Abstract
Objectives:
Despite decades of development and their status as the restorative material of choice for dentists, resin composite restoratives and adhesives exhibit a number of shortcomings that limit their long-term survival in the oral cavity. Herein we review past and current work to understand these challenges and approaches to improve dental materials and extend restoration service life.
Methods:
Peer-reviewed work from a number of researchers as well as our own are summarized and analyzed. We also include yet-unpublished work of our own. Challenges to dental materials, methods to assess new materials, and recent material improvements and research directions are presented.
Results:
Mechanical stress, host- and bacterial- biodegradation, and secondary caries formation all contribute to restoration failure. In particular, several host- and bacterial-derived enzymes degrade the resin and collagen components of the hybrid layer, expanding the marginal gap and increasing access to bacteria and saliva. Furthermore, the virulence of cariogenic bacteria is up-regulated by resin biodegradation by-products, creating a positive feedback loop that increases biodegradation. These factors work synergistically to degrade the restoration margin, leading to secondary caries and restoration failure. Significant progress has been made to produce hydrolytically stable resins to resist biodegradation, as well as antimicrobial materials to reduce bacterial load around the restoration. Ideally, these two approaches should be combined in a holistic approach to restoration preservation.
Significance:
The oral cavity is a complex environment that poses an array of challenges to long-term material success; materials testing conditions should be comprehensive and closely mimic pathogenic oral conditions.
Keywords: dental restorations, resin composite, restorative adhesive, restoration bond, dental caries, biodegradation, antimicrobial materials, enzyme inhibition, gene expression, proteins, esterase, metalloproteinase, collagenase
1. Dental materials
The oral cavity is a complex environment and presents many unique challenges for dental restorative materials. Traditionally, modem resin tooth-coloured restoratives have been rightfully designed with a focus on their mechanical and aesthetic properties while remaining easy for practitioners to place. In the meantime, research has uncovered a growing number of factors within the oral cavity that degrade and compromise these restorations, limiting their service life. This increasing understanding of the nature of the challenges presented to dental restorations has allowed the development of a number of materials adapted to this environment, with the aim of producing dental restorations that function as close as possible to natural teeth for the patient’s lifetime.
1.1. Resin composite restorative materials and their clinical performance
1.1.1. Resin composites
Over the last 50 years resin composite restorations have become by far the most common material for treating dental caries [1]. Their ease of application, variety of use-cases, and aesthetic tooth-colour matching capability, as well as concerns over the safety and environmental impact of mercury amalgam restorations, have propelled these materials to the forefront of restorative dentistry [2–4]. Resin composites restoratives broadly consist of a cross-linked polymer matrix and a solid particulate filler at 60-80% by weight [5]. The material remains malleable until the matrix is polymerized, via a blue light (480 nm) and photoinitiator system or a second chemical initiator system added to the composite at the time of application [6]. Composite restorations are typically applied in conjunction with a resin adhesive system, allowing for chemical, micromechanical, and macro-scale interlocking with the tooth [7].
1.1.2. Chemical formulation and composition
Resin composite restoration chemical formulations have varied little since their inception. In most systems, methacrylate monomers are utilized, undergoing a radical vinyl polymerization [8]. These methacrylate monomers include rigid di-methacrylate crosslinking monomers to stabilize the resin matrix, as well as diluent mono- and di-methacrylate monomers to ease in handling and, in some cases, decrease hydrophobicity of the resin during bonding to dentin [4, 9–11]. Methacrylate monomers include a side-chain or crosslinking bridge connected via an ester linkage. Common bridging groups include bisphenol-related molecules such as that in 2,2-bis [4(2-hydroxy-3-methacryloxypropoxy)- phenyl]propane (bisGMA, Figure 1), which allow stacking of pi bonds between monomers, resulting in a very rigid structure. Other common bridging groups include glycols such as those in the monomer triethylene glycol dimethacrylate (TEGDMA) to act as a diluent [12]. However, these diluent monomers also increase water sorption via their increased hydrophilicity, potentially degrading long term stability [6].
Figure 1:
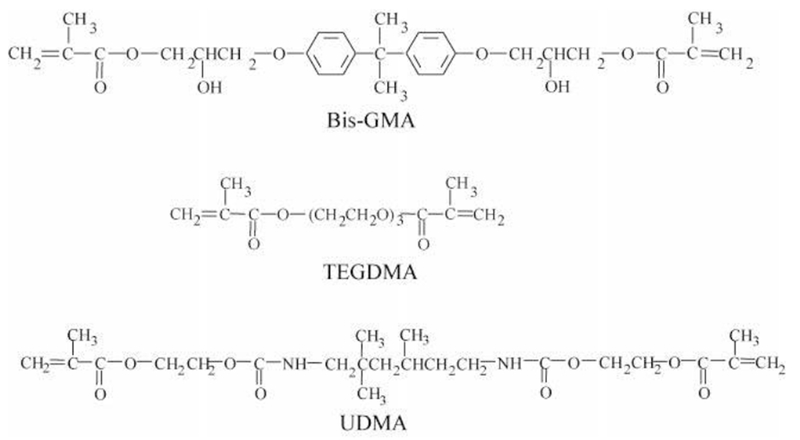
Chemical structure of bisGMA, TEGDMA and UDMA monomers [13].
Composites, by definition, also include a solid phase filler system, usually silica, quartz, or ceramic, adding a high degree of compressive strength to the composite as well as controlling the material’s aesthetic properties and reducing polymerization shrinkage [5, 6]. These filler particles come in a variety of sizes and shapes, usually a larger microscale (approximately 1 μm) irregular shaped ground glass, and a smaller nanoscale (1-100 nm) colloidal particle to fill interstitial space [4], Balance between two sizes of filler will determine the malleability of the unpolymerized system, with the higher surface area-to-volume ratio of smaller particles increasing viscosity greatly. Overall filler particle load will also determine the performance of the final material, with most systems including 60 – 80% wt. filler [5], These particles usually include surface-bound silane crosslinkers such as 3-methacryloxypropyltrimethoxysilane (gamma-MPS, Figure 2) to provide a reaction site for the polymer matrix in the form of a methacrylate vinyl group, chemically bonding the filler particles to the material through its silane group [14]
Figure 2:
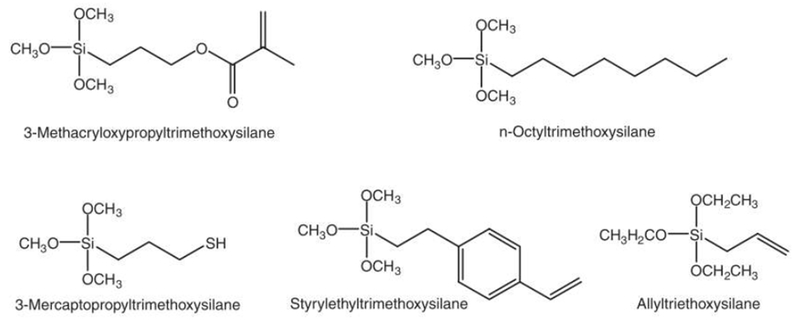
Common silane filler coupling agents used in dental composite materials [14].
1.1.3. Restoration adhesion by resin adhesives
Dental resin adhesives chemically and micromechanically bind the bulk of the restoration (the resin composite) to dentin and enamel, and critically effect the performance of the restoration [7, 15–19], Dental resin adhesives are chemically similar to resin composites, enabling them to chemically bond and polymerize with the restoration bulk. Use of photopolymerized or self-polymerizing mono- and dimethacrylate monomer adhesives is widespread, as the ability of hydrophilic mono-methacrylate monomers such as hydroxyethyl methacrylate (HEMA) to penetrate demineralized dentin and polymerize with bisGMA results in a tight micromechanically intertwined “hybrid layer’ [12, 20, 21].
Types of resin adhesive are distinguished mostly on their mode of application, number of steps involved, and suitability of various bonding substrates (Figure 3). “Total-etch” systems use a separate demineralizing acidic gel to prepare the cavity surface, by demineralizing the tooth structure and expose a network of dentin collagen fibrils [7, 22], This acidic gel is washed out with water, hence the alternate term for these systems is “etch-and-rinse”. Following this a hydrophilic primer monomer is applied to penetrate the collagen network, followed by a more hydrophobic adhesive that is chemically similar to the polymer matrix component of resin composites, such as bisGMA.
Figure 3:
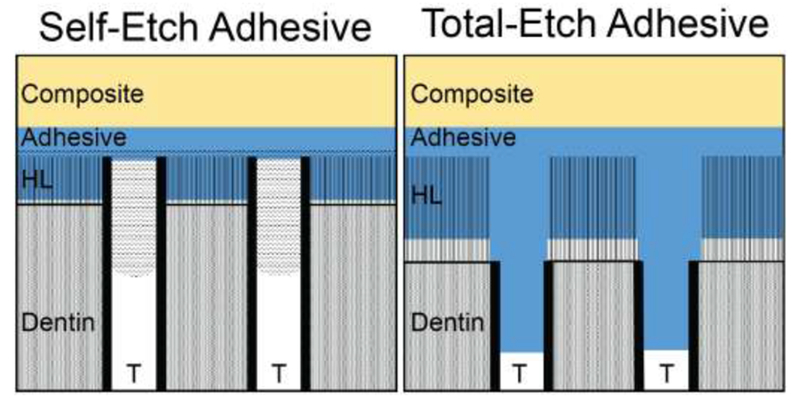
Schematic of self-etch and total-etch adhesive systems. The differences between hybrid-layer (HL) thickness and dentinal tubule (T) penetration by adhesive are displayed.
Some systems combine the primer and adhesive into a single bottle in a two-step “total-etch” system. “Selfetch” systems utilize acidic monomers or acidic primer solutions to combine the etch stage with primer or adhesive application in either a two-step prime-and-bond system or an all-in-one etch-prime-bond adhesive [7], These systems do not require rinsing of the acidic primer-adhesive. The obvious advantage of combining bonding steps is increasing convenience, which may be especially important in difficult-to-access lesions, however these systems are generally considered inferior to total-etch systems [23]. One-bottle self-etch systems seem particularly inferior, with bond-strengths significantly lower than other systems even without aging [7]. The hydrophilicity of these systems and high solvent content may lead to inadequate bonding to the composite and the formation of voids, while their low pH may interfere with polymerization of subsequent resin composites bulk [24].
1.1.4. Clinical performance
Resin composite restorations have remained popular despite performance and outcomes worse than amalgam restorations. About 70% of all resin composite restorations placed are replacements for failed resin restorations, with 5- to 7-year average lifespans, while amalgam restorations typically last twice this [1, 25–28]. Types of failure include restoration fracture, discolouration, failure of the bond to the tooth, and secondary or recurrent caries. This behaviour is in contrast with amalgam restorations, where failures typically include tooth fracture or secondary caries. High annual failure rates (up to 12.9 %) primarily due to secondary caries have been more recently confirmed in an extensive review by Chisini et al. [29].
