Abstract
Lung adenocarcinoma (LUAD) is the most common histological subtype of lung cancer. Previous studies have found that many microRNAs (miRNAs), including miRNA-126-3p, may play a critical role in the development of LUAD. However, no study of LUAD has researched the synergistic effects and co-targets of both miRNA-126-3p and miRNA-126-5p. The present study used real-time quantitative polymerase chain reaction (RT-qPCR) to explore the expression values of miRNA-126-3p and miRNA-126-5p in 101 LUAD and 101 normal lung tissues. Ten relevant microarray datasets were screened to further validate the expression levels of miRNA-126-3p and −5p in LUAD. Twelve prediction tools were employed to obtain potential targets of miRNA-126-3p and miRNA-126-5p. The results showed that both miRNA-126-3p and −5p were expressed significantly lower in LUAD. A significant positive correlation was also present between miRNA-126-3p and −5p expression in LUAD. In addition, lower expression of miRNA-126-3p and −5p was indicative of vascular invasion, lymph node metastasis (LNM), and a later tumor/node/metastasis (TNM) stage of LUAD. The authors obtained 167 targets of miRNA-126-3p and 212 targets of miRNA-126-5p; 44 targets were co-targets of both. Eight co-target genes (IGF2BP1, TRPM8, DUSP4, SOX11, PLOD2, LIN28A, LIN28B and SLC7A11) were initially identified as key genes in LUAD. The results of the present study indicated that the co-regulation of miRNA-126-3p and miRNA-126-5p plays a key role in the development of LUAD, which also suggests a fail-proof mode between miRNA-3p and miRNA-126-5p.
Keywords: miRNA-126-3p, miRNA-126-5p, lung adenocarcinoma, RT-qPCR, co-targets, bioinformatic analysis
Introduction
Lung cancer (LC) is one of the world's most widespread cancers. More than 1.5 million people are diagnosed with LC every year (1,2). Lung adenocarcinoma (LUAD) is the most common histological subtype of LC (3,4). The 5-year survival rate for LUAD patients is less than 15%, as most are diagnosed at advanced stages (5). Therefore, it is urgent to determine the molecular mechanism of LUAD and identify an effective method for early diagnosis and effective treatment.
MicroRNAs (miRNAs) are endogenous, non-coding, small RNAs. They control gene expression by combining with the messenger RNAs (mRNAs) of target genes, causing mRNA degradation or translation suppression (6,7). Numerous studies have demonstrated that the aberrant and disordered expression of miRNAs is involved in many malignant tumors, including LCs (8–12).
Previous research has confirmed that miRNAs can be divided into three different forms, as follows: primary (pri-)miRNA, precursor (pre-)miRNA and mature miRNA. The mature miRNAs designated as miRNA-3p and miRNA-5p are derived from the 3′ or 5′ arms, respectively, of their pre-miRNAs (13). Theoretically, therefore, all pre-miRNAs can produce two types of mature miRNAs. Previous research assumed that only one mature miRNA took part in regulating target mRNAs; the other was thought to be a byproduct, and it was regarded as functionally irrelevant (14). Increasingly, however, recent investigations have confirmed that both miRNA-3p and miRNA-5p originate from one pre-miRNA and can degrade various target mRNAs (15). Studies also have found a correlation and synergistic effects between miRNA-3p and −5p produced by the same pre-miRNA (16). These findings suggest that miRNA-3p and −5p both function during biological processes, implying that they may co-regulate target genes in various diseases.
miRNA-126-3p and miRNA-126-5p (miRNA-126-3p/5p) are both derived from pre-miRNA-126, which is located on human chromosome 9. miRNA-126-3p and −5p have been identified as essential biological factors in the development of certain malignancies (17). Regarding LC, studies with small sample sizes have confirmed that miRNA-126-3p is downregulated in non-small cell lung cancers (NSCLCs), including LUAD (18). Nevertheless, previous studies concerning LC have mainly focused on miRNA-126-3p; no research has demonstrated a relationship between miRNA-126-5p and LC. It is also worth noting that LUAD research has not investigated or clarified whether a correlation or synergistic effects between miRNA-126-3p and −5p exist. Based on previous research, the authors predicted that both miRNA-126-3p and −5p may be less expressed in LUAD and may co-regulate key target genes of LUAD.
The present study aimed to explore the clinical utility and investigate the correlation and synergistic effects of miRNA-126-3p and miRNA-126-3p in LUAD. A total of 202 tissues (101 LUAD tissues and 101 adjacent normal lung tissues) were collected. Real-time quantitative polymerase chain reaction (RT-qPCR) was performed to explore the respective expression values of miRNA-126-3p and −5p in the 202 tissues. A large sample size from the Gene Expression Omnibus (GEO) database was obtained so that a meta-analysis could be performed with the RT-qPCR data from the present study to further identify miRNA-126-3p and −5p expression in LUAD. In addition, 12 prediction software programs were employed to obtain the target genes of miRNA-126-3p and −5p. Then, bioinformatic analysis was conducted to identify the potential molecular mechanisms of miRNA-126-3p and −5p in LUAD.
This is the first study to investigate the co-regulation of miRNA-125-3p and −5p in LUAD. It is hoped that the present study may demonstrate the molecular function and synergistic effects of miRNA-126-3p and miRNA-126-3p. The obtained research findings may reveal the importance of these miRNAs in LUAD diagnosis and treatment.
Materials and methods
Clinical LUAD sample collection
Based on a previous study (19), 202 formalin-fixed, paraffin-embedded (FFPE) tissues were collected at the First Affiliated Hospital of Guangxi Medical University (Nanning, China). Of these 202 FFPE tissues, 101 were LUAD tissues, while the remaining 101 tissues were paired adjacent normal lung tissues. The research protocol for this study has been ratified by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. Consent from all patients was obtained at the time of the sample collection.
RT-qPCR
RT-qPCR is a sensitive technique for quantifying specific RNA targets. RNA was removed from the FFPE sample tissues using a miRNeasy FFPE kit (Qiagen, Venlo, The Netherlands) as previously described (20,21). A NanoDrop 2000 spectrophotometer (NanoDrop Technologies; Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used to ensure the purity and concentration of the extracted RNA from the FFPE tissues. The specific primers of miRNA-126-3p and miRNA-126-5p were provided by TaqMan MicroRNA Assays (4427975-000468; Applied Biosystems, Life Technologies Europe B.V., Gent, Belgium). The reverse primers were included in TaqMan MicroRNA Reverse Transcription kit (4366596; Applied Biosystems, Life Technologies Europe B.V) (22). RNU6B was used as the internal control. The forward primer sequences of RNU6B were 5′-CTCGCTTCGGCAGCACA-3′ and the reverse primer sequences were 5′-AACGCTTCACGAATTTGCGT-3′. The sequences of miRNA-126-3p were 5′-UCGUACCGUGAGUAAUAAUGCG-3′ and the sequences of miRNA-126-5p were 5′-CAUUAUUACUUUUGGUACGCG-3′. The thermocycling conditions were as follows: denaturation at 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 60 sec. The RT-qPCR was analyzed with Applied Biosystems PCR 7900 (Applied Biosystems; Thermo Fisher Scientific, Inc.) to detect miRNA expression values. Relative expression values were calculated using the 2−∆Cq method (23).
GEO data extraction
GEO (https://www.ncbi.nlm.nih.gov/gds/) is the largest fully disclosed high-throughput molecular abundance database; it is mainly used to store gene expression data. All microarray datasets related to LUAD were filtered out and downloaded from the GEO database. The microarray dataset selection criteria were as follows: i) the samples had to be human tissue; ii) the dataset had to contain LUAD and non-cancerous lung tissue groups; iii) both the LUAD and non-cancerous lung tissue groups had to include at least two samples; and iv) expression values of miRNA-126-3p or −5p had to be available.
Statistical analysis
Statistical Product and Service Solutions (SPSS) version 22.0 (IBM Corp., Armonk, NY, USA) software was applied to analyze the RT-qPCR data. A quantitative variable was computed and presented as the means ± standard deviation (SD). Student's t-test was applied to evaluate the difference between two continuous variables. A P-value of <0.05 was considered to indicate a statistically significant result. A receiver operating characteristic (ROC) curve based on the RT-qPCR data was applied to estimate the respective distinguishing impact of miRNA-126-3p and −5p on LUAD from non-cancerous tissues. Binary logistic regression and ROC curve analyses were performed for evaluating the combined distinguishing value of miRNA-126-3p and −5p (24,25). Stata statistical software (version 12.0; Stata Corp., College Station, TX, USA) was used to perform a meta-analysis of the GEO data; the results were assessed using the standard mean difference (SMD). The heterogeneity of the GEO data was assessed by I2 statistics and a Q test. An I2 >50% or a P-value <0.05 was deemed to indicate huge heterogeneity. A summary ROC (SROC) analysis based on the selected microarray datasets as well as the results from the present study was performed to determine the potential distinguishing effect of miRNA-126-3p and −5p on LUAD.
