Abstract
Galectin-3 plays crucial roles in tumor progression. However, in non-small cell lung cancer (NSCLC), it remains unclear whether the hypoxic tumor microenvironment enhances galectin-3-induced cell motility. We investigated galectin-3 expression in NSCLC cells under hypoxia, and the possible molecular mechanisms by which galectin-3 influences tumor aggressiveness. Galectin-3 levels in NSCLC cell lines under hypoxia were assessed using reverse transcription PCR and western blotting. To clarify the role of endogenous galectin-3, the effect of galectin-3 knockdown in NSCLC cells was investigated using scratch and invasion assays. The expression and clinicopathological significance of galectin-3 in 57 patients with pN0M0 invasive pulmonary adenocarcinoma were investigated by immunohistochemistry. Both mRNA and protein levels of galectin-3 in the NSCLC cell lines A549 and LK-2 were upregulated by hypoxia. As revealed by scratch and invasion assays, the cell migratory and invasive activities were significantly increased under hypoxia, but were reduced by galectin-3 knockdown. Notably, addition of galectin-3 to the media did not improve the cell motility impaired by galectin-3 knockdown. To clarify the role of endogenous galectin-3 in the enhancement of tumor cell motility under hypoxia, we focused on the function of RhoA. RhoA level in the plasma membrane, but not in the cytoplasm, was increased under hypoxia and decreased by galectin-3 knockdown. RhoA activity was significantly enhanced under hypoxia and effectively inhibited by galectin-3 knockdown. In patients with pN0M0 invasive pulmonary adenocarcinoma, higher galectin-3 expression on tumor cells was significantly associated with tumor cell invasion into microvessels and tumor recurrence after surgery. These data demonstrate that in NSCLC cells under hypoxia, upregulated galectin-3 levels increase the localization of RhoA to the plasma membrane, thus enhancing RhoA activity, which is associated with aggressive cell motility. In pN0M0 invasive pulmonary adenocarcinoma, galectin-3 is a potential biomarker for predicting tumor recurrence after radical surgery.
Keywords: non-small cell lung cancer, galectin-3, hypoxia, migration, invasion, RhoA
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer-related death worldwide (1). The prognosis of NSCLC, however, has gradually improved through advances in therapeutic strategies, such as surgery, chemotherapy, radiotherapy, molecular-targeted therapy and immunotherapy (2). For localized pN0M0 NSCLC, surgical resection offers a good prognosis, with 5-year survival rates of 70–90% (3). However, these data also imply that 10–30% of patients with pN0M0 NSCLC experience tumor recurrence within 5 years despite radical surgery. In patients with tumor recurrence after surgery, some factors or mechanisms responsible for the tumor relapse, such as local recurrence and distant metastases, would likely have already existed at the time of diagnosis with pN0M0. The identification of critical molecules responsible for tumor recurrence could contribute to the development of predictive biomarkers for tumor recurrence in early NSCLC.
Galectin-3 is a chimera-type galectin and belongs to a family of β-galactoside-binding lectins (4). It is localized in both the cytoplasm and nucleus and is secreted via a non-classical pathway that has yet to be identified (4,5). Galectin-3 is ubiquitously expressed in normal adult tissues as well as in a variety of tumor types, including lung cancer (4,5). It has been reported to promote the aggressiveness of tumors, including increased tumor cell proliferation, anti-apoptotic traits, tumor cell motility, metastatic activity and angiogenesis (4–6); however, the mechanisms by which it enhances tumor aggressiveness remain to be fully elucidated.
Hypoxia is a typical feature of the tumor microenvironment in many types of aggressively growing solid tumors, including lung cancer (7). In the tumor microenvironment, chronic hypoxia affects tumor cell motility, including its invasiveness and metastatic activities (7), contributing to a poor prognosis for cancer patients. In tumor cells under hypoxia, various transcription factors, such as hypoxia-inducible factor 1α (HIF-1α) and NF-κB, are activated, inducing a variety of downstream signals that regulate apoptosis, cell proliferation, angiogenesis and cell motility (7). Hypoxia has been reported to upregulate galectin-3 expression in both non-neoplastic cells (8) and certain cancers, including mammary tumor, glioblastoma and thyroid cancer (9–11). On the basis of these data, we hypothesized that galectin-3 would be upregulated in NSCLC cells in the hypoxic tumor microenvironment, which would promote aggressive cell motility, leading to tumor recurrence after surgery in patients with pN0M0 NSCLC.
In the present study, we report that galectin-3 expression is upregulated in NSCLC cells under hypoxia and that it contributes to tumor cell migration and invasion. Cell motility is upregulated by the function of activated RhoA, which is induced by high levels of cytoplasmic galectin-3. In addition, we demonstrated that galectin-3 is a potential biomarker for predicting tumor recurrence in pN0M0 invasive pulmonary adenocarcinoma after radical surgery.
Materials and methods
Cell culture and reagents
Human lung adenocarcinoma cell line A549 and pulmonary squamous cell carcinoma cell line LK-2 were purchased from the American Type Culture Collection (ATCC; Manassas, VA, USA) and the Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University (Sendai, Japan), respectively. All cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM; Nacalai Tesque, Kyoto, Japan), supplemented with 10% fetal bovine serum (FBS) as well as 100 U/ml penicillin G, 0.1 mg/ml streptomycin and 0.2 mg/ml amphotericin B (all from Gibco-BRL; Thermo Fisher Scientific, Inc., Tokyo, Japan), at 37°C in a humidified atmosphere of 5% CO2. For cell culture under hypoxic conditions, the AnaeroPack system (AnaeroPack-Anaero 5%; Mitsubishi Gas Chemical, Tokyo, Japan) was used in conjunction with an OXY-M oxygen monitor (JIKCO, Tokyo, Japan). To mimic a hypoxic condition, 200 µM of deferoxamine mesylate (BioVision, Inc., San Francisco, CA, USA) was added to the culture medium.
shRNA knockdown
For stable knockdown of galectin-3, plasmid vectors encoding galectin-3 shRNA or the scrambled shRNA (transOMIC Technologies, Inc., Huntsville, AL, USA) were transfected into A549 cells by electroporation with the Amaxa system (Nucleofector solution T and Nucleofector program X-01; Lonza, Basel, Switzerland) and LK-2 cells by Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer's instructions.
