Abstract
The high mortality rates associated with acute kidney injury are mainly due to extra-renal complications that occur following distant-organ involvement. Damage to these organs, which is commonly referred to as multiple organ dysfunction syndrome, has more severe and persistent effects. The brain and its sub-structures, such as the hippocampus, are vulnerable organs that can be adversely affected. Acute kidney injury may be associated with numerous brain and hippocampal complications, as it may alter the permeability of the blood-brain barrier. Although the pathogenesis of acute uremic encephalopathy is poorly understood, some of the underlying mechanisms that may contribute to hippocampal involvement include the release of multiple inflammatory mediators that coincide with hippocampus inflammation and cytotoxicity, neurotransmitter derangement, transcriptional dysregulation, and changes in the expression of apoptotic genes. Impairment of brain function, especially of a structure that has vital activity in learning and memory and is very sensitive to renal ischemic injury, can ultimately lead to cognitive and functional complications in patients with acute kidney injury. The objective of this review was to assess these complications in the brain following acute kidney injury, with a focus on the hippocampus as a critical region for learning and memory.
Keywords: Acute kidney injury, Hippocampus, Uremia, Uremic encephalopathy
Introduction
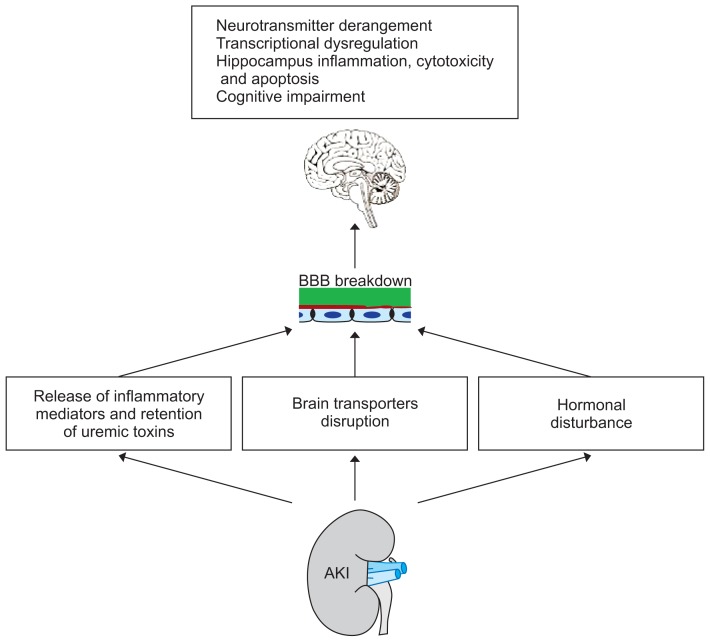
Acute kidney injury (AKI), also known as acute renal failure, with sudden loss of kidney function and retention of nitrogenous waste products such as urea and creatinine is the most common complication associated with remote organ dysfunction in critically ill patients [1]. The incidence of AKI is 34%, with an in-hospital mortality rate of around 62% [2]. Renal ischemia reperfusion is a common cause of AKI that can occur as a consequence of renal surgeries and transplantation [3]. The more obvious clinical outcomes of AKI include accumulation of waste products and electrolyte and fluid imbalance, while dysfunction of the immune system and of the cross-talk between non-renal organs make up less obvious effects. The high mortality rate associated with kidney diseases in acute and chronic conditions is mainly due to extra-renal complications and the involvement of other distant organs rather than due to the renal failure itself [4–7]. The central nervous system (CNS) is vulnerable during acute and chronic kidney diseases [8]. The pathophysiology of brain disorders has not been completely clarified. A number of known potential contributing factors for brain involvement after AKI include: 1) retention of nitrogenous end products (uremic toxins), 2) osmolality disturbance, and 3) inflammation.
A decrease in renal clearance of waste nitrogenous products accompanied with their continuous generation leads to diverse uremic retention products such as urea, creatinine, guanidine, and homocysteine. Many of these toxins affect functioning of cells and organs, resulting in endothelial vascular injury, neurotoxicity, and cognitive dysfunction [9]. In this regard, some studies were conducted on different animal models of kidney injury to distinguish the effects of uremia with (bilateral renal ischemia-reperfusion) or without renal ischemia (bilateral nephrectomy) on distant organs. While both models had unique, high levels of urea, creatinine, and organ injury and cytokine profiles with various patterns, they also had different global gene expression profiling in distant organs, which allowed them to be distinguished from each other [10–13]. These results indicated that the kidneys play an essential role in maintaining hemostasis and remote organ function.
