The C-terminal end of Cdt2 contains a PIP box for binding to PCNA to promote CRL4Cdt2 function, creating a new paradigm where the substrate receptor and substrates bind to a common multivalent docking platform for ubiquitination.
Abstract
The CRL4Cdt2 ubiquitin ligase complex is an essential regulator of cell-cycle progression and genome stability, ubiquitinating substrates such as p21, Set8, and Cdt1, via a display of substrate degrons on proliferating cell nuclear antigens (PCNAs). Here, we examine the hierarchy of the ligase and substrate recruitment kinetics onto PCNA at sites of DNA replication. We demonstrate that the C-terminal end of Cdt2 bears a PCNA interaction protein motif (PIP box, Cdt2PIP), which is necessary and sufficient for the binding of Cdt2 to PCNA. Cdt2PIP binds PCNA directly with high affinity, two orders of magnitude tighter than the PIP box of Cdt1. X-ray crystallographic structures of PCNA bound to Cdt2PIP and Cdt1PIP show that the peptides occupy all three binding sites of the trimeric PCNA ring. Mutating Cdt2PIP weakens the interaction with PCNA, rendering CRL4Cdt2 less effective in Cdt1 ubiquitination and leading to defects in Cdt1 degradation. The molecular mechanism we present suggests a new paradigm for bringing substrates to the CRL4-type ligase, where the substrate receptor and substrates bind to a common multivalent docking platform to enable subsequent ubiquitination.
Introduction
The integrity of genomic information is maintained in the cell cycle by faithful replication during the S phase and segregation of duplicated chromosomes during mitosis, which is critical for proper cell reproduction, cell function, and cell survival. In addition, cells are continuously challenged by genotoxic agents and environmental stress, and have complex mechanisms to activate DNA damage checkpoints, prevent cell-cycle progression, and repair the damaged DNA (Hoeijmakers, 2001; Branzei & Foiani, 2010). Many of the cell cycle transition events, as well as responses to DNA damage, are driven by E3 Cullin-RING ubiquitin Ligases (CRLs) that catalyse the ubiquitination and destruction of specific protein targets. Such cell cycle–regulated E3 ligases include CRL1Fbox and CRL4DCAF, which target many substrates crucial for cell cycle regulation and DNA damage responses (Cardozo & Pagano, 2004; Petroski & Deshaies, 2005; Jackson & Xiong, 2009). These CRLs comprise a scaffolding protein (cullin 1 or cullin 4 [Cul4]), an adapter protein (Skp1 and DDB1, respectively), and a RING domain protein that interacts with the E2 (such as Rbx1 or Rbx2). Finally, CRL1 and CRL4 ligases contain either an F-box or DCAF substrate recognition factor (SRF, or substrate receptor), respectively, responsible for interacting with the substrate and targeting it for ubiquitination. F-box proteins in CRL1, such as Fbw7 or β-TRCP, recognize specific degrons in substrates that often contain phosphorylated residues, whereas CRL4 include DCAFs such as DDB2, which directly recognizes UV-damaged DNA (Scrima et al, 2008).
The CRL4Cdt2 ligase uses Cdt2 as the SRF, and functions both during the S phase and after DNA damage (Abbas & Dutta, 2011; Havens & Walter, 2011; Sakaguchi et al, 2012; Stathopoulou et al, 2012). Cdt2, targets substrates such as p21 and Set8, and the DNA replication licensing factor Cdt1 for ubiquitin-mediated proteolysis, both in S phase and following DNA damage (Abbas et al, 2008; Kim et al, 2008; Nishitani et al, 2008; Centore et al, 2010; Oda et al, 2010; Tardat et al, 2010; Jorgensen et al, 2011). In addition, an increasing number of Cdt2 target proteins have been identified, including thymine DNA glycosylase, Cdc6, the DNA polymerase δ subunit p12 (Terai et al, 2013; Clijsters & Wolthuis, 2014; Shibata et al, 2014; Slenn et al, 2014), and xeroderma pigmentosum group G (XPG), a structure-specific repair endonuclease of the nucleotide excision repair pathway (Han et al, 2015).
Cdt1 and Cdt2 were originally identified as Cdc10-dependent transcript 1 and 2 in fission yeast, but have no sequence similarity (Hofmann & Beach, 1994). Cdt1 has a critical role in establishing the DNA replication licensing complex in the G1 phase: it associates with chromatin through the origin recognition complex and operates together with Cdc6 to load the MCM2-7 complex onto chromatin, thereby licensing DNA for replication (Bell & Dutta, 2002; Diffley, 2004; Nishitani & Lygerou, 2004; Blow & Dutta, 2005; Tsakraklides & Bell, 2010; Symeonidou et al, 2012).
Preventing re-licensing of replicated regions is essential (Blow & Dutta, 2005; Arias & Walter, 2007). One of the mechanisms to achieve this is by CRL1Skp2 and CRL4Cdt2 redundantly mediating Cdt1 destruction in higher organisms. CRL1Skp2 (also known as SCFSkp2) recognizes a phospho-degron motif on Cdt1 that is created at the initiation of S phase by CDKs (Li et al, 2003; Sugimoto et al, 2004; Nishitani et al, 2006). In contrast, CRL4Cdt2 recognizes Cdt1 when bound to the proliferating cell nuclear antigen (PCNA) trimer, through a binding motif (PIP box) in its N-terminal end (Arias & Walter, 2006; He et al, 2006; Higa et al, 2006; Jin et al, 2006; Nishitani et al, 2006; Ralph et al, 2006; Sansam et al, 2006; Senga et al, 2006; Kim & Kipreos, 2007). Both initiation of DNA replication and DNA damage trigger PCNA loading onto chromatin and Cdt1 association with PCNA through its PIP box (Arias & Walter, 2006; Havens & Walter, 2009; Raman et al, 2011; Shiomi et al, 2012). DNA damage–induced degradation of Cdt1 and other substrates appears to facilitate repair (Mansilla et al, 2013; Tsanov et al, 2014; Tanaka et al, 2017).
Cdt2 recruitment onto chromatin is not fully characterized: recruitment through the Cdt1 PIP box bound to PCNA and a specific basic residue downstream of the Cdt1 PIP box (Havens & Walter, 2009; Michishita et al, 2011; Havens et al, 2012) or independently of Cdt1 (Roukos et al, 2011) has been reported. Following CRL4Cdt2-mediated ubiquitination, Cdt1 is degraded, thus blocking further licensing. The N-terminal region of Cdt2 contains a WD40 repeat domain, predicted to form a substrate-recognizing propeller structure similar to the one shown for DDB2 (Scrima et al, 2008; Havens & Walter, 2011). In analogy with DDB2 (Fischer et al, 2011), the N-terminal domain of Cdt2 should bind to the DDB1 WD40 repeat domains, β-propeller A and β-propeller C, on one side. The other side would be expected to recognize the substrate, in analogy to DDB2. Higher eukaryotic Cdt2 proteins have an extended C-terminal region, not present in fission yeast. In Xenopus, the C-terminal domain of Cdt2 binds to PCNA and is important for the turnover of the Xic1 cyclin kinase inhibitor (Kim et al, 2010). Recently, we reported that Cdt2 mutated at multiple CDK consensus phosphorylation sites co-localized with PCNA throughout the S phase even when most of the substrates were degraded, also suggesting that Cdt2 interacts with PCNA independent of its substrates (Nukina et al, 2018).
To understand how Cdt2 recognizes PCNA and localizes CRL4Cdt2 activity during the S phase and following UV irradiation, we investigated the role of Cdt2 domains in localization and ubiquitination. Unexpectedly, we found a PIP box in the C-terminal end of Cdt2, and showed that it directly mediates interaction of Cdt2 with PCNA. Both Cdt1 and Cdt2 PIP box peptides bind the PCNA binding pocket in a similar manner, but Cdt2 has a significantly higher affinity for PCNA. We suggest that Cdt2 and Cdt1 could simultaneously recognize different subunits of the PCNA trimer, and we put forward a new paradigm for localizing E3 ligase activity onto the PCNA docking platform, allowing simultaneous docking of substrates in the same platform, enabling ubiquitination by proximity.
Results
Cdt2 is stably bound to UV-damaged sites independently of Cdt1
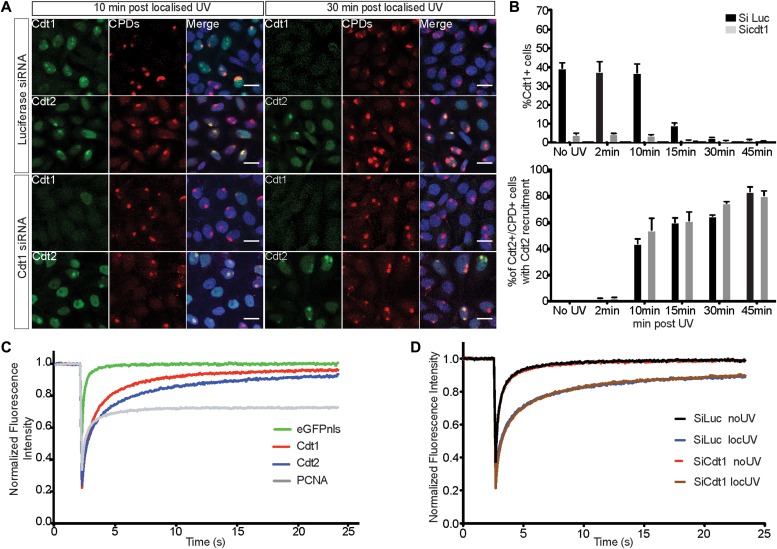
CRL4Cdt2 recognizes Cdt1 bound on PCNA and recruitment of CRL4Cdt2 to damaged chromatin was reported to require Cdt1 in Xenopus extracts (Havens & Walter, 2009). In contrast, experiments in live human cells suggested that Cdt2 was recruited to DNA damage sites independently of Cdt1 (Roukos et al, 2011). To verify whether Cdt2 binding to damaged sites requires Cdt1, we performed a time-course analysis of Cdt2 recruitment to sites of localized UV-C irradiation in control and Cdt1 depleted HeLa cells. Cells were exposed to UV-C through micropore filters, and locally induced damage was detected by the staining of cyclobutane pyrimidine dimers (CPDs). In control cells, Cdt1 was rapidly recruited to damaged sites (Fig 1A, 10 min) and proteolysed by 30 min (Fig 1A and B), consistent with earlier studies (Ishii et al, 2010; Roukos et al, 2011). Cdt2 was recruited at sites of damage by 10 min whereas cells showing Cdt2 recruitment increased at subsequent time points (30 and 45 min post-irradiation) even-though Cdt1 was degraded. Depletion of Cdt1 by siRNA had no effect on either the kinetics or the extent of Cdt2 recruitment to sites of UV-C damage (Fig 1A and B). These results show that Cdt2 is recruited to UV-C–irradiated sites independently of Cdt1 and remains there long after Cdt1 proteolysis.
Figure 1. Cdt2 binds stably to UV-damaged sites independently of Cdt1.
(A) HeLa cells were transfected with siRNAs targeted to Cdt1 (siCdt1) or control (siLuc), and locally UV-C irradiated (50 J/m2 through a micropore filter with 5-μm diameter pores), fixed at the indicated time points, and stained with antibodies against CPDs and Cdt1 or Cdt2. Nuclei were stained with Draq5. Scale bars: 20μm. (B) Percentage of cells positive for Cdt1 (top) and percentage of cells positive for CPDs and Cdt2 which show Cdt2 recruitment to sites of damage (bottom) are plotted (mean of two independent experiments with SD). (C) MCF7 cells were transfected with the indicated GFP-tagged plasmids and 24 h later they were locally UV-C irradiated (50 J/m2). 20 min following irradiation, FRAP experiments were conducted at the site of damage and in untreated cells (Fig S1). FRAP data were analysed with easyFRAP. Mean normalized fluorescence intensities at the site of damage as a function of time following photobleaching are shown. (D) MCF7 cells were synchronized in the S phase by treatment with 2-mM thymidine for 24 h. Upon release, cells were transfected with siCdt1 or control siLuc in parallel with a plasmid expressing GFP-tagged Cdt2. Cells were then synchronised in the M phase by treatment with 50 ng/ml nocodazole for 12 h, released into G1 for 5 h, and locally UV-C irradiated (50 J/m2). 20 min after irradiation, the cells were analysed by FRAP. Mean normalized fluorescence recovery curves at the sites of damage are shown.
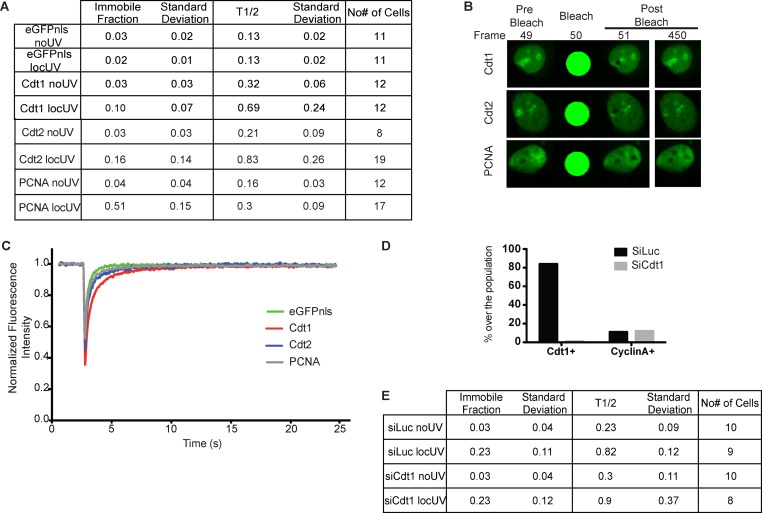
To assess the binding properties of Cdt2 at sites of UV-C damage in the presence and absence of Cdt1, fluorescence recovery after photobleaching (FRAP) was used. MCF7 cells transiently expressing Cdt1, Cdt2, or PCNA tagged with GFP were first analysed in the presence and absence of local UV-C irradiation (Figs 1C and S1A–C). All factors exhibit fast recovery in the absence of damage (Fig S1A and C), consistent with transient interactions (Xouri et al, 2007b). Following recruitment to the sites of damage, PCNA shows a significant immobile fraction, as expected for stable binding, whereas Cdt1 shows dynamic interactions at UV-C–damaged sites (Roukos et al, 2011; Rapsomaniki et al, 2015). Cdt2 has a slower fluorescence recovery rate at the site of damage than Cdt1, with a half-recovery time of 0.83 s and an immobile fraction of 16%, underlining long-term association at UV-C–damaged sites. To assess whether Cdt2 binding to sites of damage is affected by the presence of Cdt1, Cdt2 kinetics were assessed by FRAP in MCF7 cells synchronized in G1, depleted of Cdt1 and locally UV-C irradiated. As shown in Fig 1D and in Fig S1D and E, the binding kinetics of Cdt2 at the sites of damage remain the same despite the absence of Cdt1.
Figure S1. FRAP analysis of Cdt2 binding to sites of damage in the absence of Cdt1.
(A) Quantitative parameters, Immobile fraction, and half-maximal recovery time of the mobile fraction (T1/2), corresponding to the FRAP curves described in Fig 1C and showing the recovery of Cdt1-eGFP, Cdt2-eGFP, PCNA-eGFP, and eGFP-NSL as a control, in undamaged and locally UV-C–damaged cells. Mean values with SD, and the number of individual cells analysed by FRAP in each case are shown. Normalization and curve fitting of individual FRAP curves for parameter estimation was performed with easyFRAP. (B) FRAP images before, during, and after photobleaching at the sites of damage. (C) Mean normalized recovery curves of Cdt1-eGFP, Cdt2-eGFP, PCNA-eGFP, and eGFP-NSL in undamaged cells. (D) Efficiency of synchronization and Cdt1 depletion of MCF7 cells. The percentage of cells expressing Cdt1 or cyclin A is shown for MCF7 cells synchronized in G1, without (siLuc) or with (siCdt1) Cdt1 depletion, as described in Fig 1D. (E) Quantitative parameters, immobile fraction, and half-maximal recovery time of the mobile fraction (T1/2), corresponding to the FRAP curves described in Fig 1D and showing Cdt2 in the presence and absence of Cdt1 in undamaged and locally UV-C–damaged cells. Mean values with SD and the number of individual cells analysed by FRAP in each case are shown. Normalization and curve fitting of individual FRAP curves was performed with easyFRAP.
We conclude that Cdt2 binds to sites of damage stably independently of Cdt1.
