Abstract
Objectives:
To translate the Sarcopenia Quality of Life (SarQoL®) questionnaire into Dutch and to evaluate the psychometric properties of the Dutch version of this questionnaire.
Methods:
The translation was carried out using a 5-step process, with 2 initial translations, a merging of these 2 translations, 2 backwards translations, an expert committee review and a pretest of the questionnaire. Sarcopenia was diagnosed with the EWGSOP algorithm. The validation consisted of an examination of the discriminative power, internal consistency, construct validity, test-retest reliability and floor and ceiling effects.
Results:
No significant problems were encountered during the translation process. A total of 92 subjects were included in the validation part of this study, 30 of which were sarcopenic. Discriminative power between sarcopenic and non-sarcopenic subjects was found for all domains and the Overall score (median overall QoL score: 67.15 vs 79.72; p=0.003). High internal consistency was found (Cronbach’s alpha=0.883), as well as good construct validity with 75% of hypotheses confirmed. Test-retest reliability was excellent (ICC=0.976; 95% CI=0.947-0.989) and no floor or ceiling effects were observed.
Conclusion:
The Dutch version of the SarQoL® questionnaire is ready for use in clinical and research applications.
Keywords: Sarcopenia, Quality of Life, Translation, Validation, SarQoL
Introduction
As people age, their bodies undergo changes both subtle and profound. One of these is the loss of muscle mass with advancing age, which is estimated to decline at a rate of 0.26% to 0.56% per year between the ages of 20 and 70 years old. This decline accelerates to a yearly loss of 0.64% to 1.29% for men and 0.53% to 0.84% for women over 70 years of age[1].
Irwin H. Rosenberg was the first to use the term sarcopenia to describe this age-related decline in lean body mass[2]. From this, the concept of sarcopenia has evolved and has recently been defined as “a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes such as physical disability, poor quality of life, and death” by the European Working Group on Sarcopenia in Older People (EWGSOP), which also proposed an algorithm for its diagnosis: [low muscle mass AND (low muscle strength AND/OR low muscle function)][3]. However, consensus on the definition and diagnostic criteria for sarcopenia has not yet been reached, and other definitions and diagnostic criteria have been proposed[4].
Despite the absence of consensus criteria for the diagnosis of sarcopenia, the Centers for Disease Control and Prevention (CDC) have assigned an ICD-10-CM code to sarcopenia, expanding the definition from a syndrome to a pathology and opening up new avenues for research on sarcopenia[5].
Estimates of the prevalence of sarcopenia have been impacted by the lack of consensus on a single set of diagnostic criteria. Nonetheless, a meta-analysis published in 2017 which included 35 studies with community-dwelling participants aged 60 years or older and assessed sarcopenia by the EWGSOP criteria, has provided the most precise assessment yet of the prevalence of sarcopenia. The authors of this meta-analysis found, among healthy adults aged ≥60 years, an overall prevalence of 10% (95% CI=8-12%) for men and 10% (95% CI=8-13%) for women. These numbers highlight the need to treat sarcopenia as a public health problem[6]. In hospital and long-term-care settings, both the prevalence and incidence of sarcopenia are likely to be significantly higher[7,8].
Research into the quality of life of sarcopenic individuals is a relatively new field with limited data available and conflicting results on the impact of sarcopenia on quality of life. Furthermore, nearly all studies so far have used generic quality of life instruments such as the Short Form 36-item (SF-36) questionnaire and the EuroQol 5-Dimension 3-Level (EQ-5D-3L) questionnaire, which may not be sensitive enough to sarcopenia-specific factors influencing quality of life[9].
With this in mind, the Sarcopenia Quality of Life (SarQoL®) questionnaire was developed by Beaudart et al. (2015) to advance insight into the impact of sarcopenia on quality of life by providing an instrument that is specific to sarcopenia[10]. The SarQoL® questionnaire has been proven to be a valid and reliable instrument after evaluations of its psychometric properties in several language-specific versions[11-13]. It has been translated into 20 languages but, until now, a Dutch version was not available.
The purpose of this study was to translate the SarQoL® questionnaire into Dutch and to investigate its psychometric properties so as to confirm its validity and reliability as an instrument to measure quality of life in older, Dutch-speaking, community-dwelling sarcopenic individuals.
Methods
The SarQoL® questionnaire
The SarQoL® questionnaire consists of 22 questions encompassing 7 domains and 55 items, and is self-administered. The 7 domains of health-related quality of life covered in the questionnaire are: “Physical and Mental Health”, “Locomotion”, “Body Composition”, “Functionality”, “Activities of Daily Living”, “Leisure Activities” and “Fears”. Most questions (19 out of 22) use a Likert scale of frequency or intensity among which the respondents choose the answer most applicable to them. Results are presented as numerical scores between 0 and 100, both for the individual domains and for the Overall score[10].
After its development, the SarQoL® questionnaire was validated in a population of 296 subjects, 43 of which were diagnosed as sarcopenic according to the EWGSOP criteria, and it was demonstrated to be a reliable and valid instrument for assessing quality of life in older, sarcopenic, community-dwelling subjects[11]. Since then, validation studies for the English and the Romanian version of the questionnaire have confirmed its reliability and validity[12,13].
