Abstract
Background
Pneumococcal disease is a major cause of morbidity and mortality worldwide, especially in patients with comorbidities and advanced age. This study evaluated trends in epidemiology of adult pneumococcal disease in Crete, Greece, by identifying serotype distribution and antimicrobial resistance of consecutive Streptococcus pneumoniae strains isolated from adults during an 8-year time period (2009–2016) and the indirect effect of the infant pneumococcal higher-valent conjugate vaccines 10-valent pneumococcal conjugate vaccine (PCV10) and 13-valent pneumococcal conjugate vaccine (PCV13).
Materials and Methods
Antimicrobial susceptibility was performed by E-test and serotyping by Quellung reaction. Multidrug resistance (MDR) was defined as non-susceptibility to penicillin (PNSP) combined with resistance to ≥2 non-β-lactam antimicrobials.
Results
A total of 135 S. pneumoniae strains were isolated from adults during the study period. Twenty-one serotypes were identified with 17F, 15A, 3, 19A, and 11A, being the most common. The coverage rates of PCV10, and PCV13 were 17.8% and 37.8%, respectively. PCV13 serotypes decreased significantly from 68.4% in 2009 to 8.3% in 2016 (P = 0.002). The most important emerging non-PCV13 serotypes were 17F, 15A, and 11A, with 15A being strongly associated with antimicrobial resistance and MDR. Among all study isolates, penicillin-resistant and MDR strains represented 7.4% and 14.1%, respectively. Predominant PNSP serotypes were 19A (21.7%), 11A (17.4%), and 15A (17.4%). Erythromycin, clindamycin, tetracycline, trimethoprim-sulfamethoxazole, and levofloxacin resistant rates were 30.4%, 15.6%, 16.3%, 16.3%, and 1.5%, respectively.
Conclusion
Although pneumococcal disease continues to be a health burden in adults in Crete, our study reveals a herd protection effect of the infant pneumococcal higher-valent conjugate vaccination. Surveillance of changes in serotype distribution and antimicrobial resistance among pneumococcal isolates are necessary to guide optimal prevention and treatment strategies.
Keywords: Streptococcus pneumoniae, Adults, Serotyping, Antimicrobial resistance, Pneumococcal conjugate vaccine
Introduction
Streptococcus pneumoniae is a part of the commensal flora of the nasopharynx of healthy people, with the peak incidence of colonization observed in children at the age of 3 years [1]. Pneumococci can spread from the nasopharynx and cause respiratory or systemic disease or colonize other individuals. Pneumococcal diseases range from mild, non-invasive infections such as otitis media, sinusitis and pneumonia to severe, life-threatening invasive infections, including meningitis and bacteremia [2]. Children, adults with medical comorbidities and the elderly (>65 years of age), are considered at increased risk of developing pneumococcal disease [3,4]. Pneumococcal pneumonia, with or without bacteremia represents a great health burden in adults [5]. It has been estimated that pneumococcal pneumonia is responsible for more than 1.5 million deaths every year, worldwide [6]. Mortality of community-acquired pneumonia (CAP) due to S. pneumoniae has been reported to be 4% in outpatients and between 10% to 20% in patients requiring hospitalization [7].
The introduction of vaccination aimed to reduce the burden of the disease. In Greece, the 23-valent pneumococcal polysaccharide vaccine (PPV23) was first introduced in 1998 and received approval for routine immunization of adults aged ≥60 years and since 2011 it is also recommended for adults 19-50 years at increased risk for invasive pneumococcal disease (IPD) [8]. The vaccination rates for PPV23 have been estimated at 30% based on market sales. Pneumococcal conjugate vaccines (PCVs) that were introduced in infant immunization programs have been proved highly effective in preventing pneumococcal infections not only in children, but also in adults due to herd protection [9]. In Greece, PCV7 has been introduced to the pediatric national immunization program (NIP) in January 2006, and was replaced by PCV10 in 2009 and finally by PCV13 in June 2010. In addition to the pediatric indication, PCV13 has been licensed for immunization of adults >50 years in December 2011 and in 2015 was also introduced for high-risk individuals aged 19-50 years, with low coverage. Nevertheless, despite extensive vaccination programs, the burden of pneumococcal diseases continues to be high in several countries, with increased morbidity, mortality and healthcare costs [7,10].
As for pneumococcal diseases, the incidence, circulating serotypes and resistance rates to antimicrobial agents differ among countries and may vary during different periods in the same country. Pneumococcal serotypes, and antimicrobial resistance of strains isolated from adults cared for in the University Hospital of Heraklion, Crete, Greece, between 2001 and 2008, have been reported previously [11].
The present study has investigated and reports the serotype distribution and the antimicrobial susceptibilities of invasive and non-invasive S. pneumoniae isolates from adults with pneumococcal infections, over an eight-year period (2009–2016) at the University Hospital of Heraklion, Crete, Greece.
Materials and Methods
1. Bacterial isolates
S. pneumoniae clinical isolates were collected from consecutive adult patients with invasive and non-invasive pneumococcal disease admitted to the University Hospital of Heraklion, Crete, Greece, from 2009 to 2016. The University Hospital of Heraklion is a 650-bed, tertiary care hospital serving a population of around 650,000 persons. Invasive isolates were defined as those from normally sterile body sites (blood, cerebrospinal, pleural, ascitic and synovial fluid). One isolate per patient was identified and tested.
This study was approved by the Ethics Committee of the University Hospital of Heraklion, with approval to report microbiological and laboratory data as part of the routine medical care.
Identification of S. pneumoniae was based on colonial and microscopic morphology, hemolytic activity on sheep blood agar medium, catalase test, optochin susceptibility, bile solubility, and biochemical profile using the Vitek 2 automated system (BioMérieux, Marcy l' Etoile, France).
2. Serotyping
Serotyping was performed using the Pneumotest antisera latex kit and by the Quellung reaction using pneumococcal group/type and factor antisera, as recommended by the manufacturer (Statens Serum Institut, Copenhagen, Denmark).
