Abstract
Background
Our previous study showed that SUMO1 expression is closely related to progression in non‐small cell lung cancer (NSCLC); however, the function of SUMO1 in NSCLC has not yet been well elucidated.
Methods
SUMO1 was enhanced or silenced in two NSCLC cell lines by using either forced SUMO1 expression or short hairpin RNA against SUMO1 lentiviral vectors, respectively. The biological functions of SUMO1 in NSCLC were investigated through colony‐formation, cell proliferation, and invasion assays, and cell cycle analysis. NF‐κB expression was detected in the overexpressed and silenced SUMO1 cell lines. Immunohistochemistry was used to detect an association between SUMO1 and NF‐κB in the cancer and adjacent tissues of 168 patients with lung cancer.
Results
Overexpressed SUMO1 promoted the proliferation rate, colony formation ability, invasion, and NF‐κB expression in an A549 cell line. Conversely, SUMO1 depletion inhibited the cell growth rate, colony formation ability, invasion, and NF‐κB expression in a Calu‐1 cell line. SUMO1 expression was significantly correlated with NF‐κB expression in lung adenocarcinoma and squamous carcinoma patients (r > 0.5, P < 0.001).
Conclusion
Our results provide evidence that SUMO1 promotes the proliferation and invasion of NSCLC cells by regulating NF‐κB.
Keywords: NF‐κB, NSCLC, SUMO1
Introduction
Lung cancer has the highest incidence and mortality of all malignant tumors worldwide.1, 2 Non‐small cell lung cancer (NSCLC) accounts for approximately 80% of lung cancer cases. Despite comprehensive treatment strategies, including surgery, radiotherapy, and targeted therapy, the survival rate remains low.3 Therefore, the identification of effective molecular targets influencing tumor proliferation and resistance to chemotherapy are urgently required to improve lung cancer treatment.
SUMO1, a member of the ubiquitin‐like protein family, is reported to play a critical role in post‐translational modifications.4 A newly discovered oncogene, SUMO1 is a key regulator of tumor proliferation, especially in glioblastoma.5 In breast,6 ovarian,7 and liver cancers, and other tumors,8 relevant studies have shown that the SUMO1 gene could activate the tumor cell epithelial‐to‐mesenchymal transition (EMT) process via the NF‐κB signaling pathway.9, 10 Our prior study indicated that SUMO1 overexpression is significantly associated with the grade of tumor differentiation, pathological tumor node metastasis (pTNM) stage, and lymphatic metastasis in NSCLC.11 However, the exact role of SUMO1 in driving NSCLC cell carcinogenesis remains unclear.
In this study, we investigated the biological function and mechanism of SUMO1 in NSCLC cells. Stable overexpression and knockdown SUMO1 cell lines were constructed, respectively. Immunohistochemistry was used to analyze and compare the correlation between SUMO1 and NF‐κB expression in 168 NSCLC patients.
Methods
Patients and tissue sample collection
Paraffin‐embedded tissue specimens from 168 patients with confirmed NSCLC were collected from March 2007 to August 2010 at the Department of Thoracic Surgery of Tangdu Hospital. Patients who received preoperative chemotherapy, radiotherapy, or EGFR‐targeted therapy were excluded. Detailed information on the enrolled patients was obtained from the computerized registry database of medical records. Histological classification of tumors was reviewed by two pathologists and based on World Health Organization criteria. The Regional Ethics Committee for Clinical Research of the Fourth Military Medical University approved the study protocol. All subjects provided informed consent.
Immunohistochemistry
Five micrometer sections were deparaffinized and rehydrated with graded alcohol. Endogenous peroxidase activity was blocked by 3% hydrogen peroxide in methanol for 30 minutes. Antigen retrieval was performed by microwaving sections in 10 mM citrate buffer (pH 6.0) at 95°C for 20 minutes. To reduce nonspecific binding, slides were blocked with goat serum for 40 minutes. The sections were then incubated in a humidified chamber at 4°C overnight with primary anti‐SUMO1 (diluted 1:100, Y299) or anti‐NF‐κB (diluted 1:100, ab16502; Abcam, Cambridge, MA, USA) antibodies. The slides were washed three times with phosphate‐buffered saline and then were incubated for 60 minutes with a labeled polymer. EnVision + Peroxidase activity was visualized using a DAB Elite kit (Beyotime Biotechnology, Shanghai, China), and the slides were counterstained with hematoxylin.
