Abstract
Background
The Ki‐67 labeling index (LI) is a well‐known prognostic factor for primary breast cancer, but its clinical significance for metachronous axillary lymph node (ALN) recurrence has not been well documented.
Methods
Ki‐67 expression in primary tumors (PTs) and ALN metastases (ALNMs) was evaluated in 21 patients and quantified to investigate the relationship between Ki‐67 LIs in PTs and metachronous ALNMs.
Results
The median Ki‐67 LIs in the PTs and ALNMs were 25.2% (range: 2.3–80.2%) and 70% (range: 10.4–97.4%), respectively. A majority of patients had higher Ki‐67 LIs in ALNMs than in PTs (76.2%, 16/21). Disease‐specific survival was significantly better in patients with a lower‐than‐median ALNM Ki‐67 LI (P = 0.019, log‐rank test). Receiver operating characteristic curves showed a PT Ki‐67 LI of 62.8% as the optimal cutoff value and an ALNM Ki‐67 LI of 65.1%. Accordingly, we divided the patients into four groups: PT Ki‐67 LI lower than 62.8%/ALNM Ki‐67 LI lower than 65.1%, PT Ki‐67 LI lower/ALNM Ki‐67 LI higher, PT Ki‐67 LI higher/ALNM Ki‐67 LI higher, and PT Ki‐67 LI higher/ALNM Ki‐67 LI lower. Disease‐specific survival was significantly better in patients with Ki‐67 LI lower/ALNM Ki‐67 LI lower than in the other groups.
Conclusion
This is the first study to show that the Ki‐67 LI in metachronous ALNM is a prognostic factor for patients with metachronous ALN recurrence of breast cancer.
Keywords: Axillary lymph node, Ki‐67 labeling index, metachronous, metastasis, primary breast cancer
Introduction
Nuclear protein Ki‐67 expression increases during cell proliferation and the percentage of cells positive for Ki‐67, termed the Ki‐67 labeling index (LI), has been shown to be a prognostic factor in many cancers, including breast cancer.1, 2, 3 Ki‐67 is a useful marker of proliferation because it is weakly expressed in G0 but more strongly expressed in proliferating cells.4 Several studies have shown that the percentage of Ki‐67‐positive cells detected by immunohistochemical (IHC) staining of breast cancer tissue correlates with patient outcomes. For example, Ki‐67 LI has been shown to be an independent prognostic factor in patients with pathological T1N0 disease who did not receive postoperative chemotherapy;1 in menopausal patients with estrogen receptor (ER)‐positive/HER2‐negative breast cancer who received tamoxifen therapy without postoperative chemotherapy;2 and in patients with ER‐positive/HER2‐negative cancer, including those who received postoperative chemotherapy.5 These studies stratified patients based on a Ki‐67 LI higher or lower than a pre‐specified cutoff value; the outcomes were generally better in patients with a lower LI. Measurement of Ki‐67 LI has recently been incorporated into therapeutic strategies for breast cancer and the subtyping of tissue into high and low‐Ki‐67 LI categories was discussed at the St. Gallen Consensus Conference on standardizing the treatment of early breast cancer.6 However, only a few studies have compared the Ki‐67 LI of primary and metastatic breast cancer foci, most of which compared findings in primary tumors (PTs) with those in synchronous metastases.7, 8, 9, 10 The frequency of metachronous axillary lymph node recurrence (ALNR) after resection of the PT in patients with breast cancer varies depending on the surgical technique used, reportedly ranging from 0.8% to 8.6%.11, 12 The status of many biomarkers, including ER and HER2, may differ between PTs and metachronous metastatic foci, such as axillary lymph node metastases (ALNMs) obtained by dissection for ALNR.13, 14 In this paper, we present the first comparison of the Ki‐67 LI in PTs and metastatic ALNMs in breast cancer patients with ALNR. We also examine the relationship between Ki‐67 LI in primary and metachronous metastases and various patient outcomes.
Methods
Patients
We enrolled 21 women with breast cancer who had developed metachronous ALNR after resection of their PT between December 2006 and June 2017. PT samples were obtained at resection and ALNM samples by dissection or biopsy at least one year after PT resection. The Institutional Review Board of Nihon University School of Medicine approved this retrospective study and written informed consent was obtained from the patients for all procedures.
