Abstract
Non-small cell lung cancer (NSCLC) is a major type of human lung cancer and the primary cause of cancer-associated cases of mortality worldwide. Phosphatase and tensin homolog (PTEN) is a potent tumor suppressor gene in various human cancer types. The aim of the current study was to explore the role of PTEN and its associated regulatory mechanisms in NSCLC. Firstly, the expression of PTEN was detected using western blotting in a variety of NSCLC cell lines. The results revealed that compared with normal control cells, PTEN levels were significantly decreased in NSCLC cell lines (P<0.01). Short hairpin (sh)RNAs specific to PTEN were also used to knockdown endogenous PTEN in NSCLC cells. The results indicated that cell viability was significantly increased in PTEN-knockdown cells compared with those transfected with negative control shRNA (P<0.01). Conversely, overexpression of PTEN in A549 and SK-MES-1 cells significantly decreased the optical density of NSCLC cells (P<0.01). Flow cytometry was used to investigate the cell cycle; the results revealed that PTEN knockdown significantly increased the percentage of cells at G0/G1 phase (P<0.01) and decreased the number of cells at S phase (P<0.01). The molecular mechanism was further explored using western blotting and the results demonstrated that PTEN overexpression increased the levels of cleaved caspase-3 (P<0.01). These results suggest that PTEN may be a potential target gene for gene therapy in patients with NSCLCs.
Keywords: non-small cell lung cancer, phosphatase and tensin homolog deleted on chromosome 10, cell apoptosis, cell phase
Introduction
Lung cancer is a common malignancy, with the highest incidence and mortality rates of all malignant tumors worldwide (1). Clinical therapy for lung cancer typically includes surgery, chemotherapy, radiotherapy and targeted therapy (2). However, the 5-year survival rate is <15% (3), therefore, developing novel, effective methods to treat lung cancer is of great importance. Gene-targeted therapy is one of the major methods used as a therapy for lung cancers (4). It is important to clarify the molecular mechanism of lung cancer tumorigenesis. Many tumor suppressor genes and oncogenes are altered in lung cancer; these contribute to tumorigenesis, development, migration and metastasis (5,6).
The phosphatase and tensin homolog (PTEN) is a tumor suppressor gene that was first identified in 1997 (7). It has been reported that PTEN is frequently deleted or mutated in human cancer types, including breast cancer (8,9), pancreatic cancer (10), colorectal cancer (11,12), liver cancer (13), prostate cancer (14), gastric cancer (15) and non-small cell lung cancer (NSCLC) (16). PTEN serves an important role in the nucleus by maintaining genomic stability via the regulation of RAD51 (17). Loss or gene disruption of PTEN is associated with poor prognosis in human cancer types (18). Under normal circumstances, RAD51 is phosphorylated by the phosphoinositide 3-kinase (PI3K) family and dephosphorylated by the phosphatase PTEN to generate phosphatidylinositol (PI)-(4,5)-P2. However, loss of PTEN increases the levels of PI-(3,4,5)-triphosphate, which in turn activates the PI3K-protein kinase B (Akt) signaling pathway to promote cell proliferation and survival (19,20).
Elucidating the molecular mechanism of NSCLC would provide a basis for clinical therapies to treat patients with lung cancer. Lu et al (21) have previously reported that PTEN inhibits cell proliferation, promotes cell apoptosis and induces cell cycle arrest via downregulation of the PI3K/AKT/human telomerase reverse transcriptase (hTERT) pathway in lung adenocarcinoma A549 cells. In the current study, additional lung cancer cell lines, including H460, SK-MES-1, H1299 and A549, were used in order to investigate the regulatory mechanisms of NSCLC cells. The current study demonstrated that PTEN regulates cell phase progression and cell apoptosis, possibly by regulating the levels of S-phase kinase-associated protein 2 (Skp2). Future studies providing further clarification regarding the role of PTEN in human NSCLC cell proliferation may provide a basis for the development of novel gene therapies.
Materials and methods
Cell lines, shRNA, plasmid and reagents
The immortalized human bronchial epithelial cell line BEAS-2B was obtained from Shanghai Bioleaf Biotech Co., Ltd. (Shanghai, China). Human MRC-5 cells, (cat. no. AA-CELL-79) were purchased from Zhixing Biological Technology Corporation (Guangzhou, China; http://action.binzhuang.com/). A549 (cat. no. zs100735), H1299 (cat. no. as100207) and H460 (cat. no. zs101010) cells were purchased from Zishi Biotechnology Corporation (Shanghai, China; http://pozuchou1004.cn.globalimporter.net/). SK-MES-1 (cat. no. XB-0170) was purchased from Aolu Biological Corporation (Shanghai, China; http://www.chem17.com/st310034/Intro.html). The cell lines were cultured in Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (cat. no. SH30071.03; HyClone; GE Healthcare Life Sciences, Logan, UT, USA) at 37°C in a humidified atmosphere containing 5% CO2. MTT was obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The recombinant plasmid of PTEN was constructed and provided by Cyagen Biology Technology Corporation (Suzhou, China; http://www.cyagen.com/cn/zh-cn/), with open reading frames of PTEN digested with HindIII and BamHI and subcloned into a pcDNA3.1 (+) plasmid. Here, the empty pcDNA3.1 (+) plasmid was used as a negative control. PTEN short hairpin (sh)RNA (h2; cat. no. sc-44272-SH) and a scrambled control shRNA (cat. no. sc-108060) were obtained from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The A549 and SK-MES-1 cells, BEAS-2B and H460 cells were transfected with Lipofectamine 2000 (cat. no. 11668-027; Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The pc pcDNA3.1 (+) plasmid was used as a negative control of PTEN plasmid to transfect the lung cancer cells for overexpression of PTEN, and the vector already contained the shRNA sequence. A total of 0.5 µg PTEN shRNA or control shRNA were transfected using Lipofectamine 2000 in 24-well plates for 48, 72 and 96 h. An MTT assay was performed following 48, 72 or 96 h of transfection. Cell cycle determination via flow cytometry was performed 24 h following transfection. A549 cells or SK-MES-1 cells were transfected with pcDNA3.1-PTEN plasmid and control plasmid for 48 h. A total of 0.5 µg of PTEN recombinant plasmid and control plasmid were transfected using Lipofectamine 2000 in 24-well plates for 48 h. The expression of total caspase-3, cleaved caspase-3, poly ADP ribose polymerase (PARP) and cleaved PARP was assessed using western blotting.
