Abstract
Hypoxia is an important factor that results in failure of chemotherapy for the majority of solid tumor types, particularly for gastric cancer. In the present study, mesenchymal stem cells (MSCs), which have the ability to migrate to cancer tissues were used as a vehicle to supply oxygen to gastric cancer. The hemoglobin genes were transfected into MSCs as MSC-hemo groups. Subsequently, MSC-hemo groups were induced by isopropyl-b-D-thiogalactopyranoside and hemin to express hemoglobin. The hemoglobin was detected by western blotting method. Following this, the MSC-hemo groups were placed in an atmosphere containing 100% oxygen and were used to investigate the effect of the function of the oxygen-laden MSC-hemo group on gastric cancer chemotherapy with an MTT assay. As a first approach to investigate the possibility of MSCs as a vehicle to supply oxygen to anoxic cancer types, including gastric, liver, breast cancer, the results indicated that the oxygen-laden MSC-hemo group significantly enhanced the effect of chemotherapeutic treatments on gastric cancer cells. Utilizing MSCs as a svehicle to supply oxygen to the solid tumor may be a novel method to improve the hypoxia conditions of tumor tissues and improve the effect of chemotherapy on tumor cells.
Keywords: mesenchymal stem cells, hypoxia, oxygen-laden, chemotherapy, gastric cancer
Introduction
In 2012, gastric cancer was the most common malignancy globally, particularly in Eastern Asia (1). Additionally, in 2010, it was the second most common cancer and third most common cause of cancer-associated mortalities in China (2). The conventional therapies of gastric cancer, including chemotherapy and radiotherapy, have notable difficulties directly associated with hypoxia (3). Hypoxia occurs in solid tumor types, including gastric, liver, breast, pancreatic cancer, as a result of an inadequate supply of oxygen, due to exponential cellular proliferation and inefficient vascular supply; therefore, it is an adverse prognostic indicator in cancer as it is associated with resistance to radiotherapy and chemotherapy (4). The mechanisms of hypoxia-induced chemotherapy resistance are complex (5–8) due to hypoxia inducible factor-1 (HIF-1) acting as a transcription factor to upregulate numerous genes with varying functions, including the regulation of drug efflux, proliferation, angiogenesis and metabolic changes (9,10). Improving the hypoxia condition can significantly enhance the effect of gastric cancer chemotherapy (11).
With profound progress in biomedical science, numerous targeted therapeutic methods have been investigated in a number of cancer types, including gastric cancer (12). In this aspect, mesenchymal stem cells (MSCs) have a notable potential as a tool for targeted therapy, due to these cells being easily transduced in vitro and can be used as gene-delivery vehicles for gene therapy (13). To date, a large number of genes with tumor-suppressive functions have been successfully engineered into MSCs and have been tested in a number of cancer models, including interferon-α (IFN-α) in melanoma (14), IFN-γ in leukemia (15), interleukin-12 in cervical cancer (16,17), and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in breast and hepatocellular carcinoma (18,19). In the present study, the aim was to produce hemoglobin protein-expressing MSCs, and then to use these MSCs as a vehicle to supply oxygen to the hypoxic gastric cancer cells. Additionally, whether this method could enhance the effect of gastric cancer chemotherapy was investigated.
Materials and methods
Cell culture
Gastric cancer cell lines MKN-45 and SGC-7901 were preserved by the Key Laboratory of Digestive System Tumors, Lanzhou University Second Hospital (Lanzhou, China). The gastric cancer cell lines were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) in a humidified atmosphere containing 5% CO2 at 37°C. MSCs from bone marrow were preserved by the Key Laboratory of Digestive System Tumors, Lanzhou University Second Hospital and cultured in low-glucose Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS in a humidified atmosphere containing 5% CO2 at 37°C. Subsequently, the morphology of the cultured cells was observed with a light inverted phase-contrast microscope (Nikon TS-100F; ×20).
Chemicals
Cisplatin (CDDP) and 5-fluorouracil (5-fu) were obtained from Qilu Pharmaceuticals (Jinan, China) and Shanghai Xudong Haipu Pharmaceuticals Co., Ltd., (Shanghai, China), respectively. Prior to their use, CDDP and 5-fu were immediately dissolved in 0.9% sodium chloride injection/PBS/DMEM to a working concentration of 0.4 and 2 mg/ml, respectively.
Plasmid construct
The hemoglobin subunit α 2 (HBA2) and hemoglobin subunit β (HBB) genes were chemically synthetized by GenePharma Gene Technology, Co., (Shanghai, China). The sequences of HBA2 and HBB are located on GeneBank (https://cipotato.org/genebankcip/). Subsequently, the products were ligated with linearized pEX-2 Vector (GenePharma Gene Technology, Co., Shanghai, China; concentration, 500 ng/ml) using T4 DNA ligase (Takara Bio, Inc., Otsu, Japan) at 22°C for 60 min. The recombinant plasmids were transformed into Top 10 competent cells with the CaCl2 method (20). To identify the positive clones, plasmids were extracted with a mini-plasmid extract kit (Takara Bio, Inc.), according to the manufacturer's protocols. The clone was confirmed by DNA sequencing (forward primer, 5′-TCAAGCCTCAGACAGTGGTTC-3′; and reverse primer, 5′-CCTCACATTGCCAAAAGACG-3′). This was conductedon the next day after transfection. Construction plasmid helper-SL3 and envelope plasmid helper-SL4 were also purchased in GenePharma Gene Technology, Co. GFP report gene was added to mark the positive cells.