3. Susceptibility testing
Antibiotic susceptibility testing to determine the minimal inhibitory concentrations (MICs) was performed by E-test (BioMérieux), according to manufacturer's recommendations. The antimicrobials tested were: penicillin, amoxicillin, cefuroxime, cefotaxime, ceftriaxone, cefepime, imipenem, meropenem, erythromycin, clarithromycin, azithromycin, clindamycin, ciprofloxacin, levofloxacin, moxifloxacin, chloramphenicol, tetracycline, trimethoprim-sulfamethoxazole (TMP/SMX), vancomycin, linezolid, quinupristin/dalfopristin, tigecycline and daptomycin. Results were interpreted according to the 2016 Clinical and Laboratory Standards Institute criteria (CLSI) [12]. S. pneumoniae ATCC 6305 and S. pneumoniae 49619 were used as control strains. To detect M phenotype and differentiate cMLSB and iMLSB resistance phenotypes, the disk approximation test was performed by using 15 μg erythromycin disks and 2 μg clindamycin disks, as per CLSI recommendation [12]. MDR was defined as nonsusceptibility to penicillin (PNSP) combined with resistance to ≥2 non-β-lactam antimicrobials.
4. Statistical analysis
Statistical analysis was conducted by the chi-square and Fisher exact test, as appropriate. Statistical significance was set at P <0.05. All statistical analyses were performed with Graphpad Prism, V.4 (GraphPad Software Inc, San Diego, CA, USA).
Results
A total of 135 S. pneumoniae isolates were collected during the eight-year study period. All isolates were obtained from adult patients. Of the 135 patients, 106 (78.5%) were male. Their mean age was 64 years (range 21-93). Most patients (80; 59.3%) were elderly (≥65 years old).
Of all isolates, 36 were invasive and 99 non-invasive. The most common source was the bronchalveolar lavage (BAL) (51; 37.8%), followed by sputum (34; 25.2%), blood (25; 18.5%), cerebrospinal fluid (7; 5.2%), conjunctival swabs (7; 5.2%), pus (5; 3.7%), pleural fluid (2; 1.5%), middle ear aspirate (2; 1.5%), ascitic fluid (1; 0.7%) and synovial fluid 1; 0.7%).
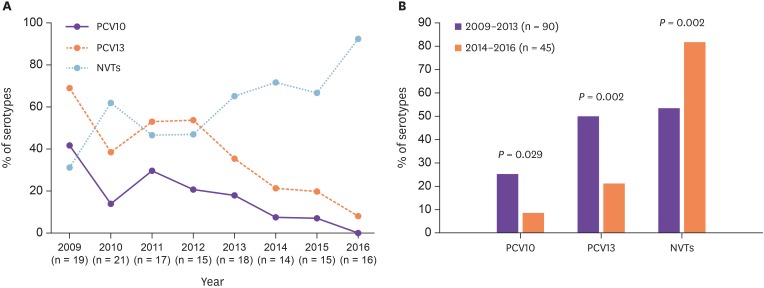
The age-stratified serotype distribution of invasive and non-invasive isolates are summarized in Table 1. The 135 isolates belonged to 21 serotypes. The most common, in decreasing order of frequency, were 17F, 15A, 3, 19A, and 11A, accounting for 49.6% of the isolates. The coverage rates of PCV7, PCV10, PCV13 and PPSV23 were 11.9%, 17.8%, 37.8% and 73.3%, respectively. Fifty-five patients were included in the range of 18 to 64 years of age and 80 in those ≥65 years. Serotype 17F was the most prevalent among adults of 18-64 years of age (7/55; 12.7%), followed by serotypes 3, 11A, 15A and 19A (each 6/55; 10.9%). Serotypes 15A and 17F (each 8/80; 10%) followed by 3, 7F and 19A (each 7/80; 8.8%) were the most frequent among the elderly ≥65 years. Among invasive isolates the major serotypes were 19A, 11A, and 15A, while among the non-invasive one serotype 17F was the most frequent. No statistical differences have been observed in serotype distribution between invasive and non-invasive isolates. Similarly, the PCVs serotypes and the 23-valent serotypes did not exhibit significant differences between invasive and non-invasive isolates (Table 1). Comparison of serotype distribution of the S. pneumoniae isolates between 2009–2013 (low childhood vaccination coverage) and 2014–2016 (high childhood vaccination coverage) showed a statistically significant decrease in the proportion of isolates included in the PCV10 and PCV13 (P = 0.029 and P = 0.002, respectively), as opposite to a significant increase in non-vaccine types (NVTs) (P = 0.002) (Fig. 1, Table 2).
Table 1. Serotype distribution of the 135 invasive and non-invasive Streptococcus pneumoniae isolates by age group.
