Abstract
Inhibiting aberrantly upregulated microRNAs (miR/miRNAs) has emerged as a novel focus for therapeutic intervention in human melanoma. Thus, identifying upregulated miRNAs is essential for identifying additional melanoma-associated therapeutic targets. In the present study, microarray-based miRNA profiling of canine malignant melanoma (CMM) tissue obtained from the oral cavity was performed and differential expression was confirmed by a reverse transcription-quantitative polymerase chain reaction (RT-qPCR). An analysis of the microarray data revealed 17 dysregulated miRNAs; 5 were upregulated and 12 were downregulated. RT-qPCR analysis was performed for 2 upregulated (miR-204 and miR-383), 3 downregulated (miR-122, miR-143 and miR-205) and 6 additional oncogenic miRNAs (oncomiRs; miR-16, miR-21, miR-29b, miR-92a, miR-125b and miR-222). The expression levels of seven of the miRNAs, miR-16, miR-21, miR-29b, miR-122, miR-125b, miR-204 and miR-383 were significantly upregulated; however, the expression of miR-205 was downregulated in CMM tissues compared with normal oral tissues. The microarray and RT-qPCR analyses validated the upregulation of two potential oncomiRs miR-204 and miR-383. The present study additionally constructed a protein interaction network and a miRNA-target regulatory interaction network using STRING and Cytoscape. In the proposed network, cyclin dependent kinase 2 was a target for miR-383, sirtuin 1 and tumor protein p53 were targets for miR-204 and ATR serine/threonine kinase was a target for both. It was concluded that miR-383 and miR-204 were potential oncomiRs that may be involved in regulating melanoma development by evading DNA repair and apoptosis.
Keywords: canine melanoma, microRNA, microarray, oncomiR, microRNA-target regulatory interaction network
Introduction
MicroRNAs (miR/miRNAs) are small endogenous non-coding RNAs that post-transcriptionally regulate the expression of target genes by binding to the 3′-untranslated regions of mRNAs, causing destabilization, degradation, or translation inhibition (1). Because dysregulation of miRNA expression has been identified in a number of cancers, some miRNAs are categorized as oncogenic miRNAs or ‘oncomiRs’, a term used to describe either tumor suppressors or oncogenes (2–5). Consequently, miRNAs have been investigated as potential therapeutic targets for several malignant cancers including melanoma (6–7). The tumor burden in mice with liver melanoma metastasis was found to be reduced by anti-miR-182 oligonucleotides that inhibited the upregulated miR-182 in the tumor cells (6). Inhibition of miR-383 over-expression suppressed the proliferation, cell cycle progression and invasion of human epithelial ovarian cancer (EOC) and immortal EOC cell lines (8). Over-expression of miR-203 sensitized malignant melanoma cells to temozolomide drug by targeting glutaminase, which opened new opportunities for chemotherapy-resistant malignant melanoma patients (9). Thus, profiling dysregulated miRNA expression in cancers is an important approach for detecting potential therapeutic targets.
Simpson et al 2013 (10) suggested significant overlapping may exist in the clinical and histopathological features of canine and human mucosal melanomas. miRNA expression has been investigated in different canine tumors, including B and T-cell lymphoma (11), lymphocytic leukemia (12), transitional cell carcinoma (13), mammary cancer (14), prostate cancer (15) and melanoma (16–18). These studies indicated that the expression patterns of specific miRNAs in specific cancers were similar to those in corresponding human cancers. For example, the upregulation of miR-21 and miR-29b in canine mammary cancer is consistent with their upregulation in human breast cancer (14,19,20) and melanoma (21,22) and miR-145, miR-203, and miR-205 were found to be downregulated in both canine malignant melanoma (CMM) and human malignant melanoma (HMM) (16,17). In the Noguchi et al (17) studies of HMM, a total of seven downregulated miRNAs were detected by microarray analysis; three of them were confirmed by quantitative reverse transcription PCR (qRT-PCR). In almost all HMM tumors that have been studied, upregulated miRNA expression has been reported, including the miR-17-92 cluster, miR-222/221, miR-21 and miR-155 (23). Therefore, it is likely that some miRNAs will be upregulated in oral CMM, similar to what Starkey et al (18) reported in canine uveal melanoma. However, until now, no upregulated miRNAs in oral CMM have been reported. To investigate this hypothesis, we examined the expression of miRNAs in CMM tissues obtained from the oral cavity using microarray and qRT-PCR analyses. Here we report the upregulation of seven miRNAs in CMM tissues. To understand the biological relevance of miRNAs it is necessary to identify the target genes with which they interact. Protein-protein interactions are essential for cells to maintain systemic biological functions such as replication of DNA, transcription, translation and signal transduction (24). Dysregulation of proteins may collapse the homeostasis process leading to complex diseases and miRNAs may act as master regulators by maintaining the stability of protein-protein interaction networks (25). So, determining the interactions between the proteins encoded by targets of dysregulated miRNAs and other proteins is very important. In this study, we drew a miRNA-target regulatory interaction network with tumor suppressor genes, which revealed miR-383 and miR-204 may play roles in the development of melanoma by avoiding DNA repair and apoptosis.