RNA extraction and reverse transcription PCR
Total RNA was extracted using the RNeasy Mini kit (Qiagen, Hilden, Germany), and cDNA was synthesized from total RNA with the Cloned AMV First-Strand cDNA Synthesis kit (Life Technologies; Thermo Fisher Scientific, Inc.). PCR was performed by incubating each cDNA with the following paired primers: Galectin-3 forward, 5′-ATGGCAGACAATTTTTCGCTCC-3′ and reverse, 5′-ATGTCACCAGAAATTCCCAGTT-3′; β-actin forward, 5′-TCAACACCCCAGCCATGTAC-3′ and reverse, 5′-CTGTGTTGGCGTACAGGTCT-3′.
Enzyme-linked immunosorbent assay (ELISA)
A549 and LK-2 cells (2.5×105 and 5×105 cells/10-cm dish, respectively) were cultured under a normoxic or hypoxic condition for 3 days. Cell culture supernatants were then harvested and centrifuged at 15,000 × g for 5 min at 4°C to remove cell debris, and the levels of galectin-3 were measured using the Human Galectin-3 ELISA kit (Abcam, Cambridge, UK) according to the manufacturer's instructions.
Cell proliferation and motility assays
For the cell proliferation assay, tumor cells (1×104/well) were cultured in 96-well plates for 24 h under a normoxic or hypoxic condition. Then, 10 µl of CellTiter 96 Aqueous One Solution Reagent (Promega Corporation, Madison, WI, USA) was added to each well, followed by incubation at 37°C for 4 h. The absorbance was measured at 492 nm using a microplate reader (Tecan Group Ltd., Männedorf, Switzerland).
For the scratch assay, A549 and LK-2 cells were grown to 80–90 and 60–70% confluence, respectively, on 24-well microplates. A linear scratch was made using a 2-mm-wide Cell Scratcher (Iwaki Glass, Chiba, Japan), and the microplates were washed twice with culture medium. Cells were incubated at 37°C for 72 h in the presence or absence of recombinant galectin-3 (ProSpec-Tany TechnoGene, Ltd., Rehovot, Israel). Immediately or 72 h after the scratch was made, the cells were fixed and stained with 0.4% crystal violet in 10% methanol. Images were obtained under a light microscope, and the area of cell migration was measured using ImageJ software [version 1.51k; National Institutes of Health (NIH), Bethesda, MD, USA; https://imagej.nih.gov/ij/].
For the invasion assay, A549 (5×104) and LK-2 (1.5×105) cells were added to BD BioCoat Matrigel Chambers (BD Biosciences, San Jose, CA, USA) and incubated at 37°C for 22 h. Then, the cells were stained with 0.4% crystal violet in 10% methanol and observed under a light microscope at ×100 magnification to identify invasive cells. The number of counted cells was averaged across three fields for each experiment.
Western blotting
Whole cell lysates were extracted using PIPA buffer (Thermo Fisher Scientific, Inc.) containing a protease inhibitor cocktail (Nacalai Tesque). The plasma membrane protein lysates were extracted using the Minute Plasma Membrane Protein Isolation kit (Invent Biotechnologies, Inc., Plymouth, MA, USA), according to the manufacturer's instructions. Protein concentrations were determined using the Bicinchoninic Acid Protein Assay kit (Pierce; Thermo Fisher Scientific, Inc.). Proteins (20 µg for galectin-3; 10 µg for RhoA and vinculin; 5 µg for β-actin; 1 µg for NaK-ATPase) were separated by SDS-PAGE (12.5% for RhoA; 10% for galectin-3 and β-actin; 7.5% for vinculin and NaK-ATPase) and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was blocked using 5% fat-free milk and incubated with rabbit polyclonal anti-galectin-3 (dilution 1:7,000; cat. no. 12733; Cell Signaling Technology, Beverly, MA, USA), mouse monoclonal anti-RhoA (dilution 1:1,000; cat. no. ab187027; Abcam), rabbit monoclonal anti-vinculin (dilution 1:10,000; cat. no. ab129002; Abcam), rabbit monoclonal anti-NaK-ATPase (dilution 1:100,000; cat. no. ab76020; Abcam), or mouse monoclonal anti-β-actin (dilution 1:5,000; cat. no. A5316; Sigma Corp., Kawasaki, Japan) antibody. The membranes were then incubated with anti-rabbit IgG horseradish peroxidase (HRP)-conjugated antibody (dilution 1:5,000; cat. no. ab6721; Abcam) or anti-mouse IgG HRP-conjugated antibody (dilution 1:5,000; cat. no. NA931; GE Healthcare, Buckinghamshire, UK). Immunolabeling was detected using an enhanced chemiluminescence system (Wako Pure Chemicals, Ltd., Tokyo, Japan).
RhoA activity assay
The RhoA activity of tumor cells was assessed by measuring the amount of RhoA-GTP using the G-LISA RhoA Activation Biochem Assay kit (Cytoskeleton, Inc., Denver, CO, USA), according to the manufacturer's instructions.
Tumor tissue samples and immunohistochemistry
Tumor tissue samples were obtained from 57 patients (Table I) with pN0M0 invasive pulmonary adenocarcinoma who underwent radical surgery at Shiga University of Medical Science Hospital between January 2008 and December 2012. This study was approved by the Institutional Review Board of Shiga University of Medical Science (#28-210) and informed consent was provided by all participating patients. Sections of formalin-fixed paraffin-embedded tumor tissues (4 µm) were stained with anti-human galectin-3 monoclonal antibody (dilution 1:100; cat. no. ab2785; Abcam) using the EnVision kit (Dako, Glostrup, Denmark), according to the manufacturer's instructions. Slides were then treated with substrate/chromogen (Dako), followed by counterstaining with hematoxylin. For negative controls, the primary antibody was omitted. The stained slides were viewed under a light microscope equipped with a digital camera (Nikon Corporation, Tokyo, Japan). Ten fields at ×400 magnification were randomly selected for each slide. The stained slides were examined under pathologists' guidance. Galectin-3 expression was described as being high if >10% of the cells were positively stained.
Table I.
