SUMMARY
Background
Acknowledging the urban-rural disparities in healthcare resources, China launched a new healthcare reform with a particular focus on improving rural care over the past decade. However, nationally representative studies comparing medical care and patient outcomes between urban and rural areas to inform healthcare policy are not available. Acute myocardial infarction, as a leading cause of mortality, can provide an ideal test condition for such an assessment.
Methods
We created a nationally representative sample of patients in China admitted for ST-segment elevation myocardial infarction (STEMI) in 2001, 2006, and 2011, using a two-stage random sampling design in 2 urban and 3 rural strata. We performed a retrospective analysis of hospital records to compare the care for patients admitted in rural and urban hospitals, and to assess the changes from 2001 to 2011.
Findings
In China, 38.7% of inpatient care for STEMI in 2001, and 35.8% in 2011, was provided by rural hospitals with lower intensity smaller volume, and poorer availability of advanced cardiac care. For instance, in 2001 most evidence-based treatments were provided more often in urban hospitals than in rural hospitals. However, these differences diminished by 2011 for reperfusion therapy (54% vs. 57%, p=000B71), and reversed for angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (66% vs. 68%, p=0·04) and early beta-blockers (56% vs. 60%, p=0·01). Despite early differences in treatment rates in 2001, the risk-adjusted rate of in-hospital death or withdrawal from treatment was not statistically different between urban and rural hospitals in any of the sampled years, with an adjusted odds ratio of 1·13 [95% confidence interval (CI) (0·77, 1·65), p=0·5] in 2001, 0·99 [95% CI (0·77, 1·27), p=0·9] in 2006, and 0·94 [95% CI (0·74, 1·19), p=0·6] in 2011.
Interpretation
While urban-rural disparities in evidence-based treatment for myocardial infarction in China have largely been eliminated, substantial gaps in care persist in both settings. Despite treatment differences, case-fatality rates for AMI were similar between urban and rural hospitals in China.
Keywords: urban-rural, regional disparity, quality of care, acute myocardial infarction
INTRODUCTION
Health care disparities between urban and rural areas have the potential to affect billions of people worldwide.1 People living in rural areas have limited access to medical services,1 may be less likely to receive evidence-based therapies,2–5 and may experience worse outcomes6–8 for high-impact conditions, such as acute myocardial infarction (AMI).
Market-based reforms in the Chinese healthcare sector, initiated in 1985,9 were deemed “generally unsuccessful” due to the inefficiencies and inequities that subsequently developed.10 In 2003, only 36% of government healthcare expenditures were allotted to hospitals in rural areas, despite the fact that they directly served 70% of the Chinese population.11 Moreover, fragmentation in social health insurance schemes has put rural residents in China at a disadvantage compared with their urban counterparts: they have lower insurance coverage (21.0% vs. 55.2%), and healthcare providers receive lower inpatient reimbursement rates (5.8% vs. 34.5%).12 Acknowledging this disparity, the Chinese government launched a new healthcare reform in 2009 that included widespread health insurance coverage, a National Essential Drug System, and improved medical care at the grassroots level.13 As a result, access to care and financial protection, particularly for the rural population, has improved substantially over the past decade.11,12,14 However, there is limited information regarding urban and rural differences in quality of care in China – including treatment patterns and patient outcomes – and how these differences have changed during this period. Understanding these differences can greatly inform healthcare policies by identifying quality gaps and laying a foundation for future quality improvement initiatives across the country.
ST-segment elevation myocardial infarction (STEMI) is an ideal condition to assess quality of care, as not only are the evidence-based treatments and their patient selection criteria codified by level A recommendations in guidelines,15,16 but timely treatments and coordinated systems-of-care are fundamental to achieving optimal outcomes.17,18 It has been the focus of multiple national quality initiatives including Hospital Compare in the US and the National Clinical Audit Programme in the UK. Accordingly, we used a nationally representative sample of hospitals in urban and rural China to compare use of evidence-based treatments, and outcomes for STEMI during this dynamic period, to evaluate government efforts to improve healthcare and reduce disparities.
METHODS
Design overview of China PEACE-Retrospective AMI study
The design of the China PEACE (Patient-centered Evaluative Assessment of Cardiac Events)-Retrospective AMI study has been published previously.19 In brief, we created a nationally representative sample of hospitalizations for AMI during 2001, 2006, and 2011 using stratified two-stage random sampling. We divided Mainland China into 5 study strata based on differences in per capita income and health services capacity across urban and rural areas, as well as 3 official economic-geographic regions: Eastern-rural, Central-rural, Western-rural, Eastern-urban, and Central/Western-urban.20 In accordance with the official statistical yearbook,14 we designated an area as urban if it was part of a downtown or suburban district within a direct-controlled municipality (Beijing, Tianjin, Shanghai, and Chongqing) or 1 of the 283 prefectural-level cities, with a median population of 0·91 million (interquartile range [IQR]: 0·61–1·49 million). Surrounding county-level regions, including counties and county-level cities, were then designated as rural, with a median population of 0·35 million (IQR: 0·20–0·58 million).20 Within this framework, Mainland China is composed of 287 urban regions and 2010 rural regions. In the first stage, we sampled representative hospitals within each stratum from 2011 so as to reflect current practices, and traced this cohort of hospitals backwards to 2006 and 2001 to characterize temporal trends. In the second stage, we sampled AMI cases based on hospitalization databases in each year using a systematic random sampling procedure.
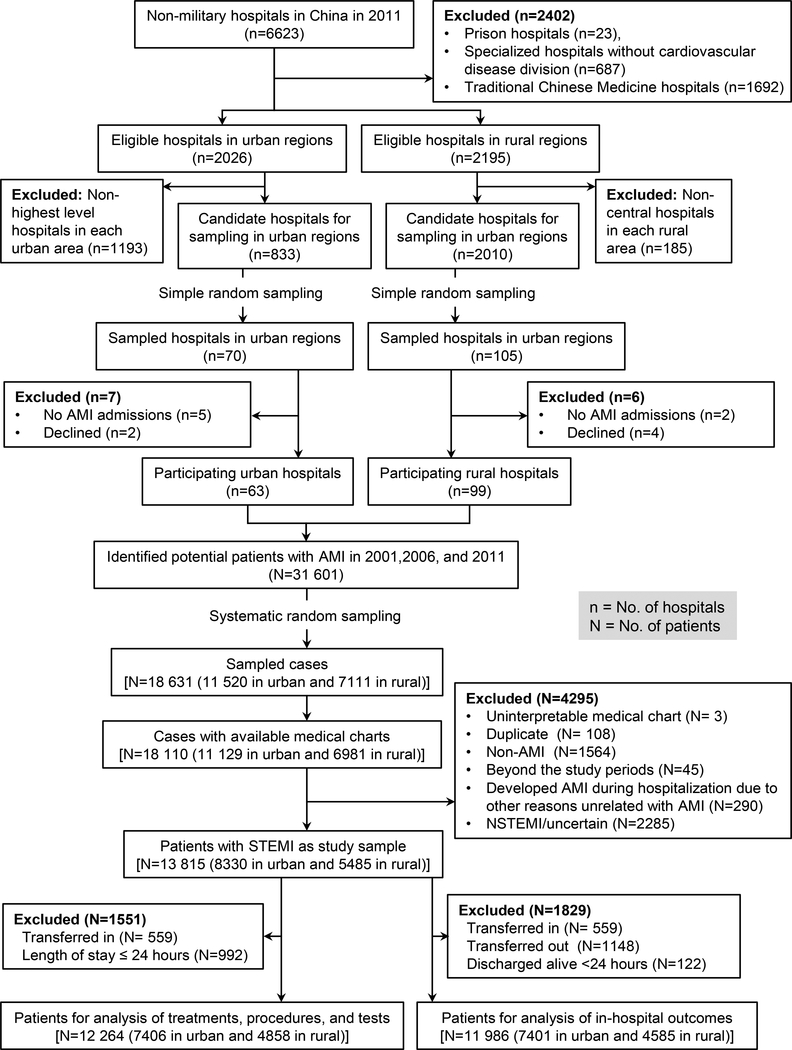
Patients with AMI were identified using International Classification of Diseases (ICD) - Clinical Modification codes when available, including versions 9 (410·xx) and 10 (I21·xx), or through principal discharge diagnosis terms. The ICD coding is done by trained medical record staff in local hospitals based on the nationwide standardized Chinese edition of ICD code dictionary. Examination of patient databases from 162 participating hospitals yielded 31 601 hospitalizations for AMI (3859 in 2001, 8863 in 2006, and 18 879 in 2011), on which basis we randomly sampled 18 631 cases and acquired medical records for 18 110 (97·2%) (Figure 1).19 Clinical information was collected through centralized medical chart abstraction using standardized data definitions. Rigorous monitoring was conducted at each stage to ensure data quality. Data abstraction quality was monitored by randomly auditing 5% of records. The medical record abstraction and data monitoring procedures have been previously described in detail.19 We also obtained information on the structural characteristics of the hospital and the department via a questionnaire from each participating hospital’s principal investigator, typically the director of the Cardiology or Internal Medicine Department (Appendix C).
Figure 1. The two-stage random sampling process in China-PEACE and the selection of the cohort for the present study.
AMI indicates acute myocardial infarction; STEMI, ST-segment elevation myocardial infarction.
The central ethics committee at the China National Center for Cardiovascular Diseases approved the China PEACE-Retrospective AMI study, with a waiver of patients’ written consent, since it was not feasible to approach those patients hospitalized several years ago in a retrospective study. All collaborating hospitals accepted the central ethics approval except for five hospitals, which obtained local approval from internal ethics committees. The study is registered at www.clinicaltrials.gov (NCT01624883).
Study sample
For this study, we included patients with a definite discharge diagnosis of STEMI, as determined by the combination of clinical discharge diagnosis and electrocardiogram (ECG) results. A total of 300 medical records were randomly selected and examined by a cardiologist, showing a 94·7% concordance between the abstractors’ designation of AMI subtype and the reviewer (Appendix D). For analyses of treatments, tests, and procedures, we excluded patients who were discharged or died within 24 hours after admission, as they might not have had the opportunity to receive therapy. We also excluded patients transferred in from other facilities because it was difficult to ascertain detailed clinical status and treatments for their initial presentation. For the analysis of in-hospital outcomes, we excluded patients who were transferred in because we sought to characterize the patients directly admitted to the hospital, as well as those transferred out, because the records of their hospitalizations were truncated. Patients discharged alive within 24 hours for reasons other than withdrawal from treatment due to terminal status were excluded under the assumption that they left against medical advice.
Variables
Hospital characteristics
Using a questionnaire completed by each hospital, we assessed the hospital’s infrastructure, including an assessment of hospital level (primary, secondary, and tertiary), teaching status (university-affiliated teaching hospital, non-university affiliated teaching hospital, and non-teaching hospital), infrastructure for advanced cardiac care (Coronary Care Unit (CCU), and catheterization laboratory), and AMI and coronary revascularization statistics (number of qualified interventionists as well as coronary artery bypass graft surgery volume performed in 2011). The Chinese government defines hospital level based on the capacity and clinical resources available within the hospital. The annual AMI inpatient volume was ascertained based on hospital databases obtained for the case sampling in each year.
Patient characteristics
Collected patient characteristics included age, sex, comorbidities, and clinical profile at presentation. Clinical profile at presentation included systolic blood pressure, heart rate, estimated glomerular filtration rate (eGFR), and cardiac arrest or cardiogenic shock at presentation. In addition, we computed a mini-GRACE score to summarize patients’ severity of disease, based on age, systolic blood pressure on admission, heart rate, ST-segment deviation, cardiac arrest and elevated cardiac biomarkers.21
Treatments, procedures and diagnostic testing
We evaluated the use of treatments recommended by the 2010 China guideline for STEMI,15 which are consistent with the 2007 United States guidelines.22 Treatments included: (1) reperfusion therapy; (2) aspirin within 24 hours of admission; (3) clopidogrel within 24 hours of admission; (4) beta-blockers within 24 hours of admission; (5) angiotensin converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB) during hospitalization; and (6) statins during hospitalization. Rates of utilization were assessed only for patients considered ideal for the treatment (i.e., those who were clinically eligible and without contraindications, as determined by detailed medical record abstraction (Appendix E)). We also evaluated use of laboratory tests (creatinine and troponin), echocardiogram, cardiovascular procedures, therapies that have been proven ineffective (e.g., magnesium sulphate), and the use of traditional Chinese medicines.
In-hospital outcomes
We measured two outcomes that are pre-specified: 1) in-hospital mortality or withdrawal from treatment due to a terminal status at discharge; and 2) in-hospital composite complications (including death, withdrawal from treatment, re-infarction, shock, ischemic stroke, or congestive heart failure [definition in Appendix F]). As withdrawal from treatment is common in China, due to reluctance to die in the hospital among terminally ill patients, we used a composite measure of in-hospital death or withdrawal from treatment, which is also used as a hospital quality measure by the Chinese government.23 Cardiologists in the coordinating centre adjudicated the clinical status of patients who withdrew from treatment, to exclude withdrawal of care for other reasons, such as financial issues. Furthermore, given the potential bias introduced by difference in lengths of stay between patients at urban and rural hospitals, as a sensitivity analysis, we assessed 7-day rates of mortality or withdrawal from treatment, to ensure the validity of comparisons.
Statistical analysis
Based on the sampling approach, which involved random identification of hospitals throughout China, we were able to calculate estimates reflecting national care patterns and outcomes. These estimates were calculated in each study year with the application of weights proportional to the inverse sampling fraction of hospitals within each stratum and the sampling fraction of patients within each hospital, to account for differences in the sampling fraction for each time period in all analyses.
We used weighted percentages to describe categorical variables, medians and IQR to describe continuous variables. To examine urban-rural differences in patient characteristics, treatments, testing, and outcomes, and to obtain crude odds ratios (OR) and 95% confidence interval (CI), we performed logistic regressions with the “urban/rural” variable as the only predictor. We performed Mann-Whitney Tests for urban-rural comparisons of continuous variables.
For temporal changes of patient characteristics and treatments in urban-rural differences across three study years (2001, 2006, and 2011), we constructed multivariable logistic regression models incorporating interaction terms for urban/rural and study periods. To compare the outcomes, we used multi-level logistic regression to account for clustering of patients within sites and adjusted for patients’ demographics (age and gender), risk factors or medical history (current smoker, hypertension, diabetes, dyslipidaemia, and history of coronary heart disease or stroke), and clinical profile at presentation (chest discomfort lasting for over 10 minutes, duration from symptom onset to admission, as well as systolic blood pressure, heart rate, cardiac arrest, and acute stroke at admission). We categorized continuous variables such as heart rate into categorical variables according to clinically meaningful cut-off values. From the multivariable model, we then computed risk-adjusted rates for each outcome of urban and rural hospitals separately. The risk-adjusted rate was calculated as the ratio of observed to predicted outcomes, multiplied by the overall unadjusted rate, a form of indirect standardization. We also compared the risk of death or withdrawal from treatment between urban and rural hospitals using time to event analyses using Cox regression models, with length of stay as time, censored for the patients without the outcome at the discharge, and a random effect of hospitals to account for patient clustering.
