Abstract
Background
Extended or combined segmentectomies are usually adapted for intersegmental pulmonary nodules. This study explored precise combined subsegmentectomy (CSS) under the guidance of three‐dimensional computed tomography bronchography and angiography (3D‐CTBA).
Methods
The definition of a pulmonary intersegmental nodule was based on a minimum distance between the nodule and the involved intersegmental veins in the preoperative 3D‐CTBA being less than the size of the nodule. Centering on the involved intersegmental vein, two adjacent subsegments belonging to the different segments were combined as a resected unit.
Results
We retrospectively reviewed the records of 47 patients (mean age 53.6 ± 12.3, range: 26–81 years) who underwent CSS. Thirty‐nine (83.0%) nodules were involved in most intersegmental locations of the upper lobes; the remainder in the lower lobes. The mean nodule size was 0.86 ± 0.32 cm; the mean margin width was 2.20 ± 0.38 cm. Pathological stages included: Tis (8 cases), T1mi (16), IA1 (T1aN0M0, 13), and IA2 (T1bN0M0, 5). Pathological diagnoses included: invasive adenocarcinoma (18 cases), minimally invasive adenocarcinoma (16), adenocarcinoma in situ (8), atypical adenomatous hyperplasia (3), and benign (2). The average operative duration was 190.8 ± 54.9 minutes; operative hemorrhage was 42.7 ± 23.0 mL; 5.8 ± 2.8 lymph nodes dissected had not metastasized; the duration of postoperative chest tube drainage was 3.0 ± 1.8 days; and the postoperative hospital stay was 5.3 ± 2.4 days.
Conclusions
Under 3D navigation, thoracoscopic CSS is a safe technique for intersegmental nodules, sparing more pulmonary parenchyma and ensuring safe margins to achieve anatomical resection.
Keywords: Bronchography and angiography, pulmonary nodule, segmentectomy, thoracoscopy, three‐dimensional computed tomography
Introduction
While segmentectomy yields the same oncological outcomes as lobectomy in stage IA non‐small cell lung cancer (NSCLC),1, 2, 3 segmentectomy has the additional advantage of preserving postoperative pulmonary function.4, 5, 6 Compared to wedge resection, anatomic segmentectomy assures a sufficient surgical margin and sampling of lymph nodes for accurate tumor N staging.7, 8
For a small pulmonary nodule at the central area of a segment, a single segmentectomy can achieve an adequate surgical margin. A study based on high‐resolution computed tomography reported that approximately 30% of c‐T1aN0M0 NSCLCs extend beyond one segment.9 Therefore, to ensure an adequate surgical margin, an extended segmentectomy is usually performed to resect additional parenchyma from the adjacent segment.10, 11, 12 For nodules located at the edge of a segment or between the adjacent segments, an extended segmentectomy is similar to an excessive wedge resection: a segmentectomy plus a wedge resection (Fig 1a–c).
Figure 1.

Schematic diagram of extended segmentectomy and combined subsegmentectomy for the intersegmental nodule. (a) A nodule was close to the intersegmental vein V2a, seated between right S1 and S2. (b) A right S1 extended segmentectomy for the nodule, a wedge resection of S2 plus an S1 segmentectomy. The intersegmental vein (V2a) could be injured. (c) A right S2 extended segmentectomy for the nodule, a wedge resection of S1 plus S2 segmentectomy. These wedge resections actually played a major role for the nodule. (d) A combined subsegmentectomy (S1a + S2a CSS) for the nodule. Centering on the involved intersegmental vein, two adjacent subsegments belonging to the different segments were combined as a resected unit. The intersubsegmental veins (V1a and V2b) could be preserved.
The intersegmental veins run along the intersegmental plane and are its natural borders.13 Three‐dimensional computed tomography bronchography and angiography (3D‐CTBA) can reveal both the segmental structures and the location of the nodule.14, 15, 16 In preoperative 3D images, we defined the nodule close to the intersegmental vein as the intersegmental nodule, which is situated at the edge of a segment or between the adjacent segments. We designed a method using combined subsegmentectomy (CSS) to treat the intersegmental nodules (Fig 1d).
The aim of this study was to evaluate the technical feasibility of CSS under 3D navigation for intersegmental nodules and to analyze the early clinical results.
