Abstract
Objectives
To evaluate P-wave dispersion (PWD) and QT dispersion (QTd) in children with congenital heart disease and pulmonary arterial hypertension (PAH-CHD) and to investigate the predictive value of both PWD and QTd for prediction of arrhythmias in such children.
Materials and methods
We included 40 children with PAH-CHD as Group I. Forty other children with CHD and no PAH were included as Group II. Forty healthy children of matched age and sex served as a Control group. Electrocardiography was performed to determine PWD and QTd. Furthermore, 24-hour Holter monitoring was performed to detect the presence of arrhythmias. Echocardiographic evaluation was also performed.
Results
QTd and PWD were significantly higher in Group I than in Group II and Control group. A significant positive correlation was present between both QTd and PWD and mean pulmonary artery pressure, right ventricular diameter, pulmonary vascular resistance (PVR), and PVR to systemic vascular resistance ratio. QTd showed 93% sensitivity, 80% specificity, and 85% accuracy for prediction of occurrence of arrhythmias in patients with PAH-CHD at a cutoff point of 61 ms, whereas PWD showed 87% sensitivity, 80% specificity, and 85% accuracy for prediction of arrhythmias at a cutoff point of 32.5 ms in such patients. Logistic regression analysis showed that both QTd and PWD were good predictors for the occurrence of arrhythmias in children with PAH-CHD (p = 0.003 and p = 0.01, respectively).
Conclusions
PWD and QTd were good predictors for the occurrence of various arrhythmias in children with PAH-CHD.
Keywords: Children, Prediction, Pulmonary hypertension, P-wave dispersion, QT dispersion
Abbreviations
- AF
atrial fibrillation
- ANOVA
analysis of variance
- ASD
atrial septal defect
- AVC
atrioventricular canal
- CHD
congenital heart disease
- ECG
electrocardiogram
- EF
ejection fraction
- GE
general electric
- HR
heart rate
- LV
left ventricle
- LVEDD
left ventricular end diastolic diameter
- LVESD
left ventricular end systolic diameter
- LVOT
left ventricular outlet
- MHz
mega hertz
- MPAP
mean pulmonary artery pressure
- MR
mitral regurge
- NPV
negative predictive value
- PAH
pulmonary arterial hypertension
- PDA
patent ductus arteriosus
- PPV
positive predictive value
- PVR
pulmonary vascular resistance
- PWD
p wave dispersion
- ROC
receiver operating characteristics
- RVD
right ventricular diameter
- RVOT
right ventricular outlet
- SVR
systemic vascular resistance
- SVT
supraventricular tachycardia
- TGA
transposition of great arteries
- TR
tricuspid regurge
- QTd
QT dispersion
- VSD
ventricular septal defect
- VTI
velocity time integral
1. Introduction
Pulmonary arterial hypertension (PAH) is a common complication of congenital heart disease (CHD) [1]. PAH increases the probability for a variety of arrhythmias to occur through changing the homogeneity of ventricular repolarization and atrial conduction by several methods such as the modulation of autonomic activity, delayed cardiac repolarization, and right ventricular myocardial ischemia [2], [3]. Attempts to characterize these abnormalities of atrial conduction and ventricular repolarization are made using 12-lead electrocardiogram (ECG) [4].
P-wave dispersion (PWD) is the difference between the maximum and minimum P-wave duration in different ECG leads. Maximum P-wave and PWD durations are used to evaluate the homogenous distribution of the sinus impulse. Moreover, they are used to evaluate both the intra- and inter-atrial conduction times, most importantly in patients with paroxysmal atrial fibrillation (AF) [5].
QT dispersion (QTd) is the difference between the maximum and minimum QT durations in different ECG leads. It is found to be an important marker of heterogeneity of ventricular repolarization duration in variable ECG leads and reflects the regional differences in the recovery periods of ventricular myocardium. The prolongation of dispersion in QT is shown to be associated with increased risk of ventricular arrhythmias and sudden cardiac death for patients with PAH, coronary heart disease, and chronic heart disease [6], [7]. We need an early prediction of arrhythmias in such children to modify the management plan in these high-risk patients. We hypothesize that there will be a greater QTd and PWD in children with PAH-CHD than in those without PAH and that a greater QTd and PWD in this group will be associated with a greater arrhythmia burden.