| Serotype | No. of isolates (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| All isolates | Invasive | I (18–64y) | I (≥65y) | Non-invasive | NI (18–64y) | NI (≥65y) | P-value | |
| 3 | 13 (9.6) | 2 (5.6) | 2 | 11 (11.1) | 6 | 5 | 0.51 | |
| 4 | 1 (0.7) | 1 (2.8) | 1 | 0.27 | ||||
| 6A | 1 (0.7) | 1 (2.8) | 1 | 0.27 | ||||
| 6B | 3 (2.2) | 1 (2.8) | 1 | 2 (2.0) | 2 | 1.00 | ||
| 7F | 8 (6.0) | 2 (4.3) | 2 | 6 (6.1) | 1 | 5 | 1.00 | |
| 8 | 8 (6.0) | 2 (5.6) | 1 | 1 | 6 (6.1) | 4 | 2 | 1.00 |
| 9V | 3 (2.2) | 2 (5.6) | 2 | 1 (1.0) | 1 | 0.17 | ||
| 9N | 3 (2.2) | 3 (3.0) | 2 | 1 | 0.56 | |||
| 10A | 3 (2.2) | 3 (3.0) | 1 | 2 | 0.56 | |||
| 11A | 12 (8.9) | 4 (11.1) | 2 | 2 | 8 (8.1) | 4 | 4 | 0.73 |
| 12F | 2 (1.5) | 2 (5.6) | 1 | 1 | 0.07 | |||
| 14 | 4 (3.0) | 1 (2.8) | 1 | 3 (3.0) | 3 | 1.00 | ||
| 15A | 14 (10.4) | 4 (11.1) | 1 | 3 | 10 (10.1) | 5 | 5 | 1.00 |
| 17F | 15 (11.1) | 3 (8.3) | 1 | 2 | 12 (12.1) | 6 | 6 | 0.76 |
| 19A | 13 (9.6) | 5 (13.9) | 3 | 2 | 8 (8.1) | 3 | 5 | 0.33 |
| 19F | 5 (3.7) | 1 (2.8) | 1 | 4 (4.0) | 1 | 3 | 1.00 | |
| 20 | 3 (2.2) | 1 (2.8) | 1 | 2 (2.0) | 2 | 1.00 | ||
| 22F | 3 (2.2) | 2 (5.6) | 1 | 1 | 1 (1.0) | 1 | 0.17 | |
| 23B | 7 (5.2) | 7 (7.1) | 2 | 5 | 0.19 | |||
| 35B | 7 (5.2) | 2 (5.6) | 2 | 5 (5.1) | 2 | 3 | 1.00 | |
| 35F | 2 (1.5) | 2 (2.0) | 2 | 1.00 | ||||
| Nontypeable | 5 (3.7) | 5 (5.1) | 5 | 0.32 | ||||
| 7-valent | 16 (11.9) | 6 (16.7) | 3 | 3 | 10 (10.1) | 1 | 9 | 0.37 |
| 10-valent | 24 (17.8) | 8 (22.2) | 3 | 5 | 16 (16.2) | 2 | 14 | 0.45 |
| 13-valent | 51 (37.8) | 16 (44.4) | 7 | 9 | 35 (35.4) | 11 | 24 | 0.42 |
| 23-valent | 99 (73.3) | 29 (8.6) | 12 | 17 | 70 (70.7) | 30 | 40 | 0.28 |
I, invasive; NI, non-invasive.
p-value compares invasive versus non-invasive isolates per serotype.
Figure 1. (A) The yearly distribution and (B) comparative analyses of prevalence of Streptococcus pneumoniae serotypes (PCV10, PCV13 and NVTs) during the two study periods of low (2009–2013) and high (2014–2016) childhood vaccination coverage. PCV10 and PCV13 serotypes significantly decreased (P = 0.029 and P = 0.002, respectively), while NVTs significantly increased (P = 0.002), over the second period.
PCV10, 10-valent pneumococcal conjugate vaccine; PCV13, 13-valent pneumococcal conjugate vaccine; NVTs, non-vaccine serotypes.
Table 2. Serotype distribution of all the Streptococcus pneumoniae isolates collected during the 2 study periods (2009–2013 and 2014–2016).
| Serotype | No. of isolates | |
|---|---|---|
| 2009–2013 | 2014–2016 | |
| 3a | 8 | 5 |
| 4a | 1 | 0 |
| 6Aa | 1 | 0 |
| 6Ba | 1 | 2 |
| 7Fa | 8 | 0 |
| 8 | 2 | 6 |
| 9Va | 2 | 1 |
| 9N | 2 | 1 |
| 10A | 2 | 1 |
| 11A | 8 | 4 |
| 12F | 1 | 1 |
| 14a | 4 | 0 |
| 15A | 8 | 6 |
| 17F | 15 | 0 |
| 19Aa | 13 | 0 |
| 19Fa | 5 | 0 |
| 20 | 1 | 2 |
| 22F | 0 | 3 |
| 23B | 6 | 1 |
| 35B | 1 | 6 |
| 35F | 0 | 2 |
a13-valent pneumococcal conjugate vaccine serotypes.
The in vitro susceptibility profile of the isolates to all antimicrobial agents tested are presented in table 3. The PNSP isolates accounted for 34.8% of all isolates (27.4% with intermediate resistance and 7.4% with high-level resistance). For other beta-lactam antibiotics tested, amoxicillin, cefuroxime, cefotaxime, ceftriaxone, cefepime, imipenem, meropenem resistance has been observed in 9.6%, 20%, 4.4%, 4.4%, 8.8%, 8.1% and 7.4%, respectively. Resistance to erythromycin was detected in 41 isolates (30.4%). Among them, M and cMLSB phenotypes were detected in 20 (48.8%) and 21 strains (51.2%) respectively. Six strains (14.6%) with the iMLSB phenotype were found. Two isolates were resistant to newer fluoroquinolones, only one to tigecycline, while all isolates were susceptible to vancomycin, linezolid and daptomycin (Table 3). The non-invasive isolates were more resistant than the invasive ones, but the difference was not statistically significant (Table 4).
Table 3. In vitro activities of the antimicrobial agents tested against the 135 Streptococcus pneumoniae isolates.
