Abstract
The addition of charged polymers, like poly-aspartic acid (pAsp), to mineralizing solutions allows for transport of calcium and phosphate ions into the lumen of collagen fibrils and subsequent crystallization of oriented apatite crystals by the so-called Polymer-Induced Liquid Precursor (PILP) mineralization process, leading to the functional recovery of artificial dentin lesions by intrafibrillar mineralization of collagen.
Objective:
To evaluate the feasibility of applying the PILP method as part of a restorative treatment and test for effectiveness to functionally remineralize artificial lesions in dentin.
Materials and Methods:
Two methods of providing pAsp to standardized artificial lesions during a restorative procedure were applied: A) pAsp was mixed into commercial RMGI (resin modified glass ionomer) cement formulations and B) pAsp was added at high concentration (25mg/ml) in solution to rehydrate lesions before restoring with a RMGI cement). All specimens were immersed in simulated body fluid for two weeks to allow for remineralization and then analyzed for dehydration shrinkage, integrity of cement-dentin interface, degree of mineralization, and changes in the nanomechanical profile (E-modulus) across the lesion.
Results:
After the remineralization treatment, lesion shrinkage was significantly reduced for all treatment groups compared to demineralized samples. Pores developed in RMGI when pAsp was added. A thin layer at the dentin-cement interface, rich in polymer formed possibly from a reaction between pAsp and the RMGI. When analyzed by SEM under vacuum, most lesions delaminated from the cement interface. EDS-analysis showed some but not full recovery of calcium and phosphorous levels for treatment groups that involved pAsp. Nanoindentations placed across the interface indicated improvement for RMGI containing 40% pAsp, and were significantly elevated when lesions were rehydrated with pAsp before being restored with RMGI. In particular the most demineralized outer zone recovered substantially in the elastic modulus, suggesting that functional remineralization has been initiated by pAsp delivery upon rehydration of air-dried demineralized dentin. In contrast, the effectiveness of the RMGI on functional remineralization of dentin was minimal when pAsp was absent.
Conclusions:
Incorporation of pAsp into restorative treatments using RMGIs promises to be a feasible way to induce the PILP-mineralization process in a clinical setting and to repair the structure and properties of dentin damaged by the caries process.
Keywords: Bioactive cement, remineralization, glass-ionomer, minimally invasive dentistry, caries, dentin
Introduction
The last decade has seen a shift in the philosophy of dental care towards prevention and minimally-invasive restorative approaches with promoting remineralization as a key element of dental treatments [1, 2]. Remineralization of enamel, which is comprised of 95wt% mineral, is an accepted phenomenon with established mechanisms [3-5]. Recent data from in vitro studies suggests that dentin remineralization is also possible but requires different approaches due to the presence of an organic matrix, e.g. collagen fibrils[6-10]. Dentin is a collagenous tissue, similar to bone, and requires apatite mineral to form both inside and outside collagen fibrils for mechanical reinforcement [11, 12]. The PILP (Polymer-Induced Liquid Precursor) method relies on the ability of highly charged macromolecules (e.g. pAsp) to stabilize calcium phosphate ions in saturated solutions, preventing spontaneous nucleation and mineral precipitation [13-15] . Instead stable nano-droplets (10-20 nm) form, comprised of a concentrated ion cloud of calcium and phosphate surrounding the charged macromolecule [7, 16-18]. These nanodroplets are considered the liquid precursor to mineral and are able to unload the ion content into the lumen of collagen fibrils, forming an amorphous calcium phosphate (ACP). Over a period of days, ACP transforms into aligned apatite crystallites oriented similarly to the nanocrystals observed in mineralized tissues like bone, dentin and cementum [19].
The fact that collagen fibrils need to be reinforced from within by intrafibrillar mineralization has often been overlooked, and approaches that successfully induced apatite mineral in enamel may only have produced superficial or extrafibrillar mineral between collagen fibrils in dentin, and therefore did not mechanically reinforce the tissue [11, 12]. In contrast, several studies have shown that when localized demineralized dentin is immersed into remineralizing solutions of calcium and phosphate comprising a process-directing agent, e.g. pAsp, the nanomechanical properties of the remineralized dentin had functionally recovered [7, 8]. Elastic modulus and hardness of the fully demineralized outer zone recovered to about 60% of normal dentin values with PILP applications of 2 weeks, while calcium phosphate solutions alone did not produce any significant mechanical recuperation [9, 20]. The restoration of mechanical properties of the tissue is critical for regaining its function, and to date the PILP-approach appears to be the prime suitable method to reintroduce mineral into collagen fibrils and restore tissue properties, either partially or fully, thus we refer to this as functional remineralization (FR) [9, 20-22]. In addition studies on commercial silicate-based and alumo-silicate-based cements have shown promising data with regards to mineral recovery in artificial lesions but lacked evidence for a functional recovery [6, 10, 23].
Based on FR observed after PILP-solution treatments, the study described herein focused on developing PILP-releasing restorative procedures that can be utilized clinically. This novel treatment aims to be integrated within a conservative minimally restorative treatment facilitating long-term collagen FR and repair of the natural tissue while in place as a restoration.
Previous studies have applied standard PILP-treatments on artificial lesions by immersion of demineralized dentin cut from tooth specimens into a solution containing 4.5mM Ca2+, 2.2 mM PO43− and between 0.1 and 1mg/ml pAsp (27kDa or 200 mer, Alamanda Polymer Inc.) for several days and weeks [9, 20]. A pure solution-based approach is not feasible for application in clinical dentistry. Therefore, we are now investigating two novel methods to deliver the PILP mineralization as part of a restorative dental treatment.
In this study, we report on A) the design of glass ionomer cements that contain pAsp and B) the use of a highly-concentrated PILP-solution that is applied to air-dried artificial lesions in dentin. The ability of these systems to produce tissue reinforcement leading to FR of the collagenous matrix of dentin is evaluated.