Cell culture
Human NSCLC cell lines SpcA‐1, A549, Calu‐1, and H838 were grown in RPMI 1640 media (Hyclone, Thermo Fisher Scientific, Beijing, China) supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), 100 units/mL penicillin, 100 μg/mL streptomycin, and 1% glutamine (Sigma‐Aldrich, St Louis, MO, USA).
Lentiviral transfection and stable cell line generation
Full‐length human SUMO1 complementary DNA was PCR amplified from total human lung cancer tissue complementary DNA, followed by insertion into the pMSCV vector immediately upstream of the internal ribosome entry site. The SUMO1 fragment was then isolated through NotI digestion and inserted into a SUMO1 retroviral vector (pMSCV/SUMO1). The NSCLC cells were infected at 10 population doublings with pMSCV/SUMO1 or the pMSCV control. After puromycin selection, the forced expression of SUMO1 was confirmed through quantitative real time (qRT)‐PCR and Western blot analyses from passages 5 to 30. Forced SUMO1 expression in the NSCLC cells used in this study were grown from passages 10 to 25 in vitro. The shRNA‐SUMO1 target sequence was: 5′‐GATCCCTCACAATACCGCA‐3′.
Western blot analysis
Protein was extracted using cell lysis buffer (Beyotime Biotechnology). Twenty‐five micrograms of protein quantified by the Bradford method was transferred to polyvinylidene fluoride (PVDF) membranes after separating by 12%
sodium dodecyl sulfate‐polyacrylamide gel electrophoresis. The PVDF membrane (Millipore, Billerica, MA, USA) was incubated at 4 °C overnight with the following antibodies: SUMO1 (1:2000, Y299), NF‐κB (1:2000, ab16502), and β‐actin (1:3000, ab8277; Abcam). Membranes were incubated with horseradish peroxidase‐coupled anti‐rabbit immunoglobulin G (1:20 000, Cell Signaling Technology, Danvers, MA, USA) at room temperature for 1.5 hours. The proteins were detected using electrochemiluminescence plus reagents (Thermo Fisher Scientific).
Quantitative RT‐PCR analysis
Total RNA was isolated using the TRIzol reagent according to the manufacturer's protocol (Thermo Fisher Scientific). Real‐time PCR was performed using SYBR Premix Ex Taq II (Takara, Dalian, China). Real‐time PCR was carried out using ABI Fast 7500 (Applied Biosystems, Foster City, CA, USA). The 2−ΔΔCt method was used to calculate the relative quantification of target genes. The primers used in this study were: SUMO‐1: 5′‐TGACCAGGAGGCAAAACCTTC‐3′ and 5′‐AATTCATTGGAACACCCTGTCTT‐3′; NF‐κB: 5′‐AACAGAGAGGATTTCGTTTCCG‐3′ and 5′‐TTTGACCTGAGGGTAAGACTTCT‐3′.
Cell proliferation assay
Non‐small cell lung cancer cells (4 × 103/well) were cultured in 96‐well tissue culture plates until they reached 50% confluence. The proliferation abilities of NSCLC cells were determined by CCK‐8 assays (Dojindo, Kumamoto, Japan). Ten microlitres of water‐soluble formazan dye was added to each well and incubated for 1.5 hours. The absorbance at 450 nm was measured by microplate reader. The absorbance of the negative control (optical density [OD]) was considered to be 0%.
Colony‐formation assay
A total of 200 NSCLC cells were seeded into each fresh six‐well plate. After seven days, the cells were rinsed with phosphate buffered saline twice, fixed with 10% formaldehyde, and stained with 0.1% crystal violet. The colonies were counted in each well.
Cell invasion assay
The invasive activity of NSCLC cells was estimated using transwells (8 μm pore size, 6.5 mm in diameter, polycarbonate membrane) coated with extracellular matrix gel (Corning, Tewksbury, MA, USA). An aliquot of 1 × 105 cells was placed in the upper chamber with 0.1 mL serum‐free medium, while the lower chamber was loaded with 0.5 mL of medium containing 10% fetal bovine serum. After incubation for 24 hours, the cells were fixed with 4% paraformaldehyde and then counterstained with 0.1% crystal violet. The cells that had migrated into the lower chamber were observed and counted under a light microscope.