Ki‐67 labeling index (LI)
Ki‐67 expression in PTs and ALNMs was detected by IHC staining with anti‐Ki‐67 clone MIB‐1 (Dako, Glostrup, Denmark), followed by colorimetric development with the peroxidase substrate 3,3′‐diaminobenzidine. The sections were counterstained with hematoxylin. The Ki‐67 proliferation index was defined as the percentage of cells with positive nuclear Ki‐67 immunostaining in a hotspot section of confirmed carcinoma and was quantified using e‐Count cell counting software (e‐path, Kanagawa, Japan). JPEG images were automatically segmented into Ki‐67‐positive and negative areas according to the cutoff point determined from a histogram of brown (Ki‐67) and blue (hematoxylin) color density in the nuclei. In cases of multiple lymph node metastases, we examined all lymph nodes and adopted the highest Ki‐67 LI. Two pathologists also microscopically reviewed the tumor samples to verify the Ki‐67‐positive and negative scores obtained by the software. The median numbers of ALNM and PT tumor cells per sample were 686 (range: 502–880) and 573 (range: 500–995), respectively. ER was measured by IHC analysis and defined as positive when ≥ 10% of the tumor cells showed positive staining. HER2 was measured by IHC or fluorescence in situ hybridization (FISH) and defined as positive when the IHC score was 3+ or FISH was positive.
Statistical methods
SPSS version 21.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis.
Local control of ALNR was assessed by univariate analysis using Pearson's χ2 test and multivariate analysis using the Cox proportional hazards regression model of the following variables: age at treatment of ALNR (≥ or < median), single or multiple ALNMs, maximum diameter of ALNMs (≥ or < median), presence or absence of metastasis to other organs at the time of ALNR treatment, immunohistological subtypes, Ki‐67 LI in ALNM (≥ or < median), higher or lower Ki‐67 LI in ALNM than in PT, and adjuvant therapy after ALNM surgery. The relationship between the time from PT resection to ALNM treatment (≥ or < median) and Ki‐67 LI in ALNM (≥ or < median) was assessed by univariate analysis using Pearson's χ2 test. Local control of ALNR was defined as a complete response when there was no evidence of recurrence in the ALNR treatment area. Tumor responses were assessed using computed tomography (CT) or echo or magnetic resonance imaging performed the day after the final ALNR treatment (median 130, range: 28–996 days). The disease‐specific survival (DSS) rates according to patient and tumor characteristics and treatment strategies were assessed by univariate analysis using Pearson's χ2 test and multivariate analysis using the Cox proportional hazards regression model. The Kaplan–Meier method was used to determine the probability of DSS from the date of surgery for PT. Patients were stratified according to the above variables and Ki‐67 LI in the PT and metachronous ALNM (≥ or < median) groups. We also established receiver operating characteristic (ROC) curves and attempted to find the optimal Ki‐67 LI cutoff value. Outcomes for these patient subgroups were compared using Mantel's log‐rank test.
Results
Table 1 summarizes the clinicopathological characteristics. The median age at ALNR treatment was 70 years (range: 29–82). The pathological stages of the PT according to the seventh edition Union for International Cancer Control Tumor Node Metastasis classification (2009) were: IA (7 patients), IIA (8 patients), IIB (4 patients), and IIIA (2 patients).
Table 1.
Characteristics of patients with breast cancer and axillary lymph node recurrence
| Characteristic | Number (%) |
|---|---|
| Patients | 21 |
| Initial surgical treatment | |
| SLN | 7 (33.3) |
| ALND | 14 (66.7) |
| Initial UICC pathological stage | |
| IA | 7 (33.3) |
| IIA | 8 (38.2) |
| IIB | 4 (19.0) |
| IIIA | 2 (9.5) |
| Age at ALNR treatment (years) | |
| Median | 70 |
| Range | 29–82 |
| ALNR treatment | |
| ALND alone | 9 (42.9) |
| ALNB alone | 2 (9.5) |
| ALND + RT | 3 (14.3) |
| ALNB + RT | 7 (33.3) |
| Number of the lymph node | |
| Single | 5 (23.8) |
| Multiple | 16 (76.2) |
| Size of the largest lymph node (mm) | |
| < 20 mm | 12 (57.1) |
| ≥ 20 mm | 9 (42.9) |
| Corresponding metastatic lesions | |
| None | 15 (71.4) |
| Lymph node | 3 (14.3) |
| Other organs | 3 (14.3) |
| Immunohistological subtype | |
| ER+/HER2‐ | 16 (76.2) |
| ER+/HER2+ | 2 (9.5) |
| ER‐/HER2‐ | 3 (14.3) |
ALNB, axillary lymph node biopsy; ALND, axillary lymph node dissection; ALNR, axillary lymph node recurrence; RT, radiotherapy; SLN, sentinel lymph node biopsy; UICC, Union for International Cancer Control.