Lentivirus packaging
293T cells (Genomeditech, Shanghai, China) were preserved by Key Laboratory of Digestive System Tumors, Lanzhou University Second Hospital, and maintained with high-glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS. The 293T cells at 90% confluence were used for lentivirus packaging. The plasmid of pEX-2 containing the hemoglobin gene coding sequence, the construction plasmid helper-SL3 and the envelope plasmid helper-SL4 were co-transfected (5 µl/1×105 cells) into 293T cells mediated by Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.). After 72 h, the culture medium containing packaged lentivirus particles were collected, filtered and stored at −80°C for further processing. The lentiviral titer was detected by cell counting using a fluorescent microscope (magnification, ×40).
Infection of MSCs with obtained lentiviral particles
MSCs were directly infected with obtained (containing hemoglobin genes and the GFP report gene) or empty lentiviral particles (only containing the GFP report gene) by adding DMEM containing 5 µg/mg polybrene (EMD Millipore, Billerica, MA, USA). The concentration of lentiviral particles was 40 µl/1×105 cells. After 72 h, the expression of GFP was observed by fluorescent microscopy (magnification, ×40) to observe transfection efficiency. Blank control was also set, which contained MSCs without any modification. The MSC-hemo-GFP, which contained hemoglobin genes and the GFP report gene, and MSC-GFP, which only contained the GFP report gene, groups were collected and washed twice with 0.5% PBS solution, and then suspended with 0.5% PBS solution for the next application.
Inducible expression of hemoglobin
Induction of hemoglobin expression was achieved by the addition of isopropyl-b-D-thiogalactopyranoside (IPTG) and hemin. The IPTG and hemin addition occurred at an optical density at 600 nm of ~0.6, with an IPTG final concentration of 0.2 mmol/L and a hemin final concentration of 25 µg/ml. Following culturing in a humidified atmosphere containing 5% CO2 at 37°C for 2 h, hemin was added again to the MSC-hemo group to a final concentration of 50 µg/ml. After 6 h at 37°C, the cells were collected for the next examination.
Observing the expression level of hemoglobin with western blotting
The cells were lysed with radio immunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Haimen, China) to prepare the protein samples for western blotting. The quantification of the extracted proteins was conducted with a BCA kit (Beyotime Institute of Biotechnology), according to the manufacturer's protocol. Subsequently, 40 µg protein samples were loaded onto a 12% SDS-PAGE gel, and transferred to a polyvinylidene difluoride (EMD Millipore) membrane. Following being blocked with 1% bovine serum albumin solution for 1 h at room temperature, the membrane was incubated with mouse anti-hemoglobin polyclonal antibody (1:1,000; cat. no. ab17925; Abcam, Shanghai, China) overnight at 4°C, and then alkaline phosphatase-conjugated goat anti-mouse IgG secondary antibodies (1:10,000; cat. no. zk9600; OriGene Technologies, Inc., Beijing, China) were applied to the membrane for 2 h at room temperature. Protein expression was detected with an electro-chemiluminescence (ECL) assay (Hypersensitive ECL chemiluminescence substrate kit; Yanxi Biotechnology, Co., Shanghai, China), according to the manufacturer's protocols (Pierce; Thermo Fisher Scientific, Inc.). The detecting system was Quantity One 4.62 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Additionally, blank control (MSCs), positive control (recombinant hemoglobin positive control; purchased from Shanghai Yu Bo Biotechnology, Co., Ltd., Shanghai, China) and β-actin (cat. no. ab8227; Abcam) were set to ensure the quality of western-blotting detection. The 3 groups used were: Non-transformed MSCs, which represented the blank control group; 0.45 µg hemoglobin as the positive control; and the MSC-hemo group, which were transfected with 40 µl/1×105 cells lentiviral particles.