Predicting miRNA-126-3p and- 5p targets
Twelve target gene prediction software programs (TargetScan, miRWalk, Microt4, miRDB, miRanda, miRBridge, miRMap, miRNAMap, PITA, PicTar2, RNA22 and RNAhybrid) (http://zmf.umm.uni-heidelberg.de/apps/zmf/mirwalk2/miRretsys-self.html) were run to obtain the miRNA-126-3p and −5p target genes. Only genes that appeared in more than two of the prediction programs were selected as candidate targets. At the same time, an R function package was used to screen all the overexpressed genes in LUAD from The Cancer Genome Atlas (TCGA) database (https://cancergenome.nih.gov/). Fold change (FC) was used to assess the level of gene expression in the TCGA data, and genes were deemed to be overexpressed if the FC >2. Then, the predicted target genes were overlapped with the overexpressed genes to obtain the miRNA-126-3p and −5p target genes in LUAD.
Bioinformatic analysis
The Database for Annotation, Visualization, and Integrated Discovery (DAVID) version 6.8 (https://david.ncifcrf.gov/) was employed for Gene Ontology (GO) analysis, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was used to analyze the function of the above-identified genes in LUAD. A protein-protein interaction (PPI) network based on the target genes of miRNA-126-3p and −5p was established using the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (http://www.string-db.org/) and Cytoscape version 3.4.0 (26) was used to determine the correlation between each target.
Results
Expression of miRNA-126-3p and miRNA-126-5p in LUAD tissues according to RT-qPCR
Co-downregulation of the expression of both miRNA-126-3p and miRNA-126-5p in LUAD
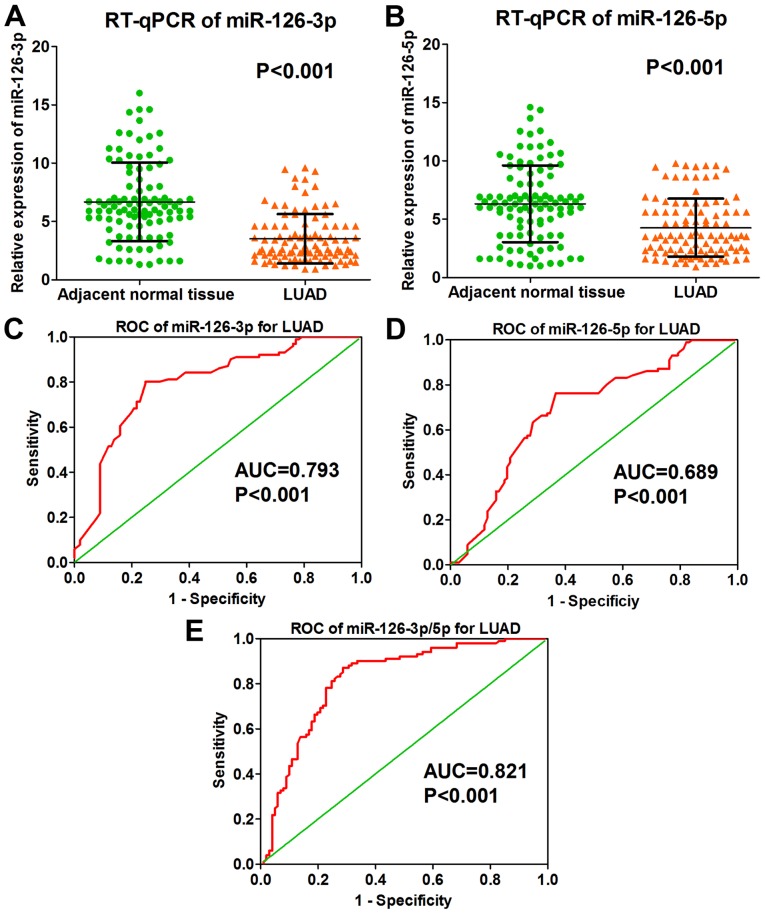
The results of the RT-qPCR performed on the 202 collected clinical samples showed that the level of miRNA-126-3p was markedly lower in LUAD tissue than it was in adjacent normal lung tissue (3.511±2.118 vs. 6.674±3.362, P<0.001; Fig. 1A and Table I); miRNA-126-5p expression was also significantly lower in the LUAD tissues (4.271±2.501 vs. 6.314±3.289, P<0.001; Fig. 1B and Table I). According to the RT-qPCR data, the ROC curve of miRNA-126-3p in LUAD tissues showed that the area under the curve (AUC) was 0.793 (P<0.001; Fig. 1C); the ROC curve of miRNA-126-5p in LUAD tissues showed an AUC of 0.689 (P<0.001; Fig. 1D). The combined ROC curve of miRNA-126-3p and −5p in LUAD tissues revealed an AUC of 0.821 (P<0.001; Fig. 1E).
Figure 1.
Diagnostic value of miRNA-126-3p and miRNA-126--5p for LUAD according to RT-qPCR. (A) Expression of miRNA-126-3p in 101 LUAD tissues and 101 adjacent normal lung tissues (3.511±2.118 vs. 6.674±3.362, P<0.001). (B) Expression of miRNA-126-5p in 101 LUAD tissues and 101 adjacent normal lung tissues (4.271±2.501 vs. 6.314±3.289, P<0.001). (C) ROC curve of miRNA-126-3p for LUAD based on the 101 LUAD tissues and 101 adjacent normal lung tissues (AUC=0.793, P<0.001). (D) ROC curve of miRNA-126-5p for LUAD based on the 101 LUAD tissues and 101 adjacent normal lung tissues (AUC=0.689, P<0.001). (E) Combined ROC curve of miRNA-126-3p and −5p for LUAD based on the 101 LUAD tissues and 101 adjacent normal lung tissues (AUC=0.821, P<0.001). LUAD, lung adenocarcinoma; AUC, area under the receiver operating characteristic (ROC) curve.
Table I.
Expression of miRNA-126-3p and miRNA-126-5p in LUAD according to RT-qPCR data.
| miRNA-126-3p | miRNA-126-5p | ||||||
|---|---|---|---|---|---|---|---|
| Clinicopathological parameters | N | Mean ± SD | t | P-value | Mean ± SD | t | P-value |
| Tissue | |||||||
| LUAD | 101 | 3.511±2.118 | −8.003 | <0.001 | 4.271±2.501 | −4.970 | <0.001 |
| Adjacent normal lung tissue | 101 | 6.674±3.362 | 6.314±3.289 | ||||
| Sex | |||||||
| Male | 56 | 3.619±2.157 | 0.572 | 0.569 | 4.487±2.359 | 0.975 | 0.332 |
| Female | 45 | 3.376±2.083 | 4.000±2.670 | ||||
| Age (years) | |||||||
| <60 | 41 | 3.549±1.897 | 0.149 | 0.881 | 4.194±2.081 | ||
| ≥60 | 60 | 3.484±2.271 | 4.323±2.535 | ||||
| Smoking history | |||||||
| + | 18 | 4.306±1.949 | 1.930 | 0.060 | 4.689±2.439 | −0.602 | 0.500 |
| − | 26 | 3.192±1.833 | 5.141±2.451 | ||||
| Tumor size (cm) | |||||||
| ≤3 | 53 | 3.373±2.250 | −0.685 | 0.495 | 4.450±2.665 | 0.755 | 0.452 |
| >3 | 48 | 3.663±1.974 | 4.073±2.319 | ||||
| Vascular invasion | |||||||
| + | 31 | 3.074±1.697 | −1.384 | 0.169 | 3.045±1.544 | −4.195 | <0.001 |
| − | 70 | 3.704±2.263 | 4.814±2.657 | ||||
| LNM | |||||||
| + | 56 | 3.161±1.679 | −1.798 | 0.076 | 3.671±2.090 | −3.197 | 0.002 |
| − | 45 | 3.946±2.514 | 5.142±2.713 | ||||
| TNM stage | |||||||
| I + II | 44 | 4.140±2.583 | 2.542 | 0.013 | 4.923±2.700 | 2.296 | 0.024 |
| III + IV | 57 | 3.025±1.527 | 3.769±2.233 | ||||
| Pathological grade | |||||||
| I | 17 | 3.482±2.323 | F=1.2 | 0.305 | 4.147±2.543 | F=1.3 | 0.271 |
| II | 61 | 3.735±2.087 | 4.565±2.629 | ||||
| III | 23 | 2.935±2.024 | 3.583±2.034 | ||||
LUAD, lung adenocarcinoma; N, number of samples; SD, standard deviation; t, t-value of Student's t-test; F, analysis of variance; LNM, lymph node metastasis; +, positive; -, negative.