1.1.5. Secondary caries
A leading cause of resin composite restoration failure, at 55%, remains secondary caries, or caries occurring at the interface between a previous restoration and the remaining tooth [25]. Caused by the demineralization and decomposition of tooth structure by bacterial acid production, secondary caries may result in staining and pitting around the restoration, or larger lesions underneath the restoration away from the surface that may compromise the mechanical integrity of the restoration [30–33]. Treatment includes the removal of the restoration and carious tissue, and re-application of a new restoration. Repeated treatment in this manner weakens the remaining tooth structure and may eventually necessitate more complex treatment modalities such as root canal therapy, or eventually result in loss of the tooth [22, 34–37].
2. The challenge of degradation in the oral cavity
2.1. Mechanical degradation
Teeth, and by extension restorations, experience a significant mechanical stress in the oral cavity. Thermocycling during food and beverage consumption, abrasion during cleaning, and mechanical stresses during mastication are the most common [10, 38, 39]. From their inception, resin composite restorations have been designed mostly around these considerations; the performance of the materials is assessed based on the ease of application, and its ability to withstand these forces and mechanically function similarly as a tooth. Most of the design considerations made during the development of these materials have addressed the mechanical challenges faced and ensure proper performance. As such, mechanical performance of adequately bonded and placed modern resin composite restorations is generally acceptable [14].
2.2. Chemical and biochemical degradation
2.2.1. Chemical degradation of resin monomers and polymers
The chief material challenges faced by resin composite restorations currently are chemical- and biodegradation of the materials over extended periods of time [7]. Although much of the physical testing described above utilizes a relatively inert environment, the oral cavity is chemically complex and bioactive, and resin composite restorations are exposed to a significant number of agents that attack their components. In modern di-vinyl resin monomers, the weak point is frequently the ester linkages attaching the methacrylate end-groups to the structurally important crosslinking bridges [10, 16, 40–43]. Hydrolysis of these ester bonds results in the degradation of the resin matrix, increasing water sorption and continued degradation. Hydrolysis may be catalyzed and accelerated via several enzymes present in the oral cavity, with human and bacterial sources [40, 41, 44, 45]. Continued breakdown of restoration resin matrix results in a decrease in material strength, as well as an increase in bacterial activity at the restoration via a number of pathways, that subsequently increases over-all risk of secondary caries formation and restoration failure [16, 41, 43, 46].
2.2.2. Short-term elution of unreacted monomers
Resin composites contain a relatively high amount of unreacted monomers after curing the restoration. About 25-50% of monomer vinyl groups remain unconverted, leading to the release of unreacted monomers that have no reacted vinyl groups in the first few hours and days after restoration placement via diffusion from the restoration [47, 48], The rate of diffusion of these monomers from resin composites is dependent on the elution media used and the monomers’ solubility in the media [48], Common in vitro experimental media includes buffers, pure water alone, and organic solvent, which are not representative of the complete chemical environment in the oral cavity. Once unreacted monomers are leached by these test media, there is little degradation of the remaining polymerized matrix [48]
2.2.3. Long-term enzymatic degradation
In contrast to the short-term challenge of unreacted monomers, the oral cavity environment possesses the ability to continually degrade most dental resins, leading to a continuous long-term release of degradation by-products. The ester bonds in several common dental resin monomers, such as bisGMA, TEGDMA, and urethane dimethacrylate (UDMA), are susceptible to hydrolysis in saliva. This degradation leads to a loss of material mass, decreased surface hardness, and increased wear [49], Degradation is increased further by the presence of unreacted vinyl groups and a low degree of conversion in dental resins. In these low-conversion materials, there is a larger population of crosslinking monomers with only one of two vinyl groups converted, and therefore only the single converted group needs to be hydrolyzed to release the structurally important cross-linking bridge [45]
2.2.4. Hvdrolase/esterase activity in the oral cavity
Human saliva is a complex media with a wide array of chemicals and molecules from several sources, fulfilling a number of different roles in the oral cavity. Human saliva has been shown to contain a variety of hydrolase activities, with the most important in relation to restorative resins being hydrolases and esterases. These have a number of sources, from human gingiva, salivary glands, immune cells, as well as the multitude of bacterial species in the mouth [10, 50, 51], These esterases cleave the vulnerable ester linkages in many common dental resins, accelerating the biodegradation of these materials [49, 52, 53], Esterase activity in human saliva has been characterized as cholesterol esterase-like (CE) and pseudocholinesterase-like (PCE), released in part by normal as well as inflamed gingiva [54, 55], These two esterases show strong ability to degrade bisGMA, TEGDMA, HEMA, and other ester-containing monomers in dental resins.
The overall hydrolytic activity in human saliva has been defined in terms of its CE-like and PCE-like activity towards known substrates. In this case, para- and ortho-nitrophenyl butyrate or acetate, and butyrylthiolcholine iodide (BTC) may be used as colorimetric substrates for CE- and PCE-like activities in saliva [55], By utilizing various side-chain lengths and positions, stoichiometric preferences of the hydrolase activity in whole saliva may be determined. For instance, although bisGMA is readily hydrolyzed by CE, urethane-modified bisGMA shows significantly higher hydrolytic stability in the presence of hydrolases, owing to the protection offered to ester bonds by hydrogen bonding urethane groups [42, 56], Saliva has been found to have strong hydrolase activity, and a preference for para-nitrophenyl butyrate and some activity towards BTC. From this, simulated human salivary esterase (SHSE) may be used to mimic saliva’s hydrolytic activity in long term in vitro studies more consistently than collecting saliva from donors [46], This is achieved by combining known activity levels of CE and PCE to match the activity of saliva measured against specific substrates.
2.3. Measuring resin degradation via quantification of by-products
To characterize the magnitude of biodegradation of restorative materials, techniques to quantify enzyme activity, the breakdown of dental resins, and the release of biodegradation by-products have been developed. The most common method to identify and quantify these by-products is liquid chromatography and UV absorbance, sometimes combined with mass spectrometry (LC or LC-MS), with methods summarized by Santerre, Jaffer and Shajii [10, 44, 57], BisGMA is a common hydrophobic monomer present in many commercial resin composite and adhesive systems, and thus its esterase biodegradation by product, 2,2-bis[4(2,3-hydroxypropoxy)phenyl]propane (bisHPPP) is a popular target molecule for quantification. Triethylene glycol (TEG), the by-product of TEGDMA breakdown, is also useful but more difficult to detect due to its lack of strong UV absorbance for LC only systems. Methacrylic acid (MA) produced by cleaving unreacted end groups from monomers may also be detected, but its detection in incubation media from biodegradation studies is limited due to evaporation. Since MA is produced only by unreacted monomers, its present cannot be regarded as a good representative of resin matrix degradation [42, 48, 55, 58], The detection of these monomers’ mono-methacrylate derivatives is also possible. The production of biodegradation by-products by saliva, CE and PCE has been studied in vitro in the months following polymerization. Amounts of both unreacted monomers and production of by-products is high at first, and decreases with time, reaching a pseudo-equilibrium as unreacted monomer is consumed by enzymes, and the degradation of the polymer surface becomes the primary substrate [58], Although degradation by-products have been identified in samples incubated with non-enzymatic buffer, their levels were significantly lower than when esterase was present. These studies represent a direct demonstration of the significant hydrolytic activity present in the mouth, and the susceptibility of certain resins to this type of degradation.
2.4. Biodegradation of the restoration-tooth interface
2.4.1. Restoration bulk vs interface
The degradation of restorative materials affects different parts of the restoration in distinct ways. The majority of the restoration surface area is composite, exposed to the oral cavity, and fulfilling an aesthetic and masticatory role [6]. As such, the degradation of this surface may roughen the surface of the material, and the released degradation by-products could affect oral bacteria [59, 60], but is unlikely to cause a catastrophic failure of the restoration. Increased porosity from the degradation of the restoration surface may, however, lead to increase staining.
In contrast, the degradation of the restoration margin, where an adhesive system is applied, may cause a critical failure of the whole restoration (fracture, detachment, or secondary caries) [7, 15–17]. The microgap formed at this interface by contracting resin matrix during polymerisation was shown to be widened by the enzymatic erosion of the adhesive surface, leading to increased saliva and bacterial penetration, undesirable staining of the restoration margin, and increase the risk of secondary caries development [16, 61]. Further, the degradation of the hybrid layer interface may weaken the overall bond to the tooth and lead to fracture and loss of the restoration [43, 46].
2.4.2. Collagenase and gelatinase activity in human dentin
Dentin contains several collagen-degrading factors that may be released or activated by the bonding procedure during restoration and may degrade the remaining demineralized tooth structure [62, 63]. This includes the collagenase matrix metalloproteinase-8 (MMP-8), which unwinds collagen fibrils and hydrolyzes their alpha chains [62, 64]. Dentin also contains the gelatinases MMP-2 and −9 [65, 66]. This activity has been extensively shown to alter the morphology of collagen fibrils within the bond hybrid layer [67]. This may modulate the fracture toughness and interface stability in vivo, however isolating the effects of dentinal MMPs in the presence of other aggravating factors such as bacterial, environmental, and host effects remains difficult [46, 68–70].
2.4.3. Salivary esterase and MMP biodegradation of the resin-dentin interface
The restoration-tooth interface and the adhesive applied there is exposed to significant stresses not present elsewhere in the restoration [7, 63]. The effects of various degrading factors on the restoration interface, fracture toughness, and mode of mechanical failure have been studied at length by our group and others [43, 46, 71–73]. Utilizing human dentin-adhesive-composite miniature short rod (mini-SR) specimens, restoration bonds may be produced, incubated, loaded and fractured in a repeatable and predictable manor, representing an improvement over traditional microtensile strength testing. Shokati et al. [43] demonstrated using these specimens the effect of human salivary derived esterases (HSDE) more broadly on the fracture toughness of the resin-dentin bond. Within 6 months, specimens exposed to HSDE showed a significant decrease in fracture toughness compared to those incubated in buffer alone, demonstrating the importance of hydrolytic stability and biodegradation on the restoration [43]. A follow-up study using SHSE [46] demonstrated that the mode of fracture for these specimens’ changes overtime and depends on the adhesive system used (self- or total-etch, demonstrated in Figure 4). The fractured area tends to progress into the resin with self-etch systems over time, suggesting a vulnerability of the resin to degradation. In contrast, total-etch bonded specimens’ failure occurred in the resin at first, and progressed into the dentin with increased incubation period, suggesting a weakening of the tooth structure or hybrid layer after restoration. The effect of an additional MMP inhibitor (galardin) was also investigated in this study. MMP inhibition appeared to modulate the degree of fracture plane area in the hybrid layer, increasing or decreasing this area depending on adhesive type, incubation media (esterase- or non-esterase containing) and incubation time, suggesting that dentinal MMPs may play a role, though minimal in causing restoration weakening and eventual failure and with no clear overall trend observed.
