Abstract
Background
Recombined humanized endostatin (Rh‐endostatin) exhibits a potent anti‐cancer effect involving multiple molecular targets and signaling pathways. HMGB1 is a highly conserved DNA‐binding protein involved in cancer development. The therapeutic effect of Rh‐endostatin on HMGB1 has not been reported, thus we investigate the effect in non‐small cell lung cancer (NSCLC) cells.
Methods
Quantitative real‐time PCR and Western blot were used to analyze the messenger RNA and protein expression of HMGB1 in A549 cancer cells, while enzyme‐linked immunosorbent assay was used to detect the release of HMGB1. Western blot was performed to evaluate HMGB1 expression in SK‐MES‐1 and H661 NSCLC cells.
Results
Rh‐endostatin inhibited the proliferation of A549 cancer cells and distinctly downregulated the expression and release of HMGB1 in dose and time dependent manners. Rh‐endostatin‐induced HMGB1 downregulation was confirmed in different types of NSCLC cells.
Conclusion
These results demonstrate the general phenomenon that Rh‐endostatin can induce HMGB1 suppression in a variety of NSCLC cells. Rh‐endostatin may suppress HMGB1 expression and release in A549 cancer cells, thus inhibiting cell proliferation.
Keywords: Cell proliferation, HMGB1, NSCLC, recombined humanized endostatin
Introduction
Non‐small cell lung cancer (NSCLC) is the most common malignancy and the leading cause of cancer‐related death.1 After recurrence, the five‐year survival rate is merely 20%.2 As standard therapeutic modalities for NSCLC, chemotherapy and radiation significantly decrease tumor recurrence and prolong patient survival. However, the use of chemotherapy and radiation in clinical practice is restricted because of toxicity. Therefore, there is an urgent need to determine a safer therapeutic approach to improve the survival of NSCLC patients.
Rh‐endostatin is a new recombinant human endostatin developed by Simcere Biopharmaceutical Co. Ltd. (Nanjing, China), different to the original endostatin identified by O'Reilly et al.3 Rh‐endostatin is designed to halt cancer progression by depriving the tumor of oxygen and nutrients for growth. Rh‐endostatin works by inhibiting angiogenesis: the proliferous formation of new blood vessels in and around the tumoral tissue. Preclinical studies have shown that this novel agent could inhibit tumor growth and shrink existing tumor blood vessels.4, 5, 6
HMGB1 is a highly conserved DNA‐binding protein present in most cell types. As a nuclear protein, HMGB1 modulates chromatin structure, promotes interaction of proteins, and plays a role as a transcription factor in gene expression regulation.7 In addition to its nuclear expression, HMGB1 can also be passively released by necrotic cells.8 It can be actively secreted into the extracellular matrix by inflammatory cells and some cancer cells, where it acts as a damage‐associated molecular pattern to mediate inflammation or plays a role as a chemoattractant factor.9 Cheng et al. showed that the induction of HMGB1 contributed to NSCLC tumorigenesis, while HMGB1 silencing suppressed cancer progression.10 Tang et al. found that HMGB1 promoted differentiation syndrome in acute promyelocytic leukemia cells.11 Considering its role as mediator of tumorigenesis, HMGB1 targeted therapy may be of meaningful value in anti‐cancer treatment.12
Given the importance of HMGB1 in tumor initiation and progression, the aim of this study was to investigate whether Rh‐endostatin can suppress the proliferation of A549 human NSCLC cells by inhibiting HMGB1 expression.
Methods
Materials
Ethyl pyruvate was purchased from Sigma Chemical (St. Louis, MO, USA). An HMGB1 enzyme‐linked immunosorbent assay (ELISA) kit was purchased from Elabscience Biotechnology Co., Ltd. (Wuhan, China). Antibodies against HMGB1 were purchased from Wanlei Life Sciences (Shenyang, China). A Cell Counting Kit‐8 (CCK‐8) was purchased from Dojindo Laboratories (Kumamoto, Japan).
Cell culture
Human lung cancer cells (A549, SK‐MES‐1, H661) were obtained from American Type Culture Collection (Manassas, VA, USA). A549 and SK‐MES‐1 cells were cultured in Dulbecco's modified Eagle medium, whereas H661 cells were incubated in RPMI‐1640 at 37°C in 5% CO2 (Gibco, Carlsbad, CA, USA). Both mediums were supplemented with 10% fetal bovine serum (Gibco), 100 μg/mL streptomycin, and 1% (v/v) penicillin.
