Abstract
This systematic review aims to categorically analyses the literature on the assessment of biceps brachii (BB) muscle activity through mechanomyography (MMG). The application of our search criteria to five different databases identified 319 studies. A critical review of the 48 finally selected records, revealed the diversity of protocols and parameters that are employed in MMG-based assessments of BB muscle activity. The observations were categorized into the following: muscle torque, fatigue, strength and physiology. The available information on the muscle contraction protocol, sensor(s), MMG signal parameters and obtained results were then tabulated based on these categories for further analysis. The review affirms that – 1) MMG is suitable for skeletal muscle activity assessment and can be employed potentially for further investigation of the BB muscle activity and condition (e.g., force, torque, fatigue, and contractile properties), 2) a majority of the records focused on static contractions of the BB, and the analysis of dynamic muscle contractions using MMG is thus a research gap, and 3) very few studies have focused on the analysis of BB muscle activity under externally stimulated contractions. Taken together, the findings of this review on BB activity assessment using MMG affirm the potential of MMG as an alternative tool.
Keywords: Mechanomyography, Muscle Assessment, Muscle Activity, Muscle Function, Biceps Brachii
Introduction
Mechanomyography (MMG) is a non-invasive tool for the recording of low-frequency lateral oscillations in active skeletal muscle fibres[1,2]. These oscillations reflect the mechanical counterpart of motor unit activity measured by electromyography (EMG)[3]. The notable benefits of MMG, such as minimal skin preparation, negligible effect of skin impedance and notably lower susceptibility to external noise, make this technique a suitable alternative to EMG[4-6]. Although EMG has been extensively used for muscle assessments for decades, it has been shown that MMG has the ability to quantify muscle function in a non-invasive manner, which strengthens its potential for clinical applications[7]. The frequency content of an MMG signal, in contrast to an EMG signal, provides valuable information on muscle contractile properties related to the muscle fibre type and composition[8]. The literature details the use of both MMG and EMG for the characterization of neuromuscular function.
Similar to EMG signals, MMG signals are sensed using transducers. Dimensional changes during isometric or dynamic muscle contractions through voluntary or stimulated motor unit activity generate mechanical oscillations or vibrations in muscles at a gross level[9]. MMG captures the oscillatory effects of these dimensional changes using transducers. The sensor weight and muscle mass are considered critical in the selection of transducers for MMG[10]. The transducers that have been used for MMG measurements include accelerometers, piezoelectric contact sensors, condenser microphones and laser displacement sensors, and amongst these, lightweight accelerometers are considered suitable because these reduce the risk of potential disturbances during the recording of surface oscillations[9].
A recorded MMG signal can depict a muscle’s motor unit activation strategy based on temporal and spectral features during both voluntary and stimulated contractions. Specifically, temporal features, such as MMG root mean square (RMS), and spectral features, such as MMG mean power frequency (MPF), median frequency (MDF) and centre frequency (CF), provide information regarding motor unit recruitment and firing[11,12]. However, at near-maximal intensities or durations, these features can exhibit opposite dynamics during these different types of contractions, suggesting an end to the recruitment of new motor units and an exponential increment in the firing rates to produce more force[13].
The current technology limits the acquisition of MMG signals to skeletal muscles (surface mechanomyogram). These muscles account for up to 40% of the body mass and are important for the movement of limbs and the body. MMG has certain limitations, and the most critical of these is crosstalk, which refers to the contamination of a myographic signal from a target muscle by a signal from a neighbouring muscle[10]. MMG has been used by many researchers to review the activity of the biceps brachii muscle (BB). These MMG studies provided evidence showing that the BB muscle is less prone to signal crosstalk, which might be due to the peculiar geometry and relatively isolated nature of the BB. In contrast, MMG-based studies of forearm[10] and leg muscles[14] have shown that the signals from these muscles are likely to be contaminated by crosstalk. Hence, MMG signals of the BB muscle are more reliable because MMG in the absence of any interference is just as reliable as EMG. Thus, the MMG-based studies of the BB muscle should be thoroughly reviewed to investigate the validity of MMG as a muscle assessment tool.
Although many MMG-based studies have assessed the BB muscle, these assessments of BB muscle activity have not yet been systematically reviewed. Hence, a systematic summary and critical analysis of the assessment of BB activity using MMG is necessary. The literature includes only a few review articles on MMG, but the present review is clearly distinct from the published articles. Specifically, a previous review[15] examined MMG frequency and amplitude responses under dynamic contraction using different experimental designs. Two other articles[16,17] discussed MMG sensors, including their development and signal processing techniques, for different muscles. In another review, the researchers provided an assessment of muscle function via MMG[6]. Muscle function using MMG was also reviewed a year later[7]. MMG feature extraction methods for clinical applications were documented in another review[18]. Another publication[19] reported advancements in MMG in terms of sensors, signal acquisition, processing and analysis. Skeletal muscle function was assessed in another study[20] that focused on the measurement reliability and sensitivity of MMG signals. Thus, there is gap in knowledge regarding the assessment of BB activity using MMG. This article reviews the published studies that assessed various activities produced in the BB muscle using MMG based on sensor type, experimental protocol and MMG signal parameters. This review is of crucial interest for aiding our understanding of the BB muscle while performing different activities. As a result, MMG-based analyses of the BB muscle can be utilized in both clinical and applied research based on its wide range of applications in sports and medicine. To the best of our knowledge, the literature does not include an article that summarizes and analyses the studies on BB muscle activity assessment using MMG.
This review aims to: (1) generate a classification of the assessment of BB muscle activity using MMG signals, (2) identify commonly selected MMG parameters addressing diverse types of BB muscle activities, and (3) analyse the results corresponding to all the compartments relevant to the protocol and BB muscle activity under consideration. Under a specific experimental protocol, the behaviour of temporal and spectral features of MMG signals is related to various muscle activities. The muscle activities that have been studied using MMG-based approaches, i.e., fatigue, strength, force and torque, provide insights regarding muscle physiology. The findings also encourage the application of MMG for clinical research in medicine, rehabilitation and prosthetic control as well as applied research in athletics and sports. Thus, MMG can be used as an alternative to EMG in various clinical applications, including rehabilitation, muscle disease and atrophy.
Methods
Identification of studies
A comprehensive literature search of studies that assessed the BB muscle using MMG was conducted. Five different databases, namely, PubMed, Science Direct, Wiley Online Library, IEEE Xplore and SCOPUS, were searched for articles on MMG-based BB muscle assessment published during the period from January 2001 to December 2017. Suitable combinations of keywords derived from the root words ‘mechanomyography’ AND ‘biceps brachii’ were used, and the identified peer-reviewed journal articles, conference publications, books, and clinical reports were reviewed for the selection of eligible records.
Study inclusion/exclusion criteria
The search of five databases using the abovementioned set of keywords yielded 315 studies. Four additional articles were obtained from other sources, such as medical journals and databases other than the five abovementioned databases. Of these 319 total studies, 60 records were excluded because they (1) were published in a language other than English, (2) described animal studies or (3) discussed a technique other than MMG. Further screening resulted in the exclusion of 199 additional articles for the following reasons: (1) the manuscript did not describe muscle assessment, (2) the study did not provide findings for the BB muscle, and (3) the article did not clearly describe the protocol used. After the removal of duplicates, extended versions and studies with insufficient information, we ultimately obtained 48 articles that met the inclusion criteria, as shown in [Figure 1].
Figure 1.
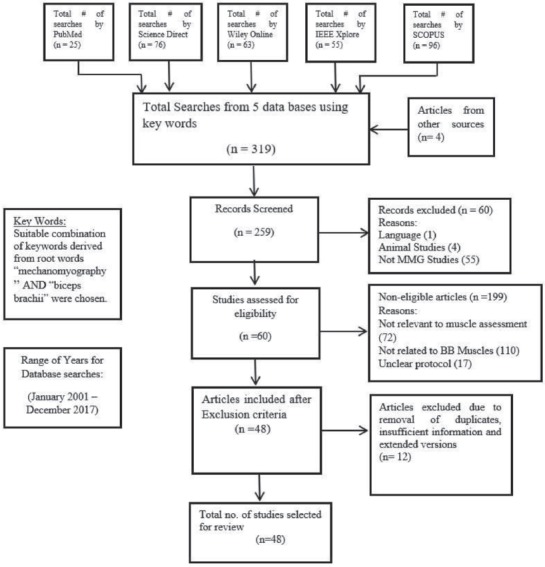
Flow chart for selection of studies.
Data extraction
The shortlisted 48 articles were analysed in detail, and the following information was extracted from each study – (1) author name and publication year, (2) sensor used, (3) details of the subjects, (4) muscle activity, (5) experimental protocol, (6) MMG parameters studied, (7) results obtained and (8) applications, implications and limitations of the study.
Data analysis
The MMG-based assessment of BB muscle activity was further categorized into comprehensive subsections, such as muscle strength, fatigue, torque and physiology.
Results
The 48 eligible records described the assessment of BB muscle activity using MMG could be classified into the following broad categories – elbow joint torque studies (10 records), muscle fatigue studies (19 records), BB muscle strength / force studies (9 records), and activity assessment based on muscle physiology (10 records).