Effect of the function of oxygen-laden MSC-hemo cells on gastric cancer cell chemotherapy
A Transwell assay was conducted, and because GFP report gene was involved in the lentivirus particles, the results were observed with afluorescent microscope (×40). In the present study, two chemotherapeutics, 5-fu and CDDP, were tested. For these chemotherapeutics, three interventions were set: Only chemotherapeutic intervention, as a control group; chemotherapeutic and non-transformed MSC interventions; and chemotherapeutic and oxygen-laden MSC-hemo interventions. These chemotherapeutics were investigated in two different gastric cancer cell lines, MKN45 and SGC-7901. The interventions were conducted as follows: Gastric cancer cells (MKN45 or SGC-7901) were seeded (1×105 cells/well) in the lower well of Transwell plates (Corning Costar, Cambridge, MA, USA), which were 6.5 mm in diameter with 8 µm pore filters and contained 600 µl DMEM (Gibco; Thermo Fisher Scientific, Inc.). Chemotherapeutics (2 mg/ml 5-fu or 0.4 mg/ml CDDP) were added into every plate of all tested groups. For the oxygen-laden MSC-hemo group, the cells were suspended in serum-free DMEM (Gibco; Thermo Fisher Scientific, Inc.) and seeded (1×105 cells/well) in the upper well of Transwell plates, and then they were placed in an atmosphere containing 100% oxygen. For the control group, 0.9% NaCl was added in the upper well of the Transwell plates. Furthermore, for the group containing the non-transformed MSCs, MSCs were suspended in serum-free DMEM and seeded (1×105 cells/well) in the upper well of the Transwell plates. Subsequently, the upper wells were placed on the lower wells. Following culturing for 24 h in a humidified atmosphere containing 5% CO2 at 37°C, the cells in the lower wells were collected to conduct an MTT assay, in order to assess the effect of the three different interventions.
MTT assay
An MTT assay was conducted as described subsequently. MTT powder (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was dissolved (final concentration, 5 mg/ml) in PBS and filtered. MTT solution was added to the cells on plates and incubation continued for 4 h at 37°C. Supernatants were removed and 200 µl 0.04 M HCl in isopropanol was added to each well. Optical densities were measured at 450 nm using Varioskan Flash (Thermo Fisher Scientific, Inc.) as the detection system. MTT assays were conducted in triplicate.
Statistical analysis
SPSS v17.0 was used to analyze data (SPSS, Inc., Chicago, IL, USA). Data were expressed as the mean ± standard deviation. Results were analyzed using one-way analysis of variance (ANOVA) to assess the statistical significance of overall differences between all treatment groups and to evaluate the MTT assay data. When the ANOVA test determined a value of P<0.05, data were further analyzed with the Student's Newman-Keuls-q test, in order to assess the statistical differences between every two groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Morphology of cultured cells
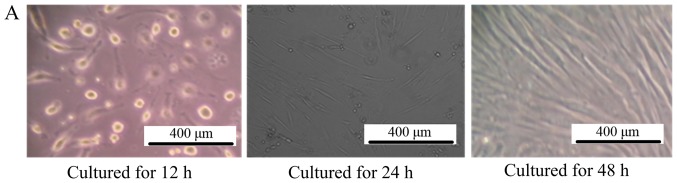
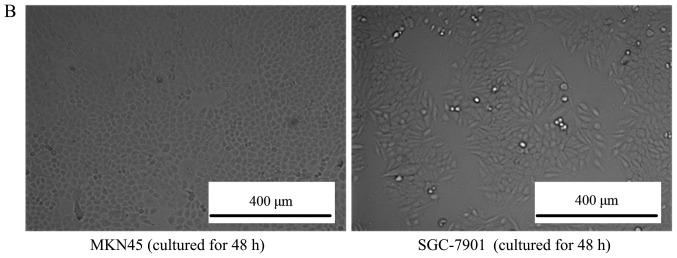
As depicted in Fig. 1A, after culturing for 48 h, the MSCs grew in a fibroblast-like shape, >80% of the cells fused together. Additionally, the gastric cell lines MKN45 and SGC-7901 also exhibited normal characteristics of gastric cancer cells, as depicted in Fig. 1B.
Figure 1.
Morphology of cultured cells. (A) The morphology of mesenchymal stem cells observed by inverted phase-contrast microscope (magnification, ×100). The left picture is the MSCs cultured for 12 h, where all the cells adhered to the bottom of the culture dish and cells were observed to be round. For the middle picture, which depicts MSCs cultured for 24 h, the cells began to grow in a fibroblast-like manner, and the right picture is the MSCs cultured for 48 h, depicting that ~80% of the cells had fused together. (B) The morphology of MKN45 and SGC-7901 cells observed by inverted phase-contrast microscope (magnification, ×100). The left picture is the MKN-45 cells cultured for 48 h, where all the cells grew in a triangle or quadrangle manner. The right picture is the SGC-7901 cells cultured for 48 h, where the cells grew in classical short shuttle-like manner. MSCs, mesenchymal stem cells.
