Abstract
Objectives:
Medication related osteonecrosis of the jaw (MRONJ) is a rare, but severe side effect of antiresorptive medications. Most animal models utilize tooth extraction as an instigating local factor to induce MRONJ, with varied results. However, these teeth are healthy, absent of dental disease, a rare finding that does not reflect clinical practices. We hypothesize that extraction of teeth with periapical inflammation leads to MRONJ in rats treated with high-dose bisphosphonates.
Materials and Methods:
Rats were pre-treated with zoledronic acid (ZA) for 1-week. Pulp Exposure (PE) was established by exposing the pulpal chamber of the first and second molars. Experimental Periapical Disease (EPD) was induced by pulp exposure and bacterial inoculation into pulp chambers of the first and second mandibular molars. The mandibular molars were extracted 4-weeks following PE or EPD, and animals euthanized 4-weeks after tooth extraction. Extraction sockets were assessed clinically, radiographically, and histologically.
Results:
Clinically, radiographically and histologically, socket healing was observed in all Veh animals, and in ZA animals after extraction of healthy teeth or teeth with PE. In contrast, bone exposure, lack of socket healing and osteonecrosis were present in the majority of the ZA animals after extraction of teeth with EPD. Bacterial presence was noted in areas of osteonecrotic alveolar bone.
Conclusion:
Our data support a synergistic contribution of severe dental disease and tooth extraction for MRONJ pathogenesis. Importantly, this model is amenable to manipulation of methodological conditions for the dissection of parameters involved in MRONJ pathogenesis.
INTRODUCTION
Anti-resorptive medications, such as bisphosphonates and denosumab, prescribed for management of bone malignancy or osteoporosis [1, 2], have rare, but serious side effects, including medication related osteonecrosis of the jaw (MRONJ). MRONJ is characterized by exposed bone in the maxillofacial region for at least 8 weeks, without a history of head and neck radiation [3]. The most common local risk factor for MRONJ development is tooth extraction [4], which in the majority of adult patients is caused by periodontal or periapical disease [5]. While prevalence is low, MRONJ decreases quality of life and can lead to serious complications [6, 7].
Even though MRONJ has been studied extensively, its pathophysiology remains elusive [6, 8]. A variety of mechanisms have been proposed, including osteoclast dysfunction, bone turnover suppression and altered wound healing [9–12]. Animal models that reproduce some, but not all, aspects of human MRONJ have been developed, utilizing two main approaches: tooth extraction or development of periapical or periodontal disease [13, 14].
In the first approach, animals treated with antiresorptives undergo tooth extraction. However, unlike in humans, the extracted teeth in these models are healthy and void of periapical or periodontal disease. This is a rare occurrence in the clinical management of adult patients, as teeth are often extracted due to severe periodontal or periapical disease. Interestingly, several studies utilizing extraction of healthy teeth in animals under high dose antiresorptive treatment report defective osseous, but normal mucosal healing [13, 15–19]. Mucosal defects are more consistently observed in animals on antiresorptives also treated with adjunctive therapies, such as steroids or chemotherapy, or during vitamin D deficiency or diabetes [17–20]. These adjuvant treatments likely compromise soft and hard tissue healing, and have a significant contribution to MRONJ development. On the other hand, others have reported defective mucosal healing in animals treated only with antiresorptive treatment [21–23]. However, there are technical differences in these studies that likely account for the disparate observations.
In the second approach toward development of MRONJ animal models, periapical or periodontal disease is induced in animals treated with antiresorptives, but no tooth extraction is performed [10, 11, 24, 25]. Such models report clinical, radiographic and histologic observations that resemble features of MRONJ. As MRONJ largely occurs following tooth extraction, the insight provided by these models is limited only to cases of spontaneous MRONJ around teeth with periodontal or periapical disease. Despite the limitations, these models provide insight into disease pathogenesis.
Here, we propose that combining the two approaches would more closely capture the clinical reality of MRONJ pathogenesis. We hypothesize that extraction of teeth with periapical inflammation, but not of healthy teeth, leads to MRONJ in rats treated with high-dose BPs.
MATERIALS AND METHODS
Animal care
Eight-week old, healthy, male Wistar-Han rats (Charles River Laboratories, Raleigh, NC) were randomly assigned to receive intraperitoneal (IP) injections of endotoxin-free saline (vehicle) or 200µg/kg zoledronic acid (ZA) (LKT Laboratories, St Paul, MN) twice weekly, in morning hours (219–261g, 236g average). Vehicle (Veh) or ZA treatment was continued throughout the duration of the experiments. Throughout the experiment, rats were housed in pathogen-free conditions (2 per cage) with a 12-hour light/dark cycle, fed a standard diet (NIH-31 Modified Open Formula, ENVIGO, Madison, WI) and given water ad libitum. Rats with retained root fragments or fractured cortical plates were excluded from analysis. All applicable institutional and/or national guidelines for the care and use of animals were followed.
Experiment 1: Extraction of teeth with Pulp Exposure (PE)
Following 1 week of pre-treatment, 24 rats (12 Veh, 12 ZA) had the crowns of their right first (M1) and second (M2) mandibular molars drilled using a 1/2 round carbide burr to create pulpal exposure. 4 weeks after pulpal exposure, the right M1 and M2 were extracted. Animals were euthanized 4 weeks following tooth extraction. 2 Veh and 2 ZA animals were excluded from analysis due to retained root fragments/fractured cortical plates.
Experiment 2: Effects of bacterial inoculation on periapical disease
After 1 week of pre-treatment, 40 rats (20 Veh, 20 ZA) had the right M1 and M2 drilled to create pulpal exposure, as described. In 10 ZA and 10 Veh treated animals, the pulpal chambers were inoculated with a solution of periapical pathogens containing 109 of each Porphyromonas gingivalis, Streptococcus gordonii, Aggregatibacter actinomycetemcomitans, and Fusobacterium nucleatum to induce experimental periapical disease (EPD). The pulpal chamber was covered with Cavit (3M ESPE, St. Paul, MN). 8 weeks after EPD, animals were euthanized.
Experiment 3: Extraction of teeth with Experimental Periapical Disease (EPD)
After 1 week of pre-treatment, EPD, described above, was induced in the right M1 and M2 of 24 Veh and 36 ZA treated animals. 24 Veh and 22 ZA treated animals, serving as controls, did not undergo any manipulation prior to extraction. 4 weeks following induction of EPD, all animals had M1 and M2 extracted. Animals were euthanized 4 weeks following tooth extraction. 4 Veh and 5 ZA animals with extraction of healthy teeth, and 8 Veh and 12 ZA animals with EPD were excluded from analysis due to retained root fragments.
Ex-vivo µCT specimen scanning & Imaging
Mandibles were harvested, then imaged using a digital microscope at 40x magnification (Keyonce VHX-1000, Osaka, Japan). Following fixation in 4% paraformaldehyde for 48 hours, Mandibles were imaged by ex-vivo µCT using SkyScan 1172 at 20 µm resolution (SkyScan, Kontich, Belgium), as described [14]. Volumetric data were converted to DICOM format and imported to generate reconstructed images. Linear and volumetric measurements of periapical bone loss, and bone volume fractionation (BV/TV) were made, as described [10]. Healing of extraction sockets was rated as complete (greater than 75% of the socket), partial (healing of 25– 75% of the socket) or absent (less than 25% of the socket), and quantified, as described [26].
Histology, TRAP staining, gram staining
Mandibles were decalcified in 15% EDTA and sectioned in a buccal-lingual fashion, in the area of bone exposure. Samples were paraffin embedded and 5µm sections were made and stained with H&E [27]. Analysis was performed using Aperio Image Scope software (Aperio Technologies, Inc., Vista, CA). The region of interest (ROI) was defined as the area of the alveolar crest to the inferior border of the mandible in the area of M1 and M2. The epithelium to alveolar crest distance, total number of osteocytic lacunae, number of empty lacunae, and osteonecrotic area were quantified [27]. Empty lacunae were those with empty or karolytic osteocytic lacunae. Osteonecrosis were identified as an area of 5 or more confluent empty lacunae. The epithelium to alveolar crest distance is the distance from the inferior part of the epithelium to the alveolar crest.