Elbow joint torque assessment
Ten records were found to describe assessment of muscle torque in the BB using MMG, as shown in [Table 1]. One of these studies[21] proposed a protocol for evaluating the effect of dehydration on torque under submaximal isometric loadings. No change in torque versus MMG RMS / MMG MPF was observed after dehydration, which suggests that dehydration does not change the filtering properties of the tissues between the MMG sensor and the BB muscle. Other researchers[22] observed the effect of the static stretching of the BB for maximal dynamic torque production. Greater torque was observed for exercises with non-stretching protocols while higher MMG amplitudes were observed for exercises with stretching protocols. The authors suggested that a greater ability to produce torque without prior stretching relates to musculotendinous stiffness rather than number of motor units recruited while a more compliant muscle (stretched) would result in reduced stiffness, increased muscle fibre oscillations and higher MMG amplitudes. Another study[23] observed a linear relationship for the MMG RMS with increase in the peak torque during isokinetic muscle actions, which suggests the possibility that motor unit control strategy could be studied through MMG amplitude analyses. Furthermore, another investigation[24] compared the isometric torque levels associated with fatigue thresholds estimated from EMG and MMG frequencies and critical torque. Critical torque is the slope coefficient of the linear relationship between isometric work and time to exhaustion. These researchers found no differences among the results obtained using these three different strategies for calculating the fatigue threshold. Another group of researchers[25] constructed an MMG torque estimator for dynamic contractions. The frequency variance was used as a significant feature for estimating torque, and this measure showed better accuracy than the linear mapping between MMG RMS and muscle contraction torque because the error in the predicted torque was reduced significantly at four different contraction frequencies. In another study[11], MMG amplitude and frequency values at different ranges of peak torque and maximal voluntary contractions were investigated, and the researchers found an increase in the MMG RMS with no meaningful change in the MMG MPF during isokinetic actions from 10% to 100% peak torque. A plateau in the MMG RMS and a decrease in the MMG MPF were also observed during isometric actions from 80% to 100% maximum voluntary contraction (MVC). The researchers claim that this finding might be due to the fusion of motor unit twitches at high intensity levels. Another study[26] found a linear increase in the MMG CF with increase in the isokinetic torque from 10% to 100% peak torque. These researchers compared and found that both the fast Fourier and continuous wavelet transforms yielded similar responses when used to examine the relationship between MMG frequency and eccentric torque. Another work[27] investigated contractile changes in the BB muscle. The muscle developed fatigue in response to transcutaneous electrical stimulation, and a strong correlation was found between the peak torque produced and the peak-to-peak MMG amplitude during fatiguing conditions. Furthermore, another investigation[28] examined the BB muscle under electrical stimulation and found a parabolic relationship between torque and the transversal displacement of the BB muscle. The behaviour of accelerometer and piezoelectric contact sensor was compared in another investigation[29] for the assessment of isokinetic and isometric muscle actions. The findings from this study suggested a linear correlation between normalized MMG amplitude and isokinetic torque when piezoelectric contact sensor and accelerometer are used as sensors. The authors observed the pattern of normalized MMG amplitude as a function of isometric torque obtained using piezoelectric sensor was flattened from 60% to 100% MVC, whereas that obtained using accelerometer continued to increase during this range of MVC. The authors observed no significant changes in isokinetic torque versus normalized MMG MPF relationships of both the sensors. In contrast, they observed normalized MMG MPF to be decreasing linearly with increase in isometric torque for accelerometer. But there was no significant relationship between normalized MMG MPF with isometric torque in contact sensor.
Table 1.
Details of records on torque assessment.
| Authors | Sensor used | Subjects | Muscle activity assessed | Experimental protocol | Parameters | Results | Application / Limitation / Future work |
|---|---|---|---|---|---|---|---|
| 21 | Piezoelectric crystal sensor | 10 (6 men, 4 women) | Effect of dehydration on muscle torque | Sub maximal isometric contractions at 25%, 50%, and 75% MVC | Torque, MMG MPF and MMG RMS | No change in the torque, RMS and MPF (both EMG & MMG) values after dehydration. | Effect of dehydration can be studied on other muscles using MMG in future. |
| 22 | Piezoelectric crystal sensor | 18 (10 men, 8 women) | Ability of muscle to produce torque after static stretching | Maximal dynamic torque production | Torque, EMG and MMG amplitudes | Torque increases while moving from stretching to non-stretching protocol. | Effect of change in muscle temperature and blood flow on muscle stiffness was not considered. |
| 23 | Piezoelectric crystal contact sensor | 12 (6 men, 6 women) | Modulation in dynamic torque production due to isokinetic muscle action | Submaximal to maximal isokinetic actions | Torque, MMG amplitude and MPF, EMG amplitude and MPF | The MMG and EMG RMS show a linear relation with increasing peak torque | The study could be extended in future to other body muscles taking MMG signal contamination into account. |
| 11 | Piezoelectric crystal sensor | 10 (5 men, 5 women) | Torque assessment during isokinetic and isometric contraction | Isometric and isokinetic testing at 10% to 90% MVC | MMG amplitude, MMG MPF and torque | MMG amplitude increases in isokinetic testing while MMG amplitude flattens and MPF decreases in isometric testing from 80% to 100% MVC. | MMG amplitude and frequency response in relation to torque can be observed for other muscles in future. |
| 26 | Piezoelectric crystal sensor | 8 (6 men, 2 women) | Eccentric torque production | Submaximal to maximal eccentric isokinetic muscle actions | MMG MPF, MDF, peak torque | MMG CF obtained for the biceps brachii muscle increases linearly with increases in eccentric isokinetic torque from 10% to 100% peak torque. | Fourier based time-frequency methods can be used in future to process MMG signal coming from other muscles as well. |
| 29 | Piezoelectric contact and accelerometer | 10 (5 men, 5 women) | Torque response under isokinetic and isometric forearm flexion | Submaximal to maximal isokinetic and isometric muscle actions | Torque, MMG amplitude for both the contact sensors and accelerometer, MMG MPF | The contact sensor and accelerometer resulted in different torque-related responses that might affect the interpretation of the motor control strategies involved. | MMG amplitude and frequency response may be compared in both contact sensor and accelerometer for muscle under fatiguing contraction. |
| 27 | Accelerometer | 10 men | Estimation of torque through MMG | Transcutaneous muscle electrical stimulation | Peak torque and MMG peak to peak amplitude | A strong correlation between peak torque and MMG peak to peak amplitude in fatigue was found. | Specialized dynamometer was not used to measure joint torque accurately instead of load cell. |
| 28 | Displacement sensor | 15 men | Torque and muscle deformations | Isometric twitch response | Delay time, Tc, half-relaxation time, Dm | Parabolic relation between transversal displacement and torque amplitude was observed. | Contractile properties have been assessed for transversal and longitudinal biceps brachii response only. Lateral response in terms of contractile properties may also be measured for same methodology in future, so that a good comparison can be made among three axis responses. |
| 24 | Accelerometer | 10 (4 men, 6 women) | Maximal non-fatigue torque level derived from MMG, EMG and critical torque | Isometric contractions at 30, 45, 60 and 75% MVIC | Critical torque, mechanomyographic fatigue threshold from frequency content, electromyographic fatigue threshold from frequency content | No differences in the isometric torque levels associated with Critical torque, mechanomyographic fatigue threshold from frequency content and electromyographic fatigue threshold from frequency content were observed. | Fatigue threshold tests can be validated by dynamic muscle actions in future. |
| 25 | Accelerometer | 7 men | Torque estimation via the MMG signal | Incremental / dynamic voluntary contractions | Muscle torque, MMG RMS, MMG MPF | The estimation obtained using the MMG torque estimator was more accurate than linear mapping. | Number of subjects considered for torque estimation experiment is very small. |
Fatigue assessment
Nineteen records reported studies of BB muscle fatigue using MMG, and these studies are summarized in [Table 2]. Five of these records discussed the indication of muscle fatigue through MMG signals[13,30-33]. The authors of one of these studies[33] considered MMG as an alternative to EMG for muscle fatigue assessment during sustained contractions at 25% MVC. The findings showed that both EMG RMS and MMG RMS increase at the onset of fatigue. In addition, the mean frequency regression slopes obtained for men and women using MMG were more similar than those obtained using EMG. Hence, this finding provides preliminary evidence showing that MMG is less sensitive to inter-sex variation. In another study[13], MMG was used to study the effects of fatigue in the BB muscle under isometric ramp contractions from 0% to 90% MVC. After the development of fatigue in the BB muscle, the MMG RMS showed a decreasing trend, while the MMG MPF values shifted to a lower scale. Another group of researchers[32] used MMG for measuring muscle fatigue and strength and estimated the motor unit activation strategy under isometric muscle contractions at 20% and 80% MVC. As indicated by these, at 20% MVC, the MMG RMS showed an initially decreasing and then increasing trend, and the MMG MPF showed the opposite trend. However, at 80% MVC, MMG RMS showed a continuous decrease, and the MMG MPF trend was unchanged. The findings revealed that fatigue induces a change in the motor unit activation strategy, as was observed by the above-mentioned changes in the MMG RMS and MMG MPF trends. In addition, the authors of this study claimed that short-time Fourier transform provides a better estimation of the MMG MPF. Similar to a previous approach, another study[31] compared Fourier and wavelet transforms for examining EMG and MMG spectral responses during maximal isokinetic muscle actions. Motor unit twitches and firing rates were assessed using different frequency parameters, including the CF, MPF and MDF. Decreases in the MPF, MDF and CF were found after the development of fatigue using both EMG and MMG. Another study[30] performed an experiment with an electret condenser microphone to observe the MMG RMS and MMG MPF during voluntary contractions and found an increase in the MMG RMS with increase in muscle fatigue and force.
Table 2.