Clinical characteristics of the patients with non-small cell lung cancer.
| Patient characteristics | Data |
|---|---|
| Total, n | 57 |
| Median age (range), years | 67 (49–83) |
| Sex, n (%) | |
| Male | 28 (49.2) |
| Female | 29 (50.8) |
| Smoking status, n (%) | |
| Never | 31 (54.4) |
| Current/former | 26 (45.6) |
| Median tumor size (range), mm | 20 (9–50) |
| T stage, n (%) | |
| T1a | 4 (7.0) |
| T1b | 24 (42.1) |
| T1c | 18 (31.6) |
| T2a | 6 (10.5) |
| T2b | 5 (8.8) |
| Histological subtype, n (%) | |
| Lepidic | 16 (28.0) |
| Papillary | 26 (45.6) |
| Acinar | 11 (19.3) |
| Solid | 3 (5.3) |
| Micropapillary | 1 (1.8) |
| Microvessel invasion, n (%) | |
| Absent | 37 (64.9) |
| Present | 20 (35.1) |
Statistical analysis
All in vitro experiments were repeated three times independently, and each was performed using duplicate or triplicate measurements. Results are expressed as the means ± standard deviation (SD). The Mann-Whitney U-test, Student's t-test, or one-way analysis of variance (ANOVA) with Turkey's post hoc test were applied to investigate significant differences between groups. Statistical analysis was performed using SPSS 22 Statistics V.22.0 software (IBM Corp., Armonk, NY, USA), with P<0.05 considered to indicate a statistically significant result.
Results
Hypoxia upregulates galectin-3 expression in human NSCLC cell lines
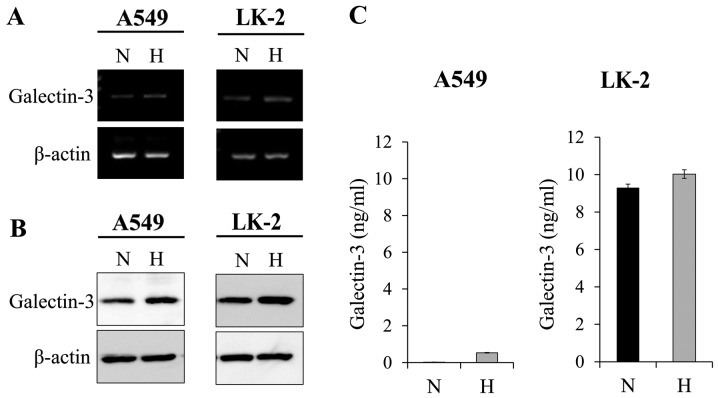
We hypothesized that in the hypoxic tumor microenvironment, galectin-3 in NSCLC cells would be responsible for promoting aggressive cell motility. To verify this hypothesis, we first evaluated whether the expression level of galectin-3 in NSCLC cells is affected by a hypoxic microenvironment in vitro. Human NSCLC cell lines A549 and LK-2 were cultured under a hypoxic (2% O2) or normoxic (21% O2) condition for 72 h. Then, the cellular mRNA and protein levels of galectin-3 were examined. We found that in both NSCLC cell lines, the mRNA (Fig. 1A) and protein (Fig. 1B) levels of galectin-3 were observably upregulated under hypoxia compared with those under normoxia. It has been reported that the galectin-3 secreted from tumor cells activates, through an autocrine mechanism, the signal transduction associated with tumor progression in several types of tumors (4,5). We focused on the mechanism and evaluated the level of secreted galectin-3 in the culture media. It was found that the level of secreted galectin-3 was not affected by the hypoxic condition (Fig. 1C). Overall, these results demonstrated that the hypoxic microenvironment increases the accumulation of cytoplasmic galectin-3 in human NSCLC cells.
Figure 1.
Hypoxia upregulates galectin-3 expression in NSCLC cells. A549 and LK-2 cells were exposed to hypoxia. The (A) mRNA and (B) protein levels of galectin-3 were increased under hypoxic conditions. (C) The levels of galectin-3 released from A549 and LK-2 cells into the culture medium were measured by ELISA. Results are expressed as the means ± SD of three independent experiments. N, normoxic condition (21% O2); H, hypoxic condition (2% O2); NSCLC, non-small cell lung cancer.
Galectin-3 promotes NSCLC cell migration and invasion under the hypoxic condition
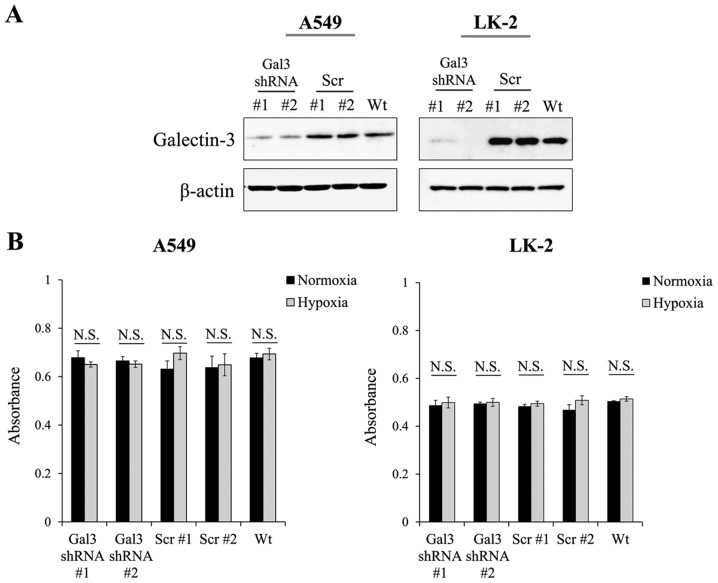
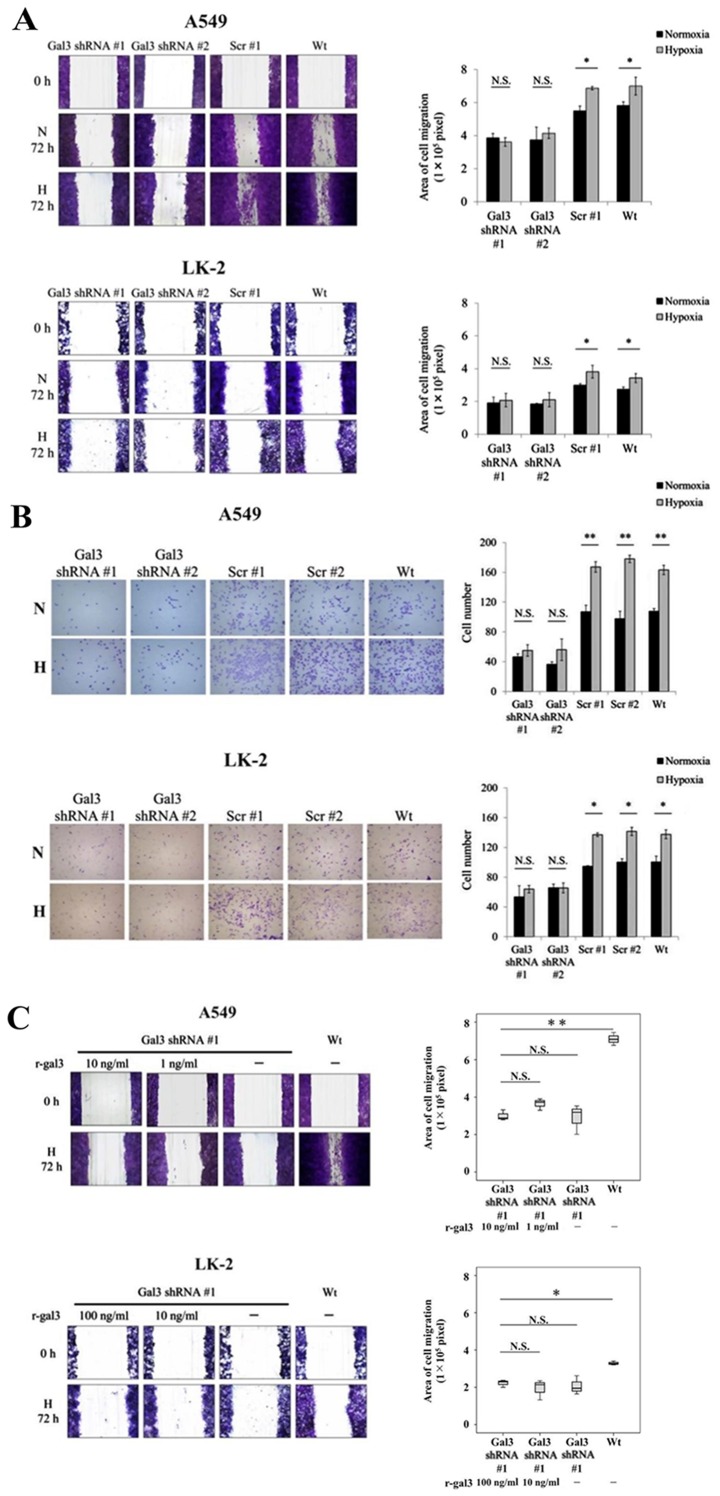