| Antibiotic | MIC50 | MIC90 | Range | S (%) | I (%) | R (%) |
|---|---|---|---|---|---|---|
| Penicillin | 0.032 | 0.75 | <0.016–4 | 65.2 | 27.4 | 7.4 |
| Amoxicillin | 0.032 | 2 | 0.016–64 | 90.4 | 5.9 | 3.7 |
| Cefuroxime | 0.047 | 2 | 0.016–12 | 80 | 6.7 | 13.3 |
| Cefotaxime | 0.047 | 0.75 | <0.016–8 | 95.6 | 2.2 | 2.2 |
| Cefepime | 0.19 | 1 | 0.016–8 | 91.2 | 4.4 | 4.4 |
| Imipenem | 0.047 | 0.125 | 0.002–3 | 91.9 | 5.9 | 2.2 |
| Meropenem | 0.047 | 0.25 | 0.002–3 | 92.6 | 5.9 | 1.5 |
| Erythromycin | 0.064 | ≥256 | 0.016–≥256 | 69.6 | - | 30.4 |
| Clindamycin | 0.064 | ≥256 | 0.016–≥256 | 84.4 | - | 15.6 |
| Ciprofloxacin | 0.75 | 1 | 0.023–≥32 | 97.8 | - | 2.2 |
| Levofloxacin | 0.75 | 1 | 0.032–≥32 | 98.5 | - | 1.5 |
| Moxifloxacin | 0.125 | 0.19 | 0.016–≥32 | 98.5 | - | 1.5 |
| Chloramphenicol | 2 | 3 | 0.19–12 | 98.5 | - | 1.5 |
| Tetracycline | 0.25 | 32 | 0.047–≥256 | 83.7 | - | 16.3 |
| TMP/SMX | 0.094 | 2 | 0.012–≥32 | 83.7 | 8.9 | 7.4 |
| Vancomycin | 0.38 | 0.5 | 0.016–1 | 100 | - | 0.0 |
| Linezolid | 0.75 | 1 | 0.023–2 | 100 | - | 0.0 |
| Quinupristin/dalfopristin | 0.38 | 1 | 0.125–4 | 91.1 | 5.9 | 3 |
| Tigecycline | 0.047 | 0.25 | 0.016–1.5 | 99.3 | - | 0.7 |
| Daptomycin | 0.094 | 0.125 | 0.016–0.75 | 100 | - | 0.0 |
S, susceptible; I, intermediate; R, resistant; TMP/SMX, trimethoprim-sulfamethoxazole.
Table 4. Comparison of antimicrobial resistance rates in invasive and non-invasive Streptococcus pneumoniae isolates over the study period.
| Antibiotic | Invasive | Non-invasive | P-value | ||||
|---|---|---|---|---|---|---|---|
| Susceptible No. (%) | Intermediate No. (%) | Resistant No. (%) | Susceptible No. (%) | Intermediate No. (%) | Resistant No. (%) | ||
| Penicillin | 25 (69.4) | 9 (25) | 2 (5.6) | 63 (63.6) | 28 (28.3) | 8 (8.1) | 0.79 |
| Amoxicillin | 33 (91.6) | 1 (2.8) | 2 (5.6) | 89 (89.9) | 7 (7.1) | 3 (3.0) | 0.52 |
| Cefuroxime | 28 (77.8) | 1 (2.8) | 7 (19.4) | 80 (80.8) | 8 (8.1) | 11 (11.1) | 0.28 |
| Cefotaxime | 33 (91.6) | 2 (5.6) | 1 (2.8) | 96 (97) | 1 (1.0) | 2 (2.0) | 0.27 |
| Cefepime | 33 (91.6) | 1 (2.8) | 2 (5.6) | 90 (90.9) | 5 (5.1) | 4 (4.0) | 0.80 |
| Imipenem | 34 (94.4) | 1 (2.8) | 1 (2.8) | 90 (90.9) | 7 (7.1) | 2 (2.0) | 0.62 |
| Meropenem | 35 (97.2) | 1 (2.8) | - | 90 (90.9) | 7 (7.1) | 2 (2.0) | 0.43 |
| Erythromycin | 29 (80.6) | - | 7 (19.4) | 65 (65.7) | - | 34 (34.3) | 0.14 |
| Clindamycin | 32 (88.9) | - | 4 (11.1) | 82 (82.8) | - | 17 (17.2) | 0.59 |
| Ciprofloxacin | 36 (100) | - | - | 96 (97) | - | 3 (3.0) | 0.56 |
| Levofloxacin | 36 (100) | - | - | 97 (98) | - | 2 (2.0) | 1.00 |
| Moxifloxacin | 36 (100) | - | - | 97 (98) | - | 2 (2.0) | 1.00 |
| Chloramphenicol | 36 (100) | - | - | 97 (98) | - | 2 (2.0) | 1.00 |
| Tetracycline | 31 (86.1) | - | 5 (13.9) | 82 (82.8) | - | 17 (17.2) | 0.79 |
| TMP/SMX | 28 (77.8) | 4 (11.1) | 4 (11.1) | 86 (86.8) | 7 (7.1) | 6 (6.1) | 0.42 |
| Vancomycin | 36 (100) | - | - | 99 (100) | - | - | NA |
| Linezolid | 36 (100) | - | - | 99 (100) | - | - | NA |
| Quinupristin/dalfopristin | 32 (88.9) | 1 (2.8) | 3 (8.3) | 91 (91.9) | 7 (7.1) | 1 (1.0) | 0.06 |
| Tigecycline | 36 (100) | - | - | 98 (99) | - | 1 (1.0) | 1.00 |
| Daptomycin | 36 (100) | - | - | 99 (100) | - | - | NA |
TMP/SMX, trimethoprim-sulfamethoxazole; NA, not applicable.
Serotypes 19A, 11A, and 15A, prevailed among penicillin-intermediate (PISP) and penicillin-resistant (PRSP) isolates (Table 5).
Table 5. Serotype distribution of Streptococcus pneumoniae isolates according to penicillin susceptibility.
| Serotype | PSSP | PISP | PRSP |
|---|---|---|---|
| 3 | 13 (14.8) | 0 | 0 |
| 4 | 1 (1.1) | 0 | 0 |
| 6A | 1 (1.1) | 0 | 0 |
| 6B | 1 (1.1) | 2 (5.4) | 0 |
| 7F | 8 (9.1) | 0 | 0 |
| 8 | 6 (6.8) | 1 (2.7) | 1 (10) |
| 9V | 3 (3.4) | 0 | 0 |
| 9N | 2 (2.3) | 0 | 1 (10) |
| 10A | 3 (3.4) | 0 | 0 |
| 11A | 4 (4.5) | 7 (18.9) | 1 (10) |
| 12F | 2 (2.3) | 0 | 0 |
| 14 | 3 (3.4) | 1 (2.7) | 0 |
| 15A | 6 (6.8) | 8 (21.6) | 0 |
| 17F | 12 (13.6) | 2 (5.4) | 1 (10) |
| 19A | 3 (3.4) | 7 (18.9) | 3 (30) |
| 19F | 2 (2.3) | 1 (2.7) | 2 (20) |
| 20 | 3 (3.4) | 0 | 0 |
| 22F | 3 (3.4) | 0 | 0 |
| 23B | 5 (5.7) | 2 (5.4) | 0 |
| 35B | 2 (2.3) | 5 (13.5) | 0 |
| 35F | 1 (1.1) | 1 (2.7) | 0 |
| Non-typeable | 4 (4.5) | 0 | 1 (10) |
Data are in the form n (%).