The C-terminal part of Cdt2 is required for recruitment to DNA damage sites
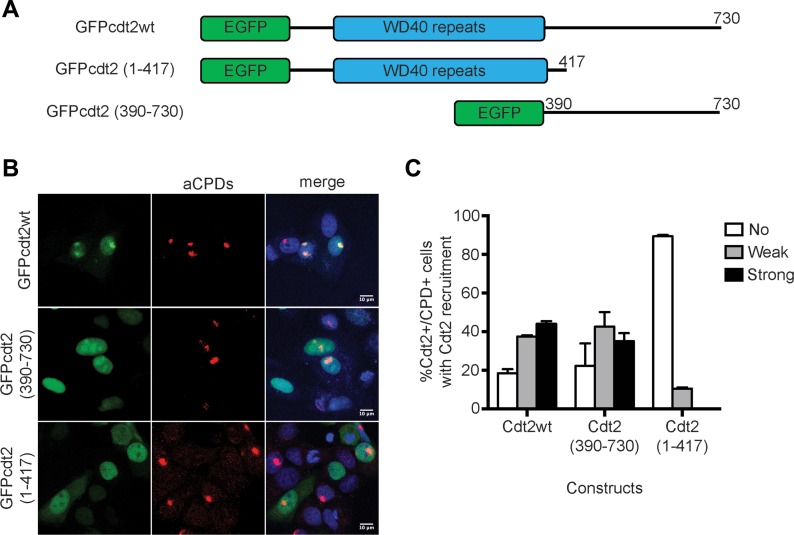
The above results suggested that Cdt2 contains a domain that mediates its association with UV-damaged sites independently of Cdt1. Human Cdt2 is a 730 amino acid polypeptide, with an N-terminal WD40 domain predicted to form a substrate receptor and a long C-terminal domain (Fig 2A). Constructs expressing Cdt21–417, which contains the N-terminal WD40 domain, the C-terminal Cdt2390–730, and the full-length Cdt21–730 as a control fused with GFP were transiently expressed in MCF7 cells, followed by localized UV-C irradiation (Fig 2B and C). Cdt21–730 was robustly detected at DNA-damage sites, as previously reported. The C-terminal Cdt2390–730 construct was also efficiently recruited to DNA-damage sites. On the contrary, there was no evidence for recruitment of Cdt21–417 to sites of damage. This suggests that the C-terminal region of Cdt2, but not the predicted N-terminal substrate receptor domain, is important for its recruitment to the sites of damage.
Figure 2. The C-terminus of Cdt2 mediates Cdt2 recruitment to sites of damage.
(A) A schematic drawing of the domain structure of Cdt2 and constructs used. (B) The C-terminal, but not the N-terminal, part of Cdt2 mediates recruitment to DNA damage sites. MCF7 cells transfected with the indicated plasmids were locally UV-C irradiated (50 J/m2 through 5 μm pore diameter micropore filters), and recruitment of each Cdt2 construct to sites of damage was examined by staining with α-CPD antibody and GFP signal. (C) The percentage of CPD and Cdt2-positive cells which showed strong, weak, or no Cdt2 signal at CPD sites is plotted (mean of two independent experiments with SD).
The C-terminal part of Cdt2 is required for interaction with PCNA and has a PIP box in its C-terminal end
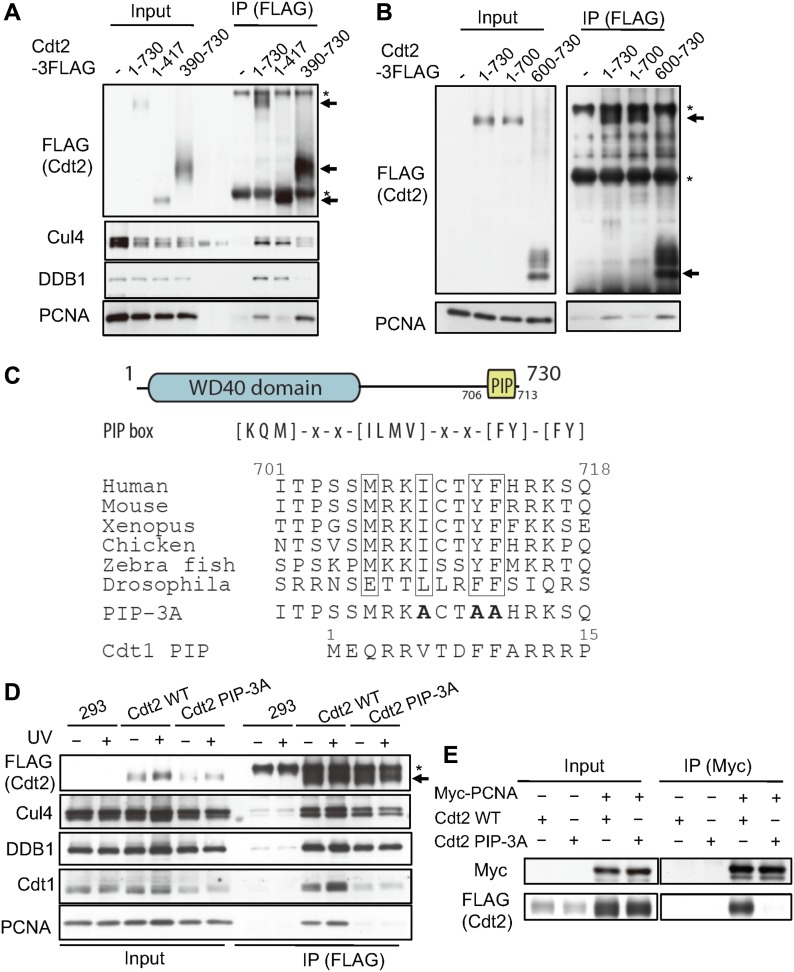
Consistent with the observation that the C-terminal region of Cdt2 is important for recruitment to the CPD-stained sites where PCNA also localizes, we identified PCNA as a Cdt2 C-terminus interacting protein in a yeast two-hybrid screening (Fig S2). To confirm the interaction with PCNA, we transiently transfected 3FLAG-tagged Cdt21–730, Cdt21–417, and Cdt2390–730 into HEK293T cells. Before cell lysate preparation, cells were cross-linked. Immunoprecipitation (IP) experiments using α-FLAG antibody showed that the C-terminal half, but not the N-terminal half, interacts with PCNA (Fig 3A). The Cdt21–417 domain, although it lost most of its affinity for PCNA, is still able to bind DDB1 as expected. In contrast, the Cdt2390–730 domain, although it still binds to PCNA, has lost its ability to bind DDB1, and therefore to form a functional CRL4 complex.
Figure S2. Yeast two-hybrid screening identified PCNA as a Cdt2 C-terminus–interacting protein.
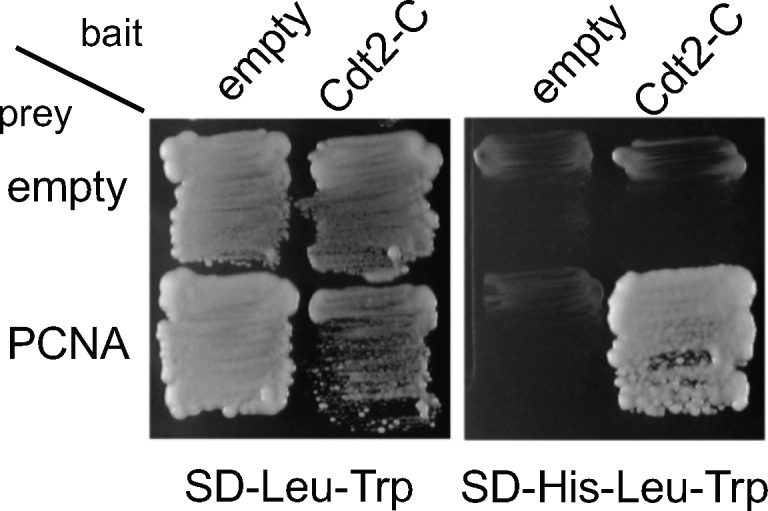
Figure 3. The C-terminal domain of Cdt2 has a conserved PIP box and interacts with PCNA.
(A) Full-length Cdt2 (Cdt21–730) and Cdt2390–730, but not Cdt21–417, interact with PCNA. HEK293 cells were transfected with plasmids expressing the indicated Cdt2 fused to a C-terminal FLAG tag, cross-linked, and precipitated with anti-FLAG antibodies (arrows). Precipitates were examined for PCNA, Cul4A, and DDB1. * indicates nonspecific bands derived from IgGs. The bands in the blank lanes for Cul4A blotting correspond to the cross-reacting bands to pre-stained marker. (B) Cdt2600–730, but not Cdt21–700, interacts with PCNA. HEK293 cells were transfected with the indicated plasmids and treated as in A. * indicates nonspecific bands derived from IgGs. (C) A schematic drawing of the Cdt2 domain structure highlighting the C-terminal PIP box and a sequence alignment of PIP boxes in various organisms. The alanine-changed PIP box mutant of Cdt2 was shown (PIP-3A) together with Cdt1 PIP box. (D) HEK293 cells were transfected with Cdt2WT or PIP box mutant (Cdt2PIP-3A) C-terminally fused to a FLAG tag, treated as in B, and Cdt2 was precipitated with anti-FLAG resin. The interaction with PCNA and DDB1 was examined by immunoblotting. * indicates nonspecific bands. (E) HEK293 cells were transfected with Myc-tagged PCNA and Cdt2WT-3FLAG or Cdt2PIP-3A-3FLAG; PCNA was immunoprecipitated by anti-Myc antibodies and the interaction with Cdt2 was examined by immunoblotting.
To narrow the region of Cdt2 required for interaction with PCNA, we made a series of additional constructs fused to a triple FLAG tag. We confirmed that when the C-terminal 30 amino acids were deleted in the Cdt21–700 construct, this was sufficient to lead to a loss of interaction with PCNA (Fig 3B). Consistently, the C-terminal 130 amino acids alone, Cdt2600–730, were sufficient to mediate the interaction with PCNA.
We postulated that the C-terminal part of Cdt2 directly interacts with PCNA via a PCNA interaction protein (PIP) box; a common motif found in PCNA-dependent DNA replication and repair factors. We used PROSITE to search the UniProtKB protein sequence database for human sequences that contained consensus PIP boxes ([KMQ]-x-x-[ILMV]-x-x-[FY]-[FY]). Our search revealed a PIP box sequence from amino acids 706 to 713 in the C-terminus of Cdt2. This motif was well conserved in vertebrates and in Drosophila (Fig 3C). We then generated a triple-mutant where the three conserved hydrophobic residues I709, Y712, and F713, also known to interact with PCNA in structures of PIP box proteins bound to PCNA, were all mutated to alanine, yielding Cdt2PIP-3A.
Introducing the Cdt2PIP-3A construct fused to a C-terminal FLAG tag to cells and subsequent IP experiments with anti-FLAG antibody after cross-linking, showed that the interaction of the Cdt2PIP-3A construct with PCNA was lost almost entirely (Fig 3D, UV - ), without significantly affecting interaction with Cul4A and DDB1. To confirm the interaction, we performed the reverse IP experiment. Myc-tagged PCNA was co-transfected with Cdt2WT or Cdt2PIP-3A, and we performed an IP with anti-Myc antibody. Cdt2WT was co-precipitated with Myc-PCNA, but Cdt2PIP-3A was not detected in the precipitates (Fig 3E).
Next, we examined the interaction of CRL4Cdt2 ligase with its target protein Cdt1, using HEK293 cells alone and cells transfected with Cdt2WT or Cdt2PIP-3A, with or without UV irradiation. Using an anti-FLAG antibody resin to precipitate Cdt2WT, both PCNA and Cdt1 were precipitated, and their amounts were notably increased after UV irradiation (Fig 3D). In contrast, neither Cdt1 nor PCNA co-precipitated well with Cdt2PIP-3A, before or after UV irradiation. These results suggested that the PIP box of Cdt2 was required for forming a stable complex with its substrates on PCNA.
A C-terminal PIP box in Cdt2 directly interacts with PCNA with high affinity
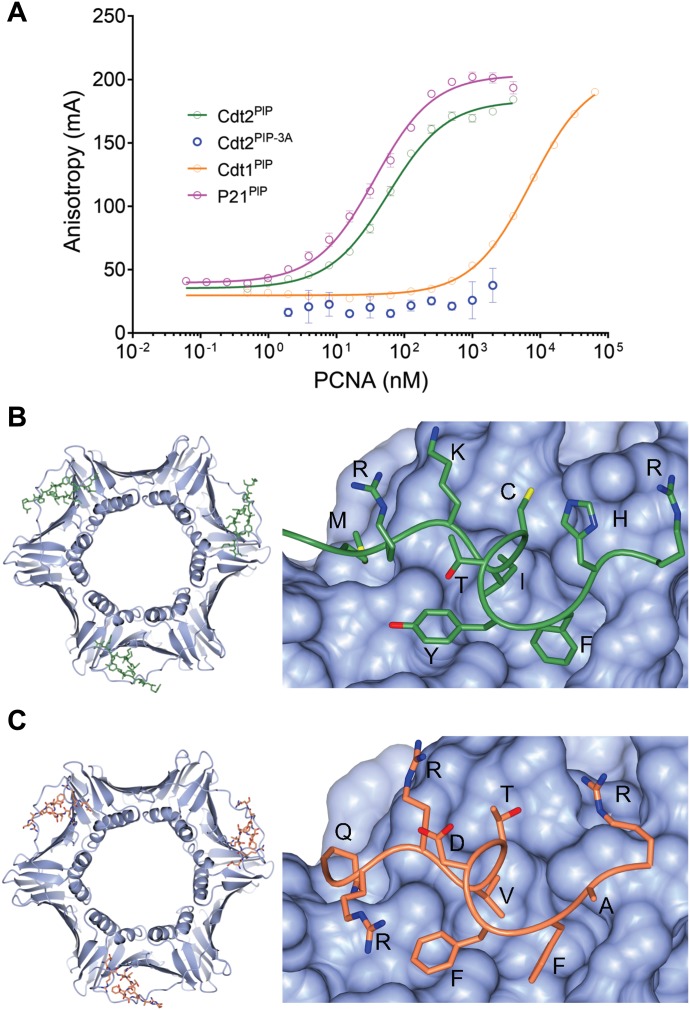
To confirm that this PIP box motif in Cdt2 interacts directly with PCNA, and to quantify that interaction, we synthesized the peptide corresponding to the human Cdt2 PIP box (704-717) (SSMRKICTYFHRKS) with a carboxytetramethylrhodamine fluorescent label at the N-terminus (Cdt2PIP) and characterized its binding to recombinant PCNA, by fluorescence polarization (FP). Cdt2PIP binds PCNA with high affinity (57 ± 3 nM, Fig 4A). The corresponding Cdt2PIP-3A peptide showed practically no detectable binding to PCNA in the same assay (Fig 4A), suggesting that the Cdt2 PIP box binds PCNA in a manner very similar to other known PCNA complexes with PIP box peptides.
Figure 4. Cdt2 PIP peptide directly interacts with PCNA.
(A) An FP assay showing that the equilibrium-binding constants of the Cdt2 PIP box peptide (Cdt2PIP), the triple mutant of key interacting residues of the classical PIP box (Cdt2PIP-3A), as well as the Cdt1 PIP box peptide (Cdt1PIP) and the p21 PIP box peptide (p21PIP). (B) Crystal structure of the Cdt2PIP peptide bound to PCNA (PDB code: 6QC0); both whole PCNA crystal structure image with Cdt2PIP and zoomed-in image are shown. PCNA is shown as a blue surface; the Cdt2 peptide backbone is shown as a green “worm” model with side chains as cylinders; oxygen, red, nitrogen, blue; and sulfur, yellow. Amino acid residues are labelled. (C) Crystal structure of whole and zoomed-in images of the Cdt1PIP bound to PCNA (PDB code: 6QCG); colors as before, but the Cdt1PIP peptide is in orange.
As Cdt1 is recruited to PCNA on chromatin during the S phase and after DNA damage through the Cdt1 N-terminal PIP box (residues 3–10) (Havens & Walter, 2009; Ishii et al, 2010; Roukos et al, 2011), we then synthesized a Cdt1PIP-TAMRA (MEQRRVTDFFARRR) peptide, to compare its affinity with PCNA. Remarkably, the affinity of the Cdt1PIP peptide to PCNA (7,200 ± 200 nM, Fig 4A) is two orders of magnitude weaker than that for the Cdt2PIP, and similar to what is reported for other peptides derived, for example, from the pol-δ p66 and FEN1 (1–60 μM) (Bruning & Shamoo, 2004). The affinity of the Cdt2PIP peptide for PCNA is similar to that reported for the tightly binding p21 PIP box peptide (50–85 nM) (Bruning & Shamoo, 2004; De Biasio et al, 2012), as it was confirmed in our assays (RRQTSMTDFYHSKR, 36 ± 2 nM, Fig 4A).