Materials and Methods:
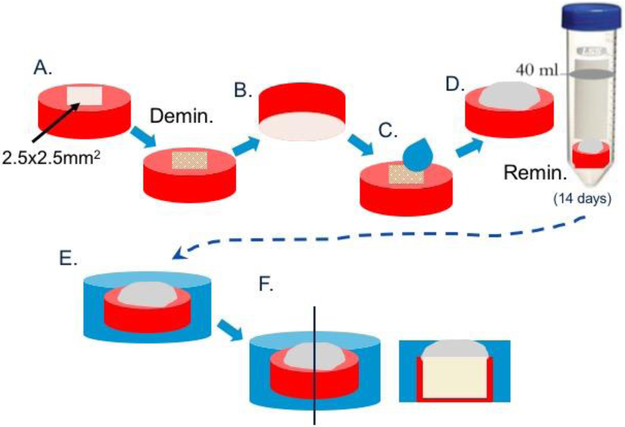
Permanent, fully-formed human molars were extracted as part of a clinical treatment plan and obtained from the UCSF dental hard tissue specimen core according to protocols approved by the UCSF Committee on Human Research. After extraction, the teeth were sterilized with gamma radiation and stored intact in de-ionized water at 4°C [24]. Dentin blocks measuring 3-4 mm in thickness were cut from the mid-coronal region of the selected teeth perpendicular to the tubule direction. A 9.5 mm core drill was used to remove the surrounding enamel and to obtain a uniform specimen of dentin, as depicted in the schematic of Figure 1. The occlusal surfaces were ground with SiC abrasive papers from 320 to 1200 grit, and then polished with aqueous diamond suspensions (Buehler, Lake Bluff, IL) of 6.0, 3.0, 1.0, and 0.25 μm particle sizes. Each specimen surface was covered with nail varnish (Revlon Nail Enamel #270, New York, NY) to prevent demineralization except for a window measuring approximately 2.5 × 2.5 mm2 (Fig.1).
Fig. 1: Specimen preparation.
Sections were cut from midcoronal dentin of third molars to about 3-4mm thickness, polished and subsequently drilled with core-drill to produce round disks of 6.5 mm diameter (A). Disks are coated with nail varnish leaving only a 2.5×2.5 mm2 window. Specimens is demineralized for 66h to create an artificial lesion (B). Nail varnish is removed from bottom side of each specimen and this side is etched with phosphoric acid gel for 30sec. Samples are then air-dried and rehydrated with deionized water or PILP-solution (rh25BC), before restored with Biocem RMGI or modified Biocem, BC20 or BC40 (C). Specimens are immersed into SBF-solution to allow for remineralization (D). Specimens are removed at 14 days, set in epoxy and stored dry (E). Specimens are sectioned through demineralized zone and cross section is polished in preparation for sample analysis (F).
Artificial carious lesions approximately 140 μm deep were induced by exposing the surface to a demineralizing solution consisting of 0.05 M acetate buffer containing 2.2 mM calcium and phosphate at pH 5.0 for 66 hours, a demineralization treatment determined by prior kinetics studies [22]. After artificial caries lesions were produced, the demineralized specimens were dried gently with compressed air for 15 sec and then rehydrated with about 20 μL of deionized water for 20 sec (except Group 4), before applying the RMGI (resin-modified glass ionomer) cement, without or with additions of pAsp, to cover exposed 2.5 × 2.5mm window (Fig. 1). The 4 groups tested include (n=3-5/group): 1.) BioCem (BC; NuSmile, Houston, TX, USA); 2.) BioCem + pAsp at 20 wt.% (BC20); 3.) BioCem + pAsp at 40 wt.% (BC40); and 4.) for which the rehydration solution was comprised of 25 mg/ml pAsp (8-12kD, Desai Chemicals Inc, China) and 4.5 mM Ca2+and 2.2 mM PO43− before restored with non-modified BioCem (rh25BC). In preparing BC20 and BC40, the BioCem tube, as received, was disassembled and the two components of the cement were weighed. Powder of pAsp was then added at 20 or 40% of that weight, respectively. Subsequently, all three components of the cement were mixed thoroughly until homogenous using a plastic spatula. The restorative material was placed onto lesion surface and light-cured for 30 seconds prior to proceeding to the next step.
Remineralization was performed by first mechanically removing nail varnish from the bottom side (opposite restorative material) of each specimen, then etching the side for 60 seconds with 37% phosphoric acid gel and rinsing with distilled water to ensure dentin tubules were free of smear layer (Fig. 1). Each sample was then placed in a 50 mL conical centrifuge tube with 40 mL of simulated body fluid (SBF) at pH 7.4. SBF was prepared according to the composition shown by Kokubo et al.11 Each group of specimens were incubated at 37 °C while being continually rocked for a period of 2 weeks. SBF infiltrates dentin through the tubules and collagen hydration and thus mimics in vivo pulpal fluid, which can reach to the dentin-cement interface.
After the remineralization period was completed, samples were removed, gently rinsed with de-ionized water and air dried. The blocks containing the lesions were embedded in room-temperature curing epoxy (Epoxicure, Buehler, Ltd, Lake Bluff, IL). Each specimen was sectioned through the 2.5 × 2.5 mm2 window. The cross section was ground with abrasive papers from 320 to 1200 grit, and then polished with aqueous diamond suspensions (Buehler, Lake Bluff, IL) of 1.0, and 0.25 μm particle size (Fig. 1).
Demineralized dentin as well as not fully mineralized dentin shrinks upon drying [25]. Therefore, to examine FR of dentin, cross sections were analyzed with reflective light microscopy using an Olympus BH-2 microscope (Olympus America Inc., San Diego, CA) to determine the amount of dentin collapsed versus recovery after the remineralization cycle. This has been shown to be a useful early indicator of effective FR [20]. The lesion depth, the distance between the original intact dentin surface protected by the nail varnish and exposed treated dentin, was measured using OMAX ToupView (Touptek Photonics, Zhejiang, China).
This data was compared to controls consisting of the artificial lesion group without remineralization (n = 3) and to normal untreated dentin from the area protected by nail varnish during demineralization. Statistical analysis was done by ANOVA with significance level P<0.05.