Next, we examined whether the motility of NSCLC cells would be enhanced by the upregulated levels of galectin-3 under hypoxia using the NSCLC cell lines A549 and LK-2 that were stably transfected with galectin-3 shRNA (A549 Gal3 shRNA #1 and #2 and LK-2 Gal3 shRNA #1 and #2; Fig. 2A). In these transfectants, the proliferative activity of the cells was not affected by galectin-3 knockdown (Fig. 2B), demonstrating that galectin-3 did not play a role in promoting the proliferation of NSCLC cells under hypoxia. Then, we examined the effect of the upregulated levels of galectin-3 on the migratory activity of NSCLC cells under hypoxia. Results of the scratch assay showed that the migration of A549 and LK-2 cells was significantly enhanced under hypoxia compared with that under normoxia (Fig. 3A). In addition, galectin-3 knockdown significantly inhibited the migration of both cell lines under hypoxia (Fig. 3A). We also examined the effect of the upregulated levels of galectin-3 on the invasive activity of NSCLC cells under hypoxia. Results of the invasion assay showed that the invasive activity of A549 and LK-2 cells was significantly enhanced under hypoxia as compared with that under normoxia (Fig. 3B). In addition, galectin-3 knockdown significantly inhibited the invasive activity of both cell lines under hypoxia (Fig. 3B). To examine whether extracellular galectin-3 is involved in regulating cell migration, A549 Gal3-shRNA and LK-2 Gal3-shRNA cells were cultured under hypoxia with recombinant galectin-3 (r-gal3) protein at a concentration equal to that measured in cell culture supernatants (1 and 10 ng/ml, respectively) or at a 10-fold excess (10 and 100 ng/ml, respectively). The results showed that exogenous supplementation of recombinant galectin-3 protein could not reverse the inhibition of cell migration induced by galectin-3 knockdown (Fig. 3C). These data demonstrated that the upregulated levels of endogenous galectin-3 under the hypoxic condition play a crucial role in cell motility, including the migratory and invasive activities of human NSCLC cells.
Figure 2.
Proliferative activity of NSCLC cells was not affected by galectin-3 knockdown. (A) Galectin-3 expression in A549 and LK-2 cells transfected with galectin-3 shRNA (Gal3 shRNA #1, #2) or scrambled control shRNA (scr #1, #2) was examined by western blot analysis. (B) Proliferation of A549 and LK-2 cells, cultured under either normoxic (21% O2) or hypoxic (1% O2) conditions, was assessed following transfection with galectin-3 shRNA or scrambled shRNA. N.S., not significant; NSCLC, non-small cell lung cancer.
Figure 3.
Galectin-3 promotes NSCLC cell migration and invasion under hypoxia. The effect of hypoxia-induced galectin-3 on Wt cells and cells transfected with galectin-3 shRNA (Gal3 shRNA #1, #2) or scrambled control shRNA (scr #1, #2) on cell migration and invasion in NSCLC cells (A549 and LK-2) was assessed by a (A) scratch and an (B) invasion assays. (C) A549 and LK-2 cells transfected with galectin-3 shRNA were cultured with the indicated concentrations of recombinant galectin-3 (r-gal3) under hypoxic conditions (H; 1% O2). Cell migration was assessed by scratch assay. The results from each assay are summarized in the accompanying histograms. *P<0.05; **P<0.01; N.S., not significant. Wt, wild-type; N, normoxic condition (21% O2); H, hypoxic condition (2% O2); NSCLC, non-small cell lung cancer.
