissue and, importantly, develops vasculature from the host that serve to sustain the grafted organoid, bypassing the often-seen central necrosis in long term in vitro organoid systems. Mansour et al. also demonstrate that the organoid establishes functional synaptic contacts with host tissue.
Organoids harbor great potential for the study of developmental and psychiatric disorders and further development of organoids will likely provide more insights into the onset of these challenging neuropsychiatric diseases, particularly when disease onset occurs in the developing brain.
Future directions
The complexity of the human brain and limited tissue availability of living human brain has challenged researchers to generate creative in vitro models which capture different aspects of developmental and psychiatric diseases. Compartmentalized systems provide many opportunities for circuit studies in vitro. Soft lithography affords an experimenter with a limitless platform for device/model design, which can capture unique elements (i.e., connectivity) of neural circuits. Most of these studies to date have been limited to either demonstrating compartmentalization as a concept or have conducted experiments using rat or mouse primary cells. How can we harness the potential of iPS cell-derived neurons to create better, humanized neural circuit models? Standardization of iPS cell protocols provides one area for further development. Excitatory and inhibitory neurons may be reliably produced, but how analogous are such cells to the brain regions we seek to model? Genotyping induced neurons with human or mouse neurons from a particular brain region will be useful. Furthermore, the ratio of glia (including microglia and oligodendrocytes, which are often not included in such models) with neurons must be considered. And while many brain nuclei house a large percentage of a single population of neurons (Cuevas-Diaz Duran et al., 2017), they often have mixtures of different cell types (e.g., inhibitory interneurons) which modulate and balance the activity of the circuit. Indeed, an imbalance between excitatory/inhibitory neuronal firing has been implicated in neuropsychiatric disorders (Nelson and Valakh, 2015; Selten et al., 2018). When taken from primary cultures, these ratios are largely preserved, but iPS cell-derived neuronal models are built from the ground up—seeding different populations of cells together into a platform, where they were previously cultured separately or mixed randomly. Comparison studies between defined brain regions (e.g., striatum) from animal models and human induced neuronal models of the striatum will likely shed light on how best balance these cell populations. There is still the concern and limitation however of rodent versus human cultures. These comparison studies will likely prove useful in the application of induced neuronal circuit models to disease studies and will hopefully aid in the development of efficacious treatments.
Regarding device design and experimental setup, a device must be designed so that it accurately models and captures the phenotypes of interest. If functional phenotypes are subtle, a device capable of electrophysiological recordings may be best. Global network activity can generally be seen in all compartmentalized devices through calcium imaging. Or if the circuit requires specific connections, as in the case of the corticostriatal circuit, directionality approaches will help to control neuronal connections.
Taking into consideration not only compartmentalized systems, no one in vitro model can fully capture every element of each disease, and they are not without their own limitations. Single cell populations of neurons/glia derived from iPS cells provide ease and reproducibility within conventional culture labware and protocols. Electrophysiology can be performed on such cultures, enabling extensive insight into the synaptic properties and neuronal firing dynamics. Expanding the culture into a 3D model, with cells grown on 3D substrates, may increase the physiological relevance of such models, but often at the cost of precise electrophysiology approaches. Two-dimensional compartmentalization adds another level of physiology into such systems. Communicating circuits, often between distinct neuronal populations can be studied. Electrophysiology, calcium imaging, or morphological analyses can provide great insight into the circuit dynamics between diseased and wild type models. Furthermore, through lithographic designs, circuits can become unidirectional, which focuses the model towards a specific circuit connection. The ability to chemically treat distinct chambers with small molecules gives such systems the ability for compound screening studies. Organoids are the most complex of the systems described above and provide the most realistic in vitro scenario which to study developmental disorders. They have some limitations, however. They are the least amenable to high throughput screening, although the formation of the SpinΩ bioreactors addresses this concern to a degree. The organoids often take weeks to develop and can only model first to second trimester brain regions (Qian et al., 2016). Continued development of the organoid system will likely extend beyond these limitations but likely not to the level of recapitulating an entire adult human brain.
Each model provides a level of insight into the nature of psychiatric and developmental disorders, and researchers should rely on each of them to provide the broadest picture of disease mechanism and progression, and to screen candidate compounds to improve therapeutic outcomes. Hopefully, such models can help bridge the gap between animal models and human disease, resulting in greater translation of candidate drugs through clinical trials.