Statistical analysis
GraphPad Prism 5.0 (GraphPad, La Jolla, CA, USA) was used for statistical analysis. All of the data are expressed as the mean ± standard error of three independent experiments. Differences between cell experiments were determined using the Student's t test. Spearman's rank correlation coefficient was used to detect the correlation between SUMO1 and NF‐κB expression. Statistical significance is represented as *P < 0.05 and **P < 0.01.
Results
Upregulation of SUMO1 enhanced the colony formation, proliferation, invasion, and cell cycle progression of non‐small cell lung cancer (NSCLC) cells
To investigate the effects of SUMO1 on NSCLC cells, we first tested the expression levels of SUMO1 in four lung cancer cell lines (Fig 1a,b). SUMO1 expression was high in Calu‐1 and H838 cells and low in spca‐1 and A549 cell lines. Stable cell lines with forced SUMO1 expression were established in A549 cells. qRT‐PCR and Western blot analysis revealed that SUMO1 expression was increased in forced SUMO1 expressed NSCLC cells compared to the control group (Fig 1c,d). We further investigated the effect of SUMO1 overexpression on the function of lung cancer cells. SUMO1 upregulation increased the colony‐formation ability (Fig 1e,f) and proliferation (Fig 1g) of NSCLC cells compared to the control. Furthermore, the number of NSCLC cells migrating through the filter was higher in the SUMO1 overexpressed group than the control (Fig 1k,l). The mobility of NSCLC cells in the wound‐healing assay was significantly increased after upregulation of SUMO1 (Fig 1h,i). Cell cycle analysis revealed that SUMO1 overexpression increased the percentage of NSCLC cells in the S phase compared to the control (Fig 1j). Collectively, these results indicated that SUMO1 upregulation enhances the proliferation and invasion of NSCLC cells in vitro.
Figure 1.
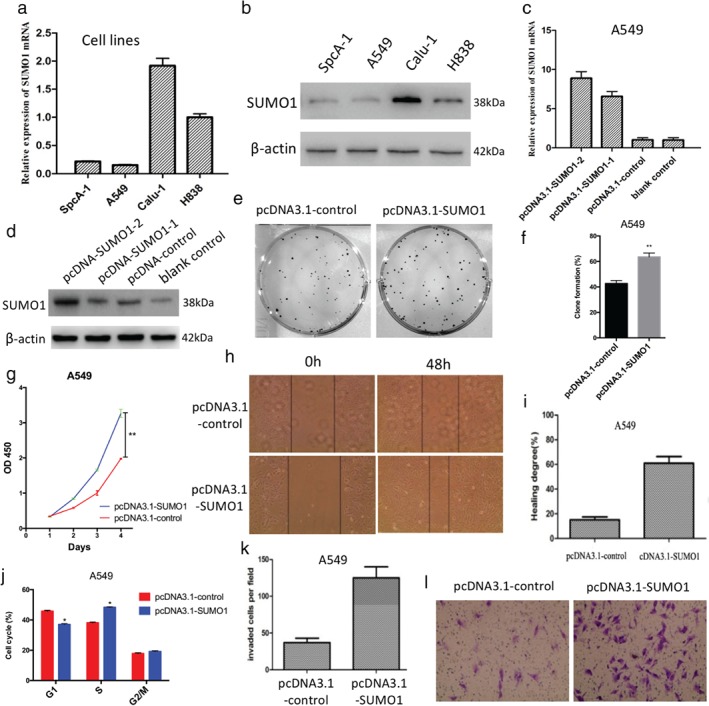
Stable forced SUMO1 expression enhanced the colony formation, proliferation, migration, cell cycle progression, and invasion of A549 cells in vitro. (a) Detection of messenger RNA (mRNA) expression of SUMO1 in different lung cancer cell lines by quantitative real time (qRT)‐PCR. (b) Similar results were obtained through Western blot analysis. (c) qRT‐PCR analysis revealed that SUMO1 mRNA expression levels were increased in SUMO1 overexpressed A549 cells compared to control cells. (d) Similar results were obtained through Western blot analysis (passages 15 and 30). Upregulation of SUMO1 enhanced the (e,f) colony‐formation ability, (g) proliferation, (h,i) migration, and (k,l) invasion of A549 cells. (j) Forced expression of SUMO1 increased the number of A549 cells in the S phase of the cell cycle. *P < 0.05, **P < 0.01. OD, optical density.
