1. Introduction
Epilepsy is a common symptom in patients with primary central nervous system (CNS) tumors. Seizures are reported in 15–95% of patients with brain tumors, depending on the type of tumor [1]. Seizures are the presenting symptom in 15–50% of patients with gliomas, and up to 75% will have at least one seizure at some point in the disease course [2].
The recognition and classification of epileptic seizures and providing effective treatment are essential to preserve the quality of life of patients with glial tumors.
In a previous study, we found that 63% of patients with glial tumors experienced seizures at some point in the disease course. Sixty two percent of seizures were focal onset, while 38% were generalized onset seizures [3]. However, considering the tumor etiology of seizures, we were unable to verify the results. Therefore, the goal of this study was to present the results of a larger cohort of patients with primary glial tumors and epilepsy based on a more rigorous examination approach.
The primary objectives of this study were to classify and quantify epileptic seizures as a symptom of onset or later in the course of the disease and the correlation between ILAE 1981 and 2017 classifications [5,6]. The secondary objectives were to quantify: 1) the cases in which epileptic seizures were reclassified; 2) patients who received antiepileptic drugs (AEDs) as monotherapy; 3) patients whose antiepileptic treatment on admission was modified; and (4) patients whose seizures were controlled with the prescribed AEDs.
2. Patients and methods
This is a prospective, descriptive, observational, clinical research study of a cohort of 82 patients with glial tumors obtained from the follow-up of 168 patients diagnosed with primary CNS tumor from January 1, 2010 through March 31, 2013 at our institution. The diagnosis of primary tumor was according to WHO classification of CNS tumors [4]. Characteristics considered include: histopathological diagnosis, epileptic seizure type according to the International League Against Epilepsy (ILAE) classification [5,6], antiepileptic treatment and response to it, as well as the modification of antiepileptic drugs (AEDs), if it has been done. For didactic and chronological purposes, we have maintained the description of the seizures according to both the 1981 and 2017 ILAE classifications. In all patients, seizure characterization and antiepileptic treatment were compared with those conducted at other medical centers. This study was approved by the Institutional Review Board.
3. Results
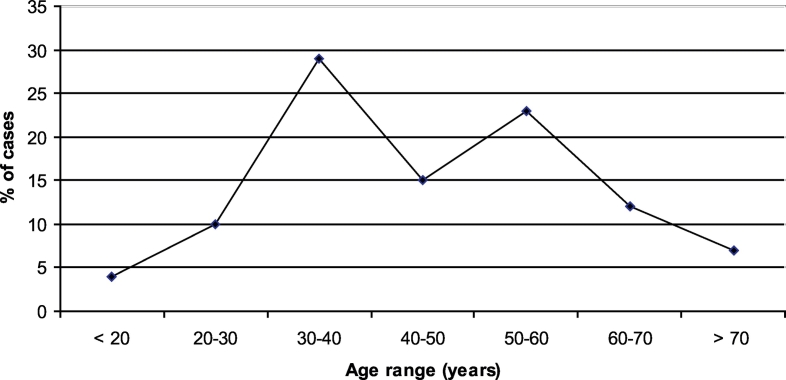
Eighty-two patients were included in the study: 49 men and 33 women. The majority of cases (29%) occurred between the third and fourth decade (Fig. 1).
Fig. 1.
Cases distribution by age.
Histological types (WHO 2007) [4] are shown in Table 1.
Table 1.
Histopathological diagnostic (WHO 2007) [4].