RhoA activation is enhanced by increased localization of galectin-3 to the plasma membrane under hypoxia
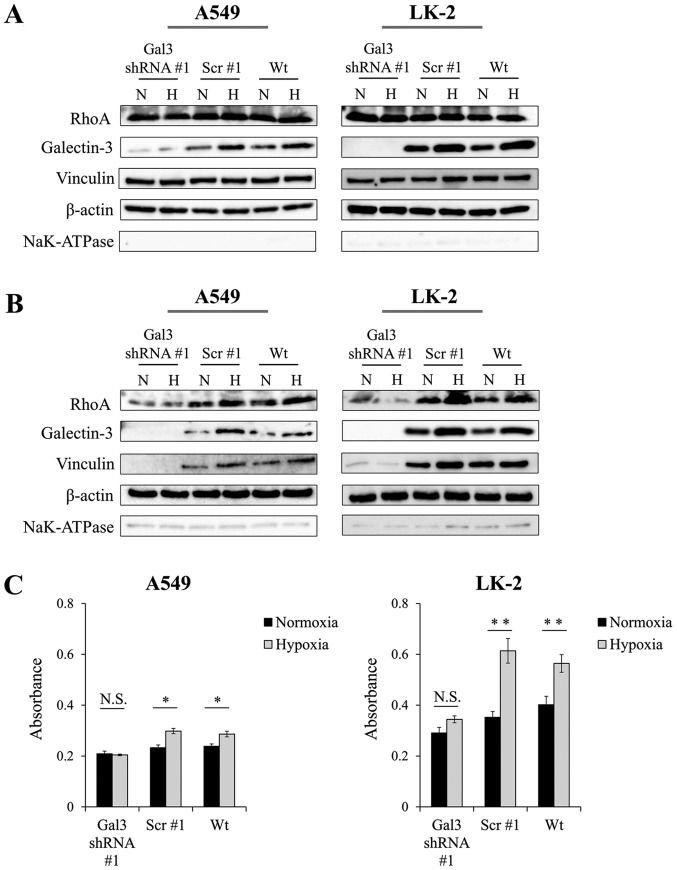
Next, we investigated how increased levels of endogenous galectin-3 would enhance the motility of NSCLC cells under hypoxic conditions. Galectin-3 has been reported to promote cell motility even under normoxic conditions (4–6); however, the details of the mechanism remain unclear. To elucidate the mechanism, we first aimed to identify the areas in which galectin-3 is upregulated in NSCLC cells under hypoxia. We found that the protein level of galectin-3 was upregulated not only in the cytoplasm (Fig. 4A) but also in the plasma membrane (Fig. 4B). Galectin-3 in the plasma membrane may be involved in cell motility, so we focused on the activation of RhoA function. Given that RhoA is known to be a member of the Rho GTPase family that controls dynamic remodeling of the actin cytoskeleton (12), we hypothesized that increased levels of galectin-3 in the plasma membrane may help activate RhoA. We evaluated the protein levels of RhoA in the cytoplasm of NSCLC cells. We also examined expression of vinculin, a cytoskeletal protein associated with cell-cell and cell-matrix junctions, which is activated by RhoA signaling (13). We found that these proteins in the cytoplasm of NSCLC cells were unaffected by hypoxia (Fig. 4A). In addition, galectin-3 knockdown did not affect the protein levels of cytoplasmic RhoA or vinculin. However, notably, the protein levels of RhoA and vinculin in the plasma membrane were increased under hypoxia compared with normoxia. Furthermore, these increases were significantly decreased by galectin-3 knockdown (Fig. 4B). In the NSCLC cells with galectin-3 knockdown, the protein levels of cytoplasmic RhoA and vinculin did not increase even under hypoxia. We also evaluated the activation of RhoA by measuring the level of RhoA-GTP and found that the level of RhoA-GTP was significantly increased under the hypoxic condition in both NSCLC cell lines; however, galectin-3 knockdown inhibited the increase in activated RhoA under hypoxia (Fig. 4C). These data demonstrated that galectin-3, which was upregulated under hypoxia, contributed to the accumulation of RhoA at the plasma membrane, leading to the activation of RhoA in NSCLC cells.
Figure 4.
Galectin-3 induces RhoA activation via the upregulation of RhoA expression in the plasma membrane. A549 and LK-2 cells transfected with galectin-3 shRNA (Gal3 shRNA #1) or scrambled control shRNA (scr #1) were cultured under normoxic (N; 21% O2) or hypoxic (H; 1% O2) conditions. The levels of RhoA protein, which is related to tumor motility, and vinculin, a cytoskeletal protein, were then measured by western blotting. Levels of RhoA and vinculin expression in (A) the cytoplasm and (B) plasma membrane. β-actin and NaK-ATPase were used as loading controls for the cytoplasm and plasma membrane, respectively. (C) RhoA activity, as measured by ELISA. Wt, wild-type; *P<0.05; **P<0.01; N.S., not significant.
