Abstract
Background
The study was conducted to investigate the clinicopathological features and prevalence of ROS1 gene fusion in Chinese patients with non‐small cell lung cancer (NSCLC).
Methods
The presence of ROS1 fusion was assessed by quantitative real‐time PCR. Associations between ROS1 fusion and clinical characteristics were analyzed.
Results
In total, 6066 patients with pathologically confirmed NSCLC and ROS1 fusion test results were enrolled. The average age was 60.89 ± 10.60 years and fusion was detected in 157 (2.59%) patients. Fusion frequency was significantly correlated with age, gender, smoking status (all P < 0.001), pathology type (P = 0.017), and lymph node metastasis stage (P = 0.027). ROS1 fusion‐positive patients were significantly younger (55.68 ± 11.34 vs. negative 61.02 ± 10.44 years; P < 0.01). Fusion frequency was higher in women (3.71% vs. men 1.81%), never‐smokers (3.33% vs. smokers 1.21%), and patients with adenocarcinoma (2.77% vs. squamous lung cancer 0.93%) and at advanced node stages (1.31%, 1.40%, 2.07%, and 3.23% for N0, N1, N2, and N3, respectively). No significant correlation between ROS1 fusion status and pathological stage was found in subgroups classified by pathological, tumor, or metastasis stage (P > 0.05). Age, smoking status, and lymph node stage were statistically significantly correlated with ROS1 fusion frequency (all P < 0.05); gender and pathology type were not significantly correlated with ROS1 fusion status after adjusting for smoking status.
Conclusion
An overall ROS1 fusion frequency of 2.59% was confirmed in this study. ROS1 fusion was more prevalent among younger patients, never‐smokers, and those at advanced node stages.
Keywords: Clinicopathological features, non‐small‐cell lung cancer, ROS1 fusion
Introduction
Lung cancer remains the leading cause of cancer death worldwide, and non‐small cell lung cancer (NSCLC) accounts for more than 80% of lung cancer cases.1, 2 During the last decade, the identification of key driver genes in NSCLC, such as EGFR and ALK, and the promising results obtained with the use of tyrosine kinase inhibitors (TKIs) that target these driver genes to treat NSCLC have rapidly facilitated the development of targeted therapy and precision medicine.3, 4, 5, 6, 7, 8, 9, 10, 11, 12 In this era of precision medicine, molecular testing has become extremely important for both the classification and treatment of lung cancer.13
ROS1 is a receptor tyrosine kinase of the insulin receptor family. It was first discovered in NSCLC in 2007.14 The ROS1 fusion partners identified in lung cancer to date include CD74, SLC34A2, GOPC, CCDC6, SDC4, TPM3, EZR, LRIG3, KDELR2, LIMA1, MSN, CLTC, TPD52L1, FIG, TMEM106B, FAM135B, and SLC6A17, with an overall prevalence of 0.9–2.6% in NSCLC15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25 and up to 3% in lung adenocarcinoma,19, 26 representing a novel molecular subgroup of NSCLC. Several important clinical studies have shown that crizotinib, an ALK inhibitor, has high activity when treating NSCLC patients harboring ROS1 fusion, with a response rate of 72–80%.27, 28 Based on these promising results, the American, Japanese, and Chinese authorities have approved crizotinib for the treatment of ROS1 fusion‐positive NSCLC patients. This development has highlighted the need for thorough investigations of ROS1 fusions in patients with NSCLC.
Similar to patients with ALK fusions, ROS1 fusion‐positive patients tend to be younger, never‐smokers, with adenocarcinoma histology.9, 10, 11, 21, 22 However, the clinical features of patients harboring a ROS1 fusion gene are not fully understood; the vast majority of studies have had small to modest sample sizes,17, 29, 30, 31, 32 which compromised the detection power of each individual study. Therefore, in this study, we performed a large‐scale, retrospective analysis to determine the prevalence and clinicopathological features of ROS1 fusion in Chinese patients with NSCLC.
Methods
Study design
This investigation was a real‐world, retrospective, multicenter, epidemiological study of ROS1 fusion prevalence in patients with NSCLC from 10 hospitals across China. The primary objective of the study was to assess the frequency of ROS1 gene fusion. The secondary objective was to investigate the correlations between ROS1 fusion status and demographic and clinical factors.