Figure 4:
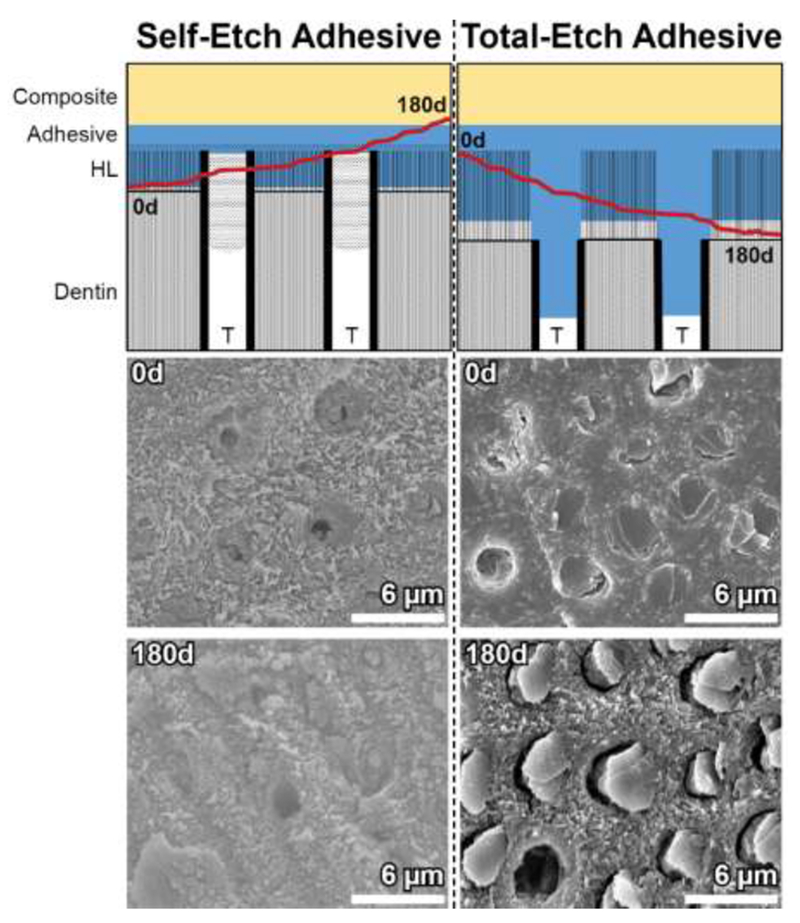
The effects of long-term incubation in esterase-containing media on the fracture mode of composite-adhesive-dentin bonded specimens, adapted from data first provided by Serkies et al. and Shokati et al. [43, 46]. Note the movement of the fracture plane (red line) over 180 days through the hybrid layer (HL) and adhesive, with different exposure of resin tags within dentinal tubules (T). Representative SEM micrographs are provided.
The presence of biodegradation by-products may also influence the action of enzymes. In one such example, the presence of leached unreacted bisGMA and TEGDMA and their degradation by-products increased the activity of MMP-8 and −9 towards test substrates [74]. TEGDMA may also increase the activity of MMP-2. This has implications for adhesive and resin composite design, as low conversion rates lead to the release of these monomers. When trapped at the restoration-tooth interface, leached monomers not broken down by hydrolases may increase the activity of MMPs in dentin at the hybrid layer and affect the strength of the bond.
2.5. Resin composite biodegradation and interactions with bacteria
2.5.1. Bacterial infiltration and marginal gap widening
The breakdown of the resin-dentin interface does not only pose a mechanical challenge but may significantly affect the ability of bacteria to infiltrate this interface. The breakdown of dental adhesive and resin composite polymer matrix and subsequent erosion of these materials widen and deepen the restoration marginal gap, allowing deeper bacteria penetration. Kermanshahi et al. [16] demonstrated the effects of CE and PCE simulated salivary degradation on an ex vivo restoration, bonded using total-etch adhesive. An image of a restoration marginal gap with bacteria is shown in Figure 5. Number and depth of penetration of the cariogenic bacteria S. mutans was monitored. Penetration increased with incubation time in hydrolytic media, while little effect was observed with buffer alone.
Figure 5:
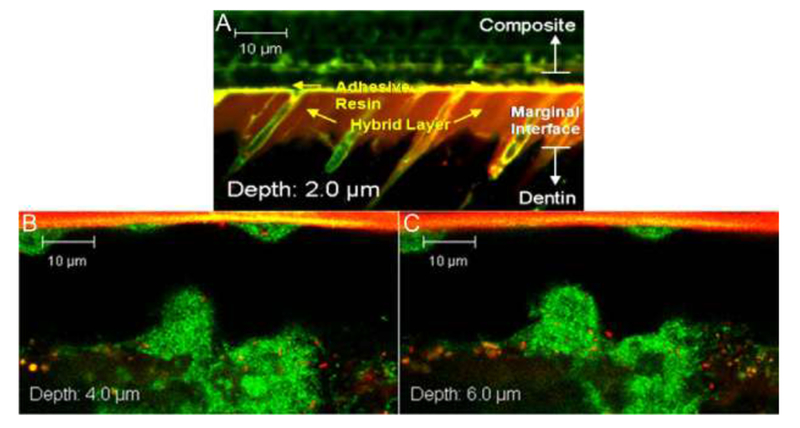
Confocal images of as prepared (A) and 90-day SHSE degraded (B and C) restoration margins, at various z-depths from the restoration margin edge, infiltrated with live S. mutans (stained green) [16]. Resin adhesive appears at the top of the gap, with dentin on the bottom.
Further study on the effect of biodegradation on bacterial ingress in the restoration margin was carried out by Huang et al. [68], using total-etch or self-etch adhesive systems, with or without SHSE and the MMP inhibitor, galardin. The results corroborated those by Kermanshahi et al. by demonstrating higher bacterial penetration in the simulated esterase environment. Furthermore, total-etch samples showed lower bacterial biomass at the restoration margin than self-etch after 90 days of SHSE biodegradation, while MMP inhibition reduced ingress in total-etch samples but not self-etch samples. These results show the importance of bonding mode and resin chemistry on biodegradation and its effect on bacterial penetration. It also demonstrated the contributing factor of MMPs on margin degradation and bacterial penetration for total-etch systems. These factors may therefore be important in secondary caries formation [34].
2.5.2. Resin composite degradation by-products affect bacterial virulence factors
In addition to allowing further penetration of cariogenic bacteria, the biodegradation of resins has significant biochemical effects on pathogenic bacteria. Numerous past studies have demonstrated concentration-dependant effects of resin monomers and biodegradation by-products on the proliferation and virulence of cariogenic bacteria [75–77]. Singh et al. demonstrated that although bisHPPP may be able to slightly inhibit the growth of S. mutans, at physiologically relevant concentrations, this by-product upregulated gtfB and yfiV genes, virulence factors contributing to S. mutans ’ attachment to smooth surfaces and survival in low-pH environments respectively [78].
Further to these studies, Sadeghinejad et al. demonstrated in a pair of studies the effects of TEG and bisHPPP on several S. mutans virulence factors and the effect environmental pH has on regulation [59, 60]. The presence of TEG at physiologically relevant concentrations greatly increased the abundance of proteins associated with caries formation, including those related to biofilm formation, carbohydrate transport and acid tolerance. The effect was most pronounced at the acidic pH of 5.5 and in the biofilm growth mode. Similarly, the upregulating effects of bisHPPP was greatest at pH 5.5 and in biofilm growth mode. These studies suggest the existence of a positive feedback loop for cariogenic and biodegradative bacteria, where the breakdown of resin polymers leads to increased cariogenic activity of the biofilm, increased bacterial proliferation and penetration, and therefore increased biodegradation.
2.5.3. The role of S. mutans in biodegradation
Bourbia et al. studied the effects of S. mutans on resin composite restorative materials and adhesives and observed a level of degradative activity comparable with human saliva [41]. Specimens were photocured and incubated with several strains of S. mutans, and bisHPPP production from the degradation of cured materials was monitored over 30 days. At 30 days the levels of bisHPPP production were significantly higher in S. mutans-containing media with Z250 composite and Easybond self-etch materials, but not the total-etch Scotchbond. These results highlight that in addition to acid production, cariogenic bacteria possess esterase activity in levels that could degrade resin composites and adhesives.
S. mutans’ role in biodegradation was further investigated by Huang et al. [51, 61, 79]. In this work, the genome of S. mutans UA159, a clinically-relevant cariogenic strain (that had its genome mapped by Ajdic et al. [80]) was searched for potential esterases. Out of several putative esterases, SMU_118c, a 280 base esterase, was chosen for investigation due to its high likelihood of degradative activity towards methacrylate resins. Using the aforementioned nitrophenyl substrates to construct an activity profile, the enzyme showed significant preferences toward para-nitrophenyl butyrate (p-NPB) under both neutral and acidic environments, similar to the whole bacterial cell activity profile measured by Bourbia et al. [41]. SMU_118c showed a high degree of stability by maintaining its activity for over 20 days under both neutral and under acidic cariogenic conditions, and demonstrated a very strong ability to degrade bisGMA in its monomer form as well as in commercial composite and adhesive resins. Correspondingly, specimens incubated with S. mutans UA159 wild type released large amounts of bisHPPP and showed clear signs of surface erosion after 30 days [61]. In contrast, SMU_118c knockout (KO) strain of S. mutans UA159 lost most of its degradative activity toward polymerized composites vs. the wild type. A complemented strain, where the SMU_118c gene was restored, regained its degradative ability. The above confirmed the role of SMU_118c esterase from this species in the degradation of dental composites.