Methods
The local institutional review board waived individual patient consent because of the retrospective design of the study. From April 2014 to August 2018, 47 segmentectomies were performed for pulmonary intersegmental nodules by a team at the First Affiliated Hospital of Nanjing Medical University.
The inclusion criterion for CSS was: a pulmonary intersegmental nodule ≤ 2 cm with a ≥ 50% ground glass opacity appearance on CT. The exclusion criterion for CSS was if preoperative 3D‐CTBA revealed that the intersegmental nodule was located in the center of the lobe. The definition of a pulmonary intersegmental nodule was based on the minimum distance between the nodule and the involved intersegmental veins in the 3D image being less than the size of the nodule.
Combined subsegmentectomies were planned and performed under the guidance of 3D navigation. The surgical procedure should achieve parenchymal resection margins ≥ 2 cm or ≥ the size of the nodule. The size of the nodule was the maximum diameter of the nodule detected on CT reconstruction lung window imaging.
Preoperative 3D‐CTBA reconstruction
All patients had undergone preoperative 3D‐CTBA examinations using dual source CT (Definition, Siemens, Munich, Germany). Patients were placed in a supine position with their hands on their heads. A 22 G intravenous indwelling needle was inserted into the median brachial or forearm vein, and approximately 60 mL of a nonionic contrast agent (ultracist, Bayer Schering, Berlin, Germany) was injected at a rate of 5 mL/second using a double tube high‐pressure injector. An additional 20 mL of saline was then added at the same rate. The patients were scanned after the contrast agent was injected for approximately 16–20 seconds. The scanning range was defined from the plane of the thoracic inlet to the diaphragmatic plane. The scanning parameters were as follows: the tube voltage was 120 kv, the effective tube current was approximately 100–150 mAs, the collimator thickness was 0.6 mm, and the reconstruction thickness was 1 mm. The reconstruction software “DeepInsight” and online software “Horos Project” (https://horosproject.org/) were used to reconstruct images of the pulmonary bronchi and blood vessels.
Surgical simulation
Surgical simulation of CSS was then performed. In dynamic 3D images, the relationship between the nodule and the intersegmental vein was observed. The intersegmental nodule was identified according to the defined standard by measuring the maximum size of the nodule and the minimum distance between the nodule and intersegmental vein. Centering on the involved intersegmental vein, two adjacent subsegments belonging to the different segments were combined as a resected unit (CSS) (Fig 2a). The involved intersegmental vein became the intrasegmental vein of the combined subsegments that would be dissected during the procedure. The intersubsegmental veins of the combined subsegments would be preserved to mark the intersubsegmental border. The targeted subsegmental arteries and bronchi were identified and targeted for dissection in the CSS.
Figure 2.
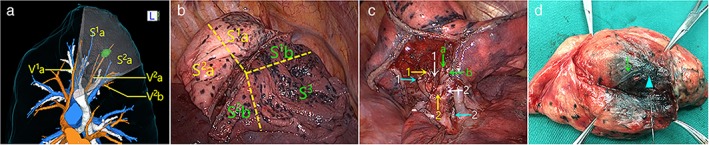
Right S1a + S2a combined subsegmentectomy for an intersegmental nodule located between S1 and S2 with the guidance of three‐dimensional (3D) navigation. (a) The pulmonary vessels, bronchi, and pulmonary nodules were reconstructed by using preoperative 3D‐computed tomography bronchography and angiography (CTBA), which revealed that a nodule was close to the intersegmental vein V2a. The intersubsegmental plane was virtualized according to the intersubsegmental veins V1a and V2b. Surgical simulation indicated an S1a + S2a CSS. The red vessels are veins, the blue vessels are arteries, and the white vessels are bronchi. (b) After the targeted vessels and bronchi were dissected, modified “inflation‐deflation” was applied to identify the intersubsegmental plane. Sharp dissection and staplers separated the intersubsegmental parenchyma. The yellow dotted lines represent intersegmental borders. (c) View of the hilum after S1a + S2a removal and depiction of the stumps of A1a (yellow arrow 1), B1a (white arrow 1), A2a (yellow arrow 2), B2a (white arrow 2), V2a (green arrow a), and the preserved B1b (blue arrow 1), B2b (blue arrow 2), intersubsegmental vein V2b (green arrow b), V1a (green arrow c). (d) The nodule (blue triangle) was in the center of the dissected specimen. The intersegmental vein (green arrow) was involved. Pathological findings confirmed the diagnosis of minimally invasive adenocarcinoma with a sufficient margin width.
