Abstract
Trophoblast cell surface antigen 2 (TROP2), a single transmembrane domain protein, is often found to be highly expressed in various types of human cancers. However, the biological function and molecular mechanism of TROP2 in glioblastoma have not been fully elucidated, particularly in regards to cell proliferation and metastasis of glioblastoma cells. In the present study, it was demonstrated that TROP2 expression was increased in glioblastoma tissues and glioblastoma cell lines by immunohistochemical analysis and western blot analysis. High TROP2 expression was significantly correlated with the poor survival of glioblastoma patients. MTT assay, BrdU incorporation assay, flow cytometry and Transwell assay were performed to demonstrate that knockdown of TROP2 in glioblastoma cells inhibited cell proliferation and metastasis. We found that the effects of TROP2-knockdown on glioblastoma cells were associated with the inhibition of JAK2 and STAT3 phosphorylation and decreased transcription of STAT3 target genes. In addition, blocking the activation of JAK2/STAT3 signaling by WP1066 negated the effects of TROP2 overexpression. Furthermore, exogenous IL-6, which functions as a potent activator of JAK2/STAT3 signaling, was able to rescue the phosphorylation of JAK2 and STAT3 in TROP2-silenced glioblastoma cells and regulate phenotypic changes in these cells. Therefore, we revealed a novel mechanism by which TROP2 activates the JAK2/STAT3 pathway to promote the growth and metastasis of glioblastoma cells. These data offer insight into the function of TROP2 in glioblastoma and indicate that TROP2 is a promising biomarker and therapeutic target for glioblastoma patients.
Keywords: TROP2, JAK2/STAT3, glioblastoma, proliferation, metastasis
Introduction
Malignant glioma is the most common and aggressive type of primary brain tumor in humans, accounting for more than 44% of all brain tumors in China and 50% of all primary central nervous system (CNS) neoplasms in the US (1). Glioblastoma, grade IV glioma, is notorious for its rapid proliferation, intensive infiltration, genetic alteration and increased angiogenesis, which makes it the most malignant glioma accounting for more than 46% of all malignant brain cancers (2–5). Despite enormous efforts in multimodal treatment approaches including typical surgical tumor resection, radiotherapy and chemotherapy, the prognosis of glioblastoma patients is undeniably grim with only a median survival time of 12–15 months (6,7). Perhaps the dismal prognosis is mainly due to the limited understanding of the molecular basis of glioblastoma development and progression. Thus, further research of the molecular mechanisms underlying glioblastoma and a thorough investigation of molecular biomarkers for prognosis and targeted therapy are crucially needed to improve glioblastoma patient outcomes.
Human trophoblast cell surface antigen 2 (TROP2), a 36-kDa transmembrane protein expressed primarily in epithelial cells and encoded by the TACSTD2 gene, was originally discovered in human placental trophoblastic tissue (8). TROP2, as a cell surface receptor, can recognize specific ligands and participate in numerous intracellular signaling pathways of cell proliferation, self-renewal and survival (9–11). Recently, it has received attention as a potential target for cancer therapy since high TROP2 expression was found to be positively associated with poor patient prognosis (12). Overexpression of TROP2 was found in several human carcinomas, including colorectal (13), lung cancer (14), glioma (15), gastric carcinoma (16), cervical (17), breast (18) and pancreatic cancer (19), underscoring the potential role of TROP2 in tumorigenesis. However, the precise mechanism and biological function of TROP2 expression in relation to glioblastoma have not been studied.
Interleukin-6 (IL-6), a well-characterized proinflammatory cytokine, has been shown to play a crucial role in driving many of the ‘hallmarks’ of cancer including activation of the signaling of cell proliferation, enhancement of migration and invasion, resistance to cell death and induction of angiogenesis through downstream activation of the Janus kinase (JAK)/signal transducer and activator of transcription 3 (STAT3) signaling pathway (20–25). Persistent activation of JAK2/STAT3 is frequently documented in human carcinomas and has emerged as an ideal target for cancer treatment (26–28). Therefore, tight regulation of the JAK2/STAT3 pathway may be effective in therapy against cancer.
In the present study, we found that downregulation of TROP2 efficiently inhibited glioblastoma cell proliferation and metastasis by regulating the JAK2/STAT3 pathway. These data offer insights into TROP2 function and suggest TROP2 as a potential target for glioblastoma patients.
Materials and methods
Cell culture and transfection
Human normal astroglia cells (SVGP12), glioblastoma cell lines [A172, LN-229, U-87 MG (cat. no. HTB-14 of unknown origin) and U-118 MG and a retroviral packaging cell line (293FT) were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured as previously described (29). All cell lines tested mycoplasma-negative. The sequences of the TROP2 short hairpin RNA (shTROP2) and GFP short hairpin RNA (shGFP) were purchased from GenePharma Co., Ltd. (Shanghai, China). Sequences of shTROP2 are provided in Table I. A vector encoding the human full-length TROP2 was cloned by PCR-based amplification and then subcloned into the pCDH-CMV-EF1-copGFP vector. Sequences of the primers used are given in Table II. The lentivirus was produced as previously described (30).
Table I.
Sequences of the TROP2-specific shRNAs.
| shTROP2-1-F | CCGGCTACTTCGAGAGGGACATCAACTCGAGTTGATGTCCCTCTCGAAGTAGTTTTTG |
| shTROP2-1-R | AATTCAAAAACTACTTCGAGAGGGACATCAACTCGAGTTGATGTCCCTCTCGAAGTAG |
| shTROP2-2-F | CCGGGAGAAAGGAACCGAGCTTGTACTCGAGTACAAGCTCGGTTCCTTTCTCTTTTTG |
| shTROP2-2-R | AATTCAAAAAGAGAAAGGAACCGAGCTTGTACTCGAGTACAAGCTCGGTTCCTTTCTC |
TROP2, trophoblast cell surface antigen 2; shRNA, short hairpin RNA; F, forward; R, reverse.
Table II.
Primer pairs for human full-length TROP2.
| TROP2-F | ATGGCTCGGGGCCCC |
| TROP2-R | CTACAAGCTCGGTTCCTTTCTCA |
TROP2, trophoblast cell surface antigen 2; F, forward; R, reverse.
