Abstract
Sirtuin-7 is an evolutionarily conserved NAD-dependent deacetylase, which serves an important role in carcinogenesis. However, the potential mechanism of sirtuin-7 in endometrial cancer has not yet been investigated. The purpose of the present study was to investigate whether sirtuin-7 exhibits inhibitory effects on endometrial cancer cells. The potential mechanisms mediated by sirtuin-7 in endometrial cancer cells were also investigated. The expression levels of sirtuin-7 in endometrial cancer cells were compared with normal endometrial cells using western blotting. The results demonstrated that sirtuin-7 is overexpressed in endometrial cancer cells compared with normal endometrial cells. The downregulation of sirtuin-7 inhibited the growth and invasiveness of endometrial cancer cells. The knockdown of sirtuin-7 was observed to increase the sensitivity of the endometrial cancer cells to cisplatin treatment in vitro. An investigation into the potential molecular mechanism demonstrated that sirtuin-7 knockdown promoted the apoptosis of endometrial cancer cells by regulating the nuclear factor (NF)-κB signaling pathway. The knockdown of sirtuin-7 inhibited NF-κB expression and resulted in a decrease in the expression of NF-κB target proteins that are anti-apoptotic: Bcl-xl, Bcl-2 and Mcl-1. Sirtuin-7 knockdown also resulted in an increase of the NF-κB target proteins that are pro-apoptotic: Caspase-3, Bad and Bax. In conclusion, the present study demonstrated that sirtuin-7 knockdown was able to markedly inhibit the growth of endometrial cancer cells, suggesting that sirtuin-7 may be a potential therapeutic target for endometrial cancer therapy.
Keywords: sirtuin-7, endometrial cancer, apoptosis, nuclear factor-κB
Introduction
Endometrial cancer is a common malignant cancer that is diagnosed in premenopausal and postmenopausal females, which occurs in the endometrial epithelium of the reproductive tract (1). In the last five years, studies have reported that endometrial cancer accounts for 20–30% of malignant tumors of the female reproductive tract and that the incidence of endometrial cancer is increasing (2,3). Although a number of anti-cancer treatment strategies are available for endometrial cancer, increasing the level of apoptosis in tumor cells has become an important method for the clinical treatment of endometrial cancer (4–6).
Sirtuin-7 is a member of the sirtuin family, which is a family of evolutionarily conserved NAD-dependent deacetylases (7). Studies have indicated that sirtuin-7 serves an important role in carcinogenesis, and the downregulation of sirtuin-7 may be a potential pharmacological strategy to increase chemoradiation-induced apoptosis in cancer cells in patients with cancer (8). A previous study has demonstrated that sirtuin-7 maintains critical features that define cancer cells by removing the acetylation mark on lysine 18 of histone H3, which has been described as a general marker of tumor prognosis and oncological treatments (9). However, the role of sirtuin-7 in endometrial cancer has not been the subject of in-depth investigations.
The inhibition of nuclear factor (NF)-κB activation can lead to the inhibition of cell growth and invasion in various types of cancer cells (10–12). A previous study indicated that the anti-malarial drug dihydroartemisinin significantly suppressed the metastasis of non-small-cell lung cancer via inhibiting NF-κB activity (13). Additionally, NF-κB has been shown to suppress apoptosis and promote the proliferation of bladder cancer cells by upregulating survivin expression in vitro and in vivo (14). Furthermore, Yi et al (15) reported that the NF-κB p53 apoptosis signaling pathway is associated with poor prognosis in colorectal cancer via regulation of cell proliferation. Furthermore, the results have concluded that NF-κB expression is overexpressed in nonmalignant endometrial tumors when compared with normal endometrial tissues (16). However, the associations between sirtuin-7 and NF-κB have not been clearly investigated in endometrial cancer cells. Therefore, in the present study, the role of sirtuin-7 was investigated, and its possible apoptotic mechanism in endometrial cancer was analyzed.
Materials and methods
Patients
A total of 5 female endometrial cancer patients were recruited at the Harbin Second Hospital (Harbin, China) between June 2014 and January 2017. The mean age of patients was 42.2 years (range, 34.2–48.5 years). All patients provided written informed consent. The Ethics Committee of Harbin Second Hospital approved the present study.
Cell lines and culture
The endometrial cancer cell lines (KLE, RL95-2, AN3CA and Ishikawa) were provided by Randy Kramer (University of California, San Francisco, CA, USA). Normal primary endometrial cells (ESCs) were derived from normal endometrial tissues that were discarded from periodontal surgical procedures, and this was approved by the Harbin Second Hospital. The 1–5th passages of ESC cells were cultured in Dulbecco's modified Eagle medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA) that was supplemented with 15% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.). The 6th passages of ESC were authenticated by Suzhou Microread Genetics Co., Ltd (Suzhou, China) and used for analysis of protein expression. KLE, RL95-2, AN3CA and Ishikawa cells were cultured in DMEM that was supplemented with 10% FBS at 37°C in a humidified incubator at 5% CO2.
