Abstract
Background
A role of Vitamin D in brain development and function has been gaining support over the last decade. There are compelling pieces of evidence that suggest vitamin D may have a neuroprotective role. The administration of vitamin D or its metabolites has been shown to reduce neurological injury and/or neurotoxicity in a variety of animal systems. The detail biochemical mechanism mediating neurons, to its ability to withstand greater oxidative stress in the presence of Vitamin D is unclear. This study was undertaken to study the biochemical effect of treatments of primary cortical neuronal cultures, with the active form of vitamin D(1,25(OH)2D3), against the induced oxidative stress.
Methods
Primary neuronal cultures from cerebral cortex were set up from neonatal (from 6 to 7 days old) Wister Rat's brain. Different doses of [1,25(OH)2D3], ranges from 0 to 1 μg/ml, was added to the culture medium and the cells were cultured in its presence for 24 h to 120 h. The effect of induced extracellular oxidative stress was measured by subjecting these cultured cells with 0.5 mM H2O2 for 2 h, prior to collection of condition medium and the cell pellet for biochemical assay. The control and H2O2 treated cultures were maintained in similar culture conditions, for similar periods of time without any [1,25(OH)2D3] treatments.
Result
The optimum concentration of [1,25(OH)2D3] for treatment of primary cortical neuronal cultures was found to be 0.25 μg/ml by Trypan exclusion assay and MTT assay. Pre-treatments of cultured neuronal cells with 0.25 μg/ml of [1,25(OH)2D3] caused significantly increased levels of reduced glutathione, accompanied by a similar increase in the enzyme levels of GST, to neutralize the induced oxidative stress by H2O2. The level of Lipid peroxidation was significantly higher in the cells treated with H2O2 alone, but it was completely reversed in the neuronal cultures pre-treated with [1,25(OH)2D3]. The levels of Catalase enzyme also significantly reduced (≥0.05) in the [1,25(OH)2D3] pre-treated neuronal cultures.
Conclusion
We concluded that the systemic treatment of primary neuronal cultures with [1,25(OH)2D3] gave better protection to neurons against the induced oxidative stress, as shown by quantitative measurements of various biomarkers of oxidative stress. This study also suggested that Vitamin D is vital for the growth, survival, and proliferation of the neurons and hence it has a potential therapeutic role against various neurodegenerative diseases.
Highlights
-
•
In-vitro studies here indicated that active metabolite of vitamin D3 helps in proliferation and health of neurons.
-
•
The neuronal protection against oxidative stress was mediated by up-regulating reduced GSH and antioxidants enzymes GST & catalase.
1. Introduction
Oxidative stress, often described as the disruption of the balance between the pro-oxidant and anti-oxidants due to the excessive accumulation of reactive oxygen species (ROS). The central nervous system is highly vulnerable to these ROS, due to its high energy demand and its high metabolic rate, in contrast to its low levels of the antioxidant defense system and its reduced capacity of cellular regeneration. In case of Parkinson's disease(PD), Alzheimer's Disease(AD) and Amyotrophic Lateral Sclerosis(ALS), several indices of ROS damage have been reported within the specific brain regions that undergo selective neurodegeneration. For example, the markers of Lipid peroxidation including 4-hydroxynonenal(4-HNE)and malondialdehyde(MDA), have been identified in the cortex and hippocampus of patients with an AD, the substantial Nigra of patients with PD and in spinal fluid from patients with ALS [[1], [2], [3], [4]]. Protein nitration, a marker of protein oxidation, has been demonstrated to be elevated in the hippocampus and neocortex of individuals with an AD, in Lewy bodies in case of PD and within motor neurons in ALS [5]. In light of the depilating effect of ROS in the pathophysiology of a brain, there is an urgent need to explore the therapeutic potential of various antioxidants. One such antioxidant which is discovered recently is Vitamin D. The role of Vitamin D in the brain development and function of neurons was suggested when the first evidence of 1α-hydroxylase an enzyme responsible of an active form of Vitamin D and receptors for vitamin D was found to be present in the brain [6]. The specific mechanisms that mediate the neuroprotective effect of vitamin D are still unclear; however, vitamin D may act in many pathways including antioxidant pathways, neuronal calcium regulation, immunomodulation and glutamatergic systems [[7], [8], [9], [10]]. A study on the biologically active vitamin D3 metabolite, Calcitriol, added to the rat primary dopamine neurons, showed a dose response increase in numbers of these neurons due to the upregulations of glial derived neurotrophic factor(GDNF) [11,12]. In the present study, the antioxidant potency of 1,25(OH)2D3 had been studied in detail using an in-vitro model of neuronal damage caused by induced oxidative stress by exposure to hydrogen peroxide. The therapeutic potential of active Vitamin D3 metabolite, on the primary cortical neurons, was investigated in detail by quantitative measurements of major antioxidants enzyme systems.