2.5.4. Effect of bacterial biodegradation on restoration-tooth bond strength
With the effect of biodegradation on dental restoration bond strength well established, as well as the degradative capabilities of several species of bacteria, it follows that bacteria should exhibit some capability to degrade the bond strength of dental restorations, not just by acids, but also by their degradative enzymes. Adebayo et al. analysed mini-SR fracture toughness specimens bonded with either a total- or self-etch system after incubating with S. mutans UA159 wild type and SMU_118c KO for up to 90 days [81]. S. mutans wild type was able to significantly degrade the interfacial fracture toughness of specimens bonded using total-etch adhesive after 30 days, when compared with both non-incubated samples, samples incubated in bacteria-free media alone or the SMU_118c KO strain, confirming the role of S. mutans whole cell and specifically the SMU_118c esterase in the biodegradation. This reduction in bond strength of the total-etch systems by this strain but not self-etch systems is in contrast to previous results showing increased bacterial bisHPPP production from self-etch adhesive over total-etch adhesive. Therefore when assessing performance, degradation of the restoration-tooth interface as a whole, and not of bulk adhesive material, may provide more clinically-relevant assessment of the adhesive. Other factors beyond esterase-catalyzed enzymatic biodegradation of the bulk are affecting bond strength. Bacteria produce many degradative factors including hydrolytic enzymes, collagenases, and acid through fermentation, and therefore likely degrade all components of the restoration-tooth interface [82].
2.5.5. The cyclic effect of resin composite biodegradation product on a cariogenic bacterium degradative esterase gene expression and protein synthesis
Just as exposure to biodegradation by-products increased the expression of several virulence factors of S. mutans, exposure to bisHPPP was shown to upregulate the expression of SMU_118c in wild-type UA159 [61]. This effect is summarized in Figure 6. Through the use of fluorescence in situ hybridization (FISH), the expression of SMU_118c gene was observed at several physiologically relevant concentrations of bisHPPP in S. mutans biofilms. Above 0.1 mM bisHPPP there was a significant increase in expression. In addition to the increase in the expression of the SMU_118c gene, the presence of bisHPPP resulted with an increase in the synthesis of the SMU_118c protein, suggesting another positive feedback loop of virulence of the bacteria described above, where the biodegradation of the materials is increased by bacterial esterase, and the resultant biodegradation by-product increases the amount of bacterial esterase that is responsible for this degradation. This could provide a possible explanation for the increase in resin composite restoration failure compared with amalgam and add another layer of complexity to proper assessment of dental materials.
Figure 6:
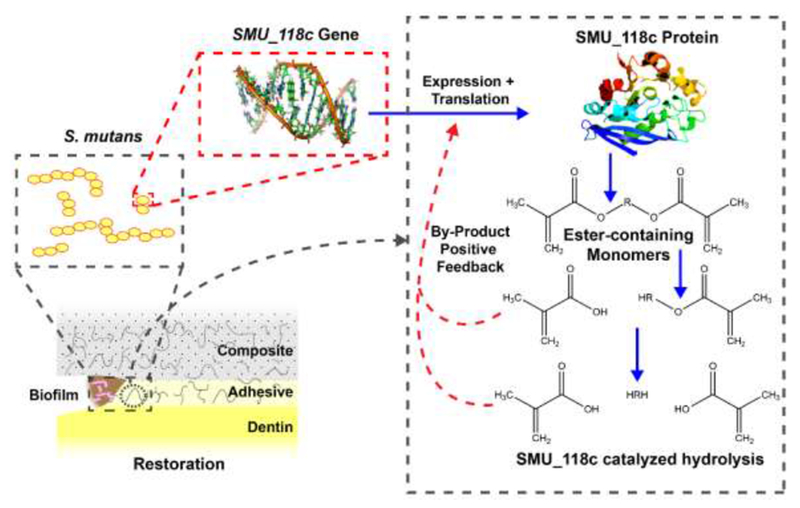
Schematic of resin degradation by bacterial esterase and the by-product/esterase activity positive feedback loop created. Adapted from Huang et al. [61].
2.5.6. Esterase and protease activity of endodontic pathogenic bacteria
Although S. mutans is one of the leading bacteriological contributors to acid production and caries, it is not the only bacterial species that may contribute to the degradation of dental materials through enzyme production. Marashdeh et al. [83] carried out several studies on Enterococcus faecalis, an oral bacterial species commonly associated with persistent endodontic infections, and as such likely also interacts with the coronal component of restorations and may have relevance to the biological interactions of restorative materials. E. faecalis was incubated with colorimetric assay substrates p-NPB and BTC and demonstrated significant CE-like hydrolase activity. Furthermore, when incubated with resin composite, and self- and total-etch adhesives, E. faecalis produced significant amounts of bisHPPP, demonstrating effectiveness at degrading common restorative resins. In another study [82], collagenase activity was also detected from E. faecalis and Micrococcus luteus. Bacteria incubated with generic and specific fluorometric collagenase assays showed significant MMP-1, 2, 8, and 9 -like activity, as well as hydroxyproline production when incubated with human dentin. SEM examination of dentin post-incubation showed visible evidence of degradation of the collagen fibril network. This activity was as high as 50-times that seen from endogenous dentinal or salivary MMP activity, suggesting bacterial breakdown of dentinal collagen at the restoration-tooth interface may me more significant to bond preservation than these host-derived activities. They also show that biodegradative activity relevant to resin restoratives and the restoration-tooth interface is not unique to S. mutans. Indeed, it is likely that other pathogenic species possess hydrolytic and collagenolytic activity able to compromise restorations and the tooth structure.
2.6. Summary of Challenges Faced by Dental Restorations
Dental resin composite restorations, especially the critical restoration-tooth interface where an adhesive is applied, face numerous challenges that limit their service life (Figure 7). The mechanical challenges these materials face are well understood, but research has identified the effects of many biochemical factors. Materials face biodegradation from host sources including salivary hydrolase activity, dentinal MMPs degrading hybrid layer collagen, and neutrophil degradative activity. Extrinsic degradation by oral bacteria, especially cariogenic and endodontic pathogens, degrade restorative materials and remaining tooth. Finally, the virulence of these bacteria is increased by restoration biodegradation by-products, creating a positive feedback loop that result with increased degradation. Restoration margin biodegradation creates an ideal environment for bacteria to proliferate, releasing acid and leading to the formation of secondary caries, thus providing a possible explanation to the higher rate of recurrent caries around these restorations.
Figure 7:
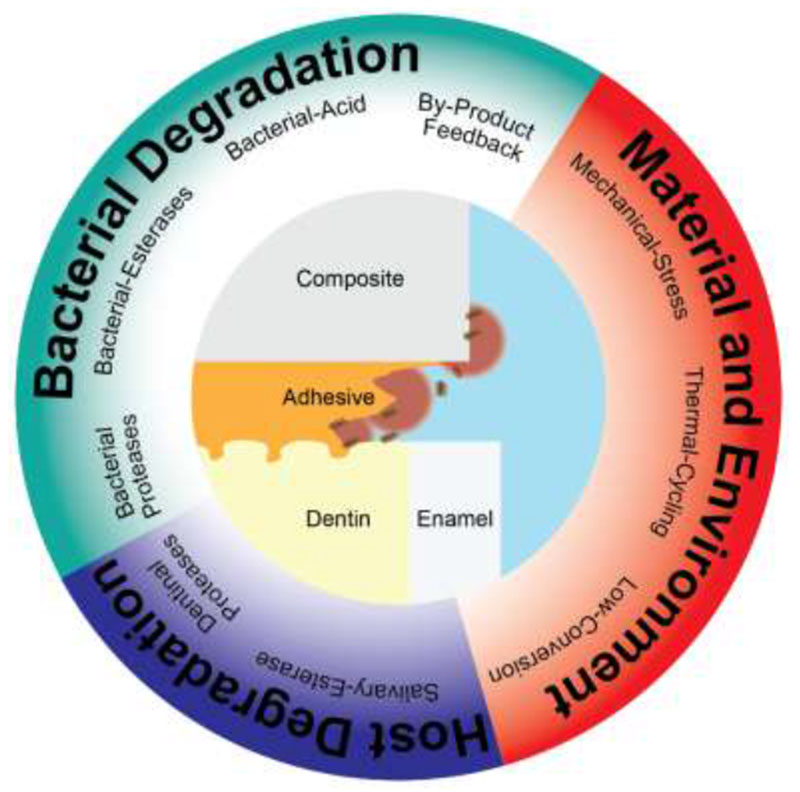
Schematic summary of the various challenges faced by resin dental restorations.
3. Current work to reduce biodegradation and secondary caries
3.1. Strategies to address clinical problems with resin restorative materials
There has been a great deal of research over the last 30 years in addressing the challenges to longevity faced by resin composite restorations in the oral cavity. With recent research giving a more complete picture of the challenges faced, rapid progress has been made towards new biostable dental restorative materials that retain the aesthetic and mechanical properties, and clinical workflows, of current systems. Most systems address either the biodegradation of resin materials bulk and/or the restoration margin, or seek to prevent bacterial ingress and proliferation to reduce secondary caries via antimicrobial capability. In this section several approaches to material design and testing are presented, with a summary of improved materialsshown in Table 1.
Table 1:
Summary of approaches taken to improve restoration service-life
| Category | Restoration Preservation Approach | Reference |
|---|---|---|
| Improvements to resin hydrolytic stability | Increasing resin monomer hydrophobicity | Kadoma et al. 2010 [84] |
| Shielding esterase bonds with fluorinated side-chains | Lagowski et al. 2017 [85] | |
| Inhibition of esterases via copper iodide nanoparticles | Renné et al. 2017 [86] | |
| Inhibition of esterase by antimicrobial monomers | Bortolatto et al. 2016 [87] | |
| MMP inhibition via quaternary ammonium monomers | Gou et al. 2018 [88] | |
| Antimicrobial non-releasing agents to inhibit biofilm formation in resin surface | Quaternary ammonium-containing monomers (eg. alkylated polyethylenimine) | Tiller et al. 2010 [89], Lin et al. 2002 [90] |
| Alkylated polyethylenimine particles | Beyth et al. 2010 [91] | |
| Quaternary ammonium monomers and self-healing resins | Yue et al. 2018 [92] | |
| Hydantoin monomers | Bortolatto et al. 2016 [87] | |
| Antimicrobial-Releasing resins to inhibit biofilm growth at material surface and within restoration margin | Chlorhexidine mixed into resin monomers | Anusavice et al 2006 [93] |
| Octenidine mixed into resin composites | Rupf et al. 2012 [94] | |
| Nitrous oxide-loaded mesoporous silica | Carpenter et al. 2013 [95] | |
| Chlorhexidine-loaded mesoporous silica | Zhang et al. 2014 [96] | |
| Octenidine-silica co-assembled particles | Stewart et al. 2018 | |
| Ciprofloxacin releasing drug-grafted-monomers | Delaviz et al. 2018 [97] | |
3.2. Methods for evaluating the biodegradation of new restorative materials
Evaluating new dental materials via relevant in vitro studies remains difficult. The complex bacteria- and enzyme-rich environment of the mouth is difficult to reproduce. However, several protocols have emerged to effectively assess new materials.
