Abstract
Objective:
This study assessed femur properties in 80 adult female rats exposed to a range of whole body vibration amplitudes at 45 Hz over five weeks. Our hypothesis was that an optimal amplitude for whole body vibration would be apparent and would result in increased bone strength.
Methods:
Animals were treated in five amplitude groups (0 g, 0.15 g, 0.3 g, 0.6 g, and 1.2 g peak), for 15 minutes per day, five days per week, for five weeks. Femur strength was assessed via: (1) three-point bending of the shaft, (2) cantilever bending of the neck, and (3) indentation of distal cancellous bone. Femoral bone mineral density, plasma prostaglandin E2 (PGE2) concentrations, cartilage thickness, and histopathologic properties were measured.
Results:
Vibration doubled (P=0.039) cancellous bone stiffness in the 0.6 g and 1.2 g groups and induced a 74% increase in PGE2 concentrations (P=0.007). However, femoral densitometry and strength of the neck and shaft were unchanged and the cancellous bone indentation strength did not differ statistically (P=0.084). Cartilage thickness of vibrated groups at the medial condyle did not increase significantly (P=0.142) and the histopathologic grade did not change. There was no definitive optimal vibration amplitude.
Conclusion:
The benefits of vibration therapy over five weeks were confined to cancellous bone.
Keywords: Vibration, Osteoporosis, Osteoarthritis, Biomechanics, Rats
Introduction
Whole body vibration (WBV) is a non-pharmaceutical therapy that has been investigated in a wide range of contexts, including gait and balance[1], lower extremity strength training[2], knee osteoarthritis[3], and fracture healing[4]. Perhaps the greatest area of interest for WBV is its potential influence on bone strength, considering the possibility that it might be used for osteoporosis prophylaxis in at-risk populations. It is possible that stimulation from low-level vibration is low enough to avoid bone damage, but large enough to stimulate bone formation[5]. This is a notion that has been investigated by a number of researchers to date. Tankasheva et al and Liphardt et al found no improvement in bone mineral density (BMD) or bone quality in postmenopausal women subjected to WBV for 6 months and 12 months respectively[6,7]. Similarly, Von Stengel et al found that WBV did not improve lumbar BMD compared to control, but did decrease fall risk[8]. Verschueren et al, on the other hand, found significant increases in hip BMD in postmenopausal women exposed to six months of WBV[9]. Oxlund et al showed that WBV over 90 days at 45 Hz could inhibit the decline in bending stress and compressive stress induced by ovariectomy in adult female rats[10]. Rubinacci et al found 30 Hz vibration at 0.6 and 3 g had anabolic effects on the cortical bone of ovariectomized rats, with the greatest effect occurring at 3 g[11]. When studying amplitudes ranging from 0 to 1.0 g at 46 Hz, Christiansen et al found a non-dose-dependent increase in trabecular bone volume at the proximal tibial metaphysis[12]. Wenger et al studied WBV at 32 Hz, and found 1.5 g to have the greatest anabolic effect based on serologic markers, and 0.5 g to have the greatest effect based on bone density[13].
These existing works shed a great deal of light on some of the potential effects of WBV, but there are some important considerations that have yet to be addressed. First, the bulk of these studies have focused on BMD as studied by computed tomography. It is established that BMD is a marker for quantity of bone tissue, as opposed to quality[14] and moreover that there is a variable relationship between BMD and bone strength. The present work makes use of both BMD and strength testing at several sites throughout the femur in order to more completely and directly study the effects of this therapy on mechanical properties of bone.
The mechanism by which vibration may influence bone properties is another aspect that remains unclear in the literature. While ordinary markers of osteoblastic and osteoclastic activities (e.g. bone specific alkaline phosphatase and tartrate-resistant acid phosphatase-5b) could be measured, the potential for vibration to influence prostaglandin E2 (PGE2) production as its mechanism of altering bone properties is an intriguing one in that past work has shown exogenous PGE2 to increase bone strength and mineral density in rat models by increasing bone formation over bone resorption[15]. Past studies with cultured osteoblast have shown an increase in the inducible enzyme responsible for PGE2 production, COX-2, with vibration exposure[16] and in vivo studies of whole body vibration at lower frequencies have shown increased serum levels of PGE2 [17]. As a result, we decided to measure plasma PGE2 levels as a preliminary investigation into this possible mechanism by which vibration might influence bone properties.
Another aspect that has been addressed in a more limited manner in the literature is the effect of vibration on cartilage[18], While some work has suggested positive effects of vibration to cartilage in humans[16] other work has suggested a potential to enhance degenerative effects[20] or no effect[21] in vivo in mouse models.