Association between miRNA-126-3p and miRNA-126-5p and the clinicopathological parameters of the LUAD samples
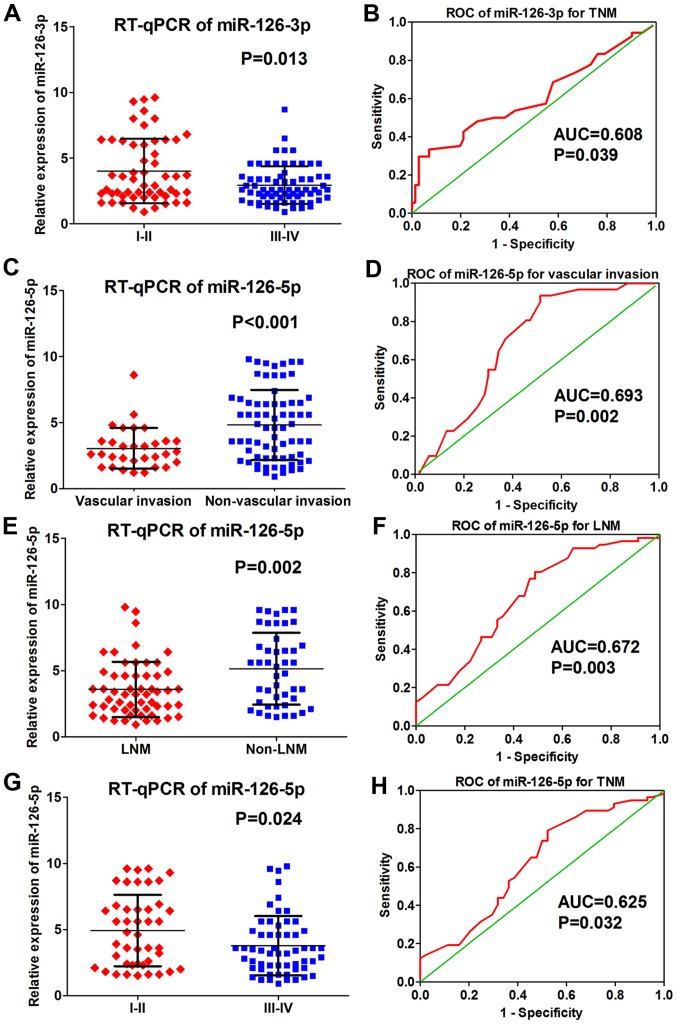
The RT-qPCR analysis determined that miRNA-126-3p levels differed significantly according to TNM stage, while miRNA-126-5p levels differed significantly in regards to vascular invasion, lymph node metastasis (LNM) and TNM stage (Table I). Compared with early TNM stages (I–II) of LUAD, miRNA-126-3p was expressed lower in later TNM stages (III–IV) of LUAD (4.140±2.583 vs. 3.025±1.527, P<0.001; Fig. 2A). The ROC of miRNA-126-3p for the TNM stages showed that the AUC was 0.608 (P=0.039; Fig. 2B). As for miRNA-126-5p, its level was significantly lower in the samples with vascular invasion (3.045±1.544 vs. 4.814±2.657, P<0.001; Fig. 2C). The ROC curve of miRNA-126-5p for vascular invasion showed that the AUC was 0.693 (P=0.002; Fig. 2D). In addition, miRNA-126-5p expression was lower in samples diagnosed with lymph node metastasis (LNM) (3.671±2.090 vs. 5.142±2.713, P=0.002; Fig. 2E) and the ROC curve relevant to LNM presented an AUC of 0.672 (P=0.003; Fig. 2F). miRNA-126-5p expression was also downregulated in later TNM stages (III–IV) of LUAD (4.923±2.700 vs. 3.769±2.233, P=0.024; Fig. 2G), while the relative ROC curve demonstrated that the AUC was 0.625 (P=0.032; Fig. 2H). No significant difference was observed between miRNA-126-3p and −5p and other clinicopathological features (all P>0.05; Table I).
Figure 2.
Correlation between miRNA-126-3p and miRNA-126-5p and clinical pathological parameters based on RT-qPCR data. (A) Expression of miRNA-126-3p in early (I–II) and late (III–IV) TNM stages of LUAD (4.140±2.583 vs. 3.025±1.527, P<0.001). (B) ROC curve of miRNA-126-3p for TNM stages of LUAD (AUC=0.608, P=0.039). (C) Expression of miRNA-126-5p in samples with vascular invasion and samples without vascular invasion (3.045±1.544 vs. 4.814±2.657, P<0.001). (D) ROC cure of miRNA-126-5p for vascular invasion of LUAD (AUC=0.693, P=0.002). (E) Expression of miRNA-126-5p in samples with LNM and samples without LNM (3.671±2.090 vs. 5.142±2.713, P=0.002). (F) ROC of miRNA-126-5p for LNM of LUAD (AUC=0.672, P=0.003). (G) Expression of miRNA-126-5p in early TNM stage (I–II) and later TNM stage (III–IV) of LUAD (4.923±2.700 vs. 3.769±2.233, P=0.024). (H) ROC of miRNA-126-5p for TNM stage of LUAD (AUC=0.625, P=0.032). LUAD, lung adenocarcinoma; AUC, area under the receiver operating characteristic (ROC) curve.
Meta-analysis of miRNA-126-3p and −5p in LUAD based on the GEO databas
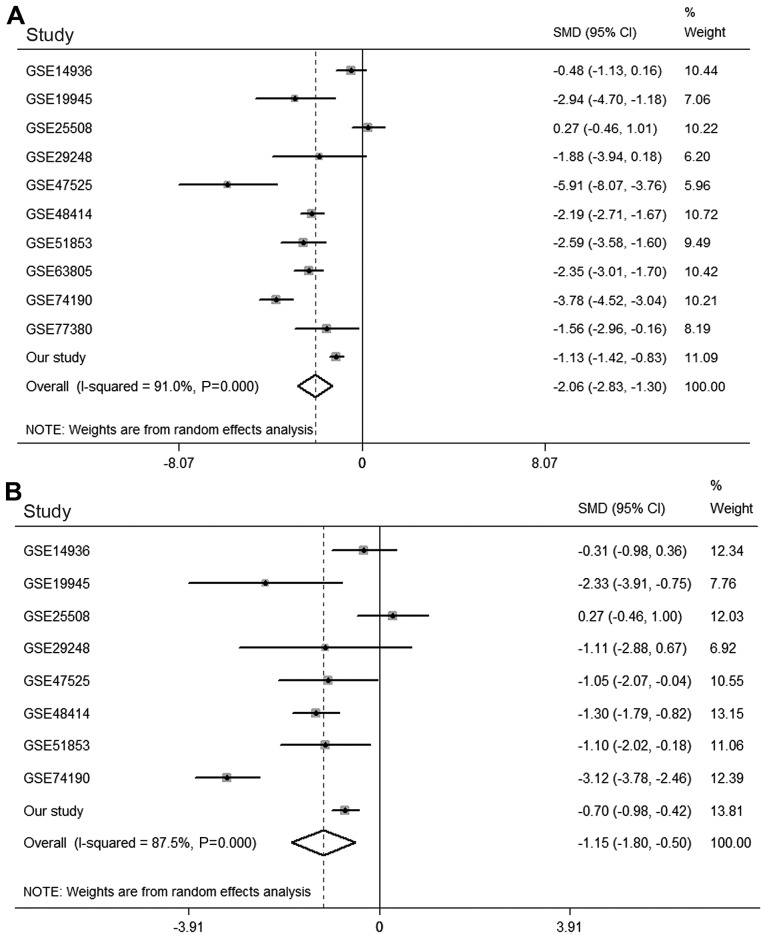
The ten relevant microarray datasets were the following: GSE14936 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14936), GSE19945 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE19945), GSE25508 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE25508), GSE29248 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE29248), GSE47525 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE47525), GSE48414 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE48414), GSE51853 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE51853), GSE63805 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE63805), GSE74190 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE74190), GSE77380 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE77380), which contained the expression values of miRNA-126-3p in 388 LUAD and 237 normal lung tissues, were identified and downloaded for meta-analysis with the results from the present study (Table II) (27–35). A random-effects model was used because major heterogeneity existed (I2=91.0%, P<0.001). The meta-analysis demonstrated that miRNA-126-3p expression was significantly lower in LUAD tissues than it was in normal lung tissues [SMD=−2.063, 95% confidence interval [CI]: −2.829 to −1.298, P<0.001; Fig. 3A].
Table II.
The 10 selected microarray datasets.
| Authors | Microarray datasets | Year | Country | LUAD tissues | Normal tissues | (Refs.) |
|---|---|---|---|---|---|---|
| Seike et al | GSE14936 | 2009 | USA | 19 | 19 | (27) |
| Ohba et al | GSE19945 | 2010 | Japan | 4 | 8 | Not published |
| Nymark et al | GSE25508 | 2011 | Finland | 10 | 26 | (28) |
| Ma et al | GSE29248 | 2011 | China | 3 | 3 | (29) |
| van Jaarsveld et al | GSE47525 | 2014 | The Netherlands | 6 | 14 | (30) |
| Bjaanaes et al | GSE48414 | 2014 | Norway | 154 | 20 | (31) |
| Arima et al | GSE51853 | 2014 | Japan | 76 | 5 | (32) |
| Robles et al | GSE63805 | 2015 | USA | 32 | 30 | (33) |
| Jin et al | GSE74190 | 2015 | China | 36 | 44 | (34) |
| Yoshimoto et al | GSE77380 | 2018 | Japan | 3 | 12 | (35) |
LUAD, lung adenocarcinoma.
Figure 3.
Forest plots of miRNA-126-3p and miRNA-126-5p based on microarray datasets. (A) Forest plot of miRNA-126-3p expression in LUAD according to 10 relevant microarray datasets and the present study. (B) Forest plot of miRNA-126-5p expression in LUAD according to 8 relevant microarray datasets and the present study. LUAD, lung adenocarcinoma; CI, confidence interval; SMD, standard mean difference.
