Abstract
Esophageal cancer staging is important for the treatment of esophageal cancer. Endoscopic ultrasonography (EUS) is a common diagnostic tool for esophageal cancer prior to surgery. However, EUS is unable to accurately discriminate the N-staging of lymph nodes. In order to distinguish an optimized standard for malignant lymph node diagnosis, the present study compared lymph nodes detected by EUS and surgery. A total of 112 patients were preoperatively examined with EUS and staged according to the 7th Edition of the American Joint Committee on Cancer Staging Manual. The results of EUS were compared with surgical findings. The critical values of long diameter, short diameter and lymph node number detected by EUS were >7.5, >5.5 mm and >2, respectively; indexes, including long diameter >7.5 mm, short diameter >5.5 mm, round, low echo, edge smooth, near lesion and detected lymph node number (>2) and T3/4 staging, met significance in the EUS group compared with the surgical group (P<0.05). Furthermore, the area under curve (AUC) value of the EUS (0.801) was superior to the conventional, surgical method (0.779). Although EUS improved the diagnostic accuracy of esophageal N staging, it was not able to satisfactorily distinguish between N2 and N3 staging. Advancements in EUS may enhance its detection ability, further improving the diagnostic accuracy of lymph node metastasis.
Keywords: esophageal cancer, endoscopic ultrasonography, American Joint Committee on Cancer, area under the curve
Introduction
Esophageal cancer is a common malignant tumor in China (1). Surgical resection remains the primary treatment method. Preoperative understanding of the invasion depth and lymph node metastasis would be helpful in treatment approach choices. The TNM system is the most widely used cancer staging system, which is based on primary tumor (T), regional lymph nodes (N) and distant metastasis (M) (2). The majority of hospitals and medical centers use the TNM system as their main method for cancer reporting. In the last 10 years, endoscopic ultrasonography has revealed the histological features of the esophagus and adjacent organs, allowing for more accurate TNM staging of esophageal cancer (3). However, there remain a number of issues that require further study.
EUS is able to accurately distinguish esophageal wall levels and cancer invasion depth (73.2–90%) (4–7). In particular, a 12–20 Hz high-frequency ultrasound results in a 0.18 mm resolution, which is suitable to judge the invasion depth of early esophageal cancer. Its accuracy in determining T1 stage esophageal cancer may exceed 90% (7). However, the endoscopic dilation balloon (applied to lesions above stage T2) can compress the esophageal wall and surrounding tissues, resulting in abnormal ultrasound images. Furthermore, EUS is unable to distinguish between tumor and inflammatory tissue (8), and is unsuitable for patients with narrow lesions. Despite these limitations, the technology remains the most accurate means of T staging diagnosis in esophageal cancer.
Additionally, there are a number of obstacles associated with the use of EUS in esophageal lymph node diagnosis. Firstly, its accuracy varies widely, ranging between 33 and 98% (9). The factors affecting accuracy may be summarized as follows: i) The majority of studies consider postoperative lymph node metastasis to be the gold standard, which disregards the consistency between lymph node positions located by EUS and surgery, thus accuracy may be overestimated; ii) it is difficult to distinguish between a benign and a malignant lymph node. A number of studies have reported high detection of lymph node metastasis (up to 80% accuracy) determined by a combination of various indexes (10). However, it is not easy to diagnose the pathological features of lymph nodes by any single index. Previous studies have demonstrated a lymph node satisfying four indexes to have a ≤80% likelihood of metastasis; nevertheless, only 25% of cases met all four criteria in this instance (11). Secondly, ultrasound-guided fine needle aspiration biopsy (EUS-FNA) may improve the accuracy of N staging. It characterizes lymph node metastasis by pathological and cytological diagnosis, although not by EUS features. However, the high cost of EUS-FNA limits its widespread use (12). Thirdly, the majority of the current reported data originates from Western countries, whose focus surrounds esophageal adenocarcinoma and squamous cell carcinoma; in China, the criteria for lymph node metastasis in esophageal cancer remains unclear. Finally, previous studies revealed the number and extent of lymph node metastases to significantly negatively correlate with the long-term survival patients with esophageal cancer following treatment, which is the theoretical basis of the 7th edition of the TNM Classification of Malignant Tumors (13,14). Therefore, accurate determination of lymph node metastasis is particularly important.
The present study retrospectively analyzed 123 patients with esophageal cancer who underwent EUS lymph node scanning at National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China), between January and June 2014. Using postoperative pathological results, the association between lymph node characteristics and pathological features was assessed with EUS technology. The study aimed to find the optimized diagnostic criteria for EUS to determine benign and malignant lymph nodes in patients with esophageal cancer.