| Astrocytics tumors | Glioblastoma | 28 |
| Diffuse astrocytoma (GII) | 22 | |
| Anaplastic astrocytoma (GIII) | 8 | |
| Brainstem glioma (not biopsied) | 2 | |
| Oligodendroglials tumors | Oligodendroglioma (GII) | 14 |
| Anaplastic oligodendroglioma (GIII) | 7 | |
| Other neuroepithelial tumors | Astroblastoma | 1 |
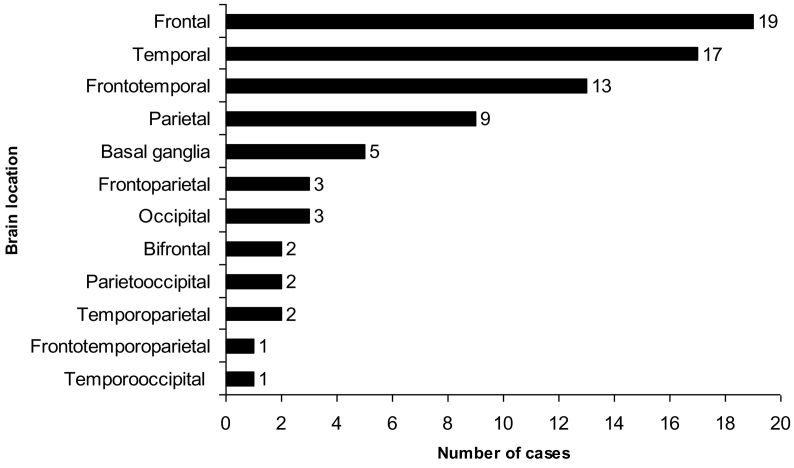
Eighty-eight percent of cases had lobular location with 59% of cases limited to a single lobe: 19 frontal, 17 temporal and 9 parietal. The involvement of two or more lobes, with frontotemporal predominance location, was observed in 29% of cases (Fig. 2). A gliomatosis cerebri growth pattern was observed in 4% of cases. The remaining cases were distributed as the following: 5 basal ganglia, 2 brainstem, 2 spinal cord and 1 cerebellum. Infratentorial cases (brainstem, cerebellum, and spinal cord) were excluded.
Fig. 2.
Cases distribution by sites.
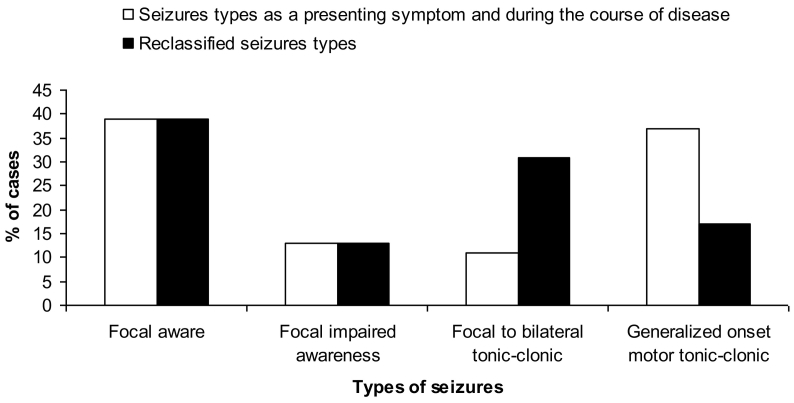
Seventy four percent (57/77) of cases had epilepsy. The types of epileptic seizures diagnosed according to the ILAE 1981 and ILAE 2017 classifications were simple partial (focal aware) 39%, partial complex seizure (focal impaired awareness) 13%, partial secondarily generalized tonic-clonic (focal to bilateral tonic-clonic) 11%, and generalized convulsive (generalized onset motor tonic-clonic) 37% (Fig. 3). In 15/57 patients (26%), the type of seizure was reclassified, and generalized onset motor seizures decreased from 37% to 17%. As a result, focal to bilateral tonic-clonic seizures increased from 11% to 31%. Thus, focal onset seizures accounts for 83% of the types of seizures.
Fig. 3.
Seizure types according to the reclassification.
As expected, some patients presented more than one type of epileptic seizure at the time of the first consultation. Table 2 shows the figures corresponding to each one of them.
Table 2.
Seizure types according to ILAE 2017 classification [6].
| Seizure types | n/% (75/100) | ||
|---|---|---|---|
| Focal onset | Aware | Motor onset | 12/16 |
| Nonmotor onset | 17/23 | ||
| Sensory | 9/12 | ||
| Autonomic | 4/5 | ||
| Cognitive | 4/5 | ||
| Impaired awareness | 10/13 | ||
| Focal to bilateral tonic-clonic | 23/31 | ||
| Generalized onset | 13/17 | ||
Seizures were the presenting symptom in 79% of patients, and in the rest during the course of the disease.