Small interfering RNA (siRNA)
Ishikawa cells were harvested when 85–95% confluence was attained. The cells were transfected the following siRNAs to silence sirtuin-7 target gene using Lipofectamine® 2000 (Invitrogen, Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The sequences are as follows: siRNA-vector, 5′-UUCUCCGAACGUGUCACGU-3′ and siRNA-sirtuin-7 siRNA, 5′-GCACCGTCCATCGTGTTTATT-3′. All siRNAs were purchased from GeneChem Inc. (Shanghai, China). Further analysis was performed 72 h after transfection.
NF-κB1 overexpression
Ishikawa cells were harvested after reaching 85–95% confluence. All the DMEM media (Thermo Fisher Scientific, Inc.) was then removed. Ishikawa cells were transfected by pCMVp-NEO-NF-κB1 (100 pmol, Invitrogen; Thermo Fisher Scientific, Inc.) or pCMVp-NEO vector (100 pmol, Invitrogen; Thermo Fisher Scientific, Inc.) using Lipofectamine® 2000 according to the manufacturer's instructions. Cells were used for further analysis 72 h after transfection.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from tumor cells using RNeasy Mini kit (Qiagen Sciences, Inc., Gaithersburg, MD, USA) in a 20 µl volume with 5 pmol of each primer and 1 µl of RNA, 0.5 µl reverse transcriptase, 2 µl buffer, 2 µl dNTPs (both Takara Biotechnology Co., Ltd., Dalian, China) and 18 µl deionized water for 2 h at 37°C according to manufacturer's protocol. The expressions levels of sirtuin-7 in cells were analyzed by RT-qPCR with β-actin as an endogenous control (17). All forward and reverse primers were synthesized by Invitrogen (Thermo Fisher Scientific, Inc.). The primer sequences are as follows: Sirtuin-7 forward, 5′-AGAAGCGTTAGTGCTGCCG-3′ and reverse, 5′-GAGCCCGTCACAGTTCTGAG-3′; β-actin forward, 5′-CGGAGTCAACGGATTTGGTC-3′ and reverse, 5′-AGCCTTCTCCATGGTCGTGA-3′. PCR amplification followed preliminary denaturation at 94°C for 2 min, followed by 45 cycles of 95°C for 30 sec, annealing temperature reduced to 56°C for 30 sec and 72°C for 10 min. The reaction was performed in a total volume of 20 µl that contains 50 ng genomic DNA, 200 µM dNTP, 2.5 U Taq DNA polymerase and 200 µM of each primer. The relative mRNA expression changes were calculated by 2−ΔΔCq (18). The results are expressed as the n-fold way compared with β-actin.
MTT cytotoxicity assays
Ishikawa cells were incubated with melittin (2.0 mg/ml) in 96-well plates for 24, 48 and 72 h in triplicate for each condition at 37°C. For the control, PBS was added instead of melittin. At each time point, 20 µl MTT (5 mg/ml) in PBS solution was added to each well and the plate was then further incubated for 4 h at 37°C. The medium was removed, and 100 µl dimethyl sulfoxide (DMSO) was added into the wells to solubilize the crystals. The optical density was analyzed with a BIO-RAD (ELISA) reader (Bio-Rad Laboratories, Inc.) at a wavelength of 490 nm.
Cell invasion and migration assays
Ishikawa cells were treated with cisplatin (5.0 mg/ml) for 24 h and non-treated cells were used as control. Migration and invasion assays of Ishikawa cells were conducted in a 6-well culture plate with chamber inserts (BD Biosciences Franklin Lakes, NJ, USA). For migration assays, Ishikawa cells (1×104/well) were placed into the upper chamber with the non-coated membrane (Transwell inserts; BD Biosciences) and DMEM supplemented with 5% PBS was plated in the lower chamber for 48 h at 37°C. For invasion assays, the cells (1×104/well) were placed into the upper chamber with the Matrigel inserts membrane for 48 h at 37°C. For migration and invasion assays, Ishikawa cells were counted in at least three randomly stained-fields using a light microscope (Olympus Corporation, Tokyo, Japan) at magnification, ×400.