Details of records on fatigue assessment.
| Authors | Sensor used | Subjects | Muscle activity assessed | Experimental protocol | Parameters | Results | Application / Limitation / Future work |
|---|---|---|---|---|---|---|---|
| 33 | Accelerometer | 18 (9 men, 9 women) | Muscle fatigue | Sustained muscle contraction at 25% MVC | RMS, MDF | From the onset of fatigue, the RMS of both EMG and MMG increases, while MDF decreases. | For future research fatigue assessment may be performed under high electrical noise for both MMG and sEMG to prove the validity of MMG as suitable alternative to sEMG. |
| 13 | Accelerometer | 10 (gender not mentioned) | Muscle fatigue | Isometric force ramp from 0% to 90% of MVC | RMS & MPF | In fatigued muscle RMS showed a decreasing trend and MPF shifted to lower values. | Application for fatigue detection and understanding of motor unit activation strategy. |
| 32 | Accelerometer | 10 men | Muscle fatigue | sustained isometric contractions at 20% and 80% MVC | RMS, MPF | At 20% MVC, the RMS first decreased and then increased, whereas the MPF increased and then remained constant. At 80% MVC, the RMS decreased, and the MPF first increased and then decreased. | Results for fatigue test may not be reliable due to: 1) Two fatigue tests at two intensities were not performed in same order for all the subjects. 2) Break between two consecutive tests was also not same among all the subjects. |
| 31 | Piezoelectric crystal sensor | 7 men | Muscle compliance and muscle fatigue | 50 consecutive isokinetic muscle actions at a velocity of 180°s-1 | CF, MPF, MDF for both MMG and EMG | Decrease in MMG MPF, MDF and CF values over repetitions were observed. | MMG frequency-based response for both wavelet and Fourier transform can be compared in future for isometric ramp contraction. |
| 43 | Accelerometer | 7 women | Effect of muscle blood flow and oxygen availability on fatigue | 10% MVC for 10 minutes | Intramuscular pressure, tissue oxygenation, RMS, MPF | Intramuscular pressure remained constant, and tissue oxygenation first increased and then returned to its resting level within 1minute. Both of these factors do not affect muscle fatigue. | Application in indication of muscle pain and disorders but the results cannot be generalized as experiment was restricted to one gender only. |
| 34 | Microphone and accelerometer | 14 men | Fatigue development trend in muscle under sustained contractions | 3 minutes isometric elbow flexion at 30% MVC | MMG absolute and normalized RMS, MPF, power spectral variance (Mc2), and skewness (µ3) | Microphone resulted in higher RMS and Mc2 values while lower MNF and µ3 values as compared to accelerometer. | MMG signal power spectrum may be studied with higher order spectral moments in future. |
| 44 | Piezoelectric crystal sensor | 12 (7 men, 5 women) | Muscle relaxation and muscle fatigue | Forearm flexions at 85% of maximum repetitions of seated preacher curl exercise with BIO & NOBIO | MMG RMS, EMG RMS | Biofeedback did not improve athlete performance due to lack of training regarding viewing the MMG signal. | The details for biofeedback used in the study are not mentioned clearly. Future work in pain control and athlete performance is recommended. |
| 30 | Electret condenser microphone | 5 (4 men, 1 woman) | Force of contraction and fatigue | Isometric contractions at 60% to 80% MVC | RMS, MDF | Increasing force of contraction & fatigue increases RMS value. | The impact of crosstalk in MMG signal, recorded through microphone from multiple muscles can be a future work. |
| 39 | Accelerometer | 5 (gender not mentioned) | Muscular effort and fatigue | Isometric contraction at 80% MVC | Noise limit and correlation dimension (D2) | Muscle activity became more dynamic at high noise levels. | This methodology can be used in future to distinguish between different muscle states. |
| 35 | MMG sensor composed of a PVDF film as the sensory material | 5 men | Investigation of muscular injury and muscular fatigue via MMG | 40% MVC with elbow flexion and extension from 45° to 90° in a 4 seconds cycle | MPF and variance (of sensor output) | Muscle fatigue decreases variance while MPF reaches its maximum value prior to fatigue. | 1) The sensor used in the study is not a commercial sensor. 2) Results of this study cannot be generalized for both genders. |
| 40 | Accelerometer | 10 men | Investigation of the influence of variation in window length for fatigued muscle | Submaximal isometric contractions of elbow joint at 70% MVC | MPF, skewness of the spectrum (µ3), zero-crossings, spectral ratio (SR) | MPF showed a negative effect on spectral energy leakage due to the nature of the algorithm used. An increase in µ3 and decrease in the MPF was interpreted as reflection of fatigue. | Influence of variation in window length can be studied on other muscles at various levels of effort in future. |
| 36 | Accelerometer | 15 men | Effect of fatigue and cooling on neuromuscular activation | Voluntary isometric contractions at 20%, 40%, 60%, 80% and 100% MVC | Latency between EMG and MMG (∆t EMG-MMG), latency between MMG and force (∆t MMG-F), electromechanical delay (EMD) | Muscle cooling increased ∆t EMG-MMG, but fatigue elongated both ∆t EMG-MMG and ∆t MMG-F. | Results cannot be generalized as subjects participated in the experiment are male only. |
| 45 | Accelerometer | 13 men | Muscle fatigue detection | Non-isometric exercises at 40% and 70% maximum dynamic strength (MDS) | Davies-Bouldin index | The optimal range of -0.775 Davies-Bouldin index can separate fatigue and non-fatigue. MMG signals. | Pseudo-wavelet method can be used in future to distinguish between fatigue and non-fatigue content of MMG signal coming from muscle under isometric contraction. |
| 37 | Accelerometer | 15 men | Change in muscle relaxation delay after fatigue | Fatigue exercise for elbow flexors at 50% MVC producing intermittent contractions | Delays from EMG cessation to onset of F decay (R-EMD), from F decay to onset of largest MMG displacement (MMG p-p) (R-∆t F-MMG), from the beginning to the end of MMGp-p (R-∆t MMGp-p), and from the end of MMGp-p to F cessation (R-∆t MMGp-p) | No prominent differences in muscle temperature before and after fatigue were observed. The total relaxation delay is mainly due to the main phase of cross-bridge detachment. | Effect of fatigue on electromechanical delay could be investigated in future for stimulated contraction in muscle. |
| 41 | Piezoelectric resonance-based active muscle stiffness sensor and MEMS accelerometer for MMG signals | 12 (10 men, 2 women) | Change in physical stiffness of muscle under fatigue condition | Isometric contractions at various levels of MVC | Temporal and spectral features respectively which obtained with muscle stiffness sensor (Sa and Sf), mean absolute value of the amplitude of the MMG and EMG signals amplitude | Stiffness caused increase in the EMG amplitude, decrease in MMG amplitude and slight variations in the temporal and spectral features respectively which obtained with muscle stiffness sensor | 1) Physiological model for stiffness changes in muscle can be developed in future to check the abnormal behaviour of MMG signal due to stiffness. 2) Muscle stiffness sensor was tested only for isometric muscle contraction. This sensor can also be tested for dynamic muscle contraction. |
| 42 | Laser displacement sensor (LDS) | 13 (gender not mentioned) | Accumulated muscle fatigue | 2-s concentric contractions, maximally fatiguing exercise | Contraction velocity (Vc), half-relaxation velocity (1/2Vr), maximal displacement (Dm), MPF and MVC | Vc, 1/2Vr and Dm decreases while MVC and MPF remained stable. | Speed of exercise was not constant while performing concentric contraction which may lead to a difference in time to fatigue occurrence among various subjects. |
| 46 | Accelerometer | 18 (gender not mentioned) | Effect of gender on muscle fatigue | 50 intermittent submaximal isometric contractions between 2 MVIC trials | MMG RMS and MMG MPF | MMG RMS increased 22.5% and 17.5% for men and women respectively. No change in MMG MPF. | Future studies may examine the intensity related gender differences for intermittent fatiguing contraction and make observation using MMG and EMG responses. |
| 47 | Accelerometer | 60 (30 men, 30 women) | Quantitative grading of muscle fatigue | Sustained contractions of both isometric and isotonic types for 3 minutes and 5 seconds respectively. | MPF, MDF and frequency ratio change | Frequency ratio change matched MPF & MDF by 87.5%. | Long-term muscle fatigue has not been taken in to account while performing experiment. It may alter the results for muscle fatigue grading |
| 38 | Accelerometer | 11(5 men, 6 women) | Effect of fatigue on muscle moment | 50 intermittent concentric muscle actions | MMG amplitude, peak moment, mean power | MMG amplitude versus mean power showed linear behaviour while moment remained same at 65% peak moment | Future research can be performed to track relation between power and MMG amplitude for diseased muscles as well as trained muscles. |
Five other studies evaluated the effect of fatigue on different MMG parameters and muscle conditions[34-38]. In one study, the researchers[35] developed an MMG sensor to measure the frequency and magnitude of muscle contraction before and after fatigue. These researchers observed stability in the sensor output and a time shift in the maximum MMG MPF value due to muscle fatigue. Another study[36] investigated the effect of fatigue and a change in temperature on electromechanical delay components comprising both electrochemical and mechanical processes. The findings revealed that fatigue increases the delays in both mechanical and electrochemical processes, whereas cooling only affected the electrochemical process. The combined effect of fatigue and cooling decreased the fibre conduction velocity, which suggests a change in the muscle fibre propagation properties. In another study[37], the researchers recorded the MMG, EMG and force during MVC from the BB muscle before and after fatigue to observe the effects of fatigue on the total relaxation delay component. They defined the total relaxation delay as the time lag from the end of neuromuscular activation to the end of force production. These researchers found that the total relaxation delay component was lengthened after fatigue. Among all the contributing components to this delay, they found the main contributor to be the largest MMG displacement duration (peak-to-peak duration of the largest MMG signal amplitude during relaxation), which incorporates the main phase of the detachment of cross-bridges and series elastic components relaxation. Another study[34] compared spectral moments of the MMG signals obtained from microphones and accelerometers under sustained contractions at 30% MVC. The general trends obtained after fatigue manifestation with both sensors were similar, but little correlation in the rate of change of spectral moments was found between these sensors. The researchers claimed that this finding was due to the characteristics of the sensors. The authors of another study[38] investigated the effect of fatigue on muscle moment and mean power and found that the MMG RMS could track the changes in the mean power. In contrast, the MMG RMS could not monitor muscle moment, probably due to the increases in muscle stiffness and change observed in the muscle activation pattern after fatigue.