More information on the SarQoL® questionnaire can be found on www.sarqol.org.
Translation
The translation strategy adopted for this study was based on the guidelines formulated by Beaton et al., and was conducted in 5 stages[14]. First, the questionnaire was independently translated from French to Dutch by two bilingual translators (AG & IB), both native Dutch speakers. Secondly, a meeting was convened between the two translators where a synthesis of the initial translations was produced. In the third phase, two bilingual translators (RB & SD), this time native French speakers, independently translated the synthesis version of the questionnaire back into French. Next, an expert committee composed of the 4 translators (AG, IB, RB & SD), a methodologist (CB) and a linguist (WV), compared the different translations and created the prefinal version of the questionnaire. In the 5th and last phase, the prefinal version of the questionnaire was administered to 14 subjects, who were afterwards interviewed about the comprehensibility of and the language used in the questionnaire. The feedback from these interviews was presented to the key investigators (AG, IB, CB & AS) who decided on the need for modifications and established the final version of the Dutch SarQoL® questionnaire.
Study population
The study sample was recruited from a database of older volunteers from the Gerontology Department of the Vrije Universiteit Brussel (VUB) and from the general public via advertisement.
Candidates were eligible for inclusion when they met the following criteria: 65 years of age or older, community-dwelling, native Dutch speaker, and able to give informed consent. Candidates were excluded for the following reasons: cognitive impairment documented as a Mini-Mental State Examination (MMSE) score of less than 24/30, the presence of an illness affecting the central nervous system (based on self-report), amputation of one or more limb(s), or the presence of any electronic implant (since this is a contra-indication for Bioelectrical Impedance Analysis)[15].
Subjects, both sarcopenic and non-sarcopenic, who had previously participated in 3 other studies at the VUB (the BUTTERFLY, SPRINT and FATPLOT studies) and for whom data on muscle mass, muscle strength, physical performance and a MMSE-score of less than 1-year old at the time of recruitment were available, were contacted by phone and sent packets with the study questionnaires by mail[16-18]. The subjects for whom physical data was not available were contacted by phone and invited to the Gerontology Department of the VUB. Additionally, 14 participants were also invited to participate in the pretest of the questionnaire and were interviewed at the VUB. Nine subjects were examined at a local service center and 1 subject was assessed at home, following the same procedures used at the VUB.
The sample size for this study was based on the recommendations of Terwee et al., which state that a sample of 100 subjects should be recruited for an instrument validation study, with at least half of them belonging to the target population of the instrument[19].
This study was approved by the Medical Ethics Committee of the Vrije Universiteit Brussel and the University Hospital Brussel (approval N° 2016/328) and all participants provided written informed consent.
Data collection
Sarcopenia measures
The diagnosis of sarcopenia was established using the algorithm of the EWGSOP, requiring the presence of low muscle mass in combination with low muscle strength and/or low physical performance to diagnose an individual as having sarcopenia. When all three elements are present, the subject is diagnosed as having severe sarcopenia[3].
Muscle mass was measured using Bioelectrical Impedance Analysis (BIA), which was calibrated before each use. In this study, the Bodystat® Quadscan 4000 (Bodystat Ltd., Isle of Man, British Isles) was used. The resistance-values at 50 kHz were converted into values for appendicular lean mass (ALM) using the following validated formula: [4.957+(0.196 * height2/resistance)+(0.060 * weight) – (2.554 * sex)]; with height in cm, weight in kg, and sex coded as 0 for men and 1 for women[20]. Muscle mass was considered as low when below at least 1 of the following cut-off values: ALM (men <19.75 kg; women <15.02 kg), ALM divided by body mass index (ALM/BMI – men <0.789; women <0.512) or the Skeletal Muscle Mass Index (SMI=ALM/height2 - men <7.26 kg/m2; women <5.5 kg/m2)[21,22].
Muscle strength was evaluated by measuring handgrip strength with a Martin-Vigorimeter (Elmed, Addison, USA), as described previously[23]. Cut-off values were -1.5 standard deviations below the sex-specific mean handgrip strength of a young and healthy reference population (n=100; 50 male and 50 female)[24]. A value of less than 70.3 kilopascal (kPa) for men and less than 46.8 kPa for women indicated low muscle strength.
Physical performance was evaluated with usual gait speed on a 4-meter track. A gait speed of <0.8 m/s was used as the threshold for identifying low gait speed[25].
Questionnaires
Together with the SarQoL® questionnaire, the following questionnaires were completed by all participants. The MMSE was administered to assess cognitive function. A general health questionnaire was used to collect the clinical and demographic characteristics of the participants. The SF-36, a multi-item generic health survey which uses 36 questions to measure functional health and wellbeing from the patient’s perspective, and the EQ-5D-3L, a standardized measure of health status developed by the EuroQol Group in 1990, were both administered once[26,27].
The Dutch version of the SarQoL® questionnaire, the focus of this study, was filled in by all participants on two occasions, with approximately 2 weeks between the administrations. Participants were blind to the results from the first administration of the SarQoL® questionnaire at the time of the second administration.