MTT assay
An MTT assay was used to determine the viability of NSCLC cells. Briefly, BEAS-2B and H460 cells, A549 and SK-MES-1 cells were transfected with PTEN-shRNA or negative control shRNA for 48, 72 or 96 h. Cells were subsequently incubated at 37°C with 20 µl of MTT (5 mg/ml) for 4 h and the purple crystals were dissolved in dimethylsulfoxide for 15 min. A total of 150 µl/well was transferred into 96-well plates and the absorbance was measured using a microplate reader at 490 nm (iMark Microplate Reader; Bio-Rad Laboratories, Inc., Hercules, CA, USA). The inhibition rate was calculated using Microsoft Excel software 2016 (Microsoft Corporation, Redmond, WA, USA).
Antibodies
Rabbit monoclonal anti-PTEN antibody (cat. no. ab32199), rabbit polyclonal anti-caspase-3 antibody (cat. no. ab44976) and rabbit polyclonal anti-active caspase-3 antibody (cat. no. ab2302) were purchased from Abcam (Cambridge, UK). Rabbit anti-PARP antibody (cat. no. 9542) was obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA). Rabbit polyclonal anti-cleaved PARP antibody (cat. no. ab4830) was obtained from Abcam and mouse monoclonal anti-β-Actin antibody (cat. no. sc-47778) was purchased from Santa Cruz Biotechnology, Inc. The Anti-SKP2 antibody (ab19877) was purchased from Abcam. The secondary antibodies included goat anti-rabbit IgG H&L horseradish peroxidase (HRP) (cat. no. ab6721; Abcam) and HRP-conjugated goat anti-mouse IgG (cat. no. sc-2005; Santa Cruz Biotechnology, Inc.).
Western blot analysis
The cell lysates were prepared using immunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Haimen, China). Protein concentration determination method BCA was performed and 20 µg/lane of total protein was added and subsequently separated by 10% SDS-PAGE. Proteins were transferred onto nitrocellulose membranes, which were subsequently blocked with 5% bovine serum albumin (Thermo Fisher Scientific, Inc.) for 30 min at room temperature. The membrane was incubated with the indicated primary and secondary antibodies. The primary antibody was diluted to 1:1,000 and incubated at 4°C overnight. The secondary antibody was diluted to 1:10,000 and incubated at 37°C for 1 h. Membranes were washed three times for 5 min with 1X Tris-buffered saline + Tween 20 buffer between each step. The bands were visualized using enhanced chemiluminescence reagents (Pierce; Thermo Fisher Scientific, Inc.). The grey values of the bands were determined and calculated by Image J software (version 1.48, National Institutes of Health, Bethesda, MD, USA).
Cell cycle analysis
Cell cycle analysis was performed using propidium iodide staining and flow cytometry analysis (Propidium Iodide Flow Cytometry kit; cat. no. ab139418, Abcam). A total of 3×106 NSCLC cells were collected by centrifugation at 300 × g for 10 min at room temperature. The medium was discarded and cells were washed twice with ice-cold PBS. Cells were subsequently fixed in 70% ethanol at 4°C overnight, washed twice with PBS and centrifuged at room temperature at 800 × g for 5 min. Cells were resuspended in PBS containing 1 mg/ml RNase A (Abcam) for 30 min at 37°C. Propidium iodide (50 µg/ml) was added into the cell suspension at 4°C for 15 min and flow cytometry analysis was performed using a BD LSRFortessa X-20 flow cytometer (BD Biosciences, San Jose, CA, USA).
Statistical analysis
Data were analyzed using SPSS v.13 (SPSS, Inc., Chicago, IL, USA) and are presented as the mean ± standard deviation. Independent samples were analyzed using independent sample t-tests and multiple comparisons were analyzed using ANOVA followed by Tukey's test. P≤0.05 was considered to indicate a statistically significant difference.
Results
PTEN levels are low in NSCLC cell lines
In order to investigate the role of PTEN in NSCLC cell proliferation, PTEN levels in NSCLC cells were assessed using western blotting (Fig. 1A). PTEN levels were significantly decreased in lung adenocarcinoma A549 (P<0.01) and H1299 (P<0.05) cell lines, lung squamous cell carcinoma cell line SK-MES-1 (P<0.01) and lung large cell carcinoma H460 cells (P<0.05) compared with the normal control BEAS-2B and MRC-5 cell lines (Fig. 1B).
Figure 1.