PSSP, penicillin-susceptible S. pneumoniae; PISP, penicillin-intermediate S. pneumoniae; PRSP, penicillin-resistant S. pneumoniae.
The rate of MDR observed among PNSP isolates was 40.4%. Multidrug resistance was higher in non-invasive than in invasive isolates. However, the difference was not statistically significant (P = 0.78). The number of MDR strains varied among serotypes. The predominant pattern of multidrug resistance was non-susceptibility to penicillin, erythromycin, clindamycin, and tetracycline (26.3%). Isolates with this phenotype belonged to serotypes 15A and 17F. The second most frequent MDR phenotypes exhibited non-susceptibility to penicillin, erythromycin, tetracycline, and TMP/SMX (15.8%), and isolates with this phenotype belonged to serotype 19A and 19F, while the third group exhibited non-susceptibility to penicillin, erythromycin, clindamycin, tetracycline, and quinupristin/dalfopristin (15.8%) and strains with this phenotype belonged to serotypes 15A, 19A and 19F.
Discussion
The present study has analyzed trends in epidemiology of pneumococcal disease in adults, including serotype distribution and antimicrobial resistance of S. pneumoniae clinical isolates from patients with IPD and non-IPD during an 8-year time period (2009–2016), and attempted to correlate epidemiological shifts as compared to a previous study period (2001–2008) [11] with the introduction of childhood higher-valent pneumococcal vaccination.
Comparison of the present results to those of a previous study from the same area, has revealed an overall 30.8% reduction of pneumococcal isolates derived from adults [11]. More specifically, IPD isolates have demonstrated a 12.2% decrease, while non-IPD ones 35.7%. Vaccination had a major impact on vaccine type (VT) infections. In particular, there was a 66.2% decrease in VT13 disease. These results are consistent with reports from other parts of the world reporting variable reductions in the overall IPD and CAP in adults, indicating that higher-valent PCVs are effective in inducing herd protection in unvaccinated population [13]. In Greece, the national meningitis reference laboratory reported a decrease in pneumococcal meningitis incidence from 0.89 per 100,000 populations in 2009 to 0.56 in 2015, in adults >60 years of age [14]. Regev-Yochay et al. in Israel found 70.1% reduction of IPD in adults covered by PCV13 within 4.5 years after the introduction of the vaccine. A >80% decrease was observed in the younger adults, while 65% was observed in older adults (≥65 years) [15]. In the UK, Waight et al. reported similar PCV impact on reducing PCV13 IPD, and Rodrigo et al. demonstrated a reduction from 24.8% to 12.6% in PCV13 CAP in adults over the last 5 years, with the largest reductions seen in those aged >85 years [16,17]. On the contrary, a study conducted in Spain evaluating S. pneumoniae serotypes of CAP in adults requiring hospitalization after the introduction of PCV13 in childhood vaccination, has shown that around 60% of pneumococcal CAPs were caused by the PCV13 serotypes, concluding that there were no significant changes from 2011 to 2014 [18]. Another recent Spanish study demonstrated that PCV13 serotypes differ across regions, depending on the different vaccine use in children, with lower percentage of cases in regions with high vaccine uptake [19]. The magnitude of the indirect protection depends on PCV coverage rates. Higher herd protection is achievable in countries with high vaccine uptake, through prevention of VT nasopharyngeal carriage and transmission. According to the WHO database, vaccination coverage in Greece was 32% from 2006-2013 and 96% in 2014–2016 [20]. The modest reduction of pneumococcal infections attributed to PCV13 serotypes during the first years of the 8-year study period is related to the low vaccination rates in children. Unlike children, there is no published data on vaccination coverage of adults for the PCV13 and PPV23. Based on data from the market sales in Greece, the estimated vaccination coverage of the adult population is relatively low, including ~ 34% and 20% vaccination rates for PCV13 in the elderly (> 65 years) and high risk adults, and a similarly low rate of vaccination coverage for PPV23 (~ 30% in the elderly). Therefore, it is reasonable to speculate that the decreased rates of pneumococcal disease observed in our study are related to a herd immunity effect. PCV13 IPD rate was 44.4% during the study period, similar to that found by investigators analyzing IPD isolates from 50 countries worldwide that have shown that 40.9% of IPD in adults ≥65 years was caused by VT in the PCV13 period [21]. Another study estimating serotype distributions of non-IPD isolates from adults ≥18 years in Europe and the USA between 2014–2015, has shown that serotype 3 was the most prevalent among isolates collected in Europe from all adults and in the USA among adults 18-64 years of age, while serotype 19A was most prevalent among adults ≥65 years from the USA [22]. In line with this study, other investigators have found serotypes 3 and 19A to be frequent causes of adult IPD in the higher-valent period [23,24,25]. Persistence of serotype 3 is probably due to the poor PCV13 efficacy against this serotype. Persistence/increase of 19A seems to be not related to vaccine pressure and might be attributed to secular trends and to antibiotic pressure leading to increased selection and spread of resistant clones [26].