Subsequently, selected specimens were studied with a transducer-based nanoindenter (Hysitron Inc, Minneapolis, MN) attached to an atomic force microscope (MultiMode, Nanoscope IIIa Controller, Bruker-Nano Inc.) using a Berkovich diamond testing probe as previously described [9, 26, 27]. Nanoindentations were spaced by four micrometer (μm) along a line moving sagittal from the interface of dentin and cement towards sound dentin, covering the entire depth of the demineralized lesion of approximately 150 μm. Two lines of nanoindents were placed for each sample and three samples per group analyzed (n=3). The location of the first data point was adjusted according to the compression that was exerted onto the lesion by the placement of the cement. The size of compression was determined by optical microscopy of fully hydrated samples.
Selected specimens were also analyzed by SEM (Quanta, FEI, Thermo Fisher Sci, USA) with elemental analysis (EDS) operating at 20 KV (X-Max, Oxford Instruments, UK). Line profiles of elemental contents were recorded for C, Si, P and Ca at a working distance at about 10-12 mm. Samples were sputter-coated with Pd-Au to about 80 nm thickness prior to analysis.
Results
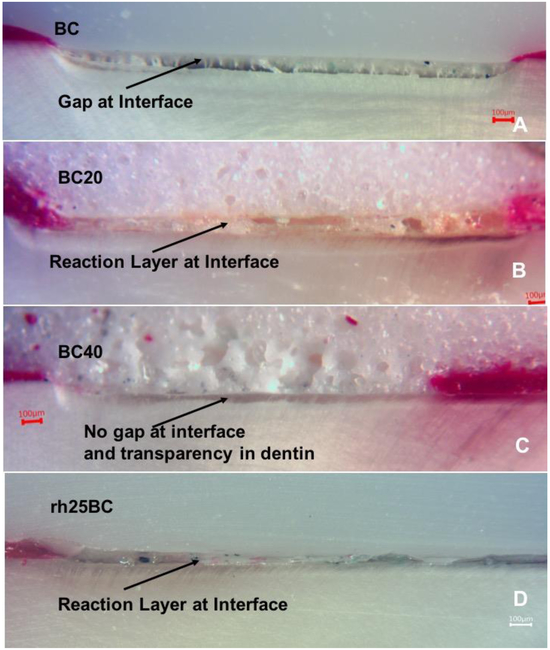
This study tested the applicability of a commercial RMGI cement, BioCem (BC), modified by the addition of pAsp to support remineralization of collagen and reinforcement of demineralized dentin, as well as the use of highly concentrated PILP-solution containing 25mg/ml applied during the rehydration step before RMGI cement application. The addition of pAsp to BC did not affect the setting behavior of the cement. BC20 and BC40 set upon lightcuring for 20 seconds. When exposed to SBF solutions the surface of the cements did not show visible signs of dissolution in the 2 weeks of remineralization treatment. Using light microscopy on polished specimens, the integrity of the cement-dentin interface and the occurrence of shrinkage of the lesion after the remineralization treatment was analyzed. While BC, as a resin-modified glass ionomer cement, largely lacks any porosity (Fig. 2A), both BC20 and BC40 showed a substantial amount of pores ranging from 1 to 20 μm in size (Figure 2B and 2C). BC, used as is, showed the largest gap developing between the cement and the lesion, which was due to a collapse of the demineralized portion of dentin upon drying.
Fig. 2: Optical micrographs.
Light microscopy of the cross-sections of specimens with restorative oriented on top. Specimens were restored with A) BC (BioCem control); B) BC20; C) BC40; D) rh25BC (rehydrated PILP and restored with BC), after 14 days of remineralization treatment. (A) Gap formation is evident in BC and associated with shrinkage of the artificial lesion upon dehydration. The gap is filled with a sticky layer, possibly polymer that was pulled from RMGI during dehydration. (B) Additions of pAsp to BC resulted in porosity of the cement. There is a small gap in the BC20, but only minor shrinkage occurred in this sample, as the cement appears to have reacted and changed the appearance of interface. The outer layer of the dentin lesion has become transparent. (C) Increased porosity in BC40 compared to BC20, but the dentin-cement interface appears intact, and the dentin surface is transparent. No shrinkage was apparent in this specimen. (D) When rehydrated with PILP-solution containing 25 mg/ml pAsp, little shrinkage was observed; instead, a wide reaction layer in the cement was observed. Erosion of the reaction layer during polishing may have led to gap formation.
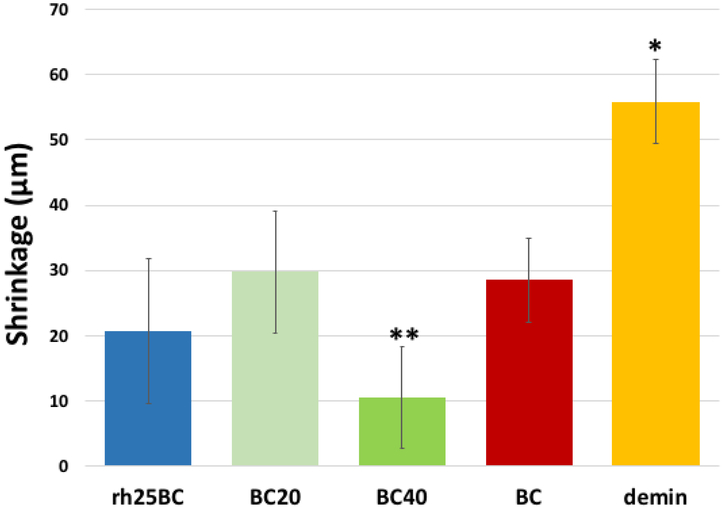
All treatment groups, BC, BC20, BC40 and rh25BC, had significantly reduced shrinkage after two weeks of remineralization when compared to demineralized dentin (Fig. 3). BC40 showed the lowest shrinkage which was significantly different from all other treatment groups (p<0.03). The shrinkage was reduced by about 50% for BC and BC20 treatments (P<0.01) Rehydration with concentrated PILP-solution or with BC40 reduced shrinkage to about 1/3 or 1/5of the original shrinkage before the remineralization treatment, respectively (P<0.001). Formation of a transparent zone became evident in all groups that involved pAsp additions, e.g. BC20, BC40 and rh25BC, indicating mineral formation inside dentin tubules [28].