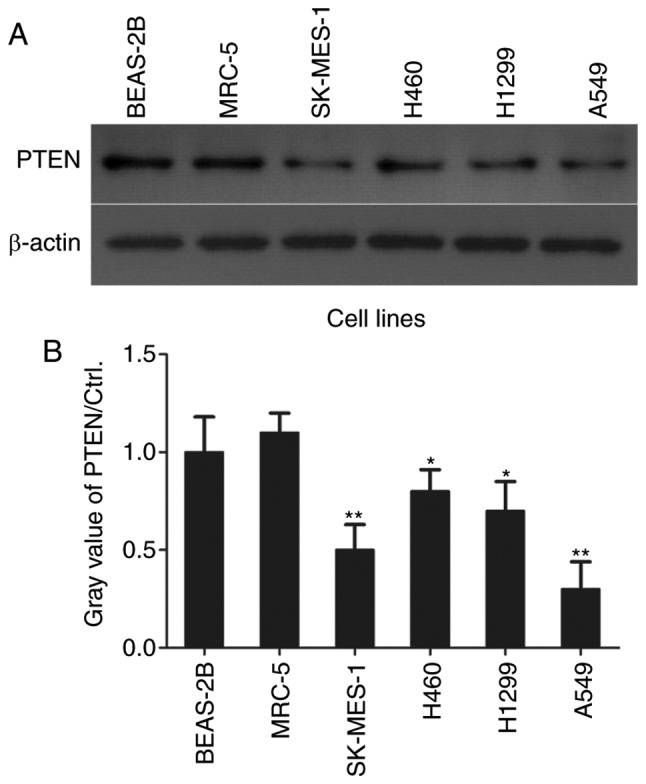
PTEN expression is low in non-small cell lung cancer cell lines. (A) PTEN expression was assessed in lung cancer H460, H1299, SK-MES-1 and A549 cell lines and normal BEAS-2B and MRC-5 control cells using western blotting and (B) quantified with β-actin as an internal reference gene. *P<0.05 and **P<0.01 vs. BEAS-2B. PTEN, phosphatase and tensin homolog; PARP, poly ADP ribose polymerase; Ctrl., control BEAS-2B cells.
PTEN knockdown increases the viability of BEAS-2B and H460 cells
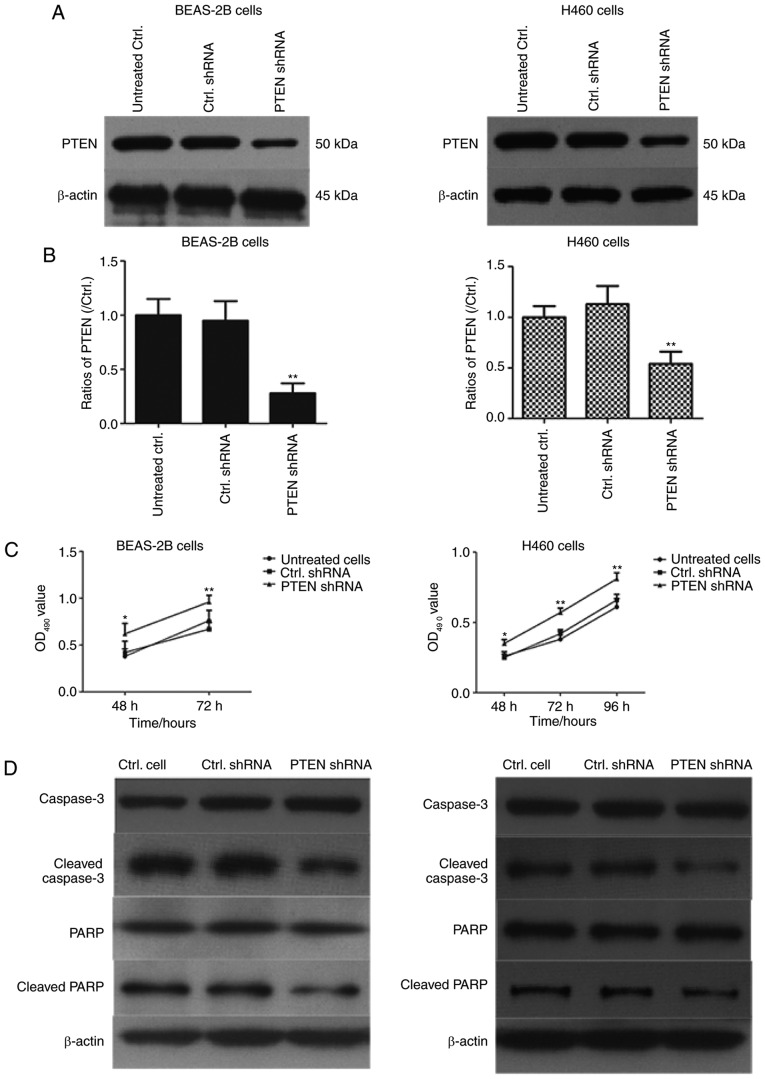
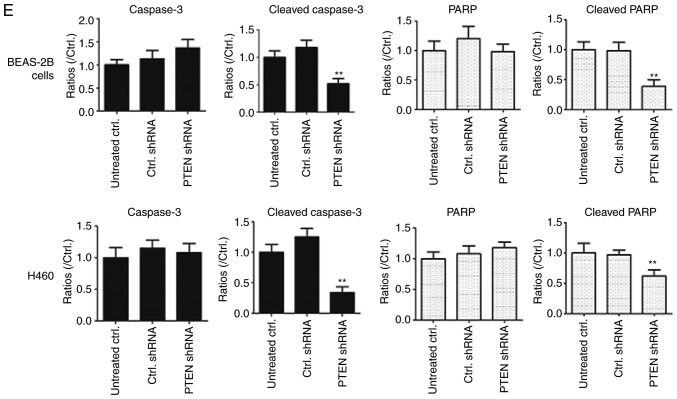
The effect of PTEN on viability in BEAS-2B and H460 cells was assessed using western blotting (Fig. 2). PTEN was significantly downregulated in cells transfected with PTEN-shRNA (P<0.01; Fig. 2B). Additionally, cell viability was determined using an MTT assay and the results demonstrated that cell viability was significantly increased in PTEN-shRNA transfected cells compared with cells transfected with control shRNA and untreated cells (P<0.05; Fig. 2C). Furthermore, western blotting was performed to assess the effects of PTEN knockdown on caspase-3 and PARP expression. As indicated in Fig. 2D and E, transfection with PTEN shRNA significantly decreased the levels of cleaved caspase-3 and cleaved PARP in BEAS-2B cells and H460 cells (P<0.01). These results demonstrated that PTEN knockdown may inhibit cell apoptosis of lung cancer cells.