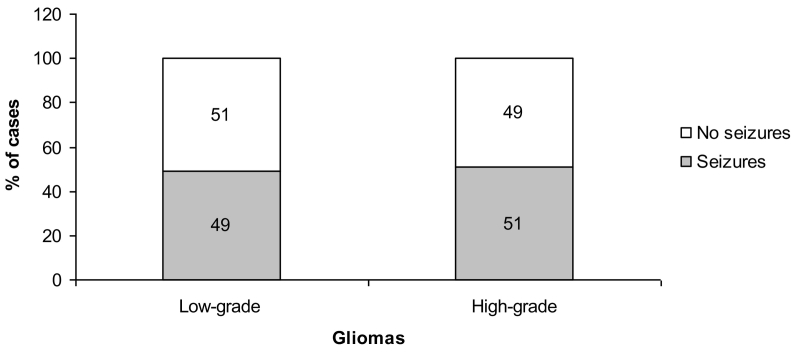
According to the histological type, epilepsy was observed in 49% and 51% of patients with low-grade and high-grade glial tumors, respectively (Fig. 4).
Fig. 4.
Seizures according to 2007 WHO grading gliomas.
Seventy five out of 77 patients (97%) were on treatment with AEDs. Of these, 20 (27%) discontinued AEDs treatment because they never experienced seizures. All neurosurgical patients were already on treatment or received prophylactic treatment with phenytoin.
Fifty five of 57 patients with seizures (96%) were on AEDs at the time of the first consultation at our institution, and 6/55 (11%) were on monotherapy with levetiracetam. The two patients who were not on treatment started treatment with levetiracetam. Among patients treated with AEDs other than levetiracetam, 31/49 (63%) were on phenytoin monotherapy. AEDs were switched to levetiracetam in 35/49 (71%) cases. Finally, 43/57 (75%) received levetiracetam at a dose range of 1000 to 2500 mg per day. During follow-up visits, 95% of patients with levetiracetam were seizure-free. Poor seizure control in 2 patients required the addition of a second and a third drug; one with the addition of lacosamide presented full seizure control, and the other with phenobarbital and lamotrigine had occasional breakthrough seizure (drug-resistant epilepsy). Ultimately, 57/77 (74%) patients had seizures and received AEDs.
4. Discussion
In this cohort of patients with gliomas we observed a higher prevalence of male cases and patients between 35 and 40 years old, similar to what was observed in a study performed previously by our neurology department in 2010 [3]. Astrocytic tumors were the most common type, especially glioblastoma.
More than half of gliomas were located within the frontal, temporal, and parietal lobes. These findings were similar to previous studies on primary tumors of the glial series [[7], [8], [9]], and also similar to our previous study [3].
Young age and long duration of illness are associated with an increased risk of secondary epileptogenesis. The exact nature of epileptogenesis in patients with brain tumors is unclear. It is probably multifactorial and caused by the different tumor types and changes in the properties of the tumor-cell membranes that generate action potentials, which affect neuronal excitability (e.g., raised concentrations of amino acids, neuroreceptors disturbance, increased gap junctions between tumor cells, and low pH microenvironment) [[10], [11], [12]].
Tumor type, histological grade and site are determining factors in the incidence and frequency of epileptic seizures. Thus, slow growing low-grade gliomas with longer survival times are more frequently associated with epilepsy than high-grade ones [10,13]. In our study, seizures were distributed almost equally among patients with high and low-grade gliomas. This finding differs from previously published figures in the literature, where seizures were observed in 65–95% of patients with low-grade gliomas and 15–34% with high-grade gliomas [1,8,9,13,14]. Nevertheless, other studies reported up to 62% seizure frequency in glioblastomas [15]. In our cohort, 28 patients had glioblastoma and 16/28 (57%) of them had seizures.