2. Method
2.1. Setting up primary cortical cell culture
Five neonatal Wister Albino rats of about 6 to 7 days old (with average weight of 20 g) were used for this study. The rats were kept at a facility of King Saud university research Centre under the strict guidelines provided by the Experimental Animal Laboratory and approved by the animal care and use committee at the College of Applied Medical Sciences at King Saud University. All procedures dealing with animals were followed in accordance with the standard ethically approved protocol.
The brains of the rats were removed immediately after being anesthetized and decapitated in a sterile condition. The meninges were removed and the midbrain (cortex) was isolated and rinsed with sterile Phosphate Buffer Saline (PBS) pH 7.4, and finely minced into small pieces. The minced tissue was then incubated with Papain solution (2 mg/ml) for 20 min at 37 °C as described previously [13].The cell suspension was filtered through the cell strainer and cell debris and big tissues were discarded. The cell suspension was centrifuged for 300 ×g for 10 min, the supernatant was discarded and the cell pellet was suspended in plating medium DMEM F12 with glutamine (Sigma Aldrich) + 10%FCS + 100 units/ml penicillin + 0.1 mg/ml of streptomycin. The cell pellet was washed twice in this medium and after that, the cells were counted using Bio-Rad automated cell counter. The cells were plated onto 12well cell culture dishes (Millipore Company) with a plating density of 5 × 106 cells/ml.
2.2. Treatment of cortical cells in culture
Different doses of [1,25(OH)2D3] that is (0.25 μg/ml, 0.5 μg/ml, 0.75 μg/ml and 1 μg/ml) was added to the cells in cultured medium just after plating. The cells were treated with different doses of [1,25(OH)2D3] in triplicate and cultured for up to 120 h. At the end of each set of incubation periods, the cells were briefly induced oxidative stress, by treating with 0.5 mM H2O2 for 2 h. The control and H2O2 treated cultures were grown without any treatments of [1,25(OH)2D3]. All the cultures were maintained in DMEM F12 medium with glutamine (Sigma Aldrich) + 10%FCS + 100 units/ml penicillin + 0.1 mg/ml of streptomycin for the selected time periods at 37 °C in an atmosphere of 95% air and 5% carbon dioxide.
2.2.1. Trypan blue exclusion assay
The cells were grown in 12 well plates, with different treatments and the cell viability is determined at different time points that is 24 h, 48 h, 72 h and 120 h by trypan blue exclusion assay. Triplicate wells of a viable cell were counted on an automated counter (BIO-RAD TC10).
2.2.2. MTT assay
For the MTT assay the MTT cell growth Kit from Millipore (CT02) was employed. The cells were plated on a 96well plates with a cell density of 1 × 104/well and treated with various concentration of [1,25(OH)2D3] that contains 100 μl of DMEM F12 medium +100 units of penicillin and streptomycin. Wells with culture medium without FBS was taken as negative control. The cells were grown for 24 h, after that they were treated with 0.5 mM H2O2 for 2 h. The control cells were plated in the same conditions without any treatments. After this 10 μl of MTT solution (2-5dipheyltetra sodium bromide in PBS) was added, and incubated in its presence for 4 h. 100 μl of isopropanol in 0.04 N HCl was added to each well. The absorbance was read at 530 nm within one hour. Each sample was done in triplicate wells.
2.2.3. Reduced glutathione assay
The estimation was carried out by the method of Beutler et al. [14]. 1 ml of cell homogenate and 1 ml of conditioned medium was used for this assay. 1.5 ml of double distilled water was added to the tissue homogenate, and conditioned medium, and treated with 0.6 ml of precipitating reagent (containing 1.67 g of glacial metaphosphoric acid, 0.2 g of EDTA and 30.0 g of NaCl made up to 100 ml with double distilled water). The above reaction mixture was centrifuged at 1200 ×g for 10 min. To 0.3 ml of supernatant, 2 ml of Na2HPO4 (0.3 M) and 0.25 ml of 5,5′ dithio-bis-2-nitrobenzoic acid (DNTB)(0.4% in 1% sodium citrate) were added, and volume was made up to 3 ml with DDW. OD was read at 412 nm against blank. Values were expressed as μg of reduced glutathione/No of cells present.