Fig. 3: Dehydration shrinkage.
Plot of average shrinkage values of the demineralized zone when fully dehydrated after restorative treatment and 14 days of remineralization. All specimens showed reduced shrinkage compared to the demineralized specimens (*). In addition, shrinkage of BC40 (**) was significantly lower (ANOVA p<0.047) compared to all other treatment groups.
The dentin-cement interface in BC20, BC40 and rh25BC was intact in the majority of these specimens, and no gap formation was observed by light microscopy after dehydration of the samples (Fig. 2B-D). However, a layer of different consistency and texture became evident at the interface in most of these specimens and appeared to be related to the interaction of the SBF solution with the cement. These layers were between 15 and 30 μm thick, showed some swelling upon hydration and appeared to be soft in nature, indicating they are not likely mineral deposits that had formed at the interface during the remineralization process. The layers were less evident and thinner in BC-only specimens (Fig. 2A), and increased in thickness when pAsp was added either to BC or during the rehydration process. The layers were further investigated by SEM/EDS.
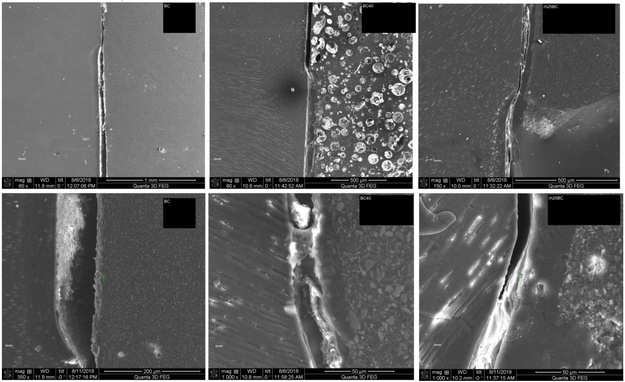
The high-vacuum of SEM created a more pronounced gap formation between the cement and demineralized dentin in specimens restored with BC only (Figs. 4A and B). The gap is associated with the dentin substrate further contracting due to dehydration in the vacuum and thus indicates that the lesion has not been fully functionally remineralized, resulting in delamination of the cement from the dentin lesion surface in BC40, and to a minor degree in rh25BC specimens (Figs. 4C to F), which was not observed upon dehydration in ambient air (Fig. 2). The interface with BC40 showed good adherence to the lesion surface (Fig. 4D). The cement, however, developed large circular pores of up to 50 μm diameter after addition of pAsp to BC (Fig. 4C). Two out of three samples showed the presence of the reaction layer (15-20 μm wide) at the interface with BC40 (Fig. 4D), and as observed by light microscopy (Fig. 2C). Specimens prepared by using PILP-solution for rehydration, rh25BC, showed a reaction layer in all three samples. There was some visible collapse of the demineralized zone in rh25BC underneath the reaction layer (Figs. 4E and F), but delamination of the cement from the dentin was minimal, suggesting that the specimens were at least partially remineralized at two weeks of treatment.
Fig. 4: SEM-analysis.
Micrographs of specimens restored with BC (A & B); BC40 (C & D); and rh25BC (D & E) after 2 weeks of remineralization. Gap formation due to shrinkage was evident in all specimens. The formation of a reaction layer was most obvious in rh25BC, clearly present in BC40, and barely detectable in BC.
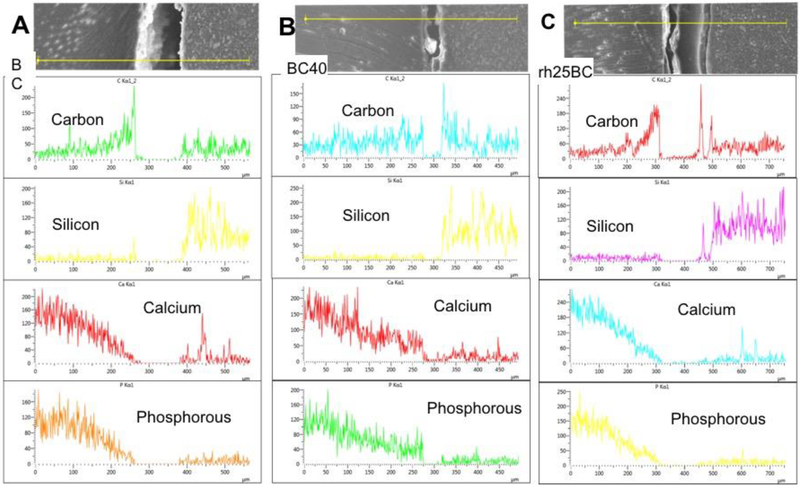
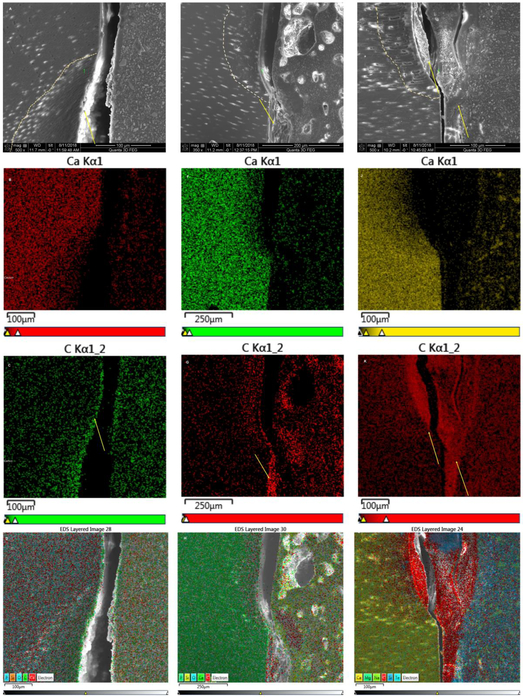
Using EDS-analysis, line profiles and elemental maps across the cement-dentin interface were generated for BC, BC40 and rh25BC (Figs. 5 and 6). All groups showed a gradual depletion of calcium and phosphorous from the lesion bottom towards the cement dentin interface, indicating that the remineralization process has not been fully completed in these specimens. The gradient associated with decreased mineral content varies between specimens and location and reaches between 100 and 150 um depth in all three groups studied. While difficult to quantify EDS line scans, BC40 appears to have the most recovery in mineralizing ions of calcium and phosphate showing a more sudden decrease in calcium and phosphorous content at the lesion surface (Fig. 5B) while both BC and rH25BC gradually decrease to zero level at the dentin surface (Fig. 5A and C). The observation of somewhat higher mineral content in BC40 restored specimens is also supported by the elemental maps, which shows a narrower zone of calcium depletion for BC40 (Fig. 6F) compared to BC (Fig. 6B) and rh25BC (Fig. 6J).