A common limitation of the existing therapeutic vibration literature is that there is a very wide range of settings available across the current devices on the market, perhaps because of the wide variety of potential applications for WBV therapy, as discussed above. Devices that are commercially available include magnitudes of vibration ranging from 0.1 g to 18.01 g, and frequencies ranging from 30 to 60 Hz[22]. However, the literature reviews reflect uncertainty about the optimal settings or populations for the application of this therapy[23-25].
The objective of this study was to evaluate the bone strength and bone density at different skeletal regions of the femur in adult female rats exposed to a range of WBV amplitudes (0, 0.15, 0.3, 0.6, or 1.2 g-pk) at 45 Hz. Our hypothesis was that an optimal amplitude for WBV would be apparent, and that this exposure would result in increased bone strength and increased BMD. A secondary objective was to evaluate the effect of vibration amplitude on plasma PGE2 levels, cartilage thickness and cartilage histopathology scores on a subset of groups that spanned the vibration amplitudes studied. Our hypothesis was that plasma PGE2 levels and cartilage thickness would be increased with vibration exposure and cartilage histopathology scores would remain unchanged. In contrast to much of the literature we did not use an ovariectomized animal model in our study but used a non-ovariectomized rat model instead so the findings would be applicable to the growing emphasis on developing interventions for maximizing bone mineral density prior to menopause[26].
Materials and methods
Animal protocol
This study consisted of 80 adult female retired-breeder Sprague-Dawley rats (mean age 24 weeks), approved for use by the UNC IACUC. Rats were caged in pairs and given ad libitum access to food and water with a 12-hr light/dark cycle (7am-7pm) throughout the study. The animals were divided into five groups of sixteen animals each with matched mean weights. Each group was assigned to a treatment amplitude of 0, 0.15, 0.3, 0.6, or 1.2 g (peak) of sinusoidal vertical vibration at 45 Hz, administered using an electromagnetic shaker platform driven by a function generator in series with a power amplifier, as previously described[27].
This treatment was administered 15 minutes per day, five days per week, for five weeks. Control animals were moved onto the shaker platform but not vibrated. As part of another study, a titanium alloy osseointegration pin was implanted transversely in the proximal tibial metaphysis bilaterally on all animals. The osseointegration study drove the decision to use 45 Hz and a timeframe of five weeks because these parameters have been shown to be effective at that setting[28]. Vibration treatments began one week after the implantation surgery. Radiographs were acquired 7 to 14 days after surgery to identify and eliminate from this dataset any animals that suffered fractures due to the pin implantation surgery. The animals were sacrificed at the end of the treatment period. Femurs were isolated, moistened with saline, wrapped in gauze, and frozen at -20°C, until mechanical testing.
Femoral densitometry
Densitometric measurements were conducted on a Hologic QDR Discovery A DXA machine, using the Small Animal – Regional High Resolution mode. As per the manufacturer’s instructions for this mode, the bones were scanned in a thin, flat-bottomed plastic container, submerged to a 25 mm depth in a saline solution. BMD was calculated for manually-positioned regions of interest (ROIs), modeled after those used previously in vitro[29] and included: the “Global” BMD, the entire proximal femur down to the lesser trochanter, and a 4.6 mm-long section of the midshaft.
Mechanical indentation of distal femoral cancellous bone
A 4-mm-long coronal section of the distal femoral metaphysis was cut 0.5 mm proximal to the femoral condyle with a low-speed saw (Isomet, Buehler Ltd., Lake Bluff, IL) under constant irrigation. A 1.6-mm-diameter cylindrical indenter with a flat testing face was slowly advanced to the center of the distal face of the section. The indenter was advanced to a depth of 2 mm into the cancellous bone at a constant displacement rate of 0.1 mm/sec, as described[15]. The maximum load and stiffness were obtained from the load-displacement curve.
Three-point bending of femoral shaft
A three-point bending test was used to determine the mechanical properties of the femoral midshaft in an anterior-posterior direction. The femurs were subjected to three-point bending to failure at a displacement rate of 0.1 mm/sec, as described[15]. The maximum load was calculated from the load-displacement curve.
Cantilever bending of femoral neck
A cantilever bending test was used to determine the mechanical properties of the femoral neck. The proximal portion of the femur, separated from the rest of the femur before the three-point bending test, was secured in a chuck fixed to the lower platen of the materials testing system as described[30]. A flat-faced stud was lowered onto the head of the femur, in a direction parallel to the axis of the femoral shaft. The stud was advanced with a constant displacement rate of 0.1 mm/sec[15]. The maximum load was calculated from the load-displacement curve.
Plasma analysis of prostaglandin E2 metabolite levels
Blood was sampled by intra-cardiac collection at the time of euthanasia and injected into EDTA treated vacutainer tubes. 10uM indomethacin was added to each tube to prevent ex vivo formation of prostaglandins. Tubes were spun at 1300G for 10 minutes at 4°C with the supernatant aspirated. Samples were stored at -80° C until analysis of PGE2 metabolite levels according to the instructions of a commercially available kit (514531, Cayman Chemical Co, Ann Arbor, MI). The 0.0, 0.15, and 1.2 g groups were analyzed.