Psychometric properties
Discriminative power
Because the SarQoL® questionnaire is an instrument designed specifically for use in sarcopenic populations, its ability to differentiate between sarcopenic and non-sarcopenic subjects on the Overall quality of life score and the different domain scores was evaluated.
Internal consistency
Internal consistency evaluates the degree of interrelatedness among the items of a questionnaire [[28]]. This property is assessed with the Cronbach’s alpha coefficient, where a value between 0.7 and 0.9 indicates good internal consistency[29].
Construct validity
The construct validity examines whether the questionnaire really measures the construct it claims to measure. It is evaluated using hypotheses on convergent and divergent validity.
Convergent validity examines correlations between the Dutch SarQoL® questionnaire and domains of other questionnaires that should, in theory, be similar. The hypotheses for this study are that strong correlations will be found between the Overall score of the Dutch SarQoL® questionnaire and the domains “Physical Functioning”, “Vitality”, and “Role Limitation due to Physical Problems” of the SF-36; as well as between the Overall score of the Dutch SarQoL® questionnaire and the Utility Index of the EQ-5D.
Divergent validity examines correlations between the SarQoL® questionnaire and domains of other questionnaires that should, in theory, be different. The hypotheses are that weak correlations will be found between the Overall score of the Dutch SarQoL® questionnaire and the domains “Mental Health” and “Role Limitation due to Emotional Problems” of the SF-36 questionnaire. We also expect to find weak correlations between the Overall score of the Dutch SarQoL® questionnaire and the questions related to Self-Care and Anxiety/Depression of the EQ-5D.
The questionnaire possesses good construct validity if at least 75% of the hypotheses are confirmed[19].
Test-retest reliability
The test-retest reliability of a questionnaire shows the extent to which the questionnaire can produce the same scores for repeated measurements in participants whose health did not change[28].
To measure this, the questionnaire is administered twice, with a preferential interval of 2 weeks between administrations. All subjects completed the second questionnaire at home and returned the study documents by mail. The two scores obtained should be highly correlated, on the condition that the subjects’ health has not changed in the period between the two administrations. To establish this, the participants were asked, before completing the SarQoL® questionnaire for the second time, whether their health had changed since the first administration of the SarQoL® questionnaire and, if this was the case, how their health had changed. Only sarcopenic participants whose health did not change between the two administrations were eligible for inclusion in the assessment of the test-retest reliability.
Floor and ceiling effects
Floor and ceiling effects are observed when more than 15% of respondents obtain either the highest score (ceiling effect) or the lowest score (floor effect) possible.
Statistical analysis
All analyses were performed with IBM SPSS for Windows, version 24.0.0 (Armonk, NY: IBM Corp.).
Normality of distribution of quantitative variables was examined by looking at the distance between mean and median, the histogram, the quantile-quantile plot and the Shapiro-Wilk test. Variables with normal distributions were reported as mean ± standard deviation. Variables that did not have normal distributions were reported as median (25th percentile - 75th percentile). Nominal variables were reported as absolute and relative frequencies (%).
The presence of significant differences between the two groups (sarcopenic and non-sarcopenic) in terms of clinical characteristics was calculated using the independent samples T-test for quantitative variables with normal distribution, Mann-Whitney’s U-test for quantitative variables without normal distribution and the Chi-squared test for nominal variables.
Results were considered statistically significant at p<0.05. The methodology used in the previous validation studies of the French and English versions of the SarQoL® questionnaire was adopted for this study and, to remain consistent with these validations, we excluded questionnaires with more than 20% missing data overall[11,12].
Discriminative power was evaluated with the independent samples T-test or the Mann-Whitney U-test, in function of the distribution of the domain and Overall score(s). Internal consistency was calculated using Cronbach’s alpha coefficient. Construct validity, both convergent and divergent, was assessed by Pearson or Spearman correlations, depending on the distribution of the variables. Test-retest reliability was measured using the Intraclass Correlation Coefficient (ICC) and its confidence interval at 95%, with the questionnaire considered reliable with an ICC value of at least 0.7[19]. Floor and ceiling effects were determined by examining the frequency tables of the Overall SarQoL® score.
Discriminative power and internal consistency were assessed in the complete sample. Construct validity, test-retest reliability and floor and ceiling effects were examined using only the sarcopenic subjects in the sample, as per the recommendations of Terwee et al.[19].
Results
Translation
No major problems were encountered during the translation process. All differences between translations were resolved by consensus, and we reached out to the developers of the SarQoL® questionnaire (CB & OB) and the linguistic expert (WV) when clarifications about the content and interpretation of the questions were needed. Some of the changes made during the expert committee review concerned the exact meaning and interpretation of words. As an example, we changed the word “figuur” in question 13 to “uiterlijk” and the word “moeilijkheden” to “moeite” in question 17. Other changes concerned grammer and style, for example we changed the sentence structure of questions 3,4 and 5 and rewrote question 19 to become “Beperkt uw spierzwakte de voldoening die u uit uw seksleven haalt?”. Lastly, we paid particular attention to the response options offered, keeping them as consistent as possible while also ensuring they matched with the question being asked. For this reason, we changed the option “veel” to “erg” in questions 15 and 18. The prefinal version was filled in by 14 subjects during the pretest. Their feedback did not indicate the need for modifications to the questionnaire.