Therefore, the aim of our study was to evaluate PWD and QTd in children with CHD and PAH (PAH-CHD), to investigate the predictive value of both PWD and QTd for prediction of arrhythmias in such children, and to correlate both PWD and QTd with various echocardiographic parameters.
2. Materials and methods
This prospective cross-sectional case–control study was conducted on 80 children with CHD who were admitted at Pediatric Cardiology Unit, Pediatric Department, Tanta University Hospital, Tanta, Egypt from January 2017 to April 2018. These children were further subdivided into two groups; 40 children with CHD and PAH as Group I and 40 other children with CHD but no PAH as Group II. Forty healthy children of matched age and sex were enrolled as a Control group. They were selected from healthy children attending Pediatric Outpatient Clinics at Tanta University Hospital. The study was approved by the Ethical Committee of the Faculty of Medicine, Tanta University. Written informed consent was signed by all participants’ parents or guardians.
Inclusion criteria included infants and children diagnosed with various CHDs either associated with PAH or not. PAH was defined as mean pulmonary artery pressure ≥25 mmHg.
Exclusion criteria included known risk factors causing prolongation of QT interval such as incomplete or complete heart block, bundle branch block, history of taking certain medications that can affect QT interval, such as macrolides, anti-arrhythmic drugs, antihistamine, antifungal, metabolic, or electrolyte disturbances, long QT syndrome, history of previous cardiac surgery, patients with heart failure, and patients with Eisenmenger syndrome.
All children enrolled in this study were subjected to the following. (1) Complete history taking and thorough clinical examination including heart rate (HR), O2 saturation, signs of CHDs and PAH, and complete local cardiac examination. (2) A 12-lead surface ECG using a three channel α 1000 apparatus. P-wave and QT interval dispersions were measured by manual method using hand-held calipers accompanied by use of magnification. PWD is calculated as the difference between the maximum and the minimum P-wave durations. QTd is calculated as the difference between the maximum and minimum QT duration in different leads of 12-lead ECG tracing. (3) A 24-hour Holter monitor to evaluate the occurrence of various arrhythmias. Arrhythmia was considered significant if symptomatic or if its duration was more than 30 seconds. Continuous ambulatory ECG monitoring using a compact digital Holter was recorded during a 24 hour period during which participants were free to practice their normal daily activities. (4) Echocardiographic assessment using Vivid 7 ultrasound machine (GE Medical System, Horten, Norway, with 7S MHz and 4S MHz multi-frequency transducers). Transthoracic echocardiographic evaluation was performed of the following parameters: type of CHD, mean pulmonary artery pressure (MPAP), left ventricle (LV) systolic function through LV ejection fraction (EF) where EF% = (LVEDD)3 − (LVESD)3/(LVEDD)3 × 100%, LV dimensions in the form of LV end systolic diameter (LVESD) and LV end diastolic diameter (LVEDD), right ventricular diameter (RVD) that was best measured at end diastole of right ventricle in apical four-chamber view, pulmonary vascular resistance (PVR), systemic vascular resistance (SVR), and PVR/SVR ratio.
PVR was measured according to the equation:
| (1) |
where VTI denotes velocity time integral of right ventricular outflow (RVOT) that can be obtained from spectral Doppler in the parasternal short axis, and V(TR) max denotes the peak tricuspid regurgitation velocity [8].
SVR was measured according to the equation:
| (2) |
where VTI denotes the velocity time integral of left ventricular outflow (LVOT) that can be obtained from spectral Doppler in the parasternal long axis view, and V(MR) max denotes the peak mitral regurgitation velocity [9].