Histology, slide scanning, digital imaging and Gram staining was performed at the Translational Pathology Core Laboratory (TPCL) at the David Geffen School of Medicine at UCLA. Gram staining was performed to identify bacteria. Bacterial quantification was measured in the ROI and normalized to the bone surface area. For osteoclast enumeration, tartrate-resistant acid phosphatase (TRAP) staining was performed utilizing the leukocyte acid phosphatase kit (387A-IKT Sigma, St. Louis, MO) and normalized to the bone surface area [14].
Statistics
Raw data were analyzed using GraphPad Prism (GraphPad Software, Inc. La Jolla, CA). Descriptive statistics were used to calculate the mean and the standard error of the mean (SEM). Data were analyzed by one and two-way ANOVA and post-hoc Tukey’s test for multiple comparisons, with statistical significance of 0.05. Socket healing was analyzed using the Fischer’s exact test.
RESULTS
Experiment 1: Extraction of teeth with pulp exposure (PE)
First, we examined the effects of PE on MRONJ development. Most Veh (10/10) and ZA (9/10) treated animals displayed no clinical bone exposure (Fig. 1A, B). Radiographically, Veh animals showed healing with indistinct extraction socket borders (Fig. 1C-C1). ZA animals also showed osseous healing. However, the original extraction socket borders were easily discernible (Fig. 1D-D1). Furthermore, ZA treatment increased the Bone Volume over Tissue Volume (BV/TV) ratio in the area of the edentulous alveolar ridge (Fig. 1G). Histologic investigation of Veh animals showed normal epithelium, submucosa and healed sockets (Fig. 1E, F). ZA treated also animals displayed soft tissue healing. However, the extraction socket showed osteonecrosis, as indicated by confluent empty osteocytic lacunae. A statistically significant increase (p<0.0001) in percent osteonecrosis was observed (Fig. 1H).
Fig. 1. Socket healing following extraction of teeth with pulpal exposure.
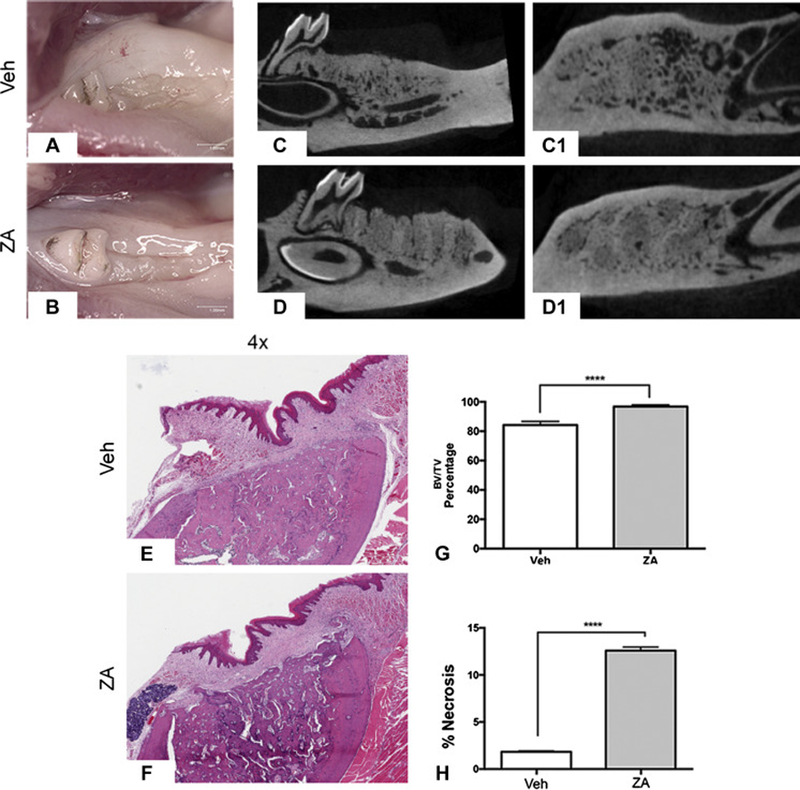
Clinical images of socket healing following extraction of teeth with pulpal exposure in Veh (a) and ZA (b) treated animals. Saggital and axial µ CT images of Veh (c, c1) and ZA (d, d1) treated animals with extraction of teeth with pulpal exposure. Representative H&E sections of extraction sockets in Veh (e) and ZA (f) treated animals with extraction of teeth with pulpal exposure. (g) Quantification of BV/TV percentage. (h) Quantification of percent osteonecrosis. Data represents mean value ± SEM. **** statistical significance, p<0.0001 (n=10 per group).
Experiment 2: Effects of bacterial inoculation on periapical disease
Because extraction of teeth with PE did not lead to MRONJ lesions clinically, we inoculated the pulpal chambers with periapical pathogens creating experimental periapical disease (EPD), and examined its effects on the periodontium prior to tooth extraction. Radiographic examination of molars with PE revealed bone loss confined to the periapical region in Veh treated animals (Fig. 2A-A1). In Veh treated animals with EPD, large, widespread radiolucencies were visualized extending to near the inferior border of the mandible (Fig. 2B-B1). In ZA treated animals with PE, minimal alveolar bone loss was noted (Fig. 2C-C1). In ZA animals with EPD, the radiographic appearance was similar, with only slight widening of the apical periodontal ligament space (Fig. 2D-D1). Quantification of the root-alveolar bone distance demonstrated a loss of apical bone in Veh animals with PE; this bone loss was significantly enhanced with EPD. The presence of EPD had no effect on the periapical bone loss in ZA treated animals (Fig. 2E). However, ZA treatment attenuated periapical bone loss. Histologic assessment paralleled radiographic findings. Veh treated animals with PE demonstrated periapical bone loss and inflammatory infiltrate around the apical area (Fig. 2F). In Veh animals with EPD, more extensive bone loss and prominent inflammatory infiltrate was noted (Fig. 2G). ZA treated animals with PE displayed minimal bone loss, with inflammatory infiltrate concentrated to the periapical region (Fig. 2H). While ZA treated animals with EPD displayed similar bone loss, a prominent inflammatory infiltrate extended beyond the periapical bone, into the surrounding alveolar bone (Fig. 2I, blue arrow).
Fig. 2. Radiographic and histologic analysis of pulp exposure & EPD prior to extraction.