BULLETPOINTS:
Compartmentalization segregates neuronal cell bodies from neurites
Most devices to date have been used to demonstrate the principal of compartmentalization in circuit dynamics
Devices need to focus design toward capturing disease-specific features
Incorporation of stem cell-derived neurons with devices can link circuit dynamics to human-specific disease elements
ACKNOWLEDGEMENTS
The authors acknowledge the following funding sources: The National Institute on Drug Abuse (NIDA) R21 5R21DA039686 and NIDA R21 5R21DA035594, and the National Institute of Alcohol Abuse and Alcoholism (NIAAA) R01AA023797.
GRANT NUMBERS: The National Institute on Drug Abuse (NIDA) R21 5R21DA039686 and NIDA R21 5R21DA035594, and the National Institute of Alcohol Abuse and Alcoholism (NIAAA) R01AA023797.
REFERENCES
- Alessandri K, Feyeux M, Gurchenkov B, Delgado C, Trushko A, Krause KH, Vignjevic D, Nassoy P, Roux A. 2016. A 3D printed microfluidic device for production of functionalized hydrogel microcapsules for culture and differentiation of human Neuronal Stem Cells (hNSC). Lab Chip 16:1593–1604. [DOI] [PubMed] [Google Scholar]
- Atwal JK, Massie B, Miller FD, Kaplan DR. 2000. The TrkB-Shc Site Signals Neuronal Survival and Local Axon Growth via MEK and PI3-Kinase. Neuron 27:265–277. [DOI] [PubMed] [Google Scholar]
- Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, Rutter M. 2009. Autism as a strongly genetic disorder: evidence from a British twin study. Psychological Medicine 25:63. [DOI] [PubMed] [Google Scholar]
- Berdichevsky Y, Sabolek H, Levine JB, Staley KJ, Yarmush ML. 2009. Microfluidics and multielectrode array-compatible organotypic slice culture method. Journal of neuroscience methods 178:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein JG, Boyden ES. 2011. Optogenetic tools for analyzing the neural circuits of behavior. Trends Cogn Sci 15:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, O’Rourke NA, Steinmetz LM, Bernstein JA, Hallmayer J, Huguenard JR, Paşca SP. 2017. Assembly of functionally integrated human forebrain spheroids. Nature 545:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennand KJ, Gage FH. 2012. Modeling psychiatric disorders through reprogramming. Dis Model Mech 5:26–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB. 1977. Local control of neurite development by nerve growth factor. Proc Natl Acad Sci U S A 74:4516–4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campenot RB, MacInnis BL. 2004. Retrograde transport of neurotrophins: fact and function. J Neurobiol 58:217–229. [DOI] [PubMed] [Google Scholar]
- Campenot RB, Martin G 2001. Construction and Use of Compartmented Cultures for Studies of Cell Biology of Neurons. 3rd ED:49–57. [Google Scholar]
- Chen LS, Baker TB, Jorenby D, Piper M, Saccone N, Johnson E, Breslau N, Hatsukami D, Carney RM, Bierut LJ. 2015. Genetic variation (CHRNA5), medication (combination nicotine replacement therapy vs. varenicline), and smoking cessation. Drug Alcohol Depend 154:278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-W, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS. 2013. Ultra-sensitive fluorescent proteins for imaging neuronal activity. Nature 499:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquinco A, Kojic L, Wen W, Wang YT, Jeon NL, Milnerwood AJ, Cynader M. 2014. A microfluidic based in vitro model of synaptic competition. Mol Cell Neurosci 60:43–52. [DOI] [PubMed] [Google Scholar]
- Crawley JN. 2012. Translational animal models of autism and neurodevelopmental disorders. Dialogues in Clinical Neuroscience 14:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Diaz Duran R, Wei H, Wu JQ. 2017. Single-cell RNA-sequencing of the brain. Clinical and Translational Medicine 6:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K 2011. Optogenetics. Nat Methods 8:26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dertinger SKW, Jiang X, Li Z, Murthy VN, Whitesides GM. 2002. Gradients of substrate-bound laminin orient axonal specification of neurons. Proceedings of the National Academy of Sciences 99:12542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lullo E, Kriegstein AR. 2017. The use of brain organoids to investigate neural development and disease. Nature Reviews Neuroscience 18:573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolle JP, Morrison B 3rd, Schloss RS, Yarmush ML 2013. An organotypic uniaxial strain model using microfluidics. Lab Chip 13:432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton M, Földy C, Sharma M, Tabuchi K, Liu X, Shamloo M, Malenka RC, Südhof TC. 2011. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. PNAS 108:13764–13769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantuzzo JA, De Filippis L, McGowan H, Yang N, Ng Y -H, Halikere A, Liu J-J, Hart RP, Wernig M, Zahn JD, Pang ZP. 2017. μNeurocircuitry: Establishing in vitro models of neurocircuits with human neurons. Technology 5:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farra N, Zhang WB, Pasceri P, Eubanks JH, Salter MW, Ellis J. 2012. Rett syndrome induced pluripotent stem cell-derived neurons reveal novel neurophysiological alterations. Molecular Psychiatry 17:1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatehullah A, Tan SH, Barker N. 2016. Organoids as an in vitro model of human development and disease. Nature Cell Biology 18:246. [DOI] [PubMed] [Google Scholar]
- Forsberg SL, Ilieva M, Maria Michel T. 2018. Epigenetics and cerebral organoids: promising directions in autism spectrum disorders. Translational Psychiatry 8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Broussard J, Haque A, Revzin A, Lin T. 2016. Functional imaging of neuron–astrocyte interactions in a compartmentalized microfluidic device. Microsystems & Nanoengineering 2:15045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladkov A, Pigareva Y, Kutyina D, Kolpakov V, Bukatin A, Mukhina I, Kazantsev V, Pimashkin A. 2017. Design of Cultured Neuron Networks in vitro with Predefined Connectivity Using Asymmetric Microfluidic Channels. Scientific Reports 7:15625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratten J, Wray NR, Keller MC, Visscher PM. 2014. Large-scale genomics unveils the genetic architecture of psychiatric disorders. Nat Neurosci 17:782–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunhanlar N, Shpak G, van der Kroeg M, Gouty-Colomer LA, Munshi ST, Lendemeijer B, Ghazvini M, Dupont C, Hoogendijk WJG, Gribnau J, de Vrij FMS, Kushner SA. 2017. A simplified protocol for differentiation of electrophysiologically mature neuronal networks from human induced pluripotent stem cells. Molecular Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman AN, Vahidi B, Kim HJ, Mismar W, Steward O, Jeon NL, Venugopalan V. 2010. Examination of axonal injury and regeneration in micropatterned neuronal culture using pulsed laser microbeam dissection. Lab Chip 10:2083–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmane S, Fournier A, Wright R, Rajbhandari L, Siddique R, Yang IH, Ramesh KT, Venkatesan A, Thakor N. 2011. Valve-based microfluidic compression platform: single axon injury and regrowth. Lab Chip 11:3888–3895. [DOI] [PubMed] [Google Scholar]
- Hyman SE. 2014. How far can mice carry autism research? Cell 158:13–14. [DOI] [PubMed] [Google Scholar]
- Hyman SE. 2016. Back to basics: luring industry back into neuroscience. Nature Neuroscience 19:1383–1384. [DOI] [PubMed] [Google Scholar]
- Jakobsson A, Ottosson M, Zalis MC, O’Carroll D, Johansson UE, Johansson F. 2017. Three-dimensional functional human neuronal networks in uncompressed low-density electrospun fiber scaffolds. Nanomedicine: Nanotechnology, Biology and Medicine 13:1563–1573. [DOI] [PubMed] [Google Scholar]
- Jensen KP, DeVito EE, Herman AI, Valentine GW, Gelernter J, Sofuoglu M. 2015. A CHRNA5 Smoking Risk Variant Decreases the Aversive Effects of Nicotine in Humans. Neuropsychopharmacology 40:2813–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesselheim AS, Hwang TJ, Franklin JM. 2015. Two decades of new drug development for central nervous system disorders. Nat Rev Drug Discov 14:815–816. [DOI] [PubMed] [Google Scholar]