Surgical methods
All of the patients were placed under general anesthesia with double lumen endotracheal intubation. The patients were positioned in the lateral decubitus position and were administered single lung ventilation using the three‐port method. During surgery, two monitors were placed before the operators: a thoracoscopic monitor and a 3D imaging system. The actual anatomical structures corresponded one‐to‐one with the virtual anatomical structures. Real‐time 3D navigation guided the dissection of the targeted bronchi and vessels.
A modified “inflation‐deflation” technique was used to identify the intersubsegmental border (Fig 2b,c). After the targeted arteries and bronchi were dissected, double lung ventilation to full expansion was initiated, followed by single lung ventilation. Approximately 10 to 15 minutes later, the preserved segments were completely deflated, but the targeted combined subsegments were kept expanded so that the intersubsegmental border was clearly identified. The inflation‐deflation interface was separated along the intersubsegmental veins up to the outer one third of the pulmonary parenchyma using electrocautery or an ultrasonic scalpel. To reduce air leakage, the residual intersubsegmental parenchyma was tailored by staplers. After the targeted combined subsegments were resected, fibrin glue was applied to cover the surface of intersubsegmental plane.
All specimens were examined by frozen section during the operation. In malignant cases, N1 and N2 lymph nodes were sampled. If there was evidence of lymph node involvement, a lobectomy and systematic lymphadenectomy was conducted. The margin width was defined as the minimum distance from the resection margin to the nodule detected in the removed specimen, which was kept semi‐inflated (Fig 2d).
Perioperative data were recorded for all cases. All patients were followed‐up at two weeks and three months postoperatively and were then monitored as outpatients at six‐month intervals. The eighth edition Union for International Cancer Control (UICC) Tumor Node Metastasis (TNM) Classification was used for staging.
Results
Clinical characteristics of the patients
The clinical characteristics of the 47 patients (13 men and 34 women, mean age 53.6 ± 12.3, range: 26–81 years) in this study are presented in Table 1. The mean nodule size was 0.86 ± 0.32 cm. The nodules were located in the right upper lobe (24, 51.1%), the left upper lobe (15, 31.9%), and the lower lobe (8, 17.0%). Pathological staging exhibited: Tis (8 cases), T1mi (16 cases), IA1 (T1aN0M0, 13 cases), and IA2 (T1bN0M0, 5 cases). The tumor pathological diagnoses included: invasive adenocarcinoma (18 cases), minimally invasive adenocarcinoma (16 cases), adenocarcinoma in situ (8 cases), atypical adenomatous hyperplasia (3 cases), and benign (2 cases).
Table 1.
Clinical characteristics of the patients
| Factor | Combined Subsegmentectomy |
|---|---|
| Age | |
| Mean (range) | 53.6 ± 12.3 (26–81 years) |
| Gender | |
| Male | 13 |
| Female | 34 |
| Comorbidity | |
| Diabetes | 4 |
| Hypertension | 8 |
| Breast carcinoma | 2 |
| Mean nodule size (cm) | 0.86 ± 0.32 |
| Nodule location | |
| RUL | 24 |
| LUL | 15 |
| LL | 8 |
| Pathological diagnoses | |
| Benign | 2 |
| AAH | 3 |
| AIS | 8 |
| MIA | 16 |
| IAC | 18 |
| TNM stage | |
| 0 (Tis) | 8 |
| T1mi | 16 |
| IA1 (T 1aN0M0) | 13 |
| IA2 (T 1bN0M0) | 5 |
AAH, atypical adenomatous hyperplasia; AIS, adenocarcinoma in situ; IAC, invasive adenocarcinoma; LL, lower lobe; LUL, left upper lobe; MIA, minimally invasive adenocarcinoma; RUL, right upper lobe; TNM, tumor node metastasis.