Patients
Eligible patients had pathologically confirmed NSCLC with ROS1 fusion detection results. The following data were collected: age, gender, smoking status, pathological type and stage, and tumor node metastasis (TNM) stage. Pathological types and stages were determined according to the 2015 World Health Organization classification.33 The TNM stage was classified according to 7th edition of the Union for International Cancer Control (UICC)/American Joint Committee on Cancer (AJCC) TNM staging.34 The Institutional Review Board of Shanghai Chest Hospital approved the study. All patients provided written informed consent before enrollment.
Detection of ROS1 fusion by quantitative real‐time PCR
Total RNAs isolated from formalin‐fixed paraffin‐embedded (FFPE) tissue from each patient were used to detect ROS1 fusion with the quantitative real‐time (qRT)‐PCR based ADx‐ARMS ROS1 Gene Fusion Detection Kit, ADx‐ARMS ALK/ROS1 Gene Fusion Joint Detection Kit, or ADx‐ARMS EGFR/ALK/ROS1 Gene Joint Detection Kit (Amoy Diagnostics Co., Ltd., Xiamen, China), according to the manufacturer's instructions (Table S1). In brief, the qRT‐PCR conditions for complementary DNA were as follows: one cycle of 95 °C for 5 minutes; 15 cycles of denaturation at 95 °C for 25 seconds, annealing at 64 °C for 20 seconds, and elongation at 72 °C for 20 seconds to ensure specificity; and up to 31 cycles of 93 °C for 25 seconds, 60 °C for 35 seconds (data collection), and 72 °C for 20 seconds. An external control for each sample and an internal control for each tube were used to check the effects of DNA insufficiency or PCR inhibitors.
Statistical analyses
A two‐tailed Student's t‐test was used to compare the ages of the ROS1 fusion positive and negative groups. Chi‐square or Fisher's exact tests were used to analyze the relationship between ROS1 fusion and other characteristics of NSCLC, including gender, smoking status, and pathological type and stage. All statistical calculations were performed using R 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria), and P < 0.05 was defined as significant with a two‐sided test. To better predict ROS1 fusion frequency, multivariate logistic regression was performed for factors with a P value < 0.05 in the univariate analysis, and the significance level was set at 1% because of the large data set.
Results
Patients
The 6066 patients eligible for this study comprised 3584 men and 2482 women, at an average age of 60.89 ± 10.60 years. The sample types for these 6066 patients were 2011 (33.15%) postoperative pathologic specimens, 181 (2.98%) cytology specimens, and 3874 (63.86%) biopsies.
Positive rate of ROS1 fusion in non‐small cell lung cancer (NSCLC) patients
ROS1 fusions were detected in 157 of the 6066 patients with NSCLC, for a 2.59% positive rate. In the subgroup with known EGFR gene and ALK fusion status, the positive rate of ROS1 was 4.36% (68/1559) in patients with EGFR wild‐type and ALK fusion‐negative status (Fig 1a).
Figure 1.
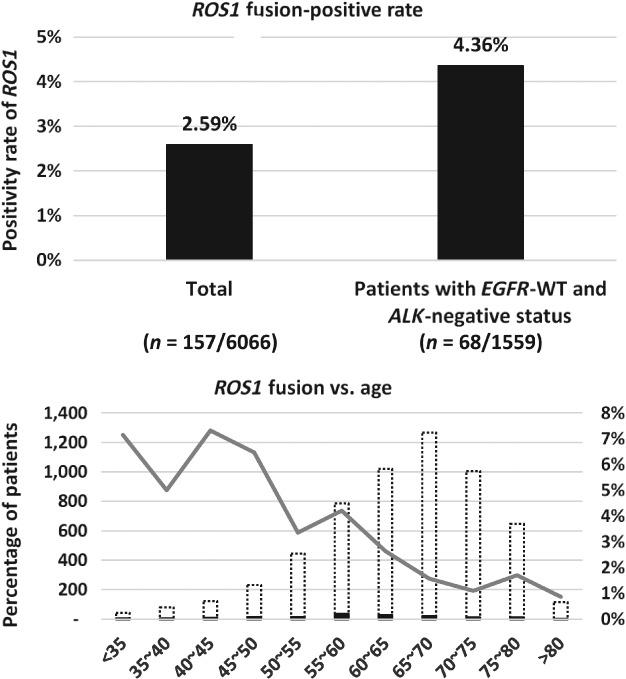
(a) The ROS1 fusion positive rate among all patients and patients with wild‐type EGFR and ALK negative status. (b) Different age groups in relation to ROS1 fusion status. ( ) ROS1 positive, (
) ROS1 positive, ( ) ROS1 negative, and (
) ROS1 negative, and ( ) ROS1 positive %.