High serum sodium concentrations and increased plasma osmolality in the brain promote the production of reactive oxygen species, resulting in endothelial injury and disruption of the blood-brain-barrier (BBB) and brain transporters [14,15]. Furthermore, immune responses after AKI lead to cytokine-induced disturbances in BBB permeability that trigger inflammatory cascades and subsequent damage in the brain with inflammation.
In addition to maintaining the acid-base and water-electrolyte balance, the kidneys have essential roles in cerebral homeostasis that include excreting toxins and drugs and changing neurotransmitter and cytokine concentrations. Unfortunately, little attention has been paid to the relationship between AKI and brain complications. Uremic encephalopathy, which progresses more in acute injury than in chronic kidney disease, is a condition that occurs following kidney damage [16,17]. Uremic encephalopathy is more prevalent, and with more complications, among patients with AKI than in those with chronic kidney damage because there is less time to adapt to uremia [18]. Neurologic complications following AKI are a major cause of mortality. Nervous system complications range in severity from fatigue to dementia, seizure, and coma. There is a higher incidence of dementia in renal failure patients than in other people [19]. A diverse range of cognitive and memory problems have been observed in patients with AKI who are undergoing hemodialysis. The hippocampus is considered an important CNS site for learning and memory consolidation. Hippocampal cellular inflammation due to the production of soluble inflammatory proteins following AKI has been reported [20]. Unfortunately, in spite of the importance brain involvement following AKI, few studies have been carried out on this subject, leaving its pathophysiology unclear. This paper will review some important mechanisms of disorders in the brain and its structures, especially the hippocampus, in AKI.
Impaired blood-brain-barrier integrity
BBB is a unique anatomical and physiological shield composed of tightly associated brain microvascular endothelial cells, astroglial foot processes, and capillary pericytes [21].
The restricted permeability of BBB is critical in maintaining cerebral hemostasis and proper neuronal functions as well as protecting the CNS against circulating toxins and pro-inflammatory molecules by filtering harmful compounds. Increased production of pro-inflammatory mediators coinciding with their decreased clearance causes the aggregation of pro-inflammatory cytokines and reactive oxygen species, which initiate systemic inflammatory responses following AKI. There has been growing evidence that systemic inflammation is associated with BBB disruption [22,23]. Unfortunately, there is a relatively limited pool of existing research investigating the association between AKI and BBB integrity. A link between microvascular permeability in a brain with cerebral edema and AKI was confirmed through an animal model of ischemia reperfusion injury [20]. In this study, pro-inflammatory chemokines in the brain structures, including the hippocampus and cerebral cortex, visualized by extravasation of Evans blue dye into the brain, suggested BBB disruption as a cause of brain inflammation and disorder after AKI.
Hormonal disturbance
Renin-angiotensin-aldosterone axis
Activation of the renin-angiotensin-aldosterone system in AKI leads to increased angiotensin II formation in peripheral circulation. Previous reports have confirmed the modulation of BBB permeability, allowing angiotensin II to cross into the brain [24,25]. Angiotensin II receptors (AT1, AT2, and AT4) or insulin-regulated membrane aminopeptidase has been detected in brain regions involved with cognitive processes, including the hippocampus [26–28]. Angiotensin II, acting either directly through related receptors or through modulatory effects on neurotransmitters, has contradictory effects on cognitive functions. Although the exact role of angiotensin II on cognitive behaviors and memory remains obscure, it appears that stimulation of the AT1 receptor via increased oxidative stress has deleterious effects on cerebellar development and cognitive functions, while conversely beneficial effects occur through AT2 receptor activation [29]. Neuronal transmission of angiotensin II has been related to mediatory effects on the excitability of presynaptic glutamatergic and gamma-aminobutyric acid-ergic neurons [30].
Cathecolamines
The proinflammatory cytokines released following AKI have widely distributed receptors in the brain micro-vasculature of the choroid plexus and subfornical and circumventricular organs that lack the BBB. Sympathetic effects of stimulating the subfornical organs [31] can lead to hemodynamic responses such as a decrease in cerebral blood flow and brain damage. Ischemic injury and edema in the medulla and cerebellum is a result of overactivity in the renal sympathetic nervous system, and ultimately leads to posterior reversible encephalopathy syndrome [31]. In addition, induction of prostaglandin E2 production by cyclooxygenase-2 activity through cytokine receptors [32] that can cross the BBB initiates brain inflammation and injury.