Fourteen patients (66.7%) received axillary lymph node dissection as initial treatment. Eleven patients (52.4%) had no lymph node metastasis at the initial pathological stage. Four patients had single lymph node metastasis and six patients had multiple lymph node metastases (range: 2–4) at the initial pathological stage. Ten patients (47.6%) received adjuvant chemotherapy. The time between PT resection and initiation of treatment for ALNR ranged from 371 to 5117 days (median 1015). The number of lymph nodes involved at the time of detection of ALNR ranged from one to more than 10 (i.e. not countable by CT). Five (23.8%) and 16 (76.2%) patients had single and multiple involved lymph nodes, respectively. The median size of the largest lymph node at ALNR was 20 mm (range 8.5–65 mm), as determined by CT. Fifteen patients (71.4%) had no other metastatic lesions at the time of ALNR, whereas three (14.3%) had lymph node metastases outside the axillary region, and another three (14.3%) had metastases to other organs. The surgical procedure for ALNR was dissection and biopsy in 12 (57.1%) and 9 (42.9%) patients, respectively. Radiation therapy (RT) to the ALN region was administered postoperatively in 10 patients (47.6%), with the median total RT dose being 60 Gy (range 50–70 Gy). With respect to ALNM subtype, 16 (76.2%) cases were ER positive/HER2 negative, two (9.5%) were ER negative/HER2 positive, and three (14.3%) were ER negative/HER2 negative.
The Ki‐67 LIs in the PTs and ALNMs were evaluated by IHC staining. The median Ki‐67 LI was 25.2% (range: 2.3–80.2%) in the PTs and 70% (range: 10.4–97.4%) in the ALNMs at recurrence.
The Ki‐67 LIs in the ALNMs at the initial pathological stage were not evaluated by IHC staining at initial treatment, thus IHC staining was performed at this time. Adequate staining of ALN tumor samples was only achieved for three patients with initial ALNM and the ALNM Ki‐67 LI was higher than in the PT in two patients and lower in one. In all three patients, the ALNM Ki‐67 LI at recurrence was higher than the ALNM Ki‐67 LI at the initial pathological stage (data not shown). The ALNM Ki‐67 LI was higher than the PT Ki‐67 LI in 16 patients (76.2%) and lower in five (23.8%), the greatest differences between the two LIs being 87.2% higher and 36.9% lower (Fig 1).
Figure 1.
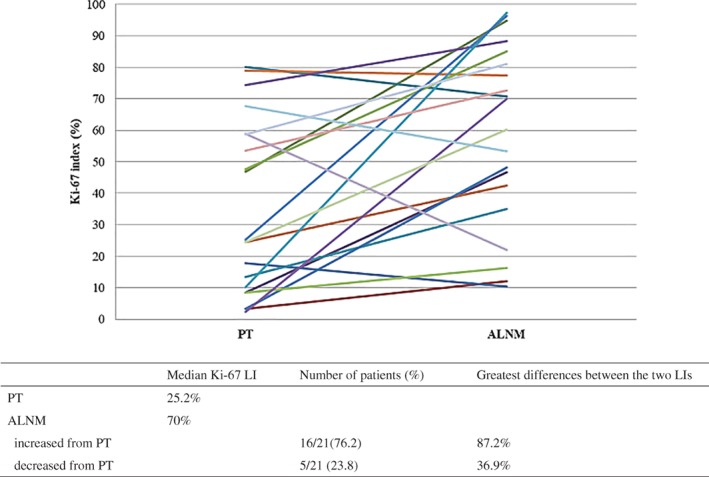
Changes in Ki‐67 LI between primary breast tumor (PT) and axillary lymph node metastasis (ALNM) at recurrence.
In the eight cases of multiple lymph node metastases at the initial or recurrent stage with successful staining, the Ki‐67 LIs varied widely among each lymph node. The median greatest difference between the LIs was 20.4% (range: 0.5–63.3%) (Table 2).
Table 2.