Intra-axial tumors with involvement of the cerebral cortex are more epileptogenic than extra-axial and deep tumors. Tumors within frontal, temporal, and parietal lobes are more linked to seizures than those with occipital location [10,13]. We believe that focal onset seizures of this location may be well underdiagnosed.
In this cohort of patients seizures were prevalent (57/77, 74%), as well as in our previous study [3]. Fifty-eight percent (45/77) had seizure as the presenting symptom of the disease, which is more than what was reported by Glantz et al. (14–51%); however, primary brain tumor and metastases were included in the meta-analysis [16]. Moreover, the study mentions that the onset of seizures during the course of the disease (10–45%) depends on factors such as tumor histology, location, age, and treatment [16]. In this study, 16% of patients developed seizures at some point in the disease course. These differences in both percentages are probably due to better information ascertainment from patients and/or witnesses as data collection was comprehensive in our study.
Another previous study that included only patients with supratentorial glioblastomas and seizures, reported seizure as the presenting symptom of the disease in 68% of cases, and 32% developed seizures at some point in the disease course [15]. This is similar to what we observed in our cohort: 62.5% and 37.5% for each group of patients, respectively. It seems that in patients with high-grade gliomas, the presence of epilepsy is more frequent than previously expected.
Focal onset seizures were the most common type in our study: focal aware, focal impaired awareness, and focal to bilateral tonic-clonic. Prior to the reclassification of epileptic seizures, the percentage of generalized onset motor tonic-clonic was 37%, similar to that obtained in a previous study [3]. However, after the implementation of a rigorous examination to patients, relatives, and/or witnesses, this number went down by 20%. Another explanation for the overestimation of generalized onset motor tonic-clonic seizures is that some patients may have experienced a rapid seizure evolution from focal to bilateral tonic-clonic; and therefore, the focal phase was unnoticed. It should be noted that from a practical point of view we had no difficulties incorporating the ILAE 2017 classification for the purpose of this study [6]. We reinforce the concept of using the actual classification.
In patients, de novo or with the diagnosis of an encephalic tumor, the priority for the patients themselves and much more for the family is to begin the specific treatment as soon as possible. The diagnosis of convulsion and AED indicated as prophylaxis or treatment, goes into the background. Several of the authors have reexamined patients and family members to better classify their epileptic seizures, especially among those to whom AEDs prophylaxis were indicated and their potential withdrawal would have resulted in great concerns by the patient and their families.
Seizure control was excellent (seizure-free) in almost all patients treated with levetiracetam. We started AED monotherapy with levetiracetam, a drug we use since the early 2000s that has been shown to be effective for focal and generalized onset seizures [3,[17], [18], [19], [20], [21]], Currently, there is no evidence of levetiracetam interaction with any known drug, especially drugs administered to neuro-oncologic patients, as it does not have deleterious effects on cognition [[22], [23], [24], [25], [26]]. Reasons why patients with seizures did not start treatment on levetiracetam (25%) were: 1) accessibility to medication; 2) poor health and death before treatment initiation; and 3) transfer of care to other medical centers.
We acknowledge that phenytoin was the most common used drug for the treatment of neuro-oncologic seizures outside our institution. Phenytoin was the most commonly discontinued AED in favour of levetiracetam, as observed in our cohort and several previous studies [9,20,27]. Also, we found that all patients without a history of epilepsy that went on to have surgery were on long-term phenytoin treatment. In patients with newly diagnosed brain tumor, AEDs are not recommended as epilepsy prophylaxis. Furthermore, AEDs tapering and discontinuing 7-10 days after neurosurgery applies for patients who did not have seizures [16,22,[28], [29], [30]].
This study is not without limitations. All cases were from a single medical center, the Department of Neurology and Neuro-Oncology University Center, Oncologic Institute “Ángel H. Roffo “, University of Buenos Aires, which might limit the generalizability of our findings. Additionally, while our studies had a relatively larger sample size compared to previous studies, it only analyzed data from 82 patients.