Natriuretic peptides
Plasma B-type natriuretic peptide, a biomarker of the congestive state, is elevated in AKI patients [33]. All types of natriuretic peptide are expressed in the CNS, and their receptors have been found in the brain. The function of natriuretic peptides in the CNS is still controversial. The binding of natriuretic peptides to related receptors lead to the formation of cyclic guanosine monophosphate, which has vasodilatory effects on blood vessels in the brain, thus increasing blood flow [34]. On the other hand, natriuretic peptides mediate nitric oxide (NO) production via stimulation of NO synthase [35]. The effect of NO on the brain varies depending on its cellular source of generation. Overproduction of NO during inflammation states such as AKI occurs through inducible or immunological NO synthase and neuronal NO synthase and has toxic consequences leading to neurodegenerative diseases and apoptosis [36].
Parathyroid hormone
Rapid mineral deregulation is an outcome of AKI. Some studies have shown that the levels of both ionized and total calcium decrease, as does the level of vitamin D, while phosphate levels increase [37,38]. Dysregulation of mineral metabolism, including that of calcium and phosphorus, along with elevation of fibroblast growth factor-23 levels as a key regulator of phosphorus excretion is accompanied with elevated parathyroid hormone (PTH) in patients with AKI [39]. PTH can cross the BBB, and its receptors are distributed throughout the human brain. Although there is a connection between AKI and abnormal PTH levels, the functional impact of PTH on cognition has not yet been conclusively determined [40]. Further studies are needed to determine the effects of PTH, vitamin D, and related factors such as calcium and phosphorus on cognitive function with AKI.
Neurotransmitter derangement and brain injury
Neurotransmitter balance in neuronal synaptic connections is essential for normal brain function. Disruption of neurotransmitter balance can lead to cerebral disorders ranging from motor activity difficulties to epilepsy and coma. AKI leads to an imbalance of excitatory and inhibitory neurotransmitters due to deregulation of transporters to across the BBB. Accumulation of guanidine compounds, including creatinine, guanidine, guanidinosuccinic acid, and methyl-guanidine, in the cerebrospinal fluid results in their efflux through the transporters into the circulation, which then has a stimulatory effect on N-methyl-D-aspartate (NMDA) glutamate receptors (GluR) and an inhibitory effect on γ-aminobutyric acid receptors [41]. Almost all peripheral inflammatory diseases are associated with wide-ranging behavioral symptoms from mood alterations to fatigue to cognitive impairments [42,43].
Interestingly, it has been demonstrated that peripheral organ inflammation can influence hippocampal synaptic transmission and enhance Schaffer collateral-induced excitatory field potentials in the cornu ammonis 1 (CA1) of the hippocampus via postsynaptic effects [44]. It seems that a relative change in the sub-units of aminomethylphosphonic acid and NMDA (GluR and NMDA receptor subtype 2B) receptors is involved in this process. Increased glutamate release and availability with down-regulation of cannabinoid receptors [45] potentiates transmitter release after systemic inflammation.
Systemic inflammation with proinflammatory cytokine production can stimulate related receptors in the BBB within circumventricular organs or on sensory afferents, which triggers a mirror inflammatory response in the brain.
In addition, disruption of NO synthesis through AKI-induced asymmetrical dimethyl arginine accumulation and reactive oxygen species can generate potent cytotoxic and proinflammatory byproducts such as proxynitrite, which can increase cerebrovascular permeability [46]. Brain catecholamine concentrations often decrease in AKI. Suppression of dopamine metabolism in the CNS is associated with motor activity disruption in uremic conditions [47].
Transcriptional dysregulation
Acute renal injury induces genomic expression in different areas of the brain, including the hippocampus. Genomic alterations that occur at an early stage of acute renal injury play an important role in initiating the pathological process of AKI.