Cartilage thickness measurement
Histological sections for cartilage assessments came from a subset of animals (n=6/group) with no surgical complications from the 0, 0.15, and 1.2 g groups. Femurs were fixed in 10% NBF for 48 hrs and decalcified in Immunocal over two weeks. Two coronal plane sections were taken through the anterior half of the left knee through the weight-bearing region of the medial condyle. Sections were 5 µm thick and 100 µm apart. Sections were stained with toluidine blue, scanned with the Aperio ScanScope XT, and viewed using Aperio Imagescope software.
In an effort to capture what was anticipated to be a small difference in mean thickness between treatment groups, an ImageJ image processing routine was developed to capture the cartilage area while eliminating most artifacts, chondrocytes, and lacunae (Figure 1). In order to standardize measurements, the images were cropped to a 1.32-mm articular surface length (the width of the weight-bearing area of the smallest femur). The cartilage area of the image was measured and divided by the articular surface length to calculate the mean thickness of this segment of articular cartilage.
Figure 1.
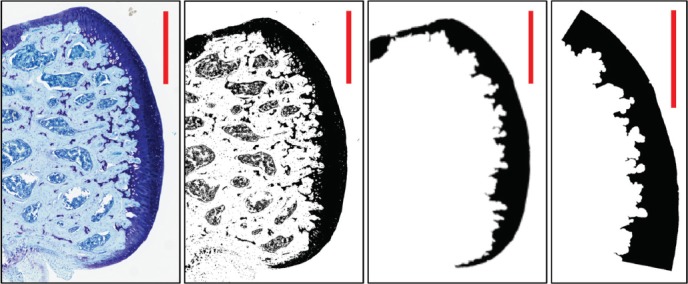
ImageJ processing routine capturing cartilage area while eliminating most artifacts. Final step cropped image to a 1.32-mm articular surface length of weight-bearing area to standardize measurements between specimens. Scale bar in each panel = 0.5mm.
Assessment of cartilage histopathology
The Osteoarthritis Research Society International (OARSI) cartilage histopathology assessment system was used. This assessment system is based on six grades, reflecting depth of the lesion and four stages reflecting extent of OA over the joint surface. The scoring system ranges from worst (score 24, deformation over 50% of area) to best (score 0, intact cartilage with OA activity over 0% of area)[31]. It was applied to detect small differences in cartilage health from one treatment group to another. The same grader blindly assessed all sections. Within an animal, two section scores were collected and averaged.
Statistical analysis
Group differences were evaluated by one-way analysis of variance followed by Dunnet’s mean comparison testing using statistical software (Sigmaplot, Systat Software Inc., San Jose, CA). Data were subjected to log10 transformation in some instances in order to pass the equality of variance test.
Results
BMD did not differ at any region of interest between treatment groups (Figure 2, Table 1). The max load of indentation of the cancellous bone did not differ statistically between treatment groups (Figure 3) despite the max load of the 0.6 and 1.2 g groups being twice that of the control. A regression analysis of max load of indentation versus vibration amplitude revealed a significant positive correlation (R=0.277, P=0.025). Corresponding with the max load indentation data, the mean indentation stiffness of the 0.6 and 1.2 g vibration groups was found to be twice that of the control group (P=0.039, Figure 3). Three-point bending strength of the femoral shaft did not differ significantly between individual treatment groups (Figure 4). Structural strength of the femoral neck under cantilever bending did not differ significantly between treatment groups (Figure 4).
Figure 2.
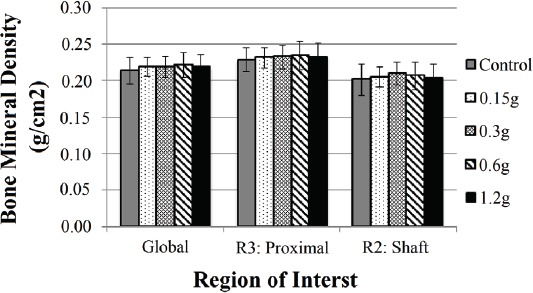
Bone mineral density results for Root Mean Square Coefficient of Variation-vetted regions of interest (ROI) the “Global” region, the entire proximal femur down to the lesser trochanter, and a 4.6mm-long section of the mid-shaft. (Mean ± SD). No significant differences were found between groups for each ROI.
Table 1.
Bone mineral density (BMD) in femora of rats that underwent 5 weeks of vibration (Mean ± SD). There were no statistically significant differences between the regions of interest: The global region (Global), the entire proximal femur down to the lesser trochanter (Proximal), and a 4.6 mm-long section of the mid-shaft (Shaft).
| Region of Interest | Control BMD (g/cm2) |
|---|---|
| Global | 0.214 ± 0.018 |
| Proximal | 0.228 ± 0.016 |
| Shaft (R2) | 0.201 ± 0.021 |
Figure 3.