Variations in Ki‐67 LI among multiple ALNMs
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Time of multiple ALNMs | Recurrent | Initial | Recurrent | Recurrent | Recurrent | Recurrent | Recurrent | Recurrent | |
| Number of lymph nodes | 4 | 3 | 2 | 2 | 3 | 4 | 4 | 2 | |
| Ki‐67 LI in ALNM | 34.6% | 14.6% | 46.5% | 20.6% | 53.8% | 53.5% | 51.5% | 73.7% | |
| 45.3% | 38.9% | 48.2% | 42.4% | 71.4% | 56.4% | 61.5% | 74.2% | ||
| 58% | 77.9% | 72.7% | 60.5% | 62.1% | |||||
| 97.4% | 70.7% | 88.4% | Median | ||||||
| Greatest differences between LIs | 62.8% | 63.3% | 1.7% | 21.8% | 18.9% | 17.2% | 36.9% | 0.5% | 20.4% |
ALNB, axillary lymph node metastasis; labeling index.
Univariate analysis showed that there was no relationship between the time from PT resection and Ki‐67 LI changes in ALNMs (data not shown).
The axillary local control rate was 66.7% (complete response in 14 patients). Univariate analysis showed that local control of ALNR was significantly better in patients with an ALNM Ki‐67 LI lower than the PT Ki‐67 LI compared to patients with an ALNM Ki‐67 LI higher than the PT Ki‐67 LI (P = 0.023) (Table 3). Similarly, multivariate analysis showed that an ALNM Ki‐67 LI lower than the PT Ki‐67 LI was associated with better local control; however, this difference was not statistically significant (P = 0.12).
Table 3.
Univariate and multivariate analyses of factors that may impact local control of ALNR
| Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|
| Characteristic and strategy | N = 21 | Odds ratio | 95% CI | P | Odds ratio | 95% CI | P |
| Age at ALNR treatment (years) | |||||||
| < 70/≥ 70 | 9/12 | 0.35 | 0.35–3.32 | 0.89 | 1.08 | 0.13–8.73 | 0.94 |
| Number of lymph nodes | |||||||
| Single/multiple | 5/16 | 0.48 | 0.15–1.53 | 0.21 | 0.50 | 0.098–2.63 | 0.42 |
| Size of the largest lymph node | |||||||
| < 20/≥ 20 mm | 9/12 | 0.67 | 0.23–1.95 | 0.47 | 3.13 | 0.43–22.8 | 0.26 |
| Corresponding metastatic lesion | |||||||
| No/Yes | 15/6 | 0.27 | 0.035–2.17 | 0.22 | 0.72 | 0.048–11.04 | 0.81 |
| Estrogen receptor | |||||||
| Positive/Negative | 16/5 | 6.01 | 0.77–46.9 | 0.087 | 9.52 | 0.56–159.81 | 0.11 |
| Ki‐67 LI | |||||||
| < Median 70%/≥ 70% | 10/11 | 0.71 | 0.24–2.08 | 0.53 | 1.36 | 0.28–6.48 | 0.69 |
| Ki‐67 LI change between PT and ALNM | |||||||
| ALNM< PT/ALNM > PT | 5/16 | 0.21 | 0.057–0.81 | 0.023 | 0.28 | 0.059–1.38 | 0.12 |
| Adjuvant chemotherapy | |||||||
| Yes/No | 15/6 | 0.69 | 0.22–2.09 | 0.51 | 0.52 | 0.061–4.55 | 0.56 |
| Adjuvant radiation therapy | |||||||
| Yes/No | 10/11 | 1.87 | 0.63–5.47 | 0.25 | 2.34 | 0.41–13.30 | 0.33 |
Bold values indicate statistical significance. ALNM, axillary lymph node metastasis; ALNR, axillary lymph node recurrence; CI, confidence interval; LI, labeling index; PT, primary tumor.
The median follow‐up from the date of surgery for PT was 2564 days. The five and 10‐year DSS rates for all 21 patients were 95.2% and 63.1%, respectively. The DSS rates according to patient and tumor characteristics and treatment strategies are presented in Table 4. Chemotherapy and radiation therapy after ALNR were not significantly associated with DSS. DSS was significantly better in patients with no other metastatic lesions than in patients with remaining lesions (P = 0.001) (Table 4). No difference in DSS was identified in the PT group when patients were stratified by the median Ki‐67 LI (25.2%, higher vs. lower; P = 0.324) (Fig 2a). However, DSS was significantly better in patients with a metachronous ALNM Ki‐67 LI lower than the median of 70% (P = 0.019) (Fig 2b). The ROC curve showed a PT Ki‐67 LI of 62.8% and an ALNM Ki‐67 LI of 65.1% as the optimal cutoff values.