Materials and methods
Sample collection
The CMM tissues used in this study were obtained from dogs (n=10) that had undergone biopsy or surgical resection for diagnosis or treatment at the Veterinary Teaching Hospital, Kagoshima University (Kagoshima, Japan). All melanoma samples were obtained from the oral cavity and were histopathologically diagnosed by two pathologists. Normal oral tissues were obtained from healthy laboratory beagle dogs (n=12). In addition to the CMM and normal oral tissues, we obtained a total of 21 canine tumors and normal tissues to use as microarray reference samples as follows: Mammary tubulopapillary carcinoma (n=4), mammary benign mixed tumor (n=4), hepatic cell carcinoma (n=1), squamous cell carcinoma (n=1), lymphoma (n=1), adenosquamous carcinoma (n=1), mast cell tumor (n=1), malignant peripheral nerve sheath tumor (n=1), normal mammary gland tissue (n=4) and normal hepatic tissue (n=3). The animal experiments were approved by the Kagoshima University's Laboratory Animal Committee (A10031).
Isolation of total RNA
All the tissues were preserved in RNAlater (Thermo Fisher Scientific Inc., Waltham, MA, USA) immediately after biopsy or surgical resection until used for RNA isolation. Total RNA was isolated from the stored tissues using a mirVana™ miRNA Isolation kit (Thermo Fisher Scientific Inc.) according to the manufacturer's instructions. RNA quantity was measured using either an ND-1000 spectrophotometer (Thermo Fisher Scientific Inc.) or a NanoPhotometer™ Pearl (Implen GmbH, München, Germany). RNA quality was verified using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) and RNA integrity numbers were determined (26).
Microarray analysis
Three assays were performed (n=3) using the miRCURY™ LNA microRNA Array, version 11.0 (Exiqon Inc., Woburn, MA, USA). In each assay, Hy3 labeled miRNAs from different CMM tissues but the same references Hy5 labeled miRNAs were used. The reference miRNAs comprised equal amounts of RNA from 21 reference samples from 10 different tissues (listed in the Sample collection section), all of which were pooled. Two-color miRNA-microarrays with 264 identical canine miRNA probes were used. Signal extraction was performed using Feature Extraction 10.7.3.1 software (Agilent Technologies). To minimize error, each miRNA was spotted at four different locations on the array and the average signal intensity value of the four spots was used and variable coefficients were calculated [standard deviation (SD) of signal intensity of four spots/average values]. miRNAs with signal intensity variable coefficients >0.5 or with low signal intensity (<100) in both the CMM and reference tissues were excluded from further analysis. The average values of the Hy3/Hy5 (fold change; FC) ratio between the CMM and reference tissues were compared using the Lowess normalization method (27). miRNAs that had FC ratios >2.0 or <0.5 were considered to be dysregulated.
qRT-PCR assays
CMM tissues (n=10) and normal oral tissues (n=12) were used in the qRT-PCRs, which were performed in duplicate using TaqMan microRNA Assays (Thermo Fisher Scientific Inc.; see Table I for assay details) with 2 ng/µl total RNA, according to the optimal reagent concentrations and reaction conditions described in the manufacturer's instructions. The canine miRNA sequences used for the PCRs were identical to the corresponding human miRNA sequences (Table I). The qRT-PCRs were carried out using an Applied Biosystems 7300 Real-Time PCR System (Thermo Fisher Scientific Inc.). RNU6B, U6 small nuclear RNA, was used as a quantitative normalization control (13,14). Relative expression levels were calculated using the comparative delta Cq method (2−ΔΔCq) (28). Cq values >36.0 were considered as absence of miRNA expression. The relative expression levels of miRNAs in the CMM tissues were calculated relative to the average values in the normal oral tissues, which were assigned a value of 1.0.