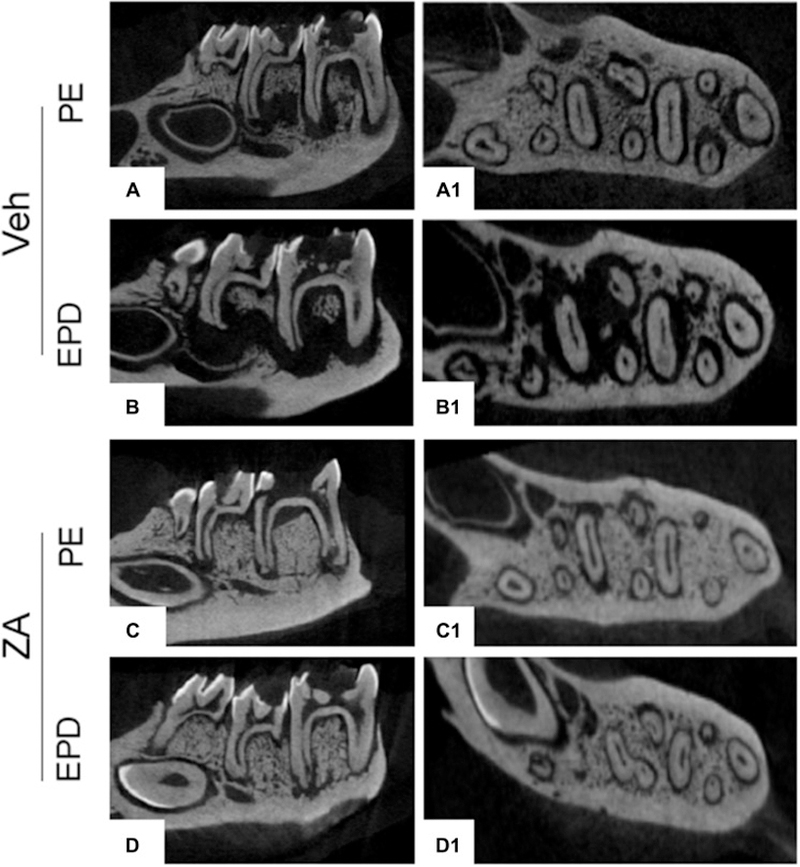
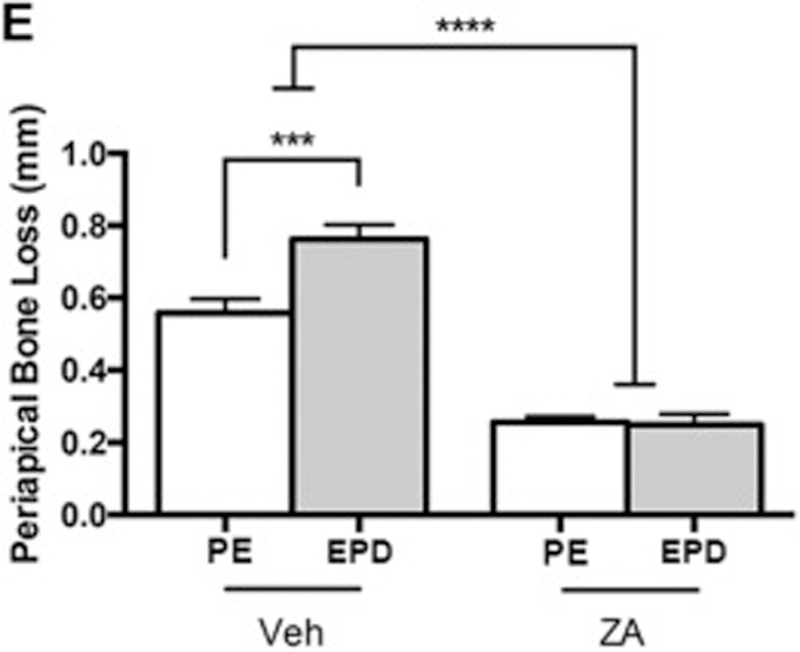
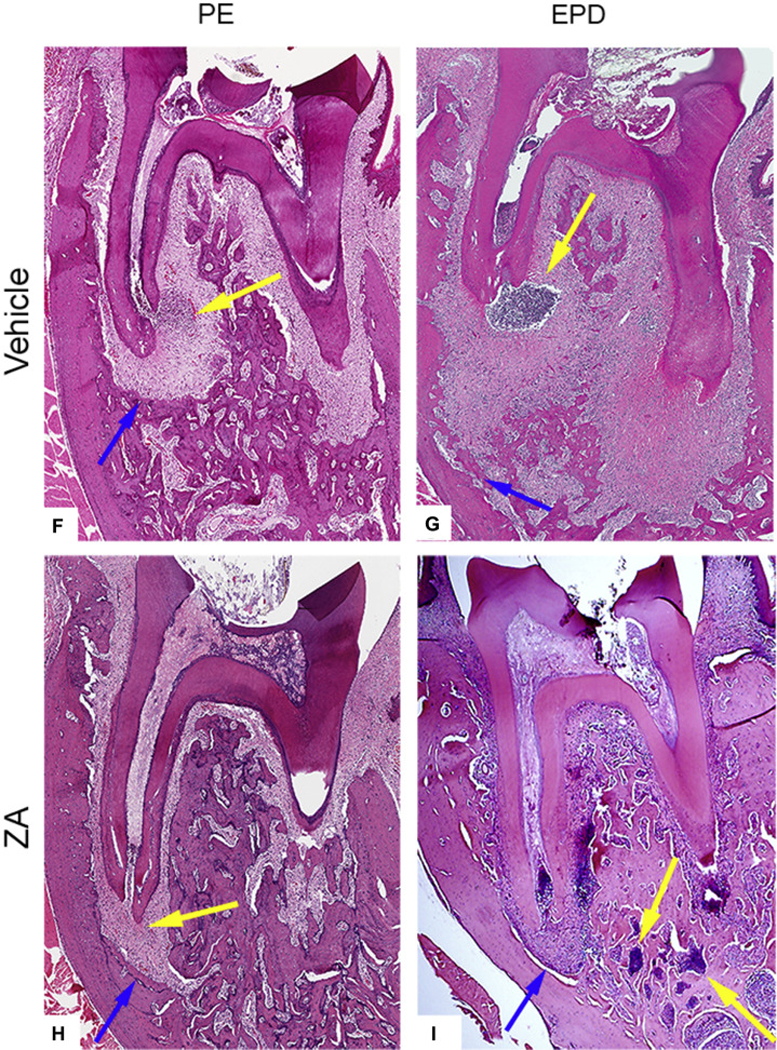
µCT assessment of vehicle treated animals in the absence (a, a1) and presence (b, b1) of bacterial inoculation (EPD), and of ZA treated animals in the absence (c, c1) and presence (d, d1) of bacterial inoculation (EPD). (e) Quantification of periapical bone loss. Data represents mean value ± SEM. **** statistical significance, p<0.0001, *** statistical significance, p<0.001, (n=10 per group). Histologic assessment of periapical disease in the absence (f, h) and presence (g, i) of bacterial inoculation (EPD) in vehicle and ZA treated animals, respectively. Blue arrows point to the extent of periapical bone loss. Yellow arrows point to areas of inflammatory infiltrate.
Experiment 3: Extraction of teeth with EPD
We then tested soft tissue and osseous healing after extraction of healthy or EPD teeth. Visual examination revealed mucosal defects in 5% (1/20) of Veh animals with extraction of healthy teeth, 0% (0/16) of Veh animals with extraction of molars with EPD, and 12% (2/17) of ZA animals with extraction of healthy teeth (Fig. 3A, B, C, white arrows and Fig. 3E). In contrast, 62.5% (15/24) of ZA treated animals with extraction of teeth with EPD showed mucosal defects and clinical bone exposure (Fig. 3D, red arrow and Fig. 3E).
Fig. 3. Clinical assessment of socket healing following extraction of teeth with EPD.