Proliferation assays
Ishikawa cells (0.5×103/well) were seeded in a six-well plate and cultured for 10 days at 37°C in a humidified incubator at 5% CO2. The cell colonies were fixed with 10% formaldehyde for 10 min at 37°C and stained for 5 min with 0.5% crystal violet for 10 min at 37°C. The number of cell colonies was then calculated using ImageJ software 3.0 (National Institutes of Health, Bethesda, MD, USA). The images were taken under a light microscope (Olympus Corporation).
Apoptosis assays
Ishikawa cells (1×106/well) were seeded in 6-well plates for 12 h at 37°C in a humidified incubator at 5% CO2. The cells, siRNA-sirtuin-7 or siRNA-vector were then incubated with cisplatin (Control, 5.0 mg/ml) or PBS (Mock) for 24 h at 37°C. The cells were removed, collected and washed with phosphate buffer solution (PBS) three times. Subsequently, Ishikawa cells were incubated with fluorescein isothiocyanate (FITC)-conjugated Annexin V and propidium iodide (PI) for 2 h at room temperature, using an Annexin V-FITC Apoptosis Detection kit (BD Biosciences) and according to the manufacturer's protocol. Flow cytometry was used to analyze the percentage of apoptotic Ishikawa cells by using FCS Express™ 4 IVD (De Novo Software, Glendale, CA, USA).
Tumor cell adhesion
Ishikawa cells (1×106/well) were cultured in six-well plate for 1.5 h at 37°C. Cells were stained with 1% Methylene blue (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for 30 min at room temperature. The number of Ishikawa cells that adhered on the monolayer was recorded using an Olympus microscope in at least three field of view (Olympus Corporation).
Western blot analysis
Western blotting was used to evaluate protein levels according to protocols as previously described by Kurien et al (19). Briefly, the cells (1×107/well) were lysed in a lysis buffer that contains 1% phenylmethanesulfonyl fluoride (PMSF) for three times and centrifuged at 8,000 × g for 10 min at 4°C. Protein concentration was measured by a BCA protein assay kit (Thermo Fisher Scientific, Inc.). The proteins (60 µg) were separated using 10% SDS-PAGE. Then, the proteins were transferred onto nitrocellulose membranes. The nitrocellulose membranes were blocked with 5% (w/v) non-fat milk powder, which was dissolved in tris-buffered saline plus Tween-20 (TBST) solution, for 2 h at 37°C. This was followed by incubation with primary rabbit anti-human antibodies: Anti-SIRT7 (dilution, 1:1,000; cat. no. ab135055), Bcl-2 (dilution, 1:1,000; cat. no. ab32124), Bcl-xl (dilution, 1:1,000; cat. no. ab32370), Mcl-1 (cat. no. ab32087), caspase-3 (cat. no. ab2171), Bad (cat. no. ab32445), Bax (cat. no. ab77566) and β-actin (dilution 1:500; cat. no. ab8226; all Abcam, Shanghai, China) for 12 h at 4°C. All antibodies were diluted at 1:1,000. The secondary goat anti-rabbit antibodies (dilution 1:2,000, cat. no. PV-6001; OriGene Technologies, Inc., Beijing, China) were incubated and the membranes were washed with PBS buffer and visualized with ECL Western blotting detection reagents (GE Healthcare, Chicago, IL, USA). The density of the bands was analyzed by Quantity One (version 4.62; Bio-Rad Laboratories, Inc.).
Immunohistochemistry
Endometrial tumors were fixed using 10% formaldehyde followed by embedding in paraffin wax. The tissue blocks were cut into serial sections with a thickness of 4 µm. The tumor sections were incubated with rabbit anti-human anti-SIRT7 (dilution, 1:1,000; cat. no. ab135055; Abcam) for 12 h at 4°C. Then, the tumor sections were treated with Goat Anti-Rabbit IgG (Alexa Fluor® 488; dilution 1:1,000; cat. no. ab150077; Abcam) for 12 h at 4°C. The sections were examined under a light microscope at a magnification of ×400.