Table 4.
Univariate Kaplan–Meier and multivariate Cox proportional hazards model analysis of five‐year disease‐specific survival rates according to the indicated factors
| Characteristic and strategy | Five‐year disease specific survival rates (%) | Univariate analysis P | Multivariate analysis P |
|---|---|---|---|
| Age at ALNR treatment (years) | |||
| < 70 | 100 | 0.821 | NS |
| ≥ 70 | 91.7 | ||
| Number of lymph nodes | |||
| Single | 100 | 0.254 | NS |
| Multiple | 93.8 | ||
| Size of the largest lymph node | |||
| < 20 mm | 88.9 | 0.517 | NS |
| ≥ 20 mm | 100 | ||
| Corresponding metastatic lesion | |||
| No | 100 | 0.001 | NS |
| Yes | 83.3 | ||
| Estrogen Receptor | |||
| Positive | 100 | 0.018 | 0.039 |
| Negative | 80 | ||
| Ki‐67 LI in the PT | |||
| < Median 25.2% | 100 | 0.324 | NS |
| ≥ 25.2% | 90.9 | ||
| Ki‐67 LI in the ALNM | |||
| < Median 70% | 100 | 0.019 | NS |
| ≥ 70% | 90.9 | ||
| Ki‐67 LI change between PT and ALNM | |||
| ALNM < PT | 100 | 0.504 | NS |
| ALNM > PT | 93.8 | ||
| Adjuvant chemotherapy for ALNM | |||
| Yes | 93.3 | 0.186 | NS |
| No | 100 | ||
| Adjuvant radiation therapy for ALNM | |||
| Yes | 100 | 0.864 | NS |
| No | 90 |
Bold values indicate statistical significance. ALNM, axillary lymph node metastasis; ALNR, axillary lymph node recurrence; LI, labeling index; NS, not significant; PT, primary tumor.
Figure 2.
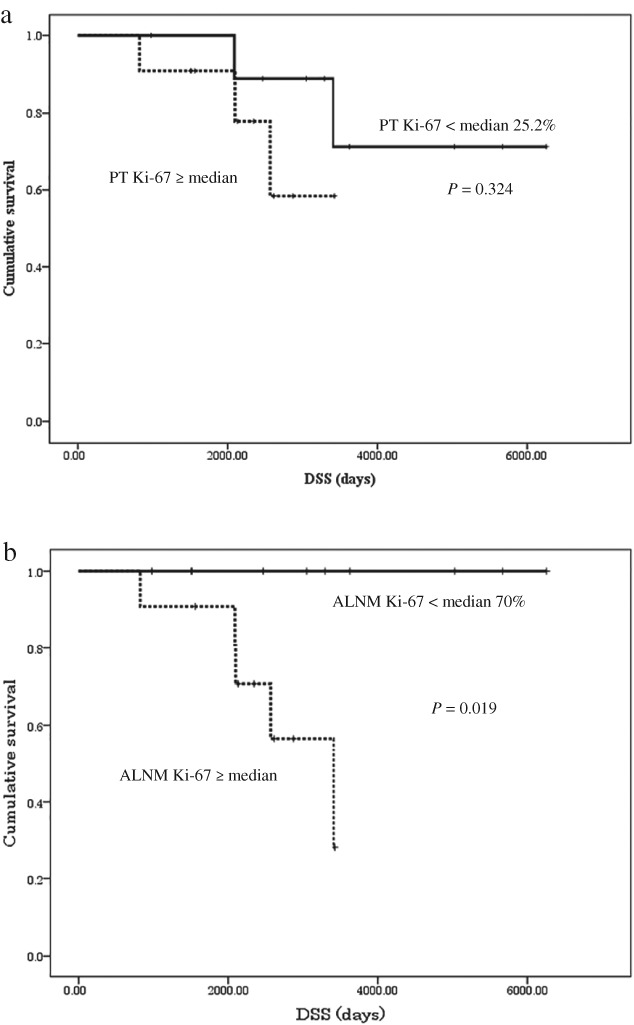
Kaplan–Meier disease‐specific survival curves. (a) Stratified by the primary tumor (PT) Ki‐67 labeling index (LI). N = 11 for ≥ and N = 10 for < median 25.2%. (b) Stratified by metachronous axillary lymph node metastasis (ALNM) Ki‐67 LI. N = 11 for ≥ and N = 10 <median 70%.