Reagents
Gibco™ fetal bovine serum (FBS) and Dulbecco's modified Eagle's medium (DMEM) were purchased from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Dimethyl sulfoxide (DMSO) and crystal violet were obtained from Sigma-Aldrich/Merck KGaA (Darmstadt, Germany). WP1066 was obtained from Selleck (Shanghai, China). The TROP2 (cat. no. ab214488) antibody, survivin (cat. no. ab76424) antibody and recombinant human IL-6 protein (cat. no. ab73197) were purchased from Abcam (Shanghai, China); phospho-JAK2 (Tyr1007/1008; cat. no. 3771), JAK2 (cat. no. 3230), phospho-STAT3 (Tyr705; cat. no. 9145), STAT3 (cat. no. 9139), cyclin D1 (cat. no. 2978), MMP2 (cat. no. 40994) and VEGF (cat. no. 9698) antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). GAPDH antibody was purchased from BD Pharmingen (Shanghai, China). All antibodies were diluted according to the instructions.
Immunohistochemical staining
Formalin-fixed and paraffin-embedded tumor specimens from patients were sliced into 5-µm thick sections that were then deparaffinized and hydrated. The procedure of endogenous antigen retrieval was performed via microwave heating for 20 min in 10 mM citric acid buffer (pH 6.0). The sections were incubated sequentially with primary antibodies overnight at 4°C and secondary antibodies for 30 min at room temperature followed by diaminobenzidine (DAB) development. All slides were examined and assessed from microscopic fields at ×20 magnification (Olympus CKX41; Olympus Corp., Tokyo, Japan). Immunohistochemical staining results were reviewed and scored as follows: 0, no staining and no background; 1+, weak staining in more than 30% of cells; 2+, moderately intense staining in more than 30% of cells but without intense staining in some cells; and 3+, staining in more than 30% of tumor cells with markedly intense staining.
Western blot analysis
Cells and fresh tissues were lysed in RIPA lysis buffer that contained a protease inhibitor cocktail. Proteins were harvested and then separated by SDS-PAGE and electrotransferred onto PVDF membranes. The membranes were incubated sequentially with primary antibodies overnight at 4°C and HRP-linked secondary antibody for 2 h at room temperature, and then visualized by a detection system (Clinx Science Instruments Co., Ltd., Shanghai, China).
Quantitative real-time PCR
Total RNA was extracted from glioblastoma cell lines using Invitrogen™ TRIzol reagent (Thermo Fisher Scientific) and then reverse transcribed into cDNA using GoScript™ Reverse Transcriptase (Promega, Madison, WI, USA). Real-time PCR was performed using specific primer pairs, cDNA and SYBR Green PCR Master Mix (Toyobo Life Science, Inc., Osaka, Japan) according to the manufacturer's instructions. All primer pairs are listed in Table III and glycerol-dehyde-3-phosphate dehydrogenase (GAPDH) served as the endogenous control. Relative quantification of mRNA expression was defined based on Cq, and the individual values were normalized to that of the GAPDH control (31).
Table III.
Primer pairs for real-time PCR.
| TROP2-F | ACAACGATGGCCTCTACGAC |
| TROP2-R | GTCCAGGTCTGAGTGGTTGAA |
| CCND1-F | GCTGCGAAGTGGAAACCATC |
| CCND1-R | CCTCCTTCTGCACACATTTGAA |
| Survivin-F | AGGACCACCGCATCTCTACAT |
| Survivin-R | AAGTCTGGCTCGTTCTCAGTG |
| MMP2-F | GATACCCCTTTGACGGTAAGGA |
| MMP2-R | CCTTCTCCCAAGGTCCATAGC |
| VEGF-F | AGGGCAGAATCATCACGAAGT |
| VEGF-R | AGGGTCTCGATTGGATGGCA |
| GAPDH-F | ACAACTTTGGTATCGTGGAAGG |
| GAPDH-R | GCCATCACGCCACAGTTTC |
TROP2, trophoblast cell surface antigen 2; CCND1, cyclin D1, MMP2, matrix metallopeptidase 2; VEGF, vascular endothelial growth factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; F, forward; R, reverse.
Cell proliferation analysis
Cell viability was examined using the MTT assay. Cells (1×103 cells/well) were seeded in 96-well plates with three replicates, and then were detected from day 0 to 6. The purple formazan was dissolved by dimethyl sulfoxide, and the absorbance was measured at a wavelength of 560 nm. All experiments were carried out independently in triplicate.
BrdU incorporation assay
Cells (2×104 cells/well) were seeded into 24-well plates and incubated overnight. Then a final concentration of 10 µg/ml BrdU (Sigma-Aldrich; Merck KGaA) was added into the media and the plates were incubated for 30 min and then fixed for 15 min. After incubation with 1 mol/l HCl for 15 min and 5% goat serum for 2 h, the cells were incubated sequentially with primary antibody overnight at 4°C and secondary antibody for 2 h at room temperature. Cell nuclei were stained with DAPI for 30 min. The BrdU-positive cells were observed and calculated from microscopy fields at ×20 magnification (Nikon 80i; Nikon Corp., Tokyo, Japan).
Flow cytometry
For cell cycle analysis, cells were harvested and then fixed in 75% ethanol at 4°C for 24 h, and then incubated with propidium iodide (PI) and RNaseA at 37°C for 30 min. Cells were examined by flow cytometry (BD Biosciences, San Jose, CA, USA) and analyzed by FlowJo software (version FlowJo 7.6; FlowJo LLC, Ashland, OR, USA).
TUNEL analysis
Cells were grown on coverslips and then stained with Click-iT plus TUNEL assay kit (Invitrogen; Thermo Fisher Scientific). After incubation with 4% paraformaldehyde for 20 min and 0.25% Triton X-100 for 15 min, the cells were incubated sequentially with TdT reaction buffer, TdT reaction mixture and TUNEL reaction cocktail according to the manufacturer's instructions. Cell nuclei were stained with DAPI for 30 min. Cells were imaged and calculated from randomly chosen microscopic fields at ×20 magnification (Nikon 80i; Nikon Corp.).
Migration and invasion assays
Boyden chambers (24-well) (8-µm pore size; Corning Inc., Corning, NY, USA) were used in the migration and invasion assays. Matrigel (BD Biosciences) was used to coat the membranes in the invasion assay. Medium with 10% FBS as a chemoattractant was added to the lower chamber, and cells with serum-free media were placed in the upper chamber. After incubation for 48 h, the cells were fixed in 4% paraformaldehyde (PFA) and then stained with crystal violet. Cells were imaged and calculated from randomly chosen microscopic fields at ×10 magnification (Nikon 80i; Nikon Corp.).