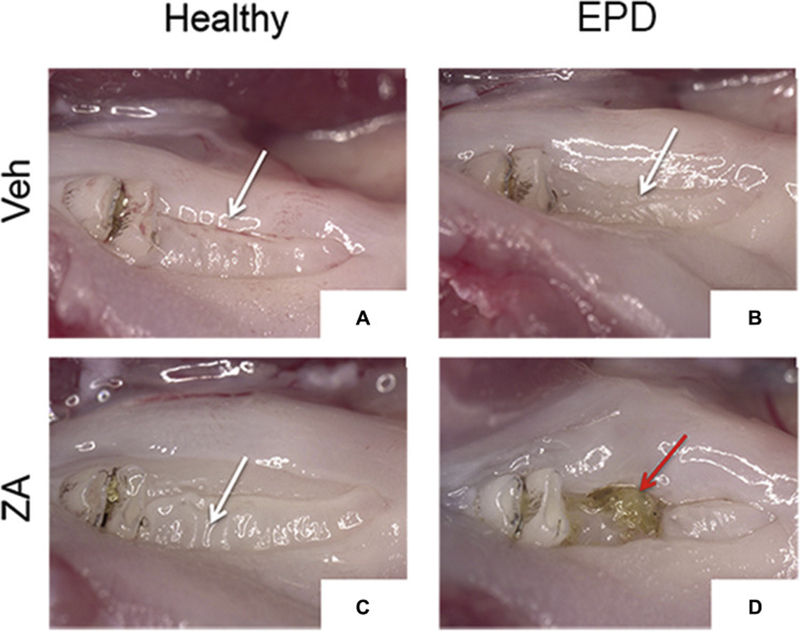
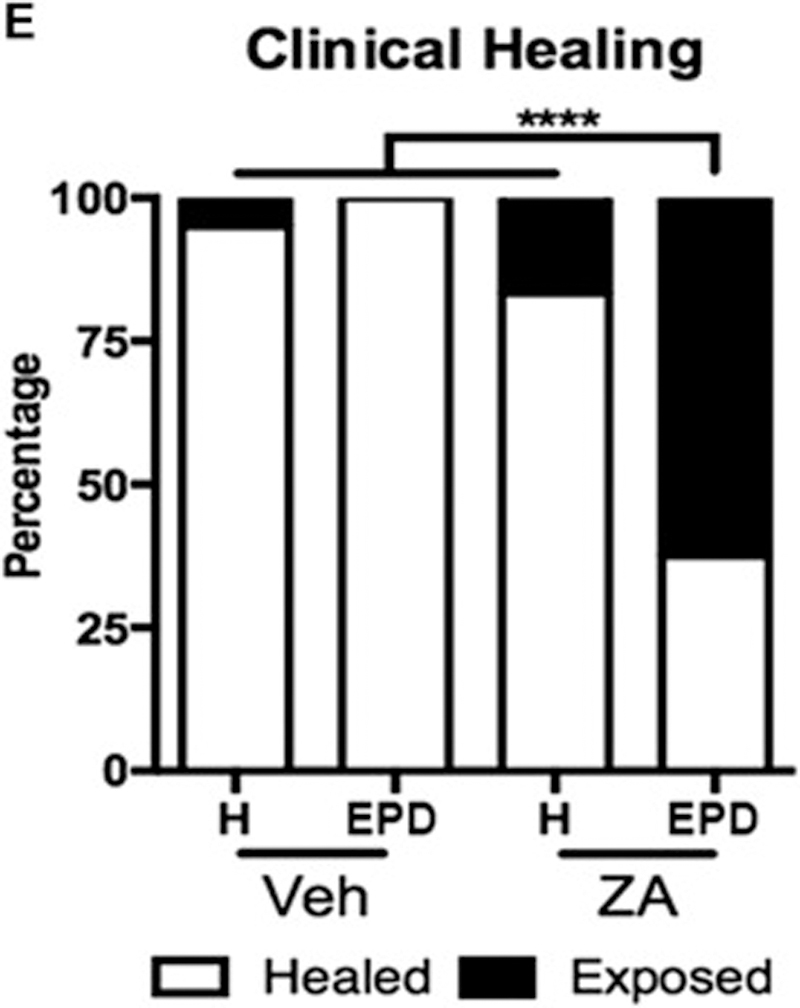
Clinical images of extraction socket healing in vehicle treated animals with the extraction of healthy teeth (a) or teeth with EPD (b), and ZA treated animals with the extraction of healthy teeth (c) and teeth with EPD (d). White arrows (a, b, c) point to healed extraction sockets. Red arrow points to an unhealed extraction socket with bone exposure (d). (e) Quantification of the percentage of animals with exposed bone or full healing. **** statistical significance, p<0.0001 (n=16–24 per group).
Radiographic assessment of the extraction sockets of Veh treated animals showed complete healing with remodeling of the socket outline (Fig. 4A-A1, B-B1, blue arrows). Most ZA treated animals with extraction of healthy teeth also showed socket with dense, woven bone, and a clear demarcation of the socket outline (Fig. 4C-C1, yellow arrows). In contrast, ZA treated animals with extraction of teeth with EPD presented partial or complete absence of osseous socket healing (Fig. 4D-D1, red arrows) and periosteal bone formation along the lingual or buccal cortices (Fig. 4D1, white arrow). Qualitative assessment showed complete osseous socket healing in the majority of Veh treated animals, regardless of treatment, as well as ZA treated animals with extraction of healthy teeth (Fig. 4E). In contrast, 50% of the ZA treated animals with extraction of teeth with EPD showed absence of osseous healing, while 20% of animals demonstrated partial healing (Fig. 4E). Quantification of bone formation in the sockets showed statistically significant decrease in BV/TV in the ZA-EPD animals compared to all other groups (Fig. 4F).
Fig. 4. Radiographic examination of extraction sockets.
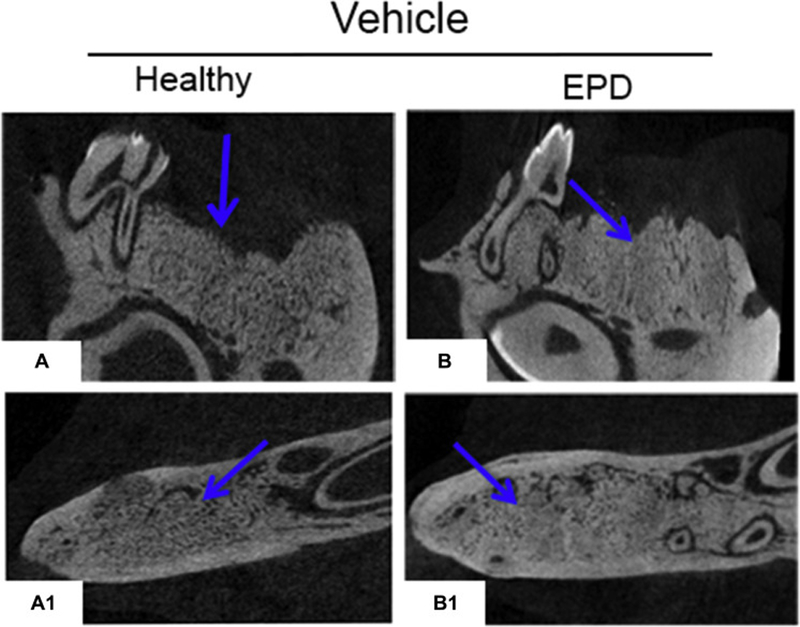
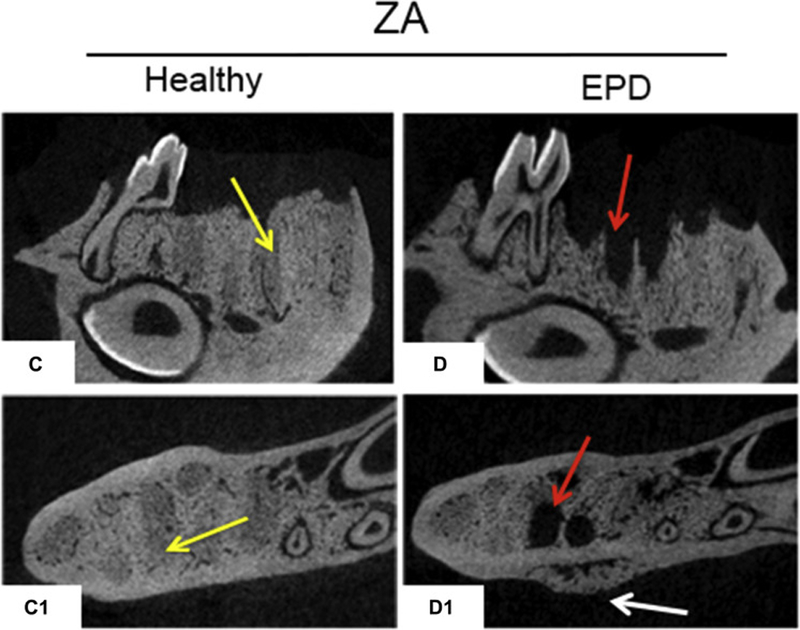
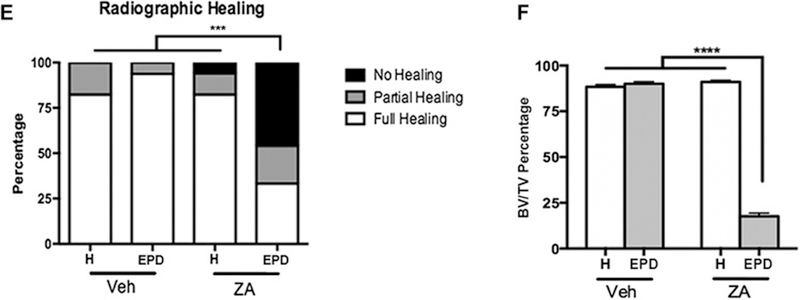
Sagittal and axial cross sections of µCT scans of vehicle treated animals with extraction of healthy teeth (a, a1) and EPD (b, b1). The blue arrows denote woven bone formation. Sagittal and axial cross sections of ZA treated animals with extraction of healthy teeth (c, c1) and EPD (d, d1). Yellow arrows point to areas of woven bone formation, demarcated from the outline of the extraction sockets. Red arrows point to empty extractions sockets. White arrow points to periosteal bone formation on the lingual cortex. (e) Quantification of radiographic healing. (f) Quantification of bone volume/tissue volume (BV/TV). Data represents mean value ± SEM. **** statistical significance, p<0.0001 (n=16–24 per group), *** statistical significance, p<0.001.