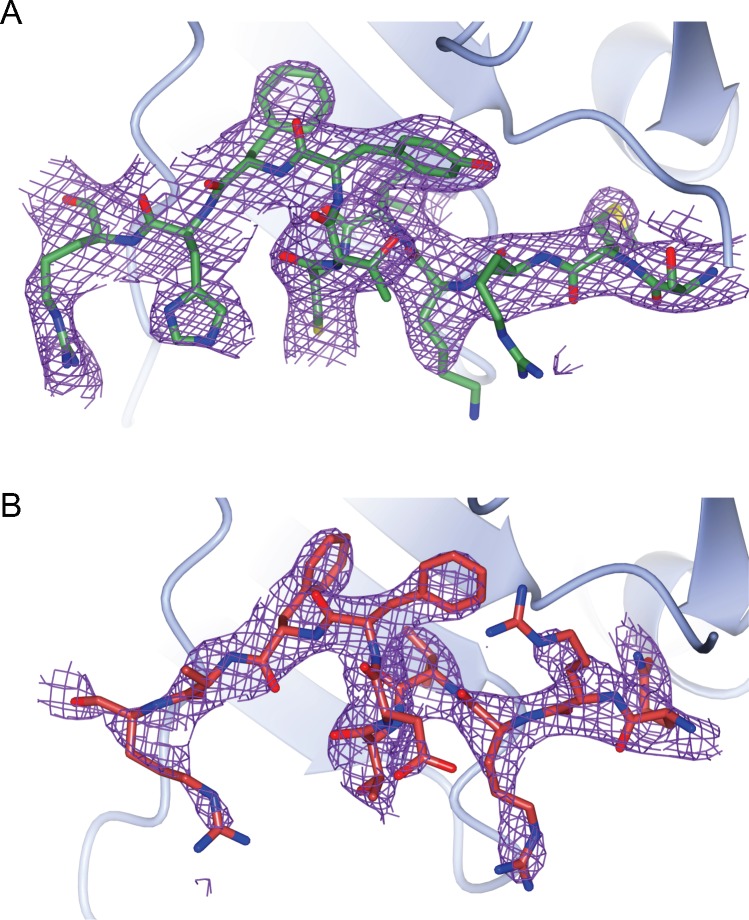
To confirm the binding mode of the Cdt2PIP and Cdt1PIP peptides to PCNA, we determined the crystal structure of both complexes by X-ray crystallography, to a 3.5- and 3.4-Å resolution, respectively. PCNA adopts its well-characterized trimer conformation, with one peptide bound per monomer. The binding mode of both the peptides was very clear (Figs 4B, C, S3A and B and Table 1, see the Materials and Methods section for details). Both peptides bind in a similar manner to other PCNA complex structures. Some notable differences in the binding mode are the conserved Phe713/Phe11 side-chain ring that rotates 180 degrees between the two structures and the Tyr712/Phe10 that repositions so as to extend more towards a hydrophilic environment in Cdt2. The Ile709 and Val7 side chains occupy a similar space in the hydrophobic recognition pocket. Albeit the peptide main chain is in a very similar conformation between the structures, some of the other side chains adopt rather different conformations. The average buried area upon the binding of the Cdt2 peptide is 692 ± 6 Å2 and average calculated energy of binding is −13 ± 0.2 kcal·mol−1, whereas the average buried area upon the binding of the Cdt1 peptide is 650 ± 4 Å2 and average calculated energy of binding is −8.3 ± 0.5 kcal·mol−1; these confirm the tighter binding of the Cdt2 peptide to PCNA.
Figure S3. Crystallography: electron density maps.
Electron density maps showing the Cdt2 (A) and Cdt1 (B) peptides electron density after structure refinement. The 2mFo-DFc map is contoured to 1.0 rms only around the modelled peptides. A characteristic monomer from the PCNA trimer is show is each case.
Table 1.
Data collection and refinement statistics.
| PDB identifier | Cdt1/6QCG | Cdt2/6QC0 |
|---|---|---|
| Data Collection | ||
| Wavelength (Å) | 1.0000 | 0.8726 |
| Resolution (Å) | 38–3.4 (3.63–3.40) | 46–3.5 (3.83–3.50) |
| Space group | P 21 21 21 | P 61 |
| Unit cell a, b, c (Å) | 76.58, 143.73, 173.59 | 151.4, 151.4, 91.49 |
| CC1/2 | 0.982 (0.647) | 0.976 (0.713) |
| Rmerge | 0.166 (0.631) | 0.289 (0.922) |
| |I/σI| | 7.6 (1.9) | 7.1 (2.1) |
| Completeness (%) | 99.3 (99.2) | 97.1 (88.1) |
| Multiplicity | 3.4 (3.5) | 8.0 (7.1) |
| Unique reflections | 26,761 | 14,830 |
| Refinement | ||
| PCNA/peptide in A.U. | 6 | 3 |
| Protein atoms | 24,564 | 12,324 |
| Averaged B-factors protein (Å2) | 48.0 | 73.0 |
| Rwork/Rfree (%) | 20.5/25.1 | 20.2/25.0 |
| Bond lengths rmsd/rmsZ (Å) | 0.0087/0.612 | 0.077/0.552 |
| Bond angles rmsd/rmsZ (°) | 1.682/0.981 | 1.709/0.997 |
| Ramachandran preferred/outliers (%) | 89.1/1.9 | 89.1/1.8 |
| Clash score (%-ile) | 97 | 97 |
| MolProbity score (%-ile) | 92 | 90 |
High-resolution shell in parentheses.
Cdt2 directly binds to PCNA on DNA through its PIP box independently of Cdt1 in vitro
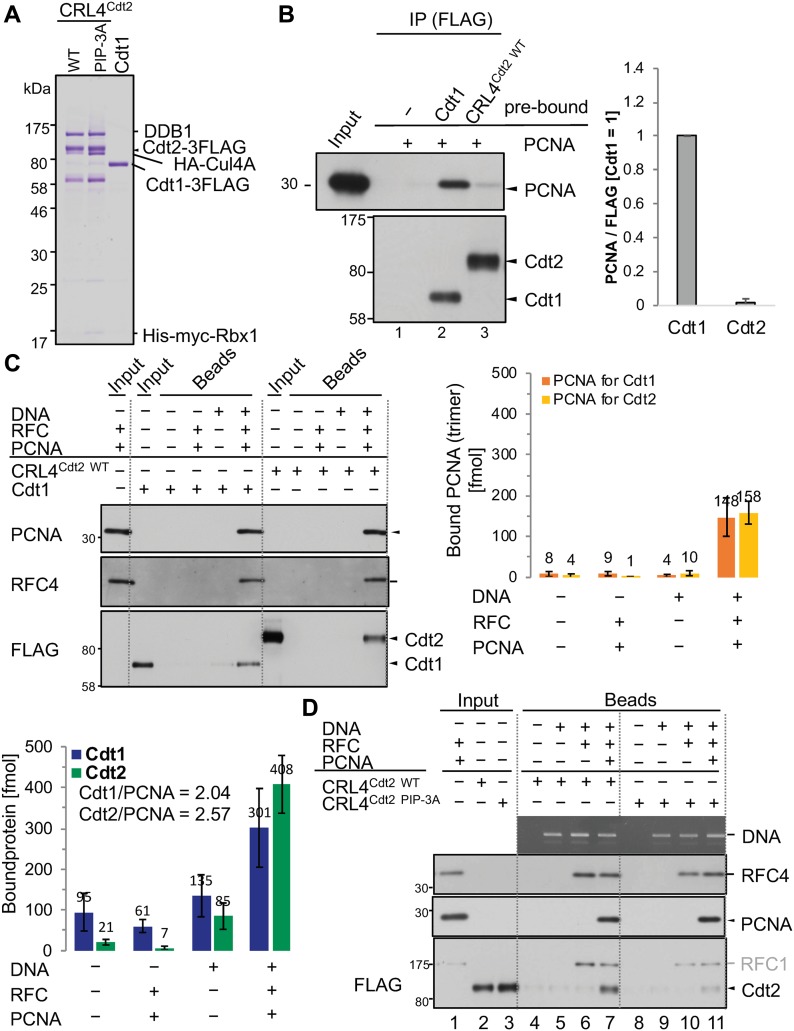
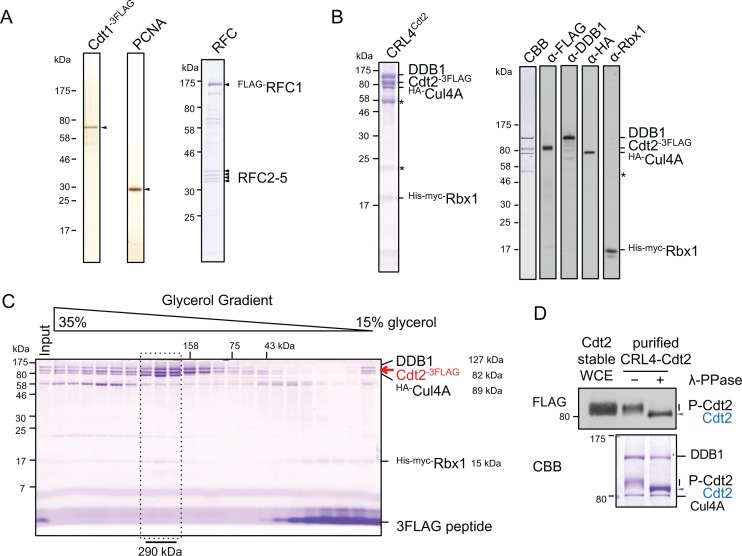
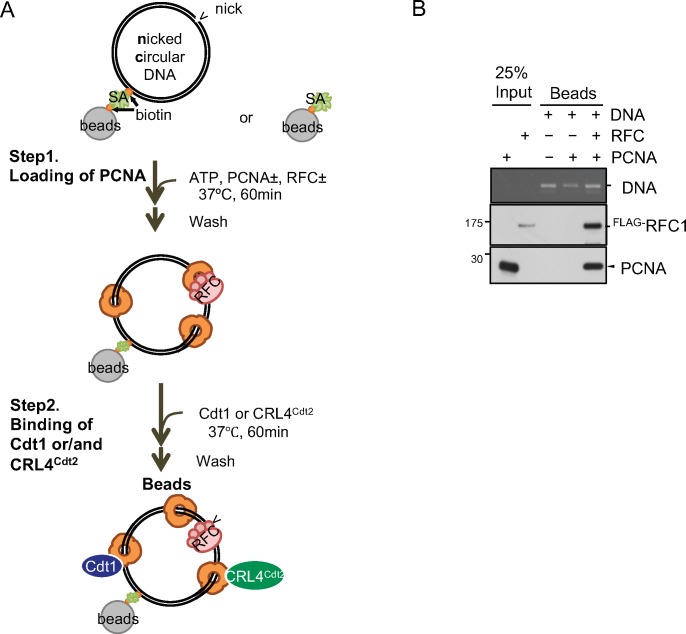
The above results suggested that Cdt2 was recruited to the PCNA sites primarily through its PIP box rather than the N-terminal substrate receptor domain, which contrasts to the model that Cdt1 and CRL4Cdt2 are sequentially recruited to PCNAon Chromatin. To investigate the mechanism of Cdt1 and CRL4Cdt2 recruitment to PCNA, we set up an in vitro analysis with purified and defined human proteins. FLAG-tagged Cdt1 (Cdt1-3FLAG) and CRL4Cdt2 (Cdt2-3FLAG) were expressed in insect cells and purified on anti-FLAG resin (Fig 5A). PCNA and its loader replication factor C (RFC) were purified as described in the experimental procedures (Fig S4). First, we examined the interaction of Cdt1 or CRL4Cdt2 with PCNA in the absence of DNA (free PCNA). The purified Cdt1 or CRL4Cdt2 were fixed on anti-FLAG beads, incubated with free PCNA, and the amount of bound PCNA was analysed. In contrast to the above-mentioned results that both Cdt1 and Cdt2 PIP box peptides bound to PCNA (Fig 4), PCNA was detected on Cdt1-beads, but much less on CRL4Cdt2-beads (Fig 5B).
Figure 5. Cdt1 and CRL4Cdt2 independently associate with PCNA loaded on DNA through PIP box in vitro.
(A) Purified CRL4Cdt2(WT), CRL4Cdt2(PIP-3A), and Cdt1 proteins. (B) Cdt1, but not CRL4Cdt2, associates with free PCNA. The purified Cdt1 and CRL4Cdt2 proteins were fixed on anti-FLAG–resins and incubated with PCNA for 2h at 4°C and the bound PCNA was analysed. The relative amounts of bound PCNA were shown, normalized with FLAG signal, and the level on Cdt1-3FLAG beads set to 1.0. Error bars represent SD from three independent experiments. (C) The ncDNA beads (DNA+) or control beads (DNA−) were loaded with PCNA by RFC or not. Then, beads were incubated with the purified Cdt1 or CRL4Cdt2, and the bound proteins were recovered for Western blotting. The amounts of PCNA (as a trimer) loaded on plasmid DNA and the amounts of bound Cdt1-3FLAG and Cdt2-3FLAG were measured and shown (f mol). The number of bound Cdt1 and Cdt2 on PCNA trimer was shown (Cdt1/PCNA and Cdt2/PCNA). Error bars represent SD from independent experiments (n = 3 or n > 3). (D) CRL4Cdt2(WT) and CRL4Cdt2(PIP-3A) were incubated with control beads, ncDNA beads, or ncDNA beads which had been pre-incubated with RFC alone or together with PCNA for 1 h at 4°C. Beads were recovered and bound proteins were analysed.
Figure S4. Purified proteins used for in vitro assay.
(A) Purified Cdt1-3FLAG, PCNA, and RFC proteins. (B) CRL4Cdt2 complex after purification on FLAG-resins. The asterisks are contaminants. The purified proteins were Western blotted with antibodies for each protein. (C) The CRL4Cdt2 complex after purification on FLAG-resins was further purified on glycerol gradient centrifugation. The peak fractions of CRL4Cdt2 tetramer (calculated molecular weight, 313 kD) are indicated by the dotted line. (D) The Cdt2 complexed with CRL4 purified from insect cells were phosphorylated. The purified CRL4Cdt2 from insect cells were treated with λ-phosphatase (λ-PPase) (+) or not (−), and run on SDS–PAGE gel, stained with CBB. The same samples were run together with whole-cell extract (WCE) prepared from Cdt2-FLAG–expressing cells and blotted with anti-FLAG antibody.
Because Cdt1 and Cdt2 bind to PCNA only when PCNA is loaded on DNA in Xenopus egg extracts (Havens & Walter, 2009), we prepared PCNA loaded on nicked circular DNA (ncDNA) with RFC (PCNAon DNA) (Fig S5A and B). In our in vitro conditions, three molecules of PCNA trimer were loaded on one plasmid DNA. Then, we analysed the binding of Cdt1 and CRL4Cdt2 to PCNAon DNA as described in Fig S5A. After incubation, Cdt1 was efficiently recovered on the PCNAon DNA beads, but not on the control beads (Fig 5C). Although we could not show CRL4Cdt2 binding to free PCNA, we show that CRL4Cdt2 binds to PCNAon DNA in the absence of Cdt1. The interaction was not mediated by RFC proteins bound on ncDNA (Fig 5D, lane 6). The molar ratio of Cdt2 bound to trimeric PCNAon DNA was the same or somewhat higher than that of Cdt1 (408 fmol of Cdt2 bound to 158 fmol trimeric PCNAon DNA, that is, 2.57 Cdt2 molecules on one PCNA trimer, while 301 fmol of Cdt1 to 148 fmol of trimeric PCNAon DNA, 2.04 Cdt1 molecules on one PCNA trimer). These results indicate that CRL4Cdt2 directly and efficiently binds to DNA-loaded PCNA without substrate, consistent with data in cells showing that Cdt2 was recruited to the sites of damage in the absence of Cdt1 (Fig 1).
Figure S5. Loading of PCNA on nc DNA beads and following binding assay.
(A) Scheme for PCNA loading on nc DNA and for following Cdt1 and CRL4Cdt2 binding assay. Nicked circular DNA beads or control beads were incubated with PCNA and RFC for 1 h at 37°C and washed (Step 1; loading of PCNA). The beads were then incubated with Cdt1 or CRL4Cdt2 (Step 2; binding). (B) RFC-dependent loading of PCNA.
To confirm that the interaction of CRL4Cdt2 with PCNAon DNA was mediated by the PIP box of Cdt2, the CRL4 complex having Cdt2PIP-3A (CRL4Cdt2(PIP-3A)) was purified like CRL4Cdt2 (Fig 5A) and used in a binding assay. As above, CRL4Cdt2 interacted with PCNAon DNA. In contrast, the binding activity of CRL4Cdt2(PIP-3A) to PCNAon DNA was severely reduced as compared with CRL4Cdt2 (Fig 5D). These results demonstrate that the PIP box of Cdt2 was directly responsible for the interaction of CRL4Cdt2 with PCNAon DNA.