Increased expression in the brain of the apoptotic Bax gene and decreased expression of the anti-apoptotic gene, B-cell lymphoma 2 (Bcl-2), was observed after renal ischemia reperfusion, indicating apoptosis of brain tissue following AKI [48]. Brain inflammation was also determined from the expression of the nuclear factor kappa B (NF-κB) pathway and cyclooxygenase-2/prostaglandin E2 downstream activation after renal ischemia reperfusion [48]. Under normal conditions, NF-κB is located in the cytosol in inactive form; however, in some abnormal situations, such as during oxidative stress after AKI, it can be activated and transferred to the nucleus, where it binds to the promoter and promotes the transcription and expression of cytokine genes.
Microarray analysis has demonstrated altered hippocampal mRNA expression in acute renal injury [18]. Some of these dysregulations are listed with their related outcomes in Table 1.
Table 1.
Some hippocampal dysregulations after acute kidney injury
| Variable | Outcome |
|---|---|
| Upregulation | |
| Macrophage scavenger receptor 1 | Inflammation |
| Cytotoxic T lymphocyte-associated protein 2 alpha & beta | Inflammation |
| Ras homolog gene family member J | Signaling disorders |
| Phospholipase A2 group III | Stress response |
| Serum amyloid A3 | Inflammation |
| Interleukin 12 receptor beta 1 | Inflammation |
| Chemokine (C–C motif) ligand 17 | Inflammation |
| B-cell leukemia/lymphoma 2 related protein A1a | Cell death |
| Downregulation | |
| ABC1, member 8a | Transporter dysfunction |
| Activin A receptor type 1C | Cell death |
| Copine V | Signaling disorders |
| Forkhead box P2 | Transcription impairment |
| Crystallin alpha B | Chaperone dysfunctions |
| G protein-coupled receptor 34 and 124 | Signaling disorders |
ABC1, ATP-binding cassette, sub-family A.
Altered gene expression and the resulting specific biological effects in the hippocampal area can lead to functional and cognitive disorders. Upregulation of Rho GTPase signaling leads to increased permeability of the BBB and inflammation through interaction with the actin cytoskeleton and phospholipid lysophosphatidic acid. Furthermore, the downregulation of claudin-1 and claudin-3 in tight junctions that occurs after AKI is associated with increasing blood-brain endothelial permeability. ATP-binding cassette transporters expressed in the BBB are involved in the efflux of toxic compounds from and influx of organic molecules into the CNS. Downregulation of these vital transporters following AKI can destroy the integrity of the BBB. Genes that are downregulated in the hippocampus after AKI and lead to accompanying hippocampal malfunction and neuronal injury include activin A receptor type 1C, which functions in cell survival, copine V, the transcription factor forkhead box P2, and the chaperone crystallin alpha B.
Hippocampus inflammation
Brain edema and dysfunction of the water transport system is a common complication of AKI. High levels of reactive oxygen species, NO, and inflammatory mediators have been detected in the hippocampus after AKI. The amount of pyknotic neurons and microglial cells or brain macrophages in the hippocampal CA1 was found to be increased after AKI. Increased levels of inflammatory markers (keratinocyte-derived chemoattractant and granulocyte-colony stimulating factor) and an increasing trend of monocyte chemoattractant protein-1 have been observed after AKI in both the hippocampus and cortex [20].
The accumulation of microglia, which are key mediators of inflammatory cascades, in the hippocampus following AKI indicate increased inflammation in the hippocampus.
The tight junction disruption induced by genomic dysregulation and the release of cytokines following AKI leads to microvascular leakage in the brain at both the soluble and cellular levels, in addition to the influx of inflammatory mediators and cytokines in the brain (Fig. 1).
Figure 1.
The hippocampus and brain complications following acute kidney injury (AKI).
BBB, blood-brain barrier.
The hippocampus is metabolically selective and vulnerable to brain edema, which ultimately leads to symptoms of cognitive impairment with hippocampal inflammation.
Hippocampus cytotoxicity and apoptosis
An imbalance between free radicals and antioxidant enzymes following the accumulation of oxidants after renal injury results in structural impairment and damage to the brain. High levels of reactive oxygen species, cytokines, and NO in the brain following AKI have been shown to correlate with neuronal cytotoxicity and apoptosis [18,20,48].
The hippocampus is one of the most sensitive regions of the brain to oxidative stress. The accumulation of free radicals in the peripheral system with impairment of BBB integrity leads to accumulation of free radicals in different parts of the brain, including the hippocampus.