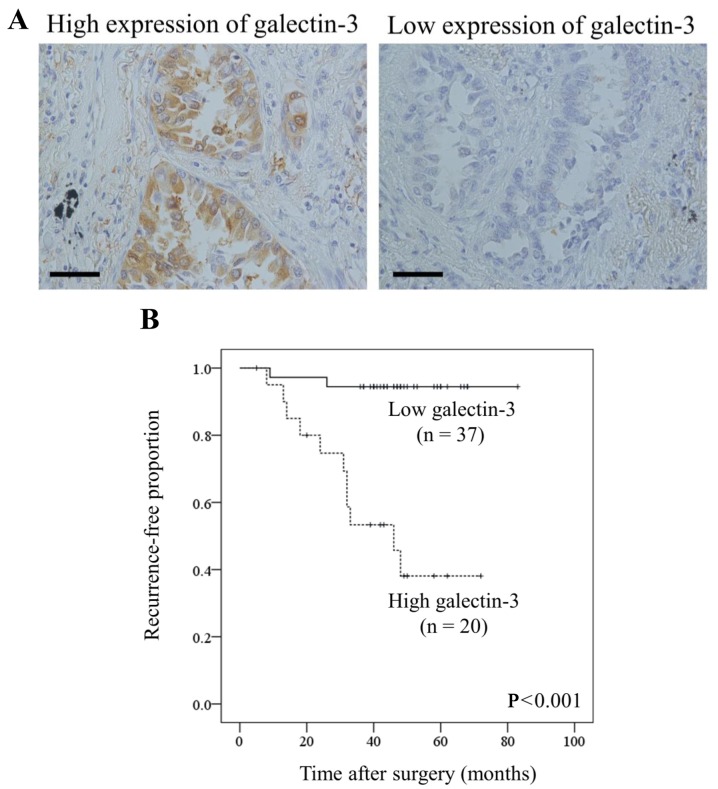
Galectin-3 expression is correlated with recurrence and microvessel invasion in human invasive pulmonary adenocarcinoma
The observed role of galectin-3 in NSCLC cells under hypoxia led us to assess the clinicopathological significance of galectin-3 in clinical samples. To evaluate the association between galectin-3 expression on tumor cells and clinicopathological findings, we performed immunohistochemical staining for 57 patients with pN0M0 invasive pulmonary adenocarcinoma who underwent radical surgery (Table I). Galectin-3 was highly expressed on tumor cells in 20 cases (35.1%; Fig. 5A and Table II). Univariate analysis showed that galectin-3 expression on tumor cells was significantly associated with the occurrence of microvessel invasion (P=0.004; Table II) as well as tumor recurrence after surgery (P<0.001). The Kaplan-Meier curve for relapse-free survival after surgery showed that patients with a high level of galectin-3 expression (n=20) had a significantly lower relapse-free survival than those with a low level of galectin-3 expression (P<0.001; Fig. 5B). Taken together, a high expression level of galectin-3 on tumor cells was associated with the microvessel invasion of tumor cells and tumor recurrence in patients with pN0M0 invasive pulmonary adenocarcinoma.
Figure 5.
Galectin-3 expression in human invasive pulmonary adenocarcinoma tissues. Galectin-3 positivity in tumor tissues was evaluated by immunohistochemical staining. (A) Examples of high and low staining at ×400 magnification. Scale bar, 100 µm. (B) Relapse-free survival curves for patients with invasive pulmonary adenocarcinoma. Patients with high galectin-3 expression had significantly poorer disease-free survival than those with low expression (P<0.001, log-rank test).
Table II.
Correlation between galectin-3 expression and clinicopathological characteristics of the patients with non-small cell lung cancer.
| Patient characteristics | n | High expression of galectin-3 n (%) | P-value |
|---|---|---|---|
| Total | 57 | 20 (35.1) | |
| Sex | |||
| Male | 28 | 11 (39.3) | 0.946 |
| Female | 29 | 9 (31.0) | |
| Smoking status | |||
| Never | 31 | 11 (35.5) | 0.551 |
| Current/former | 26 | 9 (34.6) | |
| T stage | |||
| T1a | 4 | 3 (75.0) | 0.548 |
| T1b | 24 | 8 (33.3) | |
| T1c | 18 | 5 (27.8) | |
| T2a | 6 | 2 (33.3) | |
| T2b | 5 | 2 (40.0) | |
| Histological subtype | |||
| Lepidic | 16 | 4 (25.0) | 0.201 |
| Papillary | 26 | 8 (30.8) | |
| Acinar | 11 | 6 (54.5) | |
| Solid | 3 | 1 (33.3) | |
| Micropapillary | 1 | 1 (100.0) | |
| Microvessel invasion | |||
| Absent | 32 | 6 (18.8) | 0.004 |
| Present | 25 | 14 (56.0) | |
| Tumor recurrence | |||
| No | 44 | 9 (20.5) | <0.001 |
| Yes | 13 | 11 (84.6) | |
P-values were calculated using the Mann-Whitney U test. Bold print indicates significance.
Discussion
In the present study, we examined the role of galectin-3 in the aggressive motility of non-small cell lung cancer (NSCLC) cells under hypoxia. In NSCLC cells, the level of endogenous galectin-3 was upregulated under hypoxia, which led to an increased accumulation of RhoA in the plasma membrane. This enhanced the function of RhoA, thus promoting the aggressive migratory and invasive activities of NSCLC cells. In addition, we showed that galectin-3 is a potential biomarker for predicting tumor recurrence in pN0M0 invasive pulmonary adenocarcinoma after radical surgery.
The hypoxic tumor microenvironment is a characteristic of aggressively growing tumors. Under hypoxic conditions, the expression of galectin-3 in tumor cells is upregulated by the key transcription factors HIF-1α (8) and NF-κB (5). In breast cancer, glioblastoma and thyroid cancer, tumor cells acquire more aggressive proliferative, migratory and invasive activities through the upregulation of galectin-3 under hypoxia (9–11). The motility of NSCLC cells was also expected to be enhanced by the upregulation of galectin-3 under hypoxia. In the present study, we showed that both the protein and mRNA levels of galectin-3 were upregulated and that galectin-3 knockdown inhibited the migratory and invasive activities of NSCLC cells. These data demonstrated that galectin-3 plays a crucial role in promoting cell migration and invasion under hypoxia, even in NSCLC cells.
However, the mechanism by which galectin-3 promotes cell motility under hypoxia remains unclear. Galectin-3 can be secreted from tumors (4,5) and it binds to the surface receptors of tumor cells, such as receptor tyrosine kinase and integrins. This prevents endocytosis of the receptors, leading to signal activation and tumor progression (4). Our data indicated that hypoxia does not affect the level of secreted galectin-3 from NSCLC cells. Furthermore, the addition of recombinant galectin-3 protein into the culture media did not reverse the impairment of migratory activity caused by galectin-3 knockdown in NSCLC cells. In HeLa cells and hepatocellular carcinoma cells, extracellular galectin-3 seems to influence cell motility via an autocrine process (14,15). These previous studies used higher levels of exogenous galectin-3 (15–25 µg/ml) compared with our experiments. However, the secretion level of galectin-3 in colon cancer cell culture supernatants has been reported at ≤10 ng/ml (16), which is consistent with our experiments. In addition, a previous study has shown that the median level of serum galectin-3 was ≤5 ng/ml in pancreatic cancer (17). Based on these reports, although high levels of exogenous galectin-3 could enhance tumor cell motility, the levels used in previous studies is much higher than those predicted in the tumor microenvironment. Although the sensitivity to exogenous galectin-3 may differ between tumor cell types, in NSCLC cells, the endogenous galectin-3 that is upregulated under hypoxia appears to be responsible for tumor cell motility. In this study, the scratch assay was performed to quantify tumor cell migration. However, this assay has some limitations, because the tool forming the linear wound may injure and stress the boundary cells, which could affect cell migration. A single cell motility assay will need to be conducted in our future studies.