The primary outcome was to evaluate PWD and QTd in children with PAH-CHD. The secondary outcomes were to evaluate the predictive value of both PWD and QTd for prediction of the occurrence of various arrhythmias in such children, and to correlate both PWD and QTd with various echocardiographic data.
2.1. Statistical analysis
Collected data were analyzed using SPSS version 17 (SPSS Inc., Chicago, IL, USA). Quantitative data were presented in the form of mean and standard deviation. Qualitative data were presented in the form of n (%). The Kolmogorov–Smirnov test was used to verify the normality of distribution of the obtained results. Qualitative data were compared using Chi-square test. Mean of the three groups was compared using one-way analysis of variance test. Post-hoc analysis was performed to reveal significant difference in between groups. Mean of the two groups was compared using Student t test. Correlation between variable was performed using Spearman correlation coefficient. Logistic regression analysis was performed to detect predictors of arrhythmias in our patients. Receiver operating characteristic curve was used to assess the prognostic value of QTd and PWD to predict the occurrence of arrhythmias at different cutoff points. Significance was judged when p < 0.05.
3. Results
We included 40 children, 26 male and 14 female, with PAH-CHD as Group I with a mean age of 27.3 ± 18.3 months and mean weight of 8.8 ± 3.6 kg, whereas Group II included 40 children, 20 male and 20 female, with CHD but no PAH, with a mean age of 25.6 ± 19.7 months and mean weight of 9.3 ± 3.9 kg. Forty healthy children, 22 male and 18 female, served as a Control group with a mean age of 27.5 ± 17.6 months, mean weight of 12.5 ± 4.1 kg. The patients included in Group I with PAH were diagnosed as follows: ventricular septal defect (VSD) in eight patients (20%), atrioventricular canal (AVC) in 10 patients (25%), patent ductus arteriosus (PDA) in six patients (15%), transposition of great arteries (TGA) in four patients (10%), atrial septal defect (ASD) in two patients (5%), and VSD + ASD in 10 patients (25%). Patients included in Group II without PAH were diagnosed as follows: VSD in 10 patients (25%), AVC in eight patients (20%), PDA in five patients (12.5%), TGA in five patients (12.5%), VSD + ASD in nine patients (22.5%), and ASD in three patients (7.5%). There was no statistically significant difference between the three groups with regards to age, sex, weight, or type of CHD (p > 0.05) (Table 1).
Table 1.
Comparison between the three studied groups regarding basic characteristic and demographic data.
| Variables | Group I | Group II | Control group | p |
|---|---|---|---|---|
| Age (mo) | 27.3 ± 18.3 | 25.6 ± 19.7 | 27.5 ± 17.6 | 0.356 |
| Sex (male:female) | 26:14 | 20:20 | 22:18 | 0.223 |
| Weight percentile | 0.9 ± 0.1 | 1 ± 0.1 | 1 ± 0.1 | 0.356 |
| Type of CHD | ||||
| -ASD | 2 (5) | 3 (7.5) | ||
| -VSD | 8 (20) | 10 (25) | ||
| -AVC | 10 (25) | 8 (20) | 0.342 | |
| -PDA | 6 (15) | 5 (12.5) | ||
| -TGA | 4 (10) | 5 (12.5) | ||
| -VSD + ASD | 10 (25) | 9 (22.5) |
Data are presented as n (%) or mean ± SD.
ASD = atrial septal defect; AVC = atrioventricular canal; CHD = congenital heart disease; PDA = patent ductus arteriosus; TGA = transposition of great arteries VSD = ventricular septal defect.
Regarding echocardiographic and hemodynamic data, MPAP, RVD, PVR, and PVR/SVR ratio were higher in Group I than in Group II and Control group (Table 2). Moreover, QT min, QT max, QTd, P min, P max, PWD, and average HR were significantly higher in Group I than in Group II and the Control group (Table 3).
Table 2.