In cases of missing variables, we imputed sample medians. All comparisons were 2-sided, with a p value less than 0·05 considered statistically significant. Statistical analysis was performed using SPSS version 13·0 (SPSS Inc., Chicago, USA), and SAS version 9·2 (SAS Institute, Cary, NC).
Role of the funding source
The Chinese government provided financial support for the study, but had no role in the design or conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation or approval of the manuscript.
RESULTS
Hospital and Patient Characteristics
Of the 162 participating hospitals, 63 were urban and 99 were rural. Rural hospitals differed in several important ways from urban ones, with lower levels of care intensity, smaller volumes of patients, and poorer availability of advanced cardiac care (Table 1).
Table 1:
Characteristics of urban and rural hospitals
| Hospital characteristics | Urban(n=63) | Rural(n=99) | p value |
|---|---|---|---|
| Level of hospital | < 0·001 | ||
| Tertiary | 58 (92%) | 7 (7%) | |
| Secondary or lower | 5 (8%) | 92 (93%) | |
| Type of hospital | < 0·001 | ||
| Medical college affiliated | 31 (49%) | 9 (9%) | |
| Teaching, but not medical college affiliated | 23 (37%) | 30 (30%) | |
| Non-teaching | 9 (14%) | 60 (61%) | |
| Annual AMI inpatient volume * | |||
| 2001 | 27 (8, 68) | 5 (2, 13) | < 0·001 |
| 2006 | 70 (30, 152) | 15 (6, 30) | < 0·001 |
| 2011 | 148 (58, 334) | 34 (16, 63) | < 0·001 |
| CCU in hospital in 2011 | 56 (89%) | 38 (38%) | < 0·001 |
| Catheterization laboratory in hospital in 2011 | 55 (87%) | 24 (24%) | < 0·001 |
| Number of qualified interventionists * | 4 (3,6) | 1 (0,3) | < 0·001 |
| Independent emergency department in 2011 | 60 (95%) | 91(92%) | 0·413 |
| CABG capability in 2011 | 32 (51%) | 1 (1%) | < 0·001 |
Median and interquartile range.
CCU indicates Coronary Care Unit; CABG, coronary artery bypass graft.
Within the 162 hospitals, there were a total 13 815 hospitalizations for STEMI sampled in the 3 study years (Figure 1). There were 1341, 2388, and 4601 patients sampled in urban areas in 2001, 2006, and 2011 (representing nationwide 27 746, 63 922, and 133 433 patients respectively); and 786, 1604, and 3095 in rural areas (representing nationwide 17 535, 39 825, and 74 501 patients respectively). Thus, the fraction of STEMI admissions in rural hospitals decreased from 38.7% in 2001 to 35.8% in 2011 (p=0.02).
During the medical records abstraction, the overall data accuracy exceeded 98%, and missing values were rare (0·1% for all variables). The age of patients was similar between urban and rural hospitals in 2001 [65 (56–72) vs. 65 (55–72), p=0·9] and 2006 [66 (55–73) vs. 67 (56–74), p=0·06] but, in 2011, patients treated in urban hospitals were significantly younger [63 (53–73) vs. 67 (58–76), p<0·001]. The proportion of women increased over time in rural hospitals (29%, 29%, and 33% respectively, p for trend=0·01), but remained unchanged in urban hospitals (29%, 28%, and 28% respectively, p for trend=0·3). The prevalence of cardiovascular risk factors among patients increased in both rural and urban hospitals (Table 2). After excluding patients transferred in, the pre-admission delay (time from symptom onset to hospital admission) was similar between rural and urban hospitals [14 (3–72) hours vs. 13 (3–72) hours, p=0·99] in 2001; in urban hospitals 37% of patients were admitted within 6 hours of symptom onset, compared with 35% in rural hospitals (p=0.25). However, by 2011, the pre-admission delay was significantly shorter in rural hospitals compared with urban hospitals, [8 (3–48) hours vs. 17 (4–96) hours, p<0·001], and correspondingly a larger proportion of patients were admitted within 6 hours (42% in rural vs. 32% in urban, p<0·001). The mini-GRACE score was similar between rural and urban hospitals in 2001 (139 (121, 158) vs. 139 (120, 158), p=0·8) and 2006 (142 (124, 160) vs. 141 (122, 160), p=0·2), but was significantly higher in rural hospitals in 2011 (143 (125, 161) vs. 137 (119, 158), p<0·001).
Table 2:
Characteristics of patient with ST segment elevation myocardial infarction.
| 2001 | 2006 | 2011 | p for interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Urban * | Rural * | OR (95% CI)# | Urban * | Rural * | OR (95% CI)# | Urban * | Rural * | OR (95% CI) # | ||
| n=1341 | n=786 | n=2388 | n=1604 | n=4601 | n=3095 | |||||
| Demographics | ||||||||||
| Age, yr | ||||||||||
| <60 | 428 (32%) | 267 (34%) | 0·89 (0·73, 1·10) | 810 (34%) | 514 (32%) | 1·10 (0·96, 1·25) | 1769 (39%) | 927 (29%) | 1·58 (1·43, 1·74) | <0·001 |
| 60–69 | 465 (35%) | 246 (31%) | 1·19 (0·97, 1·46) | 622 (26%) | 416 (26%) | 0·95 (0·83, 1·10) | 1124 (25%) | 811 (27%) | 0·93 (0·83, 1·03) | 0·05 |
| 70–79 | 354 (26%) | 216 (28%) | 0·94 (0·76, 1·17) | 752 (32%) | 522 (32%) | 0·98 (0·85, 1·12) | 1238 (26%) | 915 (30%) | 0·82 (0·74, 0·90) | 0·07 |
| ≥80 | 94 (7%) | 57 (7%) | 0·99 (0·68, 1·44) | 204 (9%) | 152 (9%) | 0·92 (0·74, 1·15) | 470 (10%) | 442 (14%) | 0·64 (0·56, 0·73) | 0·003 |
| Female sex | 392 (29%) | 222 (29%) | 1·02 (0·82, 1·26) | 679 (28%) | 458 (29%) | 0·96 (0·84, 1·11) | 1263 (28%) | 984 (33%) | 0·80 (0·72, 0·88) | 0·01 |
| Cardiovascular risk factors | ||||||||||
| Hypertension | 590 (45%) | 276 (36%) | 1·45 (1·19, 1·77) | 1232 (54%) | 662 (43%) | 1·56 (1·37, 1·77) | 2451 (54%) | 1439 (48%) | 1·25 (1·14, 1·37) | 0·3 |
| Diabetes | 230 (17%) | 66 (8%) | 2·27 (1·66, 3·10) | 555 (25%) | 192 (12%) | 2·50 (2·09, 2·99) | 1078 (24%) | 480 (16%) | 1·60 (1·42, 1·80) | <0·001 |
| Dyslipidaemia | 664 (52%) | 244 (31%) | 2·32 (1·89, 2·84) | 1329 (57%) | 772 (49%) | 1·35 (1·19, 1·54) | 3004 (68%) | 1839 (59%) | 1·43 (1·30, 1·58) | 0·004 |
| Current smoker | 408 (32%) | 221 (28%) | 1·16 (0·94, 1·44) | 807 (36%) | 499 (34%) | 1·08 (0·94, 1·23) | 1889 (43%) | 965 (33%) | 1·54 (1·40, 1·70) | <0·001 |
| Number of risk factors | ||||||||||
| ≥3 risk factors | 169 (13%) | 45 (6%) | 2·46 (1·71, 3·55) | 468 (22%) | 190 (13%) | 1·81 (1·52, 2·16) | 1126 (26%) | 486 (17%) | 1·76 (1·57, 1·97) | 0·2 |
| 2 risk factors | 438 (34%) | 171 (22%) | 1·83 (1·47, 2·29) | 849 (36%) | 459 (29%) | 1·38 (1·20, 1·58) | 1807 (40%) | 1071 (35%) | 1·27 (1·15, 1·39) | 0·006 |
| 1 risk factors | 496 (36%) | 326 (42%) | 0·79 (0·65, 0·96) | 767 (30%) | 623 (38%) | 0·72 (0·63, 0·82) | 1289 (26%) | 1068 (35%) | 0·66 (0·60, 0·73) | 0·09 |
| None | 238 (17%) | 244 (31%) | 0·46 (0·36, 0·58) | 304 (11%) | 332 (19%) | 0·53 (0·44, 0·63) | 379 (7%) | 470 (14%) | 0·50 (0·43, 0·59) | 0·7 |
| Medical history | ||||||||||
| Myocardial infarction | 154 (11%) | 64 (9%) | 1·37 (0·99, 1·90) | 226 (10%) | 148 (10%) | 1·06 (0·86, 1·31) | 539 (12%) | 275 (9%) | 1·37 (1·18, 1·59) | 0·4 |
| Coronary heart disease | 357 (27%) | 146 (19%) | 1·52 (1·21, 1·93) | 486 (21%) | 304 (19%) | 1·11 (0·95, 1·30) | 977 (22%) | 591 (19%) | 1·17 (1·04, 1·31) | 0·1 |
| PCI | 8 (1%) | 6 (1%) | 0·77 (0·27, 2·24) | 28 (1%) | 12 (1%) | 1·25 (0·68, 2·31) | 134 (3%) | 46 (2%) | 1·91 (1·39, 2·63) | 0·06 |
| CABG | 3 (0%) | 7 (1%) | 0·29 (0·08, 1·05) | 6 (0%) | 3 (0%) | 1·23 (0·40, 3·74) | 14 (0%) | 7 (0%) | 0·93 (0·37, 2·34) | 0·2 |
| Stroke | 126 (9%) | 72 (10%) | 0·97 (0·70, 1·34) | 276 (12%) | 145 (9%) | 1·35 (1·10, 1·67) | 562 (12%) | 335 (12%) | 1·06 (0·92, 1·22) | 0·6 |
| Clinical characteristics | ||||||||||
| Symptom onset to admission, hr ‡ | ||||||||||
| <3 | 253 (19%) | 152 (20%) | 0·94 (0·75, 1·17) | 436 (17%) | 348 (23%) | 0·70 (0·60, 0·83) | 767 (16%) | 712 (23%) | 0·63 (0·56, 0·71) | 0·003 |
| 3 to <6 | 232 (18%) | 115 (14%) | 1·29 (1·01, 1·65) | 342 (15%) | 276 (17%) | 0·83 (0·69, 0·99) | 691 (16%) | 560 (19%) | 0·82 (0·72, 0·93) | 0·006 |
| 6 to <12 | 130 (10%) | 88 (11%) | 0·89 (0·66, 1·19) | 281 (13%) | 185 (12%) | 1·03 (0·84, 1·26) | 517 (12%) | 377 (12%) | 1·00 (0·86, 1·16) | 0·6 |
| 12 to <24 | 100 (8%) | 73 (9%) | 0·85 (0·62, 1·17) | 145 (6%) | 131 (8%) | 0·75 (0·58, 0·97) | 341 (8%) | 220 (7%) | 1·05 (0·87, 1·27) | 0·1 |
| ≥24 | 599 (45%) | 348 (45%) | 1·00 (0·83, 1·19) | 1093 (49%) | 652 (40%) | 1·48 (1·29, 1·69) | 1932 (48%) | 1160 (38%) | 1·49 (1·35, 1·65) | 0·001 |
| Chest discomfort | 1244 (93%) | 726 (93%) | 1·09 (0·75, 1·58) | 2216 (93%) | 1464 (92%) | 1·28 (1·01, 1·63) | 4275 (93%) | 2843 (92%) | 1·23 (1·04, 1·47) | 0·7 |
| Cardiac arrest | 17 (1%) | 4 (0%) | 2·71 (0·77, 9·57) | 39 (2%) | 10 (1%) | 2·88 (1·48, 5·59) | 88 (2%) | 37 (1%) | 1·26 (0·88, 1·81) | 0·04 |
| Cardiogenic shock | 53 (4%) | 41 (5%) | 0·74 (0·47, 1·16) | 136 (5%) | 109 (7%) | 0·74 (0·57, 0·96) | 293 (6%) | 215 (7%) | 0·91 (0·75, 1·09) | 0·2 |
| Acute stroke | 13 (1%) | 5 (1%) | 1·43 (0·46, 4·43) | 39 (2%) | 30 (2%) | 0·98 (0·60, 1·59) | 49 (1%) | 34 (1%) | 0·78 (0·49, 1·24) | 0·3 |
| Heart rate, beat/min | ||||||||||
| <50 | 73 (5%) | 36 (5%) | 1·17 (0·75, 1·81) | 131 (5%) | 90 (5%) | 0·92 (0·69, 1·22) | 201 (4%) | 183 (7%) | 0·58 (0·47, 0·71) | 0·001 |
| 50–110 | 1190 (89%) | 698 (89%) | 0·97 (0·71, 1·31) | 2099 (89%) | 1395 (87%) | 1·15 (0·95, 1·40) | 4188 (92%) | 2729 (88%) | 1·57 (1·35, 1·82) | 0·001 |
| >110 | 78 (6%) | 52 (6%) | 0·93 (0·63, 1·39) | 158 (6%) | 119 (8%) | 0·85 (0·66, 1·08) | 212 (4%) | 183 (6%) | 0·74 (0·60, 0·91) | 0·2 |
| Systolic blood pressure, mm Hg | ||||||||||
| <90 | 87 (6%) | 67 (8%) | 0·73 (0·50, 1·05) | 132 (5%) | 132 (8%) | 0·58 (0·45, 0·75) | 216 (4%) | 192 (5%) | 0·81 (0·65, 1·00) | 0·3 |
| 90–139 | 836 (63%) | 445 (56%) | 1·31 (1·08, 1·59) | 1473 (63%) | 926 (57%) | 1·25 (1·10, 1·42) | 2855 (63%) | 1803 (57%) | 1·26 (1·15, 1·39) | 0·8 |
| ≥140 | 418 (31%) | 274 (36%) | 0·82 (0·67, 1·01) | 783 (33%) | 546 (35%) | 0·91 (0·80, 1·04) | 1530 (33%) | 1100 (37%) | 0·82 (0·74, 0·90) | 0·6 |
| eGFR† | ||||||||||
| Unmeasured | 401 (27) | 432 (54) | 0·32 (0·26, 0·39) | 344 (12%) | 452 (25%) | 0·41 (0·35, 0·48) | 275 (5%) | 343 (10%) | 0·49 (0·41, 0·58) | 0·01 |
| < 30 | 41 (3%) | 26 (3%) | 0·97 (0·56, 1·65) | 98 (4%) | 42 (3%) | 1·55 (1·08, 2·25) | 139 (3%) | 93 (3%) | 1·11 (0·85, 1·46) | 0·8 |
| 30–59 | 223 (17%) | 94 (12%) | 1·49 (1·13, 1·96) | 452 (18%) | 304 (18%) | 1·01 (0·86, 1·19) | 733 (15%) | 530 (16%) | 0·93 (0·82, 1·05) | 0·007 |
| ≥ 60 | 676 (52%) | 234 (30%) | 2·50 (2·04, 3·07) | 1494 (65%) | 806 (54%) | 1·62 (1·43, 1·85) | 3454 (76%) | 2129 (71%) | 1·33 (1·20, 1·48) | <0·001 |
| Ejection fraction † | ||||||||||
| Unmeasured | 1021 (74) | 680 (86) | 0·45 (0·35, 0·58) | 1085 (50) | 1212 (72) | 0·38 (0·33, 0·44) | 1562 (30) | 1813 (55) | 0·35 (0·32, 0·38) | 0·03 |
| <40% | 38 (3%) | 20 (3%) | 1·25 (0·70, 2·23) | 176 (8%) | 69 (4%) | 1·86 (1·39, 2·48) | 295 (7%) | 146 (5%) | 1·46 (1·19, 1·80) | 0·7 |
| ≥40% | 282 (23%) | 86 (11%) | 2·37 (1·79, 3·14) | 927 (43%) | 323 (24%) | 2·42 (2·10, 2·79) | 2744 (64%) | 1136 (41%) | 2·58 (2·35, 2·83) | 0·3 |
| Transfer | ||||||||||
| Transfer in | 27 (2%) | 10 (1%) | 1·76 (0·77, 3·99) | 91 (4%) | 12 (1%) | 4·64 (2·71, 7·95) | 353 (9%) | 66 (4%) | 2·74 (2·20, 3·41) | 0·7 |
| Transfer out | 74 (5%) | 70 (9%) | 0·58 (0·40, 0·85) | 111 (5%) | 164 (11%) | 0·39 (0·31, 0·51) | 232 (4%) | 520 (16%) | 0·21 (0·17, 0·25) | <0·001 |
OR indicates odds ratio; CI, confidence interval; PCI, percutaneous coronary intervention; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate.