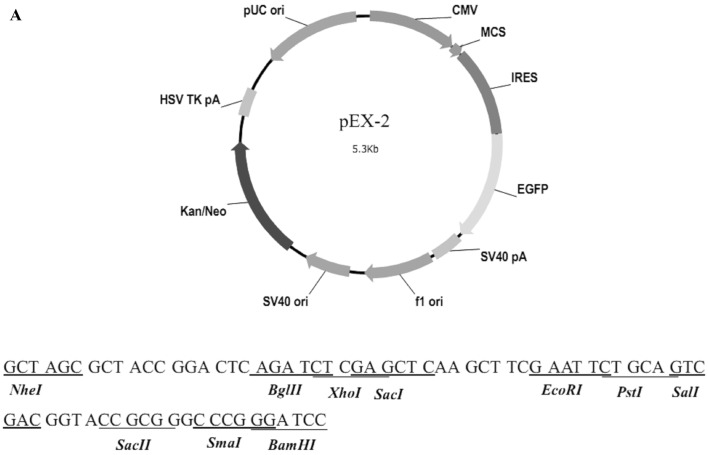
Identification of the recombinant vector
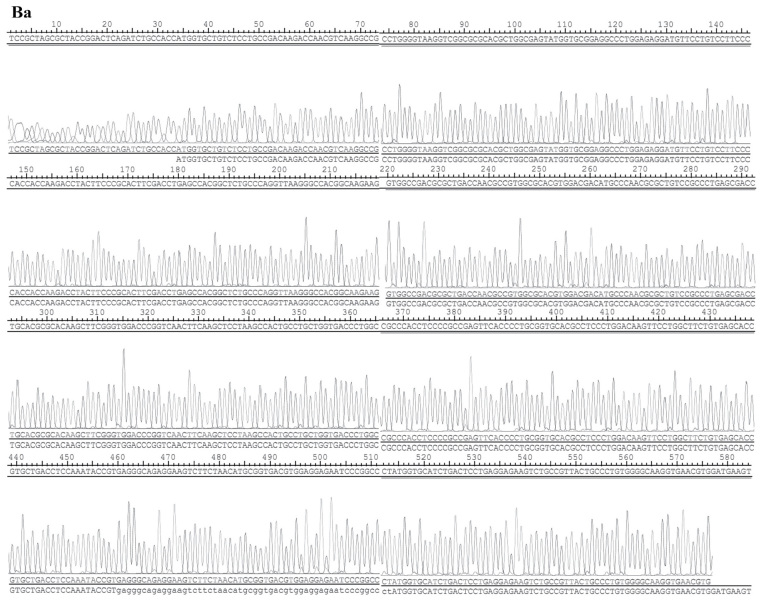
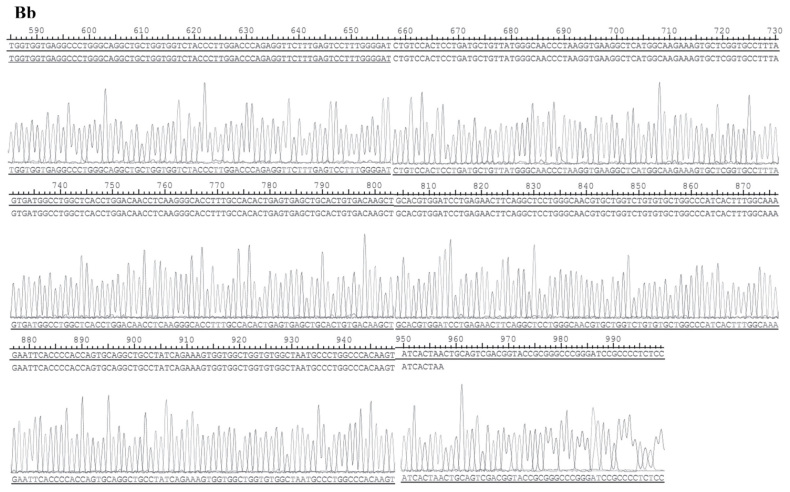
The structure of pEX-2 is depicted in Fig. 2A. The expression of hemoglobin genes in the recombinant vector was identified by sequencing, and the result demonstrated that the sequence was consistent with NM_000517 (HBA2) and NM_000518 (HBB) in Genebank (Fig. 2B).
Figure 2.
Identification results of the recombinant vector. (A) The structure of pEX-2. (B) The sequences of hemoglobin genes expressed in the recombinant vector. The result demonstrated that the sequence was consistent with (Ba) NM_000517 (HBA2). (B) The sequences of hemoglobin genes expressed in the recombinant vector. The result demonstrated that the sequence was consistent with (Bb) NM_000518 (HBB) in Genebank.
Screening of stable positive-infected MSC clones by fluorescent microscopy
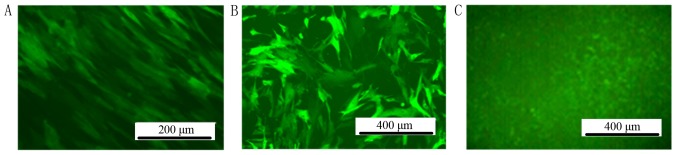
The titer of the lentiviral particles was determined to be 1×109 TU/ml. MSCs were infected by lentiviral particles in DMEM with 5 µg/ml polybrene. After 72 h infection by lentiviral particles, GFP could be observed by fluorescent microscopy in the MSC-hemo-GFP and MSC-GFP groups. No GFP expression was observed in the blank control group (Fig. 3).