2.2.4. Lipid peroxidation assay
Lipid peroxidation was calculated using the method of Garcia et al. [15], using the TBARS (Thiobarbiturate reactive substances) assay. 1 ml of Brain homogenate was incubated in a metabolic shaker at 37 °C for one hour. 1.5 ml of 20% TCA was added, and centrifuged at 600 ×g for 10 min. 1 ml of freshly prepared TBA (0.67%) was added to 1 ml of the supernatant, and the reaction mixture was heated in a boiling water bath for 10 min. Absorbance was read at 535 using a reagent blank. Values were expressed as expressed as mMoles of Malondialdehyde formed hour/ No of cells.
2.2.5. Catalase assay
Catalase activity was estimated in the cell homogenate, as well as a conditioned medium by the method of Aebie, 1984 [16]. The reaction mixture in a total volume of 3 ml contained 0.4 M sodium phosphate buffer (pH 7.2). The reaction was started by adding 1.2 ml of H2O2 and reading the change in absorbance at 240 nm for 2 min. One unit of CAT activity was defined as micromole of H2O2 decomposed per minute using the molar coefficient of H2O2 (43.6 M−1C−1).
2.2.6. Glutathione S transferase assay
The activity of Glutathione S-transferase was assayed in a reaction mixture containing 100 mM phosphate buffer, pH 6.5, 1 mM 1-Chloro-2,4-dinitrobenzene (CDNB) and 1 mM reduced GSH. The reaction was initiated by adding 10 μl of Cell lysate and formation of S-(2, 4-dinitrophenyl) glutathione (DNP-GSH) was measured spectrophotometrically as Units per minute per 5 × 106 cells [17].
2.2.7. Statistical analysis
All data are expressed as the ± Standard deviation (SD) of at least three independent replicate experiments. Students' t-test was performed to assess the difference between the control and treated groups. Comparison between the Control, H2O2 treated and vitamin D treated groups of cells were made using two-way analysis of variance (ANOVA) and Tukey's post hoc test. Values of ˂0.05 were considered to be significant.
3. Results
3.1. Effect of vitamin D treatments on the cell viability
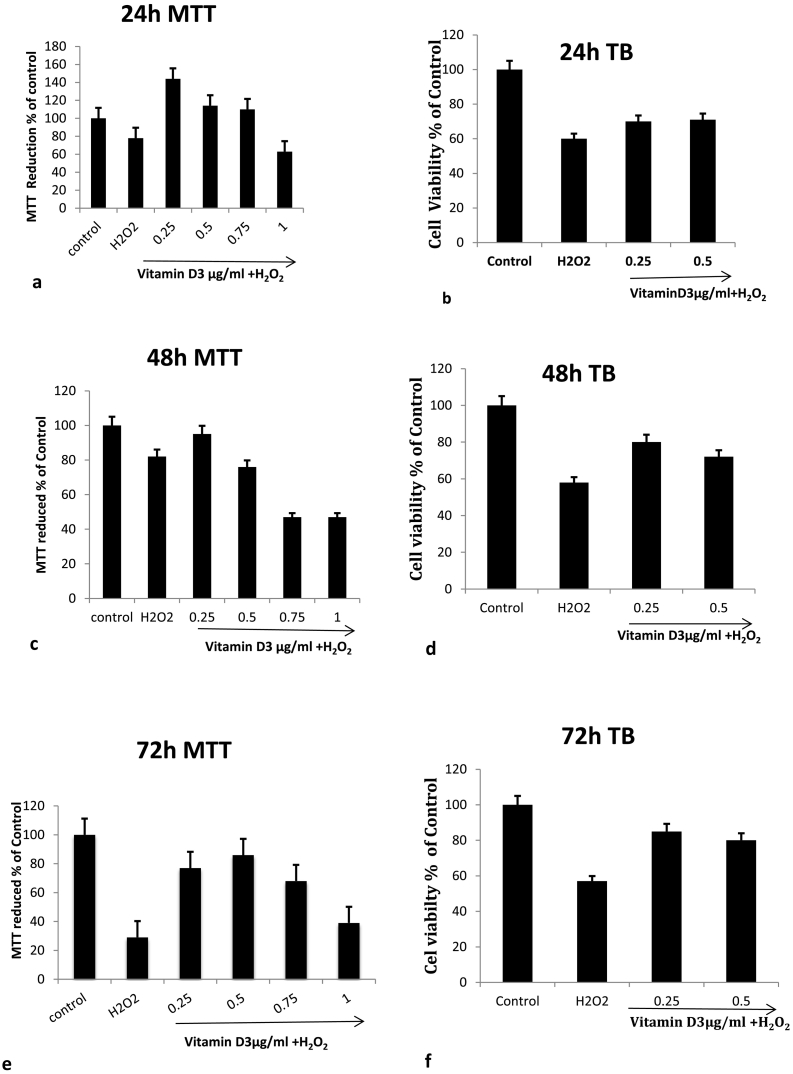