After the higher-valent PCV introduction in parallel with the decline of VT pneumococcal diseases, an increased incidence of NVT was detected (58.5%). The observed increase in the incidence of NVT pneumococcal diseases is indicative of serotype replacement [27]. The NVTs that increased in the higher-valent PCV period were 17F, 15A, 11A, 8, 23B, 35B, 9N, 10A, 20, 22F, 35F and 12F. A recent review assessing the rise in NVT after higher-valent PCV implementation, showed that 57%-84% of all IPD in adults ≥65years were caused by NVT [28]. In several countries the most frequently emerging serotypes implicated in adult IPDs since the post-PCV13 period were 22F and 8 [16,23,29,30].
Penicillin resistance was observed in almost one third of S. pneumoniae strains (7.4% resistant and 27.4% intermediately resistant). Although penicillin resistance remains a major concern for the treatment of meningitis, it does not affect the outcome of extrameningeal penicillin non-susceptible pneumococcal (PNSP) infections, because penicillin levels achieved at doses ordinarily used overcome resistance [31]. The increased resistance to penicillin is attributed to selection pressure by beta-lactam overuse. Nevertheless, a substantial decrease in penicillin-resistant and MDR strains (reduction by 6.9% and 7.4%, respectively) was noted after the introduction of the higher-valent vaccines. This change is due to the reduction of infections caused by the vaccine serotypes. Further decrease has not been observed due to persistence of the 19A, and increase of the 15A serotype that have PNSP and MDR phenotypes. Serotype 15A is among the NVT serotypes with the highest proportion of nonsusceptible isolates [32].
Despite the high non-susceptibility rates to penicillin and cefuroxime, resistance to third generation cephalosporins was low (<5%), making them a good choice for empirical treatment for suspected bacterial meningitis. Likewise, amoxicillin with good in vitro activity has been proved effective and is considered the first choice for the treatment of uncomplicated cases of community-acquired pneumonia, in many European countries [33].
Erythromycin resistance, which reached 30.5%, was mainly M-phenotype. Increased macrolide consumption is most probably the cause of increased resistance to this class of antimicrobials. Goosens et al. correlating outpatient antibiotic use with resistance in 32 countries has shown higher resistance rates to macrolides in higher consuming countries and Greece was the country with the highest macrolide use [34].
The present surveillance demonstrates that despite the extensive use of fluoroquinolones in the outpatient setting, fluoroquinolone resistance in pneumococci is still very low. In many countries resistance of S. pneumoniae to fluoroquinolones remains below 2% and seems to be unrelated to serotype switches after the PCVs introduction [35,36,37].
Resistance to TMP/SMX was 16.3%, significantly lower than that observed before higher-valent vaccine era [11]. Decreased rates of resistance to TMP/SMX in Crete are likely due to vaccination and reduced use of this antimicrobial. In South Africa, where TMP/SMX is extensively used in human immunodeficiency virus patients as prophylaxis from opportunistic infections, the rates of S. pneumoniae resistance to this drug are high. Additionally, TMP/SMX nonsusceptibility has been found to be associated with nonsusceptibility to penicillin, erythromycin, rifampicin and multidrug resistance [38]. In Malawi, S. pneumoniae TMP/SMX resistance reached a level of 96% in patients receiving treatment, due to the widespread use of this antimicrobial [39].
All isolates were susceptible to vancomycin, linezolid and daptomycin. These data suggest that these agents may be useful alternatives for treatment of penicillin-resistant and MDR pneumococcal infections.
The present study has certain limitations. First, the number of isolates tested was relatively small. Second, the results reflect on local epidemiological data from a geographical area that are not representative of the epidemiology of pneumococcal disease in the whole country, since serotype distribution and antimicrobial resistance may fluctuate among geographic regions, even in different areas of the same country. Third, detailed clinical information on the patients' clinical characteristics and outcome was not available.
The study has demonstrated that serotype distribution and antimicrobial resistance of pneumococcal isolates from adult patients have evolved after the introduction of pneumococcal conjugate higher-valent vaccination in children, indicating an indirect effect in adults, in Crete. Sustained high use of PCVs in targeted population and appropriate use of antimicrobial agents is likely to further reduce non-susceptible pneumococcal strains. Surveillance of emerging non-vaccine covered strains highly associated with antimicrobial resistance is of utmost importance, in order to guide prevention strategies and vaccine policy.
Footnotes
Conflict of Interest: No conflicts of interest.
- Conceptualization: SM.
- Data curation: VEM, DS.
- Formal analysis: GH, GS, DS.
- Writing - original draft: SM, GH, GS, VEM, DS.
- Writing - review & editing: SM, GH, GS, VEM.