Soft agar assay
To evaluate colony-forming ability, Gibco™ DMEM 2X (Thermo Fisher Scientific) containing 0.6% agarose (Sigma-Aldrich; Merck KGaA) were added to 12-well plates as a bottom layer. Then 0.4×103 cells/well in medium (10% FBS and DMEM) containing 0.3% agarose (Sigma-Aldrich; Merck KGaA) was added onto the bottom layer. After culturing for 14–21 days, colonies were imaged by the scanner (Epson Perfection V700 Photo; Epson, Beijing, China) and counted in each well.
Xenograft assay
The detailed procedures were performed as described previously (7). All studies were approved by the Animal Care and Use Committee of Southwest University, and carried out in conformity to the Guide for the Care and Use of Laboratory Animals (Ministry of Science and Technology of China, 2006).
Patient data analysis and patient tumor tissues
Kaplan-Meier analysis and survival curves were downloaded from the online database R2: microarray analysis and visualization platform (http://hgserver1.amc.nl/cgi-bin/r2/main.cgi). A total of 8 patients (5 males and 3 females, age range, 32–82, mean age 53.1) who underwent surgical resection between February 2015 and July 2015 at Daping Hospital, Chongqing were enrolled and tumor and adjacent normal tissues were collected. Prior approval was obtained from the Ethics Committee of Daping Hospital, Chongqing, China. Tissue analysis was approved by the Ethics Committee of Southwest University of China. All of the patients provided informed consent.
Statistical analysis
All experiments were carried out in triplicates, and the quantitative data are expressed as mean ± SD. Two-tailed Student's t-test was performed to calculate significance in an interval of 95% confidence level, following normal distribution with different but similar SDs. A probability (P) value of <0.05 was considered statistically significant. *P<0.05, **P<0.01, ***P<0.001 indicate different degrees of statistical significance as noted in the figures.
Results
TROP2 is upregulated in human glioblastoma and is a prognostic indicator for glioblastoma patients
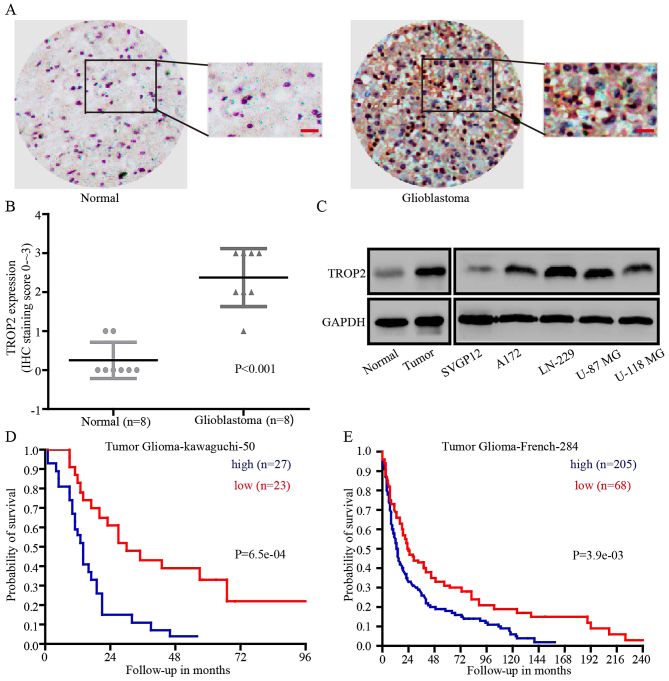
To explore whether TROP2 could be a prognostic marker for glioblastoma, immunohistochemical analysis (IHC) was performed. IHC revealed that TROP2 expression was significantly higher in glioblastoma tissues compared with that noted in the normal brain tissues in 8 paired samples (Fig. 1A and B). Then, we detected TROP2 expression at the protein level in human normal astroglial cells (SVGP12), glioblastoma cell lines (A172, LN-229, U-87 MG and U-118 MG), normal tissues and glioblastoma tissues. Compared with SVGP12 cells and normal tissues, TROP2 expression was significantly increased in all four glioblastoma cell lines and tumor tissues (Fig. 1C). To determine whether TROP2 expression level is implicated in the clinical prognosis of glioblastoma patients, we evaluated the prognostic value of TROP2 in the Tumor Glioma Kawaguchi 50 database and Tumor Glioma French 284 database from the R2 platform (genomics analysis and visualization platform). The results showed that high TROP2 expression was associated with a poor patient outcome (Fig. 1D and E). Taken together, we found that TROP2 was upregulated in glioblastoma tumors and glioblastoma cell lines, and high TROP2 expression was associated with the poor prognosis of glioblastoma patients.
Figure 1.
TROP2 is upregulated in human glioblastoma and is a prognostic indicator for glioblastoma patients. (A) Representative immunohistochemical assays of TROP2 expression in human glioblastoma (right) and adjacent normal brain tissue (left). (B) Immunohistochemical analyses of TROP2 expression in 8 paired samples of glioblastoma and normal brain tissue, P<0.001. (C) Western blot analyses were performed to detect TROP2 expression in human normal astroglial cells (SVGP12), four glioblastoma cell lines (A172, LN-229, U-87 MG and U-118 MG), normal brain tissues and glioblastoma tissues. (D and E) Kaplan-Meier analysis of progression-free survival using data from the Tumor Glioma-kawaguchi-50 database and Tumor Glioma-French-284 database with the log-rank test P-values indicated. TROP2, trophoblast cell surface antigen 2.
TROP2 promotes the migration and invasion of glioblastoma cells
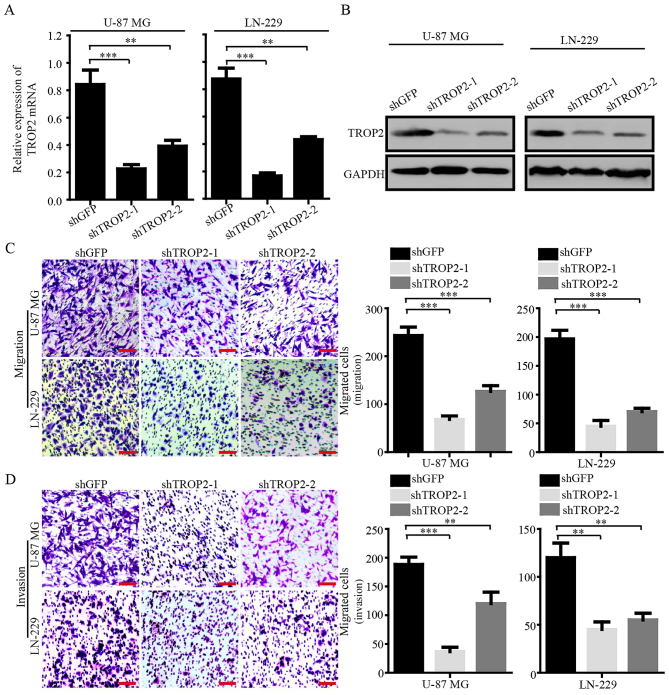
To explore the biological role of TROP2 in glioblastoma cells, we knocked down TROP2 by using two shRNA sequences (shTROP2-1 and −2) in glioblastoma cell lines U-87 MG and LN-229. Quantitative RT-PCR and western blot analysis were conducted to confirm stable knockdown of TROP2 expression in U-87 MG and LN-229 cells (Fig. 2A and B). Migration and invasion assays were performed to determine whether the expression of TROP2 was related to the metastasis of glioblastoma cells. The results showed that TROP2 knockdown significantly inhibited cell migration and invasion compared to the control cells (Fig. 2C and D). These results demonstrated that TROP2 expression is positively associated with the cell migration and invasion of glioblastoma cells.