Eight microarray datasets (GSE14936, GSE19945, GSE25508, GSE29248, GSE47525, GSE48414, GSE51853 and GSE74190) contained data concerning expression of miRNA-126-5p in 305 LUAD and 139 normal lung tissues. As major heterogeneity also existed in the meta-analysis of miRNA-126-5p (I2=87.5%, P<0.001), a random-effects model was again performed. The results showed that miRNA-126-5p also exhibited significantly low expression in LUAD tissues (SMD=−1.152, 95% CI, −1.804 to −0.499, P=0.001; Fig. 3B).
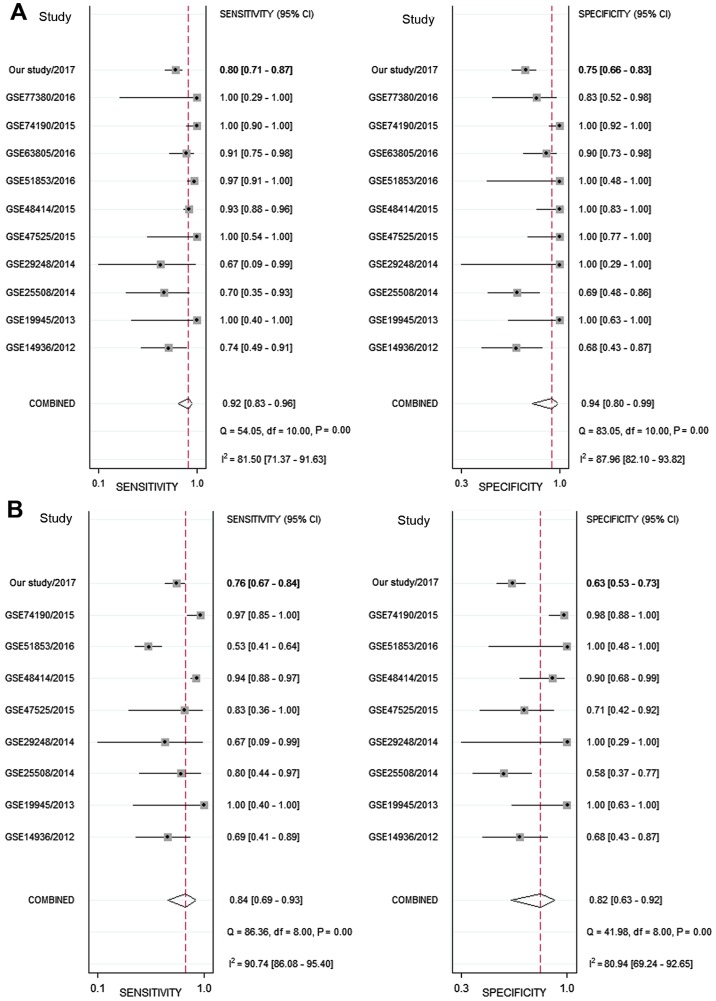
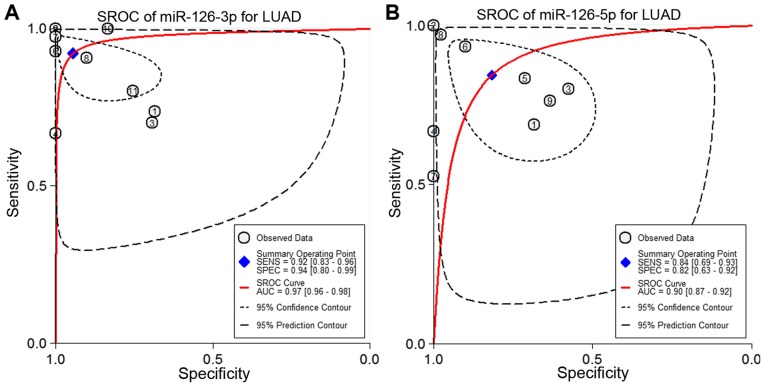
Next, the sensitivity and specificity of each microarray dataset and the present study were extracted (Fig. 4). The SROC curves of miRNA-126-3p and miRNA-126-5p, respectively, for LUAD tissues were performed using the GEO data and the present study's RT-qPCR data. The SROC of miRNA-126-3p showed an AUC of 0.97 (Fig. 5A), while the SROC of miRNA-126-5p showed an AUC of 0.90 (Fig. 5B).
Figure 4.
Sensitivity and specificity of the microarray datasets and the present study. Sensitivity and specificity of the 10 relevant microarray datasets and the present study according to the expression of (A) miRNA-126-3p and (B) miR-126-5p. CI, confidence interval.
Figure 5.
SROC of miRNA-126-3p and miRNA-126-5p for LUAD based on the microarrays and the present study. (A) SROC curve of miRNA-126-3p for LUAD according to the 10 relevant microarray datasets and the present study (AUC=0.97). (B) SROC curve of miRNA-126-5p for LUAD according to 8 relevant microarray datasets and the present study (AUC=0.90). LUAD, lung adenocarcinoma; SROC, summary ROC; AUC, area under the receiver operating characteristic (ROC) curve.
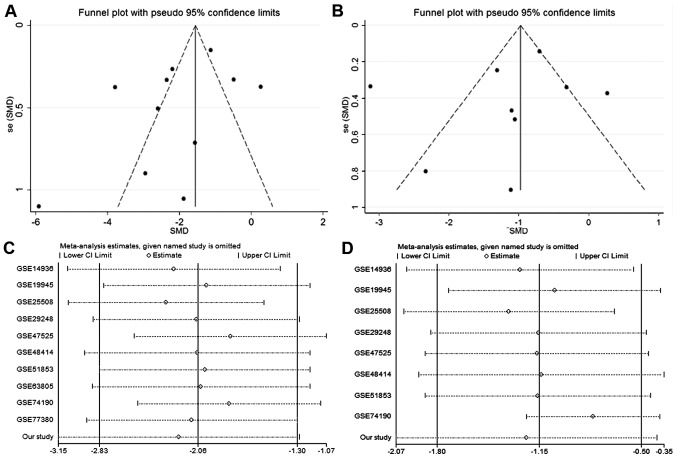
Funnel plots were employed to assess the publication bias of the two meta-analyses (Fig. 6A and B). The results of Begg's test (P=0.533 and P=0.533) and Egger's test (P=0.592, P=0.307) showed no statistically significant differences for either meta-analysis. Sensitivity analysis for these two meta-analyses revealed that significantly lower expression of miRNA-126-3p and miRNA-126-5p existed regardless of which microarray datasets were removed (Fig. 6C and D).
Figure 6.
Sensitivity analysis and publication bias of the selected microarray datasets. (A) Funnel plot of the 10 microarray datasets related to miRNA-126-3p and the present study. (B) Funnel plot of 8 microarray datasets related to miRNA-126-5p and the present study. (C) Sensitivity analysis based on the 10 microarray datasets related to miRNA-126-3p and the present study. (D) Sensitivity analysis based on 8 microarray datasets related to miRNA-126-5p and the present study. SMD, standard mean difference.
Correlation analysis between miRNA-126-3p and miRNA- 126-5p
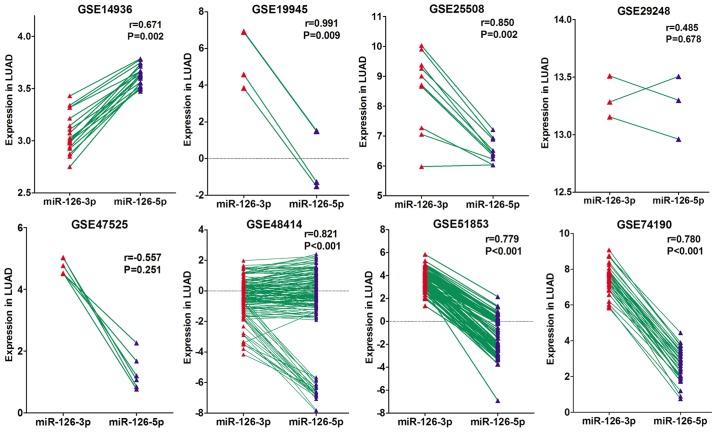
A Pearson correlation analysis was performed based on the GEO data to assess the correlation between miRNA-126-3p and miRNA-126-5p expression. Of the eight microarray datasets, six showed significant positive correlations (r=0.671–0.991, all P<0.05; Fig. 7), one suggested a tendency toward a positive correlation (r=0.486; P=0.678) and one suggested a trend toward a negative correlation (r=−0.557, P=0.251).
Figure 7.
Correlation between miRNA-126-3p and miRNA-126-5p based on GEO data. LUAD, lung adenocarcinoma.