Materials and methods
Patients
The present study entailed a retrospective analysis of 499 patients with esophageal cancer, recruited to The Chinese Academy of Medical Sciences Cancer Hospital, between January and June 2014. The inclusion criteria were as follows: i) Thoracotomy for esophageal cancer treatment at the hospital; ii) radial endoscopic ultrasonography examination at the hospital; iii) surgical and pathological confirmation of squamous cell carcinoma; and iv) clearance of lymph nodes. The exclusion criteria were as follows: i) No thoracotomy for esophageal cancer treatment at the hospital; ii) no radial endoscopic ultrasonography examination at the hospital; iii) patients who had received radiotherapy and chemotherapy prior to surgery or radial endoscopic ultrasonography examination; and iv) incomplete postoperative pathological results. A total of 123 patients were recruited, 109 males and 14 females (gender ratio 7.8:1, male:female), with an average age of 60.25±8.8 years.
Criteria of lymph nodes
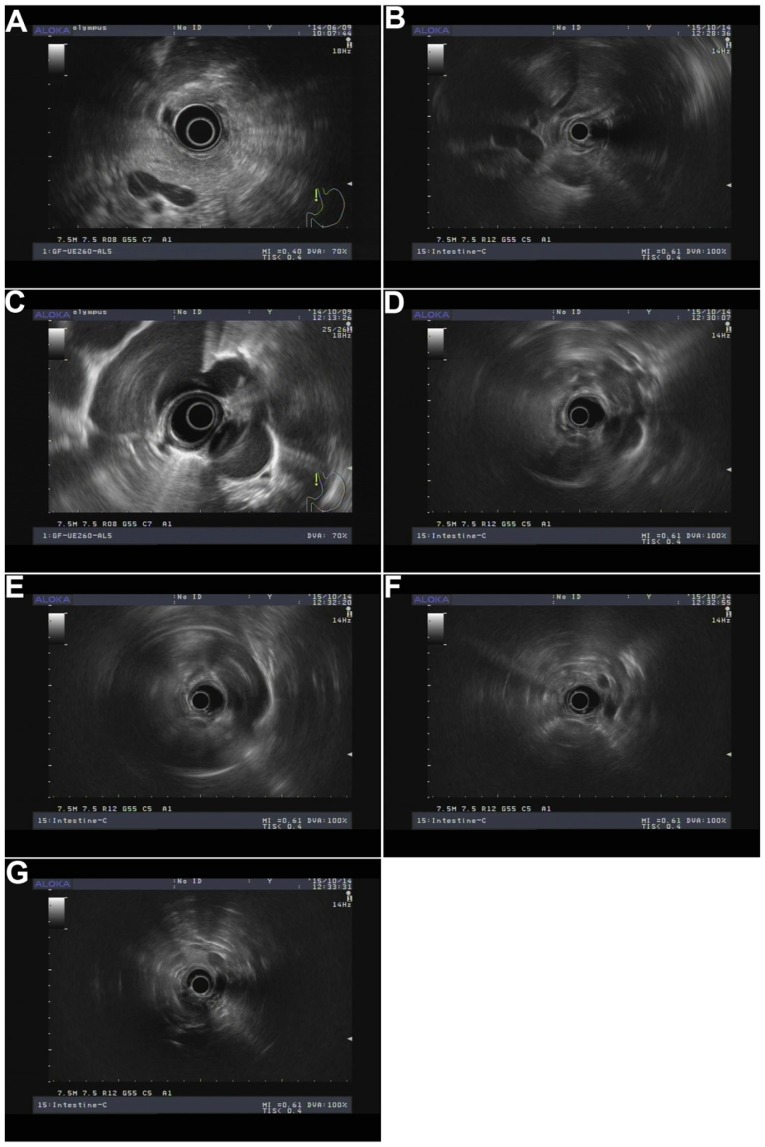
Short diameter, long diameter, short/long diameter ratio, and lymph node number were determined using AwaveAudio software (v7.2, FMJ-Software, Hagersten, Sweden). EUS clinical staging of esophageal cancer was based on the American Joint Committee on Cancer (AJCC) 2003 published guidelines (15), whilst tumor TN staging standard after surgery was based on the 7th Edition of the AJCC Manual (16). Round, low echo and smooth edge were assessed by three experienced physicians. In addition, lymph node location was determined by lymph node partition and ultrasound endoscopy of the splenic vein, the second hepatic portal, left lower pulmonary vein, carina, aortic arch, internal jugular vein, and the left thyroid area (Fig. 1): i) Para-esophageal lymph nodes near lesions; ii) left tracheal esophageal ditch, upper area of aortic arch, left esophageal, and rear-left trachea; iii) right tracheoesophageal ditch area, upper aortic arch, right esophageal, right trachea, and right rear area; iv) 4 l/5 area, carina to the aortic arch upper edge of the left front of the esophagus; v) carina, under 1 cm below the esophageal area; vi) middle of the paraesophageal area, next to the left inferior pulmonary vein and the esophageal-proximal pulmonary vein; vii) lower esophageal area, left lower pulmonary vein to the second hepatic portal; viii) pericardial lymph nodes, the second hepatic portal and the area around the inferior vena cava; ix) left gastric lymph nodes, located in the left gastric artery area; and x) retroperitoneal lymph nodes, splenic vein was marker. In addition, lymph node partition followed the standard operation of ultrasound endoscopy (Table I).