Population
A total of 111 subjects initially agreed to participate in the study, but 5 subjects did not respond after being sent the study questionnaires and 14 were excluded (Figure 1). Ultimately, a total of 92 subjects with a median age of 82 (73-85) years were included in the analysis. The sample consisted of 52 male participants (56.5%), and 30 subjects (32.6%) were diagnosed as sarcopenic, of which 8 were severely sarcopenic. No significant differences between the sarcopenic and non-sarcopenic subjects were found for age, sex, body mass index, MMSE score, number of past serious or chronic illnesses, number of medications, alcohol consumption, smoking, ALM, ALM/BMI, SMI, and gait speed. Sarcopenic subjects only significantly differed for handgrip strength (p<0.001). The complete clinical characteristics are reported in [Table 1].
Figure 1.
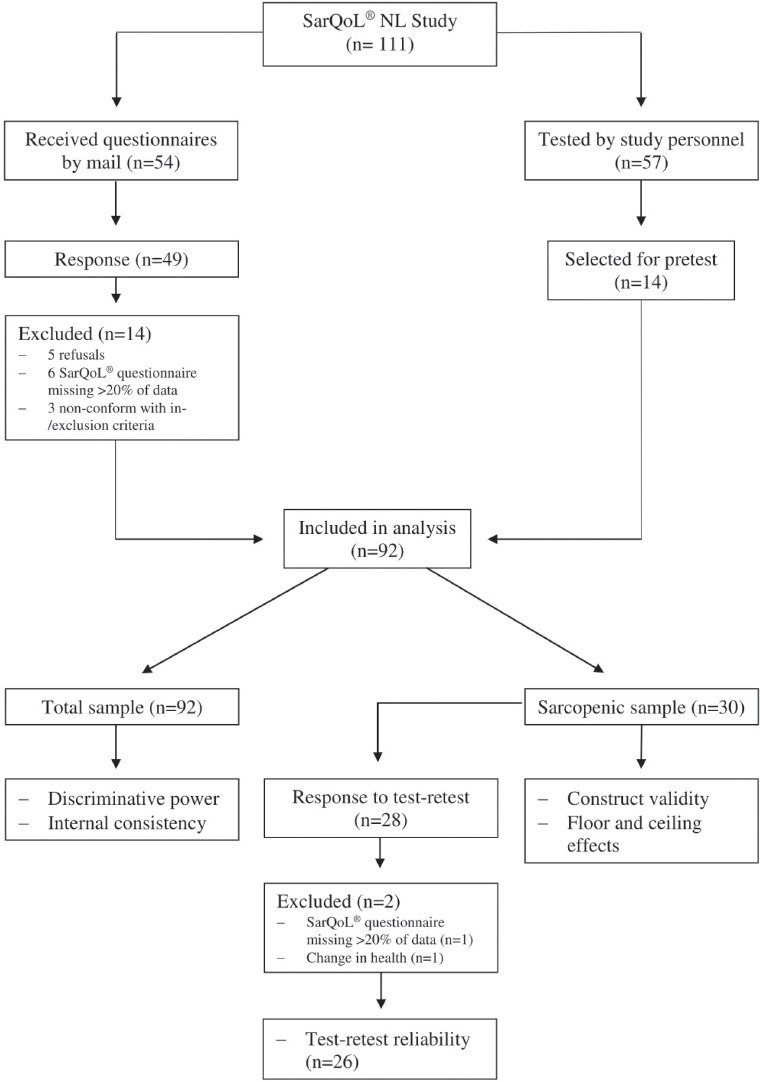
Flowchart of the validation of the Dutch-language SarQoL® questionnaire.
Table 1.
Characteristics of the study population.
| All (n=92) | No sarcopenia (n=62) | Sarcopenia (n=30) | p-value | |
|---|---|---|---|---|
| Age (years) | 82 (73-85) | 82 (71-84) | 83 (79-85) | 0.085 |
| Sex | ||||
| Female | 40 (43.5) | 27 (43.5) | 13 (43.3) | 0.984 |
| Male | 52 (56.5) | 35 (56.5) | 17 (56.7) | |
| BMI (kg/m2) | 26.19 (23.05-29.00) | 26.19 (23.10-28.72) | 26.50 (22.41-29.65) | 0.993 |
| MMSE score | 29 (28-30) | 28 (28-30) | 29 (27-29) | 0.784 |
| Number of past serious or chronic illnesses | 2 (1-3) | 2 (1-2) | 2 (1-4) | 0.069 |
| Number of drugs | 3 (1-5) | 3 (1-4) | 3 (1-4) | 0.076 |
| Alcohol consumption (units/week) | 3.0 (0-8.0) | 3.0 (0-7.5) | 3.5 (0.3-9.0) | 0.489 |
| Tobacco use | ||||
| Never | 56 (62.2) | 36 (60) | 20 (66.7) | 0.810b |
| Current | 5 (5.6) | 4 (6.7) | 1 (3.3) | |
| Past | 29 (32.2) | 20 (33.3) | 9 (30.0) | |
| ALM (kg) | 20.04 (15.64-22.64) | 20.33 (16.10-23.60) | 19.20 (14.73-21.43) | 0.076 |
| ALM/BMI | 0.751 (0.618-0.874) | 0.766 (0.628-0.905) | 0.737 (0.564-0.814) | 0.067a |
| SMI (kg/m2) | 7.00 (6.13-7.91) | 7.08 (6.13-7.93) | 6.89 (6.10-7.99) | 0.987 |
| Handgrip strength (kPa) | 60.50 (44.50-70.75) | 64.00 (50.00-74.00) | 45.00 (39.50-62.10) | <0.001 |
| Gait speed (m/s) | 0.996 (0.895-1.145) | 0.990 (0.898-1.149) | 0.999 (0.779-1.132) | 0.628a |
Independent Samples T-test, all others calculated with Mann-Whitney U-test.