The cortical neurons were cultured on 96 well plates with a cell density of 1 × 104 cells/well for 24 h, 48 h and 72 h in the presence and absence of varying doses of [1,25(OH)2D3] ranges from 0 to 1 μg/ml. After each incubation period, the cells were subjected to oxidative stress, by adding 0.5 mM of H2O2 to the culture medium for 2 h. Cell viability was measured by MTT assay as well as trypan blue exclusion assay by counting them on the automated counter as shown in Fig. 1. Both of these data showed that the optimum treatment dose was 0.25 μg/ml. Hence this dosage was used subsequently in the latter experiments. MTT assay showed that the treatment with 0.25 μg/ml of [1,25(OH)2D3] resulted in the greater protection of the neurons against the induced oxidative stress by H2O2 when compared to the H2O2 treated alone samples. These results were further supported by Trypan blue exclusion assay in which the total viability of the cells was counted after 24 h, 48 h and 72 h of the treatment with the 0.25 μg/ml and 0.5 μg/ml of [1,25(OH)2D3]. The cells which were treated with H2O2 alone lost about 40% of cell viability, as compared to the control cells. However, when the cells were pretreated with [1,25(OH)2D3], they showed improved cell viability.
Fig. 1.
Effect of Different doses of Vitamin D treatments on the viability of primary cortical neuronal cells after induced oxidative stress by 0.5mMH2O2 for 2 h after growing them in the presence and absence of [1,25(OH)2D3] for 24 h, 48 h and 72 h. The cell viability was measured either by MTT reduction for 24 h, 48 h and 72 h(a, c, e) or by Trypan blue exclusion assay (b, d, f). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
These results suggested that systemic pre-treatments with [1,25(OH)2D3] to the neuronal cultures protected them, against induced oxidative stress apoptosis.
3.1.1. Effect of vitamin D treatments on lipid peroxidation levels
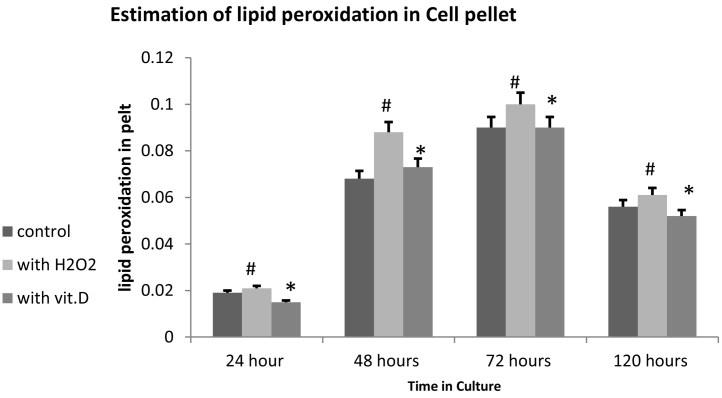
Cells under oxidative stress have a greater amount of lipid peroxidation, which can be measured by TBRAS assay, which detects the amount of Malondialdehyde (MDA) formed in the cell pellet. Our results showed that the Cells which were treated with the H2O2 alone, showed significantly increased amount of MDA. However the neuronal cells cultured for 24 h to 120 h, in the presence of [1,25(OH)2D3] showed greater tolerance to the H2O2 induced oxidative stress, as exhibited by significantly reduced MDA formation(Fig. 2).