Collecting saliva from donors for the incubation of material specimens to assess biodegradation is time consuming and does not allow for the testing of biodegradation by enzymes without bacteria and may results with inconsistent activity due to variability of the donors. Salivary esterase-like activity also decreases rapidly with time, requiring regular replenishment. Serkies et al. developed a protocol for their study of long-term incubated fracture toughness specimens using a combination of CE and PCE to mimic the hydrolase activity of saliva (SHSE) [46]. The 5-day replenishment cycle of this protocol allowed for much longer-term incubation in a degradative environment (180 days).
Studies involving bacteria and bacterial inhibition are also difficult to maintain over long periods of time. Many studies of antimicrobial materials investigate only short-term effects on a low number of species, which may not be indicative of medium- and long-term capability [98]. Many of these studies claim a potential secondary-caries reducing effect, despite not demonstrating this [99]. Ideally studies would be performed in vivo in animal models of caries, however this is not always practical, especially in the development stage of the materials, when optimization requires testing of multiple formulations under standardised conditions.
One possible approach to simulate long-term incubation with bacteria is to pre-incubate bonded restoration specimens in the SHSE described earlier before exposing them to bacteria [16, 68Huang, 2018 #61]. This allows for a simulated long-term breakdown of the restoration margin and corresponding microgap increase without needing to maintain long-term bacterial cultures. These specimens may be subsequently incubated with bacteria to assess depth of penetration and total population within the marginal gap. Marashdeh et al. and Huang et al. have used this technique with great success, analyzing the depth of penetration of E. faecalis around endodontic sealers and S. mntcms at the restoration interface, respectively [68Huang, 2018 #61, 100], These studies can identify relative susceptibility of restorative materials to marginal gap formation and bacterial penetration.
To simulate long term effects of a more realistic bacterial environment on restorative materials, a single- or multi-species environment may be used with the aid of a chemostat-based biofilm fermenter (CBBF) [16, 68, 101], By placing restoration specimens in a controlled environment able to sustain a constant bacterial population and pH, the long term and localized effects of this population on the restoration interface may be observed. These systems may even be used to reproduce caries and assess the anti-caries effects of antimicrobial dental materials.
3.3. Material biostability
The most obvious strategy to combat the biodegradation of dental resin polymer matrices is to modify the monomer to be less susceptible to hydrolase or esterase activities. However, making changes to the polymer matrix to protect or eliminate vulnerable ester bonds remains difficult without sacrificing the material’s mechanical strength and/or conversion kinetic that compromises the clinical applicability of the material. For instance, Kadoma et al. have developed a fluorinated methacrylate system that may reduce biodegradation by increasing the hydrophobicity of the matrix and increasing chemical stability [84]. These resins take significantly longer to polymerize, therefore limiting clinical relevance.
Lagowski et al. have also developed a fluorinated UDMA monomer in order to shield vulnerable ester bonds from biodegradation in composite materials [85]. Through an iterative process these monomers have been designed to match current composite mechanical performance, while enhancing hydrophobicity and vinyl group conversion. Work continues to monitor the effects of long-term biodegradation on these materials through incubation with SHSE and monitoring biodegradation by-product release.
Several studies incorporating antimicrobial properties in dental restorative materials have also investigated secondary effects on resin biostability. Al Ghanem et al. utilized copper iodide nanoparticles to inhibit bacterial growth, but noticed some bond preservation after 1-year incubation in distilled water [86]. The authors suggested that this was due to the potentially esterase-inhibiting effect of the particles and the release of copper, though the inhibition was not confirmed. Renne et al. also investigated similar polyacrylic acid-coated copper iodide particles, and corroborated previous results confirming antimicrobial efficacy [86]. They also demonstrated a strong ability of the material to inhibit collagen degradation in a simulated body fluid, but did not determine a source of this effect. This may preserve the hybrid layer in dental restorations, and subsequently preserve bond strength.
3.4. Antimicrobial monomers and non-released agents
A growing body of work has investigated the use of non-releasing additives to resin for anti-biofilm or antimicrobial effect through contact killing [102]. This may inhibit bacterial proliferation on the restorative surface or at the marginal gap where in contact with the restorative. The major advantage of these materials is the relative performance of their effect: with potentially no elution of antimicrobial substance there is no payload decrease or decrease in effect with time [103–105].
Polymers containing insoluble positively charge species such as quaternary ammoniums have shown biofilm inhibiting potential [89]. Negatively charged bacterial cells are attracted to the positively charged surface of the material. When in contact, the bacterial cell wall is disrupted, causing the cell to lyse and die [106]. Alkylated polyethylenimine either incorporated in the resin polymer matrix itself, or bound to the matrix in particle form, has shown an ability to prevent gram positive and negative bacteria adherence [90]. The particle form of these antimicrobial polymers is of particular interest, as incorporating particles may interfere less with the polymerization and mechanical properties of the resin than modifying the resin matrix [91]. However, these materials do have several limitations: antimicrobial chemistry must be compatible with the polymer system it is incorporated with, and surface killing may be self-deactivated through the build-up of extracellular material or dead cells on the resin surface [103, 107]. The interactions of these materials with a realistic biodegradative environment are also rarely studied. Most analyses are conducted in water or a neutral buffer, without the presence of hydrolytic enzymes that would normally be present in the mouth. The effect of biodegradation on many of these antimicrobial monomers is unknown, but for those containing unprotected and easily hydrolyzed bonds, it may be hypothesized that there will be release of antimicrobial monomer or monomer by-products into the oral cavity, despite their covalent attachment to the matrix.
Two recent applications of covalently bonded antimicrobial monomers included other active measures to improve the stability of the restoration bond. Yue et al. developed a quaternary ammonium-containing antimicrobial resin that also included microcapsules of unpolymerized TEGDMA and an initiator [92]. Upon fracture, the released TEGDMA was able to partially “heal” the damaged bond and seal the microcrack. In another study by Gou et al., the secondary anti-degradative effects of a quaternary ammonium monomer were investigated, and a large decrease of gelatinolytic activity was observed [88]. This may help protect the hybrid layer from collagenolytic attack. However, in both cases, the long-term effects of the secondary active protection measures were not investigated. This validation will be key to demonstrate what realistic positive impact these secondary bond-preservation and -repair effects have.
Hydantoin is another highly bactericidal molecule that shows effectiveness against common oral pathogens and limited human toxicity. Work by Bortolatto et al. to develop a hydantoin derivative antimicrobial monomer compatible with methacrylate dental resins was unique in its analysis of the effects of biodegradation on the material developed [87]. The hydantoin-containing resin composite showed a log 2.68 reduction in S. mutans biofilm viability for 72 hours with no toxicity to human gingival fibroblasts. When samples were incubated with a simulated human salivary enzyme solution, hydantoin-containing composite showed significantly lower rates of bisHPPP release, suggesting an antidegradative capability of the material. This antidegradative effect was then verified by demonstrating that free hydantoin-derived monomers showed a strong ability to inhibit the activity of cholesterol esterases against bisGMA. All-encompassing studies like this one are necessary to account for the complex degradative environment in the oral cavity, and to design materials with multiple disruptive effects on factors that damage and compromise resin restorations.
3.5. Antimicrobial releasing materials
Several groups have studied the direct mixing of antimicrobials into dental resins. Anusavice et al. mixed Chlorhexidine into urethane based resins at extremely high weight percent (33 % wt.) [93]. Although they demonstrated release for 4 months and modeled release lasting over 10 years, 50% of drug was released from samples during the first few days followed by extremely low release (sub minimum inhibitory concentration per week). Release was not studied under degradative conditions, and significant damage to specimen surfaces was seen from chlorhexidine dissolution.
In another study, Rupf et al. mixed octenidine dihydrochloride into dental composites and studied their performance in situ [94]. This study demonstrated slower release (0.2 % wt. of drug load in one week) and a reduction in in situ biofilm formation, but did not explore the long-term prospects, mechanical characteristics, or drug release kinetics of the material.
Utilizing drug-carrier particles to store and release drug without the formation of voids may circumvent these problems and extend release by limiting drug diffusion from the carrier. One promising vector is mesoporous silica nanoparticles (MSNs), porous particles that are structurally and chemically similar to the filler particles present in resin composites [108]. These particles are able to store large amounts of drug within their pores and prolong release by the limited diffusion of media into the particle, and subsequent diffusion of drug out [109]. After drug is expended, the MSN remains, occupying the void and supporting the resin structurally [108, 110]. However, the use of MSNs in long-term drug release applications is still limited by current drug loading techniques, and the speed at which drug is released [111–116]. Within the context of dental materials, Carpenter et al. utilized nitrous oxide loaded MSNs to effect a log-3 reduction in S. mutans when particles were loaded in dental resin, but release half-life was only 23 days [95]. Zhang et al. used chlorhexidine-loaded MSN adhesive to inhibit bacteria growth over 1 day, with release shown for 16 days [96]. Lee et al. used amphotericin B-loaded MSNs in a polymethyl methacrylate matrix but observed near complete release in 50 days [117]. In these studies, the limits of drug loading within MSNs has resulted in insufficient longevity. Furthermore, these studies do not account for biodegradation, its effects on drug release rates, or the effect of released drug on biodegradation.
Stewart et al. have developed an antimicrobial-drug-silica co-assembled particle system similar to MSN synthesis that is does not involve a separate drug loading step, utilizing octenidine dihydrochloride [118] (Figure 8 A). These highly loaded particles greatly improve payload and extend release when incorporated in a total-etch adhesive at 10% weight [119]. Less than 1% of drug was released over 90 days while demonstrating almost total inhibition of bacterial attachment and growth. Of importance is the relationship between the material and degradative enzymes. Biodegradation and erosion of the resin matrix increased drug release (Figure 8 B), providing a triggered release mechanism during bacterial degradation. Furthermore, the released drug octenidine dihydrochloride showed a strong ability to inhibit model and salivary enzymes’ activity towards the resin matrix [120]. Miniature short-rod specimens bonded using the test adhesive retained initial fracture toughness after 6-months of incubation in a simulated degradative environment, while the fracture toughness of control specimens was significantly reduced.