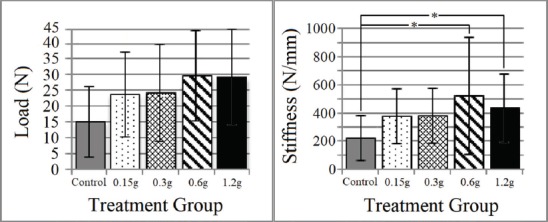
Femoral distal metaphyseal cancellous bone biomechanical property results (Mean ± SD). Max indentation load was not found to differ with treatment (F(4,60)= 2.1, P=0.084). Stiffness was found to be greater in the 0.6 and 1.2g vibration group relative to the control. * indicates significant difference (P≤0.05).
Figure 4.
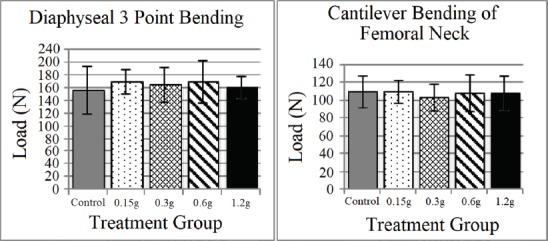
Femoral neck and shaft bending biomechanical properties (Mean ± SD). No significant differences were found between groups for either region quantified.
PGE2 metabolite levels increased with increasing levels of vibration (Figure 5). PGE2 metabolite levels were significantly greater (P=0.01) for the 1.2 g group (46.83 ± 12.40 pg/mL) as compared to the control group (27.65 ± 3.47 pg/mL). The 0.15 g group (35.58 ± 8.57 pg/mL) was not found to differ from either the control or 1.2 g group.
Figure 5.
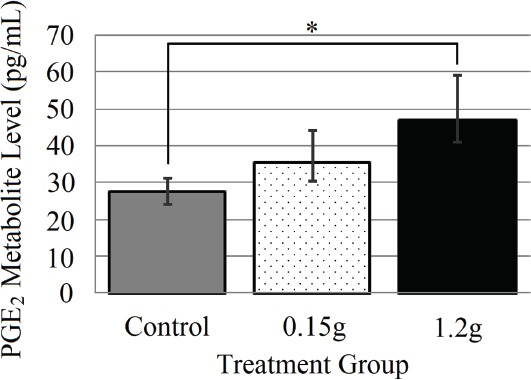
Plasma Analysis of Prostaglandin E2 metabolite level (Mean ± SD) for 0.0, 0.15, and 1.2 g groups, * indicates significant difference (P≤0.01).
Mean cartilage thickness was not found to differ between the two vibration levels (0.15 and 1.2 g) and the control group (Figure 6).
Figure 6.
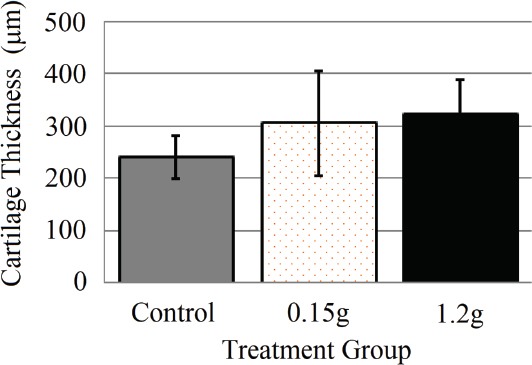
Cartilage thickness (Mean ± SD) of the weight-bearing region of medial condyle. Toluidine blue stained histological images were processed with ImageJ to isolate cartilage of 0, 0.15, and 1.2g groups. No statistical differences were found.
The OARSI histopathology scores were first compared using a Kruskal-Wallis one way ANOVA on ranks. For these data, H=0.0902 with 2 degrees of freedom (P=0.956); there was no statistical difference between these groups (Table 2).
Table 2.
Kruskal-Wallis One Way Analysis of Variance on Ranks for cartilage histopathology scores based on the Osteoarthritis Research Society International system. No statistical difference between groups were detected (P=0.956).
| Group | N | Missing | Median | 25% | 75% |
|---|---|---|---|---|---|
| Control | 6 | 0 | 7.375 | 6.750 | 10.500 |
| 0.15 g | 5 | 0 | 6.500 | 4.875 | 12.625 |
| 1.2 g | 6 | 0 | 8.125 | 3.750 | 13.000 |
Several animals were excluded from analysis. Three animals had a complete tibial fracture during the osseo-implantation surgery for the related study. Twelve other animals had smaller fractures and were also excluded. Two animals had hematomas. One was unwilling to bear weight on the operated limb. These animals were excluded, leaving the final sample size at n=14 for controls and 0.15, n=13 for 0.3 g, n=15 for 0.6 g, and n=16 for 1.2 g.