Statistical analysis
All data are expressed the mean ± standard deviation, and the experiments were performed three times. Statistical analyses were performed using one-way analysis of variance followed by Tukey's multiple comparison post-hoc tests using Graph Pad Prism software (version 5.0; GraphPad Software, Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Sirtuin-7 is overexpressed in endometrial cancer tissues
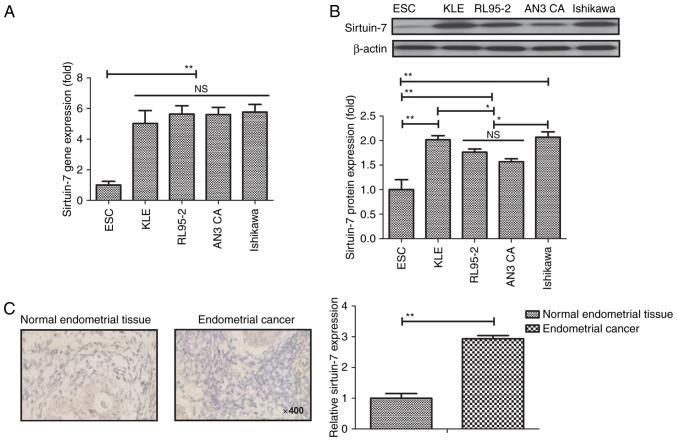
To determine the role of sirtuin-7 in endometrial cancer, the mRNA and protein levels of all sirtuin-7 were examined in endometrial cancer cell lines (KLE, RL95-2, AN3 CA and Ishikawa) and compared with ESCs. Results revealed that sirtuin-7 gene was higher in endometrial cancer cell lines compared with normal primary endometrial cells (P<0.01; Fig. 1A). Western blot analysis also demonstrated that sirtuin-7 protein expression was higher in endometrial cancer cell lines compared with normal primary endometrial cells (P<0.01; Fig. 1B). The expression of sirtuin-7 was significantly higher in endometrial cancer tissues compared with normal tissues (P<0.01; Fig. 1C). These results suggest that sirtuin-7 may be a potential target for the treatment of endometrial cancer.
Figure 1.
Sirtuin-7 is overexpressed in endometrial cancer. (A) Gene and (B) protein expression of sirtuin-7 in endometrial cancer cell lines and ESCs. (C) Sirtuin-7 protein expression in endometrial cancer tissues and normal endometrial tissues. Magnification, ×400; NS, not significant; ESC, normal primary endometrial cells. *P<0.05, **P<0.01.
Knockdown of sirtuin-7 inhibits the growth, proliferation and invasiveness of endometrial cancer cells
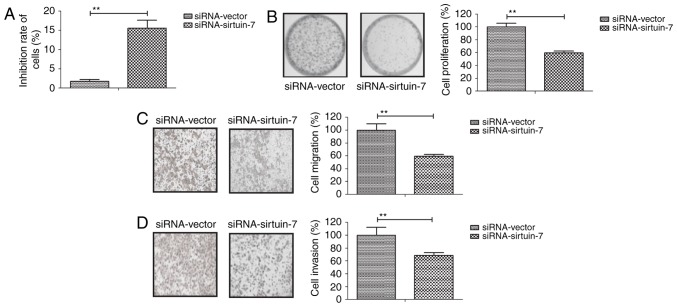
To gain further insights into the role of sirtuin-7 in endometrial cancer cells, sirtuin-7 expression was downregulated in Ishikawa cells. As indicated in Fig. 2A, siRNA-induced knockdown of sirtuin-7 was able to significantly inhibit the growth of Ishikawa cells compared with the control. The downregulation of sirtuin-7 was also able to inhibit the proliferation of Ishikawa cells (Fig. 2B). Furthermore, the knockdown of sirtuin-7 was able to suppress the migration and invasion of Ishikawa cells (Fig. 2C and D). These results suggest that the downregulation of sirtuin-7 is able to inhibit the growth and metastasis of endometrial cancer cells.
Figure 2.
Knockdown of sirtuin-7 inhibits cell growth, proliferation and invasion in endometrial cancer cells. (A) siRNA-induced knockdown of sirtuin-7 inhibits the growth of Ishikawa cells. (B) Knockdown of sirtuin-7 inhibits the (B) proliferation, (C) migration and (D) invasion of Ishikawa cells. Magnification, ×20. **P<0.01. siRNA, small-interfering RNA.
Knockdown of sirtuin-7 promotes cisplatin-induced apoptosis of endometrial cancer cells
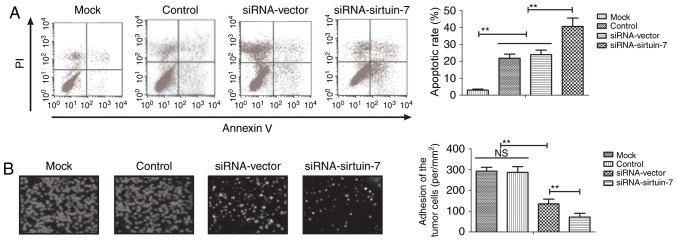
Cisplatin is the most commonly used chemotherapeutic drug for the treatment of endometrial cancer (20). Therefore, the present study investigated whether sirtuin-7 knockdown is able to enhance the apoptotic sensitivity of endometrial cancer cells to cisplatin. It was indicated that the knockdown of sirtuin-7 was able to significantly promote cisplatin-induced apoptosis of Ishikawa cells (Fig. 3A). The results indicated that the downregulation of sirtuin-7 was able to increase cisplatin-induced inhibition on the adhesion of Ishikawa cells compared with untreated controls (Fig. 3B). These results suggest that sirtuin-7 may be involved in resistance to apoptosis in endometrial cancer cells.