) ROS1 positive %.
Correlation analysis of ROS1 fusion status and characteristics in NSCLC patients
We compared age, gender, smoking history, and pathological types and stages between ROS1 fusion positive and negative patients. ROS1 fusion correlated significantly with age, gender, smoking history, pathological type, and N stage, as shown in Table 1.
Table 1.
Summary of ROS1 fusion prevalence and statistical analysis of subgroups classified by clinicopathological characteristics
| Features | All NSCLC patients | |||
|---|---|---|---|---|
| ROS1 positive | ROS1 negative | Total | P | |
| Age (years, Mean ± SD) | 56.09 ± 11.38 | 61.23 ± 10.55 | 61.11 ± 10.60 | <0.001† |
| Gender (n, %) | <0.001‡ | |||
| Female | 92 (3.71%) | 2390 (96.29%) | 2482 | |
| Male | 65 (1.81%) | 3519 (98.19%) | 3584 | |
| Smoking history (n, %) | <0.001‡ | |||
| Non‐smoker | 111 (3.33%) | 3218 (96.67%) | 3329 | |
| Smoker | 23 (1.21%) | 1880 (98.79%) | 1903 | |
| NA | 23 (2.76%) | 811 (97.24%) | 834 | |
| Pathological types (n, %) | 0.01742‡ | |||
| Adenocarcinoma | 136 (2.77%) | 4776 (97.23%) | 4912 | |
| Squamous carcinoma | 4 (0.93%) | 426 (99.07%) | 430 | |
| Others | 17 (2.35%) | 707 (97.65%) | 724 | |
| Pathological stage (n, %) | 0.6826§ | |||
| 0 | 0.00% | 16 (100.00%) | 16 | |
| I | 13 (2.19%) | 580 (97.81%) | 593 | |
| II | 6 (2.18%) | 269 (97.82%) | 275 | |
| III | 34 (3.27%) | 1006 (96.73%) | 1040 | |
| IV | 75 (2.59%) | 2824 (97.41%) | 2899 | |
| NA | 29 (2.33%) | 1214 (97.67%) | 1243 | |
| T stage (n, %) | 0.1567§ | |||
| T1 | 12 (3.20%) | 363 (96.80%) | 375 | |
| T2 | 17 (2.66%) | 623 (97.34%) | 640 | |
| T3 | 3 (1.05%) | 283 (98.95%) | 286 | |
| T4 | 23 (2.04%) | 1102 (97.96%) | 1125 | |
| NA | 102 (2.80%) | 3538 (97.20%) | 3640 | |
| N stage (n, %) | 0.0171§ | |||
| N0 | 6 (1.31%) | 451 (98.69%) | 457 | |
| N1 | 4 (1.40%) | 282 (98.60%) | 286 | |
| N2 | 18 (2.07%) | 853 (97.93%) | 871 | |
| N3 | 26 (3.23%) | 779 (96.77%) | 805 | |
| NA | 103 (2.82%) | 3544 (97.18%) | 3647 | |
| M stage (n, %) | 1‡ | |||
| M0 | 20 (2.29%) | 854 (97.71%) | 874 | |
| M1 | 34 (2.29%) | 1448 (97.71%) | 1482 | |
| NA | 103 (2.78%) | 3607 (97.22%) | 3710 | |
Two‐tailed Student's t‐test.
Fisher's exact test.
Chi‐square test for trend.
NA, not available; NSCLC, non‐small cell lung cancer; SD, standard deviation.