Fisher exact test, all others Pearson Chi-square test.
Because of missing data, absolute frequency does not add up to the full sample strength. BMI= body mass index; MMSE= mini-mental state examination; ALM= appendicular lean mass; SMI= ALM/height[2].
Discriminative power
The analysis of the discriminative power of the Dutch SarQoL® questionnaire showed significantly lower quality-of-life-scores for the sarcopenic subjects for all domains and the Overall score. Sarcopenic subjects scored a median of 67.15 (54.75-81.52) for the Overall score, significantly below the median score for the non-sarcopenic subjects at 79.72 (70.10-86.88; p=0.003). The complete results are reported in [Table 2].
Table 2.
Discriminative power of the Dutch SarQoL® questionnaire.
| No sarcopenia (n=62) | Sarcopenia (n=30) | P-value | |
|---|---|---|---|
| Domain 1: Physical and mental health | 79.97 (65.53-89.15) | 65.53 (58.04-80.56) | 0.007 |
| Domain 2: Locomotion | 83.33 (72.02-94.44) | 75.00 (46.53-86.11) | 0.020 |
| Domain 3: Body composition | 77.09 (69.79-88.54) | 64.59 (50.00-75.00) | 0.001 |
| Domain 4: Functionality | 82.14 (73.18-90.55) | 72.26 (59.14-80.77) | 0.002 |
| Domain 5: Activities of daily living | 75.00 (66.35-85.00) | 62.50 (45.42-80.83) | 0.021 |
| Domain 6: Leisure activities | 66.50 (49.88-66.50) | 49.88 (33.25-66.50) | 0.009 |
| Domain 7: Fears | 100.00 (100.00-100.00) | 87.50 (75.00-100.00) | <0.001 |
| Overall score | 79.72 (70.10-86.88) | 67.15 (54.75-81.52) | 0.003 |
Internal consistency
The Dutch SarQoL® questionnaire has a high level of internal consistency, evidenced by a Cronbach’s alpha coefficient of 0.883.
Construct validity
As shown in [Table 3], all 4 hypotheses for the convergent construct validity and 2 out of 4 for the divergent construct validity were accepted, thus confirming 75% of the pre-specified hypotheses and reflecting good construct validity.
Table 3.
Construct validity of the Dutch SarQoL® questionnaire.
| r | p | r | p | ||
|---|---|---|---|---|---|
| Convergent validity | Divergent validity | ||||
| SF-36 Physical functioning | 0.842 | <0.001a | SF-36 Social functioning | 0.426 | 0.019b |
| SF-36 Role limitation physical | 0.551 | 0.002b | SF-36 Role limitation emotional | 0.594 | 0.001b |
| SF-36 Body pain | 0.546 | 0.002b | SF-36 Mental health | 0.430 | 0.018a |
| SF-36 General Health | 0.617 | <0.001a | EQ-5D Self-care | -0.520 | 0.003b |
| SF-36 Vitality | 0.647 | <0.001a | EQ-5D Pain/discomfort | -0.418 | 0.024b |
| EQ-5D Utility score | 0.771 | <0.001b | EQ-5D Anxiety/depression | -0.225 | 0.223b |
| EQ-5D Mobility | -0.749 | <0.001b | |||
| EQ-5D Usual activities | -0.575 | 0.001b | |||
| EQ-VAS | 0.780 | <0.001a |
Pearson correlations.
Spearman correlations. EQ-VAS : EuroQol visual analogue scale.
Test-retest reliability
We received the second SarQoL® questionnaire from 28 participants, but after exclusion of 1 subject with more than 20% missing data and 1 person whose health changed between the 2 administrations, we obtained a sample of 26 subjects for the evaluation of the test-retest reliability.
An excellent agreement was found for the total score with an ICC of 0.976 (95% CI=0.947-0.989). All domains but two had an ICC greater than 0.7, indicating good reliability between the first and second SarQoL® questionnaire. A low reliability was found for domain 6 (Leisure Activities), with an ICC of 0.375 (95% CI=0.001-0.660) and domain 7 (Fears) with an ICC of 0.235 (95% CI= -0.167-0.568). The results for all domains are presented in [Table 4].
Table 4.