Fig. 5: SEM-EDS-analysis by line scans.
Elemental concentrations (counts per sec.) of Carbon, Silicon, Calcium and Phosphorous along a line as depicted in SEM image on top are shown for BC (A), BC40 (B) and rh25BC (C).
Fig. 6: SEM-EDS-analysis by elemental mapping.
Distribution of the concentration of the elements Calcium and Carbon and mixed elements are depicted as a function of location across the dentin-cement interface after restoration with BC (A to D); BC40 (E to H) and rh25BC (I to L), after 14 days of remineralization in SBF. SEM-micrographs (A, E, I); Calcium distribution (B, F, J); Carbon distribution (C, G, K); and distribution of all elements detected (D, H, L), show a reaction layer that predominantly consists of carbon.
Interestingly, EDX line scans and elemental maps revealed that the reaction layer was not rich in calcium and phosphorous either. The layers appeared to be mainly electron translucent without providing much x-ray signal and to be partially rich in carbon. The layers appeared to be very thin in BC, about 5 μm and only a narrow zone towards the dentin side of the layer showed increased intensity in carbon content (Figs 5A and6C). The reaction layer at the dentin-cement interface was a bit wider in BC40 specimens at about 15 to 20 μm (Figs. 5B and6E, 6G) but mostly did not produce much signal to the EDS detector with the exception of some carbon counts in the restorative cement directly adjacent to the dentin (Fig. 6G). In contrast, the reaction layer was much larger (30-40 μm) and sometimes multi-layered in rh25BC specimens (Fig. 5C). Line scans showed an increase in carbon content on both sides of an about 70 μm wide reaction layer within a very narrow zone, while the center of the layer did not return any x-ray signal at all. The elemental maps in Figures 6K and L, however, clearly illustrate the prevalence of carbon in these layers in specimens of the rh25BC treatment group.
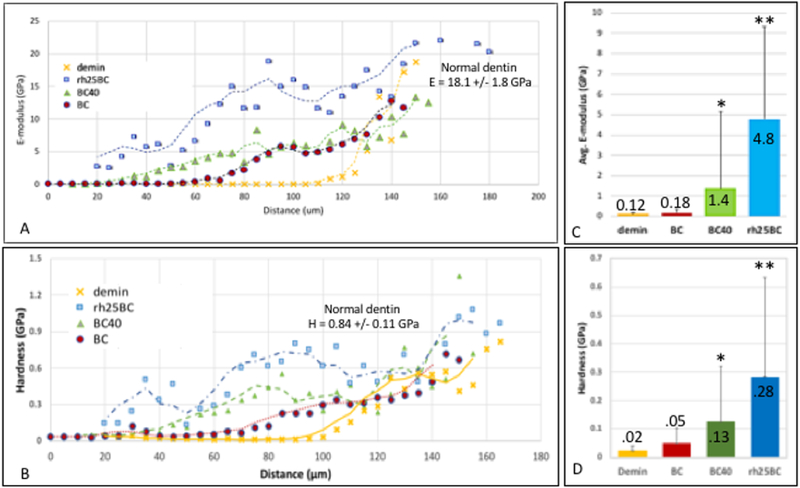
Nanoindentations were performed across the demineralized and remineralized lesions to compare the nanomechanical properties of the demineralized tissue to restored specimens BC, BC40 and rh25BC after remineralization treatments of 2 weeks (Fig. 7). Each data point in the line profiles is an average E-modulus or hardness value obtained from 6 indentations made at the specific distance from the dentin surface in three different samples (n=3). The standard deviation for each point is therefore large and is included in the line profiles of Figure S1 in the supplement. Comparing the average elastic moduli and hardness values from the surface of the lesion towards the normal dentin, a very soft outer zone to about 60 μm depth was observed in the demineralized specimens, which was associated with a fully mineral depleted collagen matrix (Figs. 7A and B), as previously reported by our group[9]. Nanomechanical properties gradually increased as residual mineral reinforced the collagen matrix at increasing levels between 80 μm and 140 μm depth, where values of normal dentin (E = 18.1 GPa; H = 0.84 GPa) were reached (Figs. 7A and B) [29]. Restoring the lesion with BC, showed little improvement from the profile of the demineralized sample. The average value of E-moduli in the outer 60 μm zone only increased marginally from 0.12 to 0.18 GPa after 2 weeks of remineralization using BC (Fig. 7C). Addition of 40% pAsp to the cement, BC40, showed some improvement of the demineralized zone with an increase in E-modulus and hardness from about 30 μm depth onward, deeper into the lesion. The graded region shifted towards the outer zone indicating that a functional remineralization process was initiated. The average E-modulus and hardness of the outer 60 μm zone was increased to 1.4 and 0.13 GPa, respectively, after BC40 restoration and remineralization (Figs. 7C and D). Interestingly, the rh25BC specimens showed the highest increase in elastic modulus and hardness after the remineralization process. Values of up to 6 and 0.4 GPa, respectively, were reached at the very outer zones. Both modulus and hardness reached normal dentin values within about 70 μm lesion depth, and remained at elevated levels all the way to the bottom of the lesion. This is a remarkable recovery of properties for a short period of two weeks of remineralization.