Figure 1.
Lymph node partition by endoscopic ultrasonography. (A) Splenic vein; (B) the second hepatic portal; (C) left lower pulmonary vein; (D) carina; (E) aortic arch; (F) internal jugular vein; (G) left thyroid area.
Table I.
Comparison of lymph node partition prior to and following surgery.
| Lymph node partition by EUS | Name | Description | Abbreviation |
|---|---|---|---|
| Left trachea and esophageal groove | Supraclavicular lymph nodes | Located on the suprasternal notch and clavicle. | 1 |
| Right trachea and esophageal groove | Right upper paratracheal lymph nodes | Located between the intersection of the trachea, the unnamed artery and the apex of the lung. | 2R |
| Left trachea and esophageal groove | Left upper tracheal lymph nodes | Located between the arch of the aorta and the apex of the lung. | 2L |
| Right trachea and esophageal groove | After mediastinal lymph nodes | Located above the bifurcation of the trachea. | 3P |
| Ultrasound endoscopy could not use | Right low paratracheal lymph nodes | Between the trachea, the unmarked arterial root and the tip of the odd vein. | 4R |
| 4L/5 area | Left low paratracheal lymph nodes | Between the arch of the aorta and the lunges. | 4L |
| 4L/5 area | Main pulmonary artery window lymph nodes | Located on the side of aortic arch, aorta and arterial catheter. | 5 |
| Ultrasound endoscopy could not use | Anterior mediastinal lymph nodes | Located in front of the ascending aorta and the unknown artery. | 6 |
| Under Carina | Subclavian lymph nodes | At the root of the tracheal bifurcation. | 7 |
| Near middle esophagus | Mid-esophageal lymph nodes | Located between the trachea and the lower pulmonary vein. | 8M |
| Near Low esophagus | Low esophageal lymph nodes | Between the root of the lower pulmonary vein and the esophagus stomach. | 8L |
| Near Low esophagus | Low lung ligament lymph nodes | Located in the lower lung ligament. | 9 |
| Ultrasound endoscopy could not use | Right tracheobronchial lymph node | Located between the head end of the odd vein and the right upper lobe bronchus. | 10R |
| Ultrasound endoscopy could not use | Left tracheobronchial lymph node | Between the bronchus and the upper left upper lobe of the bronchus. | 10L |
| Near cardia | Diaphragm lymph nodes | Between the diaphragmatic muscle and the diaphragm (diaphragm) | 15 |
| Near cardia | Cardiac around lymph nodes | Lymph nodes located around the junction of the gastroesophageal junction (septum) | 16 |
| Left gastric area | Left gastric lymph nodes | Located in the left artery of the stomach. | 17 |
| Retroperitoneal lymph nodes | Hepatic lymph node | Located in the main hepatic artery. | 18 |
| Retroperitoneal lymph nodes | Splenic lymph nodes | Located in the artery of the splenic artery. | 19 |
| Retroperitoneal lymph nodes | Celiac lymph node | Around the celiac artery | 20 |
EUS, endoscopic ultrasonography.
Lymphatic metastasis criteria
The postoperative pathological clearance of lymph nodes was used to determine metastatic status, where lymph node area had been detected by ultrasound endoscopy. i) Lymph node area was determined by ultrasound endoscopy. However, where postoperative pathology could not ascertain the cleanness of the corresponding area, lymph nodes were not statistically analyzed. ii) Postoperative pathology suggested the cleanness of the corresponding area. However, there were no lymph nodes in this area. This area was recorded as negative. iii) Postoperative pathology suggested the cleanness of the corresponding area. There were lymph nodes in this area. This area was recorded as positive. iv) If the number of lymph nodes detected by EUS was greater compared with that of pathological diagnosis after the operation, the malignant lymph nodes in this area were recorded as positive.
Statistical analysis
Statistical analysis was conducted with SPSS software, version 22.0 (IBM Corp., Armonk, NY, USA). Descriptive analysis and continuous variables are described as the mean ± standard deviation. T-test was used for single factor analysis of each clinical index. A dichotomous method was employed to determine the optimal critical values of continuous variables of the receiver operating characteristic (ROC) curve (17). Chi-square test was used to identify significant differences. Each test result on the ROC curve represents a possible diagnostic value. Youden's index is a single statistic that captures the performance of a dichotomous diagnostic test. Therefore, Youden's index was used to calculate the optimal diagnostic value. The corresponding sensitivity of each test was calculated as the Y-ordinate and the curve was plotted on the basis of the 1-specificity as the X-ordinate. In the current study, the area under the ROC curve (AUC) was used to evaluate the diagnostic efficacy of different criteria (AUC=0.9–1.0 was optimal; AUC=0.8–0.9 was good; AUC=0.7–0.8 was poor; and AUC=0.5 deemed the result invalid).