Other studies[39-43] revealed observations regarding the measurement of fatigue. The researchers of one of these studies[43] used an experimental protocol for fatiguing contractions at 10% MVC for 10 minutes. One-minute test contractions, at 5% MVC were performed before, 10 minutes after, and 30 minutes after the fatiguing contractions. The findings from this research suggested that the measurement of muscle fatigue through MMG is not affected by blood flow and oxygen availability in the BB muscle. Another research study[39] found that the fatigued MMG signal was chaotic in nature. These researchers suggested two parameters, namely, noise limit and correlation dimension, as measures of fatigue obtained using MMG. Noise limit was identified as a power indicator of chaos in a fatigued MMG signal. The nature of chaos in an MMG signal was further investigated using the correlation dimension between non-linear dynamic features of the signal originating from a fatigued BB muscle. The findings indicated that the mechanical activity of the muscle could be described using three to four degrees of freedom. Thus, the MMG signal from a fatigued BB muscle exhibits high dimensional chaos. The researchers claimed that the theory of nonlinear dynamics is useful for modelling a fatigued MMG signal. The authors of another study[40] investigated the influence of changes in the window length used for the analysis on various MMG parameters during submaximal fatiguing contractions identified by spectral skewness and a decrease in the MPF. An increase in the window length increases the capability of the algorithm to differentiate between fatigued and non-fatigued MMG signals, but a larger window size could suffer from real-time usage issues. The authors concluded that the use of a one-second window length provides optimal analysis results. The researchers of another study[41] compared the use of a stiffness sensor for MMG- and EMG-based analyses to monitor fatigue in the BB muscle. The results revealed that the signals obtained from the stiffness sensor suffers from stability issues during fatiguing contractions, indicating concerns regarding reliability and feasibility for muscle fatigue estimation. This study showed that the importance of an MMG signal for obtaining an indication of muscle fatigue was equal to that of an EMG signal. The researchers of another study[44] applied MMG feedback as a tool to observe changes in muscle relaxation during fatiguing contractions. Elbow joint flexion during a preacher curl exercise at 85% MVC was assessed with and without visual MMG biofeedback. The results revealed that MMG biofeedback enhances muscle relaxation but does not cause a delay in fatigue. This study advocates the use of MMG as a biofeedback tool similar to EMG.
Another recent study[42] determined the effect of accumulated muscle fatigue on the MMG signal. MMG, EMG and MVC were recorded from the BB muscle for 13 days before and after a fatiguing exercise. This study measured various contractile properties of the muscle, such as contraction velocity (Vc), half-relaxation velocity (½Vr) and maximal displacement (Dm). A decrease in these contractile parameters proved to be an indicator of muscle fatigue. In addition, the EMG MPF and MVC values were stable and thus do not provide insight into fatigue. Hence, it can safely be deduced that the observation of contractile properties using MMG can provide a better estimation of muscle fatigue than EMG.
In another study[45], the researchers developed a pseudo-wavelet function to classify the fatigue and non-fatigue contents of an MMG signal originating from the BB muscle. The classifier was trained using 70% of the conducted MMG trials, and the remaining 30% of the MMG data was used for testing. The authors claimed that pseudo-wavelet function enhances the classification performance of muscle fatigue by 4.7% to 16.61% compared with other wavelet functions.
The researchers of another study[46] observed the effect of gender on the MVC torque, EMG and MMG responses during intermittent fatiguing contractions in the BB muscle. A slight increase in the MMG RMS of 22.5% and 17.5% and a decrease in the EMG MPF of 20.0% and 25.3% were observed in men and women, respectively, after a fatiguing exercise. The MMG MPF, EMG RMS and amount of decrease in the MVC torque from pre- to post-test were equal for both genders suggesting muscle mass and strength differences between genders do not affect differences in fatigue resistance but rather this could be an effect of differences in fiber type composition.
Another study[47] introduced a new parameter, denoted by frequency ratio change, to quantitatively measure BB muscle fatigue during isometric and isotonic contractions. The MPF and MDF of both MMG and EMG signals were also computed to measure muscle fatigue. A high correlation was found between these features and the frequency ratio change for the evaluation of muscle fatigue. The frequency ratio change showed 87.5% similarity with the MPF and MDF for the computation of fatigue under all muscle conditions and with both myographic signals.
Strength and force assessment
Nine of the identified studies used MMG signals to assess the BB muscle strength and force, as listed in [Table 3][48-56]. In one of these studies[56], the BB muscle strength was assessed based on the MMG signal obtained from a muscle during isometric contractions using a biaxial accelerometer. An increase in the amplitude and a decrease in the frequency of the MMG signal were observed with increases in the BB muscle force. The decrease in the frequency content might be attributed to the noise in the MMG signal. However, the researchers concluded that muscle strength can be assessed using MMG parameters. Furthermore, another study[55] concluded that muscle pain induces changes in the twitch force and motor unit firing rate, and these might be observed as an increase in the MMG RMS. The muscle was under static isometric contractions. In another study, the researchers[54] characterized the motor unit activity in MMG signals from a muscle during isometric contractions at three levels, i.e., 20%, 50% and 80% MVC. The researchers observed an increase in the single-motor-unit MMG amplitude from 20% to 50% MVC, but this value remained unchanged in range of 50% to 80% MVC due to an increase in muscle stiffness. The authors of another work[53] compared the performances of an artificial neural network model and a multiple linear regression model to estimate elbow flexion force from an MMG signal. These researchers found that the artificial neural network model was more accurate with a lower normalized RMS error. The model with temporal features (RMS) produced a better estimate of force than spectral features (zero crossing). This model, which was established for one subject, was also tested on another subject for cross-subject validation. The results revealed that the within-subject model estimates were better than the cross-subject estimates, which might be due to difference in muscle morphology, stiffness and intra-muscular pressure across subjects. The authors of another study[52] utilized an artificial neural network to model the strength of the BB muscle. The frequency variance was used as a significant feature for estimating strength because it showed better accuracy than MMG RMS linear mapping. The error in the predicted strength was reduced significantly at four different contraction frequencies. Another study[51] observed an increase in the MMG RMS with increases in the BB muscle force up to 80% MVC, which is due to an increase in the isometric force after motor unit recruitment. The authors of another study[50] observed the effect of the application of direct inhibitory pressure (DIP) to the myotendinous junction of the BB muscle on the muscle force. DIP induced a decrease in the rate of force development, MVC, RMS of MMG and EMG. The study concluded that the muscle force output decreases due to the application of DIP on the myotendinous junction, and this decrease in muscle force are possibly due to the impairment of neuromuscular activation.
Table 3.
Records on muscle strength / force assessment.
| Authors | Sensor used | Subjects | Muscle activity assessed | Experimental protocol | Parameters | Results | Application / Limitation / Future work |
|---|---|---|---|---|---|---|---|
| 48 | Accelerometer | 5 men | Effect of transcranial magnetic stimulation) on the excitability of spinal motoneurons | Motor-evoked potential obtained by magnetic stimulation at 5%, 10%, 20%, 30%, 40%, 60% and 100% MVC | Motor-evoked potential area and amplitude | The peak to peak amplitude and area increases with increase in muscle contraction. | For future work, influence of motor evoked potential parameter on muscle contraction can be observed with consideration of electrical noise. |
| 56 | Accelerometer | 27 (15 men, 12 women) | Muscular strength | Isometric contractions at 20%, 40%, 60%, 80% and 100% of maximal workload performed for 8 seconds. | RMS and MPF (both parameters in both directions, i.e. perpendicular to fibres and parallel to fibres) | The RMS for both directions depicted an increasing trend in both genders, while MPF for both directions showed a decreasing trend in females. | Muscle strength may be assessed for muscle under dynamic contraction in future. |
| 55 | Accelerometer | 12 men | Change in the motor unit firing rate and twitch force due to muscle pain | Static isometric contraction (0%, 10%, 30%, 50% and 70% of MVC | MMG and EMG RMS, MMG and EMG MPF, MMG / EMG ratio | The MMG RMS increased after experimental muscle pain. | Results of this study are gender specific as all the subjects belong to male gender group only. |
| 54 | Accelerometer | 10 men | MMG and EMG (both amplitude and frequency) of a single motor unit | Contractions at three force levels (20%, 50%, and 80% MVC) | Amplitude and mean frequency | The MMG amplitude increased from 20% to 50% MVC and remained unchanged from 50% to 80% MVC. | Subjects belong to one gender only. |
| 49 | Accelerometer | 19 men | Concentric and eccentric muscle action | Unilateral forearm flexion exercise | MMG amplitude and dynamic constant external resistance (DCER) | MMG RMS as a function of the DCER showed a moderately linear relationship. | The linearity of MMG amplitude with respect to concentric DCER relationship can be investigated in future using microphone and piezoelectric MMG sensors. |
| 53 | Accelerometer | 5 men | Elbow flexion force during muscle contraction | Elbow flexions from 0% to 80% MVC | RMS, zero crossing | Both temporal and spectral features establish a non-linear relationship between MMG and force using artificial neural network model. | Future work can be done on variety of muscle activities from other muscles. Other machine learning techniques like support vector machine can be used in a future study for same methodology. |
| 52 | Accelerometer | 7 men | Contraction strength of muscle | Voluntary static contraction | MMG RMS and frequency variance | The MMG RMS increased slowly and the frequency variance decreased with increasing voluntary contractions. | Strength estimation algorithm can be tested for other muscles with various methodologies in future. |
| 51 | Accelerometer | 27 men | Muscle output force for assessing muscle contraction properties | 20%, 40%, 60%, 80%, and 100% of maximal voluntary isometric contractions (MVIC) with the elbow joint at 90ο and the palm in supine position | MMG RMS in three axes | The MMG RMS increased with increase in the muscle force in all three axes. | Muscle force gradation can be accomplished in future using a triaxial accelerometer and ultrasound for better results. |
| 50 | Accelerometer | 35 (14 men, 21 women) | Effect of direct inhibitory pressure (DIP) on BB myotendinous junction | Submaximal contractions at 20%, 40%, 60% and 80% of MVC | MMG RMS, EMG RMS and electromechanical delay | After DIP, MMG RMS and EMG RMS shifted to lower values while muscle force output decreased | 1) Reflex activity i.e. H-reflex and tendon reflex were not measured in this research. 2) Joints other than BB which possess less mechanical constriction may also be analysed to know the effect of DIP. |
In addition, another study[49] performed an unilateral forearm flexion exercise under concentric and eccentric muscle actions and reported a moderately linear behaviour for the MMG RMS as a function of the dynamic constant external resistance (DCER).
Furthermore, another study[48] investigated the effect of transcranial magnetic stimulation on the BB muscle. These researchers found that the muscle potential force obtained using an MMG signal increased with increases in the motor evoked potential (MEP) area and amplitude. The linearity in the MMG RMS and the BB force in all recordings were due to the motor unit recruitment pattern.