Fig. 2.
Estimation of Lipid peroxidation in the cell pellet after culturing the primary cortical neuron cells in the presence and absence of 0.25 μg/ml of [1,25(OH)2D3] for 24 h, 48 h, 72 h and 120 h, after that they were induced oxidative stress by exposure to 0.5 mM H2O2 for 2 h. Cell pellet was isolated and of Lipid peroxidation was estimated as described in Materials and Methods. (*) represent the values of [1,25(OH)2D3] treated samples which were significantly different (≥0.05) from the H2O2 treated alone samples.(#) represent the significant different (≥0.05) values of H2O2 treated samples compared to the control samples.
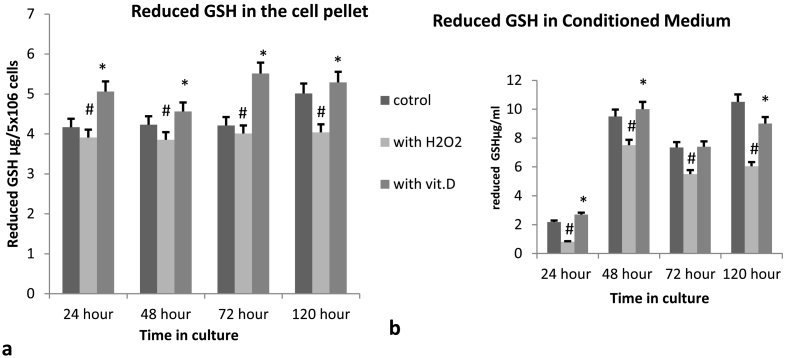
3.1.2. Effect of vitamin D on reduced glutathione levels
In order to elucidate, the state of antioxidants enzyme mechanisms, through which [1,25(OH)2D3] might confers neuroprotection. We measured the total amount of reduced GSH both, in the cell pellet as well as the conditioned medium, after various time points. Reduced GSH present in the cell pellet as well as in the conditioned medium at 24 h was elevated when compared to the H2O2 treated alone and control Cells. The same elevation of reduced GSH was observed in the cell cultures that were pretreated with [1,25(OH)2D3] (0.25 μg/ml) for 48 h, 72 h and 120 h. While the control and H2O2 treated Cells had reduced GSH levels (Fig. 3). These results further confirmed earlier studies, that one of the mechanisms through, which [1,25(OH)2D3] protects the neurons was by upregulation of antioxidant enzyme defense systems [18].
Fig. 3.
Estimation of reduced Glutathione from Cell pellet and conditioned medium derived from primary cortical neuron cultures grown in the presence and absence of 0.25 μg/ml,[1,25(OH)2D3] for 24 h, 48 h, 72 h and 120 h, after that they were exposed to 0.5 mM of H2O2 for 2 h. Conditioned medium and cell pellet was separated by centrifugation. Reduced GSH was estimated in both conditioned medium and the cell pellet as described in the methods section. (*) represent the values of Vitamin D treated groups which are significantly (≥0.05) different from the H2O2 treated alone samples. Each reading is an average of three readings from three independent sets of experiments. (#) represent the H2O2 treated samples significantly different to the control groups.
3.1.3. Effect of vitamin D on catalase enzyme
Catalase is an active enzyme which can neutralize the effect of H2O2 by converting them to water. This enzyme is the main antioxidant enzyme present in the cells which fight against the oxidative stress by rapidly breaking down H2O2 produced into the water, thus alleviating its harmful effects. The level of catalase enzyme was measured in the [1,25(OH)2D3] treated cultures for 24 h, 48 h, 72 h and 120 h. We found that the Catalase enzyme activity in the cell pellet of the [1,25(OH)2D3] treated cell cultures, was found to be reduced significantly (Fig. 4). The same observation was found in the cell pellet of the cultures derived from 48 h, 72 h and 120 h of treatment with [1,25(OH)2D3], and exposed to H2O2 induced oxidative stress, thus indicating that [1,25(OH)2D3] treatments protected the neurons from induced oxidative stress, as revealed by low levels of catalase enzyme being present. The neuronal cultures exposed to H2O2, without any pretreatments of [1,25(OH)2D3] showed increased amounts of catalase enzyme, (Fig. 3) indicating that the neurons were under direct exposure to oxidative stress by hydrogen peroxide with no protection, so increased amount of catalase enzyme released to neutralize its effect.