- le Feber J, Postma W, de Weerd E, Weusthof M, Rutten WL. 2015. Barbed channels enhance unidirectional connectivity between neuronal networks cultured on multi electrode arrays. Front Neurosci 9:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C -T, Bendriem RM, Wu WW, Shen R-F. 2017. 3D brain Organoids derived from pluripotent stem cells: promising experimental models for brain development and neurodegenerative disorders. Journal of Biomedical Science 24:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chao J, Shi Y. 2017. Modeling neurological diseases using iPSC-derived neural cells : iPSC modeling of neurological diseases. Cell Tissue Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Grace KP, Horner RL, Cortez MA, Shao Y, Jia Z. 2017. Neuroligin 3 R451C mutation alters electroencephalography spectral activity in an animal model of autism spectrum disorders. Mol Brain 10:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning PT, Johnson EM, Wilcox CL, Palmatier MA, Russell JH. 1987. MHC-specific cytotoxic T lymphocyte killing of dissociated sympathetic neuronal cultures. The American Journal of Pathology 128:395–409. [PMC free article] [PubMed] [Google Scholar]
- Marvin JS, Borghuis BG, Tian L, Cichon J, Harnett MT, Akerboom J, Gordus A, Renninger SL, Chen T -W, Bargmann CI, Orger MB, Schreiter ER, Demb JB, Gan W-B, Hires SA, Looger LL. 2013. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nature Methods 10:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley HA, Wells JM. 2017. Pluripotent stem cell-derived organoids: using principles of developmental biology to grow human tissues in a dish. Development 144:958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet LJ, Gillette MU. 2012. Over a Century of Neuron Culture: From the Hanging Drop to Microfluidic Devices. The Yale Journal of Biology and Medicine 85:501–521. [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Valakh V. 2015. Excitatory/Inhibitory balance and circuit homeostasis in Autism Spectrum Disorders. Neuron 87:684–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Hyman SE. 2010. Animal models of neuropsychiatric disorders. Nat Neurosci 13:1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oni EN, Halikere A, Li G, Toro-Ramos AJ, Swerdel MR, Verpeut JL, Moore JC, Bello NT, Bierut LJ, Goate A, Tischfield JA, Pang ZP, Hart RP. 2016. Increased nicotine response in iPSC-derived human neurons carrying the CHRNA5 N398 allele. Sci Rep 6:34341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panchision DM. 2016. Concise Review: Progress and Challenges in Using Human Stem Cells for Biological and Therapeutics Discovery: Neuropsychiatric Disorders. Stem Cells 34:523–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang ZP, Yang N, Vierbuchen T, Ostermeier A, Fuentes DR, Yang TQ, Citri A, Sebastiano V, Marro S, Sudhof TC, Wernig M. 2011. Induction of human neuronal cells by defined transcription factors. Nature 476:220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich Diana E, Altevogt Bruce M, Dunlop J, Gage Fred H, Hyman Steve E. 2014. Improving and Accelerating Drug Development for Nervous System Disorders. Neuron 84:546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JW, Vahidi B, Taylor AM, Rhee SW, Jeon NL. 2006. Microfluidic culture platform for neuroscience research. Nat Protoc 1:2128–2136. [DOI] [PubMed] [Google Scholar]
- Pastrana E 2011. Optogenetics: Controlling cell function with light. Nature Methods 8:24–25. [Google Scholar]
- Penfold ME, Armati P, Cunningham AL. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proceedings of the National Academy of Sciences 91:6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrin JM, Deleglise B, Saias L, Vignes M, Gougis P, Magnifico S, Betuing S, Pietri M, Caboche J, Vanhoutte P, Viovy JL, Brugg B. 2011. Axon diodes for the reconstruction of oriented neuronal networks in microfluidic chambers. Lab Chip 11:3663–3673. [DOI] [PubMed] [Google Scholar]
- Qian X, Nguyen Ha N, Song Mingxi M, Hadiono C, Ogden Sarah C, Hammack C, Yao B, Hamersky Gregory R, Jacob F, Zhong C, Yoon K-j, Jeang W, Lin L, Li Y, Thakor J, Berg Daniel A, Zhang C, Kang E, Chickering M, Nauen D, Ho C-Y, Wen Z, Christian Kimberly M, Shi P-Y, Maher Brady J, Wu H, Jin P, Tang H, Song H, Ming G-l. 2016. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165:1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin D, Xia Y, Whitesides GM. 2010. Soft lithography for micro- and nanoscale patterning. Nat Protoc 5:491–502. [DOI] [PubMed] [Google Scholar]