Category variables displayed as number (weighed percentage).
Excluding patients who were transferred in.
Among patients with the measurement.
With patients in rural hospital as the reference group
Treatments, tests, and procedures
We examined care processes among the 7406 patients in urban hospitals, and 4858 patients in rural hospitals (Figure 1). The reperfusion use among ideal candidates in rural hospitals in 2001 and 2006 was lower than in urban hospitals (p<0·001), but by 2011 there was no urban-rural difference in reperfusion rates (p=0·1) (Table 3). During this period, reperfusion rates increased in rural hospitals from 49% in 2001 to 57% in 2011 (p for trend=0·001), but decreased in urban hospitals from 59% in 2001 to 54% in 2011 (p for trend=0·01). In addition, given the fact that patients admitted to rural hospitals in 2011 were more likely to be ideal candidates for reperfusion due to their shorter pre-hospital delay, as compared with those in urban hospitals (54% vs. 45%, p<0·001) (Appendix G), a larger proportion of the overall study cohort in rural hospitals received recommended reperfusion therapy in 2011 (30% vs. 25%, p<0·001). Among those who received reperfusion therapy in urban hospitals in 2001, 29% received primary PCI, while at rural hospitals fibrinolytic therapy was the only method of reperfusion used. Over time, primary PCI rates increased in both rural and urban areas (p for trend<0·001 for both); in 2011, 37% of ideal reperfusion patients in urban hospitals received primary PCI, as compared with 15% in rural hospitals, wherein fibrinolytic therapy remained the principal mode of reperfusion (p<0·001).
Table 3:
Changes in treatments, tests and procedures use among patients with ST elevation myocardial infarction.
| 2001 |
2006 |
2011 |
p for interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Urban * | Rural * | OR (95% CI)# | Urban * | Rural * | OR (95% CI) # | Urban * | Rural * | OR (95% CI)# | ||
| Reperfusion Therapies | ||||||||||
| No reperfusion † | 41% | 53% | 0·60 (0·45, 0·81) | 42% | 50% | 0·72 (0·59, 0·88) | 46% | 43% | 1·11 (0·97, 1·28) | <0·001 |
| Primary PCI † | 17% | 0% | - | 25% | 6% | 4·89 (3·51, 6·82) | 37% | 15% | 3·21 (2·69, 3·83) | <0·001 |
| Fibrinolytic therapy † | 42% | 47% | 0·83 (0·62, 1·12) | 33% | 43% | 0·63 (0·52, 0·77) | 18% | 42% | 0·30 (0·26, 0·35) | <0·001 |
| Acute medications | ||||||||||
| Aspirin<=24h † | 82% | 77% | 1·37 (1·07, 1·75) | 90% | 82% | 2·08 (1·71, 2·53) | 92% | 89% | 1·53 (1·29, 1·82) | 0·7 |
| Clopidogrel<=24h † | 3% | 0% | - | 63% | 23% | 5·66 (4·86, 6·60) | 91% | 67% | 4·86 (4·25, 5·55) | 0·5 |
| Beta blockers<=24h † | 60% | 39% | 2·32 (1·71, 3·16) | 67% | 59% | 1·37 (1·11, 1·68) | 56% | 60% | 0·82 (0·71, 0·95) | <0·001 |
| Statins † | 41% | 13% | 4·45 (3·43, 5·78) | 83% | 65% | 2·64 (2·26, 3·09) | 95% | 88% | 2·35 (1·96, 2·81) | 0·4 |
| ACE inhibitors/ARB † | 64% | 58% | 1·25 (1·02, 1·54) | 74% | 66% | 1·41 (1·22, 1·64) | 66% | 68% | 0·90 (0·81, 1·00) | <0·001 |
| TCM | 44% | 78% | 0·23 (0·18, 0·28) | 50% | 81% | 0·23 (0·20, 0·27) | 61% | 82% | 0·34 (0·30, 0·39) | <0·001 |
| MgSO4 | 32% | 35% | 0·90 (0·73, 1·11) | 19% | 19% | 0·98 (0·82, 1·16) | 16% | 17% | 0·94 (0·82, 1·07) | 0·9 |
| Procedures | ||||||||||
| Cardiac Catheterization | 20% | 2% | 15·16 (8·01, 28·69) | 37% | 8% | 6·55 (5·31, 8·07) | 55% | 19% | 5·27 (4·68, 5·92) | 0·001 |
| PCI (non-primary) | 5% | 1% | 6·13 (2·51, 14·67) | 17% | 4% | 4·56 (3·45, 6·02) | 28% | 6% | 5·87 (4·92, 7·00) | 0·2 |
| CABG | 2% | 0% | - | 2% | 0% | - | 1% | 0% | - | · |
| Intra-aortic balloon pump | 1% | 0% | 5·75 (0·61, 53·97) | 2% | 0% | - | 4% | 0% | 9·27 (4·81, 17·84) | 0·5 |
| Testing | ||||||||||
| Troponin | 31% | 9% | 4·82 (3·54, 6·56) | 55% | 34% | 2·31 (2·02, 2·66) | 71% | 65% | 1·33 (1·20, 1·48) | <0·001 |
| Creatinine | 74% | 48% | 3·07 (2·49, 3·78) | 90% | 77% | 2·71 (2·25, 3·26) | 96% | 94% | 1·49 (1·20, 1·86) | <0·001 |
| Echocardiogram | 37% | 20% | 2·32 (1·84, 2·93) | 57% | 33% | 2·73 (2·37, 3·13) | 75% | 54% | 2·60 (2·35, 2·89) | 0·5 |
OR indicates odds ratio; CI, confidence interval; TCM, traditional Chinese medicine; PCI, percutaneous coronary intervention.
Category variables displayed as weighed percentage.
use of treatment among ideal patients.
with patients in rural hospital as the reference group
Aspirin and clopidogrel use within 24 hours, as well as statin use during hospitalization, were significantly higher among patients in urban hospitals (p<0·05 for all), with improvements over time in both hospital settings (Table 3). Beta-blocker use within 24 hours was more prevalent among patients treated at urban hospitals in 2001 and 2006 (p<0·05 for both); however, by 2011, rural hospitals had significantly higher use (60% in rural hospitals vs. 56% in urban hospitals, p=0·01). Similarly for ACE inhibitors or ARB rates, use in urban hospitals was higher in 2001 (64% vs. 58%, p=0·047) and 2006 (74% vs. 66%, p<0·001), but lower in 2011 (66% vs. 68%, p=0·04). The use of traditional Chinese medicines was higher in rural hospitals (p<0·001 for all), and increased in both hospital types over time (p for trend <0·001 for both).
Rural hospitals had a lower intensity of diagnostic testing, including laboratory tests (troponin and creatinine) and echocardiograms than urban hospitals (p<0·001 for all), but the urban-rural gap in the use of laboratory tests narrowed over time (p for interaction<0·001) (Table 3). Non-primary PCI and cardiac catheterization also increased over time in both urban and rural hospitals (p for trend<0·001 for all).
In-hospital outcomes
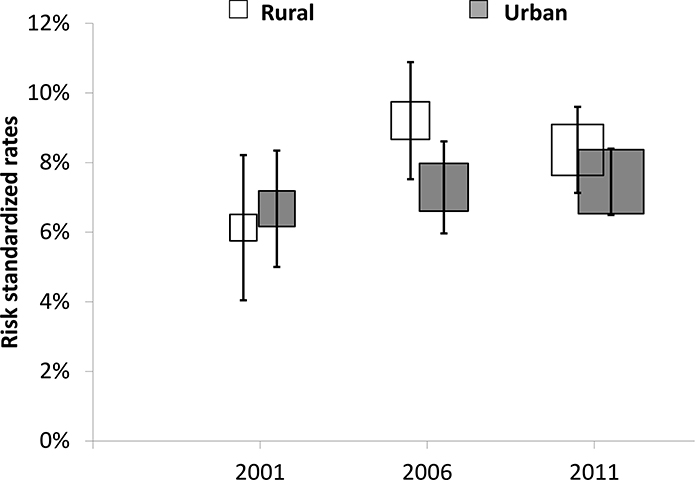
A total of 7401 urban patients and 4585 rural patients were included in the outcomes analyses (Figure 1). Compared with patients in urban hospitals, the median length of stay for patients in rural hospitals was slightly shorter in both 2001 [12 (7–17) vs. 13 (8–19), p<0·001] and 2006 [9 (5–15) vs. 12 (7–16), p<0·001], but was not significantly different in 2011 [11 (7–14) vs. 10 (7–15), p=0·07]. The unadjusted rate of in-hospital death or withdrawal from treatment was similar between patients in rural and urban hospitals in both 2001 [10·2% vs. 10·8%, p=0·7] and 2006 [13·6% vs. 11·9%, p=0·1], but was higher among patients in rural hospitals in 2011 [11·9% vs. 9·3%, p<0·001]. The unadjusted rate of composite in-hospital complications (including death, withdrawal from treatment, re-infarction, shock, ischemic stroke, or congestive heart failure) was similar between rural and urban hospitals in 2001 [16·6% vs. 18·9%, p=0·3] and 2006 [21·1% vs. 21·2%, p=0·9]; however, in 2011, complications were more common in patients in rural hospitals than those in urban hospitals [20·3% vs. 17·6%, p=0·01].
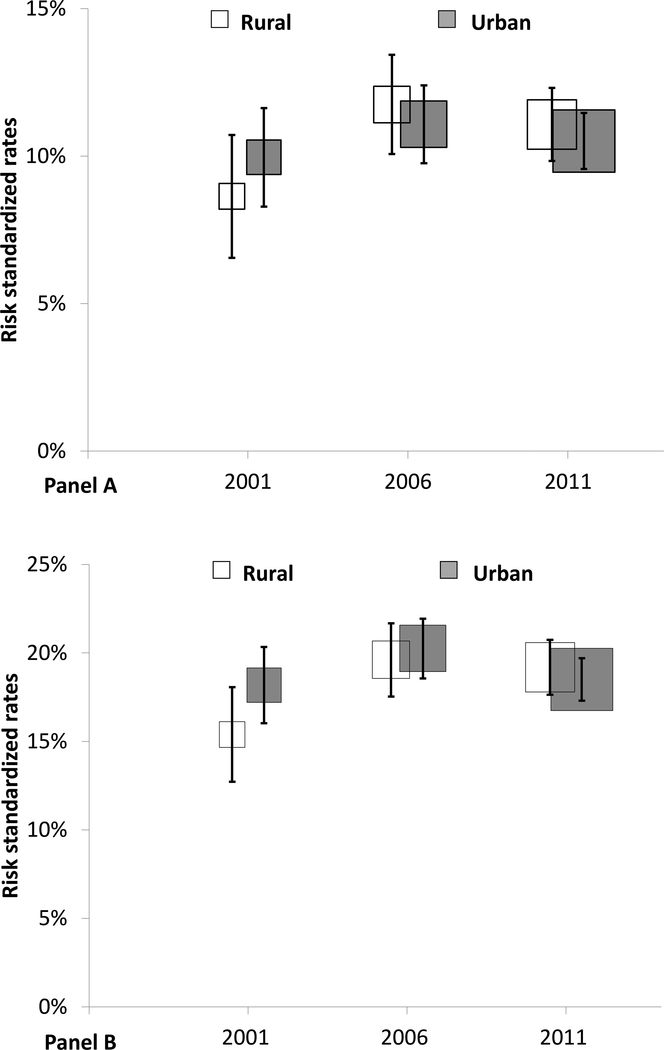
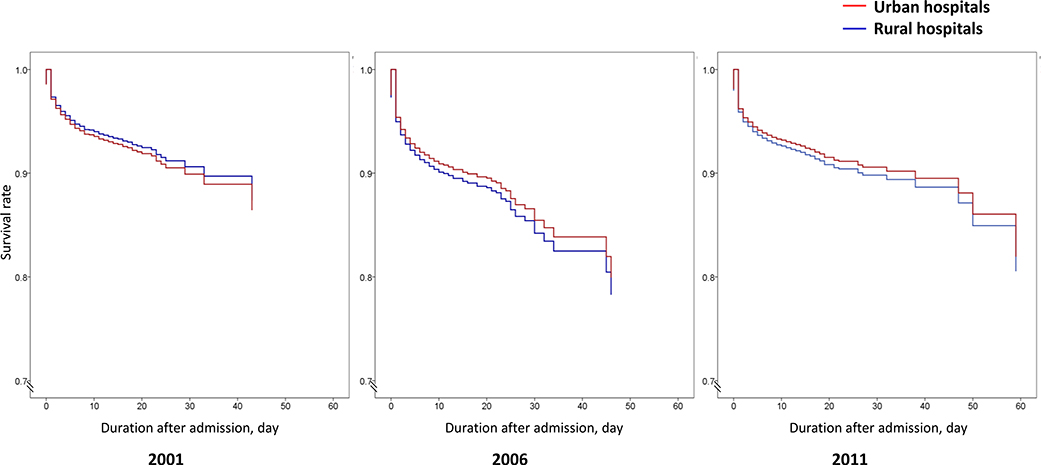
After adjustment for patient demographics and clinical characteristics, the risk of in-hospital death or withdrawal from treatment did not differ between patients in rural and urban hospitals in all 3 years, with an adjusted OR (urban vs. rural) of 1·13 [95% CI (0·77, 1·65, p=0·5] in 2001, 0·99 [95% CI (0·77, 1·27), p=0·9] in 2006, and 0·94 [95% CI (0·74, 1·19), p=0·6] in 2011 (Figure 2). Similar results were found in our sensitivity analysis using a 7-day time frame for measurement of outcomes (Appendix H). Kaplan-Meier curves for these events were similar (Appendix I). With regard to risk of in-hospital complications, there was no statistically significant difference between patients in rural and urban hospitals, with an adjusted OR (urban vs. rural) of 1·22 [95% CI (0·85, 1·75), p=0·3] in 2001, 1·15 [95% CI (0·92, 1·45), p=0·2] in 2006, and 0·99 [95% CI (0·81, 1·21), p=0·9] in 2011.