Comparison between the three studied groups regarding hemodynamic echocardiographic measurement.
| Variables | Group I | Group II | HC | p | p1 (I vs. II) |
p2 (I vs. HC) |
p3 (II vs. III) |
|---|---|---|---|---|---|---|---|
| LVEDD | 3.6 ± 0.7 | 3.8 ± 0.6 | 3.7 ± 0.7 | 0.165 | 0.142 | 0.380 | 0.223 |
| LVESD | 2.4 ± 0.4 | 2.2 ± 0.4 | 2.3 ± 0.42 | 0.297 | 0.112 | 0.352 | 0.387 |
| EF | 64.65 ± 6.35 | 63.9 ± 5.26 | 65.9 ± 5.53 | 0.541 | 0.681 | 0.493 | 0.275 |
| RVD | 4.6 ± 0.6 | 3.8 ± 0.7 | 3.5 ± 0.5 | 0.001* | 0.001* | 0.001* | 0.055 |
| MPAP | 39.6 ± 5.5 | 20.3 ± 1.3 | 20.2 ± 1 | 0.001* | 0.001* | 0.001* | 0.445 |
| PVR | 4.7 ± 1.3 | 1.49 ± 0.3 | 1.2 ± 0.3 | 0.001* | 0.001* | 0.001* | 0.294 |
| SVR | 13.3 ± 1.8 | 13.9 ± 1.9 | 13 ± 2 | 0.265 | 0.167 | 0.273 | 0.07 |
| PVR/SVR | 0.4 ± 0.1 | 0.11 ± 0.02 | 0.10 ± 0.03 | 0.001* | 0.001* | 0.001* | 0.221 |
Data are presented as mean ± SD.
EF = ejection fraction; HC = healthy control; LVEDD = left ventricular end diastolic diameter; LVESD = left ventricular end systolic diameter; MPAP = mean pulmonary artery pressure; PVR = pulmonary vascular resistance; RVD = right ventricular diameter; SVR = systemic vascular resistance.
*means significant.
Table 3.
Comparison between the three studied groups regarding electrocardiographic parameters.
| Variables | Group I | Group II | HC | p | p1 (I vs. II) |
p2 (I vs. HC) |
p3 (II vs. III) |
|---|---|---|---|---|---|---|---|
| QT max | 391.3 ± 20 | 288.7 ± 16.8 | 285.2 ± 17.5 | 0.001* | <0.001* | <0.001* | 0.261 |
| QT min | 366.8 ± 22.1 | 253.7 ± 25.7 | 248.5 ± 22.4 | 0.001* | <0.001* | <0.001* | 0.250 |
| QTd | 45.4 ± 16.4 | 32.1 ± 8.9 | 32 ± 8 | 0.001* | 0.001* | 0.001* | 0.487 |
| P max | 71.7 ± 7.1 | 56.2 ± 3 | 58.2 ± 3.9 | 0.001* | <0.001* | <0.001* | 0.306 |
| P min | 46.5 ± 6.4 | 39.3 ± 6.5 | 39.7 ± 6.4 | 0.001* | 0.001* | 0.001* | 0.423 |
| PWD | 28.6 ± 2.7 | 12 ± 3 | 13.7 ± 4.3 | 0.001* | <0.001* | <0.001* | 0.084 |
| Average HR | 154 ± 11.1 | 146 ± 10.5 | 146.2 ± 8.8 | 0.001* | 0.01* | <0.001* | 0.474 |
Data are presented as mean ± SD.
HC = healthy control; HR = heart rate; max = maximum; min = minimum; PWD = P-wave dispersion; QTd = QT dispersion.
*means significant.
There was no significant correlation between PWD or QTd with LVESD, LVEDD, SVR nor EF% in studied groups (p > 0.05), whereas there was significant positive correlation between both PWD and QTd with MPAP, RVD, PVR, and PVR/SVR ratio (p < 0.05; Table 4).
Table 4.