Histologic assessment of extraction sockets from Veh treated animals with extraction of healthy or diseased teeth showed mucosal healing with normal keratinized epithelium and submucosa, and absence of inflammatory infiltrate. The extraction sockets were filled with woven bone with reversal lines (Fig. 5A, B, white arrows); osteonecrosis was not seen. ZA treated animals with extraction of healthy teeth also showed normal epithelial and submucosal lining over the extraction sockets (Fig. 5C). Sockets healed with woven bone and reversal lines, but limited remodeling of the socket outlines (Fig. 5C, white arrows), and areas of osteonecrosis were present (Fig. 5C1, blue arrows). In contrast, ZA treated animals with extraction of teeth with EPD demonstrated mucosal healing defects, with debris accumulation and epithelial migration extending to the underlying bone. Incomplete socket healing and remodeling, presence of osteonecrosis and sequestration of necrotic bone were also present (Fig. 5D-D1).
Fig. 5. Histologic evaluation of extraction sockets following extraction.
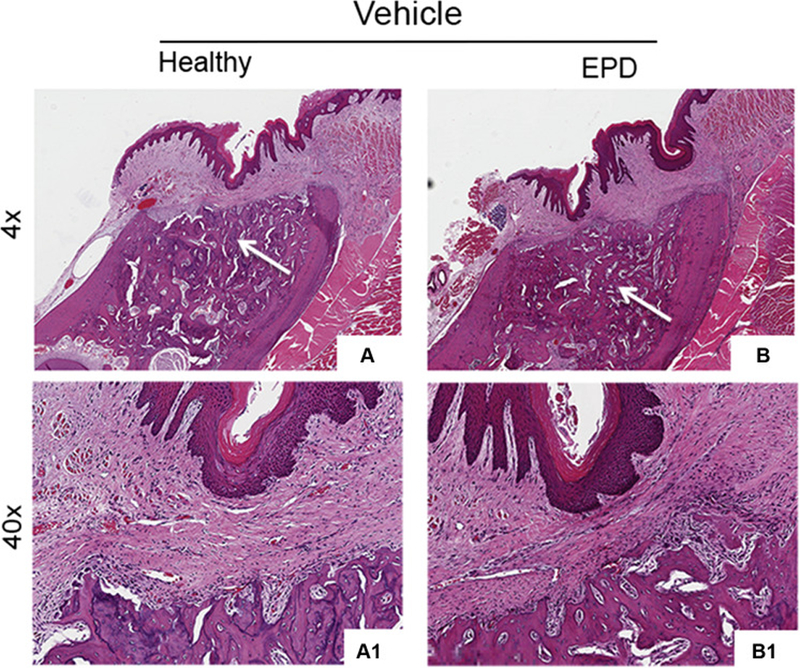
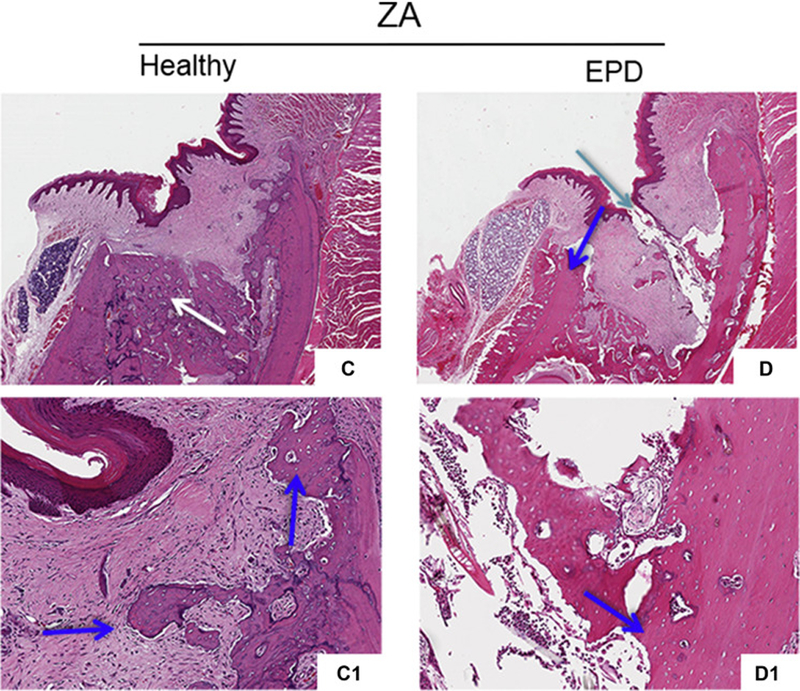
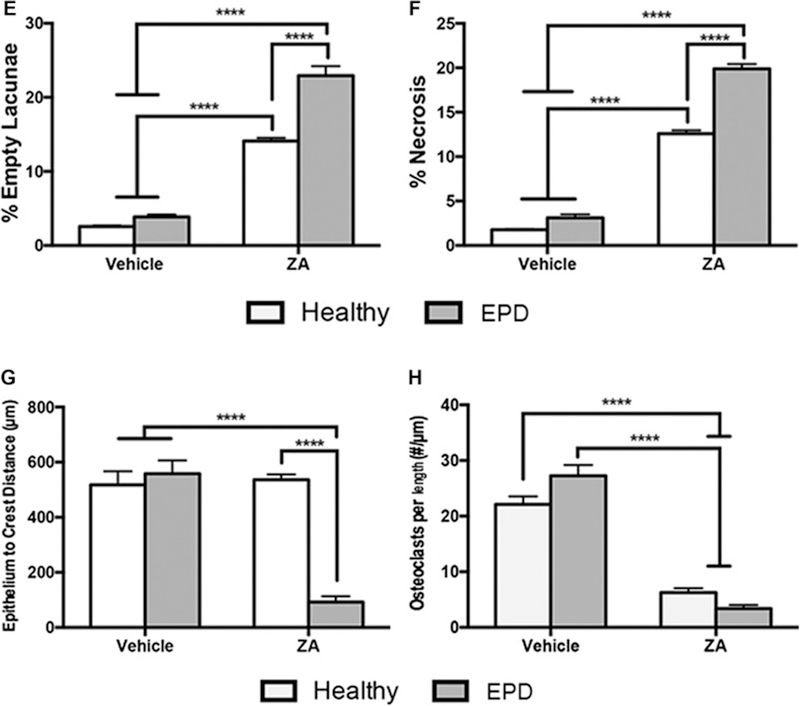
Representative H&E sections of extraction sockets in vehicle treated animals with extraction of healthy teeth (a, a1) and teeth with EPD (b, b1). White arrows point to areas of woven bone formation. H&E sections of extraction sockets in ZA treated animals with extraction of healthy teeth (c, c1) and teeth with EPD (d, d1). Blue arrows point to areas of osteonecrosis. Cyan arrow points to mucosal defect, debris and bone exposure. Quantification of (e) percent empty osteocytic lacunae, (f) percent osteonecrosis, (g) epithelium to crest distance, and (h) osteoclasts/length. Data represents mean value ± SEM. **** statistical significance, p<0.0001 (n=15 per group).