BB physiology assessment
An MMG signal can be employed for the study of muscle activity-related physiology factors during several types of muscle contractions, as summarized in [Table 4][57-66]. Three out of eight of these studies performed their assessments during sub-maximal to maximal isometric muscle contractions[64-66]. The authors of one of these studies[66] observed MMG and EMG signals in the BB and triceps brachii (TB) muscles of nine Parkinson’s disease patients and six controls under submaximal load holding and found a higher MMG RMS in agonist muscle and a lower MMG MDF in both muscles. However, the EMG RMS was equal for the muscles and both types of subjects, and the EMG MDF was increased in the TB muscle of the patients. This study showed that MMG provides better differentiation among diseased and healthy muscles than EMG. MMG also remains unaffected by a postural tremor in a limb. In another study[65], the same researchers observed the MMG and EMG responses of the BB and TB muscles in Parkinson’s disease patients and controls and found no tremor-related changes in the patients during maximal isometric contraction. No difference in the MMG and EMG signal parameters were observed between the patients and controls, which might be due to the fact that the patients were on medication. A later study[64] explored the effect of sensor location on the MMG signal using eight unidirectional accelerometers on the BB muscle. The researchers observed the maximum value of the MMG amplitude and the MPF on the belly of the muscle and found that MMG is sensitive to the muscle architecture and the territorial distribution of motor units in the muscle.
Table 4.
Records on activity related to muscle physiology.
| Authors | Sensor used | Subjects | Muscle activity assessed | Experimental protocol | Parameters | Results | Application / Limitation / Future work |
|---|---|---|---|---|---|---|---|
| 63 | Piezoelectric MMG membranes | Number of subjects not mentioned | Muscle vibration / muscle contractions | Sustained voluntary isometric contraction | Power difference, MMG and EMG amplitude | Power difference increased from 5% to 90% by rubbing skin. | The EMG and MMG probe used in experiment was not standardized and it was tested only on sustained muscle contraction. |
| 64 | Accelerometer | 10 men | Isometric muscle contractions | Isometric muscle contractions at 10%, 20%, and 40% MVC | RMS and mean power spectral frequency (MPF) | Variation in RMS and MPF with variation in sensor location. | Effect of variation in MMG sensor location was studied only for voluntary contraction. |
| 60 | Displacement sensor | 10 men | Muscle stiffness | Electrically evoked maximal single twitch | Tc, Dm | No significant change in the Tc and Dm of BB muscle was observed after bed rest. | Muscle stiffness assessment can be extended for voluntary contraction to have results closer to natural muscle condition |
| 66 | Hybrid EMG / MMG probe | 15 women (9 with Parkinson disease, 6 healthy) | Muscle stiffness and ability to carry load | Submaximal load holding | MMG Amplitude and MDF | Higher RMS in agonist muscle and lower MDF in both agonist and antagonist muscles. | Lack of normalization for EMG data. |
| 65 | Hybrid EMG / MMG probe | 20 women (10 with Parkinson’s disease, 10 healthy) | Tremor-related changes in Parkinson disease (PD) patients | Maximal isometric contraction | RMS, MDF for both PD patients and control subjects | No intergroup difference in the assessed parameters was observed due to the medication. | Maximal and submaximal load tasks were performed in different days which could alter the results of study |
| 62 | Piezoelectric transducer | 20 (10 men, 10 women) | Isometric contractions | Muscle isometric ramp and step contractions | MMG MPF, MMG total intensity, first and second principal components | The MMG MPF and first principal component showed higher values in ramp contractions than in step contractions. | Spectral properties of EMG and MMG signals can also be observed for other muscles. |
| 61 | Piezoelectric transducer | 12 (6 men, 6 women) | Contribution of BB to elbow flexion concentric / eccentric activity | Voluntary concentric and eccentric contractions at 20%, 40%, 60%, and 80% load | Elbow angle | The total intensity of MMG during eccentric contractions was higher than that obtained during concentric contractions. | Spectral properties of EMG and MMG signals can also be observed for other muscles and results may be compared to BB for generalization. |
| 59 | Laser measurement device | 19 (gender not mentioned) | Exercise hypertrophy & disuse atrophy | Electrically evoked stimulation and then strength training by bicep curl to induce muscle hypertrophy | Dm, Tc, Vc | Dm and Vc showed a decline on the same time points while Tc remained stable throughout the hypertrophic and atrophic phases. | Using dominant limb as control may change the results of contractile properties, because motor unit activity has been observed to effect homologous contralateral limb. |
| 58 | Microphone | 18 men | Change in neuromuscular efficiency and muscle stiffness after eccentric contractions | 25 contractions at 50% MVC of elbow flexion | MMG RMS and MDF | MMG RMS decreased and the MMG MDF increased during the recovery period after contractions. | Future work can be done on different intensity levels for muscle stiffness assessment. |
| 57 | Piezoelectric accelerometer | 10 men | Mechanical deformation of muscle | Resting and ramp contraction from 5% to 85% MVC | RMS correlation coefficient between the rest and contraction states, phase correlation coefficient | High similarity in bulk movement between resting and contracting muscle was observed. | Intensity variation in ramp contraction is not addressed in the study. |
The authors of another study[63] developed a differential composite probe for recording EMG and MMG data simultaneously. These researchers assessed the BB muscle under sustained voluntary contractions. The differential probe showed the same MMG and EMG amplitudes as a non-differential probe. However, the differential probe significantly reduced the artefacts produced in the muscle by rubbing the skin. A power difference higher than 90% was found between the two probes under this condition.
Another study[62] assessed muscle strength in terms of the MMG signal spectral properties through an analysis of motor unit recruitment patterns under isometric ramp and step muscle contractions. These researchers observed higher values for the MMG MPF and first principal component PCI during randomly ordered ramp contractions compared with those obtained during step contractions at the same force level, which might be due to the different motor unit recruitment strategies adopted for different patterns of muscle contractions.
Another study[61] assessed voluntary eccentric and concentric contractions in the BB muscle and investigated the spectral properties of MMG and EMG signals at different load levels. The researchers concluded that the total intensity of MMG signals obtained during eccentric contractions was higher than that obtained during concentric contractions. The observations related to EMG signals were opposite to those obtained with MMG. This finding might be attributed to an increase in physiological tremor which might not affect the MMG signal. Hence, the MMG signals were less affected by a tremor than EMG.
Two studies assessed the muscle condition under external stimulation using MMG[59,60]. The authors of one of these studies[60] observed changes in the contractile properties of the BB muscle using MMG signals after 35 days of bed rest. Various contractile properties, such as contraction time (Tc) and Dm, were measured, and no meaningful change in Tc and Dm of the BB muscle was detected. Some of the results from this study provide evidence for the use of the MMG signals from skeletal muscles to infer physiology factors through measurement of muscle stiffness based on contractile properties. Another study[59] observed parallel changes in Dm and Vc without a change in the Tc during a 16 weeks experimental period to observe the effects of exercise hypertrophy and misuse atrophy. Thus, MMG can be employed as a clinical tool to study muscle physiology and has advance applications in musculoskeletal rehabilitation.
The authors of another study[58] observed MMG signals from both the BB and TB during MVC after 25 intermittent, concentric and sub-maximal contractions. These researchers found a similar change in the MMG RMS of agonist and antagonist muscles, which suggests a common drive in agonist and antagonist activity. Another study[57] investigated the BB ramp contraction and found no change in the low-frequency (less than 30 Hz) MMG signal of resting and contracting muscles based on the RMS correlation coefficient and the phase correlation coefficient between the resting and contraction states. The similarity in the resting and contracting states at a low-frequency range is due to the bulk movement of global soft tissue, as depicted by MMG.
Discussion
The literature reveals that MMG has the potential to be an alternative tool for assessing BB muscle activity during both dynamic and isometric contractions. The MMG MPF, MDF and CF have been observed to decrease during the development of muscle fatigue. Thus, the MPF, MDF and CF demonstrate potential for monitoring muscle fatigue during physical training and sports activities. In contrast, the MMG RMS increased linearly with increases in muscle strength and torque and could thus be used to follow improvements in muscle strength during rehabilitation. Furthermore, the MMG RMS may also serve as a biofeedback parameter to monitor muscle strength during various athletic activities, specifically under conditions in which torque cannot be measured directly.
Torque assessment
Torque estimation based on MMG signals has been accomplished for several types of muscle contractions, including stimulated, voluntary, isometric and isokinetic contractions, at different intensities from submaximal to maximal levels. All the relevant selected records strongly support the application of MMG as a tool for estimating muscle torque. MMG can serve as a suitable alternative approach for measuring muscle torque, specifically under those conditions in which direct measurement of torque is not feasible. For muscles under isometric contractions, a linear relationship has been observed between torque and MMG RMS[11,21,25,36]. This linear relationship was observed up to 80% MVC but then plateaued from 80% to 100% MVC. This finding might be due to the fusion of motor unit twitches at high intensities, which further suggests variations in the motor control strategy to produce torque. Changes in torque can also be observed by an analysis of MMG frequency. A linear relationship between torque levels and MMG frequency content was observed in a previous study[21,24] for isometric contractions. Another investigation[11] showed the previously observed trend in the MMG MPF up to 80% MVC. However, the MMG MPF decreases from 80% to 100% MVC, and this change in the trend for MMG MPF could be due to the probable fusion in motor unit twitches.
Muscles under dynamic contractions show a linear relationship between the MMG RMS and torque[11,23,29]. A previous study[22] found that muscles are more able to produce torque without prior stretching, which indicates that the ability to produce torque is also related to musculotendinous stiffness, i.e., greater muscle stiffness produces more torque. Nevertheless, a linear relationship between MMG RMS and torque levels at high intensity is contrary to the typical motor control strategy[23], which suggests that minor changes in recruitment patterns might be the primary motor control strategy for increasing isokinetic torque. For muscles under electrical stimulation, previous studies found a strong correlation between MMG amplitude and torque[27,28]. In conclusion, an MMG signal and torque measurement shows a strong linear relationship. Hence, MMG appears to be a potential tool for torque estimation.