Nodule location and surgical procedure
Table 2 shows the details of the nodule locations and surgical procedures. The intersegmental nodules were located in each lobe, except the right middle lobe. The CSSs refer to various combinations of subsegments. Thirty‐nine (83.0%) nodules were involved in most intersegmental locations of the upper lobes, while the remainder was located in low lobes.
Table 2.
Details of the nodule location and surgical procedure
| Nodule Location | Combined Subsegmentectomy | Number (n = 47) |
|---|---|---|
| Right | 31 | |
| Between S1 and S2 | S1a + S2a | 5 |
| Between S1 and S3 | S1b + S3b1 | 1 |
| S1a + S3a | 1 | |
| Between S2 and S3 | S2b + S3a | 16 |
| S2b + S3a2 | 1 | |
| Between S6 and S9 | S6b + S9a | 1 |
| Between S6 and S8 | S6b + S8a | 2 |
| Between S7 and S8 | S7b + S8a | 1 |
| Between S8 and S9 | S8a + S9a | 2 |
| S8b + S9b | 1 | |
| Left | 16 | |
| Between S1 + 2 and S3 | S1+2a + S3c | 5 |
| S1+2a2 + S3b + c | 1 | |
| S1+2a + b + S3c | 3 | |
| Between S1 + 2 and S4 | S1+2c + S4a | 1 |
| Between S3 and S4 | S3a + S4a | 3 |
| S3b + S4b | 2 | |
| Between S8 and S9 | S8a + S9a | 1 |
Evaluation of intraoperative and postoperative factors
As shown in Table 3, all CSSs were performed successfully with no intraoperative conversions or complications. The mean margin width of CSS was 2.20 ± 0.38 cm. The margin width of each CSS met the margin requirement (≥ 2 cm or ≥ the size of the nodule). The average operative duration was 190.8 ± 54.9 minutes. The extent of operative hemorrhage was 42.7 ± 23.0 mL. The duration of postoperative chest tube drainage was 3.0 ± 1.8 days. The postoperative hospital stay was 5.3 ± 2.4 days. The number of dissected lymph nodes that had not metastasized was 5.8 ± 2.8.
Table 3.
Evaluation of intraoperative and postoperative factors
| Factor | Combined Subsegmentectomy |
|---|---|
| Mean margin width (cm) | 2.20 ± 0.38 |
| Intraoperative conversions and complications | |
| No | 47 |
| Yes | 0 |
| Average operative duration (minutes) | 190.8 ± 54.9 |
| Operative hemorrhage (mL) | 42.7 ± 23.0 |
| Duration of postoperative chest tub drainage (days) | 3.0 ± 1.8 |
| Postoperative hospital stay (days) | 5.3 ± 2.4 |
| Number of lymph nodes dissected | 5.8 ± 2.8 |
Discussion
The ability to obtain a sufficiently wide surgical margin is the key factor that makes segmentectomy superior to wedge resection.7, 8 For pulmonary intersegmental nodules at the edge of the involved segment or between adjacent segments, it is difficult to ensure a safe margin with a single segmentectomy. Furthermore, it may not even be possible to remove the pulmonary nodules in this situation, necessitating an extended segmentectomy.10, 11, 12 The extended segmentectomy for intersegmental nodules is essentially a wedge resection with the added potential problem of insufficient margins.
Combined subsegmentectomy can ensure that a safe margin is achieved by placing the intersegmental nodules in the central area of the involved adjacent subsegments. In our study, the margin width of all CSSs (2.20 ± 0.38 cm) met the surgical requirement. The advantage of CSS is achieving a sufficient margin for the intersegmental nodule.
Pulmonary veins run in the intersegmental planes, mark the boundary of the segments, and drain adjacent segments, thus sparing them preserves the venous drainage of adjacent segments.13 To obtain a wide enough surgical margin, the involved intersegmental vein must be dissected during extended segmentectomy,10 which theoretically can decrease the venous return of the preserved segments. With the use of 3D‐CTBA, the intersegmental vein that runs in the center of the combined subsegments becomes the intrasegmental vein, which is resected during CSS. In this group, all of the intersubsegmental veins are preserved and mark the boundaries of the intersubsegmental border. In this way, CSS does not affect the venous return of the residual subsegments.