Bioinformatic analysis based on the target genes of miRNA-126-3p and miRNA-126-5p
Prediction of the target genes
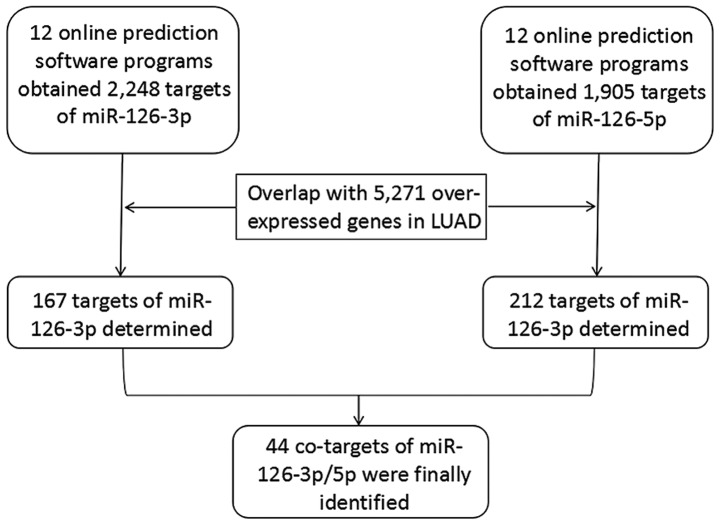
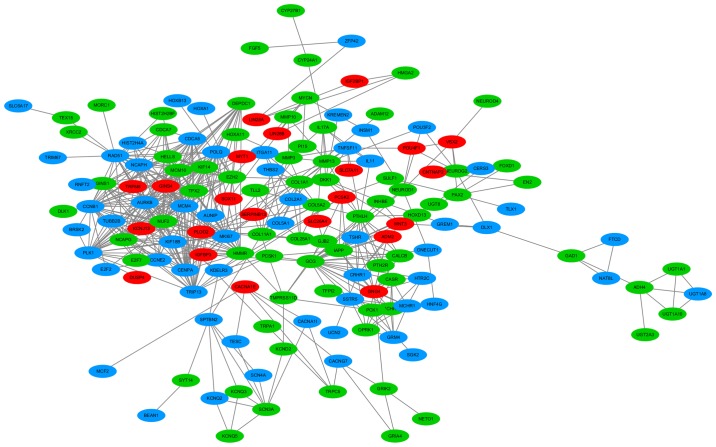
Twelve online prediction tools were employed for acquiring the potential targets of miRNA-126-3p and −5p, respectively. Initially, 2,248 genes and 1,905 genes were separately collected as the target genes of miRNA-126-3p and −5p. In addition, 5,271 upregulated genes in LUAD tissues were also obtained from the TCGA database. After the upregulated genes were overlapped with the 2,248 targets of miRNA-126-3p and the 1,905 targets of miRNA-126-5p, 167 genes and 212 genes, respectively, were identified as the targets of miRNA-126-3p and −5p in LUAD. Combining the 167 targets of miRNA-126-3p and 212 targets of miRNA-126-5p produced 335 total target genes; 44 genes were co-targets of both miRNA-126-3p and miRNA-126-5p (Fig. 8).
Figure 8.
The process of screening the targets. LUAD, lung adenocarcinoma.
GO analysis, KEGG pathways and PPI
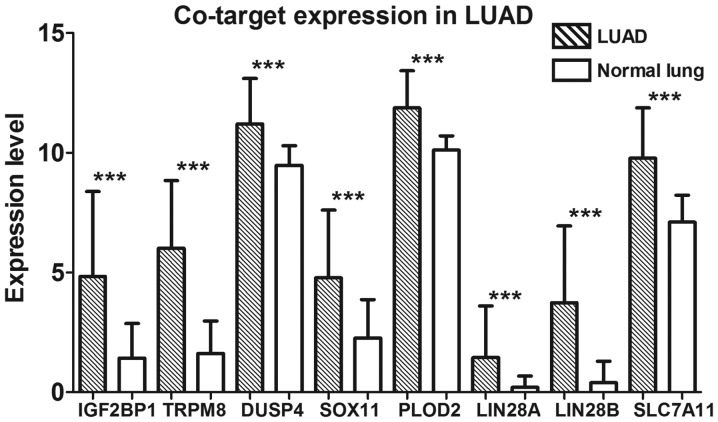
Based on these 335 target genes, GO and KEGG pathway analyses were conducted to determine their molecular biological functions in LUAD. The results of the GO analysis indicated that the targets were mainly enriched in cell-cell signaling of biological processes (BPs), which are integral to the plasma membrane of cell components (CCs) and channel activity of molecular functions (MFs) (Table III). The KEGG pathway analysis showed that target genes were significantly enriched in neuroactive ligand-receptor interaction (Table IV). In addition, a PPI network of these 335 target genes was performed (Fig. 9). Eight crucial co-target genes (IGF2BP1, TRPM8, DUSP4, SOX11, PLOD2, LIN28A, LIN28B and SLC7A11) were selected for further analysis. All were significantly highly expressed in LUAD (all P<0.001; Fig. 10).
Table III.
GO functional annotation of the 335 target genes.
| GO ID | Term | Count | P-value | Gene symbol |
|---|---|---|---|---|
| Biological process | ||||
| GO:0007267 | Cell-cell signaling | 31 | 4.43E-07 | FGF5, GRIK2, HOXA11, OPRK1, EFNA2, GLRA3, GREM1, IL11, PCSK1, KCNQ5, NPTX1, BARX1, IL17A, and others |
| GO:0045165 | Cell fate commitment | 13 | 8.31E-06 | ONECUT1, HOXA11, ONECUT2, NEUROG2, PAX2, VSX2, NR2E1, DLX1, WNT3, NEUROD1, NEUROD4, GAP43, TLX1 |
| GO:0060348 | Bone development | 11 | 7.90E-05 | PTHLH, CYP24A1, CASR, CYP27B1, TNFSF11, HOXA11, COL2A1, COL1A1, COL5A2, IGFBP3, MMP13 |
| Molecular function | ||||
| GO:0005887 | Integral to plasma membrane | 43 | 2.07E-05 | MCHR1, CASR, SCN3A, GRIK2, ENPP3, OPRK1, GLRA3, ITGA11, PCDHA1, KCNJ13, KCNQ5, UGT1A8, and others |
| GO:0034702 | Ion channel complex | 15 | 2.35E-05 | KCND2, SCN3A, GLRA3, CACNG7, CACNA1I, GABRA5, KCTD4, KCNJ13, KCNQ5, KCNQ3, CHRNA5, and others |
| GO:0031226 | Intrinsic to plasma membrane | 43 | 3.52E-05 | MCHR1, CASR, SCN3A, GRIK2, ENPP3, OPRK1, GLRA3, ITGA11, PCDHA1, KCNJ13, KCNQ5, UGT1A8, KCNQ3, and others |
| Cell component | ||||
| GO:0015267 | Channel activity | 23 | 7.61E-06 | KCND2, TRPM8, SCN3A, TRPC5, GRIK2, GLRA3, CACNG7, GABRA5, TRPA1, CACNA1I, GRIA4, CNGB3, KCTD4, and others |
| GO:0022803 | Passive transmembrane transporter activity | 23 | 7.90E-06 | KCND2, TRPM8, SCN3A, TRPC5, GRIK2, GLRA3, CACNG7, GABRA5, TRPA1, CACNA1I, GRIA4, CNGB3, KCTD4, and others |
| GO:0005216 | Ion channel activity | 22 | 9.34E-06 | KCND2, TRPM8, SCN3A, TRPC5, GRIK2, GLRA3, CACNG7, GABRA5, TRPA1, CACNA1I, GRIA4, CNGB3, KCTD4, and others |
GO, Gene Ontology.
Table IV.
KEGG pathway analysis based on the 335 target genes.
| KEGG ID | Term | Count | P-value | Gene symbol |
|---|---|---|---|---|
| hsa04080 | Neuroactive ligand-receptor interaction | 14 | 2.49E-04 | MCHR1, MCHR2, PTH2R, GRIK2, GLRA3, OPRK1, GABRA5, GRIA4, CRHR1, SSTR5, GRM4, HTR2C, TSHR, GABRP |
| hsa04512 | ECM-receptor interaction | 8 | 4.23E-04 | ITGA11, COL2A1, COL1A1, COL5A2, THBS2, COL11A1, COL5A1, HMMR |
| hsa04950 | Maturity onset diabetes of the young | 4 | 7.69E-03 | ONECUT1, IAPP, NEUROD1, HNF4G |
| hsa00140 | Steroid hormone biosynthesis | 4 | 3.97E-02 | AKR1C2, UGT1A10, UGT1A8, CYP21A2, UGT2A3, UGT1A1 |
| hsa00980 | Metabolism of xenobiotics by cytochrome P450 | 4 | 7.60E-02 | AKR1C2, UGT1A10, UGT1A8, ADH4, UGT2A3, UGT1A1 |
KEGG, Kyoto Encyclopedia of Genes and Genomes.
Figure 9.
PPI network based on 335 target genes of miRNA-126-3p and miRNA-126-5p. The blue nodes represent the target genes of miRNA-126-3p; the green nodes represent the target genes of miRNA-126-5p; the red nodes represent the co-regulated target genes of both miRNA-126-3p and miRNA-126-5p. The lines between the nodes represent the associations between the different target genes. PPI, protein-protein interaction.
Figure 10.
Expression levels of the eight key co-targets of miRNA-126-3p and miRNA-126-5p. The bars in the histogram with oblique lines represent LUAD tissues, and the blank bars represent normal lung tissues. All eight genes were expressed significantly higher in the LUAD tissues (***P<0.001). LUAD, lung adenocarcinoma.