References
- 1.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 2.Musher DM. Streptococcus pneumoniae. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and Practice of Infectious Diseases. 6th ed. New York: Elsevier, Churchill Livingstone; 2010. pp. 2623–2642. [Google Scholar]
- 3.Marrie TJ, Tyrrell GJ, Majumdar SR, Eurich DT. Effect of age on the manifestations and outcomes of invasive pneumococcal diseases in adults. Am J Med. 2018;131:100.e1–100.e7. doi: 10.1016/j.amjmed.2017.06.039. [DOI] [PubMed] [Google Scholar]
- 4.Shea KM, Edelsberg J, Weycker D, Farkouh RA, Strutton DR, Pelton SI. Rates of pneumococcal disease in adults with chronic medical conditions. Open Forum Infect Dis. 2014;1:ofu024. doi: 10.1093/ofid/ofu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Said MA, Johnson HL, Nonyane BA, Deloria-Knoll M, O'Brien KL AGEDD Adult Pneumococcal Burden Study Team. Andreo F, Beovic B, Blanco S, Boersma WG, Boulware DR, Butler JC, Carratalà J, Chang FY, Charles PG, Diaz AA, Domínguez J, Ehara N, Endeman H, Falcó V, Falguera M, Fukushima K, Garcia-Vidal C, Genne D, Guchev IA, Gutierrez F, Hernes SS, Hoepelman AI, Hohenthal U, Johansson N, Kolek V, Kozlov RS, Lauderdale TL, Mareković I, Masiá M, Matta MA, Miró Ò, Murdoch DR, Nuermberger E, Paolini R, Perelló R, Snijders D, Plečko V, Sordé R, Strålin K, van der Eerden MM, Vila-Corcoles A, Watt JP. Estimating the burden of pneumococcal pneumonia among adults: a systematic review and meta-analysis of diagnostic techniques. PLoS One. 2013;8:e60273. doi: 10.1371/journal.pone.0060273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GBD 2015 LRI Collaborators. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory tract infections in 195 countries: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:1133–1161. doi: 10.1016/S1473-3099(17)30396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welte T, Torres A, Nathwani D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax. 2012;67:71–79. doi: 10.1136/thx.2009.129502. [DOI] [PubMed] [Google Scholar]
- 8.Castiglia P. Recommendations for pneumococcal immunization outside routine childhood immunization programs in Western Europe. Adv Ther. 2014;31:1011–1044. doi: 10.1007/s12325-014-0157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klugman KP. Herd protection induced by pneumococcal conjugate vaccine. Lancet Glob Health. 2014;2:e365–e366. doi: 10.1016/S2214-109X(14)70241-4. [DOI] [PubMed] [Google Scholar]
- 10.Huang SS, Johnson KM, Ray GT, Wroe P, Lieu TA, Moore MR, Zell ER, Linder JA, Grijalva CG, Metlay JP, Finkelstein JA. Healthcare utilization and cost of pneumococcal disease in the United States. Vaccine. 2011;29:3398–3412. doi: 10.1016/j.vaccine.2011.02.088. [DOI] [PubMed] [Google Scholar]
- 11.Maraki S, Mantadakis E, Samonis G. Serotype distribution and antimicrobial resistance of adult Streptococcus pneumoniae clinical isolates over the period 2001-2008 in Crete, Greece. Chemotherapy. 2010;56:325–332. doi: 10.1159/000320152. [DOI] [PubMed] [Google Scholar]
- 12.Clinical Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-sixth informational supplement. Wayne, Pa: CLSI; 2016. [Google Scholar]
- 13.Tsaban G, Ben-Shimol S. Indirect (herd) protection, following pneumococcal conjugated vaccines introduction: A systematic review of the literature. Vaccine. 2017;35:2882–2891. doi: 10.1016/j.vaccine.2017.04.032. [DOI] [PubMed] [Google Scholar]
- 14.Tzanakaki G. National Meningitis Reference Laboratory Data 1993–2015. 2015. [Accessed 23 June 2018]. Available from: http://www.nsph.gr/files/001_Dimosias_Dioikitikis_Ygieinis/ EKAM/ApologismoiEKAM/Apol-2015-1.pdf.
- 15.Regev-Yochay G, Katzir M, Strahilevitz J, Rahav G, Finn T, Miron D, Maor Y, Chazan B, Schindler Y, Dagan R IAIPD group. The herd effects of infant PCV7/PCV13 sequential implementation on adult invasive pneumococcal disease, six years post implementation; a nationwide study in Israel. Vaccine. 2017;35:2449–2456. doi: 10.1016/j.vaccine.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 16.Waight PA, Andrews NJ, Ladhani SN, Sheppard CL, Slack MP, Miller E. Effect of the 13-valent pneumococcal conjugate vaccine on invasive pneumococcal disease in England and Wales 4 years after its introduction: an observational cohort study. Lancet Infect Dis. 2015;15:535–543. doi: 10.1016/S1473-3099(15)70044-7. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigo C, Bewick T, Sheppard C, Greenwood S, Mckeever TM, Trotter CL, Slack M, George R, Lim WS. Impact of infant 13-valent pneumococcal conjugate vaccine on serotypes in adult pneumonia. Eur Respir J. 2015;4:1632–1641. doi: 10.1183/09031936.00183614. [DOI] [PubMed] [Google Scholar]
- 18.Menéndez R, España PP, Pérez-Trallero E, Uranga A, Méndez R, Cilloniz C, Marimón JM, Cifuentes I, Méndez C, Torres A. The burden of PCV13 serotypes in hospitalized pneumococcal pneumonia in Spain using a novel urinary antigen detection test. CAPA study. Vaccine. 2017;35:5264–5270. doi: 10.1016/j.vaccine.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 19.España PP, Menendez R, Torres A, Fernández-Villar JA, Marimon JM, Martínez De La Fuente AP, Brusola AG, Reverte FM, Vasallo-Vidal F, Ercibengoa M, Cifuentes I, Méndez C. Differences in the burden of all-cause CAP due to PCV13 serotypes based in the different use of vaccine by regions in Spain (the CAPA study); 28th European Congress of Clinical Microbiology and Infectious Diseases; 2018 Apr 21-24; Madrid, Spain. [Google Scholar]
- 20.WHO. Immunization, Vaccines and Biologicals - Data, statistics and graphics. [Accessed 2 May 2018]. Available from: http://www.who.int/immunization/monitoring_surveillance/data/en/
- 21.Kazmierczak K, Hackel M, Henry LI, Kalamatas J, Hilton B, Sings H, Isturiz R. Distribution of Streptococcus pneumoniae serotypes among global population; 27th European Congress of Clinical Microbiology and Infectious Diseases; 2017 Apr 22-25; Vienna, Austria. [Google Scholar]