We divided the patients into four groups: PT Ki‐67 LI lower than 62.8%/ALNM Ki‐67 LI lower than 65.1% (9 patients, 42.9%), PT Ki‐67 LI lower/ALNM Ki‐67 LI higher (7 patients, 33.3%), PT Ki‐67 LI higher/ALNM Ki‐67 LI higher (4 patients, 19.0%), and PT Ki‐67 LI higher/ALNM Ki‐67 LI lower (1 patient, 4.8%). DSS was significantly better in patients with Ki‐67 LI lower/ALNM Ki‐67 LI lower than in the other groups (P < 0.05) (Fi3) and patients with PT Ki‐67 LI higher/ALNM Ki‐67 LI higher showed the worst DSS rate. However, only five patients had a PT Ki‐67 LI ≥ 62.8% and this cutoff value appeared to be imbalanced compared to the median value.
Figure 3.
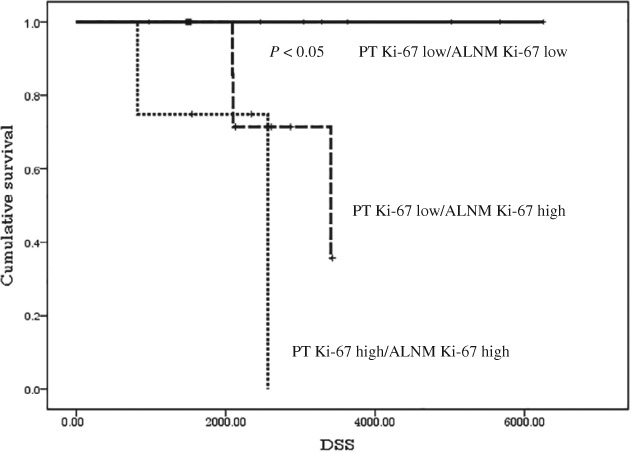
Kaplan–Meier disease‐specific survival curves stratified subjects into four groups by the primary tumor (PT) and metachronous axillary lymph node metastasis (ALNM) Ki‐67 labeling indices, according to the optimal cutoff values. ( ) PT Ki‐67 high/ALNM Ki‐67 low.
) PT Ki‐67 high/ALNM Ki‐67 low.
Discussion
In this study, we found that the mean Ki‐67 LI of metachronous ALNM was higher than that of PT; that a greater proportion of patients (76.2%) had a higher Ki‐67 LI in ALNM than in PT compared to those with lower Ki‐67 LI in ALNM than in PT; and that a higher Ki‐67 LI in metachronous ALNM was significantly correlated with poorer outcomes. To our knowledge, no previous studies have addressed the clinical significance of Ki‐67 LI for metachronous ALNM; we therefore discuss the present results in relation to previous reports on synchronous ALNM.
The few studies that have compared the Ki‐67 LI in PTs and synchronous metastatic lesions have reported inconsistent results. Some studies have reported a significantly higher Ki‐67 LI in synchronous ALNMs than in the corresponding PTs, whereas others found no difference.7, 10
Buxant et al. hypothesized that cancer cells in PTs are more stable than those in metastases and that only tumors with high Ki‐67 LI and aggressive potential can escape from the PT to form ALNM.15 Our results support the contention that the proliferative potential of PT and metachronous ALNM cancer cells may differ.
The threshold set for Ki‐67 LI may influence the concordance between PTs and ALNMs. When a Ki‐67 LI of ≥ 10% is defined as positive, the proportions of PTs and synchronous ALNMs above and below this threshold are the same, whereas the rate of concordance between PTs and synchronous ALNMs decreases to 77.5% when the threshold for Ki‐67 LI is set at ≥ 20%.8, 9 We also found that the Ki‐67 LIs varied widely among each lymph node in multiple lymph node metastases. As for metachronous pulmonary metastases, the rate of concordance between PTs and such metastases is reportedly only 57%, even when the threshold for Ki‐67 LI is set at ≥ 15%.16 The concordance rates between PTs and metachronous ALNMs in the present study were 66.7% and 71.4% when the thresholds were set at ≥ 15% and ≥ 20%, respectively. The Ki‐67 LI is a continuous variable, thus setting a threshold between PTs and ALNMs is difficult. This may reflect the fact that tumors in PTs and ALNMs have multiple genomically distinct stem cells that have various concordant and discordant responses to treatments.