Figure 3.
Knockdown of sirtuin-7 promotes cisplatin-induced apoptosis of endometrial cancer cells. (A) Knockdown of sirtuin-7 promotes cisplatin-induced apoptosis of Ishikawa cells. (B) Knockdown of sirtuin-7 increases cisplatin-induced inhibition of cell adhesion of Ishikawa cells compared with untreated controls. **P<0.01. PI, propidium iodide; NS, not significant; siRNA, small-interfering RNA.
Sirtuin-7 knockdown regulates the apoptosis of endometrial cancer cells via the NF-κB signaling pathway
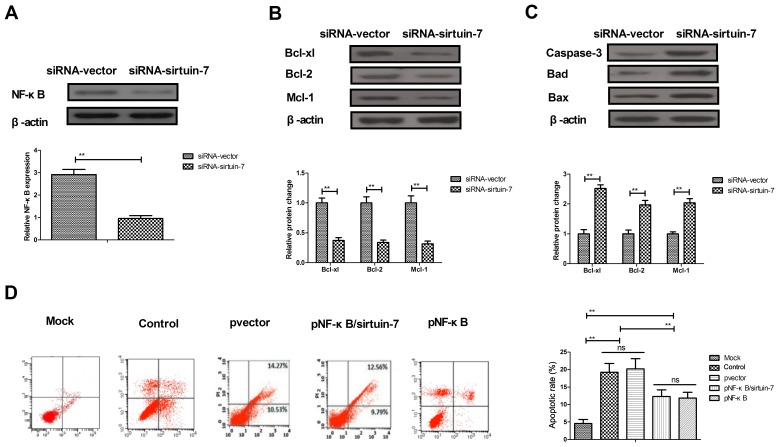
Due to the involvement of NF-κB in apoptosis of endometrial cancer cells (19), the potential mechanism of sirtuin-7 in apoptosis was further analyzed. The downregulation of sirtuin-7 was able to significantly downregulate NF-κB1 expression in Ishikawa cells (Fig. 4A). The knockdown of sirtuin-7 resulted in the downregulation of NF-κB1 target proteins (Bcl-xl, Bcl-2, and Mcl-1), which are anti-apoptotic. The knockdown of sirtuin-7 also led to the upregulation of NF-κB1 target proteins (Bad and Bax), which are pro-apoptotic (Fig. 4B and C). Furthermore, the results indicated that the overexpression of NF-κB1 inhibited sirtuin-7 knockdown and increased (pNF-κB/sirtuin-7) apoptosis of endometrial cancer cells induced by cisplatin (Fig. 4D). These results suggest that the knockdown of sirtuin-7 regulates the apoptosis of endometrial cancer cells via the NF-κB signaling pathway.
Figure 4.
Knockdown of sirtuin-7 regulates the apoptosis of endometrial cancer cells via the NF-κB signaling pathway. (A) Downregulation of sirtuin-7 decreases NF-κB expression in Ishikawa cells. (B) Western blot analysis illustrating the knockdown of sirtuin-7 decreases the expression of NF-κB target anti-apoptotic proteins (Bcl-xl, Bcl-2 and Mcl-1) in Ishikawa cells. (C) Knockdown of sirtuin-7 increases the expression of NF-κB target pro-apoptotic proteins (caspase-3, Bad and Bax) in Ishikawa cells. (D) NF-κB overexpression inhibits sirtuin-7 knockdown and increased (pNF-κB/sirtuin-7) apoptosis of endometrial cancer cells induced by cisplatin. **P<0.01. Bax, BCL2 associated × protein; Bcl-2, B-cell lymphoma 2; Mcl-1, myeloid cell leukemia 1; NS, not significant; NF-κB, nuclear factor-κB.
Discussion
Endometrial cancer is one of the most common female reproductive tract tumors worldwide (21). Although surgery, chemotherapy, radiotherapy and endocrine therapies are becoming increasingly important for the clinical treatment of endometrial cancer, the prognosis is poor for patients with recurrent or advanced stages of disease (22). Studies have suggested that sirtuin-7 is a potential therapeutic target for human cancer (23,24). In the present study, the role of sirtuin-7 in the progression of endometrial cancer and its potential molecular mechanisms were investigated. The present study reports that sirtuin-7 knockdown was able to significantly inhibit the growth, proliferation, migration and invasion of endometrial cancer cells. Additionally, sirtuin-7 knockdown was able to promote the apoptosis of endometrial cancer cells that was induced by cisplatin via downregulation of the NF-κB signaling pathway.