Discussion
In this study, we investigated how WBV at a frequency of 45 Hz delivered for 15 minutes per day, five days per week, over five weeks influences bone strength, BMD, and cartilage properties in several regions of the femur of the adult female rat. This work represents an effort to identify the optimal amplitude of vibration for improving bone strength and density with this evolving therapy, which is commercially available, but not fully understood or optimized.
We hypothesized that there would be an optimal amplitude for this therapy, and that there might exist some kind of dose-response relationship between amplitude and bone changes with unproductive effects occurring at excessively large amplitudes. We hypothesized that changes would be seen throughout the bone and would be measurable via BMD and bone mechanical properties. Our data demonstrates that WBV over a 5-week period causes a doubling in the distal femur cancellous bone strength and stiffness over a range (0.6-1.2 g) of acceleration amplitudes as measured by indentation. Moreover, our data demonstrate a significant increase in PGE2 metabolite levels in vibrated animals, with a maximum of a 70% increase in levels between the 1.2 g and control. In contrast, vibration did not influence femoral densitometry, cantilever bending strength of the femoral neck, or 3-point bending strength of the femoral shaft. While there was no definitive optimal amplitude for any of the parameters that were evaluated an optimal amplitude could exist for other vibration waveforms, daily regimens, or for vibration amplitudes higher than what were applied in this study.
These data suggest that the benefits of vibration therapy over the described time frame are confined to cancellous bone. While cancellous bone is present at the femoral neck, in the moderately aged animals of the current study the cortex remains thick and provides the greatest contribution to strength and that may be the reason no enhancement in strength was observed for the cantilever bending evaluation of the femoral neck. Changes in bone morphology due to the biomechanical stimulation of vibration are compatible with Wolff’s law. While Wolf’s law is often associated with mechanical strains present in bone, the mechanical strains induced by vibration have been found to be at a low level[41]. As speculated by others[42] it may be that inertial loading of the cell and its nucleus could be the basis of the biomechanical stimulation for vibration. The isolation of these changes to cancellous bone for our short-timeline study is logical when considering that bone remodeling is more rapid in cancellous bone than in cortical bone[32]. A longer treatment timeline might capture changes in the cortical bone, studied here via three-point bending and cantilever bending of the femoral neck.
The lack of changes in BMD is consistent with the variable response of BMD to vibration in other studies, with Liphardt most notably finding no change in BMD in a 12-month study of osteopenic postmenopausal women exposed to WBV for 10 minutes daily[6,9,33-35]. A larger study, or one using a more homogeneous sample of rats may well capture a small change in BMD. Likewise, a longer study might produce larger changes that could be more readily detected.
The finding of changes in PGE2 metabolite levels with increasing vibration is significant. Jee et al have described how PGE2 impacts both bone resorption and formation, but favors formation, resulting in increased bone mass[36]. Yang et al found exogeneous intraosseous PGE2 had a dose-dependent effect of increasing metaphyseal trabecular bone volume. Ke has correlated those changes with an increase in bone strength[15]. Furthermore, past work has shown that COX-2, the inducible enzyme responsible for PGE2 production, mediates mechanical-induced bone formation in vivo[37]. In agreement with the increased PGE2 metabolite levels found with vibration in our study, several in vitro studies of cultured osteoblast-like cells have shown vibration to increase COX-2 expression[16,38]. However, it is unclear in our study to what extent bone cells versus cell types from other tissues are responding to the WBV to cause the elevated plasma PGE2 metabolite levels found.
The lack of a significant increase in cartilage thickness in the vibrated animals compared to the controls contradicts previously reported work that vibration training can improve cartilage thickness during bed-rest immobilization[18]. From our preliminary analysis, the greater cartilage thickness observed with the vibration groups does not appear to be due to a repair response to damage as evidenced by the similar histopathology scores found in the vibrated and control groups. Future assessment of the mechanical properties of the cartilage and subchondral bone in response to vibration may be helpful in determining the nature of the changes resulting in cartilage thickening with vibration exposure. In contrast to our findings in the rat, recent in vivo work has demonstrated that WBV can induce degenerative changes in knee cartilage in the CD-1 mouse while no degenerative changes in knee cartilage were observed in the C57BL/6 mouse[20,21]. In addition, WBV has been reported to accelerate cartilage degeneration induced by anterior cruciate ligament transection in the rat, suggesting the knee joint’s response to WBV may be dependent on any pre-existing knee pathology[39].