Test-retest reliability of the SarQoL® questionnaire.
| ICC | 95% CI | |
|---|---|---|
| Domain 1: Physical and mental health | 0.820 | 0.642-0.915 |
| Domain 2: Locomotion | 0.908 | 0.793-0.959 |
| Domain 3: Body composition | 0.707 | 0.447-0.857 |
| Domain 4: Functionality | 0.948 | 0.888-0.976 |
| Domain 5: Activities of daily living | 0.875 | 0.741-0.942 |
| Domain 6: Leisure activities | 0.375 | 0.001-0.660 |
| Domain 7: Fears | 0.235 | -0.167-0.568 |
| Overall QoL score | 0.976 | 0.947-0.989 |
Floor and ceiling effects
No sarcopenic subject obtained the highest or lowest score for the questionnaire, both for the first and second SarQoL®, indicating the absence of floor and ceiling effects.
Discussion
This study translated the SarQoL® questionnaire from French to Dutch and evaluated its psychometric properties. Our results show that the Dutch SarQoL® questionnaire is ready for use since it can discriminate between sarcopenic and non-sarcopenic groups, the internal consistency is excellent, construct validity is acceptable, test-retest ability is excellent and there were no floor or ceiling effects for the Overall score.
The translation of the questionnaire was completed without significant obstacles. A rigorous methodology was used, providing safeguards against subjectivity in the translation and assuring equivalence between the original French-language SarQoL® questionnaire and the Dutch translation. Input from 14 subjects during the pretest and from the linguistic expert during and after the expert committee review confirmed that the Dutch version has the same content as the original SarQoL® questionnaire while also being comprehensible to its target audience. We were acutely aware of the regional differences in the Dutch spoken in Flanders (Belgium) and The Netherlands, and verified with the linguistic expert that the language used in the questionnaire would be interpreted in the same way in both regions.
With regards to the psychometric properties of the Dutch SarQoL® questionnaire, this study largely confirms earlier results from the French, English and Romanian validations[11-13].
An earlier analysis demonstrated that the SarQoL® questionnaire is able to discriminate between sarcopenic and non-sarcopenic subjects as long as the diagnostic criteria for sarcopenia include both muscle mass and muscle function[9]. The present study indicates that the SarQoL® questionnaire continues to be able to discriminate between the two groups irrespective of which measurement tools have been used for the assessment of muscle mass and muscle function. In both the English and French validations, muscle mass was measured by dual-energy x-ray absorptiometry (DEXA) and muscle strength with a hydraulic hand dynamometer[11,12]. In the Romanian validation, muscle mass was estimated with the Lee equation (using weight, height, gender, age and race) and muscle strength was measured with a hydraulic hand dynamometer[13]. With the addition of the data from the present study, it seems likely that the SarQoL® questionnaire can not only discriminate for several diagnostic criteria, but also when these are collected with several measurement instruments, which makes the questionnaire easier to use in different clinical settings.
With regards to the construct validity of the Dutch version of the SarQoL® questionnaire, it is noteworthy that two of the divergent hypotheses, namely the expectation of a weak correlation between the SarQoL® Overall score and the domain “Role limitation due to Emotional Problems” from the SF-36 and the question related to Self-Care from the EQ-5D, were rejected. However, these higher-than-expected correlations may be deceptive because of the homogeneity of the scores on these two domains. In fact, 22 out of 30 subjects (73%) scored 100 on the domain “Role limitation due to Emotional Problems”. Of these subjects, the minimum Overall score was 42.98 and the maximum was 94.22, a range of 51.24 points. The same phenomenon is at play for the EQ-5D domain Self-Care: 27 out of 30 subjects (90%) scored 1 (“I have no problems with self-care”), with a minimum Overall score of 43.92 and a maximum of 94.22, a range of 50.30 points.
This study has some limitations. We did not manage to recruit a sample of 100 subjects, as we set out to do, but the size of our total sample (n=92) is close to this goal. Of those 92 subjects, 30 were diagnosed as sarcopenic, which is not the 50 that is requested for these type of analyses, and is less than the French validation which had 43 sarcopenic subjects but more than the Romanian validation which recruited 13 sarcopenic subjects. The recruitment of our target sample of 50 sarcopenic subjects was complicated by its relatively low prevalence of 10% among healthy older people, and by the fact that sarcopenic individuals are less likely to volunteer for clinical studies due to their physical difficulties[6]. The sample that was recruited for this study was not a random sample and it is therefore possible that the characteristics of our sample are different from the larger population of Dutch-speaking sarcopenic individuals in Belgium. However, the Overall quality of life score as measured by the SarQoL® questionnaire in the present study is within the range found in other validation studies (unpublished data). While the evaluation of the psychometric properties should be robust with regards to volunteer bias, this might not be the case for the external validity of this study, and caution should be applied before extrapolating the quality of life scores obtained in this study to a larger population of sarcopenic individuals. A last limitation was the fact that 35 subjects completed the questionnaires at home for both administrations, while 57 subjects completed the questionnaires for the first time at the study center and 2 weeks later at home. The different circumstances in which the questionnaires were administered may have influenced the obtained results, but secondary analyses have not shown a significant difference between the two modes of administration (Supplemental Tables A and B).