Fig. 7: Elastic modulus line profiles.
A) Nanoindentations were placed from the top of the lesion towards sound dentin along two lines separated by a 4 μm spacing, and average modulus is plotted using values for those 2 line profiles on all three specimens (n = 3). Sample groups BC, BC40 and rh25BC after 2 weeks of remineralization are compared to demineralized dentin. B) Average values of E-moduli within the outer 60 μm of the lesion after remineralization treatment with BC, BC40 and rh25BC compared to demineralized lesion.
Discussion
The PILP-method has been proven to facilitate the intrafibrillar mineralization of collagen-I and to reintroduce apatite crystallites into collagenous matrices in a similar fashion as in the natural process of biomineralization [18]. A variety of anionic and cationic polymer systems have been used to remineralize artificial dentin lesions successfully [15] by immersion of the demineralized tissue into an aqueous mineralizing PILP-solution comprised of a process-directing agent and calcium and phosphate ions at metastable concentrations with regards to hydroxyapatite’s solubility. To take advantage of this remarkable ability of the PILP-method to reconstitute the structure and mechanical properties of dentin to a substantial degree by remineralization, we have evaluated two principle approaches that would allow the transfer of the PILP-method into the dental clinic as part of a restorative dental treatment. A recent study used a similar approach by adding poly-acrylic acid and a phosphorylating agent to a commercial silicate cement and reported little improvement with regards to mineral recovery in the artificial dentin lesions [10]. We tested the use of pAsp, as a process-directing agent, which was delivered a) by incorporation into a commercial RMGI cement (BioCem) or b) by rehydrating the air-dried dentin lesion with high-concentrations of PILP-solution containing 25mg/ml of pAsp. We hypothesized that in both methods pAsp would infiltrate into the collagenous matrix of demineralized dentin in the form of PILP-nanodroplets and initiate the biomineralization process by release of calcium and phosphate ions into the lumen of the collagen fibrils followed by ACP formation and subsequent intrafibrillar crystallization of apatite.
This study did not apply TEM analysis to demonstrate the presence of intrafibrillar mineral, which has been shown in previous studies by our group and others [16, 20]. Instead we tested if these approaches resulted in a mechanical reinforcement of the dentin lesion which is the most critical parameter when attempting to remineralize dentin as its function is primarily mechanical. We largely relied on the analysis of dehydration shrinkage, integrity of the dentin-cement interface and a nanomechanical evaluation of the remineralized dentin lesion as suitable indicators of functional remineralization (FR) of dentin matrices.
Biocem (BC) is a dual-cured RMGI cement which contains bioactive components including phosphate- and calcium-rich soluble glass particles[30]. There have been reports suggesting that BC regenerates tooth structure which may largely relate to the ability of RMGI cements to precipitate apatite mineral at the cement-tooth interface due to fluoride release [30]. The ability to release calcium, phosphate and fluoride ions may be very beneficial for remineralization of enamel and to also aid in establishing a strong interfacial bond between enamel and the cement, but previous studies have shown that for the remineralization of dentin a process-directing agent is critical in order to reinforce the organic matrix [8, 9, 23, 31]. When applying BC as is, without modifications to the process, we found some improvement in shrinkage (Figs. 2 and 3) which may have derived from the ability of this cement to bond to tooth structures in general, possibly through the presence of poly-acrylic acid in BioCem. In particular the optical images of Fig. 2 suggest that a polymeric phase is pulled from the cement side of the interface as the dentin lesion shrinks during dehydration. A thin layer, rich in carbon at that interface was also detected by EDS line scans and mapping and may be related to what has been described as a natural seal at the tooth-BioCem interface for this product (Figs. 5A and 6C). A severe collapse of the demineralized zone leading to large gaps between dentin lesion and cement was however observed by SEM suggesting a lack of reinforcement of dentin by mineral when BC was used to restore the specimen before remineralization (Figs. 4A and B). This observation was also supported by the low elastic modulus observed in the outer portion of the treated lesions when tested by nanoindentation (Figs. 7A and B) suggesting that FR of the dentin lesion did not occur in this treatment group.
When mixing up to 40% by weight of pAsp into the powdered portion of BC, a RMGI cement was obtained that comprised a substantial amount of the process-directing agent. pAsp can interact with calcium and other polyvalent ions released from the glass of the RMGI and also participate in the glass ionomer setting process [32]. We also assume that a large portion of pAsp did not chemically bind to the RMGI. Both BC20 and BC40 set with similar characteristics and setting times as BC without pAsp, but the formation of large pores was evident and suggests that pAsp does not integrate well within the resin matrix of the cement, and instead separates from the matrix phase forming circular domains. These phase separated domains or pores will most likely have a negative impact on the mechanical properties and bonding strength of the cement, but may act as suitable reservoirs for pAsp delivery. Shrinkage (Fig. 3) and nanomechanical data (Fig. 7) strongly suggests that FR of the artificial lesion has been initiated, as shrinkage was significantly reduced and the elastic modulus and hardness were significantly elevated at the outer portion of the lesion. While we were not able to measure the release of pAsp chemically, which is a current investigation, the obtained data suggested that pAsp was at the dentin-cement interface which initiated the PILP-mineralization process by interaction with SBF diffusing through the dentin tubules. This release may be quite slow, but release rates may not be as critical considering that for a clinical application the restoration would be interacting for the lifetime of the restoration, and possibly be able to release pAsp molecules for years.