Cell viability assay
Cell viability was assessed using a CCK‐8 kit. To obtain single cell suspension, adherent cells that reached 80% growth density were rinsed with phosphate buffered saline and digested with 0.25% trypsin. The cells were then seeded in 96‐well plates at a density of 2 × 103 cells/100μL/well. After 24 hours of incubation at concentrations of 0, 5, 10, 20, 40, 80, and 160 nmol/L, Rh‐endostatin was added to the cells for 24 hours, and a fixed concentration of 20 nmol/L Rh‐endostatin for 12, 24, 48, 72, and 96 hours, respectively. The other settings were treated with 20 nmol/L Rh‐endostatin alone and in combination with 20 ng/mL rHMGB1 or 10 mmol/L ethyl pyruvate for 24 hours. In each well, 10 μL CCK‐8 was then added and cultivated for two hours. ELISA was used to detect the absorbance of each well at 450 nm. Each experiment was conducted five times and the average values are reported.
Colony formation assay
Cells were inoculated in six‐well plates with 500 cells/well. After 24 hours of incubation, various concentrations of Rh‐endostatin (0, 5, 10, 20, 40 nmol/L) were added for 12 hours and the cells were then cultured in a normal condition for two weeks. The colonies were fixed with 4% paraformaldehyde solution and stained with 0.1% crystal violet. Colonies with > 50 cells were scored. Each test was undertaken in triplicate, and the average values are reported.
Real‐time PCR
To validate the microarray data, we selected another eight patient samples for RT‐PCR. Total RNA was isolated from thymoma and thymic cyst tissue using TRIzol Reagent (Goldenbridge Biotech, Beijing, China), according to the manufacturer's instructions. Complementary DNA was digested with DNase 1, and then synthesized via reverse transcription from RNA samples using oligo (dT) primers and TransScript RT/RI/Enzyme Mix (Takara, Beijing, China). Quantitative real‐time (qRT) PCR was used to determine the relative messenger RNA (mRNA) transcription levels of HMGB1 to the control glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) with SYBR green PCR master mix buffer (Takara) and specific primers. The qRT‐PCR primers were designed and synthesized by Sangon Biotech Company (Shanghai, China) (Table 1). PCR amplification was performed as follows: pre‐denaturation at 94°C for three minutes, followed by 35 cycles of 94°C for 30 seconds, 56–58°C for 30 seconds, 72°C for one minute, and finally, extension at 72°C for five minutes. The relative expression levels of targeted genes were normalized to GAPDH and analyzed by 2‐ΔΔCt.
Table 1.
Primers for quantitative real‐time PCR
| Gene | Forward | Reverse |
|---|---|---|
| HMGB1 | TGTGCAAACTTGTCGGGAG | TCTTTCATAACGGGCCTTGTC |
| GAPDH | ACCGAGCGCGGCTACAG | CTTAATGTCACGCACGATTTCC |
GADPH, glyceraldehyde 3‐phosphate dehydrogenase.
Western blot analysis
To extract the total protein, the harvested cells were suspended in radioimmunoprecipitation assay lysis buffer containing a protease inhibitor cocktail (Solarbio, Beijing, China). The protein expression level was examined via the bicinchoninic acid method. An aliquot of protein was separated by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (Solarbio). After blocking with 5% non‐fat milk for one hour at room temperature, the membranes were incubated overnight at 4 °C with primary antibodies against HMGB1 (1:500), followed by horseradish peroxidase‐conjugated secondary antibodies. The data were evaluated using QuantityOne software (Hercules, CA, USA). The relative expression of the target protein content was analyzed by the gray value ratio of target and β‐actin.
Enzyme‐linked immunosorbent assay
Supernatants were collected and the HMGB1 level was analyzed via ELISA using commercial kits as the standard method.
Statistical analysis
SPSS version 19.0 (IBM Corp., Armonk, NY, USA) was used for data analysis and a t‐test of independent samples for continuous variables. All data are expressed as the mean ± standard deviation. Statistical differences were assessed by Student's t‐test or one‐way analysis of variance. An alpha of P < 0.05 was used to determine statistical significance.