Fig. 4.
Estimation of Catalase enzyme and GST enzyme activity as measured in the cell pellet derived from primary cortical neurons cultures grown in the presence and absence of 0.25 μg/ml of [1,25(OH)2D3] for 24 h, 48 h, 72 h and 120 h, followed by exposure to 0.5 mM of H2O2 for 2 h. (*) represents the P value as [1,25(OH)2D3] treated samples significantly different (≥0.05) from the H2O2 treated samples. (#) represent the H2O2 treated samples compared to the control samples as significant.
3.2. Effect of vitamin D on glutathione S transferase enzyme activity
The Glutathione S transferase (GST) constitutes a family of cytosolic and membrane-associated microsomal proteins that are involved in the detoxification of electrophilic xenobiotics. Immunohistochemical studies and spectrophotometric enzyme assay suggested the presence of GST enzyme activity in Purkinje cells throughout the cerebellar cortex in the rat's brain [17]. The presence of GST enzyme in the brain suggests that it gives protection to the neurons against exogenous and endogenous neurotoxic compounds. The amount of GST enzyme was significantly lower in the neuronal culture exposed to H2O2 induced oxidative stress at 24 h, 48 h and 72 h in culture (Fig. 4). However, the presence of [1,25(OH)2D3] in the neuronal culture significantly reduced this oxidative stress by upregulating the GST enzyme levels, clearly indicating the neuroprotective potential of [1,25(OH)2D3].
4. Discussion
Vitamin D deficiency was suggested to trigger premature aging [19], enlargement of the lateral ventricle, reduction in NGF protein content, reduction in expression of a number of genes involved in neuronal structure, disruption of brain development and following persistent changes in the adult brain of animals [[7]. Limited but collateral findings in human studies were also reported. Low levels of the plasma 25hydroxyvitamin D (25OHD) were suggested to be associated with mood disorder, dementia, mild cognitive impairment, Alzheimer's disease and autism [[20], [21], [22], [23], [24]]. These studies led us to use Vitamin D in our in-vitro studies and explore its therapeutic potential in detail.
Initial studies using MTT assay and trypan blue exclusion viability assays suggested that 0.25 μg/ml was the optimum dosage while higher doses are toxic to the cells. So this dosage was used for the treatments to the cortical neurons in culture. Similar dosage of [1,25(OH)2D3] was used in other in-vitro studies [8].
Cells under oxidative stress release free radicals which cause the peroxidation of the polyunsaturated fatty acids in the form of MDA as the final product. Treatment with H2O2 alone resulted in significantly high levels of MDA, while the cells that were pretreated with [1,25(OH)2D3] and then exposed to induced oxidative stress by H2O2 were completely protected from the excess generation of free radicals as shown by the significantly low levels of MDA. The protection from oxidative stress seemed to be more and more significant in the cultures that were exposed to H2O2 after 48 h, 72 h and 120 h of pretreatment with Vitamin D.
It has been reported that treatment with [1,25(OH)2D3] can upregulate glycine –cysteine ligase catalytic subunit and Glutathione reductase and increased cellular GSH formation in cultured Monocytes [18]. Studies showed that vitamin D3 exerts its protecting effects against free radicals generation by reactive species of oxygen and nitric oxide by inhibiting the synthesis of inducible nitric oxide synthesis, and by regulating the activity of the gamma-glutamyl transpeptidase, which is a key enzyme involved in the metabolism of glutathione [25]. In the present studies, the reduced GSH was shown to be significantly upregulated with the pretreatments of neuronal cultures with [1,25(OH)2D3] after 24 h, 48 h, 72 h and 120 h. GST is one of the other antioxidant enzymes which plays a critical role in combating oxidative stress by catalyzing the conjugation of glutathione and various free radicals formed by toxins, thus helping in their elimination and maintaining the redox state of cells. In our study, neuronal cells treated with H2O2 alone caused significantly reduced levels of GST enzyme activity which agrees with our earlier finding of less amount of reduced GSH in those cultures. However with the pretreatment of these cultures with [1,25(OH)2D3] for 24 h, 48 h and 120 h substantially improved the GST enzyme activity as well as increased amount of Reduced Glutathione. These results clearly indicated the direct relationship of reduced GSH and GST enzyme levels being upregulated with the pretreatment of [1,25(OH)2D3] and their combined effect in alleviating the oxidative stress induced by H2O2.