Correlation between QT and P-wave dispersion and different studied variables in studied groups.
| Variables | QTd |
PWD |
||
|---|---|---|---|---|
| r | p | r | p | |
| MPAP | 0.731 | 0.001* | 0.498 | 0.02* |
| LVEDD | 0.073 | 0.760 | 0.194 | 0.413 |
| LVESD | 0.104 | 0.663 | 0.017 | 0.944 |
| RVD | 0.825 | 0.001* | 0.498 | 0.02* |
| EF | 0.072 | 0.763 | 0.374 | 0.104 |
| PVR | 0.305 | 0.001* | 0.291 | 0.001* |
| SVR | 0.154 | 0.517 | 0.054 | 0.820 |
| PVR/SVR | 0.944 | 0.001* | 0.708 | 0.001* |
EF = ejection fraction; LVEDD = left ventricular end diastolic diameter; LVESD = left ventricular end systolic diameter; MPAP = mean pulmonary artery pressure; PVR = pulmonary vascular resistance; PWD = P-wave dispersion; QTd = QT dispersion; RVD = right ventricular diameter; SVR = systemic vascular resistance.
*means significant.
In 24-hour ECG, 10 children in Group I developed arrhythmias, while no children in Group II or Control group had arrhythmias. Supraventricular tachycardia (SVT) occurred in six patients (15%), atrial flutter occurred in two patients (5%), whereas AF occurred in another two patients (5%). There was no episode of ventricular tachycardia in any patients of the studied groups.
Comparison of values of QTd, QT max, QT min, P max, P min, and PWD in patients with and without arrhythmias in Group I showed that these parameters were significantly higher in patients with arrhythmias than in those without arrhythmias (p < 0.05) (Table 5).
Table 5.
QT and P-wave dispersion in children with and without arrhythmias in Group I.
| Variables | Children with arrhythmias (n = 10) |
Children without arrhythmias (n = 30) |
p |
|---|---|---|---|
| QT max (ms) | 441.7 ± 25.1 | 377.2 ± 20.3 | <0.001* |
| QT min (ms) | 376.4 ± 20.8 | 337.4 ± 23.2 | 0.001* |
| QT dispersion (ms) | 65.2 ± 5.2 | 39.8 ± 4.9 | 0.001* |
| P max (ms) | 97.1 ± 9.3 | 53.5 ± 4.1 | <0.001* |
| P min (ms) | 62.5 ± 8.2 | 26.2 ± 3.8 | <0.001* |
| P-wave dispersion (ms) | 34.8 ± 1.1 | 27.5 ± 2.1 | 0.001* |
max = maximum; min = minimum.
*means significant.
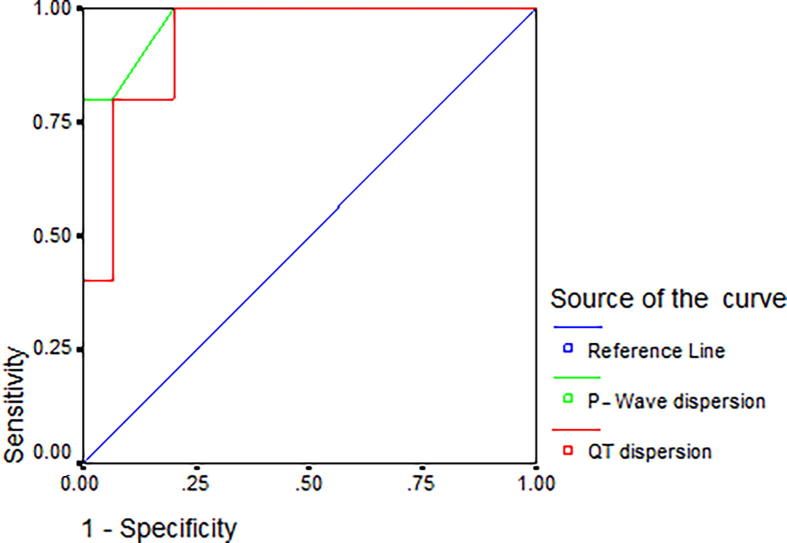
QTd showed 93% sensitivity, 80% specificity, 88% positive predictive value (PPV), 85% negative predictive value (NPV), and 85% accuracy for prediction of occurrence of arrhythmias at a cutoff point of more than 61 ms in patients with PAH-CHD, whereas PWD showed 87% sensitivity, 80% specificity, 93% PPV, 77% NPV, and 85% accuracy for prediction of arrhythmias at a cutoff point of more than 32.5 ms in these patients (Table 6 and Fig. 1).