There was a significant difference in age between ROS1 fusion positive (56.09 ± 11.38 years) and negative patients (61.23 ± 10.55 years; P < 0.001). The positive rate of ROS1 fusion was higher in women (3.71%, 92/2482) than in men (1.81%, 65/3584; P < 0.001) and in patients without a smoking history (3.33%, 111/3329) than in patients with a smoking history (1.21%, 23/1903) (P < 0.001). The Fisher's exact test revealed a significant difference in ROS1 fusion positivity among subgroups classified by pathological type (P < 0.001). The positive rate of ROS1 fusion in patients with adenocarcinoma was higher (2.77%, 136/4912) than in patients with squamous carcinoma (0.93%, 4/430).
Correlation analysis of ROS1 fusion status with pathological stage showed no significant difference between subgroups classified by P, T, or M stage (P > 0.05), whereas the ROS1 fusion positive rate in patients increased with N stage (1.31%, 1.40%, 2.07%, and 3.23% for N0, N1, N2, and N3, respectively, P < 0.05). Distant metastasis did not correlate with ROS1 fusion status (M0 vs. M1; P > 0.05).
Multivariate logistic regression (at the 5% significance level) identified age, smoking status, and N stage (all P < 0.05) as independent predictive factors for ROS1 fusion status (Table 2). Gender and pathology type were no longer significant when stratified by smoking status (Fig 2).
Table 2.
Multivariate logistic regression analysis for ROS1 fusion status
| Comparison | Variable | Regression coefficient estimate | Standard error | Odds ratio estimate (95% CI) | P |
|---|---|---|---|---|---|
| Smoking vs. age | Intercept | −1.1999 | 0.4312 | ||
| Smoking | −0.9195 | 0.2330 | 0.3987 (0.2525–0.6295) | 0.0001 | |
| Age | −0.0378 | 0.0076 | 0.9629 (0.9487–0.9774) | 0.0000 | |
| Age vs. N stage | Intercept | −2.5851 | 0.8198 | ||
| Age | −0.0311 | 0.0125 | 0.9693 (0.9459–0.9933) | 0.0126 | |
| N stage | 0.3233 | 0.1465 | 1.3817 (1.0369–1.8412) | 0.0273 | |
| Smoking vs. N stage | Intercept | −4.2088 | 0.3497 | ||
| Smoking | −1.0476 | 0.3421 | 0.3508 (0.1794–0.6859) | 0.0022 | |
| N stage | 0.3826 | 0.1467 | 1.4661 (1.0998–1.9545) | 0.0091 | |
| Smoking vs. gender | Intercept | −3.3045 | 0.1146 | ||
| Smoking | −0.9080 | 0.2695 | 0.4033 (0.2378–0.6840) | 0.0008 | |
| Gender | −0.1972 | 0.2078 | 0.8210 (0.5463–1.2338) | 0.3426 | |
| Smoking vs. pathology type | Intercept | −3.6562 | 0.5932 | ||
| Smoking | −1.0200 | 0.2480 | 0.3606 (0.2218–0.5863) | 0.0000 | |
| Pathology type | 0.3115 | 0.5944 | 1.3654 (0.4259–4.3771) | 0.6003 |
CI, confidence interval; SE, standard error.
Figure 2.
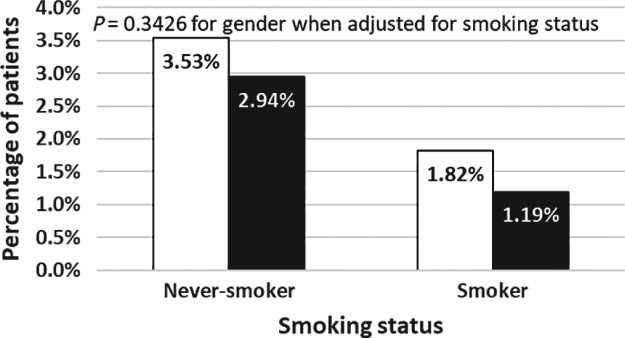
Combined effect of gender and smoking status on the frequency of ROS1 fusion. ( ) Women with ROS1 fusion positive tumors, and (
) Women with ROS1 fusion positive tumors, and ( ) Men with ROS1 fusion positive tumors.
) Men with ROS1 fusion positive tumors.