Figure 2. Differences in risk adjusted rates of in-hospital outcomes between rural and urban hospitals over time.
Panel A for in-hospital death or treatment withdrawal; panel B for in-hospital complications
DISCUSSION
Our study, the first nationally representative comparison of care and outcomes for people with AMI in urban and rural China, found that differences in the use of evidence-based treatments that were present in 2001 have largely been eliminated, but substantial opportunities for improvement persist in both settings. Despite early treatment differences, mortality between urban and rural hospitals in all three years were similar, indicating that the additional resources available to urban hospitals did not result in improved short-term benefits for patients.
In China, patients in rural hospitals were less likely to receive evidence-based STEMI care in 2001; It was similarly to the disparities identified in developed countries,2,3,5,24–30 but the rates approached parity with urban hospitals by 2011. Preferential investments and policy support for rural areas may have motivated these improvements. In 2003, only 21% of rural population were covered by health insurance, and only 6% of inpatient costs, including drugs, were reimbursed..12 By 2011, with the implementation of the New Rural Cooperative Medical Scheme, access to insurance improved considerably in rural areas, with 98% of the population covered and 44% of inpatient costs reimbursed.12 Furthermore, the National Essential Drug System established in 2009 ensured the supply of essential medications and provided full coverage for all recommended medications for AMI, with the exception of clopidogrel.31,32 While the improvements in rural hospitals over the study period are encouraging, the use of evidence-based therapies for AMI remain suboptimal in both rural and urban hospitals, compared with the 77% of contemporary patients with STEMI in the UK who receive reperfusion therapy.33 The underuse of effective strategies existed in not only expensive treatments that require advanced techniques and facilities, but also in less costly treatments such as beta-blockers. On the other hand, traditional Chinese medicines, which lack data on efficacy and safety,34 were more commonly used in rural hospitals with more constrained budgets. These examples illustrate that, apart from increasing investment, there is a need to focus on improving quality and value of care in both urban and rural hospitals in China.
In contrast to the previously demonstrated fact that patients admitted with AMI in rural hospitals may suffer worse outcomes,6–8 patients with STEMI in China had similar outcomes in rural and urban hospitals. There are several potential explanations. One possibility is that, despite more advanced facilities and greater availability of specialists, urban hospitals have not implemented treatment strategies more effectively. For instance, underuse of lifesaving reperfusion therapy was even more common in urban hospitals in 2011, as their overall reperfusion rates were lower than rural hospitals. Differences in patient characteristics do not appear to account for this finding. Contrary to expectations, the comparison of mini-GRACE scores in 2011 showed that patients admitted in rural areas had a higher average baseline risk. Hence, our findings seem to suggest that urban hospitals did not have lower AMI mortality rates nor achieved higher quality and higher value care, despite their greater access to advanced facilities and higher costs (US$3870 per AMI admission in 2011 in urban hospitals vs. US$1320 in rural hospitals).14
Only about one-third of admissions for STEMI in China were at rural hospitals in 2011, despite the fact that about half of the population lives in rural areas and these areas have a similar mortality burden from AMI as urban areas.14 The reasons may for this maybe complicated. One possibility is persistent disparities in access to hospital care: a larger proportion of patients with STEMI in rural areas may die without in-hospital care compared with those in urban areas. More limited healthcare resources in rural areas results in half as manyhospital beds, physicians, and nurses as in urban areas.35 Another explanation is that some rural patients may bypass local hospitals for distant but more advanced urban ones, even in such an acute and life-threatening condition, which could also explain lack of mortality differences between rural and urban regions. An in-depth comparison of hospital admission trends in China is warranted to better understand these patterns in order to optimize the efficiency, equity and quality of AMI healthcare resource allocation.
In summary, in addition to remarkable advances in access to health care12 and persistent gaps in quality of care for STEMI identified in prior studies,36 our study provides insights into the need for a dual strategy to improve medical care in China. For rural areas, investment efforts to improve healthcare capacity and expand insurance coverage, such as the National Essential Drug System and New Rural Cooperative Medical Scheme, have contributed substantially to improved treatment and reduced gaps in care but must continue in order to reduce the disparity in access to care between rural and urban areas. However, the fact that urban hospitals have spent more resources without achieving better outcomes suggests that increasing resources alone is not a complete solution. Rigorous and systematic quality measurement and sophisticated incentives for high-value performance are needed to optimize the quality of care.37 This study of urban-rural differences highlights the importance of studying various dimensions of care, including admission, treatments and outcomes, and can serve as a foundation for other countries seeking to eliminate such disparities.
Our findings expand on previous evaluations of the Chinese government’s efforts to improve care and reduce disparities during the most recent round of healthcare reform, and also foreshadow an important research agenda. First, in addition to generating more knowledge about the disparities in quality of care across hospitals in different areas, data on the performance of primary care centers is still largely unavailable and is essential for a more comprehensive picture of health care in China. Second, international comparisons on quality of care are of value for benchmarking performance and developing improvement-focused interventions. 33
This study should be interpreted in the context of several potential limitations. First, clinical characteristics were recorded based on documentation in medical records. There is no reason to suspect so, but it is possible that definitions of certain conditions and the completeness of documentation may have varied between urban and rural hospitals. Second, although we adjusted for a comprehensive array of patient factors including commonly measured markers of disease severity, there may be unmeasured patient factors that differed between urban and rural patients, which might have influenced our results. Finally, shorter length of stay in rural hospitals may bias the study toward lower event rates. However, we did not find this to be the case in the secondary analysis using a standardized 7-day time frame.
In conclusion, our study demonstrates diminished treatment gaps and similar patient outcomes after STEMI in urban and rural hospitals in China between 2001 and 2011, This underscores encouraging trends for achieving more equitable care, but also highlights substantial opportunities to improve the quality and value of care in both settings. In order to achieve exemplary performance and optimal outcomes, investments to improve capacity and access to care must be accompanied with the implementation of systematic quality measurements and incentive strategies.
PANEL: RESEARCH IN CONTEXT
Evidence before this study
In China, remarkable advances have been made in access to health care during the past decade,12 meanwhile important gaps persist in quality of care for STEMI, and the in-hospital mortality has not decreased.36 We searched databases of PubMed/Medline and China National Knowledge Infrastructure for articles published in English and Chinese respectively between Jan 1, 2000 and June 30, 2015 (Appendix J). We identified 14 studies on urban-rural disparities in care for AMI that were all conducted in developed countries,2,3,5–8,24–30,38 showing that patients in rural areas may be less likely to receive evidence-based therapies,2,3,5,24–30 and may experience worse outcomes.6–8 However, there were no prior studies comparing the care for AMI between urban and rural in developing countries like China.
Added value of this study
Our study extends the knowledge in evaluation of efforts by Chinese government to reduce care disparities, with the comparative assessment between urban and rural areas during a dynamic period. For the first time, to our knowledge, we demonstrates eliminated treatment gaps and similar patient outcomes after STEMI between in urban and rural hospitals, indicating markedly steps in achieving equitable care, as well as substantial opportunities to improve quality and value of care in both. The findings also provide insights into the need for a dual strategy in China and other countries – investments to improve care capacity must be accompanied with systematic quality measurement and implementation of incentive strategies.
Implications of all the available evidence
In China, additional efforts focusing on healthcare capacity and insurance coverage in rural areas are needed to reduce the disparity in care with urban areas. Moreover, rigorous and systematic quality measurement and sophisticated incentives for high-value performance may be necessary to achieve optimized outcomes of patients throughout the country.
ACKNOWLEDGMENTS
We appreciate the multiple contributions made by study teams at National Clinical Research Center of Cardiovascular Diseases and Yale-New Haven Hospital Center for Outcomes Research and Evaluation in study design and operations, particularly the data collection by Yi Pi, Jiamin Liu, Wuhanbilige Hundei, Haibo Zhang, Lihua Zhang, Xue Du, Wenchi Guan, Xin Zheng, and Yuanlin Guo. We appreciate the editing by Grace Yi and Nicholas Bergfeld. We are grateful for the support provided by the Chinese government.
FUNDING
This project was partly supported by the Research Special Fund for Public Welfare Industry of Health (201202025) from the National Health and Family Planning Commission of China, and the National Key Technology R&D Program (2013BAI09B01) from the Ministry of Science and Technology of China. Dr. Krumholz is supported by grant U01 HL105270–05 (Center for Cardiovascular Outcomes Research at Yale University) and Dr. Chan is supported by grant 1R01HL123980, both from the National Heart, Lung, and Blood Institute. The sponsors had no role in the conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation or approval of the manuscript.
Funding
National Health and Family Planning Commission of China, Ministry of Science and Technology of China.