Results
Lymph nodes detected by EUS and lymph nodes cleared by surgery
The 123 patients possessed an average of 28.3±10.8 lymph nodes following surgery (range, 10–62). Of these 3,488 lymph nodes, 288 were metastatic (metastasis rate: 8.3%; 288/3,488), in 59 patients (48.0%; 59/123). A total of 273 lymph nodes were detected by EUS prior to surgery. Locations of lymph nodes are presented in Table II. Comparison of lymph nodes detected by EUS and those cleared by surgery determined the metastatic status of 225 lymph nodes; 48 detected by EUS had not been reported by surgical clearance, and were predominantly located at the tracheal and esophagus grooves. Therefore, 225 lymph nodes (of 112 patients) were included; of these patients, lymph nodes of 57 cases (50.9%; 57 out of 112) exhibited metastasis.
Table II.
Comparison of lymph nodes detected by EUS and lymph nodes cleared by surgery.
| Sites | Lymph nodes detected by EUS | Lymph nodes cleared by surgery |
|---|---|---|
| Near lesions | 100 | 100 |
| Left gastric | 17 | 17 |
| Near cardia | 20 | 20 |
| 4L area | 4 | 4 |
| Retroperitoneal | 1 | 1 |
| Near esophagus | 57 | 57 |
| Subcarinal | 6 | 6 |
| Right trachea groove | 22 | 10 |
| Left trachea groove | 46 | 10 |
| Total | 273 | 225 |
EUS, endoscopic ultrasonography.
Detailed patient information
Patient details are summarized in Table III. The age distribution of the 112 patients was 60.25±8.8 years; the ratio of male to female was 7:1. There was no difference in the tumor location for all patients. Furthermore, 41 cases were deemed invalid due to stenosis. The accuracy of T staging was 94.6%; 28 cases were at stage N1, 18 cases were at stage N2, and 11 cases were at stage N3.
Table III.
Detailed information of patients for further study.
| Categories | Results |
|---|---|
| Age (mean age ± standard deviation) | 60.25±8.8 |
| Sex (male/female) | 98/14 |
| Tumor location in esophagus: | 112 |
| Upper section | 31 |
| Middle section | 43 |
| Lower section | 38 |
| EUS pass through the lesion (Y/N) | 71/41 |
| EUS T-staging (T1/T2/T3/T4) | 11/8/90/3 |
| Pathological T-staging after surgery (T1/T2/T3/T4) | 12/10/87/3 |
| N-staging after surgery (N0/N1/N2/N3) | 55/28/18/11 |
EUS, endoscopic ultrasonography.
Critical value of continuous variables
Short diameter, long diameter, short/long diameter ratio and lymph node number are continuous variables. The ROC method was used to determine critical values (Fig. 2). The optimal diagnostic value was determined when the Youden index (sensitivity+specificity-1) was the largest. Table IV illustrates that short/long diameter ratio had the lowest value (AUC= 0.553) for diagnosis of lymph node metastasis. The AUC values of other three indexes were all >0.6.
Figure 2.
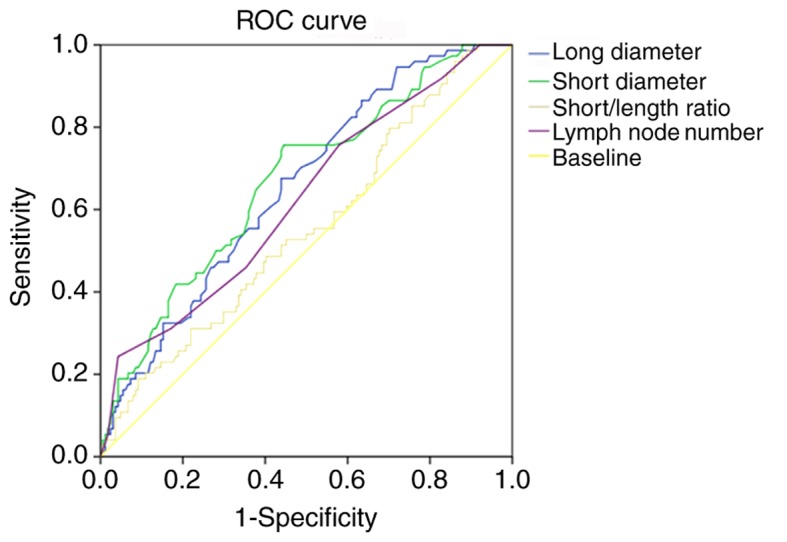
ROC analyses of four continuous variables (long diameter, short diameter, short/length ratio, and lymph node number with endoscopic ultrasonography). ROC, receiver operating characteristic; EUS, endoscopic ultrasonography.