Quantification of histologic findings confirmed the qualitative assessment. ZA treated animals demonstrated a higher incidence of empty osteocytic lacunae and area of osteonecrosis compared to the Veh treated animals. Moreover, in ZA treated animals, extraction of teeth with EPD resulted in higher levels of empty osteocytic lacunae and osteonecrotic area (Fig. 5E, F). ZA treated animals with extraction of teeth with EPD demonstrated decreased distance between the basal layer of the epithelium and the alveolar crest compared both to Veh treated animals, and ZA treated animals with extraction of healthy teeth (Fig. 5G). Finally, ZA treatment decreased the number of osteoclasts compared to the Veh treated animals (Fig. 5H).
Gram staining revealed general absence of bacteria within the submucosa or alveolar bone in Veh animals and ZA animals with extraction of healthy teeth (Fig. 6A-A1, B-B1, C-C1). In contrast, in ZA treated animals with extraction of teeth with EPD, bacteria were present around osteonecrotic areas and deeper within the alveolar bone (Fig. 6D-D2). Quantification demonstrated a significantly higher bacterial number in the ZA-EPD rats compared to all other groups (Fig. 6E).
Fig. 6. Gram staining of extraction sockets.
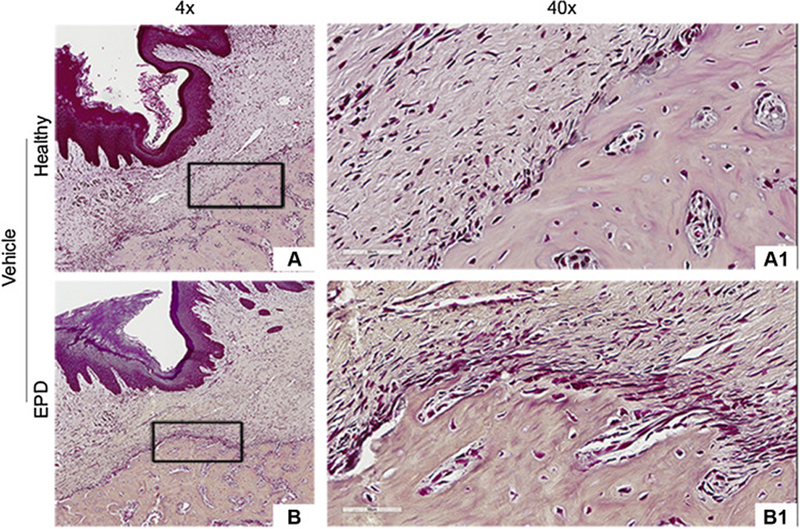
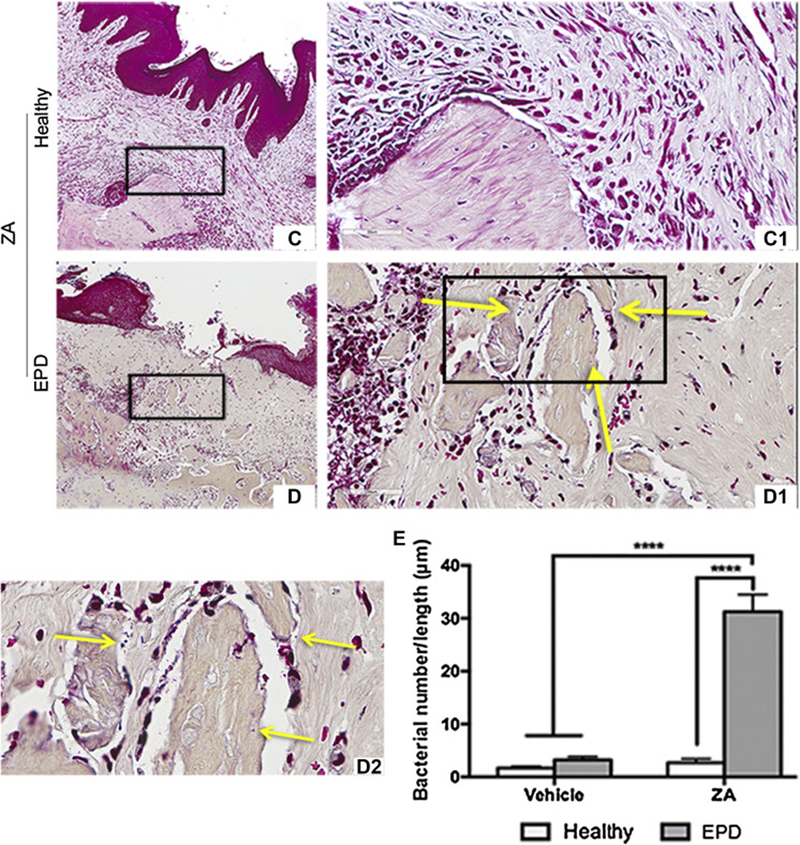
Representative gram staining of extraction sockets in vehicle treated animals with extraction of healthy teeth (a, a1) and teeth with EPD (b, b1). Representative sections of extraction sockets in ZA treated animals with extraction of healthy (c, c1) and EPD teeth (d, d1). (d2) 100x magnification of the boxed area shows visible bacteria, denoted by the yellow arrows, seen around an osteonecrotic area. (e) Quantification of bacterial colonies per length. Data represents mean value ± SEM. **** statistical significance, p<0.0001 (n=8–10 per group).
DISCUSSION
Animal models, reported by us and others, have demonstrated an association between infectious dental disease and MRONJ [10, 14, 24, 26, 28, 29]. These models utilize experimental periapical/periodontal disease or spontaneously occurring peri-radicular disease combined with antiresorptives to characterize MRONJ lesions around affected teeth. Other investigators have followed a different approach to induce MRONJ lesions, by extracting healthy molars in animals treated with antiresorptives [19, 30, 31]. Here, we combined the two, and utilized extraction of teeth with experimental periapical disease to observe the occurrence of MRONJ lesions in rats treated with high-dose ZA. We observed that extraction of teeth with EPD resulted in clinical, radiographic and histologic features of MRONJ; extraction of healthy or teeth with PE in ZA treated animals or in any Veh treated animals did not result in MRONJ lesions.
We made similar observations with spontaneous peri-radicular disease, reporting that only extraction of diseased teeth caused MRONJ lesions in mice treated with high-dose ZA [26]. Our current studies in rats, however, offer several advantages, compared to our prior mouse study. First, EPD is amenable to experimental manipulation in relation to ZA treatment and disease duration; the existence and timing of spontaneous peri-radicular cannot be controlled. Furthermore, periapical disease is a well-established model that has been extensively used to investigate various facets of apical periodontitis, including microbial infection, immune responses, and host modulation of disease [32–34]. In contrast, it is unclear if spontaneous peri-radicular disease, despite its pathologic radiographic and histologic features, parallels human disease. Finally, a larger animal model, with a more accessible oral cavity, makes intervention less technically challenging, allowing for more consistent and controlled disease development. In preliminary studies, extraction of teeth with drilled crowns in mice resulted in frequent tooth fractures and retained root fragments due to the compromised crown integrity (unpublished data). Using rats instead of mice decreased the occurrence of such procedural difficulties. Furthermore, we excluded animals with retained root fragments from analysis, as they compromise socket healing.