Figure 2.
PTEN knockdown promotes proliferation in BEAS-2B cells and H460 cells. (A) BEAS-2B cells and H460 cells were transfected with PTEN-shRNA and control shRNA for 48 h and PTEN levels were assessed using western blotting and (B) quantified **P<0.01, compared with Ctrl. shRNA group. (C) The PTEN-shRNA and Ctrl. shRNA transfected BEAS-2B cells and H460 cells were cultured for 48, 72 or 96 h and cell viability was determined using an MTT assay. *P<0.05, between PTEN shRNA and Ctrl. shRNA in 48 h; **P<0.01, between PTEN shRNA and Ctrl. shRNA in 72 h or 96 h; (D) BEAS-2B cells and H460 cells were transfected with PTEN shRNA and control shRNA for 48 h and the expression of caspase-3, cleaved caspase-3, PARP and cleaved PARP was determined using western blotting. (E) The ratios of Caspase-3, cleaved caspase-3, PARP and cleaved PARP in BEAS-2B and H460 cells were are presented in histograms *P<0.05 and **P<0.01 vs. Ctrl. shRNA-transfected cells. PTEN, phosphatase and tensin homolog 10; sh, short hairpin; Ctrl., control; PARP, poly ADP ribose polymerase; OD, optical density.
Overexpression of PTEN decreases the viability of lung cancer cell lines
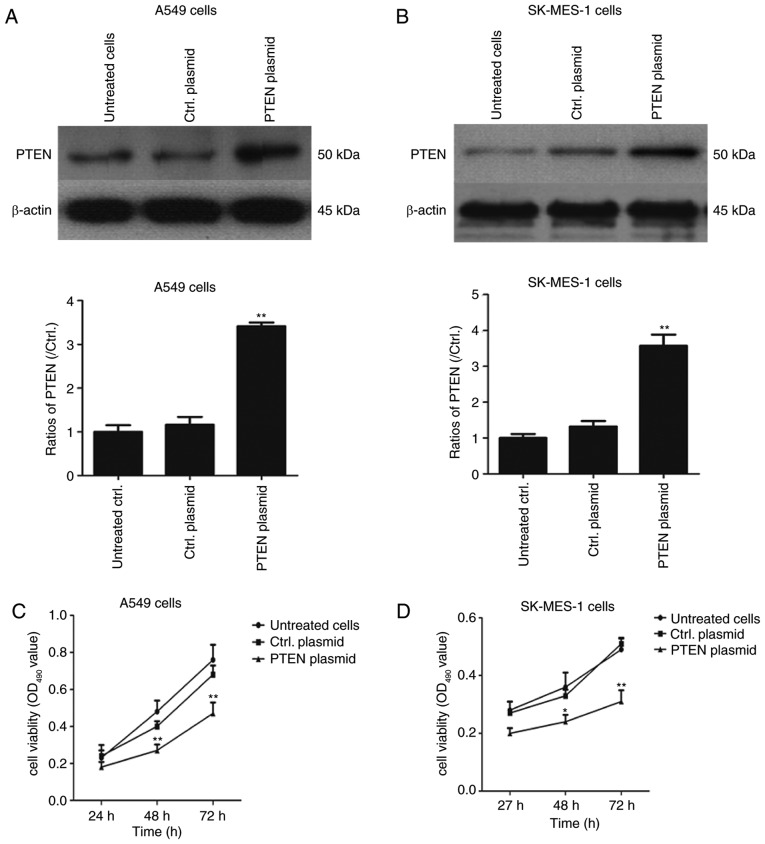
PTEN was overexpressed in NSCLC cells and the role of PTEN in cell proliferation was evaluated further. A549 and SK-MES-1 cells were transfected with pcDNA3.1-PTEN and control pcDNA3.1 (+) for 24, 48 and 72 h. The expression of PTEN was determined using western blotting and cell proliferation was determined using an MTT assay. As illustrated in Fig. 3A and B, the PTEN expression was significantly increased in PTEN plasmid-transfected A549 cells and SK-MES-1 cells compared with the control plasmid group (P<0.01), The proliferation of A549 and SK-MES-1 cells was significantly suppressed in pcDNA3.1-PTEN overexpressed lung cancer cells compared with cells transfected with the control plasmid (P<0.05; Fig. 3C) in a time-dependent manner. These data suggest that PTEN overexpression suppresses cell growth in lung cancer cell lines, with PTEN acting as a tumor suppressor gene.
Figure 3.