Downregulation of SUMO1 suppresses the colony formation, proliferation, invasion, and cell cycle progression of NSCLC cells
Quantitative RT‐PCR and Western blot were used to analyze the knockout efficiency of SUMO1 in shRNA‐SUMO1 Calu‐1 cells. SUMO1 was effectively suppressed in the shRNA‐SUMO1 Calu‐1 cell lines compared to the control (Fig 2a,b). We further investigated the effect of SUMO1 downregulation on the function of lung cancer cells. Cell counting kit 8 assay revealed that the knockout of SUMO1 expression dramatically inhibited the proliferation of NSCLC cells (Fig 2c). Downregulation of SUMO1 inhibited the colony‐formation ability compared to the control (Fig 2e,f). Mobility of NSCLC cells in the wound‐healing assay was notably decreased in shRNA‐SUMO1 cells compared to the control (Fig 2g,h). Cell invasion assay results showed that the fewer NSCLC cells migrated through the filter in the shRNA‐SUMO1 group than in the control (Fig 2i,j). Cell cycle analysis showed that downregulation of SUMO1 decreased the percentage of NSCLC cells in the S phase compared to the control (Fig 2d). These data suggested that SUMO1 downregulation inhibits the proliferation and invasion of NSCLC cells.
Figure 2.
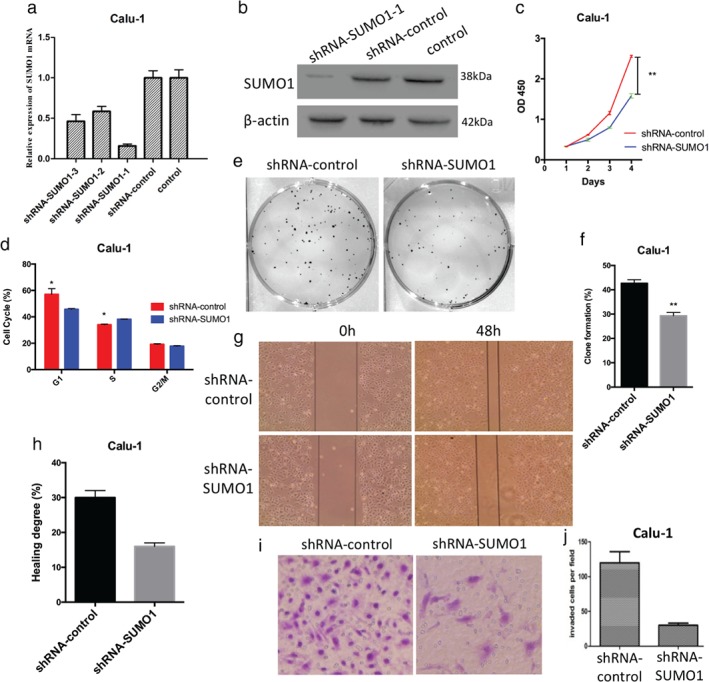
Downregulation of SUMO1 suppresses the proliferation, cell cycle progression, colony formation, migration, and invasion of Calu‐1 cells in vitro. (a) Quantitative real time‐PCR analysis revealed that the messenger RNA (mRNA) expression levels of SUMO1 in short hairpin RNA (shRNA)‐SUMO1‐1 Calu‐1 cells were significantly downregulated. (b) Similar results were obtained through Western blot analysis. (c) Downregulation of SUMO1 expression significantly inhibited the (c) proliferation, (e,f) colony‐formation ability, (g,h) migration, and (i,j) invasion of Calu‐1 cells. (d) Downregulation of SUMO1 reduced the number of Calu‐1 cells in the S phase of the cell cycle.