Discussion
In recent years, the effect of microRNAs (miRNAs) on the pathogenesis of cancers has been well investigated and clarified. However, even as the investigation of miRNAs has matured and their functions have become clearer, the co-function of miRNA-3p and −5p is rarely mentioned. The present study verified the co-downregulation of the expression of miRNA-126-3p and miRNA-126-5p in lung adenocarcinoma (LUAD) tissues using real-time quantitative polymerase chain reaction (RT-qPCR) and the GEO database. Bioinformatic analysis based on the obtained target genes of miRNA-126-3p and −5p was performed to clarify their co-regulation in LUAD.
The function of miRNA-126-3p has been articulated in previous research. miRNA-126-3p levels have been shown to be downregulated in various malignancies including colorectal, esophageal, oral, liver, breast, thyroid and renal cancers (36–42). In contrast, investigations of miRNA-126-5p in malignant tumors have been rare and limited. Several studies have reported that miRNA-126-5p is expressed at a lower level in colorectal and breast cancers (43,44). As for lung cancer (LC), several studies have reported that miRNA-126-3p expression is downregulated in non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), suggesting that it is an LC suppressor (45,46). The targets of miRNA-126-3p have also been described in previous studies. Liu and colleagues pointed out that miRNA-126-3p could inhibit the growth of LC cell lines by targeting vascular endothelial growth factor (VEGF) (47). Moreover, Song et al demonstrated that miRNA-126-3p targeted PIK3R2 and affected the PTEN/PI3K/AKT signaling pathway to suppress the growth of an NSCLC cell line (48). As for the role and effect of miRNA-126-5p in LC, available literature is scarce.
All previous studies concerning miRNA-126 in LC investigated miRNA-126-3p and ignored the function of miRNA-126-5p. To the best of our knowledge, no previous study has explored the co-function of miRNA-126-3p and −5p in LC. The present study first concentrated on the co-function and co-targets of miRNA-126-3p and −5p in LUAD. A total of 202 tissues and 11 microarray datasets were collected to identify the co-expression of miRNA-126-3p and −5p in LUAD. Bioinformatic analysis based on the potential target genes was performed in the hopes of identifying the co-regulation and synergistic effects of miRNA-126-3p and −5p in LUAD.
Both miRNA-126-3p and miRNA-126-5p are derived from precursor miRNA-126 and are associated with angiogenesis and cell proliferation in malignancies. The present study confirmed that both miRNA-126-3p and −5p have lower expression in LUAD tissues, indicating that both may be LUAD tumor suppressors. In addition, expression levels of miRNA-126-3p and −5p were significantly downregulated in late stage LUAD. The miRNA-126-5p expression levels were significantly lower in samples with vascular invasion and LNM, suggesting that miRNA-126-3p and −5p were related to the development, invasion, and metastasis of LUAD. Lower expression of miRNA-126-3p and −5p suggested a worse prognosis of LUAD.
In addition, correlation analysis showed a positive correlation between miRNA-126-3p and −5p expression, meaning that in LUAD, miRNA-126-5p levels decreased as miRNA-126-3p decreased. Thus, miRNA-126-3p and −5p are not expressed independently in LUAD; they are expressed consistently. Analysis of miRNA-126-3p and −5p levels also showed that although these two miRNAs are expressed consistently, the level of miRNA-126-5p was usually lower than that of miRNA-126-3p in the same LUAD tissue sample. This observation conforms to the rule of mature miRNA expression: When miRNA-3p and −5p are developed from the same precursor, one of them is often more highly expressed than the other (14). Previous research improperly assumed that the lower-expressed miRNA was functionally irrelevant, while the higher-expressed one played a leading role in biological processes (13,14). In fact, additional research has demonstrated the important function of both miRNA-3p and −5p (49). The present study confirmed the significantly lower expression of both miRNA-126-3p and −5p in LUAD and demonstrated their association with TNM stage, invasion and metastasis; it also confirmed that both of them perform important functions. Their consistent expression may indicate a synergistic effect in LUAD.
The target predictions further demonstrated the synergistic effects of miRNA-126-3p and −5p. Combining the 167 target genes of miRNA-126-3p with the 212 targets of miRNA-126-5p revealed that these two miRNAs share 44 co-targets. In other words, 26% (44/167) of miRNA-126-3p targets and 20% (44/212) of miRNA-126-5p targets were co-targets of both. This high target overlap provides credible evidence for their co-regulation in LUAD. The combined 335 targets were mainly enriched in neuroactive ligand-receptor interaction and ECM-receptor interaction of the KEGG pathway. However, when the 167 miRNA-126-3p targets and 212 miRNA-126-5p targets were separately considered to explore the KEGG pathway, the results also showed that the targets were mainly enriched in neuroactive ligand-receptor interaction and ECM-receptor interaction. Thus, miRNA-126-3p and −5p may influence LUAD development together via a similar signaling pathway.
More importantly, many of these co-targets have been identified as key genes in LC development. IGF2BP1 has been proven to be a target gene of miRNA-491-5p. miRNA-491-5p can downregulate IGF2BP1 to suppress the growth of NSCLC (50). TRPM8 was found to contribute to an invasive phenotype in LC (51). Furthermore, DUSP4 was shown to function as a suppressor and can inhibit the growth of EGFR-mutant LUAD (52). SOX11 was found to exhibit significantly higher expression in LC and is associated with a prognosis of neuroendocrine tumors of the lung (53). PLOD2 was proven to be regulated by the PI3K/AKT-FOXA1 axis to promote the metastasis of LC, and its high expression is indicative of a worse prognosis of LUAD (54). miRNA-98 may suppress the expression of LIN28A to decrease the invasion and metastasis of LC cells (55), while LIN28B expression is high in tumor-initiating cells from NSCLC and functions in the tumorigenesis of lung cancer cells (56). The overexpression of SLC7A11 was shown to be associated with poor prognosis of LC (57). According to TCGA data, all these co-targets play critical roles and are overexpressed in LUAD. miRNA-126-3p and −5p may directly target these key genes at the same time to co-regulate the development of LUAD. Downregulation of the expression of miRNA-126-3p and −5p reduces their combination with these targets, which decreases the degradation of these target genes and causes them to be highly expressed in LUAD. It will be meaningful to deeply research these potential targets and further validate that they are the downstream targets of miRNA-126-3p and −5p in LUAD. However, the core point of this study was to demonstrate the co-regulation as well as the synergistic effects of miRNA-125-3p and −5p in LUAD, while elucidating the downstream target genes is a secondary viewpoint of our study. Thus, we completed a preliminary identification of these potential targets. Further and intensive investigation is needed to validate them in the future.
It is worth mentioning that the co-function of miRNA-3p and −5p has only recently begun to be recognized and investigated. In fact, many miRNA-3p and −5p pairs have been found to co-regulate the same malignant tumors (49). The present study demonstrated the co-regulation of miRNA-126-3p and −5p in LUAD. The present results and the PPI network provide clear evidence of the existence of the synergistic effects between miRNA-126-3p and −5p in LUAD. The co-regulation of miRNA-126-3p and −5p may be a fail-proof mode of the regulation of miRNAs in LUAD or other malignancies, which can ensure that if either miRNA-126-3p or −5p is disabled by transcriptional inhibition or mutation, the associated biological function may still proceed stably and normally (58). In the regulation of a malignant tumor, this fail-proof mode may also exist between pairings other than miRNA-3p and −5p or even between miRNAs from two different families.
In conclusion, the present study verified the co-downregulation of the expression of miRNA-126-3p and −5p in LUAD tissues and discovered the existence of co-target genes between miRNA-126-3p and −5p, many of which have been confirmed to be closely related to the proliferation, invasion, metastasis and poor prognosis of LC. These results indicate that the co-regulation of miRNA-126-3p and −5p plays a significant role in the development of LUAD, which also implies a fail-proof mode between miRNA-3p and −5p. While miRNA-126-5p is by no means equivalent to miRNA-126-3p, the two miRNAs possess vital associations and valuable clinical significance in LUAD and various other malignancies.
Acknowledgements
Not applicable.
Funding
The present study was supported by funds from the National Natural Science Foundation of China (NSFC 81560469), the Natural Science Foundation of Guangxi, China (nos. 2017GXNSFAA198016 and 2015GXNSFCA139009), the Guangxi Medical University Training Program for Distinguished Young Scholars, Medical Excellence Award Funded by the Creative Research Development Grant from the First Affiliated Hospital of Guangxi Medical University, and the Guangxi Zhuang Autonomous Region University Student Innovative Plan (no. 201710598064).
Availability of data and materials
The datasets used and analyzed in the current study are available from the corresponding authors on reasonable request.
Authors' contributions
PC designed the study, analyzed the main data and wrote the main part of the manuscript. YYG and FCM carried out the detection of miRNA-126-3p and −5p expression in LUAD, collected the data from GEO database and contributed to the writing of the manuscript. RQH analyzed the data and revised the manuscript. ZYL completed the detection of miRNA-126-3p and −5p expression and analysed the data. GQZ and XL performed the bioinformatics analyses. XHH designed the study and revised the manuscript. LJP and GC designed the study, supervised all experiments and revised the manuscript. All authors read and approved the final manuscript and agree to be accountable for all aspects of the research in ensuring that the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Ethics approval and consent to participate
The research protocol for this study has been ratified by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. All patients content was obtained at the time of the sample collection.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests, and all authors confirm its accuracy.