Table IV.
The optimal critical value of the continuous variables.
| Indexes | AUC | Optimal critical value |
|---|---|---|
| Long diameter | 0.655 | >7.50 mm |
| Short diameter | 0.668 | >5.50 mm |
| Short/length diameter ratio | 0.553 | >0.55 |
| Lymph nodes number detected by EUS | 0.661 | >2.00 |
EUS, endoscopic ultrasonography; AUC, area under the curve.
Single factor analysis of lymph node status
In order to test the diagnostic efficacy of the nine indexes, single factor analysis (Table V) was performed. The results indicated that short/long diameter ratio did not reach statistical significance; all other indexes met statistical significance (P<0.05). Round, short diameter and low echo were significantly different between benign and malignant lymph nodes (P<0.001). Specificity, sensitivity, positive predictive value and accuracy were also calculated; Table VI displays the values of these nine indexes. Of the most significant difference indexes, specificity, sensitivity, positive predictive value and accuracy of round were 64.90, 58.90, 43.60, and 60.9%, respectively; specificity, sensitivity, positive predictive value and accuracy of short diameter were 83.80, 58.30, 49.60, and 66.7%, respectively; specificity, sensitivity, positive predictive value and accuracy of low echo were 93.20, 29.10, 39.20, and 50.2%, respectively. The results indicate that the diagnostic efficacy of single factors is not desirable.
Table V.
Single factor analysis of lymph nodes statues.
| Indexes | Benign | Ratio (n=149) (%) | Malignancy | Ratio (n=76) (%) | P-value |
|---|---|---|---|---|---|
| Long diameter >7.5 mm | 80 | 54 | 52 | 68 | 0.013 |
| Short diameter >5.5 mm | 62 | 42 | 48 | 63 | 0.001 |
| Short/length diameter ratio >0.55 | 77 | 52 | 39 | 51 | 0.810 |
| Round | 63 | 42 | 62 | 82 | <0.001 |
| Low echo | 107 | 72 | 69 | 91 | <0.001 |
| Smooth edge or not | 33 | 22 | 27 | 36 | 0.020 |
| Lymph nodes near lesion or not | 60 | 40 | 40 | 53 | 0.042 |
| Lymph nodes number with EUS >2 | 95 | 64 | 56 | 74 | 0.008 |
| T3/4 staging with EUS | 132 | 89 | 71 | 93 | 0.043 |
EUS, endoscopic ultrasonography.
Table VI.
Clinical manifestation of nine single factors.
| Indexes | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Accuracy (%) |
|---|---|---|---|---|
| Long diameter >7.5 mm | 70.30 | 47.00 | 39.40 | 50.47 |
| Short diameter >5.5 mm | 64.90 | 58.90 | 43.60 | 60.90 |
| Short/length diameter ratio >0.55 | 52.70 | 49.00 | 33.60 | 50.20 |
| Round | 83.80 | 58.30 | 49.60 | 66.70 |
| Low echo | 93.20 | 29.10 | 39.20 | 50.20 |
| Smooth edge or not | 36.50 | 78.10 | 45.00 | 64.40 |
| Lymph nodes near lesion or not | 54.10 | 60.30 | 40.00 | 58.20 |
| Lymph nodes number with EUS >2 | 75.70 | 42.10 | 37.10 | 55.60 |
| T3/4 staging with EUS | 95.90 | 12.60 | 35.00 | 40.00 |
EUS, endoscopic ultrasonography.
Index combination analysis
The indexes of conventional methods applied in clinical diagnoses include short diameter, round, low echo and edge smooth. Additionally, the present study introduced three additional indexes: i) Lymph nodes near lesions; ii) lymph node number detected by EUS; and iii) tumor T staging by EUS. The diagnostic efficacy of the conventional and the improved methods were compared, as highlighted in Fig. 3. According to the principle of the Youden index for the best critical value, the improved method had >5 indexes, whilst the conventional method had >3. AUC values for the conventional and improved methods were 0.779 and 0.801 respectively. Specificity, sensitivity, positive predictive value and accuracy of the conventional method were 63.5, 82.9, 58.8, and 74.7%, respectively. Values for the improved method were 70.3, 85.4, 59.8, and 76.1%, respectively. The improved method surpassed the conventional method for all four diagnostic efficacy indexes.
Figure 3.