A limitation to our study was that our work was confined to the femur based on the clinical significance of fractures to this skeletal site. We did not include micro-CT measurements in our study as we wanted to focus on biomechanical evaluations which we thought would be more functionally significant. A consideration in interpreting our study is that an osseointegration implantation surgery was performed as another component to this study and that this surgery may induce an inflammatory response that might influence some of our evaluation parameters.
Notably, the lack of a clearly optimal amplitude for this therapy is not altogether an adverse finding. These findings suggest that low amplitudes of vibration may be as effective as greater amplitudes for producing bone changes. Lower amplitude vibration therapy is likely to be better tolerated in clinical use, and may have better patient adherence and possibly a better safety profile compared to larger amplitudes of vibration[40].
Our findings show that under the conditions of our study there is no definitive optimal acceleration amplitude for achieving improvements in bone mechanical properties with WBV, and that these improvements are confined to isolated cancellous bone regions over a five-week treatment period.
Acknowledgements
The project described was supported by grants UL1TR000083, KL2TR000084, and TL1TR000085 from the National Center for Advancing Translational Sciences, National Institutes of Health, by Histology Core services by NIH grant P30DK034987, by NIA 5-T35-AG038047-05 - UNC-CH Summer Research in Aging for Medical Students, and by a UNC-NCSU Rehabilitation Engineering Center Pilot Grant.
Footnotes
The authors have no conflict of interest.
Edited by: A. Ireland
Author contributions
William Runge – Performed experiments, analyzed data, authored publications. David Ruppert – Helped design and orchestrate the animal protocol, helped with manuscript preparation and reviewer response. Denis Marcellin-Little – Helped design and orchestrate the densitometry work and analysis of those results. Ola Harrysson – Helped design and orchestrate the animal protocol. Laurence Dahners – Contributed to experimental design, supervised results analysis. Helped author revise manuscripts. Paul Weinhold – Supervised all efforts, and spearheaded the experimental design, results analysis, and manuscript preparation and reviewer response.
References
- 1.Bruyere O, Wuidart MA, Di Palma E, Gourlay M, Ethgen O, Richy F, et al. Controlled whole body vibration to decrease fall risk and improve health-related quality of life of nursing home residents. Arch Phys Med Rehabil. 2005;86(2):303–7. doi: 10.1016/j.apmr.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Bogaerts A, Delecluse C, Claessens AL, Coudyzer W, Boonen S, Verschueren SM. Impact of whole-body vibration training versus fitness training on muscle strength and muscle mass in older men:a 1-year randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2007;62(6):630–5. doi: 10.1093/gerona/62.6.630. [DOI] [PubMed] [Google Scholar]
- 3.Zafar H, Alghadir A, Anwer S, Al-Eisa E. Therapeutic effects of whole-body vibration training in knee osteoarthritis:a systematic review and meta-analysis. Arch Phys Med Rehabil. 2015;96(8):1525–32. doi: 10.1016/j.apmr.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Wehrle E, Wehner T, Heilmann A, Bindl R, Claes L, Jakob F, et al. Distinct frequency dependent effects of whole-body vibration on non-fractured bone and fracture healing in mice. J Orthop Res. 2014;32(8):1006–13. doi: 10.1002/jor.22629. [DOI] [PubMed] [Google Scholar]
- 5.Rubin C, Judex S, Qin YX. Low-level mechanical signals and their potential as a non-pharmacological intervention for osteoporosis. Age Ageing. 2006;35(Suppl 2):ii32–6. doi: 10.1093/ageing/afl082. [DOI] [PubMed] [Google Scholar]
- 6.Liphardt AM, Schipilow J, Hanley DA, Boyd SK. Bone quality in osteopenic postmenopausal women is not improved after 12 months of whole-body vibration training. Osteoporos Int. 2015;26(3):911–20. doi: 10.1007/s00198-014-2995-8. [DOI] [PubMed] [Google Scholar]
- 7.Tankisheva E, Bogaerts A, Boonen S, Delecluse C, Jansen P, Verschueren SM. Effects of a Six-Month Local Vibration Training on Bone Density, Muscle Strength, Muscle Mass, and Physical Performance in Postmenopausal Women. J Strength Cond Res. 2015;29(9):2613–22. doi: 10.1519/JSC.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 8.von Stengel S, Kemmler W, Engelke K, Kalender WA. Effects of whole body vibration on bone mineral density and falls:results of the randomized controlled ELVIS study with postmenopausal women. Osteoporos Int. 2011;22(1):317–25. doi: 10.1007/s00198-010-1215-4. [DOI] [PubMed] [Google Scholar]