SUMO1 expression is associated with NF‐κB in NSCLC
To verify the correlation between NF‐κB and SUMO1, we compared SUMO1 and NF‐κB in 168 patients with NSCLC who underwent surgical resection of cancer tissue by immunohistochemistry. NF‐κB and SUMO1 showed similar expression patterns (Fig 3a). There was a significant correlation between SUMO1 and NF‐κB in total NSCLC, squamous cell carcinoma, and adenocarcinoma (r > 0.5, P < 0.001) (Table 1). NF‐κB expression in different NSCLC cell lines was similar to SUMO1 (Fig 3b,c). Four pairs of human lung cancer samples, including cancer tissues and matched normal adjacent tissues were selected to test SUMO1 and NF‐κB expression by Western blot. SUMO1 and NF‐κB were upregulated in cancer tissues (Fig 3d). Overexpression of SUMO1 upregulated the expression level of NF‐κB (Fig 3e,g), while depletion of SUMO1 inhibited NF‐κB expression (Fig 3f,g). NF‐κB expression was regulated by SUMO1 in NSCLC cell lines.
Figure 3.
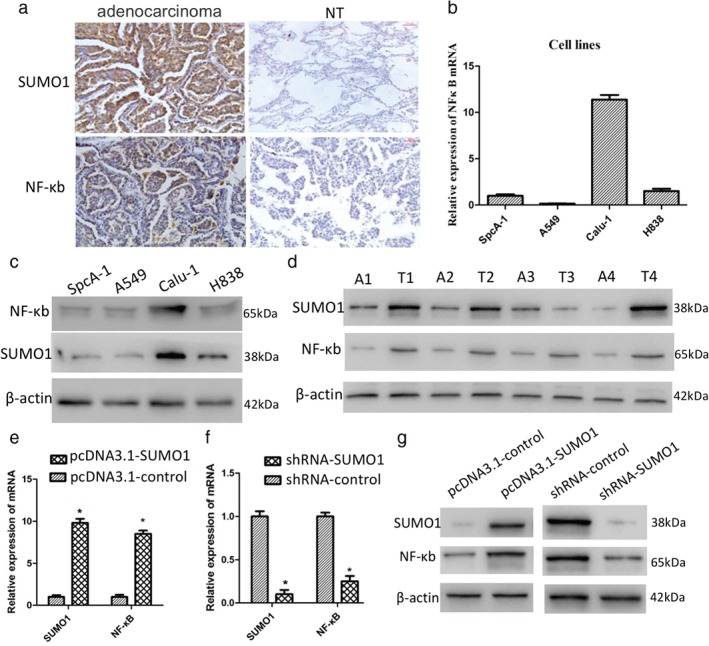
SUMO1 regulates NF‐κB expression. (a) Immunohistochemical characteristics of SUMO1 and NF‐κB in adenocarcinoma and adjacent normal tissues. (b) NF‐κB messenger RNA (mRNA) exhibited similar expression levels as SUMO1 in different lung cancer cell lines. (c) Similar results were obtained through Western blot analysis. (d) SUMO1 and NF‐κB protein levels in paired samples from four non‐small cell lung cancer patients. mRNA expression of NF‐κB was (e) increased in SUMO1‐overexpressed cells and (f) downregulated in the SUMO1 knockdown cell line. (g) Similar results were obtained through Western blot analysis. A, adjacent tissue; NT, normal tissue; T, tumor tissue.
Table 1.
Correlation analysis of SUMO1 and NF‐κB in lung cancer tissue
| Histological type | NF‐κB | SUMO1 | r | P | |||
|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | ||||
| NSCLC | − | 16 | 30 | 2 | 3 | 0.514 | <0.001 |
| + | 4 | 53 | 14 | 5 | |||
| ++ | 1 | 6 | 29 | 0 | |||
| Adenocarcinoma | − | 9 | 11 | 1 | 2 | 0.565 | <0.001 |
| + | 3 | 26 | 11 | 0 | |||
| ++ | 0 | 3 | 15 | 3 | |||
| Squamous carcinoma | − | 9 | 19 | 0 | 0 | 0.712 | <0.001 |
| + | 0 | 29 | 4 | 3 | |||
| ++ | 0 | 1 | 14 | 0 | |||
NSCLC, non‐small cell lung cancer.