Overexpression of PTEN inhibits cell proliferation in lung cancer cell lines. (A) A549 and (B) SK-MES-1 cells were transfected with pcDNA3.1-PTEN plasmid and pcDNA3.1 (+) plasmid for 24 h and PTEN expression was detected using western blotting. Cells were cultured for 24, 48 and 72 h. Cell viability was determined in (C) A549 and (D) SK-MES-1 cells. *P<0.05 and **P<0.01 vs. Ctrl. plasmid-transfected cells. PTEN, phosphatase and tensin homolog; Ctrl., control; OD, optical density.
Overexpression of PTEN downregulates Skp2 expression and induces cell-cycle arrest in G0/G1 phase
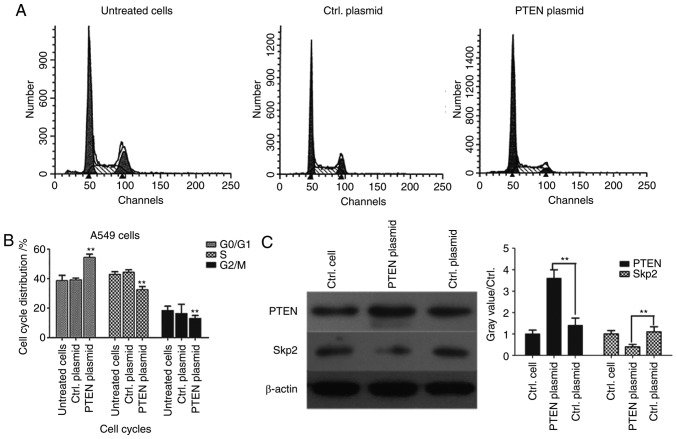
It has previously been reported that PTEN signaling regulates the assembly of the Skp, Cullin, F-box containing complex (SCF-Skp2) at the G1/S cell cycle transition (22,23). However, to the best of our knowledge, it is unclear whether Skp2 expression is deregulated in lung cancer cells. In order to further clarify the molecular mechanism of PTEN, flow cytometry was used for cell-cycle analysis in PTEN-overexpressed A549 cells. G0/G1 phase arrest was significantly induced (P<0.01) and the proportion of cells in S phase was significantly decreased in the PTEN-overexpressed group compared with the control plasmid group (P<0.01), suggesting that PTEN may regulate signaling pathways associated with cell cycle progression (Fig. 4B). In order to detect the associations between PTEN and Skp2, western blotting was performed and the results demonstrated that overexpression of PTEN significantly decreased the expression level of Skp2 (P<0.01; Fig. 4C), which suggested that Skp2 expression was likely regulated by PTEN in lung cancer cells.
Figure 4.
Overexpression of PTEN downregulates Skp2 expression and induces cell-cycle arrest at G0/G1 phase. A549 cells were transfected with pcDNA 3.1-PTEN or Ctrl. plasmids for 24 h. Untreated cells were used as negative controls. (A) Cell-cycle analysis was performed using propidium iodide staining and flow cytometry. (B) Cell cycle distribution of A549 cells. (C) PTEN and Skp2 levels were determined by western blotting. **P<0.01 vs. Ctrl. plasmid-transfected cells. PTEN, phosphatase and tensin homolog; Skp2, S-phase kinase-associated protein 2; Ctrl., control.
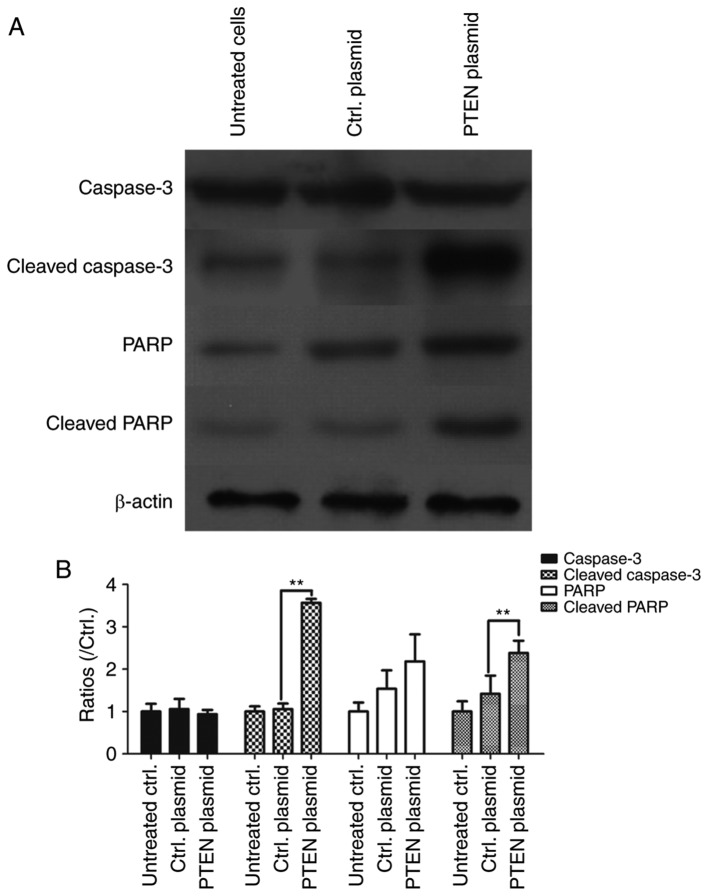
Overexpression of PTEN activates caspase-3 and promotes the cleavage of PARP
In order to assess whether PTEN regulates apoptosis in NSCLC cells, it was overexpressed in A549 cells and levels of cleaved caspase-3 and cleaved PARP were detected using western blotting. PTEN overexpression in A549 cells significantly upregulated cleaved caspase-3 expression (P<0.01), which suggests that PTEN overexpression contributes to the activation of caspase-3 (Fig. 5). The levels of PARP and cleaved PARP was also assessed using western blotting and the results demonstrated that PARP, the substrate of the activated caspase-3, was cleaved into the active form in PTEN overexpressing cells (P<0.01 vs. Ctrl. plasmid; Fig. 5). These results suggest that overexpression of PTEN increases cell apoptosis in NSCLCs.