Interestingly, we also reported defective socket healing after extraction of teeth with experimental periodontal disease in rats treated with ZA [27]. While we observed poorly formed collagen fibers, increased levels of alpha-SMA, MMP-9, and MMP-13, all indicative of impaired healing, a great majority of animals presented without mucosal defects. Similarly, following extraction of teeth with pulp exposure in mice under antiresorptive treatment, no clinical bone exposure is noted despite the presence of osteonecrotic areas [29]. Here, we made similar observations, after crown drilling and subsequent pulp exposure to the oral environment. However, we were only able to consistently induce clinical bone exposure after extraction of teeth with EPD, which were inoculated with species involved in periapical pathogenesis [35–37].
These observations place great importance on the degree of inflammation in MRONJ pathogenesis. Indeed, µCT and histologic analysis confirmed this, reflected by an increase in bone loss and prominent inflammatory presence into the periodontal tissues in inoculated animals. Interestingly, ZA similarly inhibited periapical bone loss in the absence or presence of bacterial inoculation. However, when inoculated, inflammatory infiltrate within the marrow spaces of the periapical bone was noted. Subsequent extraction of inoculated teeth led to development of MRONJ lesions with soft tissue defects. In other studies, these mucosal defects are rare, and increase in prevalence with systemic therapy, such as immunosuppression [31]. Similarly, the presence of MRONJ lesions following tooth extraction in ZA treated mice is infrequent, and becomes apparent only when animals are treated with a chemotherapeutic agent [38].
Here, only animals with extraction of teeth with EPD displayed clinical bone exposure. Importantly, bacterial presence was seen around osteonecrotic areas of unhealed extraction sockets. Bacteria were noted not only on the exposed surface, but within the necrotic bone. Our observations parallel human findings that report the existence of bone infection (osteomyelitis) prior to tooth extraction in the majority of patients on nitrogen-containing bisphosphonates that subsequently developed MRONJ [39], as well as colonization of necrotic exposed bone by a variety of bacterial morphotypes [35, 40–42]. Indeed, others have also used bacteria to induce MRONJ lesions in mice. However, in these experiments, healthy teeth were extracted, and bacteria were added in the sockets after extraction [43]. Our data, combined with such studies, demonstrate the significance of bacterial infection and associated inflammation in MRONJ pathogenesis.
Despite the resemblance to human disease, limitations of this model should be addressed. The young age of animals used in this study may affect wound healing following tooth extraction, as MRONJ largely presents in a more elderly population [44]. Here, we utilized 200 µg/kg ZA twice weekly through the experiment, to induce MRONJ lesions. Indeed, this is a higher dose than clinically prescribed for malignancies; however, this increases disease prevalence, allowing for a smaller sample size to investigate disease development. Finally, rodents, although a powerful tool, have variances in bone remodeling, when compared to humans [45].
In summary, we present a model that captures the human MRONJ clinical scenario, and provides an experimental design that can be manipulated and modified in detail to allow the dissection of parameters involved in the process of MRONJ pathogenesis. The bacterial load and types, time and dose of antiresorptives, time of experimental periapical disease, potential antibiotic treatment, endodontic treatment, and time of extraction are variables that can be easily adjusted to explore clinical outcomes. Supported by studies showing minimal MRONJ lesions in a healthy oral environment, we can begin to develop targeted therapies to prevent and treat this condition.
ACKNOWLEDGEMENTS
This work was supported by grant support from the NIH/NIDCR DE019465 (ST). DH was supported by T90/R90 DE007296 and F30 DE028171. Dr. Tetradis has received grant support from Amgen, Inc. Dr. Tetradis and Dr. Aghaloo are paid consultants for Amgen, Inc.
We gratefully thank the Translational Pathology Core Laboratory (TPCL) at the David Geffen School of Medicine at UCLA for all histology and digital imaging services provided.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All other authors state no conflict.
REFERENCES
- 1.Chen JS, Sambrook PN: Antiresorptive therapies for osteoporosis: a clinical overview. Nat Rev Endocrinol 8:81, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Wong MH, Stockler MR, Pavlakis N: Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev CD003474, 2012 [DOI] [PubMed]
- 3.Ruggiero SL, Dodson TB, Fantasia J, Goodday R, Aghaloo T, Mehrotra B, O’Ryan F, American Association of O, Maxillofacial S: American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw--2014 update. J Oral Maxillofac Surg 72:1938, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Graziani F, Vescovi P, Campisi G, Favia G, Gabriele M, Gaeta GM, Gennai S, Goia F, Miccoli M, Peluso F, Scoletta M, Solazzo L, Colella G: Resective surgical approach shows a high performance in the management of advanced cases of bisphosphonate-related osteonecrosis of the jaws: a retrospective survey of 347 cases. J Oral Maxillofac Surg 70:2501, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Chrysanthakopoulos NA: Reasons for extraction of permanent teeth in Greece: a five-year follow-up study. Int Dent J 61:19, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan AA, Morrison A, Hanley DA, Felsenberg D, McCauley LK, O’Ryan F, Reid IR, Ruggiero SL, Taguchi A, Tetradis S, Watts NB, Brandi ML, Peters E, Guise T, Eastell R, Cheung AM, Morin SN, Masri B, Cooper C, Morgan SL, Obermayer-Pietsch B, Langdahl BL, Al Dabagh R, Davison KS, Kendler DL, Sandor GK, Josse RG, Bhandari M, El Rabbany M, Pierroz DD, Sulimani R, Saunders DP, Brown JP, Compston J, International Task Force on Osteonecrosis of the J: Diagnosis and management of osteonecrosis of the jaw: a systematic review and international consensus. J Bone Miner Res 30:3, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Capocci M, Romeo U, Guerra F, Mannocci A, Tenore G, Annibali S, Ottolenghi L: Medication-related osteonecrosis of the jaws (MRONJ) and quality of life evaluation: a pilot study. Clin Ter 168:e253, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Marx RE: Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg 61:1115, 2003 [DOI] [PubMed] [Google Scholar]
- 9.Lesclous P, Abi Najm S, Carrel JP, Baroukh B, Lombardi T, Willi JP, Rizzoli R, Saffar JL, Samson J: Bisphosphonate-associated osteonecrosis of the jaw: a key role of inflammation? Bone 45:843, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Kang B, Cheong S, Chaichanasakul T, Bezouglaia O, Atti E, Dry SM, Pirih FQ, Aghaloo TL, Tetradis S: Periapical disease and bisphosphonates induce osteonecrosis of the jaws in mice. J Bone Miner Res 28:1631, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguirre JI, Akhter MP, Kimmel DB, Pingel JE, Williams A, Jorgensen M, Kesavalu L, Wronski TJ: Oncologic doses of zoledronic acid induce osteonecrosis of the jaw-like lesions in rice rats (Oryzomys palustris) with periodontitis. J Bone Miner Res 27:2130, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid IR, Bolland MJ, Grey AB: Is bisphosphonate-associated osteonecrosis of the jaw caused by soft tissue toxicity? Bone 41:318, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Williams DW, Lee C, Kim T, Yagita H, Wu H, Park S, Yang P, Liu H, Shi S, Shin KH, Kang MK, Park NH, Kim RH: Impaired bone resorption and woven bone formation are associated with development of osteonecrosis of the jaw-like lesions by bisphosphonate and anti-receptor activator of NF-kappaB ligand antibody in mice. Am J Pathol 