The presence of pyknotic neurons and pyknosis in the hippocampus following AKI demonstrates an irreversible condensation of chromatin in the hippocampal nucleus that is associated with necrosis or apoptosis [20]. Dysregulation of the Bcl-2/Bax apoptosis proteins, as described earlier, is another cause of apoptosis in the brain and related sub-structures (such as the hippocampus) [48]. Although Karimi et al [49] showed that 45 minutes of renal ischemia followed by 24 hours of reperfusion cannot induce neuronal loss and synaptic plasticity impairment in the CA1, excitability of the Schaffer collateral CA1 synapse through postsynaptic receptors indicated the role of increased plasma creatinine levels in this state.
Reduction of locomotor activity is another symptom of hippocampal cytotoxicity that has been shown 24 hours and 1 week after renal reperfusion of bilateral ischemia [50]. It seems that destruction of CA1 neurons following AKI is involved in animals’ hypo-activity [20].
Cognitive impairment in AKI
While, most studies have focused on the correlation between chronic kidney disease and dementia, it is known that there is a connection between mild kidney disease and reduced cognitive activity. Moreover, there a direct relationship has been established between glomerular filtration rate (GFR) level and cognitive function [51]. The severity of AKI and progression of cognitive decline go hand in hand with GFR < 60 mL/min/1.73 m2 in patients. During the 12-year follow-up of a cohort study, patients with AKI exhibited a higher incidence of global cognitive impairment and dementia [52]. Sickness behavior is considered to be behavioral manifestations associated with peripheral inflammation [44], including inflammation associated with AKI, that indicate CNS involvement.
It seems that cognitive impairment following acute kidney damage is a sign of functional or structural changes in the hippocampus. Albuminuria, an index of endothelial pathology, which occurs with the progression of disease with impaired glomerular endothelial function increases the risk of reduced cognitive function [53]. Unfortunately, there are few studies in this area that can suggest mechanisms detailing the association between acute renal damage and cognitive functions. In this regard, Tahamtan et al [54] prospectively assessed the cognitive outcomes of bilateral renal ischemia followed by reperfusion for 24 hours and 1 week. They observed that AKI model rats have impaired passive avoidance learning and memory capabilities only at 24 hours after reperfusion because there is less time to recover from renal dysfunction.
In addition to the direct neuronal toxicity of the uremic state, there is a significantly higher incidence of brain ischemia with prevalence of cognitive impairment and dementia in patients with kidney disease [55]. All the potential microvascular and endothelial injury factors, such as uremia, oxidative stress, and inflammatory processes after AKI, can explain the risk of cerebrovascular dysfunction and cognition deterioration following renal disease.
Conclusions
The development of remote-organ involvement during AKI increases patient morbidity and mortality while increasing length of stay in the intensive care unit, and hence consumes considerable healthcare resources. In these situations, the treatment of the kidney alone without considering distant organs cannot be efficacious.
Certainly, elucidating the mechanisms of cross-talk between organs in AKI, especially the brain and its substructures like the hippocampus, which is more vulnerable to damage and peripheral changes, is a great help in treating AKI patients with brain involvement.
Footnotes
Conflicts of interest
The author has no conflicts of interest to declare.