Figure 5.
PTEN overexpression activates caspase-3 and promotes the cleavage of PARP. A549 cells were transfected with pcDNA3.1-PTEN and Ctrl. plasmids for 48 h. (A) The expression of total caspase-3, cleaved caspase-3, PARP and cleaved PARP was assessed using western blotting. β-actin was used as an internal reference gene. (B) The levels of total caspase-3, cleaved caspase-3, PARP and cleaved PARP were shown in a histogram.**P<0.01 vs. Ctrl. plasmid-transfected cells. PTEN, phosphatase and tensin homolog; Ctrl., control; PARP, poly ADP ribose polymerase.
Discussion
Lung cancer comprises malignant lung tumors characterized by uncontrolled cell growth (24). NSCLC is the primary type of lung cancer and accounts for ~85% of all lung cancer cases (25). It has previously been reported that PTEN functions as a tumor suppressor in a variety of human cancer types (26,27). Lu et al (21) reported that PTEN inhibits cell proliferation, promotes cell apoptosis and induces cell cycle arrest via downregulation of the PI3K/AKT/hTERT pathway in lung adenocarcinoma A549 cells, which is consistent with the results of the current study. In the current study, multiple lung cancer cell lines, including H460, SK-MES-1, H1299 and A549, were used. Although the levels of PTEN were decreased in lung cancer cell lines compared with the normal control cells BEAS-2B, there was still substantial PTEN expression in NSCLC cell lines. It was probably the limited decrease in PTEN expression, not other factors, that was the major player for NSCLC progression (21).
However, there are some limitations of the current study. The current study identified that PTEN plays an important role in the proliferation and cell cycle progression of lung cancer cells. Further study would investigate how PTEN regulates the proliferation or cell cycle of cancer cells, for example, which specific cancer-related signaling pathways are regulated by PTEN or how the microRNA expression profiles are changed in the progression of lung cancer cells. Further studies should be performed in at least two other lung cancer cells lines, to validate the findings of the present study.
Levels of PTEN were markedly decreased in lung cancer cell lines compared with normal control cells. The MTT assay results in H460 cells revealed that PTEN knockdown promotes the proliferation of lung cancer cells. However, overexpression of PTEN in A549 and H460 cells significantly inhibited cell growth. This was consistent with a previous study by Li et al (13) in which overexpression of PTEN effectively inhibited proliferation of liver cancer cells and promoted their apoptosis. Furthermore, in bladder cancer cells, overexpression of PTEN suppressed growth and induced apoptosis by inhibiting the expression of surviving (28). It has also been identified that overexpression of PTEN induces cell growth arrest and apoptosis in human breast cancer ZR-75-1 cells (29) and human ovarian cancer cells (30). Together, these data suggest that PTEN functions as a tumor suppressor and may be an effective target for the regulation of NSCLC cell proliferation.
Cell apoptosis is a process of programmed cell death that occurs in multicellular organisms (31). There are two major apoptosis signaling pathways: Extrinsic (death receptor-mediated) and intrinsic (mitochondrial) (32,33). Caspase-3 is activated in the extrinsic and intrinsic pathways (34) and is therefore an effective molecule for inducing cell apoptosis to treat cancer cells (35). In the current study, overexpression of PTEN was demonstrated to increase the level of cleaved caspase-3 in NSCLC cells. In response to apoptotic signals, cleaved caspase-3, the active form of caspase-3, cleaves the 116 kDa substrate, PARP, into 85 and 25 kDa fragments (35). In the current study, the cleaved 85 kDa fragment of PARP was detected. Furthermore, PTEN overexpression was observed to increase the percentage of cells in G0/G1 phase and decrease the number of cells in S phase, suggesting that PTEN induced G0/G1 cell cycle arrest. The results of the current study suggest that PTEN is capable of inducing apoptosis and may therefore be a potential effective target for gene therapy in patients with NSCLC.
In conclusion, PTEN suppressed non-small cell lung cancer cell growth by promoting G0/G1 arrest and cell apoptosis. Taken togather, the results f the present study suggest that PTEN may be a potential target gene for gene therapy in patients with NSCLC.
Acknowledgements
Not applicable.