Figure 3.
Stable positive-infected MSC clones were detected by fluorescent microscope. (A) MSC-hemo-GFP. (B) MSC-GFP. (C) The blank control group under a fluorescent microscope exhibited no GFP expression. Scale bar, 250 µm. GFP, green fluorescent protein; MSC, mesenchymal stem cell.
Observing the expression of hemoglobin with western blotting
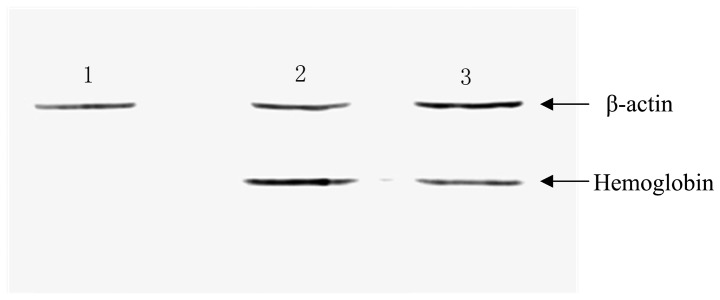
Subsequently, MSCs were infected with the constructed lentiviral vectors, and the expression of hemoglobin was detected with western blotting. As depicted in Fig. 4, hemoglobin was detected in the MSC-hemo (Lane 3) and positive control groups (Lane 2); however, hemoglobin protein was not expressed in the empty MSC control group (Lane 1).
Figure 4.
Expression of hemoglobin was observed in the MSC-hemo group with western blotting. Lane 1, non-transformed MSCs, it represented the blank control group; Lane 2, 0.45 µg hemoglobin as the positive control; and Lane 3, the MSC-hemo group, which were transfected with 40 µl/1×105 cells lentiviral particles. MSCs, mesenchymal stem cells.
Effect of oxygen-laden MSC-hemo function on the gastric cancer cell chemotherapy
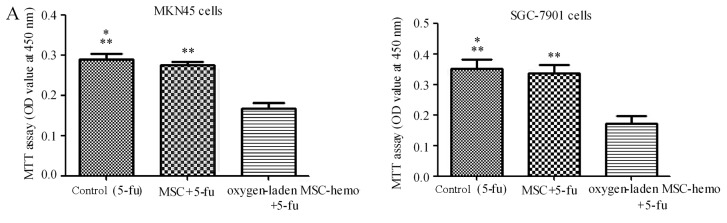
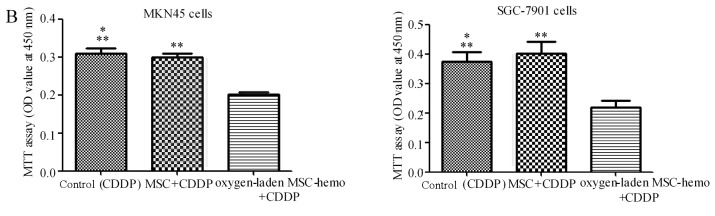
The effect of oxygen-laden MSC-hemo function on the gastric cancer cell chemotherapy was assessed with an MTT assay. As depicted in Fig. 5, compared with the 5-fu treatment group (Fig. 5A), the oxygen-laden MSC-hemo group significantly enhanced the effect of 5-fu treatment on MKN45 and SGC-7901 cells (P<0.05), while the non-transformed MSC and 5-fu group had no significant difference with the control group. In the CDDP treatment group (Fig. 5B), the identical results were produced. This data indicated that the oxygen-laden MSC-hemo group may contribute to the effect of the function of chemotherapeutics on gastric cancer cell chemotherapy.
Figure 5.
The Oxygen-laden MSC-hemo group significantly enhances the effect of chemotherapeutic treatments on gastric cancer cells. (A) 5-fu treatment group for the MKN45 and SGC-7901 cells. It demonstrated that the oxygen-laden MSC-hemo group significantly enhanced the effect of 5-fu treatment on MKN45 and SGC-7901 cells, while the MSC group had no significant difference, compared with the control group. (B) CDDP treatment group for the MKN45 and SGC-7901 cells. It demonstrated that the oxygen-laden MSC-hemo group significantly enhanced the effect of CDDP treatment on MKN45 and SGC-7901 cells, while the MSC group had no significant difference, compared with the control group. *P>0.05, compared with MSC+5-fu or MSC+CDDP; **P<0,05, compared with oxygen-laden MSC-hemo+5-fu or oxygen-laden MSC-hemo+CDDP. MSCs, mesenchymal stem cells; CDDP, cisplatin; 5-fu, 5-fluroouracil.
Discussion
Hypoxia is an integral characteristic of the tumor microenvironment and a well-documented source of therapeutic failure in clinical oncology (21). It is a direct result of a lack of oxygen, which is caused by microvascular defects that accompany the accelerated neoplastic growth and indirectly caused by alterations in the proteome/genome, angiogenesis and pH changes (4). The majority of solid tumor cases >1 mm3 in volume contain regions of hypoxia, particularly gastric cancer (22). The stomach is a hollow organ located deep within the enterocoelia, and hypoxia is more severe for gastric cancer (23).