5. Conclusions
A rigorous examination of the patient and family/witnesses undoubtedly allows for a better diagnosis and characterization of epileptic seizures in addition to the possibility of withdrawing treatment in seizure-free patients.
One fifth of epileptic patients referred with generalized onset motor tonic-clonic seizure were reclassified as focal to bilateral tonic-clonic. Focal onset seizures are the most common type in patients with glial tumors.
Levetiracetam used as monotherapy is suitable for patients with epilepsy secondary to glial tumors.
The transition of the ILAE classification from 1981 to 2017 was favorable, coherent, and with phenomenological improvement.
Source of funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
None of the authors has any conflict of interest to disclose.
Ethical standards
This study was approved by the Institutional Review Board, Oncologic Institute “Ángel H. Roffo” and conducted in accordance with the Declaration of Helsinki and current Argentine legislation.
References
- 1.Deangelis L.M. Brain tumors. N. Engl. J. Med. 2001;344:114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 2.Mohile N.A. Medical complications of brain tumors. Continuum (Minneap Minn) Neuro-Oncology. 2017;23(6):1635–1652. doi: 10.1212/CON.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 3.Cardozo Oliver J.M., Baéz A., Baéz M. Casas Parera I. Estudio de una cohorte de pacientes adultos con tumores primarios de sistema nervioso central de la serie glial. Neurol. Arg. 2010;2:240–246. [Google Scholar]
- 4.Louis D.N., Ohgaki H., Wiestler O.D. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bancaud J., Henriksen O., Rubio-Donnadieu F. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. From the commission on classification and terminology of the international league against epilepsy. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 6.Fisher R.S., Cross J.H., D'Souza C. Instruction manual for the ILAE 2017 operational classification of seizure types. Epilepsia. 2017;58:531–542. doi: 10.1111/epi.13671. [DOI] [PubMed] [Google Scholar]
- 7.Ostrom Q.T., Gittleman H., Fulop J. Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008-2012. Neuro-Oncologia. 2015;17:1–62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J.W., Wen P.Y., Hurwitz S. Morphological characteristics of brain tumors causing seizures. Arch. Neurol. 2010;67:336–342. doi: 10.1001/archneurol.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lynam L.M., Lyons M.K., Drazkowski J.F. Frequency of seizures in patients with newly diagnosed brain tumors: a retrospective review. Clin. Neurol. Neurosurg. 2007;109:634–638. doi: 10.1016/j.clineuro.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 10.Van Breemen M.S., Wilms E.B., Vecht C.J. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6:421–430. doi: 10.1016/S1474-4422(07)70103-5. [DOI] [PubMed] [Google Scholar]
- 11.Riva M. Brain tumoral epilepsy: a review. Neurol. Sci. 2005;1:S40–S42. doi: 10.1007/s10072-005-0404-y. [DOI] [PubMed] [Google Scholar]
- 12.de Groot M., Reijneveld J.C., Aronica E. Epilepsy in patients with a brain tumour: focal epilepsy requires focused treatment. Brain. 2012;135:1002–1016. doi: 10.1093/brain/awr310. [DOI] [PubMed] [Google Scholar]
- 13.Duffau H., Capelle L. Preferential brain locations of low- grade gliomas. Cancer. 2004;100:2622–2626. doi: 10.1002/cncr.20297. [DOI] [PubMed] [Google Scholar]
- 14.Arrillaga-Romany I.C., Lee E.Q., Wen P.Y. Diagnosis of brain tumors: clinical and radiographic. In: Packer R.J., Schiff D., editors. Neurology in Practice, Neuro-oncology. Wiley-Blackwell; 2012. pp. 3–12. [Google Scholar]