Table 6.
Predictive value of both QT and P-wave dispersion to predict arrhythmias in children with congenital heart disease and pulmonary arterial hypertension.
| Variables | Cutoff value (ms) | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|---|
| QT dispersion | 61 | 93 | 80 | 88 | 85 | 85 |
| P-wave dispersion | 32.5 | 87 | 80 | 93 | 77 | 85 |
NPV = negative predictive value; PPV = positive predictive value.
Fig. 1.
Receiver operating characteristic curve of the predictive value of both QT and P-wave dispersion to predict the occurrence of arrhythmias in children with congenital heart disease and pulmonary arterial hypertension.
Logistic regression analysis revealed that QTd was a good predictor for the occurrence of arrhythmias among children with PAH-CHD [odds ratio (OR): 4.3; 95% confidence interval (CI): 1.82–7.25; p = 0.003). PWD also showed a good predictive value for the occurrence of arrhythmias (OR: 2.4; 95% CI: 1.24–3.56; p = 0.01).
4. Discussion
PAH is one of the most common complications of CHD that increases the tendency for the occurrence of arrhythmias [3]. P max and PWD have been used for the evaluation of homogenous propagation of sinus impulses and consequently, the inter- and intra-atrial conduction. Dilatation of the atria, whatever the cause, is the principle cause of PWD increase [10]. Higher PWD was found in children with ASD than in the Control group and mean maximum P-wave duration and PWD were significantly correlated with ASD size and right atrial dilatation [11].
In our study, PWD duration was found to be significantly prolonged in children with PAH-CHD than in CHD patients without PAH and Control group. This was concordant with other studies [12], [13] that reported increased PWD in patients with PAH-CHD relative to those without PAH and Control group. Moreover, a cutoff value of 32.5 ms of PWD was found to predict the occurrence of arrhythmias in PAH-CHD children with a sensitivity of 87%, specificity of 80%, and accuracy of 85%. Similarly, Hallioglu et al. [14] found that PWD duration longer than 35 ms had a sensitivity of 83% and specificity of 89% for the inducibility of atrial tachyarrhythmias in patients who underwent surgical correction of Fallot tetralogy. Additionally, logistic regression analysis in our study showed that PWD was a good predictor for the occurrence of arrhythmias in children with PAH.
PWD was proven by Dilaveris et al. [15] to be a sensitive and specific ECG marker for the best separation between patients with history of paroxysmal lone AF and healthy participants. A cutoff value of 40 ms showed a sensitivity of 83% and specificity of 85% for the identification of patients with history of paroxysmal lone AF. Moreover, they revealed that the duration of P max of 110 ms or longer had a higher tendency to develop paroxysmal lone AF. This was comparable with our results that showed that the maximum duration of P wave in patients with arrhythmias was 97.1 ± 9.3.
QTd is now considered as a marker of increased risk of ventricular arrhythmias and even sudden cardiac death in variable clinical conditions. Prolongation of QTd was observed in patients with PAH due to sickle cell anaemia [4].
In our study, QTd duration was found to be significantly prolonged in children with PAH-CHD compared with those with CHD and no PAH and to the Control group. A cutoff value of 61 ms was found to predict the occurrence of arrhythmias in PAH-CHD children with a sensitivity of 93%, specificity of 80%, and accuracy of 85%. This is concordant with other investigators [12], [13] who reported increased QTd in patients with PAH-CHD relative to those without PAH and the Control group. Similarly, Goldener et al. [16] revealed that the risk of serious ventricular arrhythmia or sudden cardiac death was more obvious in patients with QTd of more than 65 ms. Moreover, logistic regression analysis in our study showed that QTd was a good predictor for the occurrence of arrhythmias in children with PAH.