These studies reveal the following – First, in some cases, MMG RMS or MPF versus torque patterns in the BB muscle may vary depending on the type of sensor used[29]. Thus, it is important to consider the MMG sensor used in the experiment for the interpretation of those patterns to describe motor control strategies which modulate torque. Second, none of the torque studies of the BB muscle considered MMG signal contamination due to adjacent muscles while establishing a torque-versus-MMG signal parameter relationship, particularly at lower torque intensities. Future studies should explore torque assessment through MMG while giving special attention to crosstalk. Third, there is a need to further explore approaches for muscle torque estimation under fatiguing conditions under which torque is not detectable, such as during sustained contractions. Nevertheless, it has been observed that MMG can be used for fatigue assessment, even if a torque measurement is not possible for a muscle (such as during external stimulation). Finally, the literature reveals that MMG signals show the same response for both time and frequency domain parameters as those observed with EMG. Therefore, MMG can serve as a suitable alternative to EMG for torque estimations.
Fatigue assessment
The phenomenon of muscle fatigue has been indicated and measured using MMG. Various temporal and spectral features of MMG have elaborated the condition of a muscle during fatigue. At a lower level of sustained muscle contraction under fatiguing conditions, such as approximately 20% MVC, the MMG RMS shows an increasing trend[32-34], and an opposite trend in the amplitude of the MMG signal was found in two previous studies[32,41]. This finding might be due to the lack of a sufficient number of motor units being recruited at the start of a contraction[32]. Nevertheless, this trend soon follows the usual increase in the MMG RMS, which could be due to the recruitment of newer motor units. In contrast, muscle stiffness causes a decrease in the MMG amplitude, as observed in a previous study[41], which suggests that a physiological model for muscle stiffness needs to be developed to understand the behaviour of MMG signals from fatigued muscles with stiffness. The increasing trend for the MMG amplitude at low-level muscle contractions was also observed to agree with the trends obtained with EMG[33,67]. Common MMG frequency parameters, including MPF, MDF and CF, have shown a decreasing trend at low-level sustained contractions for muscles under fatigue[32-34]. This consistency in the MMG signal behaviour indicates the ability for using MMG as a potential alternative to other tools for studying skeletal muscle fatigue. At higher levels, i.e., approximately 70% MVC of sustained muscle contractions during fatiguing conditions, the MMG RMS shows a decreasing trend[32]. This observation was contrary to that obtained in a previous study[30], which could be due to the very small sample size used in the study. Similarly, a decrease in the trend of the MPF was observed in two previous studies[32,40] during the fatiguing state. A decreased MPF coupled with an increase in spectral skewness was found to be indicative of muscle fatigue in a previous work[40].
For intermittent contractions under fatiguing conditions, the MMG RMS of a muscle was found to increase[46], whereas the MPF showed a stable behaviour[42,46]. The stability of MPF at low-intensity contractions could be due to the fact that central fatigue occurs more rapidly than peripheral fatigue. Hence, the MPF was observed to be unchanged at peripheral levels. Similarly, muscle fatigue could be investigated with due cognizance of its manifestation as either central fatigue or peripheral fatigue using MMG. The frequency parameters of both MMG and EMG signals, namely, MPF, MDF and CF, were observed to be decreased in a previous study[31]. This decrease in frequency components is indicative of muscle fatigue, and the decrease in CF could be due to a reduction in muscle compliance, a prolongation of motor unit twitches or a decrement in firing rates. The similar patterns for MMG CF and EMG CF found during fatiguing dynamic muscle contractions further validates the use of MMG as a muscle fatigue assessment tool. This finding was demonstrated by using MMG to improve muscle relaxation in fatigued muscles through biofeedback[44].
Contractile parameters measured using MMG have been shown to be a better indicator of muscle fatigue than those obtained using EMG[42]. However, muscle contractile properties can be assessed better when the muscle is under fatiguing conditions. However, some of these contractile parameters, including Vc and ½Vr do not have absolute values, which makes their comparison among different studies difficult. Similarly, the rate of fatigue occurrence might also be influenced by speed in concentric contractions[42], suggesting that MMG might allow a deeper understanding of muscle mechanics during dynamic contractions. Similarly, future work should consider the influence of crosstalk to validate the origins of MMG signals originating from the BB muscle. These issues are expected to become more complex at higher contraction levels without increased motor unit recruitment; instead, at these levels, the firing rates play a key role in force production.
Strength and force assessment
MMG has also been used as a tool to assess muscle strength or force under dynamic contractions[7]. A previous study[52] assessed muscle strength under static contractions and found an increasing trend in the RMS, but the sample size (seven subjects) could be considered small. The MMG RMS was observed to exhibit a linear relationship with the level of force during both stimulated[48] and voluntary contractions[49,51,52,54-56]. The MMG RMS at a low frequency range remained unchanged from 5% to 85% MVC between resting and contracting muscle states[57]. This observation contradicts the findings from other studies in the literature because low-frequency MMG signals are highly influenced by bulk movement. MMG signals should ideally be collected at a higher frequency (5 to 100 Hz) range to avoid the risk of recording signals originating from tremor, heart beat and other movement artefacts, which generally have a low frequency.
Both MMG and EMG has also been used for pain (inability to produce strength and force) assessment during BB muscle contractions[48]. The MMG spectral response depicts muscular pain. As pain increases, both the mechanical activity of a muscle and the twitch force increase, whereas the motor unit firing rate decreases[55]. These findings suggest that MMG might be a beneficial tool in sport sciences and medicine for assessing the condition of a muscle.
Taken together, these studies reveal some gaps in muscle strength assessment using MMG; for example, isokinetic and isotonic contractions should be studied to obtain a better understanding of muscle mechanics. Furthermore, the mechanisms responsible for the production of force in a muscle, including motor unit recruitment and firing rate, should be explored specifically during ramp contractions. However, in a ramp contraction study, the resolution of %MVC can be improved by performing a sustained contraction test at various intensities. In addition, the BB muscle force should be studied using MMG with various externally stimulated experiment protocols, which will improve our understanding of the muscle mechanics involved in force generation.
BB physiology assessment
The muscle physiology and condition can also be examined using MMG[11,60]. MMG has been employed to distinguish between muscles from subjects with Parkinson’s disease (PD) and normal healthy muscle, and the results revealed that MMG signals remained unaffected by physiological postural tremor from PD patients, whereas EMG signals need to be normalized[66]. Both these reasons bode well for the use of MMG for muscle assessment. The use of MMG to distinguish PD and healthy muscles was investigated in a previous study[65], and the findings indicated that MMG should not be used to test the stiffness of PD muscles during sustained muscle contractions because a postural tremor might not appear in an MMG signal[65]. The other reason for the inability to distinguish between PD and normal muscles in a previous study[65] could be that the medications taken by PD patients might affect muscle stiffness and eventually the MMG and EMG signals. Nevertheless, MMG was found to be a better tool for assessing the difference between controls and PD subjects compared with EMG because MMG signals remain unaffected by tremor-related changes in muscles[66].
MMG can also accurately detect changes in muscle contractile properties throughout hypertrophic and atrophic phases[59], which indicates the utility of MMG as a rehabilitation tool in clinical applications. Thus, MMG might be an attractive tool for future applied research in the area of gait analysis.
Of the 48 identified studies that assessed BB muscle activity through MMG, 59% utilized accelerometers, 26% used piezoelectric contact sensors, 6% employed condenser microphones, 6% worked with displacement sensors, and the remaining 2% developed composite MMG sensors. The literature reveals that displacement sensors are appropriate for measuring the contractile properties of muscles[42]. Condenser microphones provide a higher signal-to-noise ratio than accelerometers[68] and are thus preferable for MMG recordings when the mitigation of motion artefacts is crucial. Similarly, piezoelectric contact sensors are also less sensitive to motion artefacts[13]. Nevertheless, most of the identified studies employed accelerometers for the recording of MMG signals. Although ease of mounting and a miniature size make accelerometers attractive for use in MMG, there are no guidelines for the placement of these sensors, such as SENIAM for EMG[40]. Furthermore, the availability of accelerometers as triaxial sensors for measuring muscle activity separately in three directions might provide researchers additional insights into muscle activity, specifically along muscle fibres[40].
Although the above-mentioned findings appear promising, the use of the MMG technique is at its infancy stage of advancement and has some limitations. EMG, as a widely used technique for studying muscle activity, has developed standards, such as SENIAM, for signal recording. However, no similar standards have been developed for MMG, which is a newer technique. In addition, neighbouring muscles exhibit the phenomenon of crosstalk, which constitutes a limitation of MMG as a tool for analysing muscle function[10,69,70]. Hence, the crosstalk levels between proximal muscles need to be explored and quantified if MMG is to be employed as an alternative and potential measurement tool. To this effect, methods to identify, quantify and eliminate crosstalk, determine the axis of propagation of MMG signals, and the size and placement of sensors on muscles are areas that need to be standardized in MMG.
Conclusion
This review unveils a few of the critical findings regarding the application of MMG for BB muscle activity assessment. The findings from these assessments are further classified as muscle torque, fatigue, strength and physiology. Our findings strengthen the observation that MMG is a useful technique for investigating the activity of muscles, particularly the BB, through analyses of temporal and spectral parameters of MMG signals. The literature reveals that MMG is a potential alternative measurement tool to EMG because most of the main observations and findings obtained with MMG concur with those found with EMG. Hence, we can conclude that MMG signals provide valuable information regarding muscle activity and function during muscle contractions. This conclusion paves the way for the successful and potential application of MMG in future applied and clinical research. Analyses of BB muscle activity could provide insights into the muscle condition during rehabilitation and exercise. The findings from this review pertaining to force and torque have the potential to benefit the research community working in the design and control of prosthetics. The nature of the BB muscle, which is somewhat ‘isolated’ from its neighbouring muscles, provides researchers with the opportunity to study the origins of MMG signal generation due to the absence of many sources of signal contamination. To this effect, the limitations of the MMG technique, particularly with respect to crosstalk, should be further investigated on the BB muscle. In addition, we believe that the findings of this review will provide benchmarked results for the future development of novel MMG sensors because only a few sensors for MMG are currently commercially available.
Acknowledgements
The authors would like to thank Universiti Teknikal Malaysia Melaka (UTeM) for providing a conducive platform to conduct the research.