- 15.Kerkhof M., Dielemans J.C., van Breemen M.S. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro-Oncology. 2013;15:961–967. doi: 10.1093/neuonc/not057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glantz M.J., Cole B.F., Forsyth P.A. Practice parameter: anticonvulsivant prophylaxis in patients with newly diagnosed brain tumors: Report of the Quality Standard Subcommittee of the American Academy of Neurology. Neurology. 2000;54:1886–1893. doi: 10.1212/wnl.54.10.1886. [DOI] [PubMed] [Google Scholar]
- 17.Swaroop H.S., Ananya C., Nithin K. Levetiracetam: a review of its use in the treatment of epilepsy. Int. J. Med. Biomed. Res. 2013;2:166–172. http://www.ijmbr.com/reviewed/2.3.2.pdf 10.14194/ijmbr.232 Available at. [Google Scholar]
- 18.Ben-Menachem E., Falter U. Efficacy and tolerability of levetiracetam 3000 mg/d in patients with refractory partial seizures: a multicenter, double-blind, responder-selected study evaluating monotherapy. European Levetiracetam Study Group. Epilepsia. 2000;41:1276–1283. doi: 10.1111/j.1528-1157.2000.tb04605.x. [DOI] [PubMed] [Google Scholar]
- 19.Alsaadi T.M., Shatzel A., Márquez A.V. Clinical experience of levetiracetam monotherapy for adults with epilepsy: 1-year follow-up study. Seizure. 2005;14:139–142. doi: 10.1016/j.seizure.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Lim D.A., Tarapore P., Chang E. Safety and feasibility of switching from phenytoin to levetiracetam monotherapy for glioma-related seizure control following craniotomy: a randomized phase II pilot study. J. Neuro-Oncol. 2009;93:349–354. doi: 10.1007/s11060-008-9781-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lyseng-Williamson K.A. Levetiracetam: a review of its use in epilepsy. Drugs. 2011;71:489–514. doi: 10.2165/11204490-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Newton H.B., Goldlust S.A., Pearl D. Retrospective analysis of the efficacy and tolerability of levetiracetam in brain tumor patients. J. Neuro-Oncol. 2006;78:99–102. doi: 10.1007/s11060-005-9070-4. [DOI] [PubMed] [Google Scholar]
- 23.Carreño M. Levetiracetam. Drugs Today (Barc) 2007;43:769–794. doi: 10.1358/dot.2007.43.11.1136902. [DOI] [PubMed] [Google Scholar]
- 24.Fonkem E., Bricker P., Mungall D. The role of levetiracetam in treatment of seizures in brain tumor patients. Front. Neurol. 2013;4:153. doi: 10.3389/fneur.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oberndorfer S., Piribauer M., Marosi C. P450 enzyme inducing and non-enzyme inducing antiepileptics in glioblastoma patients treated with standard chemotherapy. J. Neuro-Oncol. 2005;72:255–260. doi: 10.1007/s11060-004-2338-2. [DOI] [PubMed] [Google Scholar]
- 26.Milligan T.A., Hurwitz S., Bromfield E.B. Efficacy and tolerability of levetiracetam versus phenytoin after supratentorial neurosurgery. Neurology. 2008;71:665–669. doi: 10.1212/01.wnl.0000324624.52935.46. [DOI] [PubMed] [Google Scholar]
- 27.Usery J.B., Michael L.M., 2nd, Sills A.K. A prospective evaluation and literature review of levetiracetam use in patients with brain tumors and seizures. J. Neuro-Oncol. 2010;99:251–260. doi: 10.1007/s11060-010-0126-8. [DOI] [PubMed] [Google Scholar]
- 28.Temkin N.R. Prophylactic anticonvulsants after neurosurgery. Epilepsy Curr. 2002;2:105–107. doi: 10.1046/j.1535-7597.2002.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sirven J.I., Wingerchuk D.M., Drazkowski J.F. Seizure prophylaxis in patients with brain tumors: a meta-analysis. Mayo Clin. Proc. 2004;79:1489–1494. doi: 10.4065/79.12.1489. [DOI] [PubMed] [Google Scholar]
- 30.Chassoux F., Landre E. Prevention and management of postoperative seizures in neuro-oncology. Neurochirurgie. 2017;63:197–203. doi: 10.1016/j.neuchi.2016.10.013. [DOI] [PubMed] [Google Scholar]