Catalase enzyme prevents excessive accumulation of the strongly oxidizing agent H2O2 which otherwise can do damage to the cells. Because of this preventive effect of catalase, important cellular processes which generate H2O2 as a by-product can proceed safely. Biochemical analysis of catalase has shown that it binds endogenously to 24,25(OH)2D3 [26]. In our results, the catalase enzyme activity was significantly reduced in the presence of [1,25(OH)2D3], while the cultures treated with hydrogen peroxide alone showed a marked increased level of catalase enzyme in the cell pellet. This finding again showed the antioxidant nature of Vitamin D3 metabolite which is protecting neuronal cells from the harmful effect of H2O2. In the absence of Vitamin D3 treatments to the neuronal cells, H2O2 caused a significant upregulations of catalase enzyme in order to neutralize its damaging effect to the neurons.
5. Conclusion
Oxidative stress is the main contributor to various neurodegenerating diseases, and the neuroprotective role of Vitamin D has been gaining support over the last decade. In this study, we looked at the various metabolic markers for oxidative stress such as Lipid peroxidation (levels of MDA), reduced glutathione levels and GST enzyme levels as well as catalase enzyme levels, with the long-term treatment of neuronal cultures with vitamin D. The data presented here further emphasized the therapeutic role of vitamin D3 in alleviating oxidative stress and its potential role in neuroprotection.
Acknowledgments
We are grateful to King Abdul Aziz Science and Technology and Female Research Centre of King Saud University for providing financial assistance in carrying out this research project.
References
- 1.Cui X., Gooch H., Groves N.J. Vitamin D and the brain: key questions for future research. J. Steroid Biochem. Mol. Biol. 2015;148:305–309. doi: 10.1016/j.jsbmb.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Bains J.S., Shaw C.A. Neurodegenerative disorders in humans: the role of glutathione in oxidative stress-mediated neuronal death. Brain Res. Brain Res. Rev. 1997;25(3):335–358. doi: 10.1016/s0165-0173(97)00045-3. [DOI] [PubMed] [Google Scholar]
- 3.Gianforcaro A., Hamadeh M.J. Vitamin D as a potential therapy in amyotrophic lateral sclerosis. CNS Neurosci. Ther. 2014;20(2):101–111. doi: 10.1111/cns.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kones R. Mitochondrial therapy for Parkinson's disease: neuroprotective pharmaconutrition may be disease-modifying. Clin. Pharm. 2010;2:185–198. doi: 10.2147/CPAA.S12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rimmelzwaan L.M., van Schoor N.M., Lips P. Systematic review of the relationship between vitamin D and Parkinson's disease. J. Parkinsons Dis. 2016;6(1):29–37. doi: 10.3233/JPD-150615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langub M.C., Herman J.P., Malluche H.H. Evidence of functional vitamin D receptors in rat hippocampus. Neuroscience. 2001;104(1):49–56. doi: 10.1016/s0306-4522(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 7.Wang J.Y., Wu J.N., Cherng T.L. Vitamin D(3) attenuates 6-hydroxydopamine-induced neurotoxicity in rats. Brain Res. 2001;904(1):67–75. doi: 10.1016/s0006-8993(01)02450-7. [DOI] [PubMed] [Google Scholar]
- 8.Shinpo K., Kikuchi S., Sasaki H. Effect of 1,25-dihydroxyvitamin D(3) on cultured mesencephalic dopaminergic neurons to the combined toxicity caused by l-buthionine sulfoximine and 1-methyl-4-phenylpyridine. J. Neurosci. Res. 2000;62(3):374–382. doi: 10.1002/1097-4547(20001101)62:3<374::AID-JNR7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Taniura H., Ito M., Sanada N. Chronic vitamin D3 treatment protects against neurotoxicity by glutamate in association with upregulation of vitamin D receptor mRNA expression in cultured rat cortical neurons. J. Neurosci. Res. 2006;83(7):1179–1189. doi: 10.1002/jnr.20824. [DOI] [PubMed] [Google Scholar]