With increasing age, the positive rate of ROS1 exhibited a decreasing trend. With respect to different age groups, the highest expression of ROS1 was in the age range of 55–60 years, while the ROS1 negative population was concentrated in the age range of 65–70 years. Therefore, patients with positive ROS1 fusion status are younger than those with negative ROS1 fusion status (Fig 1b).
Discussion
This study is the first real‐world, multicenter, retrospective study to investigate the prevalence and clinicopathological characteristics of ROS1 fusion in Chinese patients with NSCLC. In this study, we found that the ROS1 fusion positive rate was higher than that reported previously.15, 17, 26 We confirmed that ROS1 fusion was more prevalent in younger patients, women, never‐smokers, patients with adenocarcinoma, and patients at more advanced stages (stage III–IV). Patient age, smoking status, and N stage were independent predictive factors for ROS1 fusion status. Gender and pathology type were not significantly correlated with tumor ROS1 fusion status when the results were stratified by smoking status.
Our study provides evidence to guide prescreening in NSCLC patients to select a more enriched population who are more likely to harbor this specific fusion. ROS1 fusion is rare in patients with NSCLC. In 2012, Bergethon et al. reported that 18 of 1073 (1.67%) NSCLC tumors had a ROS1 rearrangement, and all 18 ROS1 positive tumors were adenocarcinomas (2.59%, 18/694).15 Our study showed a similar trend, with a ROS1 fusion prevalence of 2.77% in Chinese patients with adenocarcinoma and extremely rare ROS1 fusion positive results in patients with non‐adenocarcinoma. In our study, patients that were younger, female, without a smoking history, with adenocarcinoma, and at an advanced clinical stage were more likely to harbor a ROS1 fusion, and such patients should be genetically tested. The recent National Comprehensive Cancer Network Guidelines for NSCLC recommend testing for ROS1 fusion in all patients with advanced‐stage NSCLC regardless of gender, race, smoking history, or other clinical risk factors to guide patient selection for first‐line therapy with crizotinib.35
Testing methodology also plays a very important role in accurately reflecting the ROS1 fusion prevalence. In this study, ROS1 fusions were detected with qRT‐PCR kits approved by the China Food and Drug Administration (CFDA) for clinical use. Compared to qRT‐PCR, the traditional immunohistochemistry (IHC) assay is simple, inexpensive, and is routinely conducted in pathology laboratories. However, most previous studies have revealed that the IHC assay for ROS1 expression detection has significant false‐positive results because of aneuploidy leading to aberrant expression.36, 37, 38 Fluorescence in situ hybridization (FISH) can be performed even if the concrete fusion partner is unknown and has the potential to discover all ROS1 fusions in NSCLC. In the PROFILE 1001 clinical trial, FISH was used as a standard method to detect ROS1 rearrangement.28 The qRT‐PCR assay is easy to perform, highly sensitive, and relatively inexpensive. In addition, qRT‐PCR can identify concrete fusion partners, which can be confirmed by subsequent sequencing if necessary. qRT‐PCR cannot discover novel fusion partners other than the known and designed partners. In terms of data interpretation, qRT‐PCR is more objective than IHC. For the current real world study, the qRT‐PCR method was the only option to detect ROS1 fusion as there are no CFDA‐approved ROS1 IHC or FISH assays for routine clinical practice in China.
Some previous studies have reported that NSCLC patients with ROS1 fusion share many clinicopathological features with patients harboring ALK fusions.39, 40 Similar routes of pathogenesis might exist in these two subtypes of NSCLC, and this possibility is supported by both structural and functional evidence: the ALK and ROS1 kinase domains share 77% sequence homology;17, 40 and ROS1 signaling and cell viability are substantially inhibited by crizotinib, an ALK inhibitor, in cell lines expressing ROS1 fusions.15, 41 Crizotinib was the first targeted agent approved by the United States Food and Drug Administration for the treatment of advanced ROS1‐rearranged NSCLC, based on a phase II crizotinib trial. That trial demonstrated an objective response rate of 72% and median progression‐free survival of 19.2 months in advanced ROS1‐rearranged NSCLC patients.28 The Asian OO12‐01 clinical trial, the first and largest prospective phase II trial in East Asian patients with ROS1 positive advanced NSCLC, reported an overall response rate of 71.7% and median progression‐free survival of 15.9 months in ROS1 fusion patients treated with crizotinib.42 Based on these data, the Japanese Ministry of Health, Labour and Welfare approved crizotinib for the treatment of metastatic NSCLC with ROS1 fusion in early 2017, and the AmoyDx ROS1 Fusion Kit was approved simultaneously as the companion diagnostic reagent for crizotinib. This kit was the first officially approved ROS1 companion diagnostic reagent in the world. Based on evidence from the OO12‐01 clinical trial, crizotinib was then approved by the CFDA as a ROS1 TKI in late 2017. Our findings could facilitate the patient selection process for targeted therapy with ROS1 inhibitors.