APPENDIX
A. China PEACE-Retrospective AMI Study Site Investigators by Hospital
Aba Tibetan and Qiang Autonomous Prefecture People’s Hospital, ShipingWeng, ShuyingXie; Affiliated Hospital of Guiyang Medical College, Lirong Wu, Jiulin Chen; Affiliated Hospital of Hainan Medical College, Tianfa Li, Jun Wang; Affiliated Zhongshan Hospital of Dalian University, Qin Yu, Xiaofei Li; Alxa League Central Hospital, Zhong Li, ShiguoHao, Yuzhen Zhang, Xuemei Wu; Baiquan County People’s Hospital, Yachen Zhang, Zhifeng Liu; Biyang People’s Hospital, Zhongxin Wang, HaoJia; Bortala Mongol Autonomous Prefecture People’s Hospital, Bayin Bate, BadengQiqige; Changda Hospital Of Anshan, Xiang Jin, Ting Cai; Chengwu County People’s Hospital, Fengqin Liu, Dayong Xu; Chenxi County People’s Hospital, Xuejin He, Shui Yang; Chongren County People’s Hospital, Chun Yuan, Jiping Wang; County People’s Hospital of Jinning, LihuaGu, Lin Li, Shijiao Chen; Dalian Municipal Central Hospital, YongchaoZhi, Lili Sun; Dao County People’s Hospital, Shengcheng Zhou, Lingjiao Jin; Daofu County People’s Hospital, Yong Leng, Liangchuan Zhang, Tianyun Deng; Dingyuan County People’s Hospital of Anhui Province, Yuanjin Wang, Wenhua Zhang, Xinmin Ma; Dongyang People’s Hospital, Weimin Li, Liang Lu, Xuan Ge; Dulong and Nu Autonomous County People’s Hospital of Gongshan, Xiaoping Wu, Yanming He; Dunhua City Hospital of Jilin Province, FanjuMeng, Jia Li; Fenghuang County People’s Hospital, Dexi Liao, Guangyong Liu, Wen Qin; Fengshan County People’s Hospital, Wen Long, Xiangwen Chen; Fourth Hospital of Baotou City, Baohong Zhang, Yonghou Yin, Bin Tian; Fourth People’s Hospital of Zigong City, Yong Yi, Chaoyong Wu; Fugu County People’s Hospital of Shaanxi Province, Baoqi Liu, Zhihui Zhao, Haiming Li; Fujian Provincial Hospital, YansongGuo, Xinjing Chen; Fuling Center Hospital of Chongqing City, Liquan Xiang, Lin Ning; Gannan County People’s Hospital, Mei Chen, Xin Jin, Guiling Li; General Hospital of the Yangtze River Shipping, Xiuqi Li, Xing’an Wu; Gongcheng Yao Autonomous County People’s Hospital, Congjun Tan, Mingfang Feng, Meili Wang; Guangchang County People’s Hospital, Liangfa Wen, Xiang Fu, QunxingXie; Guilin People’s Hospital, Wei Zhang, Yanni Zhuang, Hua Lu;Guiping People’s Hospital, Jiaqian Lu, Yu Huang; Haerbin 242 Hospital, Yin Zhou, Qiuling Hu; Haiyan People’s Hospital, Chunhui Xiao, Xiaoli Hu; Heling Ge Er County People’s Hospital, Yongshuan Wu, Qiuli Wang; Helong Municipal People’s Hospital, Youlin Xu, Xuefei Yu; Henan Provincial People’s Hospital, Chuanyu Gao, Jianhong Zhang, You Zhang; Heze Municipal Hospital, WentangNiu, Xiaolei Ma, Yong Wang; HGKY Group Company General Hospital, Xiaowen Pan, Yanlong Liu; Hua Xin HospitalFirst Hospital of Tsinghua University, Lifu Miao, Yanping Yin, Zhiying Zhang; Huairen People’s Hospital, Shutang Feng; Huayin People’s Hospital, Aiping Wang, Jiangli Zhang, Feipeng Li; Huaying People’s Hospital, Hong Wang; Hunchun Hospital, Lijun Yu, Xinxin Zhao; Huizhou Municipal Central Hospital, Yuansheng Shen, Zhiming Li, Lizhen He; Hunan Province Mawangdui Hospital, ZhiyiRong, Wei Luo; Ji’an Municipal Central People’s hospital, Xueqiao Wang; Jianghua Yao Autonomous County People’s Hospital, Rongjun Wan, Jianglin Tang, Guanghan Wu; Jiangsu Haimen People’s Hospital, Jie Wu, Bin Xu; Jiangxi Provincial People’s Hospital, Qing Huang, Xiaohe Wu; Jiangzi County People’s Hospital, Sang Ge, Pian Pu, PingcuoDuoji; Jilin Province People’s Hospital, Hui Dai, Yuming Du, Wei Guo; Jilin Integrated Traditional Chinese & Western Medicine Hospital, Jilin Province, Jianping Shi; Jinghai County Hospital, Peihua Zhao, Jingsheng Sun; Jingxi County People’s Hospital, Hongxiang Li, Wen Liang; Jingxing County Hospital, Zhiwen Dong, Zhenhai Zhao; Jingzhou Central Hospital, Xin Li, Qin Xu; Jiuquan City People’s Hospital, Yaofeng Yuan, Zhirong Li; Jixi People’s Hospital of The Jixi Municipal People’s Hospital Medical Group, Jinbo Gao; Jize County Hospital, Qiu’eGuo; Kangbao County People’s Hospital, Ruiqing Zhao, Guangjun Song; Keshiketengqi Hospital of Chifeng City, Lize Wang, Haiyun Song; Lanping Bai and Pumi Autonomous County People’s Hospital, Jinwen He, Jinming He; Laoting County Hospital, Keyong Shang, Changjiang Liu, Kuituan Xi; Liaoyang Central Hospital, Rihui Liu, Peng Guo; Liaoyuan Central Hospital, ChaoyangGuo, Xiangjun Liu, Rujun Zhao, Zeyong Yu; Lindian County Hospital, Wenzhou Li, Xudong Jing, Huanling Wang; Linxiang People’s Hospital, Xiyuan Zhao, Chao Zhang, Long Chen; Liujiang County People’s Hospital, Meifa Wei, Yan Liu, Shengde Chen; Longyan First Hospital, Kaihong Chen, Yong Fang, Ying Liao; Luancheng County Hospital, Junli Wang, Tianyu Liu, Suzhe Cheng; Lucheng People’s Hospital, Yunke Zhou, XiaoxiaNiu, Huifang Cao; Luchuan County People’s Hospital, Zebin Feng, Min Feng; Luxi County People’s Hospital, FeilongDuan, Haiming Yi; Luyi County People’s Hospital, Yuanxun Xu, AnranGuo; Macheng People’s Hospital, Xianshun Zhou, HongzhuanCai, Peng Zheng; Mengcheng First People’s Hospital, GaofengGuo; MenglianLahudaiwa autonomous counties People’s Hospital, Xiang Li; Min County People’s Hospital, MinwuBao, Yuhong Liu; Nanjing First Hospital, Shaoliang Chen, HaiboJia, Hongjuan Peng; Nan’an Hospital, Duanping Dai, Shaoxiong Hong; Nantong Third People’s Hospital, Song Chen, Dongya Zhang, Ying Wang; Nanyang Central Hospital, Yudong Li, Jianbu Gao, Shouzhong Yang; Ningwu County People’s Hospital, Junhu An; Peking University People’s Hospital, Chenyang Shen, Yunfeng Liu; Peking University Shenzhen Hospital, Chun Wu, Huan Qu, Saiyong Chen; People’s Hospital of Jingyu, Yuhui Lin, Dehai Jiao; People’s Hospital of Yueqing City, Manhong Wang, Qiu Wang; Pianguan County People’s Hospital, YingliangXue, Ruijun Zhang; Puding County People’s Hospital, Cheng Yuan, Lei Wu; Qinghai Red Cross Hospital, Jianqing Zhang, Chunmei Wei, Yanmei Shen; Qinshui County People’s Hospital, Hehua Zhang, Hongmei Pan, Yong Gao; Qinyang People’s Hospital, Xiaowen Ma, Yanli Liang, Tianbiao Wang; Queshan County People’s Hospital, Daguo Zhao; Quzhou People’s Hospital, XiaomingTu, Zhenyan Gao; Rongjiang County People’s Hospital, Fangning Wang, Qiang Yang; Rudong County People’s Hospital, Xiaoping Kang, Jianbin Fang, Dongmei Liu; Ruyang County People’s Hospital, Chengning Shen, Mengfei Li; Shangluo Central Hospital, Yingmin Guan, Wenfeng Wang, Ting Xiao; ShangqiuChangzheng People’s Hospital, Qian Wang; Shaoyang County People’s Hospital, Fengyun Jiang, Kaiyou Wu; Shengsi People’s Hospital, Songguo Wang; Shenyang Weikang Hospital, Xujie Fu, Shu Zhang,Lifang Gao; ShougangShuicheng Iron & Steel (Group) Co·, Ltd. General Hospital, Min Zhang, Kai Fu, XiaojingDuan; Shuangshan Hospital Of Anshan, Rui Xiao, Ruixia Wu, Bin Li; Siziwang County People’s Hospital, Hongtu Zhang, Yuerong Ma, Zhonghui Cao; SunanYugur Autonomous County People’s Hospital, Zhansheng Ba, Wanhai Fu; Taizhou Hospital of Zhejiang Province, Jianjun Jiang, YafeiMi, Weiwei Zhou; The Affiliated Hospital of Beihua University, Feng Sun, Qi Zhang, Shiyu Zheng; The Fifth People’s Hospital of Dalian, Jing Zhang, Yang Zhong; The First Affiliated Hospital of Hebei North University, Fangjiang Li, Xiaoyuan Wang; The First Affiliated Hospital of Henan University of Science & Technology, Pingshuan Dong, Laijing Du, Wei Liu; The First Affiliated Hospital Of Jia Mu Si University, Zhaofa He, Meihua Jin; The First Hospital of Fuzhou City, Ting Jiang, Zhuoyan Chen; The First Hospital of Xi’an, Manli Cheng, YuqiangJi; The First People’s Hospital of Danzhou, Youhua Zhou, Jvyuan Li; The First People’s Hospital of Guangzhou, Yizhi Pan, Jian Liu; The First People’s Hospital of Guangyuan, Tianxun Wang, Ping Yang; The Fourth People’s Hospital of Shangqiu Shi, Guiyu Huang, JianjunPan,QingliangCai,Qianying Wang; The General Hospital of Yongzhou, Hunan Province, MingliLv; The people’s hospital of Wuchuan, Yuanming Yi, Xuelian Deng; The People’s Hospital of Yuanling, Wenhua Chen, RongCai; The People’s Hospital of Zhijiang City, Bing Zhang; The Second Affiliated Hospital of Harbin Medical University, Bo Yu, Yousheng Xu, Zhengqiu Wang; The Second Affiliated Hospital of Kunming Medical University, Jun Shu, Ge Zhang, Kai Li; The Second Central Hospital of Baoding City, Guang Ma, PuxiaSuo; The Second People’s Hospital of Liaoyuan City, Aimin Zhang, Yongfen Kang; Tianjin Medical University General Hospital, Zheng Wan,Yuemin Sun, Bo Bian; Tibet Autonomous Region People’s Hospital, Xuejun Hu, DawaCiren; Tongchuan Mining Bureau Central Hospital, GuojiongJia, Jieli Pan; Tongliang County People’s Hospital, Guofu Li, Hongliang Zhang, Longliang Zhan; Tongliao City Horqin District First People’s Hospital, Junping Fang, Xinli Yu; Ulanqab Central Hospital, Dacheng Wang, Dajun Liu, Xinhong Cao; Wencheng County People’s Hospital, Yi Tian, HaishengZhu,Wanchuan Liu; Wuhai People’s Hospital, Zhaohai Zhou, Lei Shi; Wuhu Second People’s Hospital, Wuwang Fang, Manxin Chen; Wulate County People’s Hospital, FuqinHan,JianyeFu,Yunmei Wang; Wuqiang County People’s Hospital, Binglu Liu, YanliangZhang,Xiupin Yuan; Wuyishan Municipal Hospital, Qingfei Lin, Yun Chen; Xiangtan County People’s Hospital, Yuliang Zhu, ZhiqiangCai; Xing County People’s Hospital, Xingping Li, LirongAo; Xingshan County People’s Hospital, Shubing Wu, Hui Zhang; Xinmi First People’s Hospital, Fusheng Zhao, Guangming Yang; Xinshao County People’s Hospital, Renfei Liu, Wenwei Ai; Xiuwu County People’s Hospital, JianbaoChang,Haijie Zhao; Xuanhan County People’s Hospital, Qijun Ran, Xuan Ma; Xupu County People’s Hospital, Shijun Jiang, Xiaochun Shu; Yanggao County People’s Hospital, Zhiru Peng, Yan Han; Yanqing County Hospital, Jianbin Wang, Li Yang; Ying County People’s Hospital, Yu Shen, Xingcun Shang; Yitong Manchu Autonomous County First People’s Hospital, Haifeng Wang; Yongxing County People’s Hospital, Hongyan Li, Zhisong Liao, Yang Cao; Yuanzhou District People’s Hospital of Guyuan City, Xiaoping Gao, MeiyingCai, Lining You; Yuncheng Central Hospital, Xuexin Li, Shuqin Li, Yingjia Li; Yunlong County People’s Hospital, Jianxun Yang, Song Ai, Jianfei Ma; Yuyao People’s Hospital, Lailin Deng; ZhangjiachuanHui Autonomous County First People’s Hospital, Keyu Wang, Shitang Gao, Jian Guan; Zhouning County Hospital, Banghua He, Youyi Lu; Zhuoni County People’s Hospital, Weirong Yang, Hong Li; Zhuozi County People’s Hospital, Zhizhong Zhang, Xiaohong Chi; Zuoyun County People’s Hospital, Ru Duan, Guangli Wang.
B. China PEACE Study Consultants
Study Consultants: Paul S. Chan, MD, MSc, Jersey Chen, MD, MPH, David J. Cohen, MD, MSc, Nihar R. Desai, MD, MPH, Kumar Dharmarajan MD, MBA, Mikhail N. Kosiborod, MD, Jing Li, MD, PhD, Xi Li, MD, PhD, Zhenqiu Lin, PhD, Frederick A. Masoudi, MD, MSPH, Jennifer Mattera, DrPH, MPH, Brahmajee K. Nallamothu, MD, MPH, Khurram Nasir, MD, MPH, Sharon-Lise T. Normand, PhD, Joseph S. Ross, MD MHS, John A. Spertus, MD, MPH, Henry H. Ting, MD, Xiao Xu, PhD
St. Luke’s Mid America Heart Institute/University of Missouri Kansas City (PSC, DJC, MNK, JAS), Kansas City, Missouri, United States; Kaiser Permanente (JC), Mid-Atlantic Permanente Research Institute, Rockville, Maryland, United States; Center for Outcomes Research and Evaluation (NRD, KD, ZL, JM, JSR, XX), Yale-New Haven Hospital, New Haven, Connecticut, United States; Division of Cardiology (KD), Department of Internal Medicine, Columbia University Medical Center, New York, New York, United States; State Key Laboratory of Cardiovascular Disease (JL, XL), China Oxford Centre for International Health Research, Fuwai Hospital, National Center for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China; Division of Cardiology (FAM), University of Colorado Anschutz Medical Campus, Aurora, Colorado, United States; Veterans Affairs Health Services Research and Development Center of Excellence (BKN), Veterans Affairs Ann Arbor Healthcare System, Ann Arbor, Michigan, United States; Department of Internal Medicine (BKN) and Center for Healthcare Outcomes and Policy (BKN), University of Michigan, Ann Arbor, Michigan, United States; Research Director, Center for Prevention and Wellness (KN), Baptist Health South Florida, Miami, Florida, United States; Department of Biostatistics (S-LTN), Harvard School of Public Health, Boston, Massachusetts, United States; Department of Health Care Policy (S-LTN), Harvard Medical School, Boston, Massachusetts, United States; Section of General Internal Medicine and the Robert Wood Johnson Clinical Scholars Program (JSR), Department of Internal Medicine, Yale University School of Medicine, Connecticut, United States; Division of Cardiovascular Diseases (HHT) and Knowledge and Evaluation Research Unit (HHT), Mayo Clinic College of Medicine, Rochester, Minnesota. United States; Department of Obstetrics, Gynecology, and Reproductive Sciences (XX), Yale School of Medicine, New Haven, Connecticut, United States
C. China PEACE hospital survey: design, conduction, and materials
Participants
In the collaborative network, we invited the principal investigator and the coordinator of each hospital to participate in the survey. The definitions of the roles were established during the planning phase of the China PEACE-Retrospective AMI Study: typically, the director of the Cardiology Department or Internal Medicine Department at each hospital served as the principal investigator, and the China PEACE study coordinator was most often a physician selected by the principal investigator.
Survey design
We organized the survey in 4 sections: personal information of the respondent (part A); general information about the hospital and the department in charge of AMI care (part B); information about hospital practices relating to the diagnosis and treatment of cardiovascular heart disease (part C); and organizational learning characteristics and quality improvement for AMI care (part D). Organizational learning culture was measured using questions from the Short-Form Learning Organization Survey (LOS-27) and the Survival after AMI (SAMI) study.
The survey was written in English and translated into Chinese. To ensure accuracy, a double translation was conducted in which the survey was translated into Chinese and then back into English independently by 2 bilingual Chinese medical researchers. Modifications were made to the Chinese translation accordingly. Participants were informed at the start of the survey that their responses would be used to study institutional characteristics and medical care patterns.
Survey conduction
The survey was piloted using a convenience sample of 6 hospitals with percutaneous coronary intervention capability. The principal investigators were invited to participate in the pilot, and one study coordinator also volunteered to participate. The responses of the 6 principal investigators (3 via in-person interviews and 3 via self-administered paper-based survey) and 1 study coordinator (via self-administered paper-based survey) were collected. The cognitive interviewing methodology, in which individual in-person interviews were conducted with each pilot participant, was used to assess understanding of the pilot survey. For paper-based pilot surveys, cognitive interviewing consisted of retrospective (post-survey) probes; for in-person interviews, concurrent (during survey) probes allowed participants to provide survey feedback in real-time. Based on the experience from the pilot, minor revisions were made to clarify the meaning of certain questions, and the sequence of questions was modified to improve logic and flow. No questions were removed or added. All data from the pilot testing were included in the final data set.