Table I.
miRs used in the reverse transcription-quantitative polymerase chain reaction assays.
| A, miRNA sequences | |||
|---|---|---|---|
| Assay name | Assay ID | Mature miRNA sequence | miRBase accession number |
| hsa-miR-16 | 000391 | UAGCAGCACGUAAAUAUUGGCG | MI0000070 |
| hsa-miR-21 | 000397 | UAGCUUAUCAGACUGAUGUUGA | MI0000077 |
| hsa-miR-29b | 000413 | UAGCACCAUUUGAAAUCAGUGUU | MI0000105 |
| hsa-miR-92a | 000431 | UAUUGCACUUGUCCCGGCCUGU | MI0000093 |
| hsa-miR-122 | 002245 | UGGAGUGUGACAAUGGUGUUUG | MI0000442 |
| hsa-miR-125b | 000449 | UCCCUGAGACCCUAACUUGUGA | MI0000446 |
| hsa-miR-143 | 002249 | UGAGAUGAAGCACUGUAGCUC | MI0000459 |
| hsa-miR-204 | 000508 | UUCCCUUUGUCAUCCUAUGCCU | MI0000284 |
| hsa-miR-205 | 000509 | UCCUUCAUUCCACCGGAGUCUG | MI0000285 |
| hsa-miR-222 | 002276 | AGCUACAUCUGGCUACUGGGU | MI0000299 |
| hsa-miR-383 | 000573 | AGAUCAGAAGGUGAUUGUGGCU | MI0000791 |
| B, Control sequences | |||
| Assay name | Assay ID | Control sequence | NCBI accession number |
| RNU6B | 001093 | CGCAAGGATGACACGCAAATTCGTGAAGCGTTCCATATTTTT | NR_002752 |
RNU6B, RNA; U6 small nuclear 6, pseudogene. miR/miRNA, microRNA.
Statistics
In the microarray experiments, P-values and false discovery rates (FDRs) were analyzed using Welch's test and the Benjamini-Hochberg correction for multiple hypotheses testing using R software (29). For the qRT-PCRs, the miRNA expression levels between CMM and normal oral tissues were analyzed using the Mann Whitney U-test. Statistical analyses were performed with JMP 10.0 (SAS Institute, Cary, USA). P<0.05 was considered to indicate a statistically significant difference.
Network construction
miRNA targets were predicted using TargetScan 7.1 (30) and 1021 human tumor suppressor genes (with basic annotations) from the Tumor Suppressor Gene Database (TSGene; http://bioinfo.uth.edu/TSGene/). A miRNA-target interaction network was drawn using Cytoscape v3.5 (http://www.cytoscape.org/) (31) and a protein-protein interaction network of tumor suppressor genes was constructed using STRING (confidence score, 0.9) (http://string-db.org/) (32). The two networks were merged within Cytoscape and interconnected nodes were separated to obtain a co-ordinate network. Analysis of basic network parameters (degree, betweenness, centroid value and Eigenvector) was done using Centiscape 2.2 (33). In the network, a node represents a protein (encoded by a target mRNA) or a miRNA and a line represents an interaction between a protein and a miRNA.
Results
Screening of differentially expressed miRNAs by microarray analysis
The microarray analysis revealed 17 dysregulated miRNAs in the CMM tissues based on the FC ratios (Table II). Of the 17 miRNAs, 5 were upregulated (FC ratios >2.0) with no significant FDRs and 12 were downregulated (FC ratios <0.5) and 4 of them had significant FDRs (P<0.05) (Table II).
Table II.