Figure 2.
TROP2 promotes migration and invasion of glioblastoma cells. (A) The expression of TROP2 mRNA in TROP2-knockdown U-87 MG and LN-229 cell lines and negative controls were detected by PCR analysis. GAPDH served as endogenous control. (B) The expression of TROP2 protein in TROP2-knockdown U-87 MG and LN-229 cell lines and negative controls were detected by western blot analysis. GAPDH served as loading control. (C) Migration and (D) invasion assays were performed in TROP2-knockdown U-87 MG and LN-229 cells and negative controls. All data are shown as the means ± SD; **P<0.01, ***P<0.001. All P-values are based on the control vs. treatment group. TROP2, trophoblast cell surface antigen 2.
TROP2 enhances proliferation and viability of glioblastoma cells
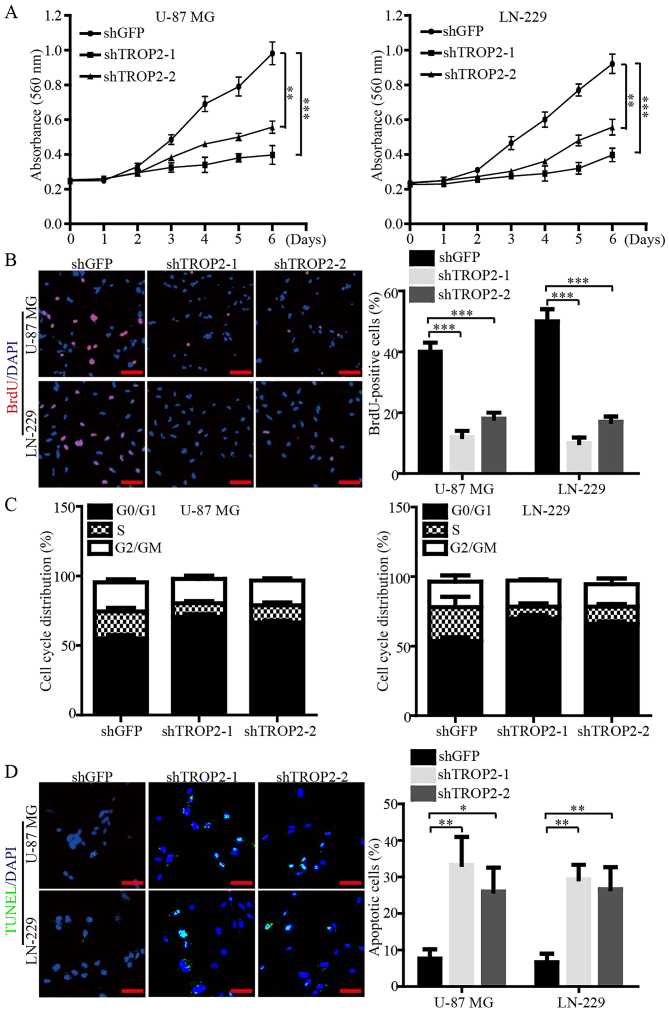
We subsequently investigated the biological role of TROP2 in cell proliferation and viability of glioblastoma cells via MTT and BrdU assays. The data demonstrated that the cell growth curve and DNA synthesis of TROP2-knockdown cells were significantly reduced when compared to the control cells (Fig. 3A and B). Flow cytometry was performed to corroborate the data of proliferation, and the results showed that TROP2 knockdown led to cell cycle arrest at the G1 phase (Fig. 3C). In addition, immunofluorescence (IF) assays of TUNEL were performed to validate that TROP2 knockdown significantly increased the cell apoptosis of both U-87 MG and LN-229 cells (Fig. 3D). Collectively, these data demonstrated that TROP2 potently promotes proliferation and viability of glioblastoma cells.
Figure 3.
TROP2 enhances proliferation and viability of glioblastoma cells. (A) Glioblastoma cell proliferation was measured by MTT assay. (B) BrdU incorporation assay was performed to examine the percentage of cells in S-phase. (C) Flow cytometric analysis of cell cycle distribution in U-87 MG and LN-229 cell lines. (D) Representative images of Tunnel assay for U-87 MG and LN-229 cell lines. All data are shown as the means ± SD; *P<0.05, **P<0.01, ***P<0.001. All P-values are based on control vs. treatment group. TROP2, trophoblast cell surface antigen 2.