Results
Recombined humanized endostatin (Rh‐endostatin) inhibits A549 human non‐small cell lung cancer (NSCLC) cell proliferation
We investigated the biological function of Rh‐endostatin on cell proliferation. CCK‐8 and colony formation assays were performed to detect the proliferation of A549 cells treated with Rh‐endostatin. As expected, Rh‐endostatin significantly suppressed cell viability and colony formation in dose and time dependent manners (Fig 1), indicating that Rh‐endostatin significantly inhibited A549 cell proliferation.
Figure 1.
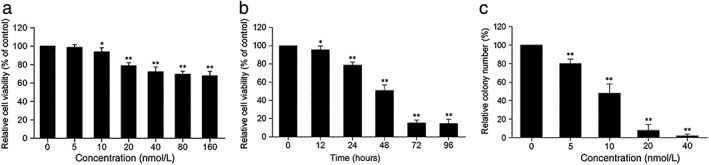
Recombined humanized endostatin (Rh‐endostatin) inhibits A549 cancer cell proliferation. A549 cells were treated with (a) the indicated concentrations of Rh‐endostatin for 24 hours (*P < 0.05 and **P < 0.01 vs. control group ([0 nmol/L]); and (b) 20 nmol/L of Rh‐endostatin for the indicated durations (*P < 0.05 and **P < 0.01 vs. control [0 hours]). Cell viability was measured using Cell Counting Kit‐8 assay. Data are expressed as mean ± standard deviation (SD) of five independent experiments. (c) A549 cells were pretreated with the indicated concentrations of Rh‐endostatin for 12 hours and then cultured at the normal condition for two weeks. Cell colony formation was measured using plate colony formation assay (**P < 0.01 vs. control [0 nmol/L]). Data are expressed as (mean ± SD) of three independent experiments.
Rh‐endostatin inhibits HMGB1 expression in A549 human NSCLC cells
To explore the effects of different concentrations and the activation time of Rh‐endostatin on the expression of HMGB1 in A549 cells, qRT‐PCR of A549 cells treated with different doses of Rh‐endostatin at different time points was conducted. As shown in Figure 2a,c, Rh‐endostatin suppressed the expression of HMGB1 mRNA in dose and time dependent manners, with significant downregulation noted as early as three hours. To analyze the profile of HMGB1 expression, we further investigated whether Rh‐endostatin‐induced HMGB1 suppression could be identified at a translational level. Western blot analyses revealed that Rh‐endostatin induced HMGB1 protein suppression in dose and time dependent manners, with significant downregulation noted as early as three hours (Fig 2b,d).
Figure 2.
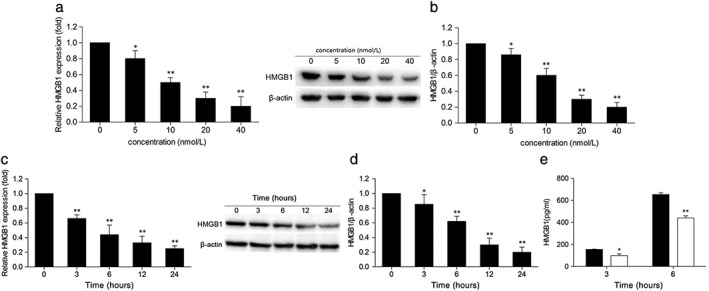
Recombined humanized endostatin (Rh‐endostatin) suppresses the expression and release of HMGB1 in A549 breast cancer cells. A549 cells were treated with the indicated concentrations of Rh‐endostatin for 24 hours, and (a) messenger RNA (mRNA) and (b) protein levels of HMGB1 were subsequently determined by quantitative real‐time (qRT) PCR and Western blot, respectively (*P < 0.05 and **P < 0.01 vs. control [0 nmol/L]). A549 cells were treated with 20 nmol/L of Rh‐endostatin for the indicated durations, and then the (c) mRNA and (d) protein levels of HMGB1 were analyzed by qRT‐PCR and Western blot, respectively (*P < 0.05 and **P < 0.01 vs. control [0 hours]); and (e) the supernatants of HMGB1 release were measured by ELISA (*P < 0.05 and **P < 0.01 vs. control (0 nmol/L). Data are expressed as the mean ± standard deviation of three independent experiments. ( ) 0 nmol/L and (
) 0 nmol/L and ( ) 20 nmol/L
) 20 nmol/L
Furthermore, we conducted ELISA analysis of supernatants to examine HMGB1 release after Rh‐endostatin treatment. Consistently, supernatant levels of HMGB1 were distinctly reduced as early as three hours after Rh‐endostatin treatment (Fig 2e). These results imply that Rh‐endostatin can potently inhibit HMGB1 expression and extracellular secretion.