Figure 8:
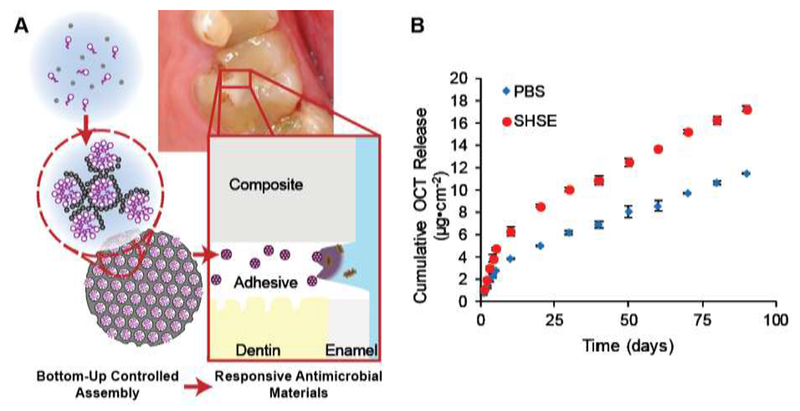
Schematic overview (A) of drug-silica co-assembled particles for responsive long-term antimicrobial dental adhesive Release of the antimicrobial drug octenidine from resin adhesive (B) is increased in the presence of biodegradative activity (simulated human salivary esterase or SHSE) [119]
Another approach is to use labile bonds for a triggered release of antibiotic from the resin polymer matrix. In this kind of system, the degradative capabilities of bacteria and saliva are used to trigger the release of drug through the hydrolysis of a susceptible bond between a traditional antibiotic molecule and a carrier monomer. Delaviz et al. grafted the antibiotic ciprofloxacin onto a dimethacrylate monomer with a labile bond to create a monomer pro-drug [97]. This new cross-linking monomer was both more hydrolytically stable than bisGMA (at its terminal methacrylate groups) as well as able to release ciprofloxacin upon biodegradation as a by-product. In this way the new material may limit biodegradation as well as respond to it through elimination of infiltrating bacteria at the restoration margin. However, significant work was first necessary to synthesize a monomer that was soluble in current resin monomer solutions and that would not interfere with polymerization. There may also be concerns over the long-term exposure of the oral cavity to an antibiotic, and the potential development of antibiotic resistance. The use of this system in conjunction with other antimicrobial strategies may mitigate this risk.
4. Conclusions
The use of resin composite restorations is ubiquitous in dentistry, but their clinical benefits come with sacrifices in longevity and stability. A large and growing body of work over the last 30 years has elucidated the effects of host and bacterial degradation of resins in the oral cavity. Furthermore, the various degradative pathways and their effects on each other have been more fully explored in recent years, tying biodegradation to a major cause of restoration failure: secondary caries. This newfound knowledge has enabled improvements to dental restorations: modification of their hydrolytic stability and mechanical properties, adding new antimicrobial components, or the use of enzyme inhibitors to prevent biodegradation. In the future, new resin composite restorative systems should aim to address many of these degradative challenges at once, breaking the feedback loops that amplify restoration degradation. This will lead to improved commercially applicable materials and increased service-life, and therefore a more positive long-term patient outcome.
Highlights:
Dental resin restoratives face biodegradative challenges from host- and bacterial-factors
Degradation at the restoration-tooth margin contributes to secondary caries
Degradation by-products increase degradative factors activity and bacterial virulence
Reproducing biodegradative effects in vitro is vital to assessing new materials
New materials should inhibit biodegradation as well as inhibit bacterial proliferation
Acknowledgments
5 Funding Sources
The work by our group that is described in this review was supported by the National Institutes of Health [R01DE021385-0]; the Canadian Institutes of Health Research [MOP 68947; MOP 115113]; Canada Foundation for Innovation John R. Evans Leaders Fund (CFI_JELF) [project #35378], and Ministry of Research and Innovation (MRI), Ontario Research Fund (ORF) [ORF-35378].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6 Declarations of interest: none
7 References
- [1].Demarco FF, Corrêa MB, Cenci MS, Moraes RR, Opdam NJM. Longevity of posterior composite restorations: not only a matter of materials. Dental materials : official publication of the Academy of Dental Materials. 2012;28:87–101. [DOI] [PubMed] [Google Scholar]
- [2].Khalichi P, Singh J, Cvitkovitch DG, Santerre JP. The influence of triethylene glycol derived from dental composite resins on the regulation of Streptococcus mutans gene expression. Biomaterials. 2009;30:452–9. [DOI] [PubMed] [Google Scholar]
- [3].Mackert JR, Berglund A. Mercury Exposure From Dental Amalgam Fillings: Absorbed Dose and the Potential for Adverse Health Effects. Critical Reviews in Oral Biology & Medicine. 1997;8:410–36. [DOI] [PubMed] [Google Scholar]
- [4].Ferracane JL. Resin composite - State of the art. Dental Materials. 2011;27:29–38. [DOI] [PubMed] [Google Scholar]
- [5].Borges BCD, Bezerra GVG, Mesquita JDA, Silva TRSF, Alves-Júnior C, Pinheiro IVDA, et al. Filler morphology of resin-based low-viscosity materials and surface properties after several photoactivation times. Acta odontologica Scandinavica. 2013;71:215–22. [DOI] [PubMed] [Google Scholar]
- [6].Deb S Polymers in dentistry. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine. 1998;212:453–64. [DOI] [PubMed] [Google Scholar]
- [7].De Munck J, Van Landuyt K, Peumans M, Poitevin A, Lambrechts P, Braem M, et al. A critical review of the durability of adhesion to tooth tissue: methods and results. J Dent Res 2005;84:118–32. [DOI] [PubMed] [Google Scholar]
- [8].Bowen R Use of epoxy resins in restorative materials. J Dent Res 1956;35:360–9. [DOI] [PubMed] [Google Scholar]
- [9].Delaviz Y, Finer Y, Santerre JP. Biodegradation of resin composites and adhesives by oral bacteria and saliva: a rationale for new material designs that consider the clinical environment and treatment challenges. Dent Mater. 2014;30:16–32. [DOI] [PubMed] [Google Scholar]
- [10].Santerre JP, Shajii L, Leung BW. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Critical Reviews in Oral Biology & Medicine. 2001;12:136–51. [DOI] [PubMed] [Google Scholar]
- [11].Smith D Posterior composite resin dental restorative materials. The Netherlands: Peter Szulc Publishing Co; 1985:47–60. [Google Scholar]
- [12].Van Landuyt KL, Snauwaert J, De Munck J, Peumans M, Yoshida Y, Poitevin A, et al. Systematic review of the chemical composition of contemporary dental adhesives. Biomaterials. 2007;28:3757–85. [DOI] [PubMed] [Google Scholar]
- [13].Barszczewska-Rybarek IM. Structure-property relationships in dimethacrylate networks based on Bis-GMA, UDMA and TEGDMA. Dental materials : official publication of the Academy of Dental Materials. 2009;25:1082–9. [DOI] [PubMed] [Google Scholar]
- [14].Cramer NB, Stansbury JW, Bowman CN. Recent advances and developments in composite dental restorative materials. Journal of dental research. 2011;90:402–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008;24:90–101. [DOI] [PubMed] [Google Scholar]
- [16].Kermanshahi S, Santerre JP, Cvitkovitch DG, Finer Y. Biodegradation of resin-dentin interfaces increases bacterial microleakage. J Dent Res 2010;89:996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Spencer P, Jonggu Park QY, Misra A, Bohaty BS, Singh V, Parthasarathy R, et al. Durable bonds at the adhesive/dentin interface: an impossible mission or simply a moving target? Braz Dent Sci 2012;15:4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Tam LE, Khoshand S, Pilliar RM. Fracture resistance of dentin-composite interfaces using different adhesive resin layers. J Dent. 2001;29:217–25. [DOI] [PubMed] [Google Scholar]
- [19].Tam LE, Pilliar RM. Fracture toughness of dentin/resin-composite adhesive interfaces. J Dent Res. 1993;72:953–9. [DOI] [PubMed] [Google Scholar]
- [20].Hashimoto M, Ohno H, Endo K, Kaga M, Sano H, Oguchi H. The effect of hybrid layer thickness on bond strength: demineralized dentin zone of the hybrid layer. Dental materials : official publication of the Academy of Dental Materials. 2000;16:406–11. [DOI] [PubMed] [Google Scholar]
- [21].Wang Y, Spencer P. Hybridization efficiency of the adhesive/dentin interface with wet bonding. Journal of dental research. 2003;82:141–5. [DOI] [PubMed] [Google Scholar]
- [22].Liu Y, Tjaderhane L, Breschi L, Mazzoni A, Li N, Mao J, et al. Limitations in Bonding to Dentin and Experimental Strategies to Prevent Bond Degradation. J Dent Res. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Peumans M, Kanumilli P, De Munck J, Van Landuyt K, Lambrechts P, Van Meerbeek B. Clinical effectiveness of contemporary adhesives: a systematic review of current clinical trials. Dental materials : official publication of the Academy of Dental Materials. 2005;21:864–81. [DOI] [PubMed] [Google Scholar]
- [24].Hashimoto M, Fujita S, Kaga M, Yawaka Y. Effect of water on bonding of onebottle self-etching adhesives. Dental materials journal. 2008;27:172–8. [DOI] [PubMed] [Google Scholar]
- [25].Kopperud SE, Tveit AB, Gaarden T, Sandvik L, Espelid I. Longevity of posterior dental restorations and reasons for failure. Eur J Oral Sci. 2012;120:539–48. [DOI] [PubMed] [Google Scholar]
- [26].Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitão J, et al. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trial. JADA. 2007;138:775–83. [DOI] [PubMed] [Google Scholar]
- [27].Murray PE, Hafez AA, Smith AJ, Cox CF. Bacterial microleakage and pulp inflammation associated with various restorative materials. Dental Materials. 2002;18:470–8. [DOI] [PubMed] [Google Scholar]
- [28].Soncini JA, Maserejian NN, Trachtenberg F, Tavares M, Hayes C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth. The Journal of the American Dental Association. 2007;138:763–72. [DOI] [PubMed] [Google Scholar]
- [29].Chisini LA, Collares K, Cademartori MG, Oliveira LJC, Conde MCM, Demarco FF, et al. Restorations in primary teeth: a systematic review on survival and reasons for failures. International Journal of Paediatric Dentistry. 2018;28:123–39. [DOI] [PubMed] [Google Scholar]
- [30].Mjor IA. Clinical diagnosis of recurrent caries. Journal of the American Dental Association (1939). 2005;136:1426–33. [DOI] [PubMed] [Google Scholar]