The Cdt2 PIP box promotes Cdt2 recruitment to UV-irradiated sites and PCNA foci
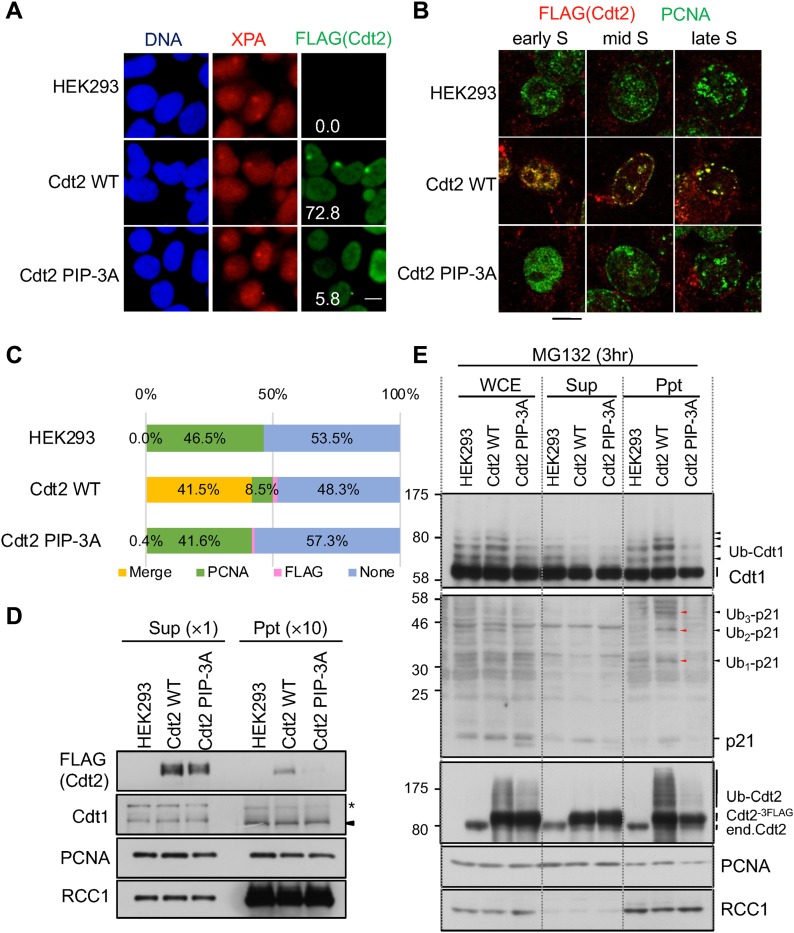
To investigate the role of the Cdt2 PIP box in cells, we isolated HEK293 cells stably expressing FLAG-tagged Cdt2PIP-3A and showed that Cdt2PIP-3A was not recruited to UV-irradiated sites (Fig 6A). XPA, a component of nucleotide excision repair, staining was used to confirm that similar extents of DNA damage were induced both in Cdt2WT- and Cdt2PIP-3A-expressing cells. Next, we verified that the recruitment of Cdt2 onto PCNA during the S phase is also dependent on its PIP box. To detect the chromatin-associated fraction of PCNA and Cdt2, asynchronous cells were pre-extracted before fixation and co-stained with PCNA and FLAG antibodies. A similar percentage of PCNA-positive S-phase cells was detected both in Cdt21–730/WT-expressing cells and Cdt2PIP-3A-expressing cells (Fig S6). Cdt2WT was co-localized with PCNA foci, corresponding to sites of DNA replication. More than 80% of PCNA-positive cells were co-stained with Cdt2WT, with the early S-phase cells displaying stronger Cdt2WT staining than the late S-phase cells as reported (Nukina et al, 2018) (Figs 6B and C, and S6A). In contrast, almost no signal was detected for Cdt2PIP-3A irrespective of the presence of PCNA. Consistent with the immunofluorescence analysis, following cell fractionation, Cdt2WT was recovered in a chromatin-containing fraction, whereas Cdt2PIP-3A was undetectable (Fig 6D).
Figure 6. The Cdt2 PIP box is important for its recruitment to DNA damage sites and replication foci and for poly-ubiquitination activity.
(A) HEK293 and stable Cdt2WT or Cdt2PIP-3A cells were locally UV irradiated, and stained for XPA and FLAG (Cdt2). XPA-positive spots were examined for FLAG signal, and its frequency was counted (%). More than 50 spots were examined. bar:10 μm. (B) HEK293 cells and stable cells were pre-extracted, fixed, and stained with PCNA and DYKDDDDK (FLAG) antibodies. Bar: 10 μm. (C) Cells stained as (B) were examined for staining with PCNA and DYKDDDDK (FLAG) antibodies and the frequency of cells with colocalized PCNA and FLAG (Cdt2) staining (Merge), PCNA staining alone (PCNA), FLAG staining alone (FLAG), or no staining (None) were plotted. (D) Whole-cell extracts were prepared from HEK293 and stably transfected cells of Cdt2WT or Cdt2PIP-3A cells, separated into soluble (Sup) and chromatin-containing insoluble (Ppt) fractions, and were examined for indicated proteins. The asterisk indicates nonspecific bands. (E) HEK293 and stably transfected cells of Cdt2WT or Cdt2PIP-3A were treated with MG132 for 3 h. Whole-cell extracts (WCE) were prepared, separated into soluble (Sup) and insoluble (Ppt) fractions, and blotted with the indicated antibodies.
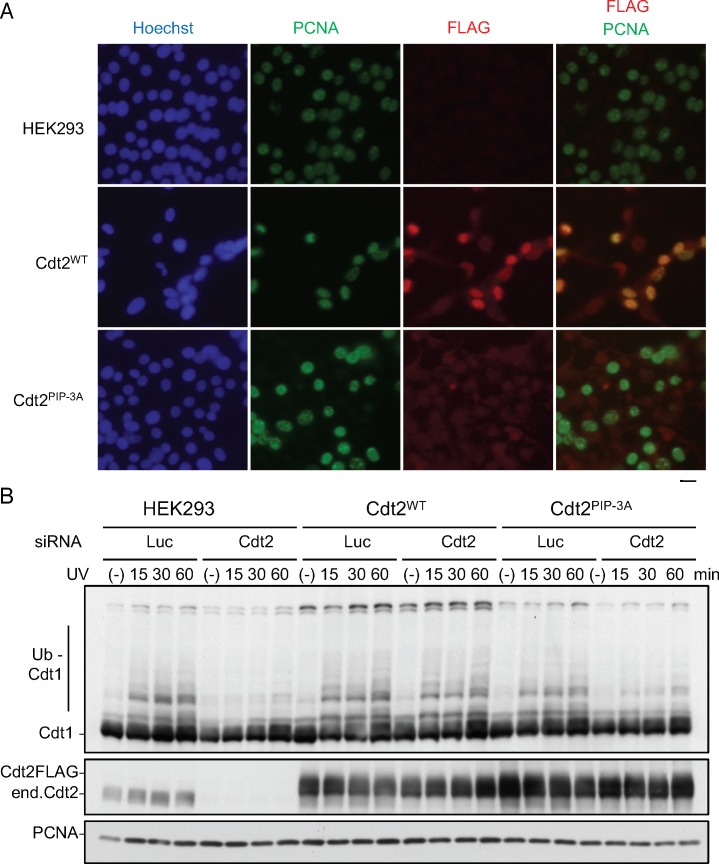
Figure S6. Cdt2 PIP box is important for its recruiting to DNA replication sites and its poly-ubiquitination activity in HEK293 cells.
(A) HEK293 cells or cells stable Cdt2WT or Cdt2PIP-3A were pre-extracted, fixed, and stained with PCNA and DYKDDDDK (FLAG) antibodies. (B) Poly-ubiquitination after UV irradiation. HEK293 and its stables expressing Cdt2WT or Cdt2PIP-3A were transfected with siCdt2 or control (siLuc). Three days later, cells were treated with MG132 (25 μM), and 10 min later irradiated with UV (20 J/m2). Cells were collected at the indicated time points, and used for Western blotting with indicated antibodies.
These results collectively suggest that although Cdt2PIP-3A is capable of forming a complex with the rest of the components of the CRL4 ligase, the Cdt2 PIP box is required for efficient recruitment of CRL4Cdt2 to the PCNA-loaded sites.
Cdt1 ubiquitination is abortive in Cdt2PIP-3A-expressing cells
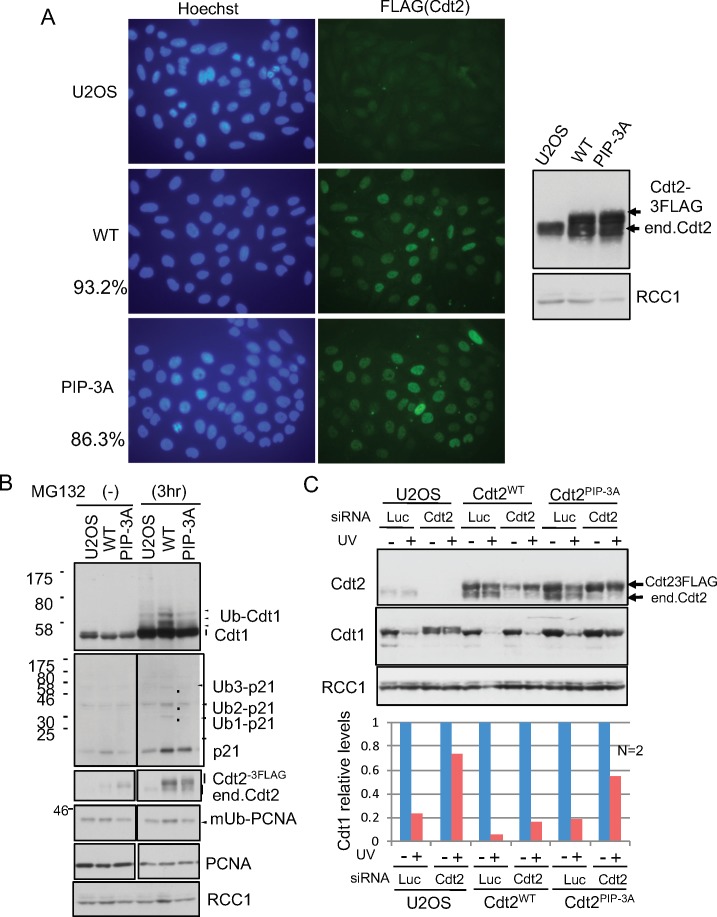
Because the recruitment of Cdt2PIP-3A to PCNA sites was compromised, it would imply that ubiquitination of Cdt1 was also abrogated. To consider this possibility, asynchronously growing control HEK293 cells and cells expressing Cdt2WT or Cdt2PIP-3A were treated with proteasome inhibitor MG132 and the levels of poly-ubiquitinated Cdt1 were examined. Although poly-ubiquitinated Cdt1 was clearly detected in Cdt2WT-expressing cells, its levels were reduced in Cdt2PIP-3A-expressing cells (Fig 6E), indicating that the ability of Cdt2PIP-3A to ubiquitinate Cdt1 was compromised. Fractionation of cell extracts revealed that most of the poly-ubiquitinated Cdt1 and p21 were recovered in the chromatin-containing precipitate fraction prepared from Cdt2WT-expressing cells, in agreement with CRL4Cdt2 operating on chromatin (Fig 6E). Cdt2PIP-3A-expressing cells however had reduced level of both poly-ubiquitinated Cdt1 and p21 on chromatin. Interestingly, Cdt2 itself is poly-ubiquitinated on chromatin, and this is severely reduced in Cdt2PIP-3A-expressing cells (Fig 6E). Increased levels of poly-ubiquitination of Cdt1 and p21 were also observed in another cell line, U2OS cells expressing Cdt2WT, but not in cells expressing Cdt2PIP-3A (Fig S7B).
Figure S7. Cdt2 PIP box is important for CRL4Cdt2 activity in U2OS cells.
(A) U2OS stable cells expressing Cdt2WT or Cdt2PIP-3A. U2OS cells were transfected with expression vectors for Cdt2WT-FLAG or Cdt2PIP-3A-FLAG, and isolated stable clones were verified by immunofluorescent analysis and Western blot analysis. Frequency of expressing cells for each construct was shown (%). (B) Poly-ubiquitination assay with U2OS stable cells. U2OS cells and Cdt2WT-3FLAG or Cdt2PIP-3A-3FLAG expressing stable U2OS cells were treated with MG132 for 3 h or not (−), and whole-cell extracts were prepared for Western blotting. (C) Cells were transfected with siCdt2 or control siLuc for 72 h, irradiated with UV (+, 5 J/m2) or not (−), and collected 1h later for Western blotting. Relative levels of Cdt1 were shown, UV (−) level set as 1.0 (average of two independent experiments).
Next, we examined the accumulation of poly-ubiquitinated Cdt1 after UV irradiation. For this experiment, the endogenous Cdt2 was depleted with an siRNA targeted to the 3′ UTR to assay for the activity of introduced Cdt2. Cells were treated with MG132, irradiated with UV and the levels of poly-ubiquitinated Cdt1 were monitored by Western blotting. In HEK293 cells treated with control siRNA, poly-ubiquitinated Cdt1 was detected 15 min after irradiation (Fig S6B). Depletion of Cdt2 blocked the poly-ubiquitination of Cdt1. The defect was rescued by Cdt2WT expression. In contrast, Cdt2PIP-3A-expressing cells were defective in poly-ubiquitination of Cdt1 (Fig S6B), suggesting that Cdt2 PIP box is required for efficient poly-ubiquitination of substrates.
The Cdt2 PIP box is required for efficient Cdt1 degradation
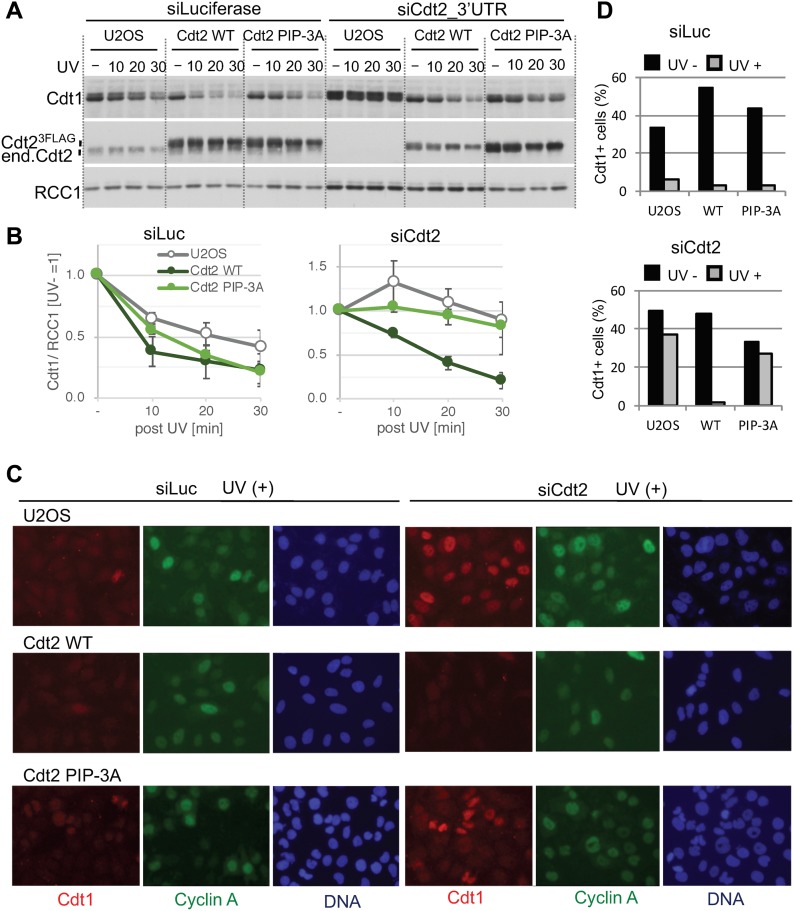
Because the poly-ubiquitination activity of Cdt2PIP-3A was reduced, we monitored the degradation of Cdt1 after UV irradiation. Asynchronously growing control U2OS cells and cells expressing Cdt2WT or Cdt2PIP-3A (Fig S7) were depleted of endogenous Cdt2 using an siRNA targeted to the 3′ UTR, and irradiated with UV. When the cells were exposed to UV (20 J/m2) radiation, degradation of Cdt1 was almost prevented in U2OS cells depleted of endogenous Cdt2 (Fig 7A and B). Ectopic expression of Cdt2WT restored Cdt1 degradation to similar kinetics to that of control siRNA-transfected U2OS cells. In contrast, Cdt2PIP-3A was much less effective in rescuing Cdt2 depletion. Similarly, at a lower dose of UV irradiation (5 J/m2), Cdt1 remained stable 1 h post UV irradiation in Cdt2PIP-3A cells, when endogenous Cdt2 was depleted, both on immunofluorescent analyses and Western blotting (Figs 7C and D, and S7C).