Funding
The work was supported by Guangdong Provincial Natural Science Foundation for key doctoral start-up project, (Grant no. S2013010016379).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LL was the guarantor for the integrity of the entire study and was responsible for study concepts and design. SH performed the literature research and performed cell cycle analysis. SM revised the manuscript for critically important intellectual content. JH undertook the clinical studies, and the experimental studies were performed by SM. Data acquisition was the responsibility of LL and LH undertook analysis of the data and statistical analysis. SC performed the western blotting experiments and prepared the manuscript. YW performed the MTT assay and reviewed the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lim JS, Soo RA. Nivolumab in the treatment of metastatic squamous non-small cell lung cancer: A review of the evidence. Ther Adv Respir Dis. 2016;10:444–454. doi: 10.1177/1753465816661091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi JG, Shao HJ, Jiang FE, Huang YD. Role of radiation therapy in lung cancer management-a review. Eur Rev Med Pharmacol Sci. 2016;20:3217–3222. [PubMed] [Google Scholar]
- 3.Condoluci A, Mazzara C, Zoccoli A, Pezzuto A, Tonini G. Impact of smoking on lung cancer treatment effectiveness: A review. Future Oncol. 2016;12:2149–2161. doi: 10.2217/fon-2015-0055. [DOI] [PubMed] [Google Scholar]
- 4.Huang CL, Yokomise H, Miyatake A. Clinical significance of the p53 pathway and associated gene therapy in non-small cell lung cancers. Future Oncol. 2007;3:83–93. doi: 10.2217/14796694.3.1.83. [DOI] [PubMed] [Google Scholar]
- 5.Bikkavilli RK, Avasarala S, Van Scoyk M, Arcaroli J, Brzezinski C, Zhang W, Edwards MG, Rathinam MK, Zhou T, Tauler J, et al. Wnt7a is a novel inducer of β-catenin-independent tumor-suppressive cellular senescence in lung cancer. Oncogene. 2015;34:5317–5328. doi: 10.1038/onc.2015.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincenten JP, Smit EF, Grünberg K, Postmus PE, Snijders PJ, Witte BI, Heideman DA, Thunnissen E. Is the current diagnostic algorithm reliable for selecting cases for EGFR- and KRAS-mutation analysis in lung cancer? Lung Cancer. 2015;89:19–26. doi: 10.1016/j.lungcan.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 8.Li B, Lu Y, Wang H, Han X, Mao J, Li J, Yu L, Wang B, Fan S, Yu X, Song B. miR-221/222 enhance the tumorigenicity of human breast cancer stem cells via modulation of PTEN/Akt pathway. Biomed Pharmacother. 2016;79:93–101. doi: 10.1016/j.biopha.2016.01.045. [DOI] [PubMed] [Google Scholar]
- 9.Sun L, Burnett J, Gasparyan M, Xu F, Jiang H, Lin CC, Myers I, Korkaya H, Liu Y, Connarn J, et al. Novel cancer stem cell targets during epithelial to mesenchymal transition in PTEN-deficient trastuzumab-resistant breast cancer. Oncotarget. 2016;7:51408–51422. doi: 10.18632/oncotarget.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gu J, Wang D, Zhang J, Zhu Y, Li Y, Chen H, Shi M, Wang X, Shen B, Deng X, et al. GFRα2 prompts cell growth and chemoresistance through down-regulating tumor suppressor gene PTEN via Mir-17-5p in pancreatic cancer. Cancer Lett. 2016;380:434–441. doi: 10.1016/j.canlet.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Ping Hai P, Bo Feng T, Li L, Hui Nan Y, Hong Z. IL-1β/NF-kb signaling promotes colorectal cancer cell growth through miR-181a/PTEN axis. Arch Biochem Biophys. 2016;604:20–26. doi: 10.1016/j.abb.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Wu QX, Yuan SX, Ren CM, Yu Y, Sun WJ, He BC, Wu K. Oridonin upregulates PTEN through activating p38 MAPK and inhibits proliferation in human colon cancer cells. Oncol Rep. 2016;35:3341–3348. doi: 10.3892/or.2016.4735. [DOI] [PubMed] [Google Scholar]
- 13.Li MF, Guan H, Zhang DD. Effect of overexpression of PTEN on apoptosis of liver cancer cells. Genet Mol Res. 2016;15 doi: 10.4238/gmr.15028120. [DOI] [PubMed] [Google Scholar]
- 14.Lotan TL, Wei W, Ludkovski O, Morais CL, Guedes LB, Jamaspishvili T, Lopez K, Hawley ST, Feng Z, Fazli L, et al. Analytic validation of a clinical-grade PTEN immunohistochemistry assay in prostate cancer by comparison with PTEN FISH. Mod Pathol. 2016;29:904–914. doi: 10.1038/modpathol.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xin R, Bai F, Feng Y, Jiu M, Liu X, Bai F, Nie Y, Fan D. MicroRNA-214 promotes peritoneal metastasis through regulating PTEN negatively in gastric cancer. Clin Res Hepatol Gastroenterol. 2016;40:748–754. doi: 10.1016/j.clinre.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Zhu DY, Li XN, Qi Y, Liu DL, Yang Y, Zhao J, Zhang CY, Wu K, Zhao S. MiR-454 promotes the progression of human non-small cell lung cancer and directly targets PTEN. Biomed Pharmacother. 2016;81:79–85. doi: 10.1016/j.biopha.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee A, Karmakar P. Attenuation of PTEN perturbs genomic stability via activation of Akt and down-regulation of Rad51 in human embryonic kidney cells. Mol Carcinog. 2013;52:611–618. doi: 10.1002/mc.21903. [DOI] [PubMed] [Google Scholar]