TROP2 promotes self-renewal and tumor growth of glioblastoma cells in vitro and in vivo
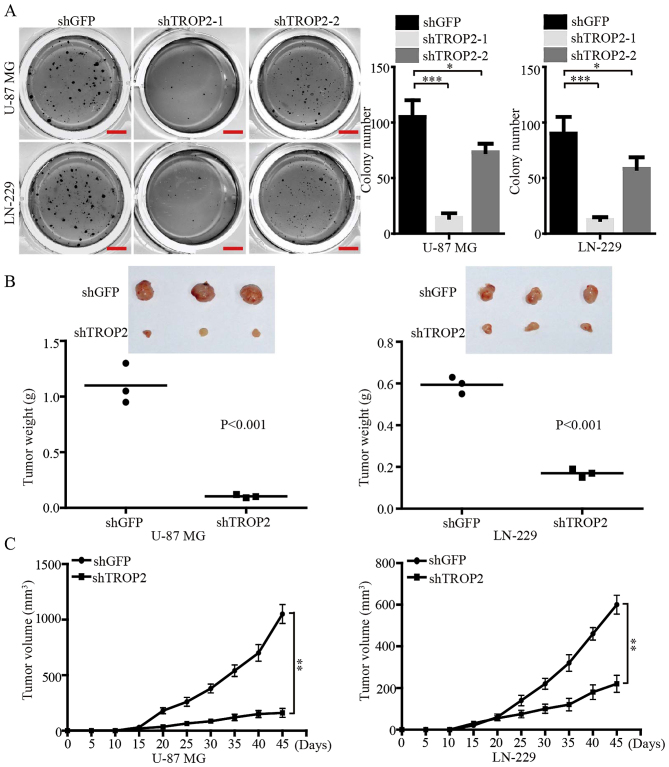
Soft agar colony formation assays were performed to investigate the effects of TROP2 expression on self-renewal of glioblastoma cells in vitro. The results demonstrated that the number and size of colonies in the TROP2-konckdown U-87 MG and LN-229 cells were reduced compared with the control cells (Fig. 4A). We also found that knockdown of TROP2 using shTROP2-1 exhibited a more powerful inhibitory effect on the colony formation of glioblastoma cells. Therefore, the highly effective shTROP2-1 was used as a representative TROP2-knockdown means for the following experiments. In order to confirm the effects of TROP2 in vivo, a subcutaneous xenograft experiment was performed. Consistent with the in vitro results, the growth of tumors derived from TROP2-knockdown cells was significantly retarded compared with the controls. At the study end point, the knockdown of TROP2 resulted in significantly decreased tumor weight and volume compared with the relevant controls (Fig. 4B and C). Taken together, these data suggest that TROP2 potently promotes the tumor growth of glioblastoma cells.
Figure 4.
TROP2 promotes self-renewal and tumor growth of glioblastoma cells in vitro and in vivo. (A) The effects of TROP2 on the colony formation in TROP2-knockdown U-87 MG and LN-229 cells. (B) Xenograft assays were performed after TROP2 knockdown in U-87 MG and LN-229 cells. The size and weight of xenograft tumors were analyzed with two-tailed Student's t-test and P-value is indicated. (C) The growth curve of xenograft tumors was analyzed with with two-tailed Student's t-test and P-value is indicated. All data are shown as the means ± SD; *P<0.05, **P<0.01, ***P<0.001. All P-values are based on control vs. treatment group. TROP2, trophoblast cell surface antigen 2.
TROP2 mediates oncogenic effects by promoting the activation of the JAK2/STAT3 pathway
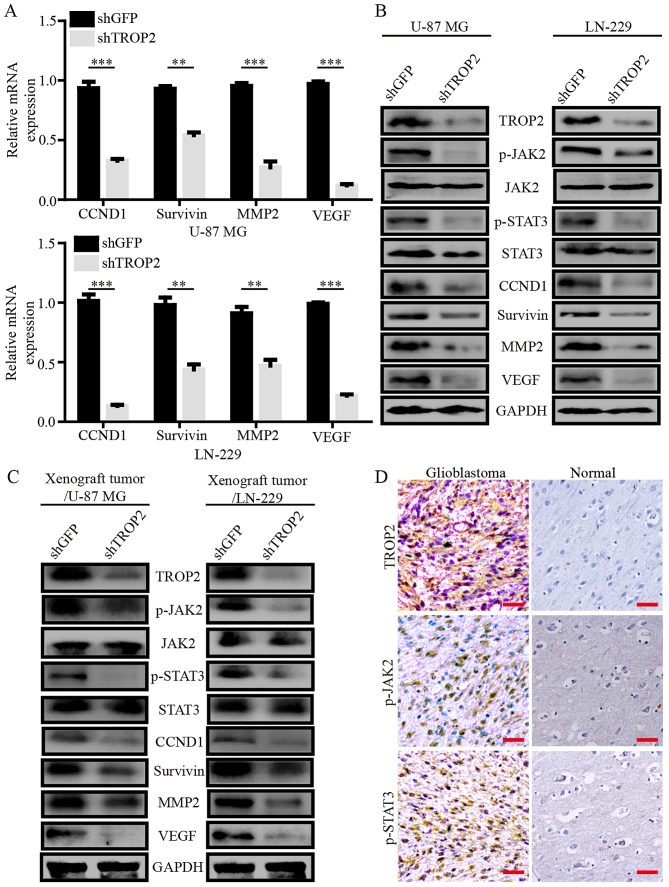
To explore the mechanisms underlying changes in TROP2-knockdown glioblastoma cells, we performed quantitative RT-PCR assays to compare the mRNA expression pattern of TROP2-knockdown glioblastoma cells with the control cells. TROP2 knockdown attenuated the expression of several genes involved in cell proliferation, apoptosis, migration and invasion, including cyclin D1 (CCND1), survivin, matrix metalloproteinase 2 (MMP2) and vascular endothelial growth factor (VEGF), which are generally considered as downstream molecules of the JAK2/STAT3 signaling pathway (Fig. 5A). Next, western blot analysis showed decreased phosphorylation of JAK2Tyr1007/1008 and STAT3Tyr705 in the TROP2-knockdown glioblastoma cells and xenograft tumors. CCND1, survivin, MMP2 and VEGF expression levels were observably decreased in the TROP2-knockdown glioblastoma cells and xenograft tumors (Fig. 5B and C). To determine the clinical relevance, we carried out immunohistochemical analysis for level of TROP2 and phosphorylation of JAK2Tyr1007/1008 and STAT3Tyr705 in human GBM specimens and normal brain tissues. The GBM samples had high TROP2 expression, which correlated with high JAK2 and STAT3 activation (Fig. 5D). These results suggest that TROP2 promotes cell proliferation and metastasis of glioblastoma cells by activating the JAK2/STAT3 pathway.
Figure 5.
TROP2 mediates oncogenic effects by promoting the activation of the JAK2/STAT3 pathway. (A) Relative mRNA expression of STAT3 target genes in TROP2-knockdown or control U-87 MG cells and LN-229 cells. Transcript levels were normalized to GAPDH. (B and C) Western blot analysis of JAK2Tyr1007/1008, total JAK2, STAT3Tyr705, total STAT3, CCND1, survivin, MMP2 and VEGF levels in TROP2-silenced U-87 MG and LN-229 cells and xenograft tumors, GAPDH was used as loading control. (D) Representative IHC staining for TROP2, p-JAK2, and p-STAT3 in GBM samples from patients. All data are shown as the means ± SD; **P<0.01, ***P<0.001. All P-values are based on control vs. treatment group. TROP2, trophoblast cell surface antigen 2; GBM, glioblastoma.