Although a previous study has suggested that sirtuin-7 mRNA expression is upregulated in patients with breast cancer (25), the expression levels of sirtuin-7 in endometrial cancer cells had not yet been investigated. In the present study, it was demonstrated that sirtuin-7 mRNA and protein expression levels are upregulated in endometrial cancer cell lines and cancer tissues compared with normal endometrial cell lines and tissues. Wang et al (8) indicated that sirtuin-7 exhibited oncogenic potential in human ovarian cancer cells, which may serve a role as an oncogene in ovarian malignancies and may be a potential therapeutic target. Additionally, a high expression of sirtuin-7 has served as a predictor of adverse outcomes in patients with breast cancer (26). In the present study, it was demonstrated that the knockdown of sirtuin-7 was able to significantly inhibit the growth and metastasis of endometrial cancer cells. In a study on pancreatic cancer, McGlynn et al (27) indicated that sirtuin-7 may be a potential novel biomarker for determining patient outcome. The findings of the present study indicate that sirtuin-7 may be a biomarker for patients with endometrial cancer.
A number of studies have suggested proapoptotic and prosurvival roles of sirtuin-7 in human cancer cells (28,29), with reports indicating that sirtuin inhibitors, sirtinol and nicotinamide, have been used to inhibit cell growth in several types of cancer, including breast and lung cancer, and oral squamous cell carcinoma (30). A study has suggested that 5-FU induces radiosensitivity via degradation of sirtuin-7 to favor the cell death pathway in targeted cancer cells (31).
In the present study, it was demonstrated that sirtuin-7 knockdown was able to enhance the apoptosis of endometrial cancer cells, which was induced by cisplatin. Previous studies have also suggested that the inhibition of NF-κB activation suppressed the production of angiogenic factors, which are involved in the apoptosis of endometrial cancer cells (32,33). The results of the present study revealed that the knockdown of sirtuin-7 was able to decrease NF-κB expression, and the apoptosis of endometrial cancer cells was promoted. However, the overexpression of NF-κB inhibited sirtuin-7 knockdown and abolished sirtuin-7 knockdown and increased apoptosis induced by cisplatin. Yang et al (34) reported that NF-κB is able to regulate the expression of Bcl-2 and caspase-3 in gastric cancer cells, which was induced by tumor necrosis factor-related apoptosis inducing ligand. The present study indicated that the knockdown of sirtuin-7 resulted in the downregulation of the NF-κB-targeted anti-apoptotic proteins (Bcl-xl, Bcl-2 and Mcl-1) and the upregulation of the NF-κB-targeted pro-apoptotic proteins (caspase-3, Bad and Bax).
In conclusion, the findings of the present study indicate a role for sirtuin-7 in the apoptosis of endometrial cancer cells, and this may be mediated via the NF-κB signaling pathway. The findings suggest that sirtuin-7 may be a potential therapeutic target for endometrial cancer therapy, however further study is required.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author upon reasonable request.
Authors' contributions
SM conducted the experiments. JM analyzed the data in the present study and HY designed the experiments.
Ethics approval and consent to participate
All patients provided written informed consent. The Ethics Committee of Harbin Second Hospital (Harbin, China) approved the present study.