In our study, Holter monitoring of the study participants recorded the occurrence of arrhythmias in children with PAH-CHD group only in the form of SVT in six patients (15%), atrial flutter in two patients (5%), and AF in another two patients (5%), whereas there was no incidence of ventricular tachyarrhythmias in any group. No arrhythmias were detected in Group II and Control group. In accordance with our results, the most common arrhythmias detected in patients with PAH were found to be sinus tachycardia, sinus bradycardia, SVT, atrial flutter, and AF, whereas the ventricular arrhythmias were relatively rare [17], [18].
In the present study, QTd and PWD were found to be positively correlated with MPAP. This was in agreement with previous studies [19], [20]. Moreover, our study showed a significant positive correlation between QTd and PWD from one side and both PVR and PVR/SVR ratio from the other side. This was in agreement with the results of Ece et al. [13], indicating increased risk of arrhythmias in patients with high PVR. Additionally, RVD was found to be significantly positively correlated with both PWD and QTd. This is in agreement with Rich et al. [21] who reported positive correlation between RVD and QT intervals in patients with PAH.
In our study, the significant prolongation of QTd and PWD in children with PAH-CHD versus children with CHD and no PAH or Control group indicated that PAH actually affects the homogenous distribution of sinus impulses and ventricular repolarization, thereby making these children more susceptible for arrhythmias, and both had a good predictive value to expect the occurrence of various arrhythmias in such children.
The limitation of the study was the relatively small number of children included. PVR and SVR were determined by echocardiography and not by catheterization as catheterization in children with CHD only or the Control group seemed unethical.
5. Conclusion
PWD and QTd were good predictors for the occurrence of various arrhythmias in children with PAH-CHD.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Farag M., El Amrousy D., El-Serogy H., Zoair A. Role of plasma asymmetric dimethyl-l-arginine levels in detection of pulmonary hypertension in children with CHD. Cardiol Young. 2018;28:1163–1168. doi: 10.1017/S1047951118001026. [DOI] [PubMed] [Google Scholar]
- 2.Rajdev A., Garan H., Biviano A. Arrhythmias in pulmonary arterial hypertension. Prog Cardiovasc Dis. 2012;55:180–186. doi: 10.1016/j.pcad.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Temple I.P. Arrhythmias in pulmonary arterial hypertension. J Congenit Cardiol. 2017;1:2. [Google Scholar]
- 4.Akgul F., Seyfeli E., Melek I., Duman S., Seydaliyeva T., Gali E. Increased QT dispersion in sickle cell disease: effect of pulmonary hypertension. Acta Haematol. 2007;118:1–6. doi: 10.1159/000100929. [DOI] [PubMed] [Google Scholar]
- 5.Aytemir K., Ozer N., Atalar E., Sade E., Aksöyek S., Övünç K. P-wave dispersion on 12-lead electrocardiography in patients with paroxysmal atrial fibrillation. Pacing Electrophysiol. 2000;23:1109–1112. doi: 10.1111/j.1540-8159.2000.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 6.Barr C.S., Naas A., Freeman M., Land C.C., Struthers A.D. QT dispersion and sudden unexpected death in chronic heart failure. Lancet. 1994;343:327–329. doi: 10.1016/s0140-6736(94)91164-9. [DOI] [PubMed] [Google Scholar]
- 7.Bulzaite I., Brazdzionyte J., Zaliunas R., Rickli H., Ammann P. QT dispersion and heart rate variability in sudden death risk stratification in patients with ischemic heart disease. Medicina Kaunas. 2006;42:450–454. [PubMed] [Google Scholar]