- 10.Longoni A., Kolling J., dos Santos T.M. 1,25-Dihydroxyvitamin D3 exerts neuroprotective effects in an ex vivo model of mild hyperhomocysteinemia. Int. J. Dev. Neurosci. 2016;48:71–79. doi: 10.1016/j.ijdevneu.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Orme R.P., Bhangal M.S., Fricker R.A. Calcitriol imparts neuroprotection in vitro to midbrain dopaminergic neurons by upregulating GDNF expression. PLoS One. 2013;8(4) doi: 10.1371/journal.pone.0062040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orme R.P., Middleditch C., Waite L. The role of vitamin D3 in the development and neuroprotection of midbrain dopamine neurons. Vitam. Horm. 2016;100:273–297. doi: 10.1016/bs.vh.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Sciarretta C., Minichiello L. The preparation of primary cortical neuron cultures and a practical application using immunofluorescent cytochemistry. Methods Mol. Biol. 2010;633:221–231. doi: 10.1007/978-1-59745-019-5_16. [DOI] [PubMed] [Google Scholar]
- 14.Beutler E., Gelbart T. Improved assay of the enzymes of glutathione synthesis: gamma-glutamylcysteine synthetase and glutathione synthetase. Clin. Chim. Acta. 1986;158(1):115–123. doi: 10.1016/0009-8981(86)90122-1. [DOI] [PubMed] [Google Scholar]
- 15.Garcia Y.J., Rodríguez-Malaver A.J., Peñaloza N. Lipid peroxidation measurement by thiobarbituric acid assay in rat cerebellar slices. J. Neurosci. Methods. 2005;144(1):127–135. doi: 10.1016/j.jneumeth.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 16.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 17.Johnson J.A., el Barbary A., Kornguth S.E. Glutathione S-transferase isoenzymes in rat brain neurons and glia. J. Neurosci. 1993;13(5):2013–2023. doi: 10.1523/JNEUROSCI.13-05-02013.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain S.K., Micinski D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP-1 and IL-8 secretion in high-glucose exposed U937 monocytes. Biochem. Biophys. Res. Commun. 2013;437(1):7–11. doi: 10.1016/j.bbrc.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcion E., Wion-Barbot N., Montero-Menei C.N. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. 2002;13(3):100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 20.Aminmansour B., Asnaashari A., Rezvani M. Effects of progesterone and vitamin D on outcome of patients with acute traumatic spinal cord injury; a randomized, double-blind, placebo controlled study. J. Spinal Cord. Med. 2016;39(3):272–280. doi: 10.1080/10790268.2015.1114224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calvello R., Cianciulli A., Nicolardi G. Vitamin D treatment attenuates neuroinflammation and dopaminergic neurodegeneration in an animal model of Parkinson's disease, shifting M1 to M2 microglia responses. J. NeuroImmune Pharmacol. 2016;12(2):327–339. doi: 10.1007/s11481-016-9720-7. (Jun) [DOI] [PubMed] [Google Scholar]
- 22.Cheng J., Rui Y., Qin L. Vitamin D combined with resveratrol prevents cognitive decline in SAMP8 mice. Curr. Alzheimer Res. 2017;14(8):820–833. doi: 10.2174/1567205014666170207093455. [DOI] [PubMed] [Google Scholar]
- 23.Kocovska E., Gaughran F., Krivoy A. Vitamin-D deficiency as a potential environmental risk factor in multiple sclerosis, schizophrenia, and autism. Front Psychiatry. 2017;8:47. doi: 10.3389/fpsyt.2017.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cannell J.J. Vitamin D and autism, what's new? Rev. Endocr. Metab. Disord. 2017;18(2):183–193. doi: 10.1007/s11154-017-9409-0. [DOI] [PubMed] [Google Scholar]
- 25.Garcion E., Sindji L., Leblondel G. 1,25-dihydroxyvitamin D3 regulates the synthesis of gamma-glutamyl transpeptidase and glutathione levels in rat primary astrocytes. J. Neurochem. 1999;73(2):859–866. doi: 10.1046/j.1471-4159.1999.0730859.x. [DOI] [PubMed] [Google Scholar]
- 26.Larsson D., Anderson D., Smith N.M. 24,25-dihydroxyvitamin D3 binds to catalase. J. Cell. Biochem. 2006;97(6):1259–1266. doi: 10.1002/jcb.20717. [DOI] [PubMed] [Google Scholar]