Consent for publication
All identifying patient information has been removed and written, informed consent for the publication of the present study was provided by all patients.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bourgin C, Saidani M, Poupon C, Cauchois A, Foucher F, Leveque J, Lavoue V. Endometrial cancer in elderly women: Which disease, which surgical management? A systematic review of the literature. Eur J Surg Oncol. 2016;42:166–175. doi: 10.1016/j.ejso.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Lee B, Suh DH, Kim K, No JH, Kim YB. Influence of positive peritoneal cytology on prognostic factors and survival in early-stage endometrial cancer: A systematic review and meta-analysis. Jpn J Clin Oncol. 2016;46:711–717. doi: 10.1093/jjco/hyw063. [DOI] [PubMed] [Google Scholar]
- 3.Lheureux S, Oza AM. Endometrial cancer-targeted therapies myth or reality? Review of current targeted treatments. Eur J Cancer. 2016;59:99–108. doi: 10.1016/j.ejca.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Guo D, Li L, Zhang H, Zheng W. Pathologic assessment and clinical impacts for endometrial cancer and precursors after progestin treatment. Zhonghua Bing Li Xue Za Zhi. 2015;44:216–220. (In Chinese) [PubMed] [Google Scholar]
- 5.Turkevich VG. Clinical evaluation of the effectiveness of radiation treatment for endometrial cancer. Vopr Onkol. 2014;60:371–374. (In Russian) [PubMed] [Google Scholar]
- 6.Karnjus-Begonja R, Vrdoljak E, Corusić A, Haller H, Jelavic TB, Matković V, Strinić T, Barisić D, Tomic S, Kukura V, et al. Clinical recommendations for diagnosing, treatment and monitoring of patients with endometrial cancer-Croatian Oncology Society and Croatian Society for Gynecology and Obstetrics as Croatian Medical Association units and Croatian Society of Gynecological Oncology. Lijec Vjesn. 2013;135:230–234. (In Croatian) [PubMed] [Google Scholar]
- 7.Jung-Hynes B, Nihal M, Zhong W, Ahmad N. Role of sirtuin histone deacetylase SIRT1 in prostate cancer. A target for prostate cancer management via its inhibition? J Biol Chem. 2009;284:3823–3832. doi: 10.1074/jbc.M807869200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang HL, Lu RQ, Xie SH, Zheng H, Wen XM, Gao X, Guo L. SIRT7 exhibits oncogenic potential in human ovarian cancer cells. Asian Pac J Cancer Prev. 2015;16:3573–3577. doi: 10.7314/APJCP.2015.16.8.3573. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Redondo P, Santos-Barriopedro I, Vaquero A. A big step for SIRT7, one giant leap for Sirtuins in cancer. Cancer Cell. 2012;21:719–721. doi: 10.1016/j.ccr.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 10.Huang X, Teng Y, Yang H, Ma J. Propofol inhibits invasion and growth of ovarian cancer cells via regulating miR-9/NF-κB signal. Braz J Med Biol Res. 2016;49:e5717. doi: 10.1590/1414-431x20165717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ji BL, Xia LP, Zhou FX, Mao GZ, Xu LX. Aconitine induces cell apoptosis in human pancreatic cancer via NF-kappaB signaling pathway. Eur Rev Med Pharmacol Sci. 2016;20:4955–4964. [PubMed] [Google Scholar]
- 12.Chen H, Huang Y, Huang J, Lin L, Wei G. Gigantol attenuates the proliferation of human liver cancer HepG2 cells through the PI3K/Akt/NF-kappaB signaling pathway. Oncol Rep. 2017;37:865–870. doi: 10.3892/or.2016.5299. [DOI] [PubMed] [Google Scholar]
- 13.Jiang J, Geng G, Yu X, Liu H, Gao J, An H, Cai C, Li N, Shen D, Wu X, et al. Repurposing the anti-malarial drug dihydroartemisinin suppresses metastasis of non-small-cell lung cancer via inhibiting NF-κB/GLUT1 axis. Oncotarget. 2016;7:87271–87283. doi: 10.18632/oncotarget.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui X, Shen D, Kong C, Zhang Z, Zeng Y, Lin X, Liu X. NF-kappaB suppresses apoptosis and promotes bladder cancer cell proliferation by upregulating survivin expression in vitro and in vivo. Sci Rep. 2017;7:40723. doi: 10.1038/srep40723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi W, Xiao E, Ding R, Luo P, Yang Y. High expression of fibronectin is associated with poor prognosis, cell proliferation and malignancy via the NF-κB/p53-apoptosis signaling pathway in colorectal cancer. Oncol Rep. 2016;36:3145–3153. doi: 10.3892/or.2016.5177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faloppa CC, Baiocchi G, Cunha IW, Fregnani JH, Osorio CA, Fukazawa EM, Kumagai LY, Badiglian-Filho L, Pinto GL, Soares FA. NF-κB and COX-2 expression in nonmalignant endometrial lesions and cancer. Am J Clin Pathol. 2014;141:196–203. doi: 10.1309/AJCPV7U7PGHOWEQG. [DOI] [PubMed] [Google Scholar]