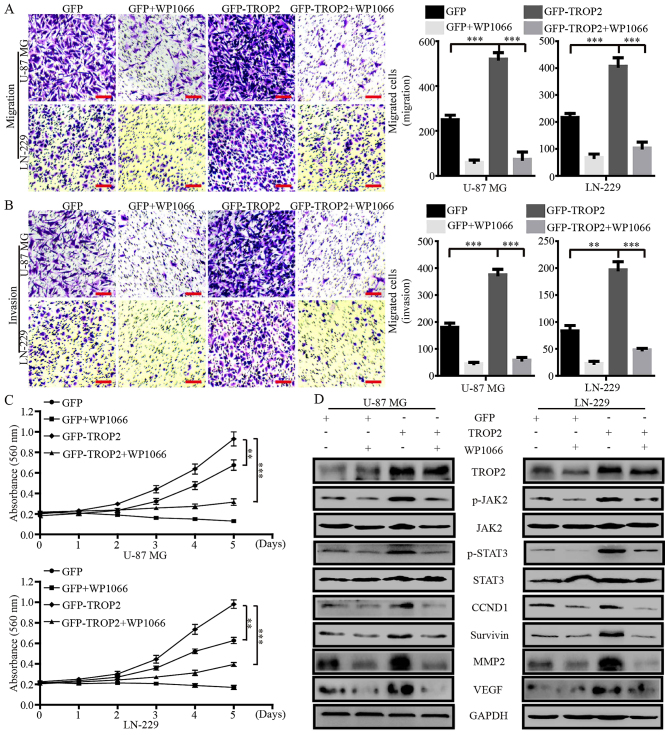
Blocking the activation of JAK2/STAT3 signaling by WP1066 negates the effects of TROP2 overexpression
WP1066 is a potent JAK2/STAT3 inhibitor that inhibits the proliferation and metastasis of cancer cells. To confirm whether the effects of TROP2 on glioblastoma cells were JAK2/STAT3 dependent, we treated TROP2-overexpressing glioblastoma cells and their controls cells with WP1066 (10 µM). Migration and invasion assays showed that WP1066 significantly decreased the accelerative effects of TROP2 on cell migration and invasion (Fig. 6A and B). MTT assays showed that WP1066 significantly reduced the promotive effects of TROP2 on cell proliferation (Fig. 6C). Western blot analysis demonstrated that the levels of JAK2 and STAT3 phosphorylation (JAK2Tyr1007/1008 and STAT3Tyr705) were altered in proportion to the alteration of total JAK2 and STAT3 protein levels in glioblastoma cells. In addition, CCND1, survivin, MMP2 and VEGF levels were markedly increased by TROP2 overexpression and were reduced by WP1066 treatment (Fig. 6D). Collectively, these data demonstrated that TROP2 promotes the activation of the JAK2/STAT3 signaling pathway.
Figure 6.
Blocking the activation of JAK2/STAT3 signaling by WP1066 negates the effects of TROP2 overexpression. (A and B) Migration and invasion assays were performed to characterize the inhibitory effects of WP1066 on the migration and invasion of TROP2-overexpressing U-87 MG and LN-229 cells. (C) MTT assay was performed to characterize the inhibitory effects of WP1066 on the proliferation of TROP2-overexpressing cells. (D) Protein expression levels of TROP2, JAK2Tyr1007/1008, JAK2, STAT3Tyr705, STAT3, CCND1, survivin, MMP2 and VEGF were analyzed by western blot analysis. All data are shown as the means ± SD; **P<0.01, ***P<0.001. All P-values are based on control vs. treatment group. TROP2, trophoblast cell surface antigen 2.
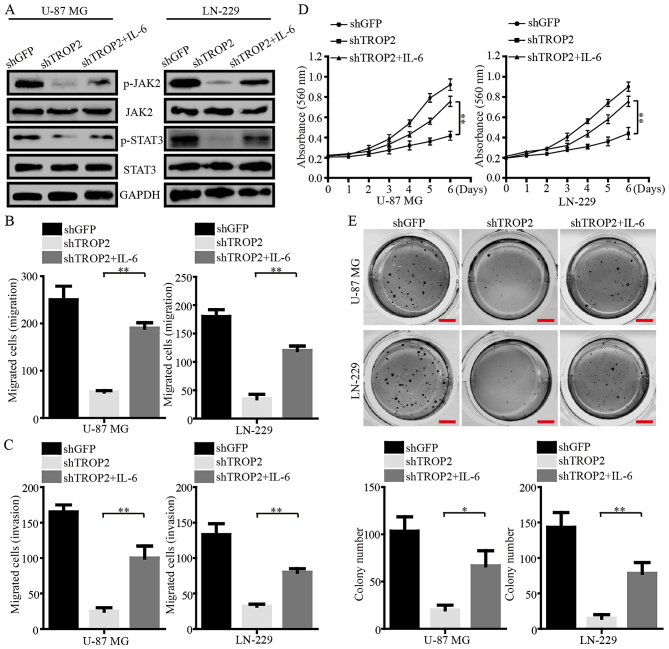
Exogenous IL-6 reverses phenotype changes of TROP2-silencing glioblastoma cells
IL-6, a potent activator of JAK2/STAT3, was used to further determine the role of TROP2 in the regulation of the JAK2/STAT3 pathway. Western blot assays demonstrated that the phosphorylation of JAK2Tyr1007/1008 and STAT3Tyr705 were increased in TROP2-knockdown glioblastoma cells after the cells were exposed to IL-6 (Fig. 7A). The migration and invasion of TROP2-knockdown cells were clearly increased after IL-6 exposure (Fig. 7B and C). In addition, MTT assays showed that cell viability was increased after IL-6 exposure (Fig. 7D). Furthermore, IL-6 exposure led to larger and more numerous colonies formed by TROP2-silenced U-87 MG and LN-229 cells than the colonies formed by these cells without exposure to IL-6 (Fig. 7E). Collectively, these data strongly suggest that TROP2 functions in an oncogenic role in glioblastoma by activating the JAK2/STAT3 signaling pathway.
Figure 7.
Exogenous IL-6 reverses phenotype changes of TROP2-silenced glioblastoma cells. (A) Western blot analysis of JAK2Tyr1007/1008, total JAK2, STAT3Tyr705 and total STAT3 levels in control cells and TROP2-knockdown cells with or without IL-6 exposure. GAPDH was used as loading control. (B and C) Qualification of the data for migration and invasion assays in control cells and TROP2-knockdown cells with or without IL-6 exposure. (D) MTT assays were performed to examine cell viability of control cells and TROP2-knockdown cells with or without IL-6 exposure. (E) Representative images of colony formation assay for control cells and TROP2-knockdown cells with or without IL-6 exposure. All data are shown as the means ± SD; *P<0.05, **P<0.01. All P-values are based on control vs. treatment group. IL-6, interleukin 6; TROP2, trophoblast cell surface antigen 2.