Suppl. Table A.
Test-retest reliability [ICC (95% CI)] for at home and at study center groups.
| At study center (n=12) | At home (n=14) | Complete sample (n=26) | |
|---|---|---|---|
| Domain 1 | 0.789 (0.422-0.934) | 0.849 (0.604-0.948) | 0.820 (0.642-0.915) |
| Domain 2 | 0.900 (0.696-0.970) | 0.921 (0.775-0.974) | 0.908 (0.793-0.959) |
| Domain 3 | 0.766 (0.293-0.930) | 0.664 (0.223-0.878) | 0.707 (0.447-0.857) |
| Domain 4 | 0.904 (0.299-0.978) | 0.969 (0.908-0.990) | 0.948 (0.888-0.976) |
| Domain 5 | 0.860 (0.581-0.958) | 0.897 (0.708-0.966) | 0.875 (0.741-0.942) |
| Domain 6 | 0.179 (-0.477-0.677) | 0.442 (-0.65-0.776) | 0.375 (0.001-0.660) |
| Domain 7 | 0.686 (0.198-0.899) | 0.054 (-0.491-0.558) | 0.235 (-0.167-0.568) |
| Overall score | 0.978 (0.926-0.994) | 0.976 (0.928-0.992) | 0.976 (0.947-0.989) |
Suppl. Table B.
Median (P25-P75) quality of life scores (2 administrations) for at home and at study center groups.
| At study center (n=12) | At home (n=14) | |||||
|---|---|---|---|---|---|---|
| Test | Retest | p-value* | Test | Retest | p-value* | |
| D1 | 64.42 (59.97-76.36) | 68.51 (56.37-87.47) | 0.666 | 69.97 (49.72-86.63) | 69.42 (61.37-84.14) | 0.422 |
| D2 | 79.17 (50.00-90.98) | 75.00 (50.00-90.98) | 0.097 | 75.00 (48.61-79.86) | 68.06 (49.31-79.17) | 0.092 |
| D3 | 62.50 (51.04-70.83) | 69.79 (56.25-82.29) | 0.032 | 69.79 (54.17-81.77) | 70.84 (65.63-83.33) | 0.725 |
| D4 | 72.26 (59.62-77.68) | 74.11 (63.46-82.55) | 0.050 | 73.15 (58.90-93.31) | 73.01 (56.25-88.12) | 0.198 |
| D5 | 65.00 (43.34-90.00) | 64.17 (53.84-81.61) | 0.824 | 60.00 (50.42-80.83) | 62.50 (50.00-80.42) | 0.859 |
| D6 | 66.50 (37.41-66.50) | 66.50 (49.88-66.50) | 0.673 | 49.88 (33.25-66.50) | 58.19 (45.72-66.50) | 0.310 |
| D7 | 87.50 (87.50-100.0) | 87.50 (78.13-100.0) | 1 | 87.50 (75.00-100.0) | 87.50 (75.00-100.0) | 0.671 |
| Overall score | 69.24 (56.51-81.97) | 68.12 (53.65-80.99) | 0.583 | 67.42 (54.04-84.33) | 67.63 (56.36-86.30) | 0.875 |
Related samples Wilcoxon signed rank test.
This study also has several strengths. We followed a standardized translation and validation protocol written by the creators of the original SarQoL® questionnaire. This ensures that our translation is of a high standard and that the results from the validation can be compared with other validation studies. The use of BIA for muscle mass assessment and the Martin-Vigorimeter for muscle strength evaluation can also be regarded as a strength of this study because the SarQoL® questionnaire had not yet been validated with these instruments. The BIA and Martin-Vigorimeter are less costly than DEXA and a hydraulic dynamometer, so being able to use these instruments could lower the threshold for clinical implementation and further research.
In conclusion, this study has confirmed that the Dutch version of the SarQoL® questionnaire is a valid and reliable instrument for the assessment of quality of life, and that it is ready for use in clinical and research populations of elderly, Dutch-speaking, community-dwelling individuals. We also provide further evidence for the psychometric properties of the SarQoL® questionnaire by validating the questionnaire in a 4th cohort, thus adding confidence in its validity, consistency and reliability.
Acknowledgements
Study concept and design: AG, AS, CB, OB & IB. Translation: AG, IB, RD, RB, WV & CB. Data acquisition and analysis: AG & AS. Manuscript: AG. Critical revision: AG, CB, OB, AS & IB. All listed authors read and approved the final manuscript. Responsible for data integrity: AG.
Footnotes
C. Beaudart, I. Bautmans and O. Bruyère are shareholders of SarQoL® sprl. None of the other authors have conflicts of interest to report.