The survey was available in 2 forms: web-based e-survey, in which each participant was able to log in with a unique password to a website where the survey was hosted, and PDF-based survey, in which subjects digitally marked their answers in PDF files and returned the files via email. We applied 2 methods to ensure the quality of the responses. We checked the response data for completeness, either by automatic verification (web-based) or by manual check by our staff (PDF-based), and on the basis of logic. For the web-based e-survey submissions, we used automatic logic check and verification while subjects were responding to the survey, and recorded total time spent on the survey. For the PDF-based survey submissions, we conducted a manual logic check, focusing on whether subjects correctly skipped inapplicable questions as indicated by the instructions in other parts of the survey. In cases of missing or illogical (e.g., questions incorrectly skipped or completed) data for PDF-based surveys, we contacted respondents by email and/or phone, informed them of which questions needed to be resolved, and asked them to resubmit the survey with the necessary changes.
Survey questionnaires
| A. | Personal information |
| A.1 | Gender: |
| ○ Male ○ Female | |
| A.2 | Education |
| ○ Junior high school ○ Senior high school (technical school or technical secondary school) ○ College (junior college) ○ Postgraduate |
|
| A.3 | Clinical job title: |
| ○ Consultant ○ Attendant ○ Resident ○ Nurse ○ Other, please specify: ___ | |
| A.4 | Senior administrative position in hospital: |
| ○ No ○ Yes, please specify: ___ | |
| A.5 | You have been working in the department for __ years. |
| B. | General Information of the hospital and the department |
| Instructions: This section focuses on characteristics of your hospital and department. For all questions, please reflect upon them during the 1-year period from 1/1/2011 to 12/31/2011 (for some of them, please consider 1/1/2001 to 12/31/2001, and 1/1/2006 to 12/31/2006, as specified). | |
| Even some questions in this section might be somewhat hard to answer immediately, especially those about the characteristics of your hospital or department in 2001 and 2006. Please try best to find the answer - as accurate as possible - to every applicable question. | |
| B.1 | Affiliated hospital of medical college: |
| ○ No ○ Yes, please specify the name of the college: ________ [Skip to B3] | |
| B.2 | Teaching hospital of medical college: |
| ○ No ○Yes, please specify the name of the college: ________ | |
| Total No. in your department | |
| In 2001 | In 2006 | In 2011 | ||
|---|---|---|---|---|
| B.3 | Beds | |||
| B.4 | Consultants | |||
| B.5 | Attendants | |||
| B.6 | Residents | |||
| B.7 | Nurses |
| B.8 | Is there any other department in your hospital providing inpatient treatment for AMI? |
| ○ No ○ Yes, please specify the name of the department: ________ | |
| B.9 | Coronary Care Unit (CCU) in hospital? |
| ○ No ○ Yes, please specify the No. of beds: ________ | |
| B.10 | Cath lab in hospital? |
| ○ No [Skip to B12] ○ Yes, please specify when started: ________ | |
| B.11 | How many qualified cardiac interventionalist there are in your hospital: ________ ○ unknown |
| B.12 | Could CABG be performed in hospital? |
| ○ No ○ Yes, please specify the No. of cases in 2011: ______ | |
| B.13 | Independent emergency department? |
| ○ No ○ Yes, please specify the No. of cardiologists in charge in emergency department normally: ______ | |
| B.14 | Formal GCP training of clinical staff in your department? |
| ○ No ○ Yes ○ Unknown | |
| B.15 | Have your apartment participated in international clinical trials? |
| ○ No ○ Yes, please specify the names of the trials: ______ ○ Unknown | |
| B.16 | SFDA certified site for CVD drug trials? |
| ○ No ○ Yes ○ Unknown | |
| B.17 | Existence of Ethics Committee in hospital? |
| ○ No ○ Yes ○ Unknown | |
| Total No. in your hospital |
| In 2001 | In 2006 | In 2011 | ||
|---|---|---|---|---|
| B.18 | Patients with stroke | |||
| B.19 | Patients with ischemic stroke | |||
| B.20 | Patients with hemorrhagic stroke |
| B.21 | Independent neurology department? |
| ○ No ○ Yes, please specify the No. of beds in the department: ______ | |
| B.22 | Carotid endarterectomy performed in hospital? |
| ○ No ○ Yes, please specify when started: ______ ○ Unknown | |
| B.23 | Carotid stenting performed in hospital? |
| ○ No ○ Yes, please specify when started: ______ ○ Unknown | |
| The average cost of the following items in your hospital |
| Items | Cost, ¥ | |
|---|---|---|
| B.24 | Biochemical test, including glucose, lipid, liver function, renal function, CRP or hsCRP | |
| B.25 | Coagulation function test | |
| B.26 | BNP or NT-proBNP | |
| B.27 | Stress test | |
| B.28 | UCG | |
| B.29 | Cardiac CT | |
| B.30 | Carotid US |
| C. | Diagnosis and treatment for CHD |
| Instructions: This section focuses on hospital processes and care of patients with AMI. For all questions, please reflect upon them during the 1-year period from 1/1/2011 to 12/31/2011. | |
| C.1 | Routine diagnostic test of CK for ACS patients after admission? |
| ○ No ○ Yes, please specify the average time delay in reporting results: ______ ○ Unknown | |
| C.2 | Routine diagnostic test of CK-MB for ACS patients after admission? |
| ○ No ○ Yes, please specify the average time delay in reporting results: ______ ○ Unknown | |
| C.3 | Routine diagnostic test of troponin for ACS patients after admission? |
| ○ No ○ Yes, please specify the average time delay in reporting results: ______ ○ Unknown | |
| C.4 | Are patients who are stable after PCI admitted to an intensive care unit? SAMI-Q25 |
| ○ Always ○ Usually ○ Sometimes ○ Rarely ○ Unknown | |
| C.5 | Did your emergency department use a uniform protocol to care for patients who arrived to the emergency department with STEMI? SAMI-Q26 |
| ○ No ○ Yes ○ Unknown | |
| C.6 | Did your emergency department use a uniform protocol to care for patients who arrived to the emergency department with Unstable Angina/NSTEMI? SAMI-Q27 |
| ○ No ○ Yes ○ Unknown | |
| C.7 | Did your hospital use simulations (i.e., trial exercises, dry-runs) to practice any of the following AMI care processes? [Check all that apply] SAMI-Q28 |
| □ Door-to-balloon or door-to-drug protocols □ Chest pain in hospitalized patients □ Inpatient codes (e.g., cardiac arrest, respiratory failure) □ None above □ Unknown |
|
| C.8 | To which patient care unit were patients who were stable with Unstable Angina/NSTEMI most likely admitted? SAMI-Q29 |
| ○ CCU ○ ICU ○ Step-down unit ○ Designated chest pain/telemetry/cardiology floor ○ General medicine floor ○ We did not have a routine method of assigning beds for patients with Unstable Angina/NSTEMI ○ Unknown | |
| C.9 | Did all, or nearly all, patients with AMI have a cardiologist as their primary attending physician? SAMI-Q30 |
| ○ No ○ Yes [Skip to C11] ○ Unknown | |
| C.10 | Were cardiology consults required for all patients with AMI? SAMI-Q30a |
| ○ No ○ Yes ○Unknown | |
| C.11 | In the intensive care unit, who was primarily responsible for the care of patients with AMI? [Check all that apply] SAMI-Q31 |
| □ Critical care physicians (i.e., intensivists) □ Cardiologist/s based exclusively in the unit □ Other cardiologists □ Other, please specify: ______ □ Unknown |
|
| C.12 | Electronic medical record? |
| ○ No [Skip to C14] ○ Yes, please specify when started: ______ ○ Unknown | |
| C.13 | Did your hospital use an electronic medical record (EMR) in the following areas? [Check all that apply]SAMI-Q34 |
| □ Emergency department □ Inpatient floors □ Critical care units □ Affiliated ambulatory offices/clinics □ None above |
|
| C.14 | On the inpatient floors, did your hospital have the following electronic capabilities? [Check all that apply] SAMI-Q35 |
| □ Computerized assisted physician order entry □ Computer prompts to alert user to potential drug-drug interactions or allergies □ Computer prompts to alert user to potential errors in dosing and information □ Computer prompts to alert user to medication order expiration □ Computer prompts to improve adherence to core measures for AMI care (e.g., beta-blocker use) □ None above |
|
| C.15 | In the emergency department, were prior ECG’s electronically available at the time of care? SAMI-Q36 |
| ○ No ○ Yes ○ Unknown | |
| C.16 | Did physicians regularly use explicit protocols or clinical pathways for patients with AMI? SAMI-Q37 |
| ○ No ○ Yes ○ Unknown | |
| C.17 | Did clinicians on the inpatient care units regularly use order sets (either paper-based or electronic) for patients with STEMI? SAMI-Q38 |
| ○ No ○ Yes ○ Unknown | |
| C.18 | Did clinicians on the inpatient care units regularly use order sets (either paper-based or electronic) for with Unstable Angina/NSTEMI? SAMI-Q39 |
| ○ No ○ Yes ○ Unknown | |
| C.19 | Which of the following types of physicians were at the hospital 24-hours/day and 7-days/week? [Check all that apply] SAMI-Q42 |
| □ Critical care physicians (i.e., intensivists) □ Non-interventional cardiologists □ Interventional cardiologists □ Cardiology fellows (including non-interventional and interventional) □ Hospitalists □ None above |
|
| C.20 | Are there any protocols used to guide nurses on when to call the attending cardiologist for patients with AMI? SAMI-Q43 |
| ○ No ○ Yes ○ Unknown | |
| C.21 | Patients with acute coronary syndrome who arrived by Emergency medical service (ambulance): |
| ○ None [Skip to C25] ○ 1–25% ○ 26–50% ○ 51–75% ○ 76–100% ○ Unknown | |
| C.22 | Emergency medical service routinely gives pre-alert calls? |
| ○ No ○ Yes ○ Unknown | |
| C.23 | Patients with acute coronary syndrome who undergo ECG en route to hospital: |
| ○ None ○ 1–25% ○ 26–50% ○ 51–75% ○ 76–100% ○ Unknown | |
| C.24 | Emergency medical service routinely tell your hospital the results of ECG? |
| ○ No ○ Yes ○ Unknown | |
| C.25 | Formal training of triage staff for assessing acute coronary syndrome? |
| ○ No ○ Yes ○ Unknown | |
| C.26 | Dedicated space in triage area for immediate ECG? |
| ○ No ○ Yes ○ Unknown | |
| C.27 | Written criteria for immediate ECG in emergency department? |
| ○ No ○ Yes ○ Unknown | |
| C.28 | Expected interval between patients’ arriving and ECG? |
| ○ ≤ 5min ○ 6–20 min ○ >20 min ○ No expected time ○ Unknown | |
| C.29 | Dedicated ECG technicians in emergency department? |
| ○ No ○ Yes, only some shifts ○ Yes, always ○ Unknown | |
| C.30 | Thrombolysis for AMI patients in hospital? |
| ○ No [Skip to C38] ○ Yes, please specify when started: _____ | |
| C.31 | Does your hospital have a set protocol to identify eligible patients for thrombolysis? |
| ○ No ○ Yes ○ Unknown | |
| C.32 | Does your hospital have a set protocol to assess contraindications of thrombolysis? |
| ○ No ○ Yes ○ Unknown | |
| C.33 | Who makes the decision about thrombolysis in your hospital? |
| ○ Emergency medicine physician alone ○ Emergency medicine physician with a cardiac consultation ○ Only Cardiologist ○ Unknown |
|
| C.34 | In your hospital, where do patients with AMI receive thrombolysis? |
| ○ In the emergency department ○ In the cardiology department (or general medicine department) ○ In the ICU or CCU ○ Unknown |
|
| C.35 | Where are the thrombolytic medicines stored and prepared? |
| ○ Stored and prepared in the department where thrombolysis is done ○ Prepared in the department where thrombolysis is done, but stored in another location ○ Stored and prepared in some location other than the department where thrombolysis is done ○Unknown |
|
| C.36 | Informed Consent before thrombolysis? |
| ○ Not necessary ○ Only orally obtained informed consent is needed ○ One written informed consent form is needed ○ More than one written informed consent form is needed ○ Unknown |
|
| C.37 | Prepayment before thrombolysis? |
| ○ No ○ Yes, please specify the average amount approximately: ___ (“−1” if unknown) ○ Unknown |
|
| C.38 | Primary PCI was performed in your hospital for STEMI patients? |
| ○ No [Skip to C60] ○ Yes, please specify when started: ___ | |
| C.39 | Activation of catheterization laboratory on weekdays? |
| ○ Emergency medicine physician with cardiologist ○ Cardiologist alone ○Emergency medicine physician alone ○ Unknown |
|
| C.40 | Activation of catheterization laboratory at night and on weekends? |
| ○ Emergency medicine physician with cardiologist ○ Cardiologist alone ○Emergency medicine physician alone ○ Unknown |
|
| C.41 | Process for activating catheterization team? |
| ○ After communicating with the emergency department, interventional cardiologist activates catheterization laboratory by calling staff or a central page operator ○ Emergency department makes at least two calls: one to the interventional cardiologist and another to a central page operator, who pages catheterization laboratory staff ○ Emergency department makes a single call to a central page operator, who then pages interventional cardiologist and catheterization laboratory staff ○ No standard approach ○ Other ○ Unknown |
|
| C.42 | Activation of on-call staff for catheterization laboratory? |
| ○ Page operator is not used ○ Page operator is used; confirmation of page receipt is required ○ Page operator is used; no confirmation of page receipt is required ○ No standard approach ○ Unknown |
|
| C.43 | First physician notified after STEMI diagnosis in emergency department? |
| ○ Cardiologist ○ Interventional cardiologist ○ Patient’s primary care physician ○ Other or variable ○ Unknown | |
| C.44 | Laboratory and radiographic results are needed to activate catheterization laboratory? |
| ○ Yes ○ No ○ No standard approach ○ Unknown | |
| C.45 | Process after emergency medical service transmits ECG results? |
| ○Emergency department waits for patient to arrive at hospital to determine whether catheterization laboratory should be activated ○ Emergency department contacts cardiologist while the patient is en route to determine whether catheterization laboratory should be activated ○ Emergency department activates catheterization laboratory while the patient is still en route to the hospital ○ No standard approach or variable approach ○ Not applicable because ECG data not transmitted en route ○ Not applicable because ECG never performed en route ○ Unknown |
|
| C.46 | Expected interval between page and arrival of staff in catheterization laboratory? |
| ○ ≤20 min ○ 21–30 min ○ >30 min ○ No expected time ○ Unknown | |
| C.47 | Expected interval between page and arrival of interventional cardiologist |
| ○ ≤20 min ○ 21–30 min ○ >30 min ○ No expected time ○ Unknown | |
| C.48 | Someone is always available to transport patients from emergency department to catheterization laboratory? |
| ○ No ○ Yes ○ Unknown | |
| C.49 | Initiation of patient transport from emergency department to catheterization laboratory? |
| ○ After catheterization laboratory notifies emergency department it is ready ○ A set interval after the decision is made regarding PCI ○ No standard approach ○ Other approach ○ Unknown |
|
| C.50 | Minimum number of nurses and technicians required in catheterization laboratory before patient is transported from emergency department? |
| ○ Interventional cardiologist must be present ○ Interventional cardiologist may not be present but need presence of 1 staff person ○ Interventional cardiologist may not be present but need presence of 2-4 staff person ○ No set number ○ Unknown |
|
| C.51 | Elective catheterization cases rescheduled for emergency PCI? |
| ○ Yes ○ No ○ It depends ○ Unknown | |
| C.52 | If interventionalist is present, number of staff required to begin PCI? |
| ○ 1 ○ 2 ○ 3 ○ 4 ○ Unknown | |
| C.53 | Catheterization laboratory is left so that next PCI can begin promptly? |
| ○ Yes ○ No ○ No standard policy ○ Unknown | |
| C.54 | Cardiology fellows participate in performing PCI? |
| ○ No ○ Yes ○ Unknown | |
| C.55 | Staff in critical care area are routinely cross-trained to cover catheterization laboratory? |
| ○ No ○ Yes ○ Unknown | |
| C.56 | Location of catheterization laboratory? |
| ○ Elevator required to travel from emergency department ○ Same floor as emergency department |
|
| C.57 | An attending cardiologist is always at the hospital? |
| ○ No ○ Yes ○ Unknown | |
| C.58 | Informed Consent before primary PCI? |
| ○ Not necessary ○ Only orally obtained informed consent is needed ○ One written informed consent form is needed ○ More than one written informed consent form is needed ○ Unknown |
|
| C.59 | Prepayment before primary PCI? |
| ○ No ○ Yes, please specify the average amount approximately ___ (“−1” if unknown) ○ Unknown |
|
| C.60 | Does your hospital measure the following time intervals? [Check all that apply] |
| □ Door to ECG □ Door to needle □ Door to balloon □ None above □ Unknown |
|
| C.61 | Do your hospital feedback the time intervals to someone? [Check all that apply] |
| □ No □ Yes, to physician staff involved in the care □ Yes, to nursing staff involved in the care □ Yes, to pharmacy staff involved in the care □ Yes, to other staff involved in the care □ Unknown |
|
| C.62 | Do your hospital report the analyze results about the time intervals regularly? [Check all that apply] |
| □ No □ Yes, to departments involved in the care (the emergency department, the cardiology department) □ Yes, to other department in your hospital □ Yes, to other institutions outside your hospital □ Unknown |
|
| D. | Organizational learning characteristics |
| Instructions: This section focuses on the organizational learning and measurements to improve AMI care, including supportive environment and leadership, experimentation and training, knowledge acquisition, reflection and performance monitoring, etc. Please draw on your own experiences in your current role working with clinical staff and administration. For all questions, please reflect upon them during the 1-year period from 1/1/2011 to 12/31/2011. | |
| Although some questions in this section look similar, there are differences between them and you should treat each one as a separate question. The best approach is to answer each question fairly quickly. That is, don’t try to count up the number of times you felt a particular way, but rather indicate the alternative that seems most reasonable. | |