Footnotes
Support from the Fundamental Research Grant Scheme (FRGS) under a grant number of FRGS/1/2015/TK04/UNIMAP/02/5 from the Ministry of Higher Education Malaysia. The authors have no conflict of interest to declare.
Edited by: G. Lyritis
References
- 1.Barry DT, Cole NM. Muscle sounds are emitted at the resonant frequencies of skeletal muscle. IEEE Trans Biomed Eng. 1990;37(5):525–31. doi: 10.1109/10.55644. [DOI] [PubMed] [Google Scholar]
- 2.Stokes M. Acoustic myography:applications and considerations in measuring muscle performance. Isokinet Exercise Sci. 1993;3(1):4–15. [Google Scholar]
- 3.Gordon G, Holbourn A. The sounds from single motor units in a contracting muscle. J Physiol. 1948;107(4):456–64. doi: 10.1113/jphysiol.1948.sp004290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma M. MMG sensor for muscle activity detection:low cost design, implementation and experimentation:a thesis presented in fulfilment of the requirements for the degree of Masters of Engineering in Mechatronics, Massey University, Auckland, New Zealand 2010 [Google Scholar]
- 5.Xie H-B, Zheng Y-P, Guo J-Y. Classification of the mechanomyogram signal using a wavelet packet transform and singular value decomposition for multifunction prosthesis control. Physiol Meas. 2009;30(5):441. doi: 10.1088/0967-3334/30/5/002. [DOI] [PubMed] [Google Scholar]
- 6.Islam MA, Sundaraj K, Ahmad RB, Ahamed NU. Mechanomyogram for muscle function assessment:a review. PloS One. 2013;8(3):e58902. doi: 10.1371/journal.pone.0058902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibitoye MO, Hamzaid NA, Zuniga JM, Wahab AKA. Mechanomyography and muscle function assessment:A review of current state and prospects. Clin Biomech. 2014;29(6):691–704. doi: 10.1016/j.clinbiomech.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Beck TW, Housh TJ, Johnson GO, Cramer JT, Weir JP, Coburn JW, et al. Does the frequency content of the surface mechanomyographic signal reflect motor unit firing rates?A brief review. J Electromyogr Kinesiol. 2007;17(1):1–13. doi: 10.1016/j.jelekin.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Orizio C, Gobbo M. Mechanomyography. Wiley Encyclopedia of Biomedical Engineering. 2006 [Google Scholar]
- 10.Islam MA, Sundaraj K, Ahmad RB, Sundaraj S, Ahamed NU, Ali MA. Cross-talk in mechanomyographic signals from the forearm muscles during sub-maximal to maximal isometric grip force. PLoS One. 2014;9(5):e96628. doi: 10.1371/journal.pone.0096628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beck TW, Housh TJ, Johnson GO, Weir JP, Cramer JT, Coburn JW, et al. Mechanomyographic amplitude and mean power frequency versus torque relationships during isokinetic and isometric muscle actions of the biceps brachii. J Electromyogr Kinesiol. 2004;14(5):555–64. doi: 10.1016/j.jelekin.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Akataki K, Mita K, Watakabe M, Ito K. Age-related change in motor unit activation strategy in force production:A mechanomyographic investigation. Muscle & Nerve. 2002;25(4):505–12. doi: 10.1002/mus.10076. [DOI] [PubMed] [Google Scholar]
- 13.Orizio C, Gobbo M, Diemont B, Esposito F, Veicsteinas A. The surface mechanomyogram as a tool to describe the influence of fatigue on biceps brachii motor unit activation strategy. Historical basis and novel evidence. Eur J Appl Physiol. 2003;90(3):326–36. doi: 10.1007/s00421-003-0924-1. [DOI] [PubMed] [Google Scholar]
- 14.Paravlić A, Zubac D, Šimunič B. Reliability of the twitch evoked skeletal muscle electromechanical efficiency:A ratio between tensiomyogram and M-wave amplitudes. J Electromyogr Kinesiol. 2017;37:108–16. doi: 10.1016/j.jelekin.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Beck TW, Housh TJ, Cramer JT, Weir JP, Johnson GO, Coburn JW, et al. Mechanomyographic amplitude and frequency responses during dynamic muscle actions:a comprehensive review. Biomed Eng online. 2005;4:67. doi: 10.1186/1475-925X-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Islam A, Sundaraj K, Ahmad B, Ahamed NU, Ali A. Mechanomyography sensors for muscle assessment:a brief review. J Phys Ther Sci. 2012;24(12):1359–65. [Google Scholar]
- 17.Islam MA, Sundaraj K, Ahmad RB, Ahamed NU, Ali MA. Mechanomyography sensor development, related signal processing, and applications:a systematic review. IEEE Sens J. 2013;13(7):2499–516. [Google Scholar]
- 18.Ibitoye MO, Hamzaid NA, Zuniga JM, Hasnan N, Wahab AKA. Mechanomyographic parameter extraction methods:an appraisal for clinical applications. Sens. 2014;14(12):22940–70. doi: 10.3390/s141222940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krueger E, Scheeren EM, Nogueira-Neto GN, Button VLdSN, Nohama P. Advances and perspectives of mechanomyography. Revista Brasileira de Engenharia Biomédica. 2014;30(4):384–401. [Google Scholar]
- 20.CèE Rampichini S, Esposito F. Novel insights into skeletal muscle function by mechanomyography:from the laboratory to the field. Sport Sci Health. 2015;11(1):1–28. [Google Scholar]
- 21.Evetovich TK, Boyd JC, Drake SM, Eschbach LC, Magal M, Soukup JT, et al. Effect of moderate dehydration on torque, electromyography, and mechanomyography. Muscle & Nerve. 2002;26(2):225–31. doi: 10.1002/mus.10203. [DOI] [PubMed] [Google Scholar]
- 22.Evetovich TK, Nauman NJ, Conley DS, Todd JB. Effect of static stretching of the biceps brachii on torque, electromyography, and mechanomyography during concentric isokinetic muscle actions. The J Strength Cond Res. 2003;17(3):484–8. doi: 10.1519/1533-4287(2003)017<0484:eossot>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 23.Beck TW, Housh TJ, Johnson GO, Weir JP, Cramer JT, Coburn JW, et al. Mechanomyographic and electromyographic time and frequency domain responses during submaximal to maximal isokinetic muscle actions of the biceps brachii. Eur J Appl Physiol. 2004;92(3):352–9. doi: 10.1007/s00421-004-1110-9. [DOI] [PubMed] [Google Scholar]
- 24.Hendrix CR, Housh TJ, Camic CL, Zuniga JM, Johnson GO, Schmidt RJ. Comparing electromyographic and mechanomyographic frequency-based fatigue thresholds to critical torque during isometric forearm flexion. J Neurosci Methods. 2010;194(1):64–72. doi: 10.1016/j.jneumeth.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 25.Lei KF, Tsai W-W, Lin W-Y, Lee M-Y. MMG-torque estimation under dynamic contractions. Systems, Man, and Cybernetics (SMC), 2011 IEEE International Conference on; 2011:IEEE [Google Scholar]
- 26.Beck TW, Housh TJ, Johnson GO, Cramer JT, Weir JP, Coburn JW, et al. Comparison of the fast Fourier transform and continuous wavelet transform for examining mechanomyographic frequency versus eccentric torque relationships. J Neurosci Methods. 2006;150(1):59–66. doi: 10.1016/j.jneumeth.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Gobbo M, Cè E, Diemont B, Esposito F, Orizio C. Torque and surface mechanomyogram parallel reduction during fatiguing stimulation in human muscles. Eur J Appl Physiol. 2006;97(1):9–15. doi: 10.1007/s00421-006-0134-8. [DOI] [PubMed] [Google Scholar]
- 28.Šimunič B, Križaj D, Narici M, Pišot R. Twitch parameters in transversal and longitudinal biceps brachii response. Annales Kinesiologiae. 2010;1(1) [Google Scholar]
- 29.Beck TW, Housh TJ, Johnson GO, Weir JP, Cramer JT, Coburn JW, et al. Comparison of a piezoelectric contact sensor and an accelerometer for examining mechanomyographic amplitude and mean power frequency versus torque relationships during isokinetic and isometric muscle actions of the biceps brachii. J Electromyogr Kinesiol. 2006;16(4):324–35. doi: 10.1016/j.jelekin.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Yang ZF, Kumar DK, Arjunan SP. Mechanomyogram for identifying muscle activity and fatigue. 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society; 2009:IEEE. doi: 10.1109/IEMBS.2009.5333666. [DOI] [PubMed] [Google Scholar]
- 31.Beck TW, Housh TJ, Johnson GO, Weir JP, Cramer JT, Coburn JW, et al. Comparison of Fourier and wavelet transform procedures for examining the mechanomyographic and electromyographic frequency domain responses during fatiguing isokinetic muscle actions of the biceps brachii. J Electromyogr Kinesiol. 2005;15(2):190–9. doi: 10.1016/j.jelekin.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Itoh Y, Akataki K, Mita K, Watakabe M, Itoh K. Time-frequency analysis of mechanomyogram during sustained contractions with muscle fatigue. Syst Comput Jpn. 2004;35(1):26–36. [Google Scholar]
- 33.Tarata MT. Mechanomyography versus electromyography, in monitoring the muscular fatigue. Biomed Eng Online. 2003;2(1):1. doi: 10.1186/1475-925X-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Madeleine P, Ge H-y, Jaskólska A, Farina D, Jaskólski A, Arendt-Nielsen L. Spectral moments of mechanomyographic signals recorded with accelerometer and microphone during sustained fatiguing contractions. Med Biol Eng Comput. 2006;44(4):290–7. doi: 10.1007/s11517-006-0036-2. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka M, Okuyama T, Saito K. Study on evaluation of muscle conditions using a mechanomyogram sensor. Systems, Man, and Cybernetics (SMC), 2011 IEEE International Conference on; 2011: IEEE [Google Scholar]
- 36.CèE Rampichini S, Agnello L, Limonta E, Veicsteinas A, Esposito F. Effects of temperature and fatigue on the electromechanical delay components. Muscle & Nerve. 2013;47(4):566–76. doi: 10.1002/mus.23627. [DOI] [PubMed] [Google Scholar]