Preserving more pulmonary parenchyma is one of the important advantages of segmentectomies. Extended segmentectomy usually resects a segment (2 or 3 subsegments) and additional parenchyma from the adjacent segment. However, during CSS, two subsegments are generally removed; therefore, CSS also preserves more pulmonary parenchyma, which is essentially equivalent to resecting one segment.5, 16
It is often difficult to distinguish the precise segment in which the nodule is located with ordinary 2D CT. In this study, 3D imaging clearly identified the pulmonary intersegmental nodules. Based on the relationship of the position between the pulmonary nodules and the intersegmental veins,14 the standard for defining the intersegmental nodules is as follows: the minimum distance between the nodule and the intersegmental vein should be less than the maximum diameter of the nodules. It is necessary to achieve a safe surgical margin with segmentectomy for early stage lung cancer. If the distance is greater than the size of nodule, a single segmentectomy is adequate. However, not all pulmonary nodules that meet this standard are suitable for CSS. For example, when the pulmonary nodule is located in the center of the lobe and the distance between the bronchial origin of the involved subsegment and the nodule is less than the size of the nodule, CSS cannot guarantee a safe surgical margin and lobectomy is necessary.
Complicated and variant anatomical structures make segmentectomy technically difficult.3, 17 Preoperative 3D‐CTBA can clearly identify the segmental structures and localize the nodules.14, 15, 16 Surgical simulation with 3D images also helps to plan the surgical approach. Through real‐time navigation during surgery actual anatomical structures correspond one‐to‐one to the virtual anatomical structures, which reduces the difficulty and improves the accuracy of the surgical technique.18, 19 In this study, we successfully completed several CSSs under 3D‐CTBA guidance and the early clinical results were satisfactory.
There are a number of limitations surrounding the use of CSS. We designed the CSS based on the relationship between the nodule and the intersegmental vein with the primary aim of obtaining a safe surgical margin. However, whether CSS achieves the recommended lymphatic drainage is unclear. The patients selected for this study were diagnosed with ground glass opacity‐dominant small cell lung cancer, which is rarely associated with lymph node metastases. Theoretically, anatomic CSS reduces the removal of pulmonary parenchyma and preserves the venous return of the remaining segments, but the outcomes of CSS should be compared with those of traditional segmentectomy in the future. Long‐term follow‐up is also needed to evaluate the efficacy of CSS for early stage lung cancer. In our sample, 39 (83.0%) nodules were involved in most intersegmental locations of the upper lobes, while the remainder was located in the lower lobes. Because this surgical method is still in the exploratory stage, it does not involve all segments of the lower lobes, and thus requires further exploration.
Considering the unique location of pulmonary intersegmental nodules, CSS is an ideal surgical approach that localizes the lesion in the center of the combined subsegments and thus ensures an adequate surgical margin and preserves the intersubsegmental vein, obtaining a sufficient margin. Moreover, 3D‐CTBA navigation contributes to the design and performance of CSS.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This work was funded by the Province Natural Science Foundation of Jiangsu (BK20151584), the Province Six Talent Peak Foundation of Jiangsu (WSW‐028), the Province Social Development Foundation of Jiangsu‐Clinical Frontier Technology (BE2016790), the Province Medical Innovation Team of Jiangsu (CXTDA2017006), and the Province 333 Talent Project of Jiangsu (BRA2017545).
Contributor Information
Quan Zhu, Email: chest2006@163.com.
Liang Chen, Email: clbright0909@njmu.edu.cn.