References
- 1.Blanco-Prieto S, Barcia-Castro L, Páez de la Cadena M, Rodríguez-Berrocal FJ, Vázquez-Iglesias L, Botana-Rial MI, Fernández-Villar A, De Chiara L. Relevance of matrix metalloproteases in non-small cell lung cancer diagnosis. BMC Cancer. 2017;17:823. doi: 10.1186/s12885-017-3842-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan Y, Wu J, Li B, Niu J, Tan H, Qiu S. Regulation of signaling pathways involved in the anti-proliferative and apoptosis-inducing effects of M22 against non-small cell lung adenocarcinoma A549 cells. Sci Rep. 2018;8:992. doi: 10.1038/s41598-018-19368-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shen J, Wang B, Zhang T, Zhu N, Wang Z, Jin J, He Y, Hu M. Suppression of non-small cell lung cancer growth and metastasis by a novel small molecular activator of RECK. Cell Physiol Biochem. 2018;45:1807–1817. doi: 10.1159/000487872. [DOI] [PubMed] [Google Scholar]
- 4.Li X, Han J, Zhu H, Peng L, Chen Z. MiR181b5p mediates TGFbeta1-induced epithelial-to-mesenchymal transition in non-small cell lung cancer stem-like cells derived from lung adenocarcinoma A549 cells. Int J Oncol. 2017;51:158–168. doi: 10.3892/ijo.2017.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denisenko TV, Budkevich IN, Zhivotovsky B. Cell death-based treatment of lung adenocarcinoma. Cell Death Dis. 2018;9:117. doi: 10.1038/s41419-017-0063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y, Zhou S. MicroRNA-29a inhibits mesenchymal stem cell viability and proliferation by targeting Roundabout 1. Mol Med Rep. 2015;12:6178–6184. doi: 10.3892/mmr.2015.4183. [DOI] [PubMed] [Google Scholar]
- 7.Zhou S, Ye W, Zhang Y, Yu D, Shao Q, Liang J, Zhang M. MiR-144 reverses chemoresistance of hepatocellular carcinoma cell lines by targeting Nrf2-dependent antioxidant pathway. Am J Transl Res. 2016;8:2992–3002. [PMC free article] [PubMed] [Google Scholar]
- 8.Gamazon ER, Trendowski MR, Wen Y, Wing C, Delaney SM, Huh W, Wong S, Cox NJ, Dolan ME. Gene and microRNA perturbations of cellular response to pemetrexed implicate biological networks and enable imputation of response in lung adenocarcinoma. Sci Rep. 2018;8:733. doi: 10.1038/s41598-017-19004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao F, Ge YZ, Zhou LH, Xu LW, Xu Z, Ping WW, Wang M, Zhou CC, Wu R, Jia RP. Identification of hub miRNA biomarkers for bladder cancer by weighted gene coexpression network analysis. Onco Targets Ther. 2017;10:5551–5559. doi: 10.2147/OTT.S146479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cochetti G, Poli G, Guelfi G, Boni A, Egidi MG, Mearini E. Different levels of serum microRNAs in prostate cancer and benign prostatic hyperplasia: Evaluation of potential diagnostic and prognostic role. Onco Targets Ther. 2016;9:7545–7553. doi: 10.2147/OTT.S119027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang L, Wei DM, Li JJ, Luo DZ, Chen G, Dang YW, Cai XY. Prognostic microRNAs and their potential molecular mechanism in pancreatic cancer: A study based on The Cancer Genome Atlas and bioinformatics investigation. Mol Med Rep. 2018;17:939–951. doi: 10.3892/mmr.2017.7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acunzo M, Romano G, Wernicke D, Croce CM. MicroRNA and cancer-a brief overview. Adv Biol Regul. 2015;57:1–9. doi: 10.1016/j.jbior.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 13.Mitra R, Lin CC, Eischen CM, Bandyopadhyay S, Zhao Z. Concordant dysregulation of miR-5p and miR-3p arms of the same precursor microRNA may be a mechanism in inducing cell proliferation and tumorigenesis: A lung cancer study. RNA. 2015;21:1055–1065. doi: 10.1261/rna.048132.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamura K, Phillips MD, Tyler DM, Duan H, Chou YT, Lai EC. The regulatory activity of microRNA* species has substantial influence on microRNA and 3′UTR evolution. Nat Struct Mol Biol. 2008;15:354–363. doi: 10.1038/nsmb.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo L, Yu J, Yu H, Zhao Y, Chen S, Xu C, Chen F. Evolutionary and expression analysis of miR-#-5p and miR-#-3p at the miRNAs/isomiRs levels. BioMed Res Int. 2015;2015:168358. doi: 10.1155/2015/168358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu T, Li J, Yan M, Liu L, Lin H, Zhao F, Sun L, Zhang Y, Cui Y, Zhang F, et al. MicroRNA-193a-3p and 5p suppress the metastasis of human non-small-cell lung cancer by downregulating the ERBB4/PIK3R3/mTOR/S6K2 signaling pathway. Oncogene. 2015;34:413–423. doi: 10.1038/onc.2013.574. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Yang P, Sun T, Li D, Xu X, Rui Y, Li C, Chong M, Ibrahim T, Mercatali L, et al. MiR-126 and miR-126* repress recruitment of mesenchymal stem cells and inflammatory monocytes to inhibit breast cancer metastasis. Nat Cell Biol. 2013;15:284–294. doi: 10.1038/ncb2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Q, Hu H, Jiao D, Yan J, Xu W, Tang X, Chen J, Wang J. miR-126-3p and miR-451a correlate with clinicopathological features of lung adenocarcinoma: The underlying molecular mechanisms. Oncol Rep. 2016;36:909–917. doi: 10.3892/or.2016.4854. [DOI] [PubMed] [Google Scholar]
- 19.Tang R, Zhong T, Dang Y, Zhang X, Li P, Chen G. Association between downexpression of miR-203 and poor prognosis in non-small cell lung cancer patients. Clin Transl Oncol. 2016;18:360–368. doi: 10.1007/s12094-015-1377-9. [DOI] [PubMed] [Google Scholar]
- 20.Verderio P, Bottelli S, Ciniselli CM, Pierotti MA, Gariboldi M, Pizzamiglio S. NqA: An R-based algorithm for the normalization and analysis of microRNA quantitative real-time polymerase chain reaction data. Anal Biochem. 2014;461:7–9. doi: 10.1016/j.ab.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Benes V, Castoldi M. Expression profiling of microRNA using real-time quantitative PCR, how to use it and what is available. Methods. 2010;50:244–249. doi: 10.1016/j.ymeth.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 22.Chen G, Umelo IA, Lv S, Teugels E, Fostier K, Kronenberger P, Dewaele A, Sadones J, Geers C, De Grève J. MiR-146a inhibits cell growth, cell migration and induces apoptosis in non-small cell lung cancer cells. PLoS One. 2013;8:e60317. doi: 10.1371/journal.pone.0060317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 24.Ivo D, Urso P, Fernando D'Urso O, Damiano Gianfreda C, Mezzolla V, Storelli C, Marsigliante S. miR-15b and miR-21 as circulating biomarkers for diagnosis of glioma. Curr Genomics. 2015;16:304–311. doi: 10.2174/1389202916666150707155610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liang XN, Guo RJ, Li S, Zheng ZM, Liang HD. Binary logistic regression analysis of solid thyroid nodules imaged by high-frequency ultrasonography, acoustic radiation force impulse, and contrast-enhanced ultrasonography. Eur Rev Med Pharmacol Sci. 2014;18:3601–3610. [PubMed] [Google Scholar]
- 26.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seike M, Goto A, Okano T, Bowman ED, Schetter AJ, Horikawa I, Mathe EA, Jen J, Yang P, Sugimura H, et al. MiR-21 is an EGFR-regulated anti-apoptotic factor in lung cancer in never-smokers. Proc Nal Acad Sci USA. 2009;106:12085–12090. doi: 10.1073/pnas.0905234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nymark P, Guled M, Borze I, Faisal A, Lahti L, Salmenkivi K, Kettunen E, Anttila S, Knuutila S. Integrative analysis of microRNA, mRNA and aCGH data reveals asbestos- and histology-related changes in lung cancer. Genes Chromosome Cancer. 2011;50:585–597. doi: 10.1002/gcc.20880. [DOI] [PubMed] [Google Scholar]
- 29.Ma L, Huang Y, Zhu W, Zhou S, Zhou J, Zeng F, Liu X, Zhang Y, Yu J. An integrated analysis of miRNA and mRNA expressions in non-small cell lung cancers. PLoS One. 2011;6:e26502. doi: 10.1371/journal.pone.0026502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Jaarsveld MT, Wouters MD, Boersma AW, Smid M, van Ijcken WF, Mathijssen RH, Hoeijmakers JH, Martens JW, van Laere S, Wiemer EA, Pothof J. DNA damage responsive microRNAs misexpressed in human cancer modulate therapy sensitivity. Mol Oncol. 2014;8:458–468. doi: 10.1016/j.molonc.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjaanaes MM, Halvorsen AR, Solberg S, Jørgensen L, Dragani TA, Galvan A, Colombo F, Anderlini M, Pastorino U, Kure E, et al. Unique microRNA-profiles in EGFR-mutated lung adenocarcinomas. Int J Cancer. 2014;135:1812–1821. doi: 10.1002/ijc.28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arima C, Kajino T, Tamada Y, Imoto S, Shimada Y, Nakatochi M, Suzuki M, Isomura H, Yatabe Y, Yamaguchi T, et al. Lung adenocarcinoma subtypes definable by lung development-related miRNA expression profiles in association with clinicopathologic features. Carcinogenesis. 2014;35:2224–2231. doi: 10.1093/carcin/bgu127. [DOI] [PubMed] [Google Scholar]