- [31].Arends J, Ruben J, Dijkman AG. Effect of fluoride release from a fluoride-containing composite resin on secondary caries: an in vitro study. Quintessence Int. 1990;21:671–4. [PubMed] [Google Scholar]
- [32].Eriksen HM, Bjertness E, Hansen BF. Cross-sectional clinical study of quality of amalgam restorations, oral health and prevalence of recurrent caries. Community Dent Oral Epidemiol. 1986;14:15–8. [DOI] [PubMed] [Google Scholar]
- [33].Takahashi N, Nyvad B. The role of bacteria in the caries process: ecological perspectives. J Dent Res. 2011;90:294–303. [DOI] [PubMed] [Google Scholar]
- [34].Spencer P, Ye Q, Park J, Topp EM, Misra A, Marangos O, et al. Adhesive/Dentin interface: the weak link in the composite restoration. Ann Biomed Eng. 2010;38:1989–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Anusavice KJ. Criteria for placement and replacement of dental restorations. Fla Dent J. 1988;59:30–1. [PubMed] [Google Scholar]
- [36].Brantley CF, Bader JD, Shugars DA, Nesbit SP. Does the cycle of rerestoration lead to larger restorations? Journal of the American Dental Association (1939). 1995;126:1407–13. [DOI] [PubMed] [Google Scholar]
- [37].Hunter AR, Treasure ET, Hunter AJ. Increases in cavity volume associated with the removal of class 2 amalgam and composite restorations. Oper Dent. 1995;20:2–6. [PubMed] [Google Scholar]
- [38].Bouillaguet S BIOLOGICAL RISKS OF RESIN-BASEDMATERIALS TO THE DENTIN-PULP COMPLEX. Crit Rev Oral Biol Med. 2004;15:47–60. [DOI] [PubMed] [Google Scholar]
- [39].Li Y, Carrera C, Chen R, Li J, Lenton P, Rudney JD, et al. Degradation in the dentin-composite interface subjected to multi-species biofilm challenges. Acta Biomaterialia. 2014;10:375–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bean TA, Zhuang WC, Tong PY, Eick JD, Yourtee DM. Effect of esterase on methacrylates and methacrylate polymers in an enzyme simulator for biodurability and biocompatibility testing. J Biomed Mater Res. 1994;28:59–63. [DOI] [PubMed] [Google Scholar]
- [41].Bourbia M, Ma D, Cvitkovitch DG, Santerre JP, Finer Y. Cariogenic bacteria degrade dental resin composites and adhesives. J Dent Res. 2013;92:989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Finer Y, Santerre JP. The influence of resin chemistry on a dental composite’s biodegradation. J Biomed Mater Res A. 2004;69:233–46. [DOI] [PubMed] [Google Scholar]
- [43].Shokati B, Tam LE, Santerre JP, Finer Y. Effect of salivary esterase on the integrity and fracture toughness of the dentin-resin interface. Journal of biomedical materials research Part B, Applied biomaterials. 2010;94:230–7. [DOI] [PubMed] [Google Scholar]
- [44].Jaffer F, Finer Y, Santerre JP. Interactions between resin monomers and commercial composite resins with human saliva derived esterases. Biomaterials. 2002;23:1707–19. [DOI] [PubMed] [Google Scholar]
- [45].Santerre JP, Shajii L, Tsang H. Biodegradation of commercial dental composites by cholesterol esterase. Journal of dental research. 1999;78:1459–68. [DOI] [PubMed] [Google Scholar]
- [46].Serkies KB, Garcha R, Tam LE, De Souza GM, Finer Y. Matrix metalloproteinase inhibitor modulates esterase-catalyzed degradation of resin dentin interfaces. Dental Materials. 2016;32:1513–23. [DOI] [PubMed] [Google Scholar]
- [47].Ferracane JL, Condon JR. Rate of elution of leachable components from composite. Dental Materials. 1990;6:282–7. [DOI] [PubMed] [Google Scholar]
- [48].Finer Y, Santerre JP. Influence of silanated filler content on the biodegradation of bisGMA/TEGDMA dental composite resins. J Biomed Mater Res A. 2007;81:75–84. [DOI] [PubMed] [Google Scholar]
- [49].Freund M, Munksgaard EC. Enzymatic degradation of BISGMA/TEGDMA-polymers causing decreased microhardness and greater wear in vitro. European Journal of Oral Sciences. 1990;98:351–5. [DOI] [PubMed] [Google Scholar]
- [50].Chauncey HH. Salivary enzymes. Journal of the American Dental Association (1939). 1961;63:360–8. [DOI] [PubMed] [Google Scholar]
- [51].Huang B, Siqueira WL, Cvitkovitch DG, Finer Y. Esterase from a Cariogenic Bacterium Hydrolyzes Dental Resins. Acta Biomaterialia. 2018;71:330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Winkler MM, Greener EH, Lautenschlager EP. Non-linear in vitro wear of posterior composites with time. Dent Mater. 1991;7:258–62. [DOI] [PubMed] [Google Scholar]
- [53].Larsen IB, Freund M, Munksgaard EC. Change in surface hardness of BisGMA/TEGDMA polymer due to enzymatic action. J Dent Res. 1992;71:1851–3. [DOI] [PubMed] [Google Scholar]
- [54].Lappin DF, Koulouri O, Radvar M, Hodge P, Kinane DF. Relative proportions of mononuclear cell types in periodontal lesions analyzed by immunohistochemistry. J Clin Periodontol. 1999;26:183–9. [DOI] [PubMed] [Google Scholar]
- [55].Finer Y, Santerre JP. Salivary esterase activity and its association with the biodegradation of dental composites. J Dent Res. 2004;83:22–6. [DOI] [PubMed] [Google Scholar]
- [56].Santerre JP, Labow RS. The effect of hard segment size on the hydrolytic stability of polyether-urea-urethanes when exposed to cholesterol esterase. J Biomed Mater Res. 1997;36:223–32. [DOI] [PubMed] [Google Scholar]
- [57].Shajii L, Paul Santerre J. Effect of filler content on the profile of released biodegradation products in micro-filled bis-GMA/TEGDMA dental composite resins. Biomaterials. 1999;20:1897–908. [DOI] [PubMed] [Google Scholar]
- [58].Finer Y, Jaffer F, Santerre JP. Mutual influence of cholesterol esterase and pseudocholinesterase on the biodegradation of dental composites. Biomaterials. 2004;25:1787–93. [DOI] [PubMed] [Google Scholar]
- [59].Sadeghinejad L, Cvitkovitch DG, Siqueira WL, Merritt J, Santerre JP, Finer Y. Mechanistic, genomic and proteomic study on the effects of BisGMA-derived biodegradation product on cariogenic bacteria. Dent Mater. 2017;33:175–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Sadeghinejad L, Cvitkovitch DG, Siqueira WL, Santerre JP, Finer Y. Triethylene Glycol Up-regulates Virulence-associated Genes and Proteins in Streptococcus mutans. PLOS ONE. 2016;in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Huang B, Sadeghinejad L, Adebayo OA, Xiao YH, Siqueira WL, Cvitkovitch DG, et al. Gene Expression and Protein Synthesis of an Esterase from Streptococcus mutans is affected by Biodegradation By-product from Resin Composites and Adhesives. To be submitted to Acta Biomaterialia. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Nagase H, Fushimi K. Elucidating the function of non catalytic domains of collagenases and aggrecanases. Connect Tissue Res. 2008;49:169–74. [DOI] [PubMed] [Google Scholar]
- [63].Sano H, Takatsu T, Ciucchi B, Russell CM, Pashley DH. Tensile properties of resin-infiltrated demineralized human dentin. J Dent Res. 1995;74:1093–102. [DOI] [PubMed] [Google Scholar]
- [64].Sulkala M, Tervahartiala T, Sorsa T, Larmas M, Salo T, Tjaderhane L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch Oral Biol. 2007;52:121–7. [DOI] [PubMed] [Google Scholar]
- [65].Martin-De Las Heras S, Valenzuela A, Overall CM. The matrix metalloproteinase gelatinase A in human dentine. Arch Oral Biol. 2000;45:757–65. [DOI] [PubMed] [Google Scholar]
- [66].Mazzoni a, Mannello F, Tay FR, Tonti GaM, Papa S, Mazzotti G, et al. Zymographic analysis and characterization of MMP-2 and −9 forms in human sound dentin. Journal of dental research. 2007;86:436–40. [DOI] [PubMed] [Google Scholar]
- [67].Pashley DH, Tay FR, Yiu C, Hashimoto M, Breschi L, Carvalho RM, et al. Collagen degradation by host-derived enzymes during aging. 2004. p. 216–21. [DOI] [PubMed] [Google Scholar]
- [68].Huang B, Cvitkovitch DG, Santerre JP, Finer Y. Biodegradation of resin–dentin interfaces is dependent on the restorative material, mode of adhesion, esterase or MMP inhibition. Dental Materials. 2018. [DOI] [PubMed] [Google Scholar]
- [69].Carrilho M, Geraldeli S, Tay F, de Goes MF, Carvalho RM, Tjäderhane L, et al. In vivo preservation of the hybrid layer by chlorhexidine. Journal of dental research. 2007;86:529–33. [DOI] [PubMed] [Google Scholar]
- [70].Carrilho MRO, Carvalho RM, de Goes MF, di Hipólito V, Geraldeli S, Tay FR, et al. Chlorhexidine Preserves Dentin Bond in vitro. Journal of dental research. 2007;86:90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Shono Y, Terashita M, Shimada J, Kozono Y, Carvalho RM, Russell CM, et al. Durability of resin-dentin bonds. J Adhes Dent. 1999;1:211–8. [PubMed] [Google Scholar]
- [72].Wataha JC, Rueggeberg FA, Lapp CA, Lewis JB, Lockwood PE, Ergle JW, et al. In vitro cytotoxicity of resin-containing restorative materials after aging in artificial saliva. Clin Oral Investig. 1999;3:144–9. [DOI] [PubMed] [Google Scholar]
- [73].Burrow MF, Satoh M, Tagami J. Dentin bond durability after three years using a dentin bonding agent with and without priming. Dent Mater. 1996;12:302–7. [DOI] [PubMed] [Google Scholar]
- [74].Zaman S, Finer Y. Effects of Resin Monomers and their Degradation By-products and the Long-Term Inhibitory activity of Galardin on Human Dentinal Matrix Metalloproteinases. World Biomaterials Congress. Montreal, Canada2015. [Google Scholar]
- [75].Khalichi P, Cvitkovitch DG, Santerre JP. Effect of composite resin biodegradation products on oral streptococcal growth. Biomaterials. 2004;25:5467–72. [DOI] [PubMed] [Google Scholar]
- [76].Hansel C, Leyhausen G, Mai UE, Geurtsen W. Effects of various resin composite (co)monomers and extracts on two caries-associated micro-organisms in vitro. Journal of dental research. 1998;77:60–7. [DOI] [PubMed] [Google Scholar]
- [77].Kawai K, Tsuchitani Y. Effects of resin composite components on glucosyltransferase of cariogenic bacterium. Journal of Biomedical Materials Research. 2000;51:123–7. [DOI] [PubMed] [Google Scholar]