References
- 1.Malek M, Nematbakhsh M. The preventive effects of diminazene aceturate in renal ischemia/reperfusion injury in male and female rats. Adv Prev Med. 2014;2014 doi: 10.1155/2014/740647. 740647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang CH, Fan PC, Chang MY, et al. Acute kidney injury enhances outcome prediction ability of sequential organ failure assessment score in critically ill patients. PLoS One. 2014;9:e109649. doi: 10.1371/journal.pone.0109649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev. 2015;4:20–27. doi: 10.12861/jrip.2015.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yap SC, Lee HT. Acute kidney injury and extrarenal organ dysfunction: new concepts and experimental evidence. Anesthesiology. 2012;116:1139–1148. doi: 10.1097/ALN.0b013e31824f951b. [DOI] [PubMed] [Google Scholar]
- 5.Chertow GM, Lazarus JM, Paganini EP, Allgren RL, Lafayette RA, Sayegh MH. Predictors of mortality and the provision of dialysis in patients with acute tubular necrosis. The Auriculin Anaritide Acute Renal Failure Study Group. J Am Soc Nephrol. 1998;9:692–698. doi: 10.1681/ASN.V94692. [DOI] [PubMed] [Google Scholar]
- 6.Malek M, Hassanshahi J, Fartootzadeh R, Azizi F, Shahidani S. Nephrogenic acute respiratory distress syndrome: a narrative review on pathophysiology and treatment. Chin J Traumatol. 2018;21:4–10. doi: 10.1016/j.cjtee.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly KJ. Acute renal failure: much more than a kidney disease. Semin Nephrol. 2006;26:105–113. doi: 10.1016/j.semnephrol.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Doi K, Rabb H. Impact of acute kidney injury on distant organ function: recent findings and potential therapeutic targets. Kidney Int. 2016;89:555–564. doi: 10.1016/j.kint.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Vanholder R, Pletinck A, Schepers E, Glorieux G. Biochemical and clinical impact of organic uremic retention solutes: a comprehensive update. Toxins (Basel) 2018;10 doi: 10.3390/toxins10010033. pii: E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoke TS, Douglas IS, Klein CL, et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol. 2007;18:155–164. doi: 10.1681/ASN.2006050494. [DOI] [PubMed] [Google Scholar]
- 11.Rabb H, Wang Z, Nemoto T, Hotchkiss J, Yokota N, Soleimani M. Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int. 2003;63:600–606. doi: 10.1046/j.1523-1755.2003.00753.x. [DOI] [PubMed] [Google Scholar]
- 12.Karimi Z, Ketabchi F, Alebrahimdehkordi N, Fatemikia H, Owji SM, Moosavi SM. Renal ischemia/reperfusion against nephrectomy for induction of acute lung injury in rats. Ren Fail. 2016;38:1503–1515. doi: 10.1080/0886022X.2016.1214149. [DOI] [PubMed] [Google Scholar]
- 13.Hassoun HT, Grigoryev DN, Lie ML, et al. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am J Physiol Renal Physiol. 2007;293:F30–F40. doi: 10.1152/ajprenal.00023.2007. [DOI] [PubMed] [Google Scholar]
- 14.Chi OZ, Hunter C, Liu X, Tan T, Weiss HR. Effects of VEGF on the blood-brain barrier disruption caused by hyperosmolarity. Pharmacology. 2008;82:187–192. doi: 10.1159/000151433. [DOI] [PubMed] [Google Scholar]
- 15.Sadik NA, Mohamed WA, Ahmed MI. The association of receptor of advanced glycated end products and inflammatory mediators contributes to endothelial dysfunction in a prospective study of acute kidney injury patients with sepsis. Mol Cell Biochem. 2012;359:73–81. doi: 10.1007/s11010-011-1001-4. [DOI] [PubMed] [Google Scholar]
- 16.Burn DJ, Bates D. Neurology and the kidney. J Neurol Neurosurg Psychiatry. 1998;65:810–821. doi: 10.1136/jnnp.65.6.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Deyn PP, Saxena VK, Abts H, et al. Clinical and pathophysiological aspects of neurological complications in renal failure. Acta Neurol Belg. 1992;92:191–206. [PubMed] [Google Scholar]
- 18.Chou AH, Lee CM, Chen CY, et al. Hippocampal transcriptional dysregulation after renal ischemia and reperfusion. Brain Res. 2014;1582:197–210. doi: 10.1016/j.brainres.2014.07.030. [DOI] [PubMed] [Google Scholar]
- 19.Fukunishi I, Kitaoka T, Shirai T, Kino K, Kanematsu E, Sato Y. Psychiatric disorders among patients undergoing hemodialysis therapy. Nephron. 2002;91:344–347. doi: 10.1159/000058418. [DOI] [PubMed] [Google Scholar]