Discussion
Non‐small cell lung cancer is a highly malignant tumor with poor clinical prognosis.2 In recent years, significant progress has been made to improve the survival rate of NSCLC patients; however, to successfully manage NSCLC patients,12, 13 biomarkers with high sensitivity and specificity to predict the severity of NSCLC are still urgently required.14, 15, 16 We previously reported that SUMO1 is related to the clinical features of lung cancer and could be used as a molecular marker for diagnosis and prognosis;11 however the exact molecular mechanisms are unclear.
SUMO1 is attached to other proteins in a generic, reversible manner. The aggregation of SUMO1 (sumoylation) affects the subcellular localization and stability of the substrate, thus affecting its transcriptional activity.17, 18 Many transcriptional regulatory proteins have been shown to be substrates for the ubiquitination of this class; however, it is widely believed that a number of substrates have not been detected.19 SUMO1 plays an important role in tumor development;20, 21 however, the molecular mechanism underlying the role of SUMO1 in cancer progression is not completely understood.22 SUMO1 at least partially regulates cell cycle progression through the transcriptional regulation of NF‐κB, a tumor promoter in inflammation‐associated cancers.23, 24 Silencing of NF‐κB expression has been implicated as a key event in NSCLC progression.25, 26
In the present study, we found that forced expression of SUMO1 promoted the proliferation rate, colony formation ability, invasion, and NF‐κB expression in NSCLC cell lines. Conversely, depletion of SUMO1 inhibited the cell growth rate, colony formation ability, invasion, and NF‐κB expression. In lung adenocarcinoma and squamous carcinoma patients, NF‐κB expression is significantly correlated with SUMO1 (r > 0.5, P < 0.001). Based on these findings, we hypothesize that SUMO1 promotes the proliferation and invasion of NSCLC cells, perhaps partly by altering NF‐κB expression. However, the exact mechanism underlying the role SUMO1 in the progression of NSCLC requires further study.
Our results and those of previous studies indicate that SUMO1 promotes the proliferation and migration of NSCLC cells by regulating the expression of NF‐κB. Therefore, SUMO1 might be a potential diagnostic marker and therapeutic target for NSCLC.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (81802784), Fundamental Research Funds for the Central Universities, Xi'an Jiaotong University (xjj2017075), Special Research Funds for Talents Training of the Second Affiliated Hospital of Xi'an Jiaotong University (YJQN201612) and the Key Research and Development Program of Shaanxi Province of China (2017ZDCXL‐SF‐02‐05).
Contributor Information
Kai Zhu, Email: zk8350@xjtu.edu.cn.
Xiaofei Li, Email: 13402988668@163.com.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Reungwetwattana T, Weroha SJ, Molina JR. Oncogenic pathways, molecularly targeted therapies, and highlighted clinical trials in non‐small‐cell lung cancer (NSCLC). Clin Lung Cancer 2012; 13: 252–66. [DOI] [PubMed] [Google Scholar]
- 3. Polanski J, Jankowska‐Polanska B, Rosinczuk J, Chabowski M, Szymanska‐Chabowska A. Quality of life of patients with lung cancer. Onco Targets Ther 2016; 9: 1023–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilkinson KA, Henley JM. Mechanisms, regulation and consequences of protein SUMOylation. Biochem J 2010; 428: 133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bellail AC, Olson JJ, Hao C. SUMO1 modification stabilizes CDK6 protein and drives the cell cycle and glioblastoma progression. Nat Commun 2014; 5: 4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Park MA, Seok YJ, Jeong G, Lee JS. SUMO1 negatively regulates BRCA1‐mediated transcription, via modulation of promoter occupancy. Nucleic Acids Res 2008; 36: 263–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singh S, Pradhan AK, Chakraborty S. SUMO1 negatively regulates the transcriptional activity of EVI1 and significantly increases its co‐localization with EVI1 after treatment with arsenic trioxide. Biochim Biophys Acta 2013; 1833: 2357–68. [DOI] [PubMed] [Google Scholar]