- 8.Abbas A., Fortuin F., Petal O., Moreno C.A., Schiller N.B., Lester S.J. Noninvasive determination of systemic vascular resistance using Doppler echocardiography. J Am Soc Echocardiogr. 2004;17:834–838. doi: 10.1016/j.echo.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Abbas A., Fortuin F., Schiller N., Appleton C.P., Moreno C.A., Lester S.J. A simple method for noninvasive estimation of pulmonary vascular resistance. Am J Cardiol. 2003;41:1021–1027. doi: 10.1016/s0735-1097(02)02973-x. [DOI] [PubMed] [Google Scholar]
- 10.Bu’Lock F., Mott M., Martin R. Left ventricular diastolic function in children measured by Doppler echocardiography: normal values and relation with growth. Br Heart J. 1995;73:334–339. doi: 10.1136/hrt.73.4.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ho T., Chia E., Yip W., Chan K.Y. Analysis of P-wave and P dispersion in children with secundum atrial septal defect. Ann Noninvasive Electrocardiol. 2001;6:305–309. doi: 10.1111/j.1542-474X.2001.tb00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sap F., Karatas Z., Altin H., Alp H., Oran B., Baysal T. Dispersion durations of P-wave and QT interval in children with congenital heart disease and pulmonary arterial hypertension. Pediatr Cardiol. 2013;34:591–596. doi: 10.1007/s00246-012-0503-5. [DOI] [PubMed] [Google Scholar]
- 13.Ece I., Uner A., Balli S., Oflaz M.B., Kibar A.E., Sal E. P-wave and QT interval dispersion analysis in children with Eisenmenger syndrome. Arch Turk Soc Cardiol. 2014;42:154–160. doi: 10.5543/tkda.2014.68704. [DOI] [PubMed] [Google Scholar]
- 14.Hallioglu O., Aytemir K., Celiker A. The significance of P-wave duration and P-wave dispersion for risk assessment of atrial tachyarrhythmias in patients with corrected Tetralogy of Fallot. Ann Noninvasive Electrocardiol. 2007;9:339–344. doi: 10.1111/j.1542-474X.2004.94569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dilaveris P., Gialafos E., Sideris S., Theopistou A.M., Andrikopoulos G.K., Kyriakidis M. Simple electrocardiographic markers for the prediction of paroxysmal idiopathic atrial fibrillation. Am Heart J. 1998;135:733–738. doi: 10.1016/s0002-8703(98)70030-4. [DOI] [PubMed] [Google Scholar]
- 16.Goldener B., Brandspiegel H., Horwitz L., Jadonath R., Cohen T.J. Utility of QT dispersion combined with the signal-averaged electrocardiogram in detecting patients susceptible to ventricular tachyarrhythmia. Am J Cardiol. 1995;76:1192–1194. doi: 10.1016/s0002-9149(99)80337-3. [DOI] [PubMed] [Google Scholar]
- 17.Kanemoto N., Sasamoto H. Arrhythmias in primary pulmonary hypertension. Jpn Heart J. 1979;20:765–775. doi: 10.1536/ihj.20.765. [DOI] [PubMed] [Google Scholar]
- 18.Tongers J., Schwerdtfeger B., Klein G., Kempf T., Schaefer A., Knapp J.M. Incidence and clinical relevance of supraventricular tachyarrhythmias in pulmonary hypertension. Am Heart J. 2007;153:127–132. doi: 10.1016/j.ahj.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Hong-liang Z., Qin L., Zhi-hong L., Zhi-hui Z., Chang-ming X., Xin-hai N. Heart rate-corrected QT interval and QT dispersion in patients with pulmonary hypertension. Wien Klin Wochenschr. 2009;121:330–333. doi: 10.1007/s00508-009-1184-9. [DOI] [PubMed] [Google Scholar]
- 20.Taooka Y., Takezawa G., Sutani A., Isobe T. QTc prolongation in pulmonary hypertension cases due to lung diseases. Respir Med. 2016;2:67–73. [Google Scholar]
- 21.Rich J., Thenappan T., Freed B., Patel A.R., Thisted R.A., Childres R. QTc prolongation is associated with impaired right ventricular function and predicts mortality in pulmonary hypertension. Int J Cardiol. 2013;167:669–676. doi: 10.1016/j.ijcard.2012.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]