| The definition of “workgroup” below is the department, unit, ward, or group caring AMI patients that you are working at. | |
| This section adopts 7-point (from highly inaccurate to highly accurate). If you think the options are difficult to understand or distinguish, please grade the accuracy here using actual numbers, while 1 is the lowest (highly inaccurate), 7 is the highest (highly accurate), then choose the corresponding option. | |
| D.1 | In this workgroup, people value new ideas. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.2 | Clinicians are encouraged to creatively solve problems related to AMI care processes. (60) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.3 | Innovative ideas about AMI care are shared widely in the hospital. (61) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.4 | Differences in opinions are welcomed in this workgroup. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.5 | In this workgroup, people are open to alternative ways of getting work done. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.6 | People in this workgroup are eager to share information about what doesn’t work as well as to share information about what does work. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.7 | This workgroup frequently compares its performance to: Best-in-class organizations. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.8 | This workgroup frequently compares its performance to: Other similar workgroups. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.9 | This workgroup consistently collects information on technological trends. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.10 | If you make a mistake in this workgroup, it is often held against you. (Among clinicians taking care of patients with AMI, there is a tendency to blame individuals for errors in patient care). (66) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.11 | Clinicians caring for patients with AMI are easily able to address problems and tough issues with their department heads/chiefs. (56) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.12 | Department heads/chiefs are easily able to address problems and tough issues with senior level administration.(57) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.13 | Nurses are comfortable checking with physicians if they have concerns about patient care.(65) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.14 | Clinicians involved in the care of patients with AMI value each others’ skills and talents (e.g., physicians value nurses’ skills and talents and vice-versa).(58) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.15 | Clinicians involved in the care of patients with AMI avoid sharing responsibility for medical errors. ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always. (59) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.16 | Were physicians explicitly encouraged to disclose medical errors to patients or their family members? (7) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.17 | This workgroup engages in productive conflict and debate during discussions. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.18 | In this workgroup, we frequently identify and discuss underlying assumptions that might affect key decisions. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.19 | The hospital has the resources and information it needs to reduce 30-day mortality rates in patients with AMI. (51) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.20 | Senior-level administration is supportive of efforts to improve AMI care. (52) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.21 | There is simply no time for reflection in this workgroup. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.22 | In this workgroup, people are too busy to invest time in improvement. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.23 | My manager(s) establish(es) forums for and provide(s) time and resources for identifying problems and organizational challenges. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.24 | My manager(s) establish(es) forums for and provide(s) time and resources for reflecting and improving on past performance. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.25 | My manager(s) listen(s) attentively. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.26 | My manager(s) invite(s) input from others in discussions. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.27 | This workgroup experiments frequently with new product/service offerings. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.28 | This workgroup experiments frequently with new ways of working. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.29 | This workgroup frequently employs pilot projects or simulations when trying our new ideas. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.30 | This workgroup has a formal process for conducting and evaluating experiments or new ideas. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.31 | Experienced employees in this workgroup receive training when new initiatives are launched. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.32 | Experienced employees in this workgroup receive training when shifting to a new position. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.33 | Newly hired employees in this workgroup receive adequate training. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.34 | Did your hospital provide training to EMS providers about AMI care? (17) |
| ○ Yes, about monthly ○ Yes, about quarterly ○ Yes, about annually ○ Yes, other: _________ ○ No ○ Unknown |
|
| D.35 | This workgroup has forums for meeting with and learning from: Experts from outside the organization. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.36 | This workgroup has forums for meeting with and learning from: Experts from other departments/teams/divisions. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.37 | This workgroup has forums for meeting with and learning from: Customers/clients. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.38 | This workgroup regularly conducts post-audits, after-action reviews, and debriefings. |
| ○ highly inaccurate ○ inaccurate ○ somewhat inaccurate ○ not sure ○ somewhat accurate ○ accurate ○ highly accurate | |
| D.39 | Did your hospital have regular ‘morbidity and mortality’ conferences (or another educational session) for discussing individual cases involving patients with AMI? (5) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.40 | Did your hospital review the deaths of patients with AMI? (4a) |
| ○ No, we did not review these cases (go to D44) ○ Yes, we reviewed only deaths with potential quality issues (i.e., unexpected deaths) ○ Yes, we reviewed all deaths ○ Other, please specify: ___________ ○ Unknown |
|
| D.41 | Did your hospital have a designated person or group to review the deaths of patients with AMI (i.e., on an individual case level) that occurred during hospitalization? (4) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.42 | How long after the occurrence of the death were the cases typically reviewed? (4b) |
| ○ Within one week of the death ○ Within one month of the death ○ Within 3 months of the death ○ Other, please specify: ____________________________ ○ We did not have a set timeframe for reviewing these cases ○ Unknown |
|
| D.43 | Who usually reviewed these cases? (4c) |
| a. Senior management of the hospital ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always b. Cardiology chiefs ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always c. Nursing directors ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always d. Other physicians participating in the care of patients with AMI ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always e. Quality Improvement/Quality Management department staff ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always |
|
| D.44 | Did your hospital have a designated person or group to review any of the following adverse events in patients with AMI (i.e., on an individual case level)? (6) |
| a. Sentinel events (unexpected occurrence involving death or serious physical or psychological injury) that occurred during hospitalization ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always b. Unexpected transfers from a floor (non-monitored unit) to an intensive are unit ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always c. Catastrophic complications that occurred immediately after discharge from the hospital ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always |
|
| D.45 | How long after the occurrence of these adverse events were the cases typically reviewed? (6a) |
| ○ Within one week of the adverse event ○ Within one month of the adverse event ○ Within 3 months of the adverse event ○ Other, please specify: ____________ ○ We did not have a set timeframe for reviewing these cases ○ Unknown |
|
| D.46 | Who usually reviewed these cases? (6b) |
| a. Senior management of the hospital ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always b. Cardiology chiefs ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always c. Nursing directors ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always d. Other physicians participating in the care of patients with AMI ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always e. Quality Improvement/Quality Management department staff ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always f. Other, please specify: ___ |
|
| D.47 | Did your hospital use root cause analysis or a similar method to understand the following problems in AMI care? |
| a. Poor adherence to the core medication (i.e., anti-platelet agents) measures ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always b. Delay to fibrinolytic therapy or percutaneous coronary intervention (PCI) ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always |
|
| D.48 | Did your hospital review data on 30-day mortality rates (deaths occurring within 30 days of admission, including both inpatient and post-discharge deaths) in patients admitted with AMI (Check all that apply) (10) |
| □ Yes, through the medical insurance data system □ Yes, through a regional database system □ Yes, we internally collect our own data on deaths □ Yes, other, please specify: ___ □ No [Skip to D52] □ Unknown |
|
| D.49 | How quickly were mortality rates in patients with AMI available to your hospital (i.e., what was the most current data available to your hospital)? (10a) |
| ○ Within 6 months of care delivery ○ 6 months to 1 year after care delivery ○ 1 – 2 years after care delivery ○ Less frequently than 2 years of care delivery ○ Unknown |
|
| D.50 | Did your hospital regularly compare its performance to other hospitals on either inpatient in patients with AMI? (14) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.51 | Did your hospital have efforts to improve any of the following inpatient acute myocardial infarction (AMI) quality measures? (1) |
| a. Adherence to the core medication (i.e., anti-platelet agents) measures ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always b. Time to fibrinolytic therapy or percutaneous coronary intervention (PCI) ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always |
|
| D.52 | Beyond these quality measures, did your hospital initiate efforts to improve any of the following in patients admitted with AMI? (2) |
| a. Inpatient mortality in patients with AMI ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always b. Post-discharge mortality (death occurring after discharge, but within 30 days of admission) in patients with AMI ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always c. Readmission within 30 days from prior admission in patients with AMI ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always |
|
| D.53 | Did your hospital have a quality improvement team(s) devoted to improving: (3) |
| a. Inpatient mortality in patients with AMI ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always b. Post-discharge mortality (death occurring after discharge, but within 30 days of admission) in patients with AMI |
|
| D.54 | 3a. Please indicate members of either the inpatient or post-discharge mortality team(s). |
| a. Senior management of the
hospital ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always b. Hospital governing board ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always c. Chief of cardiology ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always d. Nursing directors ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always e. Other physicians participating in the care of patients with AMI ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always f. Quality Improvement/Quality Management department staff ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always g. Other please specify: ________________________ |
|
| D.55 | Nurses are engaged in efforts to improve AMI care. (53) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.56 | Cardiologists are engaged in efforts to improve AMI care. (54) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.57 | Emergency medicine physicians are engaged in efforts to improve AMI care. (55) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.58 | Did your hospital have one or more physician champions focused on improving either inpatient or 30-day mortality in patients with AMI? (12) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.59 | Did your hospital have one or more nurse champions focused on improving either inpatient or 30-day mortality in patients with AMI? (13) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.60 | After we make changes to improve AMI care, we fail to evaluate their effectiveness. (67) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.61 | Did cardiology and emergency department staff meet together to review care for patients with AMI? (15) |
| ○ Yes, about monthly ○ Yes, about quarterly ○ Yes, about annually ○ Yes, other: ____________________ ○ No [Skip to D63] ○ Unknown |
|
| D.62 | What was typically discussed at these meetings? (15a). |
| a. Care of patients with ST-elevation myocardial infarction (STEMI) ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always b. Care of patients with Unstable Angina/non-STEMI (NSTEMI) ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always c. Care of patients with chest pain, in general ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always |
|
| D.63 | Did clinicians from your hospital meet with emergency medical system (EMS) providers to review the care of patients with AMI? (16) |
| ○ Yes, about monthly ○ Yes, about quarterly ○ Yes, about annually ○ Yes, other: ____________________ ○ No ○ Unknown |
|
| D.64 | There is good coordination among the different departments involved with the care of patients with AMI. (62) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.65 | Departments caring for patients with AMI (e.g., cardiology, emergency medicine) communicate easily with each other.(64) |
| ○ Yes, about monthly ○ Yes, about quarterly ○ Yes, about annually ○ Yes, other: ____________________ ○ No [Skip to D63] ○ Unknown |
|
| D.66 | Clinicians caring for patients with AMI share new evidence-based approaches with the AMI team.(63) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
| D.67 | Which best describes the quality of your interaction with hospitals that referred patients to you with AMI?(18) |
| ○ Very collaborative (we shared data along with strategies for improving AMI care) ○ Somewhat collaborative (we communicated regularly, but we did not share data and strategies) ○ Not collaborative (we had no or minimal contact with the referring hospital/s) ○ Not applicable [Skip to D69] |
|
| D.68 | Did your hospital routinely give feedback to the referring hospital/s on any of the following? (18a.) |
| a. Time to transfer ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always b. AMI-related procedures performed ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always c. Patient outcome ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always d. Other please specify: _________________________ |
|
| D.69 | Which best describes the quality of your interaction with hospitals that you referred patients to with AMI? (19) |
| ○ Very collaborative (we shared data along with strategies for improving AMI care) ○ Somewhat collaborative (we communicated regularly, but we did not share data and strategies) ○ Not collaborative (we had no or minimal contact with hospitals in our region) ○ Not applicable |
|
| D.70 | Was your hospital part of a regional effort or consortium of hospitals to improve AMI care? (20) |
| ○ Never ○ Rarely ○ Sometimes ○ Usually ○ Always | |
D. Validation of Acute Myocardial Infarction (AMI) Type (ST-segment elevation myocardial infarction or non ST-segment elevation myocardial infarction)
A total of 300 medical records were randomly selected and examined by a senior cardiologist from the Yale Center for Outcomes Research and Evaluation. The review aimed at determining the concordance between the abstracted results of AMI subtypes and the first available electrocardiogram (ECG) or ECG description in medical records. Our review showed that there was a 94·7% concordance in the selected cases. Details of the results are shown in the following table.
| Review Results of AMI Types | N (%) |
|---|---|
| Consistent | 284 (94.7%) |
| Consistent with ECG graph | 246 (82.0%) |
| Consistent with ECG description in records | 38 (12.7%) |
| Inconsistent | 12 (4.0%) |
| Unavailable (either ECG graph or ECG description in medical records) | 4 (1.3 %) |
E. Ideal Candidates for the Treatments
For the reperfusion therapy, we included patients who were admitted within 12 hours of symptom onset and did not receive reperfusion therapy before hospital presentation. Then we excluded patients without any contraindications (history of hemorrhagic stroke, active bleeding at presentation, or any other physician documented contraindications for fibrinolytic therapy if the patient was treated in non-percutaneous coronary intervention(PCI) capable hospital; allergy to contrast agents or any other documented contraindication to PCI if the patient was treated in PCI-capable hospital).