- 20.Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol. 2008;19:1360–1370. doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serlin Y, Shelef I, Knyazer B, Friedman A. Anatomy and physiology of the blood-brain barrier. Semin Cell Dev Biol. 2015;38:2–6. doi: 10.1016/j.semcdb.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ratliff BB, Rabadi MM, Vasko R, Yasuda K, Goligorsky MS. Messengers without borders: mediators of systemic inflammatory response in AKI. J Am Soc Nephrol. 2013;24:529–536. doi: 10.1681/ASN.2012060633. [DOI] [PubMed] [Google Scholar]
- 23.Patel JP, Frey BN. Disruption in the blood-brain barrier: the missing link between brain and body inflammation in bipolar disorder? Neural Plast. 2015;2015 doi: 10.1155/2015/708306. 708306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleegal-DeMotta MA, Doghu S, Banks WA. Angiotensin II modulates BBB permeability via activation of the AT(1) receptor in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29:640–647. doi: 10.1038/jcbfm.2008.158. [DOI] [PubMed] [Google Scholar]
- 25.Biancardi VC, Stern JE. Compromised blood-brain barrier permeability: novel mechanism by which circulating angiotensin II signals to sympathoexcitatory centres during hypertension. J Physiol. 2016;594:1591–1600. doi: 10.1113/JP271584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis CJ, Kramár E, De A, et al. AT4 receptor activation increases intracellular calcium influx and induces a non-N-methyl-D-aspartate dependent form of long-term potentiation. Neuroscience. 2006;137:1369–1379. doi: 10.1016/j.neuroscience.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 27.von Bohlen und Halbach O, Albrecht D. Mapping of angiotensin AT1 receptors in the rat limbic system. Regul Pept. 1998;78:51–56. doi: 10.1016/S0167-0115(98)00109-8. [DOI] [PubMed] [Google Scholar]
- 28.AbdAlla S, Lother H, el Missiry A, et al. Angiotensin II AT2 receptor oligomers mediate G-protein dysfunction in an animal model of Alzheimer disease. J Biol Chem. 2009;284:6554–6565. doi: 10.1074/jbc.M807746200. [DOI] [PubMed] [Google Scholar]
- 29.Labandeira-Garcia JL, Rodríguez-Perez AI, Garrido-Gil P, Rodriguez-Pallares J, Lanciego JL, Guerra MJ. Brain renin-angiotensin system and microglial polarization: implications for aging and neurodegeneration. Front Aging Neurosci. 2017;9:129. doi: 10.3389/fnagi.2017.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pan HL. Brain angiotensin II and synaptic transmission. Neuroscientist. 2004;10:422–431. doi: 10.1177/1073858404264678. [DOI] [PubMed] [Google Scholar]
- 31.Wei SG, Zhang ZH, Beltz TG, Yu Y, Johnson AK, Felder RB. Subfornical organ mediates sympathetic and hemodynamic responses to blood-borne proinflammatory cytokines. Hypertension. 2013;62:118–125. doi: 10.1161/HYPERTENSIONAHA.113.01404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Konsman JP, Vigues S, Mackerlova L, Bristow A, Blomqvist A. Rat brain vascular distribution of interleukin-1 type-1 receptor immunoreactivity: relationship to patterns of inducible cyclooxygenase expression by peripheral inflammatory stimuli. J Comp Neurol. 2004;472:113–129. doi: 10.1002/cne.20052. [DOI] [PubMed] [Google Scholar]
- 33.Jeong EG, Nam HS, Lee SM, An WS, Kim SE, Son YK. Role of B-type natriuretic peptide as a marker of mortality in acute kidney injury patients treated with continuous renal replacement therapy. Ren Fail. 2013;35:1216–1222. doi: 10.3109/0886022X.2013.823870. [DOI] [PubMed] [Google Scholar]
- 34.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of C-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci U S A. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Costa MD, Bosc LV, Majowicz MP, Vidal NA, Balaszczuk AM, Arranz CT. Atrial natriuretic peptide modifies arterial blood pressure through nitric oxide pathway in rats. Hypertension. 2000;35:1119–1123. doi: 10.1161/01.HYP.35.5.1119. [DOI] [PubMed] [Google Scholar]
- 36.Toda N, Ayajiki K, Okamura T. Cerebral blood flow regulation by nitric oxide: recent advances. Pharmacol Rev. 2009;61:62–97. doi: 10.1124/pr.108.000547. [DOI] [PubMed] [Google Scholar]