To examine the association between endogenous galectin-3 and tumor cell motility, we focused on the function of RhoA. RhoA is a member of the Rho GTPase family that regulates a variety of cellular processes, including migration, cell adhesion, cell polarity, cell proliferation and apoptosis (12). RhoA is well known to control the actin cytoskeleton, promoting cell motility (12,18). Most Rho GTPases (90–95%) are found in the cytosol in an inactive state, bound to Rho-specific guanine nucleotide dissociation inhibitors (18). During activation, RhoA is geranylgeranylated and transferred to the plasma membrane, where guanine nucleotide exchange factors induce the conversion of inactive RhoA-GDP to active RhoA-GTP (18). It is therefore important to retain RhoA in the plasma membrane to activate it. Previous studies have indicated that galectin-3 upregulates RhoA activation in hepatocellular and thyroid cancer cells (10,15). However, whether galectin-3 enhances RhoA activation in NSCLC and the mechanism by which galectin-3 regulates the activation of RhoA remain unclear. In the present study, we found that the level of RhoA in the plasma membrane was increased under hypoxia and was decreased by galectin-3 knockdown, whereas RhoA expression in the cytoplasm was unchanged. We also found that RhoA activity was significantly enhanced under hypoxic conditions and was effectively inhibited by galectin-3 knockdown in NSCLC cells. In addition, more vinculin was localized to the plasma membrane under hypoxia, and galectin-3 knockdown inhibited this localization. These findings indicate that galectin-3 may also help establish RhoA anchorage in the plasma membrane, contributing to the activation of RhoA function that is associated with cell motility.
Galectin-3 was more highly expressed in LK-2 cells than in A549 cells, which was associated with the effect of galectin-3 knockdown on RhoA activation. However, in terms of tumor cell migration and invasion, A549 cells were more strongly inhibited by galectin-3 knockdown than LK-2 cells. Previous reports have verified that galectin-3 enhances invasion of KRAS mutant tumor cells, such as pancreatic adenocarcinoma cells, via binding to K-Ras protein (19,20). A549 cells carry a KRAS mutation (G12S) and LK-2 cells harbor wild-type KRAS. Therefore, in this study, the K-Ras status may have also influenced tumor cell motility. However, a limitation of this study is that we have not confirmed the association between K-Ras and galectin-3, and we plan to examine the expression of K-Ras and galectin-3 in NSCLC patients in the future.
Previous studies have reported galectin-3 overexpression in several human carcinomas and a correlation with the clinical aggressiveness of the tumors, including pancreatic cancers and melanoma (21,22). However, in laryngeal and renal cell cancers, a low expression of galectin-3 was associated with poor prognosis (23,24). In NSCLC, the association between galectin-3 expression and the prognosis of patients remains controversial (25–27). However, these studies included advanced pN1-3 or M1 patients with several histological subtypes, which may have affected the study results; therefore, here we exclusively analyzed patients with the same histological subtype and early stage disease. Immunohistochemistry showed that the galectin-3-expressing tumor cells were particularly located around regions of necrosis and scarring, which are thought to be hypoxic, and that a high level of galectin-3 expression was significantly associated with the occurrence of tumor cell invasion into microvessels. These clinical data were corroborated by the results of the cell-based assays in the present study. Even at the pN0M0 stage, patients with galectin-3-expressing invasive adenocarcinoma had a risk of tumor recurrence after radical surgery due to the migratory and invasive activities of tumor cells.
In conclusion, in human NSCLC, hypoxia-induced upregulation of galectin-3 in tumor cells enhanced RhoA activity by increasing the level of RhoA in the plasma membrane, thereby promoting the migratory and invasive activities of tumor cells. In pN0M0 invasive pulmonary adenocarcinomas, galectin-3 is a potential biomarker for predicting tumor recurrence after radical surgery and may be an attractive therapeutic target.
Acknowledgements
The authors would like to thank Mitsuaki Ishida at the Department of Pathology and Laboratory Medicine in Kansai Medical University for immunohistochemical analysis support.
Glossary
Abbreviations
- NSCLC
non-small cell lung cancer
- HIF-1α
hypoxia-inducible factor 1α
- ELISA
enzyme-linked immunosorbent assay
- HRP
horseradish peroxidase
- SD
standard deviation
Funding
The present study was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS KAKENHI; grant nos. 16K19976, 16K10677 and 18K16415).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
YK, YO, KT and TI designed the study; YK performed the experiments; TI assisted with the immunohistochemical analysis of the results; TA assisted with the statistical analyses. YK, JH and YO wrote the manuscript. KT, TA and JH reviewed and edited the manuscript. All authors read and approved the manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board of Shiga University of Medical Science, Shiga, Japan (#28-210).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Reck M, Rabe KF. Precision diagnosis and treatment for advanced non-small-cell lung cancer. N Engl J Med. 2017;377:849–861. doi: 10.1056/NEJMra1703413. [DOI] [PubMed] [Google Scholar]