For aspirin, we excluded patients with any contraindications for aspirin (allergy to aspirin, active bleeding on admission, history of hemorrhagic stroke, or other documented contraindications).
For clopidogrel, we excluded patients who participated the ClOpidogrel and Metoprolol in Myocardial Infarction Trial(COMMIT) or patients with any contraindications for clopidogrel (allergy to clopidogrel, active bleeding on admission, history of hemorrhagic stroke, or other documented contraindications).
For beta-blockers, we excluded patients who participated the ClOpidogrel and Metoprolol in Myocardial Infarction Trial(COMMIT) or patients with any contraindications for beta-blockers (allergy to beta-blockers, cardiogenic shock on admission, heart failure on admission, second or third degree atrioventricular block with no pacemaker implanted, systolic blood pressure <100mmHg on admission, bradycardia [heart rate <60 beats/min] on admission without taking a beta-blocker, or other documented contraindications).
For angiotensin converting enzyme(ACE) inhibitors or angiotensin receptor blockers(ARB), we excluded patients with any contraindications for ACE inhibitors (allergy to ACE inhibitors, hyperkalemia (serum potassium >5·5mmol/L during hospitalization), creatinine >265 umol/L during hospitalization, pregnancy or breast feeding, or other documented contraindications).
For statins, we excluded patients with any contraindications for statins (allergy to statins).
F. Definition of In-hospital Complications
1). Re-infarction
Indicate if there is physician documentation of recurrent myocardial infarction during hospitalization.
2). Cardiogenic shock
Indicate if there is physician documentation of cardiogenic shock during hospitalization.
3). Ischemic stroke
Indicate if there are physician documentations of new-onset ischemia stroke and stroke-related symptoms during hospitalization. The stroke-related symptoms include: trouble walking/loss of balance/incoordination, one-sided numbness or hemi-anesthesia, one-sided facial numbness or hemi-anesthesia, mouth askew and drooling, dysarthria or slurred speech, loss of vision or blurred version in one or both eyes, dizziness with vomiting, severe headache and vomiting, unconsciousness, and hyperspasmia.
4). Congestive heart failure
Indicate if there is physician documentation of heart failure during hospital stay. This include those without a history of heart failure but develop heart failure during hospitalization, and those with a history of heart failure as a chronic comorbidity and develop worsening heart failure during hospitalization.
G. Proportions of Patients Ideal for Treatments by Year
| Treatment | 2001 |
2006 |
2011 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Rural | Urban | p value | Rural | Urban | p value | Rural | Urban | p value | |
| Reperfusion therapy | 46% | 46% | 0.8 | 50% | 44% | 0.03 | 54% | 45% | <0.001 |
| Aspirin≤ 24 h | 98% | 98% | 0.4 | 98% | 98% | 0.2 | 98% | 97% | 0.3 |
| Clopidogrel ≤ 24 h | 94% | 90% | 0.01 | 98% | 98% | 0.4 | 98% | 98% | 0.06 |
| Beta-blockers ≤ 24 h | 46% | 40% | 0.01 | 46% | 43% | 0.2 | 46% | 48% | 0.3 |
| Statins | 100% | 100% | - | 100% | 100% | - | 100% | 100% | 0.4 |
| ACE inhibitors/ARB | 97% | 97% | 0.9 | 98% | 96% | 0.05 | 98% | 97% | 0.04 |
ACE: angiotensin converting enzyme, ARB: angiotensin receptor blockers.
H. Differences in risk adjusted rate of 7-day death or treatment withdrawal between rural and urban hospitals over time
I. Survival plot in Cox regression on in-hospital death or withdrawal from treatment between urban and rural hospitals
J. Searching strategy in PubMed
((urban[Title/Abstract] OR rural[Title/Abstract]) AND (“registry”[Title/Abstract] OR “Health Care Quality, Access, and Evaluation”[Mesh] OR “treatment”[Title/Abstract] OR “treatments”[Title/Abstract] OR “therapy”[Title/Abstract] OR “therapies”[Title/Abstract] OR “therapeutic”[Title/Abstract] OR “care”[Title/Abstract] OR “healthcare”[Title/Abstract] OR “pattern”[Title/Abstract] OR “patterns”[Title/Abstract] OR “quality”[Title/Abstract] OR “disparities”[Title/Abstract] OR “disparity”[Title/Abstract] OR “gap”[Title/Abstract] OR “gaps”[Title/Abstract] OR “measure”[Title/Abstract] OR “measurements”[Title/Abstract] OR Inequity[Title/Abstract] OR equity[Title/Abstract]) AND “myocardial infarction”[Mesh] AND (“2000/01/01”[PDAT] : “2015/6/30”[PDAT]) AND (“humans”[MeSH Terms] AND “adult”[MeSH Terms]) NOT (gene[Title/Abstract] OR mutation[Title/Abstract] OR rat[Title/Abstract] OR mice[Title/Abstract] OR cell[Title/Abstract] OR Molecular[Title/Abstract]))
Footnotes
DISCLOSURES
There are no relevant conflicts of interest. Dr. Krumholz reports contract with Centers for Medicare & Medicaid Services to develop and maintain performance measures, research agreements with Johnson & Johnson (Janssen) and Medtronic to develop methods of clinical trial data sharing, personal fees from UnitedHealth as chair of cardiac scientific advisory board. Dr. Masoudi reports contract with American College of Cardiology for the role as Senior Medical Officer, National Cardiovascular Data Registries.
REFERENCES
- 1.Scheil-Adlung X Global evidence on inequities in rural health protection: New data on rural deficits in health coverage for 174 countries. Geneva: International Labour Organization, 2015. [Google Scholar]
- 2.Baldwin LM, Chan L, Andrilla CH, Huff ED, Hart LG. Quality of care for myocardial infarction in rural and urban hospitals. J Rural Health 2010; 26(1): 51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sheikh K, Bullock C. Urban-rural differences in the quality of care for medicare patients with acute myocardial infarction. Arch Intern Med 2001; 161(5): 737–43. [DOI] [PubMed] [Google Scholar]
- 4.Keeler EB, Rubenstein LV, Kahn KL, et al. Hospital characteristics and quality of care. JAMA 1992; 268(13): 1709–14. [PubMed] [Google Scholar]
- 5.Joynt KE, Orav E, Jha AK. Mortality rates for medicare beneficiaries admitted to critical access and non–critical access hospitals, 2002–2010. JAMA 2013; 309(13): 1379–87. [DOI] [PubMed] [Google Scholar]
- 6.Bhuyan SS, Yang W, Opoku S, Ge L. Rural-urban differences in acute myocardial infarction mortality: Evidence from Nebraska. Journal of Cardiovascular Disease Research 2013; 4(4): 209–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vu HD, Heller RF, Lim LL-Y, D’Este C, O’Connell RL. Mortality after acute myocardial infarction is lower in metropolitan regions than in non-metropolitan regions. Journal of Epidemiology and Community Health 2000; 54(8): 590–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Svensson L, Karlsson T, Nordlander R, Wahlin M, Zedigh C, Herlitz J. Safety and delay time in prehospital thrombolysis of acute myocardial infarction in urban and rural areas in Sweden. Am J Emerg Med 2003; 21(4): 263–70. [DOI] [PubMed] [Google Scholar]
- 9.Ministry of Health of People’s Republic of China. Report about Some Policy Issues on the Healthcare Reform. 1985.
- 10.Bo J Yanfeng Ge: Reflection on the Healthcare Reform in China. China Economic Times. 2005. June-6. [Google Scholar]
- 11.Ministry of health of the people’s republic of China. China public health statistical yearbook 2004 Beijing: Peking union medical college publishing house; 2004. [Google Scholar]
- 12.Meng Q, Xu L, Zhang Y, et al. Trends in access to health services and financial protection in China between 2003 and 2011: a cross-sectional study. Lancet 2012; 379(9818): 805–14. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z Launch of the health-care reform plan in China. Lancet 2009; 373(9672): 1322–4. [DOI] [PubMed] [Google Scholar]
- 14.Ministry of Health of the People’s Republic of China. China public health statistical yearbook 2012. Beijing: Peking union medical college publishing house; 2012. [Google Scholar]
- 15.China Society of Cardiology of Chinese Medical Association, Editorial Board of Chinese Journal of Cardiology. Guideline for diagnosis and treatment of patients with ST-elevation myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi 2010; 38(8): 675–90. [PubMed] [Google Scholar]
- 16.O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. JACC 2013; 61(4): e78–e140. [DOI] [PubMed] [Google Scholar]
- 17.Bradley EH, Curry LA, Spatz ES, et al. Hospital strategies for reducing risk-standardized mortality rates in acute myocardial infarction. Ann Intern Med 2012; 156(9): 618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nallamothu BK, Normand SLT, Yongfei W, et al. Relation between door-to-balloon times and mortality after primary percutaneous coronary intervention over time: a retrospective study. Lancet 2015; 385(14): 1114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dharmarajan K, Li J, Li X, Lin Z, Krumholz HM, Jiang L. The China Patient-centered Evaluative Assessment of Cardiac Events (China PEACE) Retrospective Study of Acute Myocardial Infarction: Study Design. Circ Cardiovasc Qual Outcomes 2013; 6(6): 732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Bureau of Statistics of China. China Statistical Yearbook 2012. Beijing: China: Statistics Press; 2012. [Google Scholar]
- 21.Simms AD, Reynolds S, Pieper K, et al. Evaluation of the NICE mini-GRACE risk scores for acute myocardial infarction using the Myocardial Ischaemia National Audit Project (MINAP) 2003–2009: National Institute for Cardiovascular Outcomes Research (NICOR). Heart 2013; 99(1): 35–40. [DOI] [PubMed] [Google Scholar]
- 22.Antman EM, Hand M, Armstrong PW, et al. 2007. focused update of the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Group to Review New Evidence and Update the ACC/AHA 2004 Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction). Circulation 2008; 117(2): 296–329. [DOI] [PubMed] [Google Scholar]
- 23.Ministry of Health of the People’s Republic of China. The qualification standard of secondary general hospital (version 2012). 2012. http://www.nhfpc.gov.cn/yzygj/s3586q/201201/b8dda05b1d23413c94150b5c17b5cc6f.shtml (accessed Mar 6 2014).
- 24.Joynt KE, Harris Y, Orav EJ, Jha AK. Quality of care and patient outcomes in critical access rural hospitals. JAMA 2011; 306(1): 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris DE, Aboueissa AM, Hartley D. Myocardial infarction and heart failure hospitalization rates in Maine, USA - variability along the urban-rural continuum. Rural and remote health 2008; 8(2): 980. [PubMed] [Google Scholar]
- 26.Baldwin LM, MacLehose RF, Hart LG, Beaver SK, Every N, Chan L. Quality of care for acute myocardial infarction in rural and urban US hospitals. J Rural Health 2004; 20(2): 99–108. [DOI] [PubMed] [Google Scholar]
- 27.Kilbourne BJ, Levine RS, Lambert W, Brown D, Hennekens CH. Geographic Variations in Percutaneous Coronary Interventions and Coronary Artery Bypass Graft Surgery Among Tennessee Elders. Southern medical journal 2011; 104(6): 389–96. [DOI] [PubMed] [Google Scholar]
- 28.Loslier J, Vanasse A, Niyonsenga T, Courteau J, Orzanco G, Hemiari A. Myocardial infarction in Québec rural and urban populations between 1995 and 1997. Canadian journal of rural medicine : the official journal of the Society of Rural Physicians of Canada = Journal canadien de la medecine rurale : le journal officiel de la Societe de medecine rurale du Canada 2007; 12(2): 95. [PubMed] [Google Scholar]
- 29.Hassan A, Pearce NJ, Veugelers P, Hirsch G, Cox J The effect of place of residence on access to invasive cardiac services following acute myocardial infarction. Canadian Journal of Cardiology 2009; 25(4): 207–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herrin J, Miller LE, Turkmani DF, et al. NAtional performance on door-in to door-out time among patients transferred for primary percutaneous coronary intervention. Archives of Internal Medicine 2011; 171(21): 1879–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ministry of Health of People’s Republic of China. National Essential Drug List (Version 2009). Beijing; 2009. [Google Scholar]
- 32.Ministry of Human Resources and Social Security of the People’s Republic of China. Notification on drug lists for national basic medical insurance, occupational injury insurance, and maternity insurance. 2009. http://www.mohrss.gov.cn/gkml/xxgk/201407/t20140717_136141.htm (accessed Sept 30 2015).
- 33.Chung S-C, Gedeborg R, Nicholas O, et al. Acute myocardial infarction: a comparison of short-term survival in national outcome registries in Sweden and the UK. Lancet 2014; 383(9925): 1305–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu T, Ni J, Wei J. Danshen (Chinese medicinal herb) preparations for acute myocardial infarction. Cochrane database syst Rev 2008; (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ministry of Health of the People’s Republic of China. China public health statistical yearbook 2013. Beijing: Peking union medical college publishing house; 2013. [Google Scholar]
- 36.Li J, Li X, Wang Q, et al. ST-segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE-Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet 2015; 385: 441–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang L, Krumholz HM, Li X, Li J, Hu S. Achieving best outcomes for patients with cardiovascular disease in China by enhancing the quality of medical care and establishing a learning health-care system. The Lancet 2015; 386(10002): 1493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.James P, Li P, Mm. Myocardial infarction mortality in rural and urban hospitals: Rethinking measures of quality of care. Annals of Family Medicine 2007; 5(2): 105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]