- 9.Verschueren SM, Roelants M, Delecluse C, Swinnen S, Vanderschueren D, Boonen S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women:a randomized controlled pilot study. J Bone Miner Res. 2004;19(3):352–9. doi: 10.1359/JBMR.0301245. [DOI] [PubMed] [Google Scholar]
- 10.Oxlund BS, Ortoft G, Andreassen TT, Oxlund H. Low-intensity, high-frequency vibration appears to prevent the decrease in strength of the femur and tibia associated with ovariectomy of adult rats. Bone. 2003;32(1):69–77. doi: 10.1016/s8756-3282(02)00916-x. [DOI] [PubMed] [Google Scholar]
- 11.Rubinacci A, Marenzana M, Cavani F, Colasante F, Villa I, Willnecker J, et al. Ovariectomy sensitizes rat cortical bone to whole-body vibration. Calcif Tissue Int. 2008;82(4):316–26. doi: 10.1007/s00223-008-9115-8. [DOI] [PubMed] [Google Scholar]
- 12.Christiansen BA, Silva MJ. The effect of varying magnitudes of whole-body vibration on several skeletal sites in mice. Ann Biomed Eng. 2006;34(7):1149–56. doi: 10.1007/s10439-006-9133-5. [DOI] [PubMed] [Google Scholar]
- 13.Wenger KH, Freeman JD, Fulzele S, Immel DM, Powell BD, Molitor P, et al. Effect of whole-body vibration on bone properties in aging mice. Bone. 2010;47(4):746–55. doi: 10.1016/j.bone.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 14.Fonseca H, Moreira-Goncalves D, Coriolano HJ, Duarte JA. Bone quality:the determinants of bone strength and fragility. Sports Med. 2014;44(1):37–53. doi: 10.1007/s40279-013-0100-7. [DOI] [PubMed] [Google Scholar]
- 15.Ke HZ, Shen VW, Qi H, Crawford DT, Wu DD, Liang XG, et al. Prostaglandin E2 increases bone strength in intact rats and in ovariectomized rats with established osteopenia. Bone. 1998;23(3):249–55. doi: 10.1016/s8756-3282(98)00102-1. [DOI] [PubMed] [Google Scholar]
- 16.Bacabac RG, Smit TH, Van Loon JJ, Doulabi BZ, Helder M, Klein-Nulend J. Bone cell responses to high-frequency vibration stress:does the nucleus oscillate within the cytoplasm? FASEB J. 2006;20(7):858–64. doi: 10.1096/fj.05-4966.com. [DOI] [PubMed] [Google Scholar]
- 17.Adams JA, Bassuk J, Wu D, Grana M, Kurlansky P, Sackner MA. Periodic acceleration:effects on vasoactive, fibrinolytic, and coagulation factors. J Appl Physiol (1985) 2005;98(3):1083–90. doi: 10.1152/japplphysiol.00662.2004. [DOI] [PubMed] [Google Scholar]
- 18.Liphardt AM, Mundermann A, Koo S, Backer N, Andriacchi TP, Zange J, et al. Vibration training intervention to maintain cartilage thickness and serum concentrations of cartilage oligometric matrix protein (COMP) during immobilization. Osteoarthritis Cartilage. 2009;17(12):1598–603. doi: 10.1016/j.joca.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Fam AG, Kolin A. Unusual metacarpophalangeal osteoarthritis in a jackhammer operator. Arthritis Rheum. 1986;29(10):1284–8. doi: 10.1002/art.1780291016. [DOI] [PubMed] [Google Scholar]
- 20.McCann MR, Patel P, Pest MA, Ratneswaran A, Lalli G, Beaucage KL, et al. Repeated exposure to high-frequency low-amplitude vibration induces degeneration of murine intervertebral discs and knee joints. Arthritis Rheumatol. 2015;67(8):2164–75. doi: 10.1002/art.39154. [DOI] [PubMed] [Google Scholar]
- 21.Kerr GJ, McCann MR, Branch JK, Ratneswaran A, Pest MA, Holdsworth DW, et al. C57BL/6 mice are resistant to joint degeneration induced by whole-body vibration. Osteoarthritis Cartilage. 2017;25(3):421–5. doi: 10.1016/j.joca.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Alizadeh-Meghrazi M, Zariffa J, Masani K, Popovic MR, Craven BC. Variability of vibrations produced by commercial whole-body vibration platforms. J Rehabil Med. 2014;46(9):937–40. doi: 10.2340/16501977-1868. [DOI] [PubMed] [Google Scholar]
- 23.Wysocki A, Butler M, Shamliyan T, Kane RL. Whole-body vibration therapy for osteoporosis:state of the science. Ann Intern Med. 2011;155(10):W206–13. doi: 10.7326/0003-4819-155-10-201111150-00006. 680,6. [DOI] [PubMed] [Google Scholar]