Discussion
Glioblastoma is the most common and aggressive malignant cancer, exhibiting high rates of proliferation and infiltration. Despite advances in multimodal treatments involving surgical resection, chemotherapy and radiotherapy, patients with glioblastoma have a median overall survival (OS) that rarely exceeds two years, and the rate of 5-year survival is <5% (32). Molecular-targeted therapy has become a promising method in cancer treatment, especially for gastric and lung cancer (33,34). However, a lack of suitable molecular-targeted drugs is perhaps the key challenge for combating glioblastoma. TROP2, a single transmembrane domain protein, has been found to be highly expressed in various human carcinomas, including breast cancer, lung cancer, gastric cancer, cervical cancer, ovarian cancer and nasopharyngeal carcinoma (35). Previous studies concerning the biological roles of TROP2 in human carcinomas were mainly focused on the adhesion between cancer cells, and were also involved in cell signal transduction and the growth of cancer cells (11). However, the biological function and molecular mechanism of TROP2 in glioblastoma have not been fully elucidated, especially in regards to the cell proliferation and metastasis of glioblastoma cells.
In the present study, we first used immunohistochemistry and western blot analysis to assess the TROP2 protein pattern in glioblastoma tissues and glioblastoma cell lines. We observed that TROP2 was highly expressed in human glioblastoma samples and the levels of TROP2 were positively associated with the overall survival of glioblastoma patients. In addition, TROP2 was also overexpressed in glioblastoma cell lines compared with that noted in human normal astroglial cells (SVGP12). As to the biological study, we observed that silencing of TROP2 in glioblastoma cells inhibited cell proliferation and metastasis of glioblastoma cells. These results suggest that TROP2 functions as an oncogene in glioblastoma cells.
The molecular mechanisms of TROP2 in carcinogenesis and tumor progression remain largely unknown. TROP2 has been shown to regulate the activation of several cancer-related factors, such as retinoblastoma (RB) protein, Jun and nuclear factor (NF)-κB (36). Our results demonstrated that TROP2 knockdown attenuated the expression of various genes involved in cell proliferation (CCND1), apoptosis (survivin), metastasis (MMP2) and angiogenesis (VEGF), all of which are downstream molecules of the JAK2/STAT3 signaling pathway. We found that TROP2 knockdown was associated with the inhibition of JAK2 and STAT3 phosphorylation in glioblastoma cells. In addition, the effects of TROP2 overexpression on glioblastoma cells was negated by JAK2/STAT3 inhibitor WP1066. IL-6, as a potent activator of JAK2/STAT3 signaling, increased the phosphorylation of JAK2 and STAT3 in TROP2-knockdown glioblastoma cells and regulated phenotypic changes in these cells. Thus, we demonstrated for the first time that TROP2 could promote the activation of the JAK2/ STAT3 signaling pathway.
In conclusion, we discovered a novel mechanism by which TROP2 activates the JAK2/STAT3 signaling pathway to promote glioblastoma growth and metastasis. These results provide new insights into the functions of TROP2 and indicate that TROP2 is a promising biomarker and therapeutic target for glioblastoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key Research and Development Program of China (nos. 2016YFC1302204 and 2017YFC1308600), the Fundamental Research Funds for the Central Universities (XDJK2017D186), the Graduate Scientific Research Foundation of Chongqing (CYB17068), the National Natural Science Foundation of China (81672502) and the Chongqing University Innovation Team Building Program funded projects (CXTDX201601010).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JH was responsible for the collection and assembly of data, data analysis and interpretation, and manuscript writing. AL and QD carried out the collection and assembly of data and the data analysis. GZ was responsible for the collection and assembly of data, and revised the manuscript. XH carried out the collection and assembly of data, wrote and reviewed the manuscript. HC was responsible for the conception and design of the study, data analysis and interpretation, manuscript revision and financial support. All authors read and approved the final manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
All animal studies were approved by the Animal Care and Use Committee of Southwest University, and carried out in conformity to the Guide for the Care and Use of Laboratory Animals (Ministry of Science and Technology of China, 2006). Primary human tumor specimen collection was granted prior approval from the Ethics Committee of Daping Hospital, Chongqing, China. Tissue analysis was approved by the Ethics Committee of Southwest University of China, Chongqing, China. All of the patients provided informed consent to participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare no competing interests, and all authors confirm its accuracy.