Edited by: G. Lyritis
References
- 1.Visser M. Epidemiology of muscle mass loss with age. In: Cruz-Jentoft AJ, Morley JE, editors. Sarcopenia. Chichester: John Wiley & Sons, Ltd; 2012. pp. 1–7. [Google Scholar]
- 2.Rosenberg IH. Epidemiologic and methodologic problems in determining nutrional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21 1988. Am J Clin Nutr. 1989;50(5):1121–235. [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, et al. Sarcopenia:European consensus on definition and diagnosis. Age Ageing. 2010;39(4):412–23. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dupuy C, et al. Searching for a relevant definition of sarcopenia:results from the cross-sectional EPIDOS study. J Cachexia Sarcopenia Muscle. 2015;6:144–54. doi: 10.1002/jcsm.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anker SD, et al. Welcome to the ICD-10 code for sarcopenia. J Cachexia Sarcopenia Muscle. 2016;7(5):512–4. doi: 10.1002/jcsm.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafiee G, et al. Prevalence of sarcopenia in the world:a systematic review and meta- analysis of general population studies. J Diabetes Metab Disord. 2017;16(1):21. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martone AM, et al. The incidence of sarcopenia among hospitalized older patients:Results from the Glisten study. J Cachexia Sarcopenia Muscle. 2017;8(6) doi: 10.1002/jcsm.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckinx F, et al. Prevalence of sarcopenia in a population of nursing home residents according to their frailty status:Results of the SENIOR cohort. J Musculoskelet Neuronal Interact. 2017;17(3):209–17. [PMC free article] [PubMed] [Google Scholar]
- 9.Beaudart C, et al. Current review of the SarQoL®:a health-related quality of life questionnaire specific to sarcopenia. Expert Rev Pharmacoeconomics Outcomes Res. 2017;17(4):335–41. doi: 10.1080/14737167.2017.1360768. [DOI] [PubMed] [Google Scholar]
- 10.Beaudart C, et al. Development of a self-administrated quality of life questionnaire for sarcopenia in elderly subjects:The SarQoL. Age Ageing. 2015;44(6):960–6. doi: 10.1093/ageing/afv133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaudart C, et al. Validation of the SarQoL, a specific health-related quality of life questionnaire for Sarcopenia. J Cachexia Sarcopenia Muscle. 2017;8(2):238–44. doi: 10.1002/jcsm.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beaudart C, et al. English translation and validation of the SarQoL®, a quality of life questionnaire specific for sarcopenia. Age Ageing. 2017;46(2):271–7. doi: 10.1093/ageing/afw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ildiko GA, et al. Psychometric performance of the Romanian version of the SarQoL®, a health-related quality of life questionnaire for sarcopenia. Arch Osteoporos. 2017;12(103):103. doi: 10.1007/s11657-017-0397-1. [DOI] [PubMed] [Google Scholar]
- 14.Beaton DE, et al. Guidelines for the process of cross-cultural adaptation of self-report measures. Spine (Phila Pa 1976) 2000;25(24):3186–91. doi: 10.1097/00007632-200012150-00014. [DOI] [PubMed] [Google Scholar]
- 15.Tombaugh TN, et al. The Mini-Mental State Examination:A Comprehensive Review. J Am Geriatr Soc. 1992;40:922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- 16.De Dobbeleer L, et al. Force-time characteristics during sustained maximal handgrip effort according to age and clinical condition. Exp Gerontol. 2017;98:192–8. doi: 10.1016/j.exger.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 17.Cao Dinh H, et al. Association between Immunosenescence Phenotypes and pre-frailty in Older Subjects:Does Cytomegalovirus Play a Role? Journals Gerontol Ser A Biol Sci Med Sci. 2018 doi: 10.1093/gerona/gly135. [DOI] [PubMed] [Google Scholar]
- 18.Cao Dinh H, et al. Strength endurance training but not intensive strength training reduces senescence-prone T-cells in peripheral blood in community-dwelling elderly women. J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/gly229. [DOI] [PubMed] [Google Scholar]
- 19.Terwee C, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60(1):34–42. doi: 10.1016/j.jclinepi.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Scafoglieri A, et al. Predicting appendicular lean and fat mass with bioelectrical impedance analysis in older adults with physical function decline –The PROVIDE study. Clin Nutr. 2017;36(3):869–75. doi: 10.1016/j.clnu.2016.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Studenski SA. et al. The FNIH sarcopenia project:Rationale, study description, conference recommendations, and final estimates. Journals Gerontol - Ser A Biol Sci Med Sci. 2014;69(5):547–58. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baumgartner RN, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 23.Bautmans I, et al. A fatigue resistance test for elderly persons based on grip strength:reliability and comparison with healthy young subjects. Aging Clin Exp Res. 2005;17(3):217–22. doi: 10.1007/BF03324600. [DOI] [PubMed] [Google Scholar]
- 24.Bautmans I, et al. Grip work estimation during sustained maximal contraction:Validity and relationship with dependency and inflammation in elderly persons. J Nutr Heal Aging. 2011;15(8):731–6. doi: 10.1007/s12603-010-0317-1. [DOI] [PubMed] [Google Scholar]
- 25.Lauretani F, et al. Age-associated changes in skeletal muscles and their effect on mobility:an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95(5):1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 26.Ware J, et al. The MOS 36-Item Short Form Health Survey (SF-36):I Conceptual Framework and Item Selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 27.Oppe M. EQ-5D-3L User Guide. 2015 [Google Scholar]
- 28.Mokkink LB, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63(7):737–45. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 29.De Vet HCW, et al. Measurements in Medicine. Cambridge University Press; 2011. [Google Scholar]