- 17.Xiao S, Wang J, Xiao N. MicroRNAs as noninvasive biomarkers in bladder cancer detection: a diagnostic meta-analysis based on qRT-PCR data. Int J Biol Markers. 2016;31:e276–e285. doi: 10.5301/jbm.5000199. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Kurien BT, Scofield RH. Validating antibody specificities for immunohistochemistry by protein blotting methods. Methods Mol Biol. 2017;1554:61–73. doi: 10.1007/978-1-4939-6759-9_3. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Kyo S, Nakamura M, Mizumoto Y, Maida Y, Bono Y, Takakura M, Fujiwara H. Imatinib sensitizes endometrial cancer cells to cisplatin by targeting CD117-positive growth-competent cells. Cancer Lett. 2014;345:106–114. doi: 10.1016/j.canlet.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 21.van der Steen-Banasik E, Christiaens M, Shash E, Coens C, Casado A, Herrera FG, Ottevanger PB. European Organisation for Research and Treatment of Cancer, Gynaecological Cancer Group (EORTC-GCG): Systemic review: Radiation therapy alone in medical non-operable endometrial carcinoma. Eur J Cancer. 2016;65:172–181. doi: 10.1016/j.ejca.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Mouka V, Tsili AC, Messinis T, Papoudou-Bai A, Kamina S, Argyropoulou MI. Solitary adrenal metastasis from early-stage dedifferentiated endometrial carcinoma: CT findings and review of the literature. J Obstet Gynaecol. 2016;36:881–882. doi: 10.1080/01443615.2016.1188275. [DOI] [PubMed] [Google Scholar]
- 23.Tsai YC, Greco TM, Cristea IM. Sirtuin 7 plays a role in ribosome biogenesis and protein synthesis. Mol Cell Proteomics. 2014;13:73–83. doi: 10.1074/mcp.M113.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hubbi ME, Hu H, Kshitiz Gilkes DM, Semenza GL. Sirtuin-7 inhibits the activity of hypoxia-inducible factors. J Biol Chem. 2013;288:20768–20775. doi: 10.1074/jbc.M113.476903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aljada A, Saleh AM, Alkathiri M, Shamsa HB, Al-Bawab A, Nasr A. Altered Sirtuin 7 expression is associated with early stage breast cancer. Breast Cancer (Auckl) 2015;9:3–8. doi: 10.4137/BCBCR.S23156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng Q, Peng H, Chen F, Luo R, Li R. High expression of Sirt7 served as a predictor of adverse outcome in breast cancer. Int J Clin Exp Pathol. 2015;8:1938–1945. [PMC free article] [PubMed] [Google Scholar]
- 27.McGlynn LM, McCluney S, Jamieson NB, Thomson J, MacDonald AI, Oien K, Dickson EJ, Carter CR, McKay CJ, Shiels PG. SIRT3 & SIRT7: Potential novel biomarkers for determining outcome in pancreatic cancer patients. PLoS one. 2015;10:e0131344. doi: 10.1371/journal.pone.0131344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paredes S, Villanova L, Chua KF. Molecular pathways: Emerging roles of mammalian Sirtuin SIRT7 in cancer. Clin Cancer Res. 2014;20:1741–1746. doi: 10.1158/1078-0432.CCR-13-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Han Y, Liu Y, Zhang H, Wang T, Diao R, Jiang Z, Gui Y, Cai Z. Hsa-miR-125b suppresses bladder cancer development by down-regulating oncogene SIRT7 and oncogenic long non-coding RNA MALAT1. FEBS Lett. 2013;587:3875–3882. doi: 10.1016/j.febslet.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Alhazzazi TY, Kamarajan P, Joo N, Huang JY, Verdin E, D'Silva NJ, Kapila YL. Sirtuin-3 (SIRT3), a novel potential therapeutic target for oral cancer. Cancer. 2011;117:1670–1678. doi: 10.1002/cncr.25676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang M, Lu X, Zhang C, Du C, Cao L, Hou T, Li Z, Tu B, Cao Z, Li Y, et al. Downregulation of SIRT7 by 5-fluorouracil induces radiosensitivity in human colorectal cancer. Theranostics. 2017;7:1346–1359. doi: 10.7150/thno.18804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang J, Song H, Lu Y, Chen H, Jiang S, Li L. Effects of estradiol on VEGF and bFGF by Akt in endometrial cancer cells are mediated through the NF-kappaB pathway. Oncology reports. 2016;36:705–714. doi: 10.3892/or.2016.4888. [DOI] [PubMed] [Google Scholar]
- 33.Davies S, Dai D, Feldman I, Pickett G, Leslie KK. Identification of a novel mechanism of NF-κB inactivation by progesterone through progesterone receptors in Hec50co poorly differentiated endometrial cancer cells: Induction of A20 and ABIN-2. Oncol Rep. 2004;94:463–470. doi: 10.1016/j.ygyno.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 34.Yang LQ, Fang DC, Wang RQ, Yang SM. Effect of NF-kappaB, survivin, Bcl-2 and Caspase3 on apoptosis of gastric cancer cells induced by tumor necrosis factor related apoptosis inducing ligand. World J Gastroenterol. 2004;10:22–25. doi: 10.3748/wjg.v10.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author upon reasonable request.