Solid cancer types with hypoxia-induced phenotypes are frequently resistant to chemotherapy and have a poor prognosis (24). In particular, cancer cells with reduced levels of oxygenation are more resistant to a number of chemotherapeutic agents, including 5-fu and CDDP (25). This cellular response to hypoxia, primarily mediated by HIF-1, may increase the aggressiveness of cancer cells and contribute to poor responses to treatment (26). With regards to the resistance of gastric cancer cells to 5-fu, Nakamura et al (27) reported that the expression of HIF-1α in gastric cancer tissue was an independent prognostic factor in patients who were administered 5-fu adjuvant treatment following resection. These authors also demonstrated that transfection of the HIF-1α gene into gastric cancer cells increased their resistance to 5-fu in vitro and in vivo. Xuan et al (3) also confirmed that in the MKN45 and AGS cell lines, HIF-1α expression is dependent on hypoxic conditions and that the genetic enhancement of HIF-1α under normoxic or hypoxic conditions can eliminate the sensitivity to 5-fu. Improvementof hypoxia in cancer is therefore a prime target for the development of novel gastric cancer therapeutics. Reduced treatment dosages and increased benefits for the patient are envisaged as a consequence of these investigations. In conclusion, the ability to increase oxygenation of tumors will revolutionize contemporary cancer treatment. Successful treatment of hypoxic cells has the potential to not only improve local control but also impact overall patient survival.
Generally, oxygen is transported in the blood by hemoglobin (28). Recombinant human erythropoietin (rHuEPO) is currently administered to patients with cancer, in order to protect against chemotherapy or tumor-associated anemia, and clinical trial results indicate numerous beneficial effects of rHuEPO treatment on the therapeutic outcome of patients (29). In the present study, a novel method was developed to increase the oxygen supply for the gastric cancer cells to overcome the therapeutic resistance of MKN45 and SGC-7901 cell lines to 5-fu and CDDP.
MSCs are a type of marrow stroma cells, which exist in numerous tissues and are easy to obtain and amplify. Examples include the bone marrow, umbilical cord blood and umbilical cord (30). Due to their notable differentiation potential, they are used in regenerative medicine (31). In the last decade, increasing numbers of researchers have determined another beneficial characteristic of MSCs, that MSCs are able to home in on tumor sites (32–34). This means that MSCs are ideal vehicles for targeted gene therapies. At the same time, MSCs can avoid immune rejection (35,36), which provides further convenience for the use of MSCs as a tool for targeted therapy. In the present study, MSCs were used as a vehicle for hemoglobin, with MSCs infected by lentivirus vectors delivering the HBA2 and HBB genes. Additionally, the present results demonstrated that these MSC-hemo cells could continuously release hemoglobin protein following induction with IPTG and hemin. Following placing in an atmosphere containing 100% oxygen, the effect of MSC-hemo on gastric cancer chemotherapy was evaluated. A total of two groups were set according to the therapeutic drugs used (5-fu or CDDP). Subsequently, three different intervention measures were conducted on MKN45 and SGC-7901 cells in every group. The first intervention measure was 0.9% NaCl and the therapeutic drug as a control, the second measure was non-transformed MSCs and the therapeutic drug and the final measure was the oxygen-laden MSC-hemo group and the therapeutic drug. The results depicted in Fig. 5 indicated that the oxygen-laden MSC-hemo group could significantly improve the effect of chemotherapy, compared with the control group and the non-transformed MSC group (P<0.05). Furthermore, the non-transformed MSC+5-fu or MSC+CDDP groups demonstrated no significant difference with the control group (P>0.05). These results indicated that this method can successfully supply oxygen to the gastric cancer cells and enhance the effect of chemotherapeutics. This provides a novel method of thinking to reduce the resistance to chemotherapy of gastric cancer and improve the overall patient survival in clinical work. Furthermore, this is the initial step and this requires further study to test this method in vivo and in preclinical experiments.
To conclude, improving the tumor hypoxia environment could provide notable benefit for cancer treatments, particularly for gastric cancer. Using MSCs as a vehicle carrying oxygen to the tumor microenvironment is a direct and effective method. With the profound progress of biomedical science, targeted therapy had been improved in the technology and security aspects (37). The present study demonstrated the potential of MSCs as an effective delivery system that targets tumors and reduces the resistance of anticancer drugs. This may represent a prospective method for the treatment of gastric cancer types.
Acknowledgements
The authors would like to thank Professor Liang Qiao (University of Sydney, Sydney, Australia) for his suggestions.