- 18.Bohn BA, Mina S, Krohn A, Simon R, Kluth M, Harasimowicz S, Quaas A, Bockhorn M, Izbicki JR, Sauter G, et al. Altered PTEN function caused by deletion or gene disruption is associated with poor prognosis in rectal but not in colon cancer. Hum Pathol. 2013;44:1524–1533. doi: 10.1016/j.humpath.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Zheng Z, Zhang Y, Zhang Z, Yang Y, Song T. Effect of miR-106b on invasiveness of pituitary adenoma via PTEN-PI3K/AKT. Med Sci Monit. 2017;23:1277–1285. doi: 10.12659/MSM.900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim HJ, Wang X, Crowe P, Goldstein D, Yang JL. Targeting the PI3K/PTEN/AKT/mTOR pathway in treatment of sarcoma cell lines. Anticancer Res. 2016;36:5765–5771. doi: 10.21873/anticanres.11160. [DOI] [PubMed] [Google Scholar]
- 21.Lu XX, Cao LY, Chen X, Xiao J, Zou Y, Chen Q. PTEN inhibits cell proliferation, promotes cell apoptosis, and induces cell cycle arrest via downregulating the PI3K/AKT/hTERT pathway in lung adenocarcinoma A549 cells. Biomed Res Int. 2016;2016:2476842. doi: 10.1155/2016/2476842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jonason JH, Gavrilova N, Wu M, Zhang H, Sun H. Regulation of SCF(SKP2) ubiquitin E3 ligase assembly and p27(KIP1) proteolysis by the PTEN pathway and cyclin D1. Cell Cycle. 2007;6:951–961. doi: 10.4161/cc.6.8.4104. [DOI] [PubMed] [Google Scholar]
- 23.Mamillapalli R, Gavrilova N, Mihaylova VT, Tsvetkov LM, Wu H, Zhang H, Sun H. PTEN regulates the ubiquitin-dependent degradation of the CDK inhibitor p27(KIP1) through the ubiquitin E3 ligase SCF(SKP2) Curr Biol. 2001;11:263–267. doi: 10.1016/S0960-9822(01)00065-3. [DOI] [PubMed] [Google Scholar]
- 24.Giannopoulou E, Nikolakopoulos A, Kotsirilou D, Lampropoulou A, Raftopoulou S, Papadimitriou E, Theocharis AD, Makatsoris T, Fasseas K, Kalofonos HP. Epidermal growth factor receptor status and Notch inhibition in non-small cell lung cancer cells. J Biomed Sci. 2015;22:98. doi: 10.1186/s12929-015-0196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vesel M, Rapp J, Feller D, Kiss E, Jaromi L, Meggyes M, Miskei G, Duga B, Smuk G, Laszlo T, et al. ABCB1 and ABCG2 drug transporters are differentially expressed in non-small cell lung cancers (NSCLC) and expression is modified by cisplatin treatment via altered Wnt signaling. Respir Res. 2017;18:52. doi: 10.1186/s12931-017-0537-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Nostrand JL, Brisac A, Mello SS, Jacobs SB, Luong R, Attardi LD. The p53 target gene SIVA enables non-small cell lung cancer development. Cancer Discov. 2015;5:622–635. doi: 10.1158/2159-8290.CD-14-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deben C, Wouters A, de Beeck Op K, van Den Bossche J, Jacobs J, Zwaenepoel K, Peeters M, Van Meerbeeck J, Lardon F, Rolfo C, et al. The MDM2-inhibitor Nutlin-3 synergizes with cisplatin to induce p53 dependent tumor cell apoptosis in non-small cell lung cancer. Oncotarget. 2015;6:22666–22679. doi: 10.18632/oncotarget.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu ZX, Song TB, Li DM, Zhang XT, Wu XL. Overexpression of PTEN suppresses growth and induces apoptosis by inhibiting the expression of survivin in bladder cancer cells. Tumour Biol. 2007;28:9–15. doi: 10.1159/000097041. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Lin G, Wu B, Zhou X, Zhou K. Overexpression of PTEN induces cell growth arrest and apoptosis in human breast cancer ZR-75-1 cells. Acta Biochim Biophys Sin (Shanghai) 2007;39:745–750. doi: 10.1111/j.1745-7270.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- 30.Yan X, Fraser M, Qiu Q, Tsang BK. Over-expression of PTEN sensitizes human ovarian cancer cells to cisplatin-induced apoptosis in a p53-dependent manner. Gynecol Oncol. 2006;102:348–355. doi: 10.1016/j.ygyno.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 31.Terra LF, Garay-Malpartida MH, Wailemann RA, Sogayar MC, Labriola L. Recombinant human prolactin promotes human beta cell survival via inhibition of extrinsic and intrinsic apoptosis pathways. Diabetologia. 2011;54:1388–1397. doi: 10.1007/s00125-011-2102-z. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, Zeng F, Liu X, Li Y, Zhou J, Huang Y, Wang Y, Zhou S, Zhu W, Shu E, et al. Chan-Yu-Bao-Yuan-Tang induces apoptosis in NSCLC and SCLC cell lines via a mitochondria-mediated pathway. Xenobiotica. 2011;41:593–602. doi: 10.3109/00498254.2011.565818. [DOI] [PubMed] [Google Scholar]
- 33.Kim MJ, Kwon SB, Kim MS, Jin SW, Ryu HW, Oh SR, Yoon DY. Trifolin induces apoptosis via extrinsic and intrinsic pathways in the NCI-H460 human non-small cell lung-cancer cell line. Phytomedicine. 2016;23:998–1004. doi: 10.1016/j.phymed.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 34.Elankumaran S, Rockemann D, Samal SK. Newcastle disease virus exerts oncolysis by both intrinsic and extrinsic caspase-dependent pathways of cell death. J Virol. 2006;80:7522–7534. doi: 10.1128/JVI.00241-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin J, Wang M, Jin C, Qi Q. miR-101 sensitizes A549 NSCLC cell line to CDDP by activating caspase 3-dependent apoptosis. Oncol Lett. 2014;7:461–465. doi: 10.3892/ol.2013.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.