Figure 7. The Cdt2 PIP box plays an important role for rapid Cdt1 degradation after UV irradiation.
(A) U2OS cells and U2OS cells stably expressing Cdt2WT-3FLAG or Cdt2PIP-3A-3FLAG were transfected with siCdt2 targeted to the 3′ UTR or control siLuc for 48 h, irradiated with UV (+, 20 J/m2) or not (−), and collected at the indicated time points (min) for Western blotting. (B) The protein levels were measured and the relative amounts of Cdt1, normalized to RCC1, after UV irradiation were shown, UV (−) level set as 1.0. Error bars represent SD from three independent experiments. (C) Cells were transfected with siCdt2 or control siLuc for 72 h, irradiated with UV (+, 5 J/m2) or not (−) (not shown), and 1h later fixed for staining with Cdt1 and cyclinA antibodies. (D) The frequency of Cdt1-positive (+) cells among the total cells in (C) was shown (%) (average of two independent experiments).
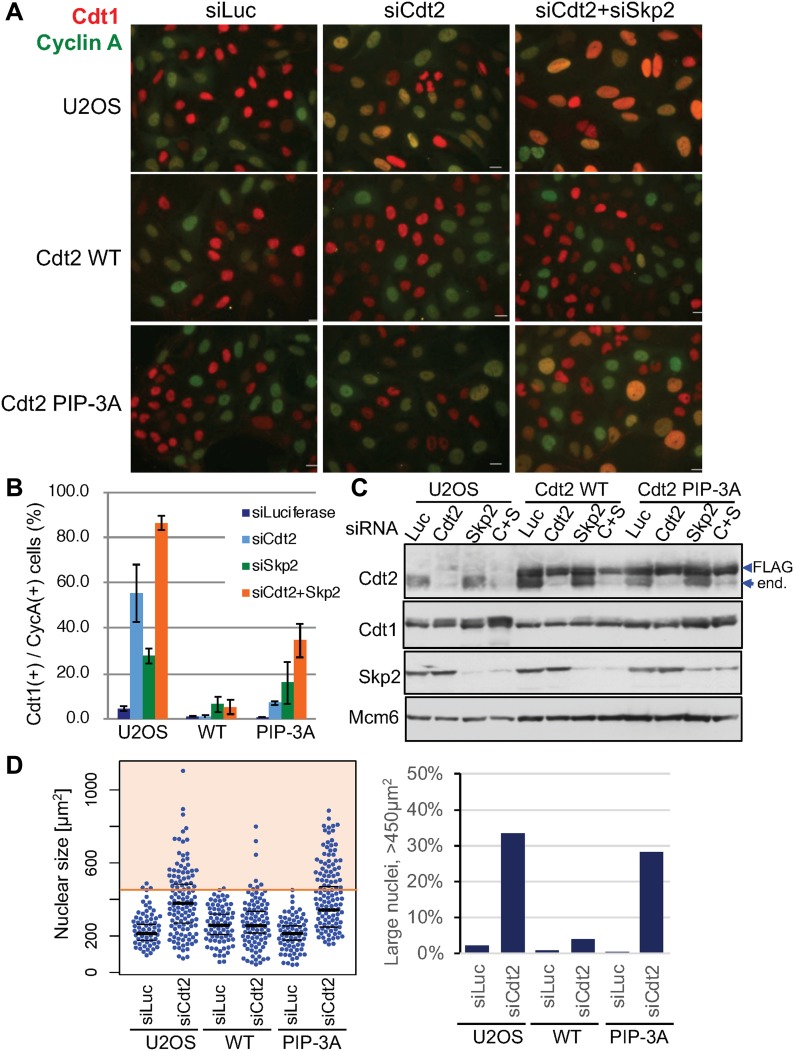
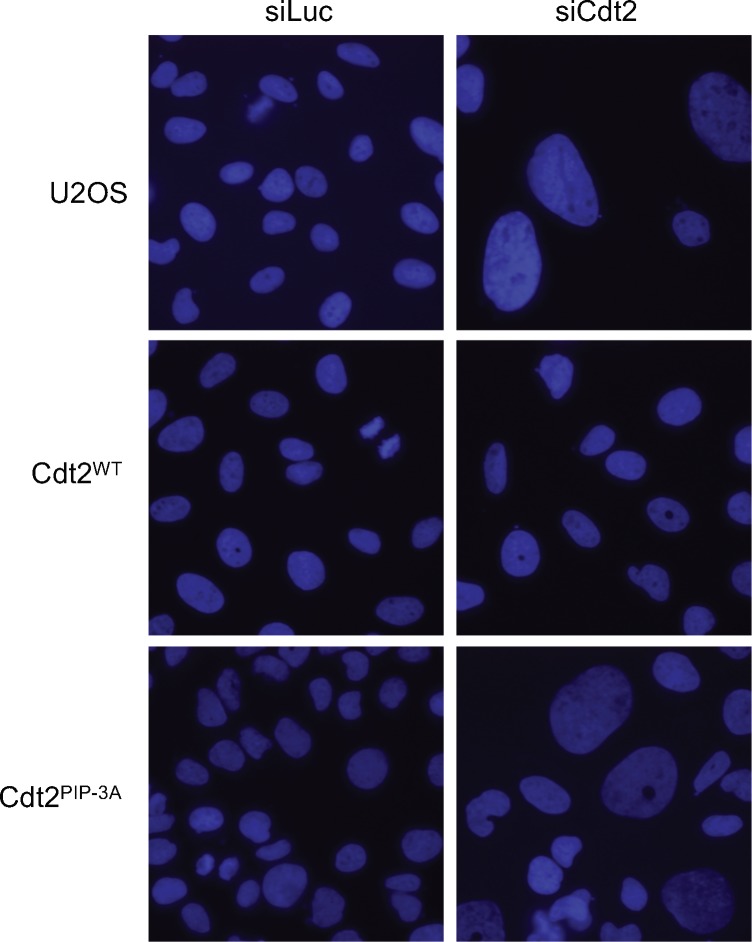
Next, we examined Cdt1 degradation after the onset of the S phase in the Cdt2PIP-3A-expressing cells. Cyclin A is present in the S and G2 phase, when Cdt1 should be degraded. Thus, Cdt1 should be present in cells that are negative for cyclin A, and absent in cells that are positive for cyclin A (Nishitani et al, 2006; Xouri et al., 2007a, 2007b). Cdt1 degradation after the onset of the S phase is carried out by two ubiquitin ligases, CRL1Skp2 and CRL4Cdt2. We depleted the substrate recognition subunits, Cdt2 and/or Skp2, by siRNA, and the percentage of Cdt1 positive cells among cyclin A–positive cells was assessed by immunofluorescence. In control siRNA-transfected U2OS cells, ∼2% of cyclin A–positive cells stained positive for Cdt1. In contrast, in Cdt2-depleted, Skp2-depleted, and Cdt2/Skp2 co-depleted cells, 50%, 30%, and more than 80% of cyclin A–positive cells stained positive for Cdt1, respectively (Fig 8A–C), consistent with earlier work (Nishitani et al, 2006). Then, we depleted Cdt2 and Skp2 and examined Cdt1 degradation in Cdt2WT or Cdt2 PIP-3A-expressing cells. Ectopic expression of Cdt2WT fully complemented the depletion of Cdt2, and also the co-depletion of Skp2. This was probably because the levels of ectopically expressed Cdt2WT were higher than the endogenous Cdt2 levels. On the other hand, Cdt1 degradation was not fully rescued by the expression of Cdt2PIP-3A protein both in Cdt2-depleted and Cdt2 and Skp2 co-depleted cells (Fig 8A and B). Upon depletion of endogenous Cdt2, many cells exhibited significantly enlarged nuclei, consistent with re-replication. This phenotype was rescued by ectopic expression of Cdt2WT, but not of Cdt2PIP-3A (Figs 8D and S8), suggesting that the PIP box of Cdt2 is essential to prevent re-replication.
Figure 8. The Cdt2 PIP box plays an important role for Cdt1 degradation during cell cycle.
(A, B) U2OS cells and U2OS cells stably expressing Cdt2WT-3FLAG or Cdt2PIP-3A-3FLAG were transfected with siCdt2 and/or siSkp2 or control siLuc for 72 h. Cells were fixed and stained with Cdt1 and cyclinA antibodies. The frequency of Cdt1-positive (+) cells among cyclin A–positive (+) cells was calculated (n = 3). (C) Cells transfected with siRNAs as above (C+S; siRNAs for Cdt2 and Skp2) were examined for indicated protein levels. (D) Cells treated with siCdt2 or control siLuc were measured for their nuclear size (n = 200 for each), and are shown in a scatter plot. Frequency of cells with nuclear size larger than 450 μm2 is shown (%).
Figure S8. Cdt2PIP-3A mutant shows enlarged nuclei.
Cells transfected with siLuc or siCdt2 for 72 h were stained with Hoechst 33258.
Taken together, our in vitro and in vivo analyses show that the Cdt2 PIP box brings CRL4 ubiquitin ligase to PCNA sites and is required for efficient substrate degradation both during the S phase and following UV irradiation, to maintain genome stability.
Discussion
Cdt2 substrates are degraded rapidly as cells enter the S phase or when cells are exposed to DNA-damaging agents. Cdt2 of higher eukaryotic cells have an extended C-terminal region. We demonstrate that Cdt2 has a PIP box in its C-terminal end that recognizes chromatin-bound PCNA with high affinity. This interaction is important for CRL4Cdt2 function in recognizing its targets before their ubiquitin-dependent degradation: this previously unnoticed Cdt2 PIP box is required for recruitment of CRL4Cdt2 to chromatin-bound PCNA, and indispensable for efficient Cdt1 ubiquitination, thus for prompt degradation of Cdt1 both in the S phase and after UV irradiation. The Cdt2 PIP box peptide directly interacts with PCNA with high affinity (about 50 nM) in vitro, similar to the unusually high-affinity p21 PIP box peptide, and significantly tighter than the Cdt1 PIP box peptide. Molecular modelling experiments suggest that both the Cdt1 and Cdt2 PIP boxes bind PCNA in the classical way and their binding to PCNA occurs independently of each other (Fig 4). Furthermore, the purified CRL4Cdt2 ligase interacted directly with the PCNA loaded on DNA via Cdt2 PIP box (Fig 5). Consistently, the C-terminal domain of Cdt2 has been shown to bind to PCNA in Xenopus egg extracts (Kim et al, 2010). Our analysis now shows that the PIP box is conserved in Xenopus, and thus likely to be responsible for this interaction.
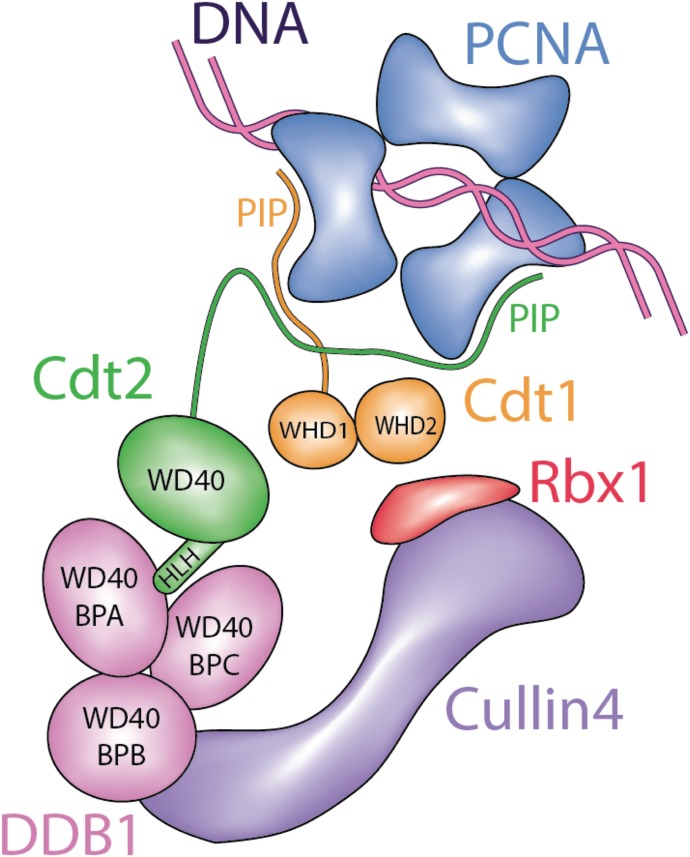
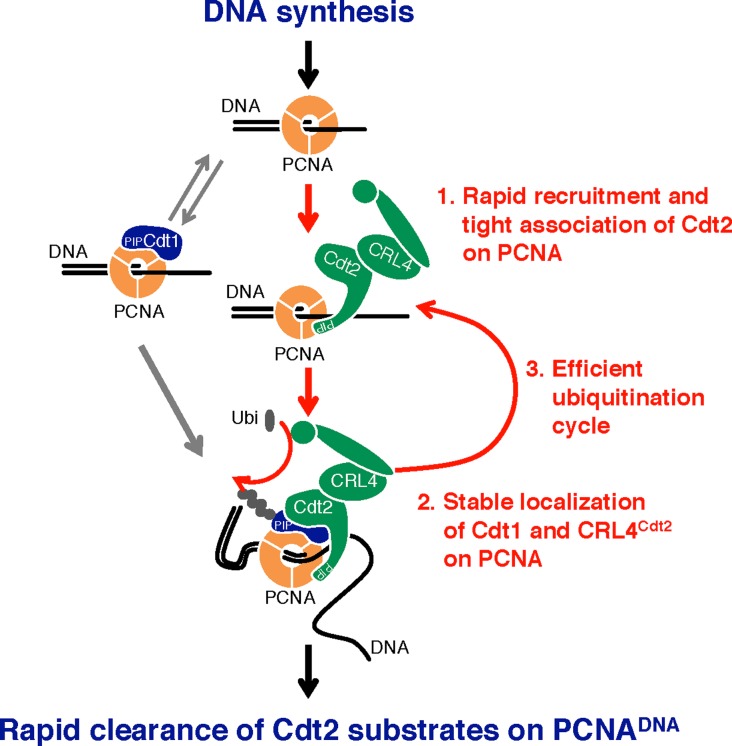
PCNA is a trimer, hence it offers three PIP box binding sites that are proposed to be asymmetric when PCNA is loaded onto DNA (Ivanov et al, 2006). Consequently, both Cdt1 and Cdt2 can be simultaneously recruited onto PCNA, effectively utilizing PCNA as a common docking platform for bringing the CRL4Cdt2 ligase and the Cdt1 substrate in proximity, allowing more efficient transfer of a ubiquitin moiety from the E2 to Cdt1 (Fig 9). This Cdt2 PIP box assisted ubiquitination is likely to be used by other Cdt2 substrates, as p21 poly-ubiquitination was also defective in Cdt2PIP-3A-expressing cells (Figs 6E and S7B). This mechanism, where co-localization onto the same protein platform brings the E3 ubiquitin ligase and its substrate in proximity, is a new paradigm in ubiquitin conjugation. This model fits recent results which show that Cdt2 itself can be auto-ubiquitinated by CRL4Cdt2 (Abbas et al, 2013): CRL4Cdt2 and Cdt2 (or a second CRL4Cdt2) may both bind PCNA through the Cdt2 PIP box and catalyse this auto-ubiquitination event. Consistently, the poly-ubiquitination of Cdt2 which was observed on chromatin in Cdt2WT-expressing HEK293 cells was severely reduced in Cdt2PIP-3A-expressing cells (Fig 6E).
Figure 9. Model.
A model showing how both CRL4Cdt2 and Cdt1 are likely recruited to the chromatin-bound PCNA through their PIP boxes, for efficient ubiquitination.
The mono-ubiquitination (mUb) of PCNA is carried out by Rad6-Rad18, but CRL4Cdt2 also contributes to it. In non-damaged cells, mUb of PCNA is present at a basal level and its levels increase in response to DNA damage (Terai et al., 2013). The Cdt2 PIP box may also be important for mUb of PCNA, as mUb-PCNA levels appeared decreased in Cdt2PIP-3A in comparison with Cdt2wt-expressing cells (Fig S7B). Therefore, Cdt2 PIP box may be required for various events driven by CRL4Cdt2 in the cell; degradation of PIP-degron proteins, auto-ubiquitination of Cdt2, and PCNA mUb.