Glossary
Abbreviations
- MSCs
mesenchymal stem cells
- IPTG
isopropyl-b-D-thiogalactopyranoside
- HIF-1
hypoxia inducible factor-1
- IFN-α
interferon-α
- IFN-γ
interferon-γ
- FBS
fetal bovine serum
- DMEM
Dulbecco's modified Eagle's medium
- CDDP
cisplatin
- 5-fu
5-fluorouracil
- GFP
green fluorescent protein
- MOI
multiplicity of infection
- ANOVA
one-way analysis of variance
- rHuEPO
recombinant human erythropoietin
Funding
This study was partly supported by the National Natural Science Foundation of China (grant no. 31270532).
Availability of data and materials
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
YL conceived and designed the study. YZ was a major contributor in acquiring data, data analysis and writing the manuscript. WH helped with the acquisition of data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Power DG, Kelsen DP, Shah MA. Advanced gastric cancer-slow but steady progress. Cancer Treat Rev. 2010;36:384–392. doi: 10.1016/j.ctrv.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Xuan Y, Hur H, Ham IH, Yun J, Lee JY, Shim W, Kim YB, Lee G, Han SU, Cho YK. Dichloroacetate attenuates hypoxia-induced resistance to 5-fluorouracil in gastric cancer through the regulation of glucose metabolism. Exp Cell Res. 2014;321:219–230. doi: 10.1016/j.yexcr.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Willers H, Azzoli CG, Santivasi WL, Xia F. Basic mechanisms of therapeutic resistance to radiation and chemotherapy in lung cancer. Cancer J. 2013;19:200–207. doi: 10.1097/PPO.0b013e318292e4e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown LM, Cowen RL, Debray C, Eustace A, Erler JT, Sheppard FC, Parker CA, Stratford IJ, Williams KJ. Reversing hypoxic cell chemoresistance in vitro using genetic and small molecule approaches targeting hypoxia inducible factor-1. Mol Pharmacol. 2006;69:411–418. doi: 10.1124/mol.105.015743. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo C, Han S, Liu J, Sun S, Han Z, et al. Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci. 2008;99:121–128. doi: 10.1111/j.1349-7006.2007.00643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hao J, Song X, Song B, Liu Y, Wei L, Wang X, Yu J. Effects of lentivirus-mediated HIF-1alpha knockdown on hypoxia-related cisplatin resistance and their dependence on p53 status in fibrosarcoma cells. Cancer Gene Ther. 2008;15:449–455. doi: 10.1038/cgt.2008.4. [DOI] [PubMed] [Google Scholar]
- 8.Nardinocchi L, Puca R, Sacchi A, D'Orazi G. Inhibition of HIF-1alpha activity by homeodomain-interacting protein kinase-2 correlates with sensitization of chemoresistant cells to undergo apoptosis. Mol Cancer. 2009;8:1. doi: 10.1186/1476-4598-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Semenza GL. Hypoxia, clonal selection, and the role of HIF-1 in tumor progression. Crit Rev Biochem Mol Biol. 2000;35:71–103. doi: 10.1080/10409230091169186. [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL. HIF-1 and tumor progression: Pathophysiology and therapeutics. Trends Mol Med. 2002;8(4 Suppl):S62–S67. doi: 10.1016/S1471-4914(02)02317-1. [DOI] [PubMed] [Google Scholar]
- 11.Mayer A, Vaupel P. Hypoxia, lactate accumulation, and acidosis: Siblings or accomplices driving tumor progression and resistance to therapy? Adv Exp Med Biol. 2013;789:203–209. doi: 10.1007/978-1-4614-7411-1_28. [DOI] [PubMed] [Google Scholar]
- 12.Wafik SE, editor. Impact of genetic targets on cancer therapy. Springer; New York: 2013. [Google Scholar]
- 13.Serakinci N, Fahrioglu U, Christensen R. Mesenchymal stem cells, cancer challenges and new directions. Eur J Cancer. 2014;50:1522–1530. doi: 10.1016/j.ejca.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Ren C, Kumar S, Chanda D, Chen J, Mountz JD, Ponnazhagan S. Therapeutic potential of mesenchymal stem cells producing interferon-alpha in a mouse melanoma lung metastasis model. Stem Cells. 2008;26:2332–2338. doi: 10.1634/stemcells.2008-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Lu Y, Huang W, Xu H, Chen X, Geng Q, Fan H, Tan Y, Xue G, Jiang X. In vitro effect of adenovirus-mediated human Gamma Interferon gene transfer into human mesenchymal stem cells for chronic myelogenous leukemia. Hematol Oncol. 2006;24:151–158. doi: 10.1002/hon.779. [DOI] [PubMed] [Google Scholar]