An even simpler approach to integrate the PILP-process into a clinical delivery system constitutes the application of highly concentrated pAsp solutions to rehydrate the lesions (rh25BC), as this approach does not affect the cement chemistry. When demineralized dentin is dried with compressed air most of the water (or pulpal fluid) is blown off and removed and the dentin matrix will collapse at this point. When the lesion is rehydrated with PILP-solution, collagen fibrils will float in the aqueous suspension and pAsp nanodroplets will infiltrate the demineralized matrix rather quickly during the rehydration process. Once rehydrated, excess solution is carefully wicked off and the RMGI cement, e.g. BC, is applied as in a standard restorative procedure and light-cured for setting of the cement, sealing in the high-content of pAsp and PILP-nanodroplets into the lesion. When immersed into SBF solution, calcium and phosphate ions in SBF will further interact with pAsp to promote the PILP-process. At this point it is not clear what concentration of pAsp will be optimal. Previous studies found that only 0.1 mg/ml pAsp were sufficient to remineralize artificial lesions in dentin when PILP was applied in solution, but those studies used between 40 and 200 ml of PILP-solution. Here only about 20 μl are applied once without replenishment of PILP-solution. Hence this approach tries to maximize the pAsp delivery in a single application during the rehydration process. The solubility of pAsp in water is above 50 mg/ml. It has been found that highly concentrated pAsp-solutions can inhibit the PILP-process possibly by “overstabilizing” the liquid precursor phase and not allowing for infiltration or for a transition of ACP into intrafibrillar apatite mineral [33, 34]. Initially we associated the observation of a thick reaction layer at the dentin-cement interface in rh25BC samples with the presence of ACP in these samples, but elemental analysis suggests there is no or very little calcium and phosphorous in these layers. Instead they appear to be comprised of carbon and are mainly x-ray translucent. The layers tend to swell with hydration and are extremely soft according to nanoindentation analysis. Therefore, the nature of these layers seems to be associated with the presence of pAsp, which may have interacted with the RMGI or its dissolved species to form some low density polymeric network at the interface. The thickness of this layer is substantially increased in rh25BC compared to the other groups. While it is not clear what effect the layer may have on the long-term mechanical stability of the dentin-cement interface, this study determined that rehydration treatments with 25mg/ml pAsp had an impressive effect on the nanomechanical properties. There was a substantial recovery of the modulus within two weeks of remineralization which came close to the results previously reported for PILP-mineralization performed in solution [8, 20]. The performance of nanoindentations on these specimens is tedious and time consuming. Therefore an n = 3 was used for this initial study to examine the feasibility of the approach. A major challenge is to determine the exact position of the lesion surface with the nanoindenter, which is affected by the amount of compression the lesion experiences when the cement is placed. The compression will inhibit the ability of the collagen matrix to expand fully and it will remain compressed by a few micrometers. We have taken great care to adjust for this compression in these indentation experiments, and we repeated these measurements multiple times with confirming results. Despite the fact that there was substantial variability and the standard deviation of the elastic modulus was high for the rh25BC group (Fig. S1), nanoindentation profiles clearly suggest that collagen fibrils have been reinforced by mineralization at this early time point of treatment and that FR did occurred in these artificial lesions. Longer remineralization times will be evaluated in subsequent studies, as well as the use of deeper lesions that more closely mimic natural lesions in dentin, to better assess the usefulness of this method in vitro for remineralization before applying the method to natural caries in dentin that involves much deeper lesion with varies zones and bacterial contamination.
Conclusions
This initial study provided supporting data that integration of the PILP-process in a restorative dental treatment is feasible and can promote functional remineralization of demineralized dentin. The use of these modified cementation techniques could improve current approaches in minimally-invasive dentistry by adding a reliable method to repair dentin damaged by the caries process and may further promote the ongoing paradigm shift in dentistry [2, 35]. This damage reversal is essential for the conservation of dental tissue, which will be critical for the long-term survival of the tooth.
Supplementary Material
Acknowledgments
This work was supported in by NIH/NIDCR Grant RO1-DE016849, by the UCSF Catalyst Award "PILP Treatment for the Repair of Dental Caries" and by the Center for Dental, Oral & Craniofacial Tissue & Organ Regeneration (C-DOCTOR) with the support of NIH NIDCR (U24DE026914). Additional support was provided by the IADR Innovation in Oral Care Award, 2017.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Innes NP, Frencken JE, Schwendicke F. Don't Know, Can't Do, Won't Change: Barriers to Moving Knowledge to Action in Managing the Carious Lesion. J Dent Res. 2016;95:485–6. [DOI] [PubMed] [Google Scholar]
- [2].Schwendicke F, Frencken JE, Bjorndal L, Maltz M, Manton DJ, Ricketts D, et al. Managing Carious Lesions: Consensus Recommendations on Carious Tissue Removal. Adv Dent Res. 2016;28:58–67. [DOI] [PubMed] [Google Scholar]
- [3].Sullivan RJ, Fletcher R, Bachiman R, Penugonda B, LeGeros RZ. Intra-oral comparison and evaluation of the ability of fluoride dentifrices to promote the remineralization of caries-like lesions in dentin and enamel. J Clin Dent. 1995;6:135–8. [PubMed] [Google Scholar]
- [4].ten Cate JM, Featherstone JD. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit Rev Oral Biol Med. 1991;2:283–96. [DOI] [PubMed] [Google Scholar]
- [5].Mount GJ, Ngo H. Minimal intervention: early lesions. Quintessence Int. 2000;31:535–46. [PubMed] [Google Scholar]