- Quadrato G, Brown J, Arlotta P. 2016. The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nature Medicine 22:1220. [DOI] [PubMed] [Google Scholar]
- Rees E, O’Donovan MC, Owen MJ. 2015. Genetics of schizophrenia. Current Opinion in Behavioral Sciences 2:8–14. [Google Scholar]
- Renault R, Durand JB, Viovy JL, Villard C. 2016. Asymmetric axonal edge guidance: a new paradigm for building oriented neuronal networks. Lab Chip 16:2188–2191. [DOI] [PubMed] [Google Scholar]
- Renault R, Sukenik N, Descroix S, Malaquin L, Viovy JL, Peyrin JM, Bottani S, Monceau P, Moses E, Vignes M. 2015. Combining microfluidics, optogenetics and calcium imaging to study neuronal communication in vitro. PLoS One 10:e0120680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccone SF, Hinrichs AL, Saccone NL, Chase GA, Konvicka K, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau O, Swan GE, Goate AM, Rutter J, Bertelsen S, Fox L, Fugman D, Martin NG, Montgomery GW, Wang JC, Ballinger DG, Rice JP, Bierut LJ. 2007. Cholinergic nicotinic receptor genes implicated in a nicotine dependence association study targeting 348 candidate genes with 3713 SNPs. Hum Mol Genet 16:36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Mei A, Paquola ACM, Stern S, Bardy C, Klug JR, Kim S, Neshat N, Kim HJ, Ku M, Shokhirev MN, Adamowicz DH, Marchetto MC, Jappelli R, Erwin JA, Padmanabhan K, Shtrahman M, Jin X, Gage FH. 2018. Efficient Generation of CA3 Neurons from Human Pluripotent Stem Cells Enables Modeling of Hippocampal Connectivity In Vitro. Cell Stem Cell 22:684–697.e689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selten M, van Bokhoven H, Nadif Kasri N. 2018. Inhibitory control of the excitatory/inhibitory balance in psychiatric disorders. F1000Research 7:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda Y, Sadakata T, Furuichi T. 2013. Animal Models of Autism Spectrum Disorder (ASD): A Synaptic-Level Approach to Autistic-Like Behavior in Mice. Experimental Animals 62:71–78. [DOI] [PubMed] [Google Scholar]
- Singh Dolt K, Hammachi F, Kunath T. 2017. Modeling Parkinson’s disease with induced pluripotent stem cells harboring α‐synuclein mutations. Brain Pathology 27:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman MA, Aboharb F, Zeltner N, Studer L. 2017. Pluripotent stem cells in neuropsychiatric disorders. Molecular Psychiatry 22:1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. 2004. CaV1.2 Calcium Channel Dysfunction Causes a Multisystem Disorder Including Arrhythmia and Autism. Cell 119:19–31. [DOI] [PubMed] [Google Scholar]
- Stachowiak EK, Benson CA, Narla ST, Dimitri A, Chuye LEB, Dhiman S, Harikrishnan K, Elahi S, Freedman D, Brennand KJ, Sarder P, Stachowiak MK. 2017. Cerebral organoids reveal early cortical maldevelopment in schizophrenia—computational anatomy and genomics, role of FGFR1. Translational Psychiatry 7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. 2006. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. [DOI] [PubMed] [Google Scholar]
- Takayama Y, Kida YS. 2016. In Vitro Reconstruction of Neuronal Networks Derived from Human iPS Cells Using Microfabricated Devices. PLoS One 11:e0148559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammimaki A, Herder P, Li P, Esch C, Laughlin JR, Akk G, Stitzel JA. 2012. Impact of human D398N single nucleotide polymorphism on intracellular calcium response mediated by alpha3beta4alpha5 nicotinic acetylcholine receptors. Neuropharmacology 63:1002–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Kim J, Zhou L, Wengert E, Zhang L, Wu Z, Carromeu C, Muotri AR, Marchetto MCN, Gage FH, Chen G. 2016. KCC2 rescues functional deficits in human neurons derived from patients with Rett syndrome. Proceedings of the National Academy of Sciences of the United States of America 113:751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. 2005. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nature methods 2:599–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Jeon NL. 2011. Microfluidic and Compartmentalized Platforms for Neurobiological Research. Critical Reviews in Biomedical Engineering 39:185–200. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Rhee SW, Tu CH, Cribbs DH, Cotman CW, Jeon NL. 2003. Microfluidic Multicompartment Device for Neuroscience Research. Langmuir 19:1551–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Network and Pathway Analysis Subgroup of Psychiatric Genomics Consortium. 2015. Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nat Neurosci 18:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. 2010. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463:1035–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikman KS, Backström E, Kristensson K, Hill RH. 2001. A two-compartment in vitro model for studies of modulation of nociceptive transmission. Journal of Neuroscience Methods 105:175–184. [DOI] [PubMed] [Google Scholar]
- Virlogeux A, Moutaux E, Christaller W, Genoux A, Bruyere J, Fino E, Charlot B, Cazorla M, Saudou F. 2018. Reconstituting Corticostriatal Network on-a-Chip Reveals the Contribution of the Presynaptic Compartment to Huntington’s Disease. Cell Rep 22:110–122. [DOI] [PubMed] [Google Scholar]
- Wen Z, Christian KM, Song H, Ming GL. 2016. Modeling psychiatric disorders with patient-derived iPSCs. Curr Opin Neurobiol 36:118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wevers NR, van Vught R, Wilschut KJ, Nicolas A, Chiang C, Lanz HL, Trietsch SJ, Joore J, Vulto P. 2016. High-throughput compound evaluation on 3D networks of neurons and glia in a microfluidic platform. Scientific Reports 6:38856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization ROfE. 2018. Data and Resources. Accessed 5/7/18.Available at: http://www.euro.who.int/en/health-topics/noncommunicable-diseases/mental-health/data-and-resources.In.
- Xia Y, Whitesides GM. 1998. Soft Lithography. Annual Review Material Science 28:153–184. [Google Scholar]
- Xu M, Lee EM, Wen Z, Cheng Y, Huang W -K, Qian X, Tcw J, Kouznetsova J, Ogden SC, Hammack C, Jacob F, Nguyen HN, Itkin M, Hanna C, Shinn P, Allen C, Michael SG, Simeonov A, Huang W, Christian KM, Goate A, Brennand KJ, Huang R, Xia M, Ming G-l, Zheng W, Song H, Tang H 2016. Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen. Nature Medicine 22:1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang IH, Siddique R, Hosmane S, Thakor N, Hoke A. 2009. Compartmentalized microfluidic culture platform to study mechanism of paclitaxel-induced axonal degeneration. Exp Neurol 218:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Chanda S, Marro S, Ng YH, Janas JA, Haag D, Ang CE, Tang Y, Flores Q, Mall M, Wapinski O, Li M, Ahlenius H, Rubenstein JL, Chang HY, Buylla AA, Sudhof TC, Wernig M. 2017. Generation of pure GABAergic neurons by transcription factor programming. Nat Methods 14:621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Danko T, Botelho SC, Patzke C, Pak C, Wernig M, Sudhof TC. 2016. Autism-associated SHANK3 haploinsufficiency causes Ih channelopathy in human neurons. Science 352:aaf2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z -N, Freitas BC, Qian H, Lux J, Acab A, Trujillo CA, Herai RH, Nguyen Huu VA, Wen JH, Joshi-Barr S, Karpiak JV, Engler AJ, Fu X-D, Muotri AR, Almutairi A. 2016. Layered hydrogels accelerate iPSC-derived neuronal maturation and reveal migration defects caused by MeCP2 dysfunction. Proceedings of the National Academy of Sciences 113:3185–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Chen X -Q, Han E, Hu Y, Paik P, Ding Z, Overman J, Lau AL, Shahmoradian SH, Chiu W, Thompson LM, Wu C, Mobley WC. 2016. TRiC subunits enhance BDNF axonal transport and rescue striatal atrophy in Huntington’s disease. Proceedings of the National Academy of Sciences 113:E5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Zhou Y, Weissmiller AM, Pearn ML, Mobley WC, Wu C. 2014. Real-time Imaging of Axonal Transport of Quantum Dot-labeled BDNF in Primary Neurons. Journal of Visualized Experiments : JoVE:51899. [DOI] [PMC free article] [PubMed] [Google Scholar]