References
- 1. Landreneau RJ, Normolle DP, Christie NA et al Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage i non‐small‐cell lung cancer: A propensity‐matched analysis. J Clin Oncol 2014; 32: 2449–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhong C, Fang W, Mao T, Yao F, Chen W, Hu D. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy for small‐sized stage ia lung cancer. Ann Thorac Surg 2012; 94: 362–7. [DOI] [PubMed] [Google Scholar]
- 3. Yamashita S, Tokuishi K, Anami K et al Thoracoscopic segmentectomy for t1 classification of non‐small cell lung cancer: A single center experience. Eur J Cardiothorac Surg 2012; 42: 83–8. [DOI] [PubMed] [Google Scholar]
- 4. Harada H, Okada M, Sakamoto T, Matsuoka H, Tsubota N. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005; 80: 2041–5. [DOI] [PubMed] [Google Scholar]
- 5. Yoshimoto K, Nomori H, Mori T, Ohba Y, Shiraishi K, Ikeda K. Combined subsegmentectomy: Postoperative pulmonary function compared to multiple segmental resection. J Cardiothorac Surg 2011; 6: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wisnivesky JP, Henschke CI, Swanson S et al Limited resection for the treatment of patients with stage ia lung cancer. Ann Surg 2010; 251: 550–4. [DOI] [PubMed] [Google Scholar]
- 7. Tsutani Y, Miyata Y, Nakayama H et al Appropriate sublobar resection choice for ground glass opacity‐dominant clinical stage ia lung adenocarcinoma: Wedge resection or segmentectomy. Chest 2014; 145: 66–71. [DOI] [PubMed] [Google Scholar]
- 8. Paoletti L, Pastis NJ, Denlinger CE, Silvestri GA. A decade of advances in treatment of early‐stage lung cancer. Clin Chest Med 2011; 32: 827–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Horinouchi H, Nomori H, Nakayama T et al How many pathological t1n0m0 non‐small cell lung cancers can be completely resected in one segment? Special reference to high‐resolution computed tomography findings. Surg Today 2011; 41: 1062–6. [DOI] [PubMed] [Google Scholar]
- 10. Schuchert MJ, Pettiford BL, Pennathur A et al Anatomic segmentectomy for stage i non‐small‐cell lung cancer: Comparison of video‐assisted thoracic surgery versus open approach. J Thorac Cardiovasc Surg 2009; 138: 1318–25 e1. [DOI] [PubMed] [Google Scholar]
- 11. Oizumi H, Kanauchi N, Kato H et al Total thoracoscopic pulmonary segmentectomy. Eur J Cardiothorac Surg 2009; 36: 374–7. [DOI] [PubMed] [Google Scholar]
- 12. Okada M, Tsutani Y, Ikeda T et al Radical hybrid video‐assisted thoracic segmentectomy: Long‐term results of minimally invasive anatomical sublobar resection for treating lung cancer. Interact Cardiovasc Thorac Surg 2012; 14: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stanley C. Fell; Segmental Resection In: Patterson GA, Cooper JD, Deslauriers J, AEMR L, Luketich JD, Rice TW. (eds). Pearson's Thoracic and Esophageal Surgery; Vol 2 set, 3rd edn. Churchill Livingstone, New York: 2008; 73.887–93. [Google Scholar]
- 14. Iwano S, Yokoi K, Taniguchi T, Kawaguchi K, Fukui T, Naganawa S. Planning of segmentectomy using three‐dimensional computed tomography angiography with a virtual safety margin: Technique and initial experience. Lung Cancer 2013; 81: 410–5. [DOI] [PubMed] [Google Scholar]
- 15. Chan EG, Landreneau JR, Schuchert MJ et al Preoperative (3‐dimensional) computed tomography lung reconstruction before anatomic segmentectomy or lobectomy for stage i non‐small cell lung cancer. J Thorac Cardiovasc Surg 2015; 150: 523–8. [DOI] [PubMed] [Google Scholar]
- 16. Oizumi H, Kanauchi N, Kato H et al Anatomic thoracoscopic pulmonary segmentectomy under 3‐dimensional multidetector computed tomography simulation: A report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011; 141: 678–82. [DOI] [PubMed] [Google Scholar]
- 17. Gossot D, Ramos R, Brian E, Raynaud C, Girard P, Strauss C. A totally thoracoscopic approach for pulmonary anatomic segmentectomies. Interact Cardiovasc Thorac Surg 2011; 12: 529–32. [DOI] [PubMed] [Google Scholar]
- 18. Wu WB, Xu XF, Wen W et al Three‐dimensional computed tomography bronchography and angiography in the preoperative evaluation of thoracoscopic segmentectomy and subsegmentectomy. J Thorac Dis 2016; 8: S710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu WB, Xu XF, Wen W, Xu J, Zhu Q, Chen L. Thoracoscopic pulmonary sub‐subsegmentectomy based on three‐dimensional images. Ann Thorac Surg 2016; 102: e389–91. [DOI] [PubMed] [Google Scholar]