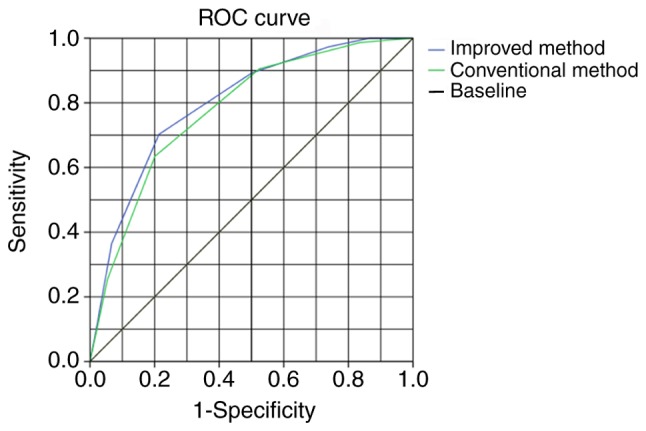
Evaluation of the diagnostic efficacy of the conventional and improved methods. ROC, receiver operating characteristic.
Accuracy of N staging diagnosed by the two methods
In the present study, the accuracy of the conventional method for esophageal cancer lymph node diagnosis was 67.9% (N0: 40; N1-3: 36; 76 out of 112); whilst the accuracy of the improved method was 74.1% (N0: 46; N1-3: 37; 83 out of 112). However, the accuracy of both methods was poor for N2 and N3 staging. For N2 staging, the accuracy of the conventional and improved methods was 11.1 and 22.2%, respectively; accuracy for N3 staging was 0.0% for the two methods. Esophageal stenosis did not allow for efficient endoscopic ultrasound, which affected the esophageal N staging diagnosis. Therefore, 41 cases with esophageal stenosis were excluded prior to subsequent reanalysis. The results indicated that the accuracy of the conventional method for esophageal cancer lymph node diagnosis was 70.4% (N0: 24; N1-3: 26; 50 out of 71), while that of the improved method was 76.1% (N0: 29; N1-3: 25; 54 out of 71). The accuracy of the conventional and improved methods for N2 staging was 22.2 and 44.4%, respectively; accuracy of N3 staging was 0.0% for both methods. This result confirms that the two methods are poor indicators of N2 and N3 staging.
Discussion
The accurate staging of esophageal cancer may not only determine patient prognosis, but also serve an important role in treatment choice. EUS used for T staging of esophageal cancer has been previously reported, where its accuracy is consistently high (73.2–90%) (4–7). However, EUS accuracy of N staging between esophageal cancer studies is more varied (33–98%) (9). Therefore, optimizing EUS may be valuable in improving the accuracy of lymph node diagnosis. In the present study, EUS was employed to determine the region of the lymph node, and postoperative pathological clearance of a corresponding metastatic lymph node was used to judge lymph node metastasis. Our results indicated that lymph nodes detected by EUS matched those detected with surgical clearance of lymph node. However, lymph nodes located in the tracheal and esophageal groove were difficult to diagnose. It may be speculated that this phenomenon was caused by surgical difficulties. However, lymph nodes located in the tracheal and esophageal groove were frequently subject to metastasis, which may indicate an association between location and metastatic capacity (18). The present study determined the optimized critical value of long diameter (>7.5 mm) and short diameter of lymph nodes (>5.5 mm). Of the AUC curve analyses, a short diameter of >5.5 mm was selected as the determining criterion for lymph node metastasis, which differs from a previous report (lymph node diameter >10 mm) (19). Due to the high resolution of EUS, lymph nodes with a short diameter of ≤0.3 mm were frequently harvested. The metastasis rate of these lymph nodes with a <10 mm short diameter should not be ignored. In the present study, ~16.7% of lymph nodes with ≤5.5 mm short diameter exhibited metastasis. In addition, although the standard lymph node size for metastasis was reduced, the AUC of the four indexes remained <0.7, suggesting that size is an insufficient determinant of lymph node metastasis.
A previous study suggested that a combination of multiple indexes (including size, shape and smooth edge) may improve the diagnostic accuracy of lymph node metastasis (20). Lymph nodes adjacent to a tumor are more likely to be affected (21); therefore in the present study, lymph nodes located near cancerous tissue were used as a marker of lymph node metastasis. In addition, the number of lymph nodes identified by EUS in esophageal cancer correlated positively with incidence of metastasis (12). Thus, lymph node number was also considered an identifying index. In the current study, the short/length diameter ratio was not sufficient to determine lymph node pathology due to poor diagnostic accuracy. Short/long diameter ratio denotes lymph node shape and it was identified that benign lymph nodes often exhibited larger ratios. Differences were identified between the other indexes. However, the positive predictive value and the accuracy of any other single index were inadequate to apply in clinical practice. The combination of multiple indexes to determine lymph node status is common in clinical study (22). Generally, size, echo, round and smooth edge are used to determine lymph node metastasis; the improved method had an additional three indexes (lymph nodes near lesion, lymph nodes number with EUS and T3/4 staging). ROC curve analysis demonstrated that the accuracy and positive predictive value of the improved method was increased in comparison with the conventional method, with an AUC of 0.801. This result indicated that the improved method may promote diagnostic accuracy for malignant lymph nodes. The present study also employed the two methods for N-staging diagnosis; the accuracy of the improved method was better than that of the conventional method (74.1 vs. 67.9%). Of esophageal stricture cases, the accuracy of the two methods was increased for lymph node metastasis (76.1 vs. 70.4%). However, these methods were poor for N2 staging and N3 staging, which suggests that diagnostic standards require improvement. There may be a number of possible reasons for this. Efficient N and N3 staging is effected by invasion of lymph node tumors. The invasion pattern may not alter the lymph node structure, thus may not be recognized by EUS. In addition, the number of lymph nodes detected by EUS was between two and five. Therefore, the two methods were insufficient to diagnose N3 staging.