- [6].Qi YP, Li N, Niu LN, Primus CM, Ling JQ, Pashley DH, et al. Remineralization of artificial dentinal caries lesions by biomimetically modified mineral trioxide aggregate. Acta Biomater. 2012;8:836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Niu LN, Zhang W, Pashley DH, Breschi L, Mao J, Chen JH, et al. Biomimetic remineralization of dentin. Dental materials : official publication of the Academy of Dental Materials. 2014;30:77–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Habelitz S, Hsu T, Hsiao P, Saeki K, Chien YC, Marshall GW Jr., The Natural Process of Biomineralization and In Vitro Remineralization of Dentin Lesions In: McKittrick J, Narayan R, editors. Advances in Bioceramics and Biotechnologies II: The Amrican Ceramic Society, John Wiley & Sons; 2014. p. 13–23. [Google Scholar]
- [9].Burwell AK, Thula-Mata T, Gower LB, Habelitz S, Kurylo M, Ho SP, et al. Functional remineralization of dentin lesions using polymer-induced liquid-precursor process. PLoS One. 2012;7:e38852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Li X, De Munck J, Yoshihara K, Pedano M, Van Landuyt K, Chen Z, et al. Re-mineralizing dentin using an experimental tricalcium silicate cement with biomimetic analogs. Dental materials : official publication of the Academy of Dental Materials. 2017;33:505–13. [DOI] [PubMed] [Google Scholar]
- [11].Bertassoni LE, Habelitz S, Kinney JH, Marshall SJ, Marshall GW Jr., Biomechanical perspective on the remineralization of dentin. Caries Res. 2009;43:70–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kinney JH, Habelitz S, Marshall SJ, Marshall GW. The importance of intrafibrillar mineralization of collagen on the mechanical properties of dentin. J Dent Res. 2003;82:957–61. [DOI] [PubMed] [Google Scholar]
- [13].Olszta MJ, Odom DJ, Douglas EP, Gower LB. A new paradigm for biomineral formation: mineralization via an amorphous liquid-phase precursor. Connect Tissue Res. 2003;44 Suppl 1:326–34. [PubMed] [Google Scholar]
- [14].Olszta MJ, Cheng XG, Jee SS, Kumar R, Kim YY, Kaufman MJ, et al. Bone structure and formation: A new perspective. Materials Science & Engineering R-Reports. 2007;58:77–116. [Google Scholar]
- [15].Niu LN, Jee SE, Jiao K, Tonggu L, Li M, Wang L, et al. Collagen intrafibrillar mineralization as a result of the balance between osmotic equilibrium and electroneutrality. Nat Mater. 2017;16:370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nudelman F, Lausch AJ, Sommerdijk NA, Sone ED. In vitro models of collagen biomineralization. J Struct Biol. 2013;183:258–69. [DOI] [PubMed] [Google Scholar]
- [17].Gu L, Kim YK, Liu Y, Ryou H, Wimmer CE, Dai L, et al. Biomimetic Analogs for Collagen Biomineralization. J Dent Res. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nudelman F, Pieterse K, George A, Bomans PH, Friedrich H, Brylka LJ, et al. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat Mater. 2010;9:1004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gower LB. Biomimetic Model Systems for Investigating the Amorphous Precursor Pathway and Its Role in Biomineralization. Chem Rev. 2008;108:4551–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Saeki K, Chien YC, Nonomura G, Chin AF, Habelitz S, Gower LB, et al. Recovery after PILP remineralization of dentin lesions created with two cariogenic acids. Arch Oral Biol. 2017;82:194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nurrohman H, Saeki K, Carneiro K, Chien YC, Djomehri S, Ho SP, et al. Repair of dentin defects from DSPP knockout mice by PILP mineralization. J Mater Res. 2016;31:321–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chien YC, Burwell AK, Saeki K, Fernandez-Martinez A, Pugach MK, Nonomura G, et al. Distinct decalcification process of dentin by different cariogenic organic acids: Kinetics, ultrastructure and mechanical properties. Arch Oral Biol. 2016;63:93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim YK, Yiu CK, Kim JR, Gu L, Kim SK, Weller RN, et al. Failure of a glass ionomer to remineralize apatite-depleted dentin. J Dent Res. 2010;89:230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Brauer DS, Saeki K, Hilton JF, Marshall GW, Marshall SJ. Effect of sterilization by gamma radiation on nano-mechanical properties of teeth. Dental materials : official publication of the Academy of Dental Materials. 2008;24:1137–40. [DOI] [PubMed] [Google Scholar]
- [25].Marshall GW Jr., Chang YJ, Saeki K, Gansky SA, Marshall SJ. Citric acid etching of cervical sclerotic dentin lesions: an AFM study. J Biomed Mater Res. 2000;49:338–44. [DOI] [PubMed] [Google Scholar]
- [26].Habelitz S, Marshall GW Jr., Balooch M, Marshall SJ. Nanoindentation and storage of teeth. J Biomech. 2002;35:995–8. [DOI] [PubMed] [Google Scholar]
- [27].Bertassoni LE, Habelitz S, Marshall SJ, Marshall GW. Mechanical recovery of dentin following remineralization in vitro--an indentation study. J Biomech. 2011;44:176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kinney JH, Nalla RK, Pople JA, Breunig TM, Ritchie RO. Age-related transparent root dentin: mineral concentration, crystallite size, and mechanical properties. Biomaterials. 2005;26:3363–76. [DOI] [PubMed] [Google Scholar]
- [29].Marshall GW, Habelitz S, Gallagher R, Balooch M, Balooch G, Marshall SJ. Nanomechanical properties of hydrated carious human dentin. J Dent Res. 2001;80:1768–71. [DOI] [PubMed] [Google Scholar]
- [30].Webman M, Mulki E, Roldan R, Arevalo O, Roberts JF, Garcia-Godoy F. A Retrospective Study of the 3-Year Survival Rate of Resin-Modified Glass-Ionomer Cement Class II Restorations in Primary Molars. J Clin Pediatr Dent. 2016;40:8–13. [DOI] [PubMed] [Google Scholar]
- [31].Alves FB, Hesse D, Lenzi TL, Guglielmi Cde A, Reis A, Loguercio AD, et al. The bonding of glass ionomer cements to caries-affected primary tooth dentin. Pediatr Dent. 2013;35:320–4. [PubMed] [Google Scholar]
- [32].Marshall GW, marshall SJ, Nurrohman H, Saeki K, Habelitz S, Gower L. Compositions for the remineralization of dentin. In: UCSF, editor. Internat Patent2017. [Google Scholar]
- [33].Thula TT, Svedlund F, Rodriguez DE, Podschun J, Pendi L, Gower LB. Mimicking the Nanostructure of Bone: Comparison of Polymeric Process-Directing Agents. Polymers (Basel). 2011;3:10–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jee SS, Thula TT, Gower LB. Development of bone-like composites via the polymer-induced liquid-precursor (PILP) process. Part 1: influence of polymer molecular weight. Acta Biomater. 2010;6:3676–86. [DOI] [PubMed] [Google Scholar]
- [35].Whitehouse JA. Adopting a new philosophy: minimal invasion. Dent Today. 2006;25:102, 4–5. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.