Rh‐endostatin downregulates HMGB1 expression in various human NSCLC cells
To investigate whether Rh‐endostatin‐induced suppression of HMGB1 occurred in other NSCLC cells, Western blot analyses were conducted with SK‐MES‐1 and H661 NSCLC cells treated with Rh‐endostatin for 24 hours. Interestingly, Rh‐endostatin suppressed the expression of HMGB1 in all cancer cells tested (Fig 3). These data demonstrate that Rh‐endostatin could widely downregulate the expression of HMGB1 in various human NSCLC cells.
Figure 3.
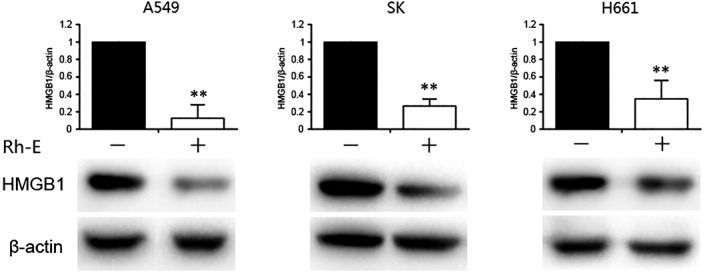
Recombined humanized endostatin (Rh‐endostatin)‐induced HMGB1 suppression occurs in various human cancer cells. Various NSCLC were treated with Rh‐endostatin (20 nmol/L) for 24 hours. The level of HMGB1 expression was assayed by Western blot (**P < 0.01 vs. control [0 nmol/L]). Data are expressed as the mean ± standard deviation of three independent experiments.
Discussion
Various factors involved in different signal pathways take part in the development of cancer. HMGB1 is a conserved nuclear protein and plays a critical role in nucleosome stabilization and gene transcription. Moreover, HMGB1 upregulation has recently been proven to act as the link between tumor‐associated inflammation and tumorigenesis.13 HMGB1 is stably expressed in the nucleus of quiescent cells and HMGB1 secretion has been confirmed as its translocation from the nucleus to the cytoplasm.9 Recently, many reports have shown that overexpression of extracellular HMGB1 contributes to cancer carcinogenesis and metastasis by promoting apoptotic evasion; mediating tumor‐associated inflammation; and increasing tumor cell proliferation, migration, and angiogenesis.14 Enhanced HMGB1 expression has been observed in patients with various cancers, including breast cancer,12 cervical carcinoma,15 metastatic prostate cancer,16 and hepatocellular carcinoma.17
This research explored the effect of Rh‐endostatin on HMGB1 expression and the possible mechanisms of proliferation in A549 cells. We confirmed that Rh‐endostatin inhibits A549 cell proliferation (Fig 1). Our findings also suggest that the expression and release of HMGB1 are significantly suppressed by Rh‐endostatin treatment in A549 cells (Fig 2). Downregulation of HMGB1 by Rh‐endostatin may be a general phenomenon in other types of cancer cells (Fig 3). Additionally, NF‐κB is of crucial importance in the synthesis of mediators involved in tumor development and progression. Binding to TLR4, HMGB1 activates the NF‐kB signaling pathway, which promotes the expression of various genes taking part in tumor cell proliferation, migration, and invasion.18, 19 Recent research has shown that the macrophage migration inhibitory factor promotes cancer metastasis by activating the HMGB1/TLR4/NF‐κB signaling pathway.20 Therefore, we recommend that future research should be directed toward a better understanding of the relationship between the key gene and NSCLC and the mechanism and role of the HMGB1/TLR4/NF‐κB pathway in NSCLC occurrence and development.
In summary, we have shown that Rh‐endostatin significantly inhibits the proliferation of A549 NSCLC cells, and this therapeutic effect is a result of the downregulation of HMGB1 expression and release. Our results revealed an interesting mechanism of NSCLC and identified a novel therapeutic drug for NSCLC treatment.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 81601411).
Contributor Information
Zhi‐Yu Guan, Email: guanzy69@163.com.
Jun Zhang, Email: cns2008@163.com.