Whether ROS1 gene alterations influence patient survival remains controversial. In our study, we found that the ROS1 fusion positive rate was higher in patients with nodal metastasis. Jin et al. reported that ROS1 fusion positive status was highly associated with micropapillary component and aerogenous spread, which has been identified as a marker of aggressive tumor biology.43 In addition, our study also found that distant metastasis did not correlate with ROS1 fusion status. However, because of the limited prognostic information, we could not evaluate the clinical implications of ROS1 rearrangement. Further study is required to evaluate the clinical significance of ROS1 fusion.
Rare cases of double‐positive lung cancer have been reported. In 2017, two patients harboring concomitant ROS1 and ALK fusions were reported in the literature.44, 45 In our study, we found only one patient with co‐occurring ROS1 and ALK fusions, suggesting that the co‐occurrence is rare in Chinese NSCLC patients. Currently, there is no consensus on standard therapy for tumors with double‐positive mutations or fusions. If concurrent driver mutations are identified, molecular diagnosis should be confirmed before proceeding with targeted therapy. ROS1 fusion was more prevalent in EGFR negative and ALK negative patients (4.36%), indicating that combined detection of EGFR mutations and ALK and ROS1 fusions would increase patient benefits from targeted therapy. With the 15 wide use of ROS1 inhibitors expected in the near future, 16 accurate and extensive diagnosis of ROS1 fusions in NSCLC is essential for clinical practice.
In summary, the positive rate of ROS1 fusion in Chinese patients with NSCLC was 2.59%, whereas in EGFR wild‐type and ALK negative patients, the positive rate of ROS1 fusion was 4.36%. Our results showed that ROS1 fusion was more prevalent in patients that were younger, female, without a smoking history, with adenocarcinoma, and at advanced stages. The prevalence of ROS1 gene fusion was 2.77% in patients with adenocarcinoma and was significantly lower (0.93%) in patients with squamous carcinoma. The observed frequency of tumor ROS1 fusion in demographic and clinical subgroups of Chinese patients suggests that ROS1 fusion testing should be considered for all NSCLC patients with stage IIIB/IV adenocarcinoma. Such an approach will help ensure the optimal identification and treatment of patients whose tumors harbor a ROS1 fusion.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1. ROS1 fusion genes detectable by the AmoyDx assay.
Contributor Information
Qing Zhang, Email: zqbinli@163.com.
Dongmei Lin, Email: lindm3@163.com.