- 22.Kazmierczak K, Hackel M, Kalamatas J, Hall-Murray C, Sings H, Isturiz R. Distribution of Streptococcus pneumoniae serotypes among non-sterile isolates from adults 18 years and older in Europe and the United States, 2014-2015; 27th European Congress of Clinical Microbiology and Infectious Diseases; 2017 Apr 22-25; Vienna, Austria. [Google Scholar]
- 23.Horácio AN, Silva-Costa C, Lopes JP, Ramirez M, Melo-Cristino J Portuguese Group for the Study of Streptococcal Infections. Serotype 3 remains the leading cause of invasive pneumococcal disease in adults in Portugal (2012-2014) despite continued reductions in other 13-valent conjugate vaccine serotypes. Front Microbiol. 2016;7:1616. doi: 10.3389/fmicb.2016.01616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corcoran M, Vickers I, Mereckiene J, Murchan S, Cotter S, Fitzgerald M, McElligott M, Cafferkey M, O'Flanagan D, Cunney R, Humphreys H. The epidemiology of invasive pneumococcal disease in older adults in the post-PCV era. Has there been a herd effect? Epidemiol Infect. 2017;145:2390–2399. doi: 10.1017/S0950268817001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Linden M, Falkenhorst G, Perniciaro S, Imöhl M. Effects of infant pneumococcal conjugate vaccination on serotype distribution in invasive pneumococcal disease among children and adults in Germany. PLoS One. 2015;10:e0131494. doi: 10.1371/journal.pone.0131494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izurieta P, Bahety P, Adegbola R, Clarke C, Hoet B, Andrews NJ, Waight PA, Burbidge P, Pearce E, Roalfe L, Zancolli M, Slack M, Ladhani SN, Miller E, Goldblatt D. Public health impact of pneumococcal conjugate vaccine infant immunization programs: assessment of invasive pneumococcal disease burden and serotype distribution. Expert Rev Vaccines. 2018;17:479–493. doi: 10.1080/14760584.2018.1413354. [DOI] [PubMed] [Google Scholar]
- 27.Feikin DR, Kagucia EW, Loo JD, Link-Gelles R, Puhan MA, Cherian T, Levine OS, Whitney CG, O'Brien KL, Moore MR Serotype Replacement Study Group. Serotype-specific changes in invasive pneumococcal disease after pneumococcal conjugate vaccine introduction: a pooled analysis of multiple surveillance sites. PLoS Med. 2013;10:e1001517. doi: 10.1371/journal.pmed.1001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hausdorff WP, Hanage WP. Interim results of an ecological experiment - Conjugate vaccination against the pneumococcus and serotype replacement. Hum Vaccin Immunother. 2016;12:358–374. doi: 10.1080/21645515.2015.1118593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, Miller L, Scherzinger K, Thomas A, Farley MM, Zell ER, Taylor TH, Jr, Pondo T, Rodgers L, McGee L, Beall B, Jorgensen JH, Whitney CG. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15:301–309. doi: 10.1016/S1473-3099(14)71081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hays C, Vermee Q, Agathine A, Dupuis A, Varon E, Poyart C, Ploy MC, Raymond J and the ORP IIe de France Quest. Demonstration of the herd effect in adults after the implementation of pneumococcal vaccination with PCV13 in children. Eur J Clin Microbiol Infect Dis. 2017;36:831–838. doi: 10.1007/s10096-016-2868-5. [DOI] [PubMed] [Google Scholar]
- 31.Falcó V, Burgos J, Pahissa A. The spectrum of invasive pneumococcal disease in adults in the XXI century. Clin Pulm Med. 2013;20:214–220. [Google Scholar]
- 32.Dagan R, Juergens C, Trammel J, Patterson S, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Efficacy of 13-valent pneumococcal conjugate vaccine (PCV13) versus that of 7-valent PCV (PCV7) against nasopharyngeal colonization of antibiotic-nonsusceptible Streptococcus pneumoniae . J Infect Dis. 2015;211:1144–1153. doi: 10.1093/infdis/jiu576. [DOI] [PubMed] [Google Scholar]
- 33.Woodhead M, Blasi F, Ewig S, Garau J, Huchon G, Ieven M, Ortqvist A, Schaberg T, Torres A, van der Heijden G, Read R, Verheij TJ Joint Taskforce of the European Respiratory Society and European Society for Clinical Microbiology and Infectious Diseases. Guidelines for the management of adult lower respiratory tract infections--full version. Clin Microbiol Infect. 2011;17(Suppl 6):E1–59. doi: 10.1111/j.1469-0691.2011.03672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goossens H, Ferech M, Vander Stichele R, Elseviers M ESAC Project Group. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365:579–587. doi: 10.1016/S0140-6736(05)17907-0. [DOI] [PubMed] [Google Scholar]
- 35.Imöhl M, Reinert RR, van der Linden M. Antibiotic susceptibility rates of invasive pneumococci before and after the introduction of pneumococcal conjugate vaccination in Germany. Int J Med Microbiol. 2015;305:776–783. doi: 10.1016/j.ijmm.2015.08.031. [DOI] [PubMed] [Google Scholar]
- 36.Mendes RE, Costello AJ, Jacobs MR, Biek D, Critchley IA, Jones RN. Serotype distribution and antimicrobial susceptibility of USA Streptococcus pneumoniae isolates collected prior to and post introduction of 13-valent pneumococcal conjugate vaccine. Diagn Microbiol Infect Dis. 2014;80:19–25. doi: 10.1016/j.diagmicrobio.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Hauser C, Kronenberg A, Allemann A, Mühlemann K, Hilty M. Serotype/serogroup-specific antibiotic non-susceptibility of invasive and non-invasive Streptococcus pneumoniae, Switzerland, 2004 to 2014. Euro Surveill. 2016;21(21) doi: 10.2807/1560-7917.ES.2016.21.21.30239. [DOI] [PubMed] [Google Scholar]
- 38.Soeters HM, von Gottberg A, Cohen C, Quan V, Klugman KP. Trimethoprim-sulfamethoxazole prophylaxis and antibiotic nonsusceptibility in invasive pneumococcal disease. Antimicrob Agents Chemother. 2012;56:1602–1605. doi: 10.1128/AAC.05813-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cornick JE, Harris SR, Parry CM, Moore MJ, Jassi C, Kamng'ona A, Kulohoma B, Heyderman RS, Bentley SD, Everett DB. Genomic identification of a novel co-trimoxazole resistance genotype and its prevalence amongst Streptococcus pneumoniae in Malawi. J Antimicrob Chemother. 2014;69:368–374. doi: 10.1093/jac/dkt384. [DOI] [PMC free article] [PubMed] [Google Scholar]