- 37.Leaf DE, Waikar SS, Wolf M, Cremers S, Bhan I, Stern L. Dysregulated mineral metabolism in patients with acute kidney injury and risk of adverse outcomes. Clin Endocrinol (Oxf) 2013;79:491–498. doi: 10.1111/cen.12172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haycock GB. Management of acute and chronic renal failure in the newborn. Semin Neonatol. 2003;8:325–334. doi: 10.1016/S1084-2756(03)00044-7. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Hsu R, Hsu CY, et al. FGF-23 and PTH levels in patients with acute kidney injury: a cross-sectional case series study. Ann Intensive Care. 2011;1:21. doi: 10.1186/2110-5820-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lourida I, Thompson-Coon J, Dickens CM, et al. Parathyroid hormone, cognitive function and dementia: a systematic review. PLoS One. 2015;10:e0127574. doi: 10.1371/journal.pone.0127574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scaini G, Ferreira GK, Streck EL. Mechanisms underlying uremic encephalopathy. Rev Bras Ter Intensiva. 2010;22:206–211. doi: 10.1590/S0103-507X2010000200016. [DOI] [PubMed] [Google Scholar]
- 42.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Mello C, Swain MG. Liver-brain inflammation axis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G749–G761. doi: 10.1152/ajpgi.00184.2011. [DOI] [PubMed] [Google Scholar]
- 44.Riazi K, Galic MA, Kentner AC, Reid AY, Sharkey KA, Pittman QJ. Microglia-dependent alteration of glutamatergic synaptic transmission and plasticity in the hippocampus during peripheral inflammation. J Neurosci. 2015;35:4942–4952. doi: 10.1523/JNEUROSCI.4485-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu H, Ho W, Mackie K, Pittman QJ, Sharkey KA. Brain CB1 receptor expression following lipopolysaccharide-induced inflammation. Neuroscience. 2012;227:211–222. doi: 10.1016/j.neuroscience.2012.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watson CP, Pazarentzos E, Fidanboylu M, Padilla B, Brown R, Thomas SA. The transporter and permeability interactions of asymmetric dimethylarginine (ADMA) and L-arginine with the human blood-brain barrier in vitro. Brain Res. 2016;1648:232–242. doi: 10.1016/j.brainres.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adachi N, Lei B, Deshpande G, et al. Uraemia suppresses central dopaminergic metabolism and impairs motor activity in rats. Intensive Care Med. 2001;27:1655–1660. doi: 10.1007/s001340101067. [DOI] [PubMed] [Google Scholar]
- 48.Zhang N, Cheng GY, Liu XZ, Zhang FJ. Expression of Bcl-2 and NF-κB in brain tissue after acute renal ischemia-reperfusion in rats. Asian Pac J Trop Med. 2014;7:386–389. doi: 10.1016/S1995-7645(14)60061-4. [DOI] [PubMed] [Google Scholar]
- 49.Karimi N, Haghani M, Noorafshan A, Moosavi SMS. Structural and functional disorders of hippocampus following ischemia/reperfusion in lower limbs and kidneys. Neuroscience. 2017;358:238–248. doi: 10.1016/j.neuroscience.2017.06.058. [DOI] [PubMed] [Google Scholar]
- 50.Tahamtan M, Moosavi SM, Sheibani V, Nayebpour M, Esmaeili-Mahani S, Shabani M. Erythropoietin attenuates motor impairments induced by bilateral renal ischemia/reperfusion in rats. Fundam Clin Pharmacol. 2016;30:502–510. doi: 10.1111/fcp.12226. [DOI] [PubMed] [Google Scholar]
- 51.Elias MF, Elias PK, Seliger SL, Narsipur SS, Dore GA, Robbins MA. Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant. 2009;24:2446–2452. doi: 10.1093/ndt/gfp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai HH, Yen RF, Lin CL, Kao CH. Increased risk of dementia in patients hospitalized with acute kidney injury: a nationwide population-based cohort study. PLoS One. 2017;12:e0171671. doi: 10.1371/journal.pone.0171671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barzilay JI, Gao P, O’Donnell M ONTARGET and TRANSCEND Investigators, et al. Albuminuria and decline in cognitive function: the ONTARGET/TRANSCEND studies. Arch Intern Med. 2011;171:142–150. doi: 10.1001/archinternmed.2010.502. [DOI] [PubMed] [Google Scholar]
- 54.Tahamtan M, Sheibani V, Shid Moosavi SM, et al. Pre-treatment with erythropoietin attenuates bilateral renal ischemia-induced cognitive impairments. Iran J Pharm Res. 2018;17:601–612. [PMC free article] [PubMed] [Google Scholar]
- 55.Bugnicourt JM, Godefroy O, Chillon JM, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. J Am Soc Nephrol. 2013;24:353–363. doi: 10.1681/ASN.2012050536. [DOI] [PubMed] [Google Scholar]