- 16.Gao P, Ding Q, Wu Z, Jiang H, Fang Z. Therapeutic potential of human mesenchymal stem cells producing IL-12 in a mouse xenograft model of renal cell carcinoma. Cancer Lett. 2010;290:157–166. doi: 10.1016/j.canlet.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Seo SH, Kim KS, Park SH, Suh YS, Kim SJ, Jeun SS, Sung YC. The effects of mesenchymal stem cells injected via different routes on modified IL-12-mediated antitumor activity. Gene Ther. 2011;18:488–495. doi: 10.1038/gt.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grisendi G, Bussolari R, Cafarelli L, Petak I, Rasini V, Veronesi E, De Santis G, Spano C, Tagliazzucchi M, Barti-Juhasz H, et al. Adipose-derived mesenchymal stem cells as stable source of tumor necrosis factor-related apoptosis-inducing ligand delivery for cancer therapy. Cancer Res. 2010;70:3718–3729. doi: 10.1158/0008-5472.CAN-09-1865. [DOI] [PubMed] [Google Scholar]
- 19.Zhang B, Shan H, Li D, Li ZR, Zhu KS, Jiang ZB. The inhibitory effect of MSCs expressing TRAIL as a cellular delivery vehicle in combination with cisplatin on hepatocellular carcinoma. Cancer Biol Ther. 2012;13:1175–1184. doi: 10.4161/cbt.21347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan WT, Verma CS, Lane DP, Gan SK. A comparison and optimization of methods and factors affecting the transformation of Escherichia coli. Biosci Rep. 2013;33(pii):e00086. doi: 10.1042/BSR20130098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ji RC. Hypoxia and lymphangiogenesis in tumor microenvironment and metastasis. Cancer Lett. 2014;346:6–16. doi: 10.1016/j.canlet.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29:297–307. doi: 10.1016/S0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 23.Bubnovskaya L, Osinsky D, Trachevsky V, Naleskina L, Kovelskaya A, Gumenyuk L. Premorphological alterations in gastric mucosa in patients with gastric cancer: Hypoxia level assessed by 31P NMR spectroscopy. Exp Oncol. 2014;36:271–275. [PubMed] [Google Scholar]
- 24.Höckel M, Vaupel P. Tumor hypoxia: Definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 25.Teicher BA. Hypoxia and drug resistance. Cancer Metastasis Rev. 1994;13:139–168. doi: 10.1007/BF00689633. [DOI] [PubMed] [Google Scholar]
- 26.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura J, Kitajima Y, Kai K, Hashiguchi K, Hiraki M, Noshiro H, Miyazaki K. HIF-1alpha is an unfavorable determinant of relapse in gastric cancer patients who underwent curative surgery followed by adjuvant 5-FU chemotherapy. Int J Cancer. 2010;127:1158–1171. doi: 10.1002/ijc.25129. [DOI] [PubMed] [Google Scholar]
- 28.Di Caprio G, Stokes C, Higgins JM, Schonbrun E. Single-cell measurement of red blood cell oxygen affinity. Proc Natl Acad Sci USA. 2015;12:9984–9989. doi: 10.1073/pnas.1509252112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dicato M, Duham C, Berchem G, Ries F. Clinical benefit from erythropoietin. Curr Opin Oncol. 2000;12:297–302. doi: 10.1097/00001622-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 30.Väänänen HK. Mesenchymal stem cells. Ann Med. 2005;37:469–479. doi: 10.1080/07853890500371957. [DOI] [PubMed] [Google Scholar]
- 31.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: Environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. doi: 10.1038/emm.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loebinger MR, Kyrtatos PG, Turmaine M, Price AN, Pankhurst Q, Lythgoe MF, Janes SM. Magnetic resonance imaging of mesenchymal stem cells homing to pulmonary metastases using biocompatible magnetic nanoparticles. Cancer Res. 2009;69:8862–8867. doi: 10.1158/0008-5472.CAN-09-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasportas LS, Kasmieh R, Wakimoto H, Hingtgen S, van de Water JA, Mohapatra G, Figueiredo JL, Martuza RL, Weissleder R, Shah K. Assessment of therapeutic efficacy and fate of engineered human mesenchymal stem cells for cancer therapy. Proc Natl Acad Sci USA. 2009;106:4822–4827. doi: 10.1073/pnas.0806647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang B, Wu X, Mao Y, Bao W, Gao L, Zhou P, Xie R, Zhou L, Zhu J. Dual-targeted antitumor effects against brainstem glioma by intravenous delivery of tumor necrosis factor-related, apoptosis-inducing, ligand-engineered human mesenchymal stem cells. Neurosurgery. 2009;65:610–624. doi: 10.1227/01.NEU.0000350227.61132.A7. [DOI] [PubMed] [Google Scholar]
- 35.Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: Implications in transplantation. Transplantation. 2003;75:389–397. doi: 10.1097/01.TP.0000045055.63901.A9. [DOI] [PubMed] [Google Scholar]
- 36.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, Risso M, Gualandi F, Mancardi GL, Pistoia V, Uccelli A. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107:367–372. doi: 10.1182/blood-2005-07-2657. [DOI] [PubMed] [Google Scholar]
- 37.Tsimberidou AM. Targeted therapy in cancer. Cancer Chemother Pharmacol. 2015;76:1113–1132. doi: 10.1007/s00280-015-2861-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the present study are available from the corresponding author on reasonable request.