Jie Zhang, Email: 18017321562@163.com.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017; 67: 7–30. [DOI] [PubMed] [Google Scholar]
- 2. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 3. Lynch TJ, Bell DW, Sordella R et al Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129–39. [DOI] [PubMed] [Google Scholar]
- 4. Paez JG, Jänne PA, Lee JC et al EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497–500. [DOI] [PubMed] [Google Scholar]
- 5. Pao W, Miller V, Zakowski M et al EGF receptor gene mutations are common in lung cancers from "never smokers" and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004; 101: 13306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mok TS, Wu YL, Thongprasert S et al Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57. [DOI] [PubMed] [Google Scholar]
- 7. Zhou C, Wu YL, Chen G et al Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735–42. [DOI] [PubMed] [Google Scholar]
- 8. Sequist LV, Yang JC, Yamnoto N et al Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutation. J Clin Oncol 2013; 31: 3327–34. [DOI] [PubMed] [Google Scholar]
- 9. Park K, Tan EH, O'Byrne K et al Afatinib versus gefitinib as first‐line treatment of patients with EGFR mutation‐positive non‐small‐cell lung cancer (LUX‐Lung 7): A phase 2B, open‐label, randomised controlled trial. Lancet Oncol 2016; 17: 577–89. [DOI] [PubMed] [Google Scholar]
- 10. Jänne PA, Yang JC, Kim DW et al AZD9291 in EGFR inhibitor‐resistant non‐small‐cell lung cancer. N Engl J Med 2015; 372: 1689–99. [DOI] [PubMed] [Google Scholar]
- 11. Goss G, Tsai CM, Shepherd FA et al Osimertinib for pretreated EGFR Thr790Met‐positive advanced non‐small‐cell lung cancer (AURA2): A multicentre, open‐label, single‐arm, phase 2 study. Lancet Oncol 2016; 17: 1643–52. [DOI] [PubMed] [Google Scholar]
- 12. Solomon BJ, Mok T, Kim DW et al First‐line crizotinib versus chemotherapy in ALK‐positive lung cancer. N Engl J Med 2014; 371: 2167–77. [DOI] [PubMed] [Google Scholar]
- 13. Popper HH, Ryska A, Tímár J, Olszewski W. Molecular testing in lung cancer in the era of precision medicine. Transl Lung Cancer Res 2014; 3: 291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rikova K, Guo A, Zeng Q et al Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007; 131: 1190–203. [DOI] [PubMed] [Google Scholar]
- 15. Bergethon K, Shaw AT, Ou SH et al ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol 2012; 30: 863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takeuchi K, Soda M, Togashi Y et al RET, ROS1 and ALK fusions in lung cancer. Nat Med 2012; 18: 378–81. [DOI] [PubMed] [Google Scholar]
- 17. Davies KD, Le AT, Theodoro MF et al Identifying and targeting ROS1 gene fusions in non‐small cell lung cancer. Clin Cancer Res 2012; 18: 4570–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rimkunas V, Crosby K, Kelly M et al Frequencies of ALK and ROS in NSCLC FFPE tumor samples utilizing a highly specific immunohistochemistry‐based assay and FISH analysis. J Clin Oncol 2010; 28 (15): Suppl): Abstract 10536. [Google Scholar]
- 19. Rimkunas VM, Crosby KE, Li D et al Analysis of receptor tyrosine kinase ROS1‐positive tumors in non‐small cell lung cancer: Identification of a FIG‐ROS1 fusion. Clin Cancer Res 2012; 18: 4449–57. [DOI] [PubMed] [Google Scholar]
- 20. Govindan R, Ding L, Griffith M et al Genomic landscape of non‐small cell lung cancer in smokers and never‐smokers. Cell 2012; 150: 1121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seo JS, Ju YS, Lee WC et al The transcriptional landscape and mutational profile of lung adenocarcinoma. Genome Res 2012; 22: 2109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cancer Genome Atlas Research Network . Comprehensive molecular profiling of lung adenocarcinoma. (Published erratum appears in Nature 2014; 514: 514.). Nature 2014; 511: 543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ou SH, Chalmers ZR, Azada MC et al Identification of a novel TMEM106B‐ROS1 fusion variant in lung adenocarcinoma by comprehensive genomic profiling. Lung Cancer 2015; 88: 352–4. [DOI] [PubMed] [Google Scholar]
- 24. Zhu VW, Upadhyay D, Schrock AB, Gowen K, Ali SM, Ou SH. TPD52L1‐ROS1, a new ROS1 fusion variant in lung adenosquamous cell carcinoma identified by comprehensive genomic profiling. Lung Cancer 2016; 97: 48–50. [DOI] [PubMed] [Google Scholar]