- 8. Ma KW, Au SW, Waye MM. Over‐expression of SUMO‐1 induces the up‐regulation of heterogeneous nuclear ribonucleoprotein A2/B1 isoform B1 (hnRNP A2/B1 isoform B1) and uracil DNA glycosylase (UDG) in hepG2 cells. Cell Biochem Funct 2009; 27: 228–37. [DOI] [PubMed] [Google Scholar]
- 9. Desterro JM, Rodriguez MS, Hay RT. SUMO‐1 modification of IkappaBalpha inhibits NF‐kappaB activation. Mol Cell 1998; 2: 233–9. [DOI] [PubMed] [Google Scholar]
- 10. Vatsyayan J, Qing G, Xiao G, Hu J. SUMO1 modification of NF‐kappaB2/p100 is essential for stimuli‐induced p100 phosphorylation and processing. EMBO Rep 2008; 9: 885–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ke C, Zhu K, Sun Y, Zhang Z, Ni Y, Li X. SUMO‐1 expression modulates non‐small cell lung cancer progression. Int J Clin Exp Med 2018; 11: 5638–47. [Google Scholar]
- 12. Fumagalli C, Bianchi F, Raviele PR et al Circulating and tissue biomarkers in early‐stage non‐small cell lung cancer. Ecancermedicalscience 2017; 11: 717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raso MG, Wistuba II. Molecular pathogenesis of early‐stage non‐small cell lung cancer and a proposal for tissue banking to facilitate identification of new biomarkers. J Thorac Oncol 2007; 2: S128–35. [DOI] [PubMed] [Google Scholar]
- 14. Romero‐Ventosa EY, Blanco‐Prieto S, Gonzalez‐Pineiro AL, Rodriguez‐Berrocal FJ, Pineiro‐Corrales G, Paez de la Cadena M. Pretreatment levels of the serum biomarkers CEA, CYFRA 21‐1, SCC and the soluble EGFR and its ligands EGF, TGF‐alpha, HB‐EGF in the prediction of outcome in erlotinib treated non‐small‐cell lung cancer patients. Springerplus 2015; 4: 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luo H, Ge H, Cui Y et al Systemic inflammation biomarkers predict survival in patients of early stage non‐small cell lung cancer treated with stereotactic ablative radiotherapy – A single center experience. J Cancer 2018; 9: 182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murray N. The challenge of using biomarkers and molecularly targeted drugs to improve cure rate in early stage non‐small cell lung cancer. J Thorac Dis 2015; 7: 230–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mukherjee S, Thomas M, Dadgar N, Lieberman AP, Iniguez‐Lluhi JA. Small ubiquitin‐like modifier (SUMO) modification of the androgen receptor attenuates polyglutamine‐mediated aggregation. J Biol Chem 2009; 284: 21296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dohmen RJ. SUMO protein modification. Biochim Biophys Acta 2004; 1695: 113–31. [DOI] [PubMed] [Google Scholar]
- 19. Johnson ES. Protein modification by SUMO. Annu Rev Biochem 2004; 73: 355–82. [DOI] [PubMed] [Google Scholar]
- 20. Seeler JS, Dejean A. SUMO and the robustness of cancer. Nat Rev Cancer 2017; 17: 184–97. [DOI] [PubMed] [Google Scholar]
- 21. Eifler K, Vertegaal ACO. SUMOylation‐mediated regulation of cell cycle progression and cancer. Trends Biochem Sci 2015; 40: 779–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Han ZJ, Feng YH, Gu BH, Li YM, Chen H. The post‐translational modification, SUMOylation, and cancer (review). Int J Oncol 2018; 52: 1081–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nagai S, Davoodi N, Gasser SM. Nuclear organization in genome stability: SUMO connections. Cell Res 2011; 21: 474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mabb AM, Miyamoto S. SUMO and NF‐kappaB ties. Cell Mol Life Sci 2007; 64: 1979–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen W, Li Z, Bai L, Lin Y. NF‐kappaB in lung cancer, a carcinogenesis mediator and a prevention and therapy target. Front Biosci (Landmark Ed) 2011; 16: 1172–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bivona TG, Hieronymus H, Parker J et al FAS and NF‐kappaB signalling modulate dependence of lung cancers on mutant EGFR. Nature 2011; 471: 523–6. [DOI] [PMC free article] [PubMed] [Google Scholar]