- 3.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V, et al. The IASLC lung cancer staging project: Proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed H, AlSadek DM. Galectin-3 as a potential target to prevent cancer metastasis. Clin Med Insights Oncol. 2015;9:113–121. doi: 10.4137/CMO.S29462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cardoso AC, Andrade LN, Bustos SO, Chammas R. Galectin-3 determines tumor cell adaptive strategies in stressed tumor microenvironments. Front Oncol. 2016;6:127. doi: 10.3389/fonc.2016.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung LY, Tang SJ, Wu YC, Sun GH, Liu HY, Sun KH. Galectin-3 augments tumor initiating property and tumorigenicity of lung cancer through interaction with β-catenin. Oncotarget. 2015;6:4936–4952. doi: 10.18632/oncotarget.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semenza GL. Hypoxia-inducible factors: Mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33:207–214. doi: 10.1016/j.tips.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zeng Y, Danielson KG, Albert TJ, Shapiro IM, Risbud MV. HIF-1 alpha is a regulator of galectin-3 expression in the intervertebral disc. J Bone Miner Res. 2007;22:1851–1861. doi: 10.1359/jbmr.070620. [DOI] [PubMed] [Google Scholar]
- 9.de Oliveira JT, Ribeiro C, Barros R, Gomes C, de Matos AJ, Reis CA, Rutteman GR, Gärtner F. Hypoxia up-regulates galectin-3 in mammary tumor progression and metastasis. PLoS One. 2015;10:e0134458. doi: 10.1371/journal.pone.0134458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng J, Lu W, Wang C, Xing Y, Chen X, Ai Z. Galectin-3 induced by hypoxia promotes cell migration in thyroid cancer cells. Oncotarget. 2017;8:101475–101488. doi: 10.18632/oncotarget.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikemori RY, Machado CM, Furuzawa KM, Nonogaki S, Osinaga E, Umezawa K, de Carvalho M, Verinaud L, Chammas R. Galectin-3 up-regulation in hypoxic and nutrient deprived microenvironments promotes cell survival. PLoS One. 2014;9:e111592. doi: 10.1371/journal.pone.0111592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hetmanski JH, Schwartz JM, Caswell PT. Modelling GTPase dynamics to understand RhoA-driven cancer cell invasion. Biochem Soc Trans. 2016;44:1695–1700. doi: 10.1042/BST20160184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brew CT, Aronchik I, Kosco K, McCammon J, Bjeldanes LF, Firestone GL. Indole-3-carbinol inhibits MDA-MB-231 breast cancer cell motility and induces stress fibers and focal adhesion formation by activation of Rho kinase activity. Int J Cancer. 2009;124:2294–2302. doi: 10.1002/ijc.24210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao X, Balan V, Tai G, Raz A. Galectin-3 induces cell migration via a calcium-sensitive MAPK/ERK1/2 pathway. Oncotarget. 2014;5:2077–2084. doi: 10.18632/oncotarget.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serizawa N, Tian J, Fukada H, Baghy K, Scott F, Chen X, Kiss Z, Olson K, Hsu D, Liu FT, et al. Galectin 3 regulates HCC cell invasion by RhoA and MLCK activation. Lab Invest. 2015;95:1145–1156. doi: 10.1038/labinvest.2015.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumont P, Berton A, Nagy N, Sandras F, Tinton S, Demetter P, Mascart F, Allaoui A, Decaestecker C, Salmon I. Expression of galectin-3 in the tumor immune response in colon cancer. Lab Invest. 2008;88:896–906. doi: 10.1038/labinvest.2008.54. [DOI] [PubMed] [Google Scholar]
- 17.Gaida MM, Bach ST, Günther F, Baseras B, Tschaharganeh DF, Welsch T, Felix K, Bergmann F, Hänsch GM, Wente MN. Expression of galectin-3 in pancreatic ductal adenocarcinoma. Pathol Oncol Res. 2012;18:299–307. doi: 10.1007/s12253-011-9444-1. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Mata R, Boulter E, Burridge K. The ‘invisible hand’: Regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12:493–504. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shalom-Feuerstein R, Plowman SJ, Rotblat B, Ariotti N, Tian T, Hancock JF, Kloog Y. K-ras nanoclustering is subverted by overexpression of the scaffold protein galectin-3. Cancer Res. 2008;68:6608–6616. doi: 10.1158/0008-5472.CAN-08-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song S, Ji B, Ramachandran V, Wang H, Hafley M, Logsdon C, Bresalier RS. Overexpressed galectin-3 in pancreatic cancer induces cell proliferation and invasion by binding Ras and activating Ras signaling. PLoS One. 2012;7:e42699. doi: 10.1371/journal.pone.0042699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimamura T, Sakamoto M, Ino Y, Shimada J, Kosuge T, Sato Y, Tanaka K, Sekihara H, Hirohashi S. Clinicopathological significance of galectin-3 expression in ductal adenocarcinoma of the pancreas. Clin Cancer Res. 2002;8:2570–2575. [PubMed] [Google Scholar]
- 22.Prieto VG, Mourad-Zeidan AA, Melnikova V, Johnson MM, Lopez A, Diwan AH, Lazar AJ, Shen SS, Zhang PS, Reed JA, et al. Galectin-3 expression is associated with tumor progression and pattern of sun exposure in melanoma. Clin Cancer Res. 2006;12:6709–6715. doi: 10.1158/1078-0432.CCR-06-0758. [DOI] [PubMed] [Google Scholar]
- 23.Piantelli M, Iacobelli S, Almadori G, Iezzi M, Tinari N, Natoli C, Cadoni G, Lauriola L, Renalletti FO. Lack of expression of galectin-3 is associated with a poor outcome in node-negative patients with laryngeal squamous-cell carcinoma. J Clin Oncol. 2002;20:3850–3856. doi: 10.1200/JCO.2002.01.078. [DOI] [PubMed] [Google Scholar]
- 24.Merseburger AS, Kramer MW, Hennenlotter J, Serth J, Kruck S, Gracia A, Stenzl A, Kuczyk MA. Loss of galectin-3 expression correlates with clear cell renal carcinoma progression and reduced survival. World J Urol. 2008;26:637–642. doi: 10.1007/s00345-008-0294-8. [DOI] [PubMed] [Google Scholar]
- 25.Mathieu A, Saal I, Vuckovic A, Ransy V, Vereerstraten P, Kaltner H, Gabius HJ, Kiss R, Decaestecker C, Salmon I, Remmelink M. Nuclear galectin-3 exprXession is an independent predictive factor of recurrence for adenocarcinoma and squamous cell carcinoma of the lung. Mod Pathol. 2005;18:1264–1271. doi: 10.1038/modpathol.3800416. [DOI] [PubMed] [Google Scholar]
- 26.Szöke T, Kayser K, Trojan I, Kayser G, Furak J, Tiszlavicz L, Baumhäkel JD, Gabius HJ. The role of microvascularization and growth/adhesion-regulatory lectins in the prognosis of non-small cell lung cancer in stage II. Eur J Cardiothorac Surg. 2007;31:783–787. doi: 10.1016/j.ejcts.2007.01.072. [DOI] [PubMed] [Google Scholar]
- 27.Kosacka M, Piesiak P, Kowal A, Gołecki M, Jankowska R. Galectin-3 and cyclin D1 expression in non-small cell lung cancer. J Exp Clin Cancer Res. 2011;30:101. doi: 10.1186/1756-9966-30-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.