We show that the Cdt2 PIP box is crucial for recruitment of the CRL4Cdt2 ligase onto PCNA in human cells. However, further interactions of Cdt2 domains, mediated by its N-terminal WD40 repeats, with the PCNA-bound Cdt1 PIP box (the PIP degron) also enhance the affinity of the CRL4Cdt2 (Havens & Walter, 2009). Here, we propose a synergistic mechanism where the Cdt2 PIP box and the Cdt1 PIP-degron operate in concert to promote CRL4Cdt2-dependent target ubiquitination (Figs 9 and S9). It is also noteworthy that the binding of Cdt1 onto PCNA is transient in cells, whereas Cdt2 interactions are more stable (Roukos et al, 2011 and Fig 1B): a synergistic mechanism would imply that interactions of pre-bound Cdt2 with transiently formed Cdt1 PIP-degron, are important for localizing CRL4Cdt2 and Cdt1 onto PCNA. The property that affinity of Cdt2 PIP box to PCNA is higher than that of Cdt1 PIP box (Fig 4A) is compatible with such a model. In addition, weaker affinity of Cdt1 PIP box can help to release Cdt1 after poly-ubiquitination, whereas CRL4Cdt2 could remain on PCNA to trap the next substrate, leading to an efficient degradation cycle of substrates (Fig S9). In the absence of Cdt2 PIP box, the process needs to be performed sequentially; transient Cdt1 binding to PCNA and recognition by Cdt2 via its N-terminal WD40 repeats, which would be, however, less effective for Cdt1 degradation.
Figure S9. Model; PIP box of Cdt2 promotes rapid clearance of substrate Cdt1 on PCNAon DNA.
Both Cdt1 and CRL4Cdt2 can bind independently to PCNAon DNA through their PIP boxes, when DNA synthesis starts. Based on the fact that the binding of Cdt1 onto PCNA is transient in cells, whereas Cdt2 interactions are more stable, a model is shown, illustrating that Cdt2 PIP box promotes ubiquitination of Cdt1 on PCNA trimer through multiple steps. CRL4Cdt2 binds to PCNAon DNA tightly and independently of Cdt1, and catches and localizes Cdt1 when it is recruited to PCNA, forming a stable complex on PCNA trimer for ubiquitination. After poly-ubiquitinated Cdt1 was released, CRL4Cdt2 catches next Cdt1, creating an efficient ubiquitination cycle.
Our biochemical analysis demonstrated that CRL4Cdt2 interacts more strongly with PCNAon DNA than with PCNA that is not associated with DNA (free PCNA) (Fig 5B and C). Because Cdt2 PIP peptide can bind to free PCNA (Fig 4), there is a mechanism that enhances the affinity of CRL4Cdt2 to the PCNAon DNA. This is compatible with models that suggest that a conformational change upon DNA binding can modulate affinity. It is tempting to speculate that the high-affinity CRL4Cdt2 complex docking into DNA-bound PCNA triggers changes that allow the transient loading of low-affinity substrates such as Cdt1, enabling rapid ubiquitination molecules in the vicinity. On the other hand, we reproducibly observed that Cdt2 was recovered, although at lower levels, on DNA beads in the absence of PCNA (Fig 5C), suggesting that CRL4Cdt2 has a DNA-binding activity. Furthermore, the RFC1 complex may have a role connecting PCNA and Cdt2, as Cdt2 PIP-3A was detected to some more levels in the presence of the PCNA and RFC1 complex (Fig 5D). As reported, PCNA loaders appear to have an additional role in the CRL4Cdt2-mediated ubiquitination after loading PCNA (Shiomi et al, 2012). The extended C-terminal region of Cdt2 might have an additional domain involved in such a regulation, or an unknown factor might be involved. The exact molecular mechanisms that interplay to regulate these interactions need to be investigated further.
Although the Cdt2 PIP box mediates direct interaction with PCNAon DNA, the affinity of Cdt2 to PCNA decreases as cells progress into and complete the S phase. The chromatin levels of Cdt2 are reduced during the late S phase (Rizzardi et al, 2015), and the co-localization of Cdt2WT with PCNA was mostly observed in the early S phase cell nuclei (Fig 6B). It was suggested that the CDK-dependent phosphorylation of Cdt2 down-regulates Cdt2 interaction with PCNA, as Cdk1 inhibition recovered the chromatin interaction of Cdt2 at the late S phase, and Cdt2 mutated at CDK phosphorylation sites displayed strong interaction with PCNA throughout the S phase (Rizzardi et al, 2015; Nukina et al, 2018). We propose that the C-terminal region of Cdt2 contributes to regulating the activity of CRL4Cdt2 in the cell cycle by controlling its affinity to PCNA; Cdt2 PIP box–mediated binding to PCNA is important for rapid substrate degradation at the onset of the S phase, whereas the phosphorylation of the C-terminal region of Cdt2 inhibits its PCNA interaction and thus reduces ubiquitination activity during the late S phase, leading to substrate re-accumulation. It remains, however, to be elucidated how phosphorylation of Cdt2 affects its interaction to PCNA. We noticed that our Cdt2 preparation from insect cells was phosphorylated (Fig S4D) at levels found in human cells. The assay with de-phosphorylated Cdt2 and kinase-treated Cdt2 could help to understand how the phosphorylation on Cdt2 regulate its binding activity.
CRL-type ligases are thought to use the “SRF” or “substrate receptor” subunit to recognize a specific degron in the substrate itself. However, CRL4-type ligases can deviate from that mechanism. For example, CRL4DDB2 recognizes specific UV-damaged bases in DNA, creating a “ubiquitination zone” around the repair site, to ubiquitinate specific substrates (Fischer et al, 2011). Here, we show a novel specific mechanism, where the ligase and substrate recognize the same molecule of a cellular platform, to bring the enzyme and substrate into close proximity and thus to facilitate substrate trapping: our data constitute a new paradigm for the use of a docking platform in ubiquitin conjugation. Creating such a three-component system offers many possibilities for regulation and robustness of the ubiquitination response: the recognition of PCNA by both substrate and Cdt2 through specialized PIP boxes likely enables further interactions between substrate and Cdt2 (as, for example, the recognition of the PIP degron by its N-terminal WD40 repeat domain), creating a set of redundant molecular recognition events that regulate this system in the cellular context.
Recently published work is consistent with and supports our data (Leng et al, 2018).
Experimental Procedures
Cell culture
HeLa cells, HEK293 cells, 293T cells, and U2OS cells were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum and 5% CO2. MCF7 cells were cultured in Dulbecco's modified Eagle's medium with 20% fetal bovine serum and 5% CO2 at 37°C. HEK293 cells and U2OS cells stably expressing Cdt2WT(1–730)-3FLAG, Cdt21–417-3FLAG, FLAG-Cdt2390–730, or Cdt2PIP-3A-FLAG were isolated using plasmids pCMV-HA-Cdt2WT(1–730)-3FLAG, pCMV-HA-Cdt21–417-3FLAG, p3FLAG-3NLS-myc-Cdt2390–730, or pCMV-HA-Cdt2PIP-3A-3FLAG. Proteasome inhibitor MG132 was used at 25 μM. UV-C (254 nm) irradiation of whole cells in dishes was performed at 5 to 100 J/m2 using a UV lamp (SUV-16, As One, Japan), a UV cross-linker (FS-800, Funakoshi) or a UV box equipped with a UV-C lamp and a radiometer 254 nm.
Plasmids
The Cdt2WT(1–730)-3FLAG–expressing plasmid, pCMV-HA-Cdt2WT(1–730)-3FLAG was constructed by cloning the PCR-amplified HA-Cdt2WT(1–730) fragment into NcoI-NotI-cut pCMV-3FLAG. The Cdt21–417-3FLAG–expressing plasmid, pCMV-HA-Cdt21–417-3FLAG, and pCMV-HA-Cdt21–700-3FLAG were constructed in the same way by PCR-amplifying the corresponding region. FLAG-Cdt2390–730, and Cdt2600–730-expressing plasmids were constructed by PCR-amplifying the corresponding regions, (390–730, and 600–730) and cloning into the p3FLAG-3NLS-myc plasmid which was prepared by cutting out the p21 gene from the p3FLAG-3NLS-myc-p21 plasmid (Nishitani et al, 2008). pcDNA-6myc-PCNA was constructed by ligating the PCR-amplified 6myc and PCNA. To construct the expression plasmid of PIP box mutant of Cdt2 (Cdt2PIP-3A-FLAG), pCMV-HA-Cdt2PIP-3A-3FLAG, Quick Change site-directed mutagenesis method (StrateGene) was performed using primers: AGCTCCATGAGGAAAGCCTGCACAGCCGCCCATAGAAAGTCCCAGGAG and CTGGGACTTTCTATGGGCGGCTGTGCAGGCTTTCCTCATGGAGCTGGG. The baculovirus expression plasmids for HA-Cul4A and His-myc-Rbx1 were as described (Nishitani et al, 2008). The pBP8-Cdt2WT(1–730)-3FLAG plasmid was constructed by ligating the BamH1-Cdt2-Kpn1 fragment from pBP8-Cdt2 and the Kpn1-Cdt2-3FLAG-Sma1 fragment from pCMV-HA-Cdt2-3FLAG into pBacPAK8 between BamH1 and Sma1 sites. pBP9-DDB1 was constructed by ligating the PCR-amplified BamH1-DDB1-EcoR1 fragment and the EcoR1-DDB1-Xho1 fragment into pBacPAK9 at BamH1-Xho1 sites. pBP9-Cdt1-3FLAG was constructed by cloning the PCR-amplified Sac1-Cdt1-3FLAG-Xho1 fragment using pCMV-Cdt1-3FLAG as a template into pBacPAK9. The GFP-Cdt2wt, GFP-Cdt2(1–417), and GFP-Cdt2(390–730) expression plasmids were constructed by subcloning of the respective FLAG-tagged constructs into pEGFP-C3 (Clontech). For the GFP-Cdt2wt–expressing plasmid, pCMV-HA-Cdt2WT(1–730)-3FLAG was cut with NcoI and KpnI, KpnI and XmaI. To produce blunt ends for the NcoI sites, Klenow was used. The fragment was then cloned into the HindIII and XmaI sites of pEGFP-C3. The ends produced by HindIII were made blunt by Klenow. For the GFP-Cdt2(1–417), the pCMV-HA-Cdt21–417-3FLAG construct was cut by NcoI and XmaI. Klenow was used for the NcoI ends. The fragment was then cloned into the HindIII and XmaI sites of pEGFP-C3. The ends produced by HindIII were made blunt by Klenow. To achieve a better nuclear localization for the GFP-Cdt2wt and GFP-Cdt2(1–417) constructs, these were sublconed into p3FLAG-3NLS-myc by XhoI and BamHI. Then, they were subcloned again into pEGFP-C3 by using the HindIII-BamHI sites. For the GFP-Cdt2(390–730) expression plasmid, the p3FLAG-3NLS-myc Cdt2(390–730) construct was cut with HindIII and BamHI. The fragment produced was cloned into the HindIII-BamHI sites of pEGFP-C3.
Antibodies, Western blotting, and immunofluorescence
For Western blotting, whole-cell lysates were prepared by lysing cell pellets directly in SDS–PAGE buffer. For immunofluorescence, U2OS or HEK293 cells were fixed in 4% paraformaldehyde (PFA; WAKO) for 10 min, permeabilized in 0.25% (vol/vol) Triton X-100 in PBS, and stained with the indicated antibodies as described previously. For double-staining with PCNA and Cdt2WT or Cdt2PIP-3A, cells were permeabilized in 0.1% Triton X-100 for 1 min on ice, and were fixed in 4% PFA for 10 min at room temperature, followed by fixation in ice-cold methanol for 10 min. For cyclobutane pyrimidine dimer (CPDs) staining, MCF7 and HeLa cells were fixed in 4% PFA for 10 min, permeabilized in 0.3% Triton X-100 in PBS, and then incubated in 0.5 N NaOH for 5 min for DNA denaturation before immunofluorescence. Alexa488-conjugated anti-mouse and Alexa592-conjugated anti-rabbit antibodies were used as secondary antibodies with Hoechst 33258 to visualize DNA. The following primary antibodies were used: Cdt1 (Nishitani et al, 2006), Cdt2 (Nishitani et al, 2008), cyclin A (mouse, Ab-6; Neomarkers; rabbit, H-432; Santa Cruz), Myc (mouse, 9E10; Santa Cruz), FLAG (F3165, F7425; Sigma-Aldrich), PCNA (PC10; Santa Cruz; rabbit serum, a gift from Dr. Tsurimoto), XP-A (FL-273; Santa Cruz), RCC1 (Shiomi et al, 2012), DDB1 (Bethyl Laboratories), Rbx1 (ROC1, Ab-1; Neomarkers), Cul4A (Bethyl Laboratories), RFC4 (H-183; Santa Cruz Biotechnology), DYKDDDDK Tag (Cell Signaling), and CPDs (CosmoBio). Protein levels were analysed by ImageJ software.
Micropore UV irradiation assay
We used a method to induce DNA photoproducts within localized areas of the cell nucleus as described in Ishii et al (2010). To perform micropore UV irradiation, cells were cultured on cover slips, washed twice with PBS, and subsequently covered with an isopore polycarbonate membrane filter (Millipore) with a pore size of either 3 or 5 μm in diameter, and irradiated. UV irradiation was achieved using a UV lamp (SUV-16, As One, Japan) with a dose rate of 0.4 J/m2·s, which was monitored with a UV radiometer (UVX Radiometer, UVP) at 254 nm, or with a UV box equipped with a UV-C lamp monitored with a VLX-3W radiometer (Vilber Lourmat) at 254 nm (CX-254 sensor). The filter was removed and cells were either fixed or cultured for the indicated time before fixation, and processed for immunofluorescence. For FRAP analysis, cells were cultured for 20 min to allow recruitment to sites of damage before photobleaching.
IP from Cdt2-FLAG–expressing cells
Control or Cdt2-FLAG expressing 293 cells that were UV irradiated (50 J/m2), or not UV irradiated, or in the middle S phase (5 h after release from aphidicolon arrest) were fixed with 0.02% formaldehyde for 10 min, lysed using 0.1% Triton X-100-containing modified cytoskeleton (mCSK) buffer (10 mM Pipes, pH 7.9, 100 mM NaCl, 300 mM sucrose, 0.1% [vol/vol] Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 10 mM β-glycerophosphate, 1 mMNa3VO4, 10 mM NaF), and then sonicated. After centrifugation (120,000g, Beckman Type 45Ti rotor, for 20 min at 4°C), the supernatants were mixed with anti-FLAG antibody-conjugated magnetic beads (M8823; Sigma-Aldrich) for 1 h at 4°C to obtain immunoprecipitates. The precipitate was washed with ice-cold 0.1% Triton X-100 containing mCSK buffer and subsequently suspended in SDS sample buffer.
RNAi knockdown experiments
Double-stranded RNAs were transfected at 100 nM using Oligofectamine (Invitrogen) or HiPerFect (QIAGEN). Twenty-four hours after the first transfection, a second transfection was performed and cells were cultured for two more days. The following siRNAs were made by Dharmacon: Skp2; GCAUGUACAGGUGGCUGUU, Cdt2_3′UTR; GCUGAGCUUUGGUCCACUA. The siRNA for siLuc, known as GL2, was used as a control siRNA. For Cdt1 silencing in Fig 1A and B, unsychronized HeLa cells were transfected twice with 200 nM of Cdt1 siRNA or control luciferase siRNA using Lipofectamine 2000 with a time interval of 24 h and were analysed 48 h after the second transfection. In synchronized MCF7 cells (Fig 1D), a single transfection with 200 nM of Cdt1 siRNA or control luciferase siRNA using Lipofectamine 2000 5 h after release from thymidine and before adding nocodazole was carried out. The Cdt1 siRNA was made by MWG: AACGUGGAUGAAGUACCCGACTT.
Chromatin fractionation
Cell extracts were prepared using 0.1% Triton X-100–containing mCSK buffer (200 μg of total protein in 400–800 μl). After centrifugation (17,900g for 10 min at 4°C), soluble and chromatin-containing pellet fractions were obtained. The pellets were washed with ice-cold 0.1% Triton X-100–containing mCSK buffer and subsequently suspended in SDS sample buffer.
Protein purification
PCNA
PCNA was purified essentially as a trimer as described (Fukuda et al, 1995). Cell lysate was prepared from pT7-PCNA–transformed Rosetta(DE3) and sequentially subjected to Resource Q, Superdex 200, and Mono Q (GE Healthcare Life Science) column chromatography.