As for the prognostic value of the Ki‐67 LI for synchronous ALNM, patient outcomes have been found to be poor when the Ki‐67 LI values are high in both PT and ALNM or when the PT LI is low and the ALNM LI is high. Furthermore, outcomes correlate with the ALNM Ki‐67 LI, with high LIs indicating poorer outcomes.7, 8 Patients with a lower Ki‐67 LI in synchronous ALNM than in the corresponding PT or a lower Ki‐67 LI in residual PT following adjuvant chemotherapy also have better survival rates than those who do not.17, 18 Our findings show that DSS is better in patients with a lower rather than a higher Ki‐67 LI in ALNM, consistent with the aforementioned studies on synchronous ALNM or residual PT.
Whether radiotherapy (RT) should be added to surgery when treating ALNR is controversial because there is contradictory evidence indicating that RT both improves and worsens outcomes.11, 19 We found no association between DSS and the administration of RT. Previous studies reported five‐year DSS and five and 10‐year overall survival rates of 57.9%, 49.3%, and 56%, respectively, for patients with ALNR overall.11, 19, 20 In our cohort of 21 patients, the five and 10‐year DSS rates were 95.2% and 63.1%, respectively, which are better than those reported elsewhere. One possible reason for this discrepancy is that we measured survival from the date of surgery for the PT.
A strict Ki‐67 LI threshold of 14% was set at the 2011 St. Gallen Consensus Meeting to assist physicians to decide whether chemotherapy should be administered to patients with ER‐positive/HER2‐negative disease6 however, the methods for calculating Ki‐67 LI vary considerably.21 An international breast cancer working group has made recommendations to overcome the considerable interobserver variation between pathologists.22 In the present study, we used cell counting software, which analyzes immunostaining based on pixel intensity in nuclei images, in an attempt to reduce operator bias in determining the Ki‐67 LI. We previously reported a correlation between Ki‐67 LI determined using this method and responses to RT in small‐cell lung cancer.23
This study has the following limitations: the number of cases was small, and the status of the biomarkers ER and HER2 varied among the patients. We intend to investigate the significance of Ki‐67 LI in ALNR further by accumulating more cases with sufficient data on ER and HER2 subtypes.
In conclusion, despite the limitations of this small cohort study, our results suggest that Ki‐67 LI of metachronous ALNM has prognostic potential, which is consistent with the fact that cancer cells in metachronous ALNM have already demonstrated their ability to survive and reflect the most recent characteristics of an individual patient's cancer cells.
Disclosure
No authors report any conflict of interest.
Acknowledgment
We thank Yoshinori Takekawa, Yokosuka City Hospital, for performing the pathological assessments.
References
- 1. Synnestvedt M, Borgen E, Russnes HG et al Combined analysis of vascular invasion, grade, HER2 and Ki67 expression identifies early breast cancer patients with questionable benefit of systemic adjuvant therapy. Acta Oncol 2013; 52: 91–101. [DOI] [PubMed] [Google Scholar]
- 2. Bjerre C, Knoop A, Bjerre K et al Association of tissue inhibitor of metalloproteinases‐1 and Ki67 in estrogen receptor positive breast cancer. Acta Oncol 2013; 52: 82–90. [DOI] [PubMed] [Google Scholar]
- 3. Stuart‐Harris R, Caldas C, Pinder SE, Pharoah P. Proliferation markers and survival in early breast cancer: A systematic review and meta‐analysis of 85 studies in 32,825 patients. Breast 2008; 17: 323–34. [DOI] [PubMed] [Google Scholar]
- 4. Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 1983; 15: 13–20. [DOI] [PubMed] [Google Scholar]
- 5. Niikura N, Masuda S, Kumaki N et al Prognostic significance of the Ki67 scoring categories in breast cancer subgroups. Clin Breast Cancer 2014; 14: 323–9. [DOI] [PubMed] [Google Scholar]