- 25. Zehir A, Benayed R, Shah RH et al Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. (Published erratum appears in Nat Med 2017; 23: 1004.). Nat Med 2017; 23: 703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen YF, Hsieh MS, Wu SG et al Clinical and the prognostic characteristics of lung adenocarcinoma patients with ROS1 fusion in comparison with other driver mutations in East Asian populations. J Thorac Oncol 2014; 9: 1171–9. [DOI] [PubMed] [Google Scholar]
- 27. Mazières J, Zalcman G, Crinò L et al Crizotinib therapy for advanced lung adenocarcinoma and a ROS1 rearrangement: Results from the EUROS1 cohort. J Clin Oncol 2015; 33: 992–9. [DOI] [PubMed] [Google Scholar]
- 28. Shaw AT, Ou SH, Bang YJ et al Crizotinib in ROS1‐rearranged non‐small‐cell lung cancer. N Engl J Med 2014; 371: 1963–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li C, Fang R, Sun Y et al Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. PLoS One 2011; 6: e28204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mescam‐Mancini L, Lantuéjoul S, Moro‐Sibilot D et al On the relevance of a testing algorithm for the detection of ROS1‐rearranged lung adenocarcinomas. Lung Cancer 2014; 83: 168–73. [DOI] [PubMed] [Google Scholar]
- 31. Pan Y, Zhang Y, Li Y et al ALK, ROS1 and RET fusions in 1139 lung adenocarcinomas: A comprehensive study of common and fusion pattern‐specific clinicopathologic, histologic and cytologic features. Lung Cancer 2014; 84: 121–6. [DOI] [PubMed] [Google Scholar]
- 32. Jin Y, Sun PL, Kim H et al ROS1 gene rearrangement and copy number gain in non‐small cell lung cancer. Virchows Arch 2015; 466: 45–52. [DOI] [PubMed] [Google Scholar]
- 33. Travis WD, Brambilla E, Nicholson AG et al The 2015 World Health Organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol 2015; 10 (9): 1243–60. [DOI] [PubMed] [Google Scholar]
- 34. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. American Joint Committee on Cancer. Cancer Staging Manual, 7th edn. Springer, New York: 2009. [Google Scholar]
- 35. National Comprehensive Cancer Network . (NCCN) Clinical Practice guidelines in oncology. Non Small Cell Lung Cancer. version 4. 2017. [Cited 18 Jan 2017.] Available at URL: www.nccn.org.
- 36. Bhattaeharjee A, Richards WG, Staunton J et al Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci U S A 2001; 98: 13790–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bild AH, Yao G, Chang JT et al Oncogenic pathway signatures in human cancers as a guide to targeted therapies. Nature 2006; 439: 353–7. [DOI] [PubMed] [Google Scholar]
- 38. Garber ME, Troyanskaya OG, Schluens K et al Diversity of gene expression in adenocarcinoma of the lung. Proc Natl Acad Sci U S A 2001; 98: 13784–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shaw AT, Yeap BY, Mino‐Kenudson M et al Clinical features and outcome of patients with non‐small‐cell lung cancer who harbor EML4‐ALK. J Clin Oncol 2009; 27: 4247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Solomon B. Validating ROS1 rearrangements as a therapeutic target in non‐small‐cell lung cancer. J Clin Oncol 2015; 33: 972–4. [DOI] [PubMed] [Google Scholar]
- 41. McDermott U, Iafrate AJ, Gray NS et al Genomic alterations of anaplastic lymphoma kinase may sensitize tumors to anaplastic lymphoma kinase inhibitors. Cancer Res 2008; 68: 3389–95. [DOI] [PubMed] [Google Scholar]
- 42. Wu YL, Yang JC, Kim DW et al Phase II Study of Crizotinib in East Asian patients with ROS1‐positive advanced non‐small‐cell lung cancer. J Clin Oncol 2018; 36: 1405–11. [DOI] [PubMed] [Google Scholar]
- 43. Jin Y, Sun P‐L, Park SY et al Frequent aerogenous spread with decreased E‐cadherin expression of ROS1‐rearranged lung cancer predicts poor disease‐free survival. Lung Cancer 2015; 89: 343–9. [DOI] [PubMed] [Google Scholar]
- 44. Song ZB, Zheng YH, Zhang YP. ALK and ROS1 rearrangements, coexistence and treatment in EGFR‐wild type lung adenocarcinoma: A multicenter study of 732 cases. J Thorac Oncol 2017; 12 (1 Suppl): s1160–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uguen A, Schick U, Quéré G. A rare case of ROS1 and ALK double rearranged non‐small cell lung cancer. J Thorac Oncol 2017; 12: e71–2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ROS1 fusion genes detectable by the AmoyDx assay.