- 24.Prisby RD, Lafage-Proust MH, Malaval L, Belli A, Vico L. Effects of whole body vibration on the skeleton and other organ systems in man and animal models:what we know and what we need to know. Ageing Res Rev. 2008;7(4):319–29. doi: 10.1016/j.arr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Komrakova M, Sehmisch S, Tezval M, Ammon J, Lieberwirth P, Sauerhoff C, et al. Identification of a vibration regime favorable for bone healing and muscle in estrogen-deficient rats. Calcif Tissue Int. 2013;92(6):509–20. doi: 10.1007/s00223-013-9706-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu J, Lombardi G, Jiao W, Banfi G. Effects of Exercise on Bone Status in Female Subjects, from Young Girls to Postmenopausal Women:An Overview of Systematic Reviews and Meta-Analyses. Sports Med. 2016;46(8):1165–82. doi: 10.1007/s40279-016-0494-0. [DOI] [PubMed] [Google Scholar]
- 27.Sandhu E, Miles JD, Dahners LE, Keller BV, Weinhold PS. Whole body vibration increases area and stiffness of the flexor carpi ulnaris tendon in the rat. J Biomech. 2011;44(6):1189–91. doi: 10.1016/j.jbiomech.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Guan X, Liu T, Wang X, Yu M, Yang G, et al. Whole body vibration improves osseointegration by up-regulating osteoblastic activity but down-regulating osteoblast-mediated osteoclastogenesis via ERK1/2 pathway. Bone. 2015;71:17–24. doi: 10.1016/j.bone.2014.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Sievanen H, Kannus P, Jarvinen M. Precision of measurement by dual-energy X-ray absorptiometry of bone mineral density and content in rat hindlimb in vitro. J Bone Miner Res. 1994;9(4):473–8. doi: 10.1002/jbmr.5650090406. [DOI] [PubMed] [Google Scholar]
- 30.Alam I, Sun Q, Liu L, Koller DL, Fishburn T, Carr LG, et al. Identification of a quantitative trait locus on rat chromosome 4 that is strongly linked to femoral neck structure and strength. Bone. 2006;39(1):93–9. doi: 10.1016/j.bone.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology:grading and staging. Osteoarthritis Cartilage. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 32.Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–9. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butezloff MM, Zamarioli A, Leoni GB, Sousa-Neto MD, Volpon JB. Whole-body vibration improves fracture healing and bone quality in rats with ovariectomy-induced osteoporosis. Acta Cir Bras. 2015;30(11):727–35. doi: 10.1590/S0102-865020150110000002. [DOI] [PubMed] [Google Scholar]
- 34.Minematsu A, Nishii Y, Imagita H, Takeshita D, Sakata S. Whole-body vibration can attenuate the deterioration of bone mass and trabecular bone microstructure in rats with spinal cord injury. Spinal Cord. 2015 doi: 10.1038/sc.2015.220. [DOI] [PubMed] [Google Scholar]
- 35.Yang F, King GA, Dillon L, Su X. Controlled whole-body vibration training reduces risk of falls among community-dwelling older adults. J Biomech. 2015;48(12):3206–12. doi: 10.1016/j.jbiomech.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 36.Jee WS, Ma YF. The in vivo anabolic actions of prostaglandins in bone. Bone. 1997;21(4):297–304. doi: 10.1016/s8756-3282(97)00147-6. [DOI] [PubMed] [Google Scholar]
- 37.Forwood MR. Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res. 1996;11(11):1688–93. doi: 10.1002/jbmr.5650111112. [DOI] [PubMed] [Google Scholar]
- 38.Uzer G, Manske SL, Chan ME, Chiang FP, Rubin CT, Frame MD, et al. Separating Fluid Shear Stress from Acceleration during Vibrations in Vitro:Identification of Mechanical Signals Modulating the Cellular Response. Cell Mol Bioeng. 2012;5(3):266–76. doi: 10.1007/s12195-012-0231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qin J, Chow SK, Guo A, Wong WN, Leung KS, Cheung WH. Low magnitude high frequency vibration accelerated cartilage degeneration but improved epiphyseal bone formation in anterior cruciate ligament transect induced osteoarthritis rat model. Osteoarthritis Cartilage. 2014;22(7):1061–7. doi: 10.1016/j.joca.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Thompson WR, Yen SS, Rubin J. Vibration therapy:clinical applications in bone. Curr Opin Endocrinol Diabetes Obes. 2014;21(6):447–53. doi: 10.1097/MED.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Judex S, Lei X, Han D, Rubin C. Low-magnitude mechanical signals that stimulate bone formation in the ovariectomized rat are dependent on the applied frequency but not on the strain magnitude. J Biomech. 2007;40(5):1333–1339. doi: 10.1016/j.jbiomech.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 42.Garman R, Rubin C, Judex S. Small oscillatory accelerations, independent of matrix deformations, increase osteoblast activity and enhance bone morphology. PLoS ONE. 2007;7:E653. doi: 10.1371/journal.pone.0000653. [DOI] [PMC free article] [PubMed] [Google Scholar]