Cdt1-3FLAG
The baculoviruses for Cdt1-3FLAG expression were infected into Sf21 insect cells, and cultured at 27°C for 60 h. Cell lysate was prepared with 0.5 M NaCl–containing buffer B (50 mM Tris–HCl, pH 8.0, 0.15 M NaCl, 1 mM EDTA, 10% glycerol, 1× Protease inhibitor cocktail [Roche Applied Science], 1 mM PMSF, 2 μg/ml Leupeptin), and passed on Diethylaminoethyl (DEAE) column. The flowthrough fraction was mixed with anti-FLAG resin (Sigma-Aldrich) and Cdt1-3FLAG was eluted with 200 µg/ml 3× FLAG peptides (Sigma-Aldrich).
RFC complex
The baculoviruses for FLAG-Rfc1, Rfc2, Rfc3, Rfc4, and Rfc5 were co-infected to High Five cells, expressed, and purified as described (Shiomi et al, 2000).
CRL4Cdt2
The baculoviruses for Cdt2-3FLAG (WT or PIP-3A), DDB1, HA-Cul4, and His-myc-Rbx1 were co-infected into Sf21 cells, and purified as described (Hayashi et al, 2014). Briefly, the cell lysate was prepared with 0.5 M NaCl and 0.5% NonidetP-40–containing buffer B, clarified by centrifugation, and passed on a DEAE column. The flowthrough fraction was mixed with anti-FLAG resin and CRL4Cdt2-3FLAG was eluted with buffer B containing 0.1 M NaCl, 0.1% NP-40 and 200 μg/ml 3FLAG peptide (Sigma-Aldrich). If necessary, CRL4Cdt2-3FLAG was loaded on 15–35% glycerol gradient in buffer G (25 mM Hepes, pH 7.8, 0.1 M NaCl, 1 mM EDTA, 0.01% NP-40) and subjected to ultra-centrifugation (Beckman TLS-55) at 214,000 g for 18 h at 4°C. 100 μl fractions were collected.
In vitro binding assay for Cdt1 and CRL4Cdt2 with PCNA
Cdt1-3FLAG (WT) and CRL4Cdt2-3FLAG (WT) beads were prepared as follows (Fig 5B): Cleared lysates were made from insect cells infected with baculoviruses for Cdt1-3FLAG (WT) or CRL4Cdt2-3FLAG (WT); incubated with anti-FLAG magnetic beads pre-blocked with buffer B containing 50% FBS, 0.5 M NaCl, and 0.5% NP-40; and washed with 0.5 M NaCl and 0.5% NP-40–containing buffer B and then with 0.1 M NaCl and 0.1% NP-40–containing buffer B. The protein beads prepared as above were incubated with PCNA protein at 4°C for 2 h, washed, and subjected to Western blotting.
Preparation of ncDNA beads
Closed circular (cc) DNA beads were prepared as described (Higashi et al, 2012). Briefly, single-stranded DNA templates prepared using pBluescript II KS(−) were annealed with a biotinated oligonucleotide primer (5′-CGCCTTGATCGT [biotin-dT]GGGAACCGGAGCTGAATGAAGC-3′). After second-strand synthesis and ligation, covalently cc double-stranded DNA was purified by CsCl/EtBr density gradient centrifugation, incubated with streptavidin, and bound to biotin-sepharose beads to produce the cc DNA beads (around 100 ng per 1 μl bed volume of beads). A single nick was introduced by Nb.BbvCI (New England Biolabs) treatment to produce the ncDNA beads. As control beads, biotin-sepharose beads were incubated with streptavidin alone (sa-beads).
PCNA loading and binding assay with purified Cdt1 and CRLCdt2
For loading of PCNA on DNA beads, 2 μl bed volume of nc DNA beads were incubated with 280 ng of RFC and 200 ng of PCNA in 20 μl of 2mM ATP–containing HBS buffer (10 mM Hepes, pH 7.4, 10 mM MgCl2, 0.2 mM EDTA, 0.05 % Tween 20, and 0.15 M NaCl) at 37°C for 1 h and washed with HBS buffer three times. The sa-beads were treated in the same way and used as control beads. The beads were incubated with Cdt1 or CRL4Cdt2 alone at 4°C for 1 h and washed with HBS buffer. The bound proteins were analysed on Western blotting. To measure the amounts of nc DNA bound to beads, nc DNA was released from beads after incubation in a proteinase K buffer (10 mM Tris–HCl, pH 7.5, 50 mM NaCl, 1 mM EDTA, 0.75 % SDS, and 2 mg/ml proteinase K) at 50°C for 1 h, extracted with phenol chloroform and ethanol-precipitated. The purified nc DNA was run on 0.8% agarose gel in 1× TBE (Tris-borate-EDTA) buffer and detected with ethidium bromide.
In vitro binding of PIP box peptides to PCNA
The expression plasmid for full-length human PCNA has been described (Hibbert & Sixma, 2012). Human PCNA was expressed in Bl21 (DE3) Escherichia coli cells (Merck KGaA) and purified as described (Hibbert & Sixma, 2012). The peptides Cdt2PIP (704–717), Cdt2PIP-ΤΑΜRΑ (704–717), Cdt1PIP (1–14), and Cdt1PIP-ΤΑΜRΑ (1–14) were synthesized in-house using Fmoc synthesis.
FP was monitored using a PheraStar plate reader (BMG Labtech) in non-binding 96-well plates (Corning), using an excitation wavelength of 540 nm and an emission wavelength of 590 nm. Experiments were performed in a buffer containing 10 mM Hepes, pH 7.5, 125 mM NaCl, and 0.5 mM Tris (2-carboxyethyl) phosphine hydrochloride. In the direct binding experiments, 1 nM of labelled peptide was mixed with PCNA at final concentrations ranging from 0 to 2 μM. Error bars represent standard deviations from triplicate experiments. The data were fitted according to standard equations (Littler et al, 2010).
X-ray crystallography structure determination
The purified PCNA was co-crystallized with Cdt2 and Cdt1 peptides, using the sitting drop vapour diffusion method in MRC 3-well crystallization plates (Swissci), with standard screening procedures (Newman et al, 2005). The protein solution at 17 mg·ml−1 was pre-incubated with an equimolar amount of peptide, and 0.1 μl of this solution was mixed with 0.1 μl of reservoir solution and equilibrated against a 30 μl reservoir. Crystals for the Cdt2 complex were obtained in 10% (wt/vol) PEG 6000 and 100 mM MES/HCl, pH 6.0. Crystals for the Cdt1 complex were obtained in 15% (wt/vol) PEG 400, 100 mM calcium acetate, and 100 mM MES/HCl, pH 6.0. Crystals appeared at 4°C within 72 h. Crystals were briefly transferred to a cryo-protectant solution containing the reservoir solution and 25% (wt/vol) PEG200 and vitrified by dipping in liquid nitrogen.
Data collection and structure refinement
X-ray data were collected on beamline ID29 at the European Synchrotron Radiation Facility. The images were integrated with XDS (Kabsch, 2010) and merged and scaled with AIMLESS (Evans, 2011). The starting phases were obtained by molecular replacement using PHASER (McCoy et al, 2007; Winn et al, 2011) with an available PCNA structure (PDB, 1AXC) as the search model. The models were built using COOT (Emsley et al, 2010) and refined with REFMAC (Murshudov et al, 2011) in iterative cycles. Model re-building and refinement parameter adjustment were performed in PDB-REDO (Joosten et al, 2014); homology-based hydrogen bond restraints (van Beusekom et al, 2018) were used at some stages of the procedure. The quality of the models was evaluated by MOLPROBITY (Chen et al, 2010). Data collection and refinement statistics are presented in Table 1. PISA, “Protein Interfaces, Surfaces and Assemblies” service PISA at the European Bioinformatics Institute (http://www.ebi.ac.uk/pdbe/prot_int/pistart.html) (Krissinel & Henrick, 2007) was used to calculate binding energies for Cdt2 PIP and Cdt1 PIP.
FRAP experiments and data analysis
Cells (HeLa or MCF7) were plated on 35-mm glass-bottom dishes in phenol-red–free medium (Invitrogen). FRAP experiments were conducted as previously described (Xouri et al, 2007b; Giakoumakis et al, 2017). A Leica TCS SP5 microscope equipped with a 63x magnification 1.4 NA oil-immersion lens and FRAP booster were used. During FRAP, cells were maintained at 37°C and 5% CO2. A defined circular region of interest with 2 μm diameter (ROI1) was placed in the nucleus and at the recruitment sites. GFP was excited using a 488-nm argon laser line. 50 pre-bleach images were acquired with 3% of the 488-nm line at 60% argon laser intensity, followed by a single bleach pulse on ROI1 using 476-nm and 488-nm laser lines combined at maximum power. In this manner, at least 60% of the fluorescence in ROI1 was bleached successfully. Next, 400 post-bleach images with a 0.052 s interval were recorded. Mean intensities of ROI1, the whole nucleus (ROI2) and an area outside of the nucleus (ROI3) were quantified and exported as .csv format files. Data analysis was performed using easyFRAP (Rapsomaniki et al, 2012; Giakoumakis et al, 2017; Koulouras et al, 2018).
Supplementary Material
Acknowledgements
This work was financially supported by JSPS KAKENHI grant numbers JP25131718 and JP26291025 (to H Nishitani), 13J07320 (to A Hayashi), the European Research Council (ERC-StG 281851 and ERC-PoC 755284), the project Bioimaging-GR, MIS 5002755 under the Operational Program “Competitiveness, Enterpreneurship, and Innovation” co-financed by Greece and the EU (to Z Lygerou) and a Greek state scholarship (to A Perrakis). We thank the Advanced Light Microscopy Facility of the University of Patras.
Author Contributions
A Hayashi: data curation, formal analysis, funding acquisition, and investigation.
NN Giakoumakis: data curation, formal analysis, and investigation.
T Heidebrecht: data curation, formal analysis, and investigation.
T Ishii: data curation, formal analysis, and investigation.
A Panagopoulos: data curation, formal analysis, and investigation.
C Caillat: data curation, formal analysis, and investigation.
M Takahara: data curation, formal analysis, and investigation.
RG Hibbert: data curation, formal analysis, and investigation.
N Suenaga: data curation, formal analysis, and investigation.
M Stadnik-Spiewak: data curation, formal analysis, and investigation.
T Takahashi: methodology.
Y Shiomi: validation and investigation.
S Taraviras: validation and investigation.
E von Castelmur: validation and investigation.
Z Lygerou: supervision, funding acquisition, investigation, and writing—review and editing.
A Perrakis: supervision, funding acquisition, investigation, and writing—original draft.
H Nishitani: supervision, funding acquisition, investigation, and writing—review and editing.
Conflict of Interest Statement
The authors declare that they have no conflict of interest.
References
- Abbas T, Dutta A (2011) CRL4Cdt2: Master coordinator of cell cycle progression and genome stability. Cell Cycle 10: 241–249. 10.4161/cc.10.2.14530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas T, Mueller AC, Shibata E, Keaton M, Rossi M, Dutta A (2013) CRL1-FBXO11 promotes Cdt2 ubiquitylation and degradation and regulates Pr-Set7/Set8-Mediated cellular migration. Mol Cell 49: 1147–1158. 10.1016/j.molcel.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas T, Sivaprasad U, Terai K, Amador V, Pagano M, Dutta A (2008) PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev 22: 2496–2506. 10.1101/gad.1676108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias EE, Walter JC (2006) PCNA functions as a molecular platform to trigger Cdt1 destruction and prevent re-replication. Nat Cell Biol 8: 84–90. 10.1038/ncb1346 [DOI] [PubMed] [Google Scholar]
- Arias EE, Walter JC (2007) Strength in numbers: Preventing rereplication via multiple mechanisms in eukaryotic cells. Genes Dev 21: 497–518. 10.1101/gad.1508907 [DOI] [PubMed] [Google Scholar]
- Bell SP, Dutta A (2002) DNA replication in eukaryotic cells. Annu Rev Biochem 71: 333–374. 10.1146/annurev.biochem.71.110601.135425 [DOI] [PubMed] [Google Scholar]
- Blow JJ, Dutta A (2005) Preventing re-replication of chromosomal DNA. Nat Rev Mol Cell Biol 6: 476–486. 10.1038/nrm1663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzei D, Foiani M (2010) Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol 11: 208–219. 10.1038/nrm2852 [DOI] [PubMed] [Google Scholar]
- Bruning JB, Shamoo Y (2004) Structural and thermodynamic analysis of human PCNA with peptides derived from DNA polymerase-delta p66 subunit and flap endonuclease-1. Structure 12: 2209–2219. 10.1016/j.str.2004.09.018 [DOI] [PubMed] [Google Scholar]
- Cardozo T, Pagano M (2004) The SCF ubiquitin ligase: Insights into a molecular machine. Nat Rev Mol Cell Biol 5: 739–751. 10.1038/nrm1471 [DOI] [PubMed] [Google Scholar]
- Centore RC, Havens CG, Manning AL, Li JM, Flynn RL, Tse A, Jin J, Dyson NJ, Walter JC, Zou L (2010) CRL4(Cdt2)-mediated destruction of the histone methyltransferase Set8 prevents premature chromatin compaction in S phase. Mol Cell 40: 22–33. 10.1016/j.molcel.2010.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen VB, Arendall WB 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC (2010) MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 66: 12–21. 10.1107/s0907444909042073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clijsters L, Wolthuis R (2014) PIP-box-mediated degradation prohibits re-accumulation of Cdc6 during S phase. J Cell Sci 127: 1336–1345. 10.1242/jcs.145862 [DOI] [PubMed] [Google Scholar]
- De Biasio A, Campos-Olivas R, Sanchez R, Lopez-Alonso JP, Pantoja-Uceda D, Merino N, Villate M, Martin-Garcia JM, Castillo F, Luque I, et al. (2012) Proliferating cell nuclear antigen (PCNA) interactions in solution studied by NMR. PLoS One 7: e48390 10.1371/journal.pone.0048390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley JF. (2004) Regulation of early events in chromosome replication. Curr Biol 14: R778–R786. 10.1016/j.cub.2004.09.019 [DOI] [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of coot. Acta Crystallogr D Biol Crystallogr 66: 486–501. 10.1107/s0907444910007493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans PR. (2011) An introduction to data reduction: Space-group determination, scaling and intensity statistics. Acta Crystallogr D Biol Crystallogr 67: 282–292. 10.1107/s090744491003982x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer ES, Scrima A, Bohm K, Matsumoto S, Lingaraju GM, Faty M, Yasuda T, Cavadini S, Wakasugi M, Hanaoka F, et al. (2011) The molecular basis of CRL4DDB2/CSA ubiquitin ligase architecture, targeting, and activation. Cell 147: 1024–1039. 10.1016/j.cell.2011.10.035 [DOI] [PubMed] [Google Scholar]
- Fukuda K, Morioka H, Imajou S, Ikeda S, Ohtsuka E, Tsurimoto T (1995) Structure-function relationship of the eukaryotic DNA replication factor, proliferating cell nuclear antigen. J Biol Chem 270: 22527–22534. 10.1074/jbc.270.38.22527 [DOI] [PubMed] [Google Scholar]
- Giakoumakis NN, Rapsomaniki MA, Lygerou Z (2017) Analysis of protein kinetics using fluorescence recovery after photobleaching (FRAP). Methods Mol Biol 1563: 243–267. 10.1007/978-1-4939-6810-7_16 [DOI] [PubMed] [Google Scholar]
- Han C, Wani G, Zhao R, Qian J, Sharma N, He J, Zhu Q, Wang QE, Wani AA (2015) Cdt2-mediated XPG degradation promotes gap-filling DNA synthesis in nucleotide excision repair. Cell Cycle 14: 1103–1115. 10.4161/15384101.2014.973740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens CG, Shobnam N, Guarino E, Centore RC, Zou L, Kearsey SE, Walter JC (2012) Direct Role for proliferating cell nuclear antigen (PCNA) in substrate recognition by the E3 Ubiquitin ligase CRL4-Cdt2. J Biol Chem 287: 11410–11421. 10.1074/jbc.M111.337683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens CG, Walter JC (2009) Docking of a specialized PIP Box onto chromatin-bound PCNA creates a degron for the ubiquitin ligase CRL4Cdt2. Mol Cell 35: 93–104. 10.1016/j.molcel.2009.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens CG, Walter JC (2011) Mechanism of CRL4Cdt2, a PCNA-dependent E3 ubiquitin ligase. Genes Dev 25: 1568–1582. 10.1101/gad.2068611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi A, Suenaga N, Shiomi Y, Nishitani H (2014) PCNA-dependent ubiquitination of Cdt1 and p21 in mammalian cells. Methods Mol Biol 1170: 367–382. 10.1007/978-1-4939-0888-2_19 [DOI] [PubMed] [Google Scholar]