- 33.Robles AI, Arai E, Mathé EA, Okayama H, Schetter AJ, Brown D, Petersen D, Bowman ED, Noro R, Welsh JA, et al. An integrated prognostic classifier for stage I lung adenocarcinoma based on mRNA, microRNA, and DNA methylation biomarkers. J Thorac Oncol. 2015;10:1037–1048. doi: 10.1097/JTO.0000000000000560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin Y, Liu Y, Zhang J, Huang W, Jiang H, Hou Y, Xu C, Zhai C, Gao X, Wang S, et al. The expression of miR-375 is associated with carcinogenesis in three subtypes of lung cancer. PLoS One. 2015;10:e0144187. doi: 10.1371/journal.pone.0144187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoshimoto T, Motoi N, Yamamoto N, Nagano H, Ushijima M, Matsuura M, Okumura S, Yamaguchi T, Fukayama M, Ishikawa Y. Pulmonary carcinoids and low-grade gastrointestinal neuroendocrine tumors show common MicroRNA expression profiles, different from adenocarcinomas and small cell carcinomas. Neuroendocrinology. 2018;106:47–57. doi: 10.1159/000461582. [DOI] [PubMed] [Google Scholar]
- 36.Xiong Y, Kotian S, Zeiger MA, Zhang L, Kebebew E. MiR-126-3p inhibits thyroid cancer cell growth and metastasis, and is associated with aggressive thyroid cancer. PLoS One. 2015;10:e0130496. doi: 10.1371/journal.pone.0130496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du C, Lv Z, Cao L, Ding C, Gyabaah OA, Xie H, Zhou L, Wu J, Zheng S. MiR-126-3p suppresses tumor metastasis and angiogenesis of hepatocellular carcinoma by targeting LRP6 and PIK3R2. J Transl Med. 2014;12:259. doi: 10.1186/s12967-014-0259-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu W, Chen H, Wong N, Haynes W, Baker CM, Wang X. Pseudohypoxia induced by miR-126 deactivation promotes migration and therapeutic resistance in renal cell carcinoma. Cancer Lett. 2017;394:65–75. doi: 10.7150/jca.16739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J, Ping JL, Ma B, Chen YR, Li LQ. Deregulation of miR-126-3p in basal-like breast cancers stroma and its clinical significance. Pathol Res Pract. 2017;213:922–928. doi: 10.1016/j.prp.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 40.Fiala O, Pitule P, Hosek P, Liska V, Sorejs O, Bruha J, Vycital O, Buchler T, Poprach A, Topolcan O, Finek J. The association of miR-126-3p, miR-126-5p and miR-664-3p expression profiles with outcomes of patients with metastatic colorectal cancer treated with bevacizumab. Tumour Biol. 2017;39:1010428317709283. doi: 10.1177/1010428317709283. [DOI] [PubMed] [Google Scholar]
- 41.Kong R, Ma Y, Feng J, Li S, Zhang W, Jiang J, Zhang J, Qiao Z, Yang X, Zhou B. The crucial role of miR-126 on suppressing progression of esophageal cancer by targeting VEGF-A. Cell Mol Biol Lett. 2016;21:3. doi: 10.1186/s11658-016-0004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasahira T, Kurihara M, Bhawal UK, Ueda N, Shimomoto T, Yamamoto K, Kirita T, Kuniyasu H. Downregulation of miR-126 induces angiogenesis and lymphangiogenesis by activation of VEGF-A in oral cancer. Br J Cancer. 2012;107:700–706. doi: 10.1038/bjc.2012.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paszek S, Gabło N, Barnaś E, Szybka M, Morawiec J, Kołacińska A, Zawlik I. Dysregulation of microRNAs in triple-negative breast cancer. Ginekol Pol. 2017;88:530–536. doi: 10.5603/GP.a2017.0097. [DOI] [PubMed] [Google Scholar]
- 44.Jinushi T, Shibayama Y, Kinoshita I, Oizumi S, Jinushi M, Aota T, Takahashi T, Horita S, Dosaka-Akita H, Iseki K. Low expression levels of microRNA-124-5p correlated with poor prognosis in colorectal cancer via targeting of SMC4. Cancer Med. 2014;3:1544–1552. doi: 10.1002/cam4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng Z, Pan L, Niu Z, Li W, Dang X, Wan L, Zhang R, Yang S. Identification of microRNAs as potential biomarkers for lung adenocarcinoma using integrating genomics analysis. Oncotarget. 2017;8:64143–64156. doi: 10.18632/oncotarget.19358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jusufović E, Rijavec M, Keser D, Korošec P, Sodja E, Iljazović E, Radojević Z, Košnik M. let-7b and miR-126 are down-regulated in tumor tissue and correlate with microvessel density and survival outcomes in non-small-cell lung cancer. PLoS One. 2012;7:e45577. doi: 10.1371/journal.pone.0045577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu B, Peng XC, Zheng XL, Wang J, Qin YW. MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer. 2009;66:169–175. doi: 10.1016/j.lungcan.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Song L, Li D, Gu Y, Wen ZM, Jie J, Zhao D, Peng LP. MicroRNA-126 targeting PIK3R2 inhibits NSCLC A549 cell proliferation, migration, and invasion by regulation of PTEN/PI3K/AKT pathway. Clin Lung Cancer. 2016;17:e65–e75. doi: 10.1016/j.cllc.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 49.Huang CJ, Nguyen PN, Choo KB, Sugii S, Wee K, Cheong SK, Kamarul T. Frequent co-expression of miRNA-5p and −3p species and cross-targeting in induced pluripotent stem cells. Int J Med Sci. 2014;11:824–833. doi: 10.7150/ijms.8358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gong F, Ren P, Zhang Y, Jiang J, Zhang H. MicroRNAs-491-5p suppresses cell proliferation and invasion by inhibiting IGF2BP1 in non-small cell lung cancer. Am J Transl Res. 2016;8:485–495. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Du GJ, Li JH, Liu WJ, Liu YH, Zhao B, Li HR, Hou XD, Li H, Qi XX, Duan YJ. The combination of TRPM8 and TRPA1 expression causes an invasive phenotype in lung cancer. Tumour Biol. 2014;35:1251–1261. doi: 10.1007/s13277-013-1167-3. [DOI] [PubMed] [Google Scholar]
- 52.Lin H, Qiu S, Xie L, Liu C, Sun S. Nimbolide suppresses non-small cell lung cancer cell invasion and migration via manipulation of DUSP4 expression and ERK1/2 signaling. Biomed Pharmacother. 2017;92:340–346. doi: 10.1016/j.biopha.2017.05.072. [DOI] [PubMed] [Google Scholar]
- 53.Walter RF, Mairinger FD, Werner R, Ting S, Vollbrecht C, Theegarten D, Christoph DC, Zarogoulidis K, Schmid KW, Zarogoulidis P, Wohlschlaeger J. SOX4, SOX11 and PAX6 mRNA expression was identified as a (prognostic) marker for the aggressiveness of neuroendocrine tumors of the lung by using next-generation expression analysis (NanoString) Future Oncol. 2015;11:1027–1036. doi: 10.2217/fon.15.18. [DOI] [PubMed] [Google Scholar]
- 54.Du H, Chen Y, Hou X, Huang Y, Wei X, Yu X, Feng S, Wu Y, Zhan M, Shi X, et al. PLOD2 regulated by transcription factor FOXA1 promotes metastasis in NSCLC. Cell Death Dis. 2017;8:e3143. doi: 10.1038/cddis.2017.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu WL, Chang JM, Chong IW, Hung YL, Chen YH, Huang WT, Kuo HF, Hsieh CC, Liu PL. Curcumin inhibits LIN-28A through the activation of miRNA-98 in the lung cancer cell line A549. Molecules. 2017;22:E929. doi: 10.3390/molecules22060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, Soh BS, Sun LL, Tai BC, Nga ME, et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012;148:259–272. doi: 10.1016/j.cell.2011.11.050. [DOI] [PubMed] [Google Scholar]
- 57.Cohen AS, Khalil FK, Welsh EA, Schabath MB, Enkemann SA, Davis A, Zhou JM, Boulware DC, Kim J, Haura EB, Morse DL. Cell-surface marker discovery for lung cancer. Oncotarget. 2017;8:113373–113402. doi: 10.18632/oncotarget.23009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choo KB, Soon YL, Nguyen PN, Hiew MS, Huang CJ. MicroRNA-5p and −3p co-expression and cross-targeting in colon cancer cells. J Biomed Sci. 2014;21:95. doi: 10.1186/s12929-014-0095-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed in the current study are available from the corresponding authors on reasonable request.