The limitations of the present study are as follows. Firstly, this analysis is of a single-center retrospective study. The results require further prospective multi-center analyses to verify accuracy and operability. Secondly, the gold standard applied in this study was unable to fully confirm the association between lymph nodes detected by EUS and pathological lymph nodes after surgical clearance. Thirdly, some indicators lack the quantitative standards in the clinic. Finally, the accuracy and positive predictive value of the improved method remains inadequate. Therefore, it is necessary to find or add further independent criteria to improve diagnostic accuracy, such as the recent development of elastography. This technology can be used to map the elasticity and stiffness of soft tissue, which are properties that provide diagnostic information regarding the presence or status of disease. For example, cancerous tumors will often be harder compared with the surrounding tissue and diseased livers are typically stiffer compared with healthy livers (23,24).
EUS has become the preferred method for gastrointestinal cancer staging, particularly for the depth of tumor invasion (T staging). In the present study, ROC curve and χ2 tests were used to analyze specific parameters in order to optimize standards for malignant lymph node diagnosis. The results were as follows. The best critical values of long diameter, short diameter and the lymph nodes number detected by EUS were >7.5, >5.5 mm and >2, respectively. Long diameter >7.5 mm, short diameter >5.5 mm, round, low echo, edge smooth, near lesion and detected lymph nodes number (>2), T3/4 staging reached significance. The AUC value of the improved method (0.801) surpassed that of the conventional method (0.779). The optimized method may improve esophageal N staging accuracy. However, further optimization is required. Detection ability has improved with EUS development, including endoscopic elastography and endoscopic ultrasound imaging technology. It is considered that these technologies may further improve the diagnostic accuracy of lymph node metastasis.
Acknowledgements
Not applicable.
Funding
The authors would like to thank CAMS Innovation Fund for Medical Sciences (CIFMS), (grant no. 2016-12M-001), the National Key Research and Development Program of China (grant no. 2016YFC1302800), the National Natural Science Foundation of China (grant no. 61372192), the PUMC Youth Fund and the Fundamental Research Funds for the Central Universities (grant no. 2017320012) for their financial support.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
GW, YZ and SH were the guarantors of the integrity of the entire study. GW, YZ, SH and LD were involved in the study conception and study design. GW was involved in the definition of intellectual content. YL, YK and XY conducted the clinical studies. LD, YL, ZW and GW performed literature research. YZ, SH, LD, XY, YL, YK, XY and ZW conducted the experimental studies. YL, ZW and GW were involved in data acquisition. SH and GW performed the data analysis. YZ, LD, SH, XY, ZW and GW were involved in manuscript preparation and revised the final version manuscript.
Ethics approval and consent to participate
The Research Ethics Committee of Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China) approved the collection of tissue samples for research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen W, Zheng R, Zeng H, Zhang S. The incidence and mortality of major cancers in China, 2012. Chin J Cancer. 2016;35:73. doi: 10.1186/s40880-016-0137-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong SJ, Kim TJ, Nam KB, Lee IS, Yang HC, Cho S, Kim K, Jheon S, Lee KW. New TNM staging system for esophageal cancer: What chest radiologists need to know. Radiographics. 2014;34:1722–1740. doi: 10.1148/rg.346130079. [DOI] [PubMed] [Google Scholar]
- 3.Lennon AM, Penman ID. Endoscopic ultrasound in cancer staging. Br Med Bull. 2007;84:81–98. doi: 10.1093/bmb/ldm033. [DOI] [PubMed] [Google Scholar]
- 4.Heidemann J, Schilling MK, Schmassmann A, Maurer CA, Büchler MW. Accuracy of endoscopic ultrasonography in preoperative staging of esophageal carcinoma. Dig Surg. 2000;17:219–224. doi: 10.1159/000018838. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y, Fu JH, Rong TH, Xu GL, Li XD, Zhang PY, Yang H, Zhu ZH, Zhang SY. Application of endoscopic ultrasonography to preoperative clinical staging of esophageal cancer. Ai Zheng. 2005;24:1358–1362. (In Chinese) [PubMed] [Google Scholar]