- He YJ, McCall CM, Hu J, Zeng Y, Xiong Y (2006) DDB1 functions as a linker to recruit receptor WD40 proteins to CUL4-ROC1 ubiquitin ligases. Genes Dev 20: 2949–2954. 10.1101/gad.1483206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbert RG, Sixma TK (2012) Intrinsic flexibility of ubiquitin on proliferating cell nuclear antigen (PCNA) in translesion synthesis. J Biol Chem 287: 39216–39223. 10.1074/jbc.m112.389890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa LA, Banks D, Wu M, Kobayashi R, Sun H, Zhang H (2006) L2DTL/CDT2 interacts with the CUL4/DDB1 complex and PCNA and regulates CDT1 proteolysis in response to DNA damage. Cell Cycle 5: 1675–1680. 10.4161/cc.5.15.3149 [DOI] [PubMed] [Google Scholar]
- Higashi TL, Ikeda M, Tanaka H, Nakagawa T, Bando M, Shirahige K, Kubota Y, Takisawa H, Masukata H, Takahashi TS (2012) The prereplication complex recruits XEco2 to chromatin to promote cohesin acetylation in Xenopus egg extracts. Curr Biol 22: 977–988. 10.1016/j.cub.2012.04.013 [DOI] [PubMed] [Google Scholar]
- Hoeijmakers JH. (2001) Genome maintenance mechanisms for preventing cancer. Nature 411: 366–374. 10.1038/35077232 [DOI] [PubMed] [Google Scholar]
- Hofmann JF, Beach D (1994) cdt1 is an essential target of the Cdc10/Sct1 transcription factor: Requirement for DNA replication and inhibition of mitosis. Embo J 13: 425–434. 10.1002/j.1460-2075.1994.tb06277.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Shiomi Y, Takami T, Murakami Y, Ohnishi N, Nishitani H (2010) Proliferating cell nuclear antigen-dependent rapid recruitment of Cdt1 and CRL4Cdt2 at DNA-damaged sites after UV irradiation in HeLa cells. J Biol Chem 285: 41993–42000. 10.1074/jbc.m110.161661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov I, Chapados BR, McCammon JA, Tainer JA (2006) Proliferating cell nuclear antigen loaded onto double-stranded DNA: Dynamics, minor groove interactions and functional implications. Nucleic Acids Res 34: 6023–6033. 10.1093/nar/gkl744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S, Xiong Y (2009) CRL4s: The CUL4-RING E3 ubiquitin ligases. Trends Biochem Sci 34: 562–570. 10.1016/j.tibs.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Arias EE, Chen J, Harper JW, Walter JC (2006) A family of diverse Cul4-Ddb1-interacting proteins includes Cdt2, which is required for S phase destruction of the replication factor Cdt1. Mol Cell 23: 709–721. 10.1016/j.molcel.2006.08.010 [DOI] [PubMed] [Google Scholar]
- Joosten RP, Long F, Murshudov GN, Perrakis A (2014) The PDB_REDO server for macromolecular structure model optimization. IUCrJ 1: 213–220. 10.1107/s2052252514009324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen S, Eskildsen M, Fugger K, Hansen L, Larsen MS, Kousholt AN, Syljuasen RG, Trelle MB, Jensen ON, Helin K, et al. (2011) SET8 is degraded via PCNA-coupled CRL4(CDT2) ubiquitylation in S phase and after UV irradiation. J Cell Biol 192: 43–54. 10.1083/jcb.201009076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W. (2010) Xds. Acta Crystallogr D Biol Crystallogr 66: 125–132. 10.1107/s0907444909047337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Budhavarapu VN, Herrera CR, Nam HW, Kim YS, Yew PR (2010) The CRL4Cdt2 ubiquitin ligase mediates the proteolysis of cyclin-dependent kinase inhibitor Xic1 through a direct association with PCNA. Mol Cell Biol 30: 4120–4133. 10.1128/mcb.01135-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Kipreos ET (2007) The Caenorhabditis elegans replication licensing factor CDT-1 is targeted for degradation by the CUL-4/DDB-1 complex. Mol Cell Biol 27: 1394–1406. 10.1128/mcb.00736-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Starostina NG, Kipreos ET (2008) The CRL4Cdt2 ubiquitin ligase targets the degradation of p21Cip1 to control replication licensing. Genes Dev 22: 2507–2519. 10.1101/gad.1703708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulouras G, Panagopoulos A, Rapsomaniki MA, Giakoumakis NN, Taraviras S, Lygerou Z (2018) EasyFRAP-web: A web-based tool for the analysis of fluorescence recovery after photobleaching data. Nucleic Acids Res 46: W467–W472. 10.1093/nar/gky508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel E, Henrick K (2007) Inference of macromolecular assemblies from crystalline state. J Mol Biol 372: 774–797. 10.1016/j.jmb.2007.05.022 [DOI] [PubMed] [Google Scholar]
- Leng F, Saxena L, Hoang N, Zhang C, Lee L, Li W, Gong X, Lu F, Sun H, Zhang H (2018) Proliferating cell nuclear antigen interacts with the CRL4 ubiquitin ligase subunit CDT2 in DNA synthesis-induced degradation of CDT1. J Biol Chem 293: 18879–18889. 10.1074/jbc.RA118.003049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhao Q, Liao R, Sun P, Wu X (2003) The SCF(Skp2) ubiquitin ligase complex interacts with the human replication licensing factor Cdt1 and regulates Cdt1 degradation. J Biol Chem 278: 30854–30858. 10.1074/jbc.c300251200 [DOI] [PubMed] [Google Scholar]
- Littler DR, Alvarez-Fernandez M, Stein A, Hibbert RG, Heidebrecht T, Aloy P, Medema RH, Perrakis A (2010) Structure of the FoxM1 DNA-recognition domain bound to a promoter sequence. Nucleic Acids Res 38: 4527–4538. 10.1093/nar/gkq194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansilla SF, Soria G, Vallerga MB, Habif M, Martinez-Lopez W, Prives C, Gottifredi V (2013) UV-triggered p21 degradation facilitates damaged-DNA replication and preserves genomic stability. Nucleic Acids Res 41: 6942–6951. 10.1093/nar/gkt475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A. (2007) Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr D Biol Crystallogr 63 (Pt 1): 32–41. 10.1107/S0907444906045975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita M, Morimoto A, Ishii T, Komori H, Shiomi Y, Higuchi Y, Nishitani H (2011) Positively charged residues located downstream of PIP box, together with TD amino acids within PIP box, are important for CRL4(Cdt2) -mediated proteolysis. Genes Cell 16: 12–22. 10.1111/j.1365-2443.2010.01464.x [DOI] [PubMed] [Google Scholar]
- Murshudov GN, Skubak P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA (2011) REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr 67: 355–367. 10.1107/s0907444911001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman J, Egan D, Walter TS, Meged R, Berry I, Ben Jelloul M, Sussman JL, Stuart DI, Perrakis A (2005) Towards rationalization of crystallization screening for small- to medium-sized academic laboratories: The PACT/JCSG+ strategy. Acta Crystallogr D Biol Crystallogr 61: 1426–1431. 10.1107/s0907444905024984 [DOI] [PubMed] [Google Scholar]
- Nishitani H, Lygerou Z (2004) DNA replication licensing. Front Biosci 9: 2115–2132. 10.2741/1315 [DOI] [PubMed] [Google Scholar]
- Nishitani H, Shiomi Y, Iida H, Michishita M, Takami T, Tsurimoto T (2008) CDK inhibitor p21 is degraded by a proliferating cell nuclear antigen-coupled Cul4-DDB1Cdt2 pathway during S phase and after UV irradiation. J Biol Chem 283: 29045–29052. 10.1074/jbc.m806045200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani H, Sugimoto N, Roukos V, Nakanishi Y, Saijo M, Obuse C, Tsurimoto T, Nakayama KI, Nakayama K, Fujita M, et al. (2006) Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1 for proteolysis. EMBO J 25: 1126–1136. 10.1038/sj.emboj.7601002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukina K, Hayashi A, Shiomi Y, Sugasawa K, Ohtsubo M, Nishitani H (2018) Mutations at multiple CDK phosphorylation consensus sites on Cdt2 increase the affinity of CRL4(Cdt2) for PCNA and its ubiquitination activity in S phase. Genes Cell 23: 200–213. 10.1111/gtc.12563 [DOI] [PubMed] [Google Scholar]
- Oda H, Hubner MR, Beck DB, Vermeulen M, Hurwitz J, Spector DL, Reinberg D (2010) Regulation of the histone H4 monomethylase PR-Set7 by CRL4(Cdt2)-mediated PCNA-dependent degradation during DNA damage. Mol Cell 40: 364–376. 10.1016/j.molcel.2010.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroski MD, Deshaies RJ (2005) Function and regulation of cullin-RING ubiquitin ligases. Nat Rev Mol Cell Biol 6: 9–20. 10.1038/nrm1547 [DOI] [PubMed] [Google Scholar]
- Ralph E, Boye E, Kearsey SE (2006) DNA damage induces Cdt1 proteolysis in fission yeast through a pathway dependent on Cdt2 and Ddb1. EMBO Rep 7: 1134–1139. 10.1038/sj.embor.7400827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman M, Havens CG, Walter JC, Harper JW (2011) A genome-wide screen identifies p97 as an essential regulator of DNA damage-dependent CDT1 destruction. Mol Cell 44: 72–84. 10.1016/j.molcel.2011.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapsomaniki MA, Cinquemani E, Giakoumakis NN, Kotsantis P, Lygeros J, Lygerou Z (2015) Inference of protein kinetics by stochastic modeling and simulation of fluorescence recovery after photobleaching experiments. Bioinformatics 31: 355–362. 10.1093/bioinformatics/btu619 [DOI] [PubMed] [Google Scholar]
- Rapsomaniki MA, Kotsantis P, Symeonidou IE, Giakoumakis NN, Taraviras S, Lygerou Z (2012) easyFRAP: An interactive, easy-to-use tool for qualitative and quantitative analysis of FRAP data. Bioinformatics 28: 1800–1801. 10.1093/bioinformatics/bts241 [DOI] [PubMed] [Google Scholar]
- Rizzardi LF, Coleman KE, Varma D, Matson JP, Oh S, Cook JG (2015) CDK1-dependent inhibition of the E3 ubiquitin ligase CRL4CDT2 ensures robust transition from S Phase to Mitosis. J Biol Chem 290: 556–567. 10.1074/jbc.m114.614701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roukos V, Kinkhabwala A, Colombelli J, Kotsantis P, Taraviras S, Nishitani H, Stelzer E, Bastiaens P, Lygerou Z (2011) Dynamic recruitment of licensing factor Cdt1 to sites of DNA damage. J Cell Sci 124: 422–434. 10.1242/jcs.074229 [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Takami T, Yasutani Y, Maeda T, Morino M, Ishii T, Shiomi Y, Nishitani H (2012) Checkpoint kinase ATR phosphorylates Cdt2, a substrate receptor of CRL4 ubiquitin ligase, and promotes the degradation of Cdt1 following UV irradiation. PLoS One 7: e46480 10.1371/journal.pone.0046480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansam CL, Shepard JL, Lai K, Ianari A, Danielian PS, Amsterdam A, Hopkins N, Lees JA (2006) DTL/CDT2 is essential for both CDT1 regulation and the early G2/M checkpoint. Genes Dev 20: 3117–3129. 10.1101/gad.1482106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrima A, Konickova R, Czyzewski BK, Kawasaki Y, Jeffrey PD, Groisman R, Nakatani Y, Iwai S, Pavletich NP, Thoma NH (2008) Structural basis of UV DNA-damage recognition by the DDB1-DDB2 complex. Cell 135: 1213–1223. 10.1016/j.cell.2008.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senga T, Sivaprasad U, Zhu W, Park JH, Arias EE, Walter JC, Dutta A (2006) PCNA is a cofactor for Cdt1 degradation by CUL4/DDB1-mediated N-terminal ubiquitination. J Biol Chem 281: 6246–6252. 10.1074/jbc.m512705200 [DOI] [PubMed] [Google Scholar]
- Shibata E, Dar A, Dutta A (2014) CRL4Cdt2 E3 ubiquitin ligase and proliferating cell nuclear antigen (PCNA) cooperate to degrade thymine DNA glycosylase in S phase. J Biol Chem 289: 23056–23064. 10.1074/jbc.m114.574210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi Y, Hayashi A, Ishii T, Shinmyozu K, Nakayama J, Sugasawa K, Nishitani H (2012) Two different replication factor C proteins, Ctf18 and RFC1, separately control PCNA-CRL4Cdt2-mediated Cdt1 proteolysis during S phase and following UV irradiation. Mol Cell Biol 32: 2279–2288. 10.1128/mcb.06506-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi Y, Usukura J, Masamura Y, Takeyasu K, Nakayama Y, Obuse C, Yoshikawa H, Tsurimoto T (2000) ATP-dependent structural change of the eukaryotic clamp-loader protein, replication factor C. Proc Natl Acad Sci USA 97: 14127–14132. 10.1073/pnas.97.26.14127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slenn TJ, Morris B, Havens CG, Freeman RM Jr, Takahashi TS, Walter JC (2014) Thymine DNA glycosylase is a CRL4Cdt2 substrate. J Biol Chem 289: 23043–23055. 10.1074/jbc.m114.574194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulou A, Roukos V, Petropoulou C, Kotsantis P, Karantzelis N, Nishitani H, Lygerou Z, Taraviras S (2012) Cdt1 is differentially targeted for degradation by anticancer chemotherapeutic drugs. PLoS One 7: e34621 10.1371/journal.pone.0034621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto N, Tatsumi Y, Tsurumi T, Matsukage A, Kiyono T, Nishitani H, Fujita M (2004) Cdt1 phosphorylation by cyclin A-dependent kinases negatively regulates its function without affecting geminin binding. J Biol Chem 279: 19691–19697. 10.1074/jbc.m313175200 [DOI] [PubMed] [Google Scholar]
- Symeonidou IE, Taraviras S, Lygerou Z (2012) Control over DNA replication in time and space. FEBS Lett 586: 2803–2812. 10.1016/j.febslet.2012.07.042 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Takahara M, Nukina K, Hayashi A, Sakai W, Sugasawa K, Shiomi Y, Nishitani H (2017) Mismatch repair proteins recruited to ultraviolet light-damaged sites lead to degradation of licensing factor Cdt1 in the G1 phase. Cell Cycle 16: 673–684. 10.1080/15384101.2017.1295179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardat M, Brustel J, Kirsh O, Lefevbre C, Callanan M, Sardet C, Julien E (2010) The histone H4 Lys 20 methyltransferase PR-Set7 regulates replication origins in mammalian cells. Nat Cell Biol 12: 1086–1093. 10.1038/ncb2113 [DOI] [PubMed] [Google Scholar]
- Terai K, Shibata E, Abbas T, Dutta A (2013) Degradation of p12 subunit by CRL4Cdt2 E3 ligase inhibits fork progression after DNA damage. J Biol Chem 288: 30509–30514. 10.1074/jbc.c113.505586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsakraklides V, Bell SP (2010) Dynamics of pre-replicative complex assembly. J Biol Chem 285: 9437–9443. 10.1074/jbc.m109.072504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov N, Kermi C, Coulombe P, Van der Laan S, Hodroj D, Maiorano D (2014) PIP degron proteins, substrates of CRL4Cdt2, and not PIP boxes, interfere with DNA polymerase eta and kappa focus formation on UV damage. Nucleic Acids Res 42: 3692–3706. 10.1093/nar/gkt1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beusekom B, Touw WG, Tatineni M, Somani S, Rajagopal G, Luo J, Gilliland GL, Perrakis A, Joosten RP (2018) Homology-based hydrogen bond information improves crystallographic structures in the PDB. Protein Sci 27: 798–808. 10.1002/pro.3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn MD, Ballard CC, Cowtan KD, Dodson EJ, Emsley P, Evans PR, Keegan RM, Krissinel EB, Leslie AG, McCoy A, et al. (2011) Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr 67: 235–242. 10.1107/s0907444910045749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xouri G, Dimaki M, Bastiaens PI, Lygerou Z (2007a) Cdt1 interactions in the licensing process: A model for dynamic spatiotemporal control of licensing. Cell Cycle 6: 1549–1552. 10.4161/cc.6.13.4455 [DOI] [PubMed] [Google Scholar]
- Xouri G, Squire A, Dimaki M, Geverts B, Verveer PJ, Taraviras S, Nishitani H, Houtsmuller AB, Bastiaens PI, Lygerou Z (2007b) Cdt1 associates dynamically with chromatin throughout G1 and recruits Geminin onto chromatin. EMBO J 26: 1303–1314. 10.1038/sj.emboj.7601597 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.