- 6. Goldhirsch A, Wood WC, Coates AS et al Strategies for subtypes‐‐dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011; 22: 1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Park D, Kåresen R, Noren T, Sauer T. Ki‐67 expression in primary breast carcinomas and their axillary lymph node metastases: Clinical implications. Virchows Arch 2007; 451: 11–8. [DOI] [PubMed] [Google Scholar]
- 8. Tawfik K, Kimler BF, Davis MK, Fan F, Tawfik O. Ki‐67 expression in axillary lymph node metastases in breast cancer is prognostically significant. Hum Pathol 2013; 44: 39–46. [DOI] [PubMed] [Google Scholar]
- 9. Nogami T, Shien T, Tanaka T et al Expression of ALDH1 in axillary lymph node metastases is a prognostic factor of poor clinical outcome in breast cancer patients with 1‐3 lymph node metastases. Breast Cancer 2014; 21: 58–65. [DOI] [PubMed] [Google Scholar]
- 10. Zhao S, Xu L, Liu W et al Comparison of the expression of prognostic biomarkers between primary tumor and axillary lymph node metastases in breast cancer. Int J Clin Exp Pathol 2015; 8: 5744–8. [PMC free article] [PubMed] [Google Scholar]
- 11. Shen SC, Liao CH, Lo YF et al Favorable outcome of secondary axillary dissection in breast cancer patients with axillary nodal relapse. Ann Surg Oncol 2012; 19: 1122–8. [DOI] [PubMed] [Google Scholar]
- 12. McKinna F, Gothard L, Ashley S, Ebbs SR, Yarnold JR. Lymphatic relapse in women with early breast cancer: A difficult management problem. Eur J Cancer 1999; 35: 1065–9. [DOI] [PubMed] [Google Scholar]
- 13. Kuukasjärvi T, Kononen J, Helin H, Holli K, Isola J. Loss of estrogen receptor in recurrent breast cancer is associated with poor response to endocrine therapy. J Clin Oncol 1996; 14: 2584–9. [DOI] [PubMed] [Google Scholar]
- 14. Nakamura R, Yamamoto N, Onai Y, Watanabe Y, Kawana H, Miyazaki M. Importance of confirming HER2 overexpression of recurrence lesion in breast cancer patients. Breast Cancer 2013; 20: 336–41. [DOI] [PubMed] [Google Scholar]
- 15. Buxant F, Anaf V, Simon P, Fayt I, Noël JC. Ki‐67 immunostaining activity is higher in positive axillary lymph nodes than in the primary breast tumor. Breast Cancer Res Treat 2002; 75: 1–3. [DOI] [PubMed] [Google Scholar]
- 16. Nogami T, Shien T, Tanaka T et al The discordance between primary breast cancer lesions and pulmonary metastatic lesions in expression of aldehyde dehydrogenase 1‐positive cancer cells. Breast Cancer 2014; 21: 698–702. [DOI] [PubMed] [Google Scholar]
- 17. Diaz‐Botero S, Espinosa‐Bravo M, Gonçalves VR et al Different prognostic implications of residual disease after neoadjuvant treatment: Impact of Ki 67 and site of response. Ann Surg Oncol 2016; 23: 3831–7. [DOI] [PubMed] [Google Scholar]
- 18. Montagna E, Bagnardi V, Viale G et al Changes in PgR and Ki‐67 in residual tumour and outcome of breast cancer patients treated with neoadjuvant chemotherapy. Ann Oncol 2015; 26: 307–13. [DOI] [PubMed] [Google Scholar]
- 19. Konkin DE, Tyldesley S, Kennecke H, Speers CH, Olivotto IA, Davis N. Management and outcomes of isolated axillary node recurrence in breast cancer. Arch Surg 2006; 141: 867–74. [DOI] [PubMed] [Google Scholar]
- 20. Wright FC, Walker J, Law CH, McCready DR. Outcomes after localized axillary node recurrence in breast cancer. Ann Surg Oncol 2003; 10: 1054–8. [DOI] [PubMed] [Google Scholar]
- 21. Niikura N, Sakatani T, Arima N et al Assessment of the Ki67 labeling index: A Japanese validation ring study. Breast Cancer 2016; 23: 92–100. [DOI] [PubMed] [Google Scholar]
- 22. Dowsett M, Nielsen TO, A'Hern R et al Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst 2011; 103: 1656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ishibashi N, Maebayashi T, Aizawa T, Sakaguchi M, Nishimaki H, Masuda S. Correlation between the Ki‐67 proliferation index and response to radiation therapy in small cell lung cancer. Radiat Oncol 2017; 12: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]