- 6.Mennigen R, Tuebergen D, Koehler G, Sauerland C, Senninger N, Bruewer M. Endoscopic ultrasound with conventional probe and miniprobe in preoperative staging of esophageal cancer. J Gastrointest Surg. 2008;12:256–262. doi: 10.1007/s11605-007-0300-2. [DOI] [PubMed] [Google Scholar]
- 7.Shimpi RA, George J, Jowell P, Gress FG. Staging of esophageal cancer by EUS: Staging accuracy revisited. Gastrointest Endosc. 2007;66:475–482. doi: 10.1016/j.gie.2007.03.1051. [DOI] [PubMed] [Google Scholar]
- 8.Sun F, Chen T, Han J, Ye P, Hu J. Staging accuracy of endoscopic ultrasound for esophageal cancer after neoadjuvant chemotherapy: A meta-analysis and systematic review. Dis Esophagus. 2015;28:757–771. doi: 10.1111/dote.12274. [DOI] [PubMed] [Google Scholar]
- 9.Puli SR, Reddy JB, Bechtold ML, Antillon D, Ibdah JA, Antillon MR. Staging accuracy of esophageal cancer by endoscopic ultrasound: A meta-analysis and systematic review. World J Gastroenterol. 2008;14:1479–1490. doi: 10.3748/wjg.14.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Catalano MF, Sivak MV, Jr, Rice T, Gragg LA, Van Dam J. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc. 1994;40:442–446. doi: 10.1016/S0016-5107(94)70206-3. [DOI] [PubMed] [Google Scholar]
- 11.Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc. 1997;45:474–479. doi: 10.1016/S0016-5107(97)70176-7. [DOI] [PubMed] [Google Scholar]
- 12.Vazquez-Sequeiros E. Nodal staging: Number or site of nodes? How to improve accuracy? Is FNA always necessary? Junctional tumors-what's N and what's M? Endoscopy. 2006;38(Suppl 1):S4–S8. doi: 10.1055/s-2006-946642. [DOI] [PubMed] [Google Scholar]
- 13.Edge SB, Byrd DR, Carducci MA, Compton CC, Fritz AG, Greene F, Trotti A, editors. American Joint Committee on Cancer (AJCC): Cancer staging manual, corp-author. 7th edition. Springer; New York, NY: 2009. [Google Scholar]
- 14.Sobin LH, Gospodarowicz MK, Wittekind C, editors. International Union Against Cancer (UICC): TNM classification of malignant tumors, corp-author. 7th edition. Wiley-Blackwell; Oxford: 2009. [Google Scholar]
- 15.AJCC Cancer Staging Manual. 6th edition. Springer; Chicago, IL: 2002. [Google Scholar]
- 16.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: Esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17:1721–1724. doi: 10.1245/s10434-010-1024-1. [DOI] [PubMed] [Google Scholar]
- 17.Zou KH, O'Malley AJ, Mauri L. Receiver-operating characteristic analysis for evaluating diagnostic tests and predictive models. Circulation. 2007;115:654–657. doi: 10.1161/CIRCULATIONAHA.105.594929. [DOI] [PubMed] [Google Scholar]
- 18.Li X, Zhao J, Liu M, Zhai F, Zhu Z, Yu F, Zhang M, Han L, Zhao Y, Wang H. Determination of radiotherapeutic target zones for thoracic esophageal squamous cell cancer with lower cervical lymph node metastasis according to CT-images. Oncotarget. 2016;7:35865–35873. doi: 10.18632/oncotarget.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li S, Chen S. Diagnostic criteria of metastatic lymph node in a different site of the mediastinum in esophageal carcinoma: A research on pathology and computed tomography. Int J Radia Oncol Biol Phy. 2017;99(Suppl 1):E166. doi: 10.1016/j.ijrobp.2017.06.996. [DOI] [Google Scholar]
- 20.Moon SH, Kim HS, Hyun SH, Choi YS, Zo JI, Shim YM, Lee KH, Kim BT, Choi JY. Prediction of occult lymph node metastasis by metabolic parameters in patients with clinically N0 esophageal squamous cell carcinoma. J Nucl Med. 2014;55:743–748. doi: 10.2967/jnumed.113.130716. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Liu S, Pan J, Zheng X, Zhu K, Zhu J, Xiao J, Ying M. The pattern and prevalence of lymphatic spread in thoracic oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg. 2009;36:480–486. doi: 10.1016/j.ejcts.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 22.Vazquez-Sequeiros E. Looking for new features to improve accuracy of EUS lymph node criteria. J Gastrointestin Liver Dis. 2009;18:135–136. [PubMed] [Google Scholar]
- 23.Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound elastography: Review of techniques and clinical applications. Theranostics. 2017;7:1303–1329. doi: 10.7150/thno.18650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jeong WK, Lim HK, Lee HK, Jo JM, Kim Y. Principles and clinical application of ultrasound elastography for diffuse liver disease. Ultrasonography. 2014;33:149–160. doi: 10.14366/usg.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.