References
- 1. Masters GA, Temin S, Azzoli CG et al Systemic therapy for stage IV non–small‐cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol 2017; 33: 932–7. [DOI] [PubMed] [Google Scholar]
- 2. Goldstraw P, Chansky K, Crowley J et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016, 11: 39–51. [DOI] [PubMed] [Google Scholar]
- 3. O'Reilly MS, Boehm T, Shing Y et al Endostatin: An endogenous inhibitor of angiogenesis and tumor growth. Cell 1997; 88: 277–85. [DOI] [PubMed] [Google Scholar]
- 4. Ling Y, Yang Y, Lu N et al Endostar,a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF‐induced tyrosine phosphorylation of KDR/Flk‐1 of endothelial cells. Biochem Biophys Res Commun 2007; 361: 79–84. [DOI] [PubMed] [Google Scholar]
- 5. Lu N, Ling Y, Gao Y et al Endostar suppress esinvasion through downregulating the expression of matrix metalloproteinase‐2/9 in MDA‐MB‐435 human breast cancer cells. Exp Biol Med 2008; 233: 1013–20. [DOI] [PubMed] [Google Scholar]
- 6. Ling Y, Lu N, Gao Y et al Endostar induces apoptotic effects in HUVECs through activation of caspase‐3 and decrease of Bcl‐2. Anticancer Res 2009; 29: 411–7. [PubMed] [Google Scholar]
- 7. Chen M, Huang W, Wang C et al High‐mobility group box 1 exacerbates CCl(4)‐induced acute liver injury in mice. Clin Immunol 2014; 153: 56–63. [DOI] [PubMed] [Google Scholar]
- 8. Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002; 418: 191–5. [DOI] [PubMed] [Google Scholar]
- 9. Wang C, Nie H, Li K et al Curcumin inhibits HMGB1 releasing and attenuates concanavalin A‐induced hepatitis in mice. Eur J Pharmacol 2012; 697: 152–7. [DOI] [PubMed] [Google Scholar]
- 10. Ni P, Zhang Y, Liu Y et al HMGB1 silence could promote MCF‐7 cell apoptosis and inhibit invasion and metastasis. Int J Clin Exp Pathol 2015; 8: 15940–6. [PMC free article] [PubMed] [Google Scholar]
- 11. Tang L, Chai W, Ye F et al HMGB1 promotes differentiation syndrome by inducing hyperinflammation via MEK/ERK signaling in acute promyelocytic leukemia cells. Oncotarget 2017; 8: 27314–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun S, Zhang W, Cui Z et al High mobility group box‐1 and its clinical value in breast cancer. Onco Targets Ther 2015; 8: 413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu J, Luo J, Li Y et al HMGB1 induces human non‐small cell lung cancer cell motility by activating integrin alphavbeta3/FAK through TLR4/NF‐kappaB signaling pathway. Biochem Biophys Res Commun 2016; 480 (4): 522–7. [DOI] [PubMed] [Google Scholar]
- 14. Chung HW, Lim JB. High‐mobility group box‐1 contributes tumor angiogenesis under interleukin‐8 mediation during gastric cancer progression. Cancer Sci 2017; 108: 1594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cheng H, Wang W, Zhang Y et al Expression levels and clinical significance of hepsin and HMGB1 proteins in cervical carcinoma. Oncol Lett 2017; 14: 159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate 2005; 64: 92–100. [DOI] [PubMed] [Google Scholar]
- 17. Shi W, Su L, Li Q et al Suppression of toll‐like receptor 2 expression inhibits the bioactivity of human hepatocellular carcinoma. Tumour Biol 2014; 35: 9627–37. [DOI] [PubMed] [Google Scholar]
- 18. Van Beijnum JR, Buurman WA, Griffioen AW. Convergence and amplification of toll‐like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 2008; 11: 91–9. [DOI] [PubMed] [Google Scholar]
- 19. Gong W, Wang ZY, Chen GX, Liu YQ, Gu XY, Liu WW. Invasion potential of H22 hepatocarcinoma cells is increased by HMGB1‐induced tumor NF‐kappaB signaling via initiation of HSP70. Oncol Rep 2013; 30: 1249–56. [DOI] [PubMed] [Google Scholar]
- 20. Lv W, Chen N, Lin Y et al Macrophage migration inhibitory factor promotes breast cancer metastasis via activation of HMGB1/TLR4/NF kappa B axis. Cancer Lett 2016; 375: 245–55. [DOI] [PubMed] [Google Scholar]