- [78].Singh J, Khalichi P, Cvitkovitch DG, Santerre JP. Composite resin degradation products from BisGMA monomer modulate the expression of genes associated with biofilm formation and other virulence factors in Streptococcus mutans. J Biomed Mater Res A. 2009;88:551–60. [DOI] [PubMed] [Google Scholar]
- [79].Huang B, Ma D, Bourbia M, Cvitkovitch DG, Santerre JP, Finer Y. Identification and Characterization of Degradative Activities of Esterase Isolated from Streptococcus mutans the 45th Annual Meeting of the AADR and the 40th Annual Meeting of the CADR. Los Angeles, CA2016. [Google Scholar]
- [80].Ajdić D, McShan WM, McLaughlin RE, Savić G, Chang J, Carson MB, et al. Genome sequence of <em>Streptococcus mutans</em> UA159, a cariogenic dental pathogen. Proceedings of the National Academy of Sciences. 2002;99:14434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Adebayo OA, Tam LE, Finer Y. Cariogenic Bacteria Compromise Resin-Dentin Interfacial Integrity 43rd Annual Meeting & Exhibition of the AADR. Charlotte, NC, USA2014. [Google Scholar]
- [82].Marashdeh QM, Gitalis R, Lévesque C, Finer Y. Endodontic Pathogens Possess Collagenolytic Activities that Degrade Human Dentin Collagen Matrix. International Endodontic Journal. 2018;in revision. [DOI] [PubMed] [Google Scholar]
- [83].Marashdeh MQ, Gitalis R, Levesque C, Finer Y. Enterococcus faecalis Hydrolyzes Dental Resin Composites and Adhesives. Journal of endodontics. 2018;44:609–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kadoma Y Kinetic polymerization behavior of fluorinated monomers for dental use. Dent Mater J. 2010;29:602–8. [DOI] [PubMed] [Google Scholar]
- [85].Lagowski M, Meilin Y, Finer Y, Santerre JP. Influence of Fluorinated Divinyl Urethane Monomers on Resin Composite Chemical Biostability and Physical Properties 33rd Annual Meeting Of The Canadian Biomaterials Society. Winnipeg, MB, Canada2017. [Google Scholar]
- [86].Alghanem A, Fernandes G, Visser M, Dziak R, Renné WG, Sabatini C. Biocompatibility and bond degradation of poly-acrylic acid coated copper iodide-adhesives. Dental Materials. 2017;33:e336–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bortolatto JF, Finer Y. Covalently bonded antimicrobial monomer for increased longevity of resin composites. World Biomaterials Congress Montreal, Canada 2016 p. 2118. [Google Scholar]
- [88].Gou YP, Meghil MM, Pucci CR, Breschi L, Pashley DH, Cutler CW, et al. Optimizing resin-dentin bond stability using a bioactive adhesive with concomitant antibacterial properties and anti-proteolytic activities. Acta Biomater. 2018. [DOI] [PubMed] [Google Scholar]
- [89].Tiller JC, Lee SB, Lewis K, Klibanov AM. Polymer surfaces derivatized with poly(vinyl-N-hexylpyridinium) kill airborne and waterborne bacteria. Biotechnology and Bioengineering. 2002;79:465–71. [DOI] [PubMed] [Google Scholar]
- [90].Lin J, Qiu S, Lewis K, Klibanov AM. Bactericidal properties of flat surfaces and nanoparticles derivatized with alkylated polyethylenimines. Biotechnology Progress. 2002;18:1082–6. [DOI] [PubMed] [Google Scholar]
- [91].Beyth N, Yudovin-Farber I, Perez-Davidi M, Domb AJ, Weiss EI. Polyethyleneimine nanoparticles incorporated into resin composite cause cell death and trigger biofilm stress in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22038–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Yue S, Wu J, Zhang Q, Zhang K, Weir MD, Imazato S, et al. Novel dental adhesive resin with crack self-healing, antimicrobial and remineralization properties. Journal of Dentistry. 2018. [DOI] [PubMed] [Google Scholar]
- [93].Anusavice KJ, Zhang NZ, Shen C. Controlled release of chlorhexidine from UDMA-TEGDMA resin. J Dent Res. 2006;85:950–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Rupf S, Balkenhol M, Sahrhage TO, Baum A, Chromik JN, Ruppert K, et al. Biofilm inhibition by an experimental dental resin composite containing octenidine dihydrochloride. Dental Materials. 2012;28:974–84. [DOI] [PubMed] [Google Scholar]
- [95].Carpenter AW, Reighard KP, Saavedra JE, Schoenfisch MH. O2-Protected Diazeniumdiolate-Modified Silica Nanoparticles for Extended Nitric Oxide Release from Dental Composites. Biomater Sci. 2013;1:456–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zhang JF, Wu R, Fan Y, Liao S, Wang Y, Wen ZT, et al. Antibacterial dental composites with chlorhexidine and mesoporous silica. J Dent Res. 2014;93:1283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Delaviz Y, Nascimento MA, Laschuk MW, Liu TW, Yang M, Santerre JP. Synthesis and characterization of Ciprofloxacin-containing divinyl oligomers and assessment of their biodegradation in simulated salivary esterase. Dent Mater. 2018. [DOI] [PubMed] [Google Scholar]
- [98].Cocco AR, de Oliveira da Rosa WL, da Silva AF, Lund RG, Piva E. A systematic review about antibacterial monomers used in dental adhesive systems: Current status and further prospects. Dental Materials. 2015;31:1345–62. [DOI] [PubMed] [Google Scholar]
- [99].do Amaral GS, de Cassia Negrini T, Maltz M, Arthur RA. Restorative materials containing antimicrobial agents: Is there evidence for their antimicrobial and anti-caries effects? - A systematic-review. Aust Dent J. 2015:6–15. [DOI] [PubMed] [Google Scholar]
- [100].Marashdeh M Exploration of Degradative Activities of Enterococcus faecalis and Determinants of Bacterial Biofilm Proliferation within the Sealer-Dentin Interfacial Margins. Toronto: University of Toronto; 2017. [Google Scholar]
- [101].Kinniment SL, Wimpenny JW, Adams D, Marsh PD. Development of a steady-state oral microbial biofilm community using the constant-depth film fermenter. Microbiol-Sgm. 1996;142 ( Pt 3:631–8. [DOI] [PubMed] [Google Scholar]
- [102].Imazato S, Ma S, Chen J- H, Xu HHK. Therapeutic polymers for dental adhesives: Loading resins with bio-active components. Dental materials : official publication of the Academy of Dental Materials. 2013;30:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Timofeeva L, Kleshcheva N. Antimicrobial polymers: mechanism of action, factors of activity, and applications. Applied microbiology and biotechnology. 2011;89:475–92. [DOI] [PubMed] [Google Scholar]
- [104].Wicht MJ, Haak R, Kneist S, Noack MJ. A triclosan-containing compomer reduces Lactobacillus spp. predominant in advanced carious lesions. Dental Materials. 2005;21:831–6. [DOI] [PubMed] [Google Scholar]
- [105].Beyth N, Farah S, Domb AJ, Weiss EI. Antibacterial dental resin composites. React Funct Polym. 2014;75:81–8. [Google Scholar]
- [106].Kawabata N, Nishiguchi M. Antibacterial activity of soluble pyridinium-type polymers. Applied and Environmental Microbiology. 1988;54:2532–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Siedenbiedel F, Tiller JC. Antimicrobial Polymers in Solution and on Surfaces: Overview and Functional Principles. Polymers-Basel. 2012;4:46. [Google Scholar]
- [108].Praveen S, Sun Z, Xu J, Patel A, Wei Y, Ranade R, et al. Compression and Aging Properties of Experimental Dental Composites Containing Mesoporous Silica as Fillers. Mol Cryst Liq Cryst. 2006;448:223/[825]–231/[833]. [Google Scholar]
- [109].Yamamoto E, Kuroda K. Colloidal Mesoporous Silica Nanoparticles. B Chem Soc Jpn. 2016;89:501–39. [Google Scholar]
- [110].Samuel SP, Li S, Mukherjee I, Guo Y, Patel AC, Baran G, et al. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dent Mater. 2009;25:296–301. [DOI] [PubMed] [Google Scholar]
- [111].Izquierdo-Barba I, Vallet-Regi M, Kupferschmidt N, Terasaki O, Schmidtchen A, Malmsten M. Incorporation of antimicrobial compounds in mesoporous silica film monolith. Biomaterials. 2009;30:5729–36. [DOI] [PubMed] [Google Scholar]
- [112].Lu J, Liong M, Zink JI, Tamanoi F. Mesoporous silica nanoparticles as a delivery system for hydrophobic anticancer drugs. Small. 2007;3:1341–6. [DOI] [PubMed] [Google Scholar]
- [113].Han N, Zhao Q, Wan L, Wang Y, Gao Y, Wang P, et al. Hybrid lipid-capped mesoporous silica for stimuli-responsive drug release and overcoming multidrug resistance. ACS Appl Mater Interfaces. 2015;7:3342–51 [DOI] [PubMed] [Google Scholar]
- [114].He Q, Shi J, Chen F, Zhu M, Zhang L. An anticancer drug delivery system based on surfactant-templated mesoporous silica nanoparticles. Biomaterials. 2010;31:3335–46. [DOI] [PubMed] [Google Scholar]
- [115].He Q, Gao Y, Zhang L, Zhang Z, Gao F, Ji X, et al. A pH-responsive mesoporous silica nanoparticles-based multi-drug delivery system for overcoming multi-drug resistance. Biomaterials. 2011;32:7711–20. [DOI] [PubMed] [Google Scholar]
- [116].Izquierdo-Barba I, Martinez A, Doadrio AL, Perez-Pariente J, Vallet-Regi M. Release evaluation of drugs from ordered three-dimensional silica structures. Eur J Pharm Sci. 2005;26:365–73. [DOI] [PubMed] [Google Scholar]
- [117].Lee J- H, El-Fiqi A, Jo J- K, Kim D- A, Kim S- C, Jun S- K, et al. Development of long-term antimicrobial poly(methyl methacrylate) by incorporating mesoporous silica nanocarriers. Dental Materials. 2016;32:1564–74. [DOI] [PubMed] [Google Scholar]
- [118].Stewart CA, Finer Y, Hatton BD. Drug self-assembly for synthesis of highlyloaded antimicrobial drug-silica particles. Scientific Reports. 2018;8:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Stewart CA, Hong JH, Hatton BD, Finer Y. Responsive antimicrobial dental adhesive based on drug-silica co-assembled particles. Acta Biomater. 2018;76:283–94. [DOI] [PubMed] [Google Scholar]
- [120].Stewart CA, Hong JH, Hatton BD, Finer Y. Preservation of Restoration Bond Through Interfacial Delivery of Antidegradative Agents International Associationof Dental Research. London, UK2018. [Google Scholar]