184:3084, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Molon RS, Cheong S, Bezouglaia O, Dry SM, Pirih F, Cirelli JA, Aghaloo TL, Tetradis S: Spontaneous osteonecrosis of the jaws in the maxilla of mice on antiresorptive treatment: a novel ONJ mouse model. Bone 68:11, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi Y, Hiraga T, Ueda A, Wang L, Matsumoto-Nakano M, Hata K, Yatani H, Yoneda T : Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Miner Metab 28:165, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Kuroshima S, Sasaki M, Nakajima K, Tamaki S, Hayano H, Sawase T: Transplantation of Noncultured Stromal Vascular Fraction Cells of Adipose Tissue Ameliorates Osteonecrosis of the Jaw-Like Lesions in Mice. J Bone Miner Res 33:154, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Kikuiri T, Kim I, Yamaza T, Akiyama K, Zhang Q, Li Y, Chen C, Chen W, Wang S, Le AD, Shi S: Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J Bone Miner Res 25:1668, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hokugo A, Christensen R, Chung EM, Sung EC, Felsenfeld AL, Sayre JW, Garrett N, Adams JS, Nishimura I: Increased prevalence of bisphosphonate-related osteonecrosis of the jaw with vitamin D deficiency in rats. J Bone Miner Res 25:1337, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sonis ST, Watkins BA, Lyng GD, Lerman MA, Anderson KC: Bony changes in the jaws of rats treated with zoledronic acid and dexamethasone before dental extractions mimic bisphosphonate-related osteonecrosis in cancer patients. Oral Oncol 45:164, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Zhang Q, Yu W, Lee S, Xu Q, Naji A, Le AD: Bisphosphonate Induces Osteonecrosis of the Jaw in Diabetic Mice via NLRP3/Caspase-1-Dependent IL-1beta Mechanism. J Bone Miner Res 30:2300, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howie RN, Borke JL, Kurago Z, Daoudi A, Cray J, Zakhary IE, Brown TL, Raley JN, Tran LT, Messer R, Medani F, Elsalanty ME: A Model for Osteonecrosis of the Jaw with Zoledronate Treatment following Repeated Major Trauma. PLoS One 10:e0132520, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marino KL, Zakhary I, Abdelsayed RA, Carter JA, O’Neill JC, Khashaba RM, Elsalanty M, Stevens MR, Borke JL: Development of a rat model of bisphosphonate-related osteonecrosis of the jaw (BRONJ). J Oral Implantol 38 Spec No:511, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Biasotto M, Chiandussi S, Zacchigna S, Moimas S, Dore F, Pozzato G, Cavalli F, Zanconati F, Contardo L, Giacca M, Di Lenarda R: A novel animal model to study non-spontaneous bisphosphonates osteonecrosis of jaw. J Oral Pathol Med 39:390, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Aghaloo TL, Cheong S, Bezouglaia O, Kostenuik P, Atti E, Dry SM, Pirih FQ, Tetradis S: RANKL inhibitors induce osteonecrosis of the jaw in mice with periapical disease. J Bone Miner Res 29:843, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aghaloo TL, Kang B, Sung EC, Shoff M, Ronconi M, Gotcher JE, Bezouglaia O, Dry SM, Tetradis S: Periodontal disease and bisphosphonates induce osteonecrosis of the jaws in the rat. J Bone Miner Res 26:1871, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soundia A, Hadaya D, Esfandi N, de Molon RS, Bezouglaia O, Dry SM, Pirih FQ, Aghaloo T, Tetradis S: Osteonecrosis of the jaws (ONJ) in mice after extraction of teeth with periradicular disease. Bone 90:133, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soundia A, Hadaya D, Esfandi N, Gkouveris I, Christensen R, Dry SM, Bezouglaia O, Pirih F, Nikitakis N, Aghaloo T, Tetradis S: Zoledronate Impairs Socket Healing after Extraction of Teeth with Experimental Periodontitis. J Dent Res 22034517732770, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Molon RS, Shimamoto H, Bezouglaia O, Pirih FQ, Dry SM, Kostenuik P, Boyce RW, Dwyer D, Aghaloo TL, Tetradis S: OPG-Fc but Not Zoledronic Acid Discontinuation Reverses Osteonecrosis of the Jaws (ONJ) in Mice. J Bone Miner Res 30:1627, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song M, Alshaikh A, Kim T, Kim S, Dang M, Mehrazarin S, Shin KH, Kang M, Park NH, Kim RH: Preexisting Periapical Inflammatory Condition Exacerbates Tooth Extraction-induced Bisphosphonate-related Osteonecrosis of the Jaw Lesions in Mice. J Endod 42:1641, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S, Williams DW, Lee C, Kim T, Arai A, Shi S, Li X, Shin KH, Kang MK, Park NH, Kim RH: IL-36 Induces Bisphosphonate-Related Osteonecrosis of the Jaw-Like Lesions in Mice by Inhibiting TGF-beta-Mediated Collagen Expression. J Bone Miner Res 32:309, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bi Y, Gao Y, Ehirchiou D, Cao C, Kikuiri T, Le A, Shi S, Zhang L: Bisphosphonates cause osteonecrosis of the jaw-like disease in mice. Am J Pathol 177:280, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stashenko P, Wang CY, Tani-Ishii N, Yu SM: Pathogenesis of induced rat periapical lesions. Oral Surg Oral Med Oral Pathol 78:494, 1994 [DOI] [PubMed] [Google Scholar]
- 33.Stashenko P, Yu SM: T helper and T suppressor cell reversal during the development of induced rat periapical lesions. J Dent Res 68:830, 1989 [DOI] [PubMed] [Google Scholar]
- 34.Tani-Ishii N, Wang CY, Tanner A, Stashenko P: Changes in root canal microbiota during the development of rat periapical lesions. Oral Microbiol Immunol 9:129, 1994 [DOI] [PubMed] [Google Scholar]
- 35.Wei X, Pushalkar S, Estilo C, Wong C, Farooki A, Fornier M, Bohle G, Huryn J, Li Y, Doty S, Saxena D : Molecular profiling of oral microbiota in jawbone samples of bisphosphonate-related osteonecrosis of the jaw. Oral Dis 18:602, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siqueira JF Jr., Rocas IN, Souto R, de Uzeda M, Colombo AP: Actinomyces species, streptococci, and Enterococcus faecalis in primary root canal infections. J Endod 28:168, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Narayanan LL, Vaishnavi C: Endodontic microbiology. J Conserv Dent 13:233, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuroshima S, Yamashita J: Chemotherapeutic and antiresorptive combination therapy suppressed lymphangiogenesis and induced osteonecrosis of the jaw-like lesions in mice. Bone 56:101, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Saia G, Blandamura S, Bettini G, Tronchet A, Totola A, Bedogni G, Ferronato G, Nocini PF, Bedogni A: Occurrence of bisphosphonate-related osteonecrosis of the jaw after surgical tooth extraction. J Oral Maxillofac Surg 68:797, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Schipmann S, Metzler P, Rossle M, Zemann W, von Jackowski J, Obwegeser JA, Gratz KW, Jacobsen C: Osteopathology associated with bone resorption inhibitors - which role does Actinomyces play? A presentation of 51 cases with systematic review of the literature. Journal of Oral Pathology & Medicine 42:587, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Sedghizadeh PP, Yooseph S, Fadrosh DW, Zeigler-Allen L, Thiagarajan M, Salek H, Farahnik F, Williamson SJ: Metagenomic investigation of microbes and viruses in patients with jaw osteonecrosis associated with bisphosphonate therapy. Oral Surg Oral Med Oral Pathol Oral Radiol 114:764, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Bruyn L, Coropciuc R, Coucke W, Politis C: Microbial population changes in patients with medication-related osteonecrosis of the jaw treated with systemic antibiotics. Oral Surg Oral Med Oral Pathol Oral Radiol, 2017 [DOI] [PubMed]
- 43.Mawardi H, Giro G, Kajiya M, Ohta K, Almazrooa S, Alshwaimi E, Woo SB, Nishimura I, Kawai T: A role of oral bacteria in bisphosphonate-induced osteonecrosis of the jaw. J Dent Res 90:1339, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gosain A, DiPietro LA: Aging and wound healing. World J Surg 28:321, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Frost HM, Jee WS: On the rat model of human osteopenias and osteoporoses. Bone Miner 18:227, 1992 [DOI] [PubMed] [Google Scholar]