References
- 1.Fuller GN, Scheithauer BW. The 2007 Revised World Health Organization (WHO) Classification of Tumours of the Central Nervous System: Newly codified entities. Brain Pathol. 2007;17:304–307. doi: 10.1111/j.1750-3639.2007.00084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS Statistical Report: Primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro-oncol. 2015;17(Suppl 4):iv1–iv62. doi: 10.1093/neuonc/nov189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee I. Advances in surgical approaches in glioblastoma (GBM) Linchuang Zhongliuxue Zazhi. 2017;6:42. doi: 10.21037/cco.2017.07.04. [DOI] [PubMed] [Google Scholar]
- 4.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115:3–8. doi: 10.3171/2011.2.JNS10998. [DOI] [PubMed] [Google Scholar]
- 5.Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch SJ, Holland E, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]
- 6.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359:492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 7.Hou J, Deng Q, Zhou J, Zou J, Zhang Y, Tan P, Zhang W, Cui H. CSN6 controls the proliferation and metastasis of glioblastoma by CHIP-mediated degradation of EGFR. Oncogene. 2017;36:1134–1144. doi: 10.1038/onc.2016.280. [DOI] [PubMed] [Google Scholar]
- 8.Lipinski M, Parks DR, Rouse RV, Herzenberg LA. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc Natl Acad Sci USA. 1981;78:5147–5150. doi: 10.1073/pnas.78.8.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin JC, Wu YY, Wu JY, Lin TC, Wu CT, Chang YL, Jou YS, Hong TM, Yang PC. TROP2 is epigenetically inactivated and modulates IGF-1R signalling in lung adenocarcinoma. EMBO Mol Med. 2012;4:472–485. doi: 10.1002/emmm.201200222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ripani E, Sacchetti A, Corda D, Alberti S. Human Trop-2 is a tumor-associated calcium signal transducer. Int J Cancer. 1998;76:671–676. doi: 10.1002/(SICI)1097-0215(19980529)76:5<671::AID-IJC10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 11.Shvartsur A, Bonavida B. Trop2 and its overexpression in cancers: Regulation and clinical/therapeutic implications. Genes Cancer. 2015;6:84–105. doi: 10.18632/genesandcancer.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cubas R, Li M, Chen C, Yao Q. Trop2: A possible therapeutic target for late stage epithelial carcinomas. Biochim Biophys Acta. 2009;1796:309–314. doi: 10.1016/j.bbcan.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Ohmachi T, Tanaka F, Mimori K, Inoue H, Yanaga K, Mori M. Clinical significance of TROP2 expression in colorectal cancer. Clin Cancer Res. 2006;12:3057–3063. doi: 10.1158/1078-0432.CCR-05-1961. [DOI] [PubMed] [Google Scholar]
- 14.Zheng Z, Dong XJ. Clinical value of serum trophoblast cell surface protein 2 (TROP2) antibody in non-small-cell lung cancer patients. Biomarkers. 2016;21:1–4. doi: 10.1080/1354750X.2016.1201532. [DOI] [PubMed] [Google Scholar]
- 15.Ning S, Liang N, Liu B, Chen X, Pang Q, Xin T. TROP2 expression and its correlation with tumor proliferation and angiogenesis in human gliomas. Neurol Sci. 2013;34:1745–1750. doi: 10.1007/s10072-013-1326-8. [DOI] [PubMed] [Google Scholar]
- 16.Zhao W, Zhu H, Zhang S, Yong H, Wang W, Zhou Y, Wang B, Wen J, Qiu Z, Ding G, et al. Trop2 is overexpressed in gastric cancer and predicts poor prognosis. Oncotarget. 2016;7:6136–6145. doi: 10.18632/oncotarget.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu X, Li S, Yi F. Trop2 gene: A novel target for cervical cancer treatment. J Cancer Res Clin Oncol. 2014;140:1331–1341. doi: 10.1007/s00432-014-1696-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Son S, Shin S, Rao NV, Um W, Jeon J, Ko H, Deepagan VG, Kwon S, Lee JY, Park JH. Anti-Trop2 antibody-conjugated bioreducible nanoparticles for targeted triple negative breast cancer therapy. Int J Biol Macromol. 2018;110:406–415. doi: 10.1016/j.ijbiomac.2017.10.113. [DOI] [PubMed] [Google Scholar]
- 19.Mao Y, Wang X, Zheng F, Wang C, Tang Q, Tang X, Xu N, Zhang H, Zhang D, Xiong L, et al. The tumor-inhibitory effectiveness of a novel anti-Trop2 Fab conjugate in pancreatic cancer. Oncotarget. 2016;7:24810–24823. doi: 10.18632/oncotarget.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, Wels J, Theilen T, Granitto S, Zhang X, et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15:848–862. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Müller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/bj20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanda N, Seno H, Konda Y, Marusawa H, Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y, et al. STAT3 is constitutively activated and supports cell survival in association with survivin expression in gastric cancer cells. Oncogene. 2004;23:4921–4929. doi: 10.1038/sj.onc.1207606. [DOI] [PubMed] [Google Scholar]
- 23.Hajimoradi M, Mohammad Hassan Z, Ebrahimi M, Soleimani M, Bakhshi M, Firouzi J, Samani FS. STAT3 is overactivated in gastric cancer stem-like cells. Cell J. 2016;17:617–628. doi: 10.22074/cellj.2016.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rokavec M, Öner MG, Li H, Jackstadt R, Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et al. Corrigendum. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest. 2015;125:1362. doi: 10.1172/JCI81340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung SS, Giehl N, Wu Y, Vadgama JV. STAT3 activation in HER2-overexpressing breast cancer promotes epithelial-mesenchymal transition and cancer stem cell traits. Int J Oncol. 2014;44:403–411. doi: 10.3892/ijo.2013.2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cutolo M, Meroni M. Clinical utility of the oral JAK inhibitor tofacitinib in the treatment of rheumatoid arthritis. J Inflamm Res. 2013;6:129–137. doi: 10.2147/JIR.S35901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vyas D, O'Dell KM, Bandy JL, Boyce EG. Tofacitinib: The First Janus Kinase (JAK) inhibitor for the treatment of rheumatoid arthritis. Ann Pharmacother. 2013;47:1524–1531. doi: 10.1177/1060028013512790. [DOI] [PubMed] [Google Scholar]
- 28.Hashizume M, Tan SL, Takano J, Ohsawa K, Hasada I, Hanasaki A, Ito I, Mihara M, Nishida K. Tocilizumab, a humanized anti-IL-6R antibody, as an emerging therapeutic option for rheumatoid arthritis: Molecular and cellular mechanistic insights. Int Rev Immunol. 2015;34:265–279. doi: 10.3109/08830185.2014.938325. [DOI] [PubMed] [Google Scholar]
- 29.Zhang D, Wang F, Pang Y, Zhao E, Zhu S, Chen F, Cui H. ALG2 regulates glioblastoma cell proliferation, migration and tumorigenicity. Biochem Biophys Res Commun. 2017;486:300–306. doi: 10.1016/j.bbrc.2017.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang MY, Xuan F, Liu W, Cui HJ. MINA controls proliferation and tumorigenesis of glioblastoma by epigenetically regulating cyclins and CDKs via H3K9me3 demethylation. Oncogene. 2017;36:387–396. doi: 10.1038/onc.2016.208. [DOI] [PubMed] [Google Scholar]
- 31.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al. European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group: Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Yuan H, Li Y, Han Y. The role of HER3 in gastric cancer. Biomed Pharmacother. 2014;68:809–812. doi: 10.1016/j.biopha.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 34.Smith J. Erlotinib: Small-molecule targeted therapy in the treatment of non-small-cell lung cancer. Clin Ther. 2005;27:1513–1534. doi: 10.1016/j.clinthera.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Xu P, Zhao Y, Liu K, Lin S, Liu X, Wang M, Yang P, Tian T, Zhu YY, Dai Z. Prognostic role and clinical significance of trophoblast cell surface antigen 2 in various carcinomas. Cancer Manag Res. 2017;9:821–837. doi: 10.2147/CMAR.S147033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerra E, Trerotola M, Aloisi AL, Tripaldi R, Vacca G, La Sorda R, Lattanzio R, Piantelli M, Alberti S. The Trop-2 signalling network in cancer growth. Oncogene. 2013;32:1594–1600. doi: 10.1038/onc.2012.151. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.