- 37.CèE Rampichini S, Venturelli M, Limonta E, Veicsteinas A, Esposito F. Electromechanical delay components during relaxation after voluntary contraction:reliability and effects of fatigue. Muscle & Nerve. 2015;51(6):907–15. doi: 10.1002/mus.24466. [DOI] [PubMed] [Google Scholar]
- 38.Hill EC, Housh TJ, Smith CM, Schmidt RJ, Johnson GO. Mechanomyographic amplitude tracks fatigue-induced changes in mean power, but not moment production. Isokinet Exercise Sci. 2017;(Preprint):1–7. [Google Scholar]
- 39.Xie H-B, Guo J-Y, Zheng Y-P. Uncovering chaotic structure in mechanomyography signals of fatigue biceps brachii muscle. J Biomech. 2010;43(6):1224–6. doi: 10.1016/j.jbiomech.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 40.Nogueira-Neto G, Scheeren E, Krueger E, Nohama P, Button VLS. The Influence of Window Length Analysis on the Time and Frequency Domain of Mechanomyographic and Electromyographic Signals of Submaximal Fatiguing Contractions. Open J Biophys. 2013;3(03):178. [Google Scholar]
- 41.Han H, Jo S, Kim J. Comparative study of a muscle stiffness sensor and electromyography and mechanomyography under fatigue conditions. Med Biol Eng Comput. 2015;53(7):577–88. doi: 10.1007/s11517-015-1271-1. [DOI] [PubMed] [Google Scholar]
- 42.Tosovic D, Than C, Brown J. The effects of accumulated muscle fatigue on the mechanomyographic waveform:implications for injury prediction. Eur J Appl Physiol. 2016:1–10. doi: 10.1007/s00421-016-3398-7. [DOI] [PubMed] [Google Scholar]
- 43.Blangsted A, Vedsted P, Sjøgaard G, Søgaard K. Intramuscular pressure and tissue oxygenation during low-force static contraction do not underlie muscle fatigue. Acta physiologica scandinavica. 2005;183(4):379–88. doi: 10.1111/j.1365-201X.2005.01411.x. [DOI] [PubMed] [Google Scholar]
- 44.Evetovich TK, Conley DS, Todd JB, Rogers DC, Stone TL. Effect of mechanomyography as a biofeedback method to enhance muscle relaxation and performance. The J Strength Cond Res. 2007;21(1):96–9. doi: 10.1519/00124278-200702000-00018. [DOI] [PubMed] [Google Scholar]
- 45.Al-Mulla MR, Sepulveda F. Novel pseudo-wavelet function for mmg signal extraction during dynamic fatiguing contractions. Sens. 2014;14(6):9489–504. doi: 10.3390/s140609489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hill E, Housh TJ, Smith C, Cochrane KC, Jenkins N, Cramer JT, et al. Effect of sex on torque, recovery, EMG, and MMG responses to fatigue. J Musculoskelet Nuronal interact. 2016;16(4):310. [PMC free article] [PubMed] [Google Scholar]
- 47.Okkesim Ş, Coşkun K. Features for muscle fatigue computed from electromyogram and mechanomyogram:A new one. Proceedings of the Institution of Mechanical Engineers, Part H:Journal of Engineering in Medicine. 2016;230(12):1096–105. doi: 10.1177/0954411916675640. [DOI] [PubMed] [Google Scholar]
- 48.Reza MF, Ikoma K, Chuma T, Mano Y. Mechanomyographic response to transcranial magnetic stimulation from biceps brachii and during transcutaneous electrical nerve stimulation on extensor carpi radialis. J Neurosci Methods. 2005;149(2):164–71. doi: 10.1016/j.jneumeth.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 49.Stock M, Beck T, DeFreitas J, Dillon M. Linearity and reliability of the mechanomyographic amplitude versus dynamic constant external resistance relationships for the biceps brachii. Physiol Meas. 2010;31(11):1487. doi: 10.1088/0967-3334/31/11/006. [DOI] [PubMed] [Google Scholar]
- 50.CèE Longo S, McCoy E, Bisconti AV, Tironi D, Limonta E, et al. Acute effects of direct inhibitory pressure over the biceps brachii myotendinous junction on skeletal muscle activation and force output. J Electromyogr Kinesiol. 2017;37:25–34. doi: 10.1016/j.jelekin.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Jotta B, Garcia MAC, Pino AV, de Souza MN. Characterization of the mechanomyographic signal of three different muscles and at different levels of isometric contractions. Acta Bioeng Biomech. 2015;17(4):73–84. [PubMed] [Google Scholar]
- 52.Lei K, Cheng S, Lee M, Lin W. Measurement and estimation of muscle contraction strength using mechanomyography based on artificial neural network algorithm. Biomed Eng Appl Basis Commun. 2013;25(02):1350020. [Google Scholar]
- 53.Youn W, Kim J. Feasibility of using an artificial neural network model to estimate the elbow flexion force from mechanomyography. J Neurosci Methods. 2011;194(2):386–93. doi: 10.1016/j.jneumeth.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 54.Cescon C, Sguazzi E, Merletti R, Farina D. Non-invasive characterization of single motor unit electromyographic and mechanomyographic activities in the biceps brachii muscle. J Electromyogr Kinesiol. 2006;16(1):17–24. doi: 10.1016/j.jelekin.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 55.Madeleine P, Arendt-Nielsen L. Experimental muscle pain increases mechanomyographic signal activity during sub-maximal isometric contractions. J Electromyogr Kinesiols. 2005;15(1):27–36. doi: 10.1016/j.jelekin.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Matta TTd, Perini TA, Oliveira GLd, Ornellas JdS, Louzada AA, Magalhães J, et al. Interpretation of the mechanisms related to the muscular strength gradation through accelerometry. Revista Brasileira de Medicina do Esporte. 2005;11(5):306–10. [Google Scholar]
- 57.Kawamoto T, Yamazaki N. Bulk movement included in multi-channel mechanomyography:Similarity between mechanomyography of resting muscle and that of contracting muscle. J Electromyogr Kinesiol. 2012;22(6):923–9. doi: 10.1016/j.jelekin.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Jaskólski A, Andrzejewska R, Marusiak J, Kisiel-Sajewicz K, Jaskólska A. Similar response of agonist and antagonist muscles after eccentric exercise revealed by electromyography and mechanomyography. J Electromyogr Kinesiol. 2007;17(5):568–77. doi: 10.1016/j.jelekin.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 59.Than C, Tosovic D, Seidl L, Brown JM. The effect of exercise hypertrophy and disuse atrophy on muscle contractile properties:a mechanomyographic analysis. Eur J Appl Physiol. 2016;116(11-12):2155–65. doi: 10.1007/s00421-016-3469-9. [DOI] [PubMed] [Google Scholar]
- 60.Pišot R, Narici MV, Šimunič B, De Boer M, Seynnes O, Jurdana M, et al. Whole muscle contractile parameters and thickness loss during 35-day bed rest. Eur J Appl Physiol. 2008;104(2):409–14. doi: 10.1007/s00421-008-0698-6. [DOI] [PubMed] [Google Scholar]
- 61.Qi L, Wakeling JM, Ferguson-Pell M. Spectral properties of electromyographic and mechanomyographic signals during dynamic concentric and eccentric contractions of the human biceps brachii muscle. J Electromyogr Kinesiol. 2011;21(6):1056–63. doi: 10.1016/j.jelekin.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 62.Qi L, Wakeling JM, Green A, Lambrecht K, Ferguson-Pell M. Spectral properties of electromyographic and mechanomyographic signals during isometric ramp and step contractions in biceps brachii. J Electromyogr Kinesiol. 2011;21(1):128–35. doi: 10.1016/j.jelekin.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Gregori B, Galie E, Accornero N. Surface electromyography and mechanomyography recording:a new differential composite probe. Med Biol Eng Comput. 2003;41(6):665–9. doi: 10.1007/BF02349974. [DOI] [PubMed] [Google Scholar]
- 64.Cescon C, Farina D, Gobbo M, Merletti R, Orizio C. Effect of accelerometer location on mechanomyogram variables during voluntary, constant-force contractions in three human muscles. Med Biol Eng Comput. 2004;42(1):121–7. doi: 10.1007/BF02351021. [DOI] [PubMed] [Google Scholar]
- 65.Marusiak J, Jaskólska A, Jarocka E, Najwer W, Kisiel-Sajewicz K, Jaskólski A. Electromyography and mechanomyography of elbow agonists and antagonists in Parkinson disease. Muscle & Nerve. 2009;40(2):240–8. doi: 10.1002/mus.21250. [DOI] [PubMed] [Google Scholar]
- 66.Marusiak J, Jaskólska A, Kisiel-Sajewicz K, Yue GH, Jaskólski A. EMG and MMG activities of agonist and antagonist muscles in Parkinson's disease patients during absolute submaximal load holding. J Electromyogr Kinesiol. 2009;19(5):903–14. doi: 10.1016/j.jelekin.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 67.Hussain J, Sundaraj K, Low YF, Lam C, Sundaraj S, Ali MA. A systematic review on fatigue analysis in triceps brachii using surface electromyography. Biomed Signal Process and Control. 2018;40:396–414. [Google Scholar]
- 68.Posatskiy A, Chau T. The effects of motion artifact on mechanomyography:A comparative study of microphones and accelerometers. J Electromyogr Kinesiol. 2012;22(2):320–4. doi: 10.1016/j.jelekin.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 69.Islam MA, Sundaraj K, Ahmad RB, Sundaraj S, Ahamed NU, Ali MA. Longitudinal, lateral and transverse axes of forearm muscles influence the crosstalk in the mechanomyographic signals during isometric wrist postures. PloS One. 2014;9(8):e104280. doi: 10.1371/journal.pone.0104280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Islam A, Sundaraj K, Ahmad RB, Sundaraj S, Ahamed NU, Ali M. Analysis of crosstalk in the mechanomyographic signals generated by forearm muscles during different wrist postures. Muscle & Nerve. 2015;51(6):899–906. doi: 10.1002/mus.24454. [DOI] [PubMed] [Google Scholar]