Dysregulated miRNAs identified in canine malignant melanoma tissues by microarray analysis.
| A, Upregulated miRNAs | ||
|---|---|---|
| Upregulated (FC>2.0) | ||
| miRNA | FC | FDR |
| miR-9 | 2.420 | >0.05 |
| miR-149 | 2.022 | >0.05 |
| miR-204 | 2.781 | >0.05 |
| miR-326 | 2.056 | >0.05 |
| miR-383 | 3.581 | >0.05 |
| B, Downregulated miRNAs | ||
| Downregulated (FC<0.5) | ||
| miRNA | FC | FDR |
| miR-10 | 0.486 | <0.05 |
| miR-101 | 0.446 | >0.05 |
| miR-122 | 0.060 | <0.05 |
| miR-142 | 0.385 | >0.05 |
| miR-143 | 0.244 | <0.05 |
| miR-195 | 0.391 | >0.05 |
| miR-200c | 0.382 | >0.05 |
| miR-205 | 0.100 | <0.05 |
| miR-328 | 0.299 | >0.05 |
| miR-487b | 0.430 | >0.05 |
| miR-652 | 0.457 | >0.05 |
FC, fold change; FDR, false discovery ratio; miR/miRNA, microRNA.
Confirmation of differentially expressed miRNAs by qRT-PCR
qRT-PCRs were performed to validate some of the dysregulated miRNAs from the microarray analysis (Table II). Because none of the upregulated miRNAs had significant FDRs, we selected the two most highly upregulated miRNAs, miR-204 and miR-383, for validation. From among the downregulated miRNAs, we selected three miRNAs (miR-122, miR-143 and miR-205) that had the most significant FDRs. We also selected six other miRNAs (miR-16, miR-21, miR-29b, miR-92a, miR-125b and miR-222) for validation because they were reported to be dysregulated in cancers other than CMM (13,14,34–36).
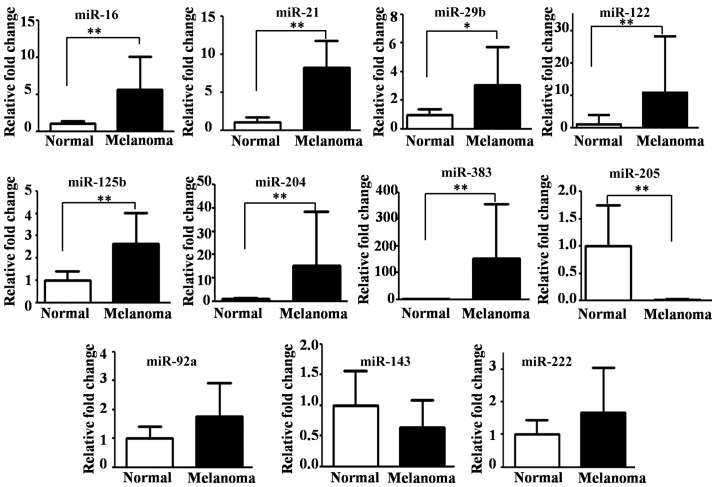
We found that seven miRNAs were significantly upregulated [P-values from 0.0001 (miR-21) to 0.025 (miR-29b)], but miR-205 was the only significantly downregulated miRNA (P<0.0001) in the CMM tissues compared with normal oral tissues (Fig. 1). No significant differences were detected in the expression of miR-92a, miR-143 and miR-222 between the CMM and normal oral tissues (Fig. 1).
Figure 1.
Reverse transcription-quantitative polymerase chain reaction validation of five dysregulated miRNAs from the microarray assays and six other cancer-associated miRNAs. Relative expression levels in CMM tissues (Melanoma) and normal oral tissues (Normal) are shown. The mean expression levels of the Normal samples were set to 1.0. P-values were determined by the Mann Whitney U-test (*P<0.05, **P<0.01). The bars indicate standard error. miR/miRNA, microRNA; CMM, canine malignant melanoma.
Of the 17 dysregulated miRNAs identified by microarray analysis (Table II), only miR-204, miR-383 and miR-205 were found to be highly differentially expressed by qRT-PCR. The average FCs for miR-204 and miR-383 were 15.3 and 152.7, respectively, but for miR-205 the average FC was 0.01 (Fig. 1).
The relative expression patterns of miR-204, miR-383 and miR-205 were consistent between the qRT-PCR and microarray results, but there were discrepancies for some of the other miRNAs. For example, miR-122 was downregulated (FC<0.5) in the microarray analysis but significantly upregulated in the qRT-PCR analysis and miR-143 was downregulated (FC of 0.244) in the microarray analysis but was not found to be significantly differentially expressed by qRT-PCR (Fig. 1).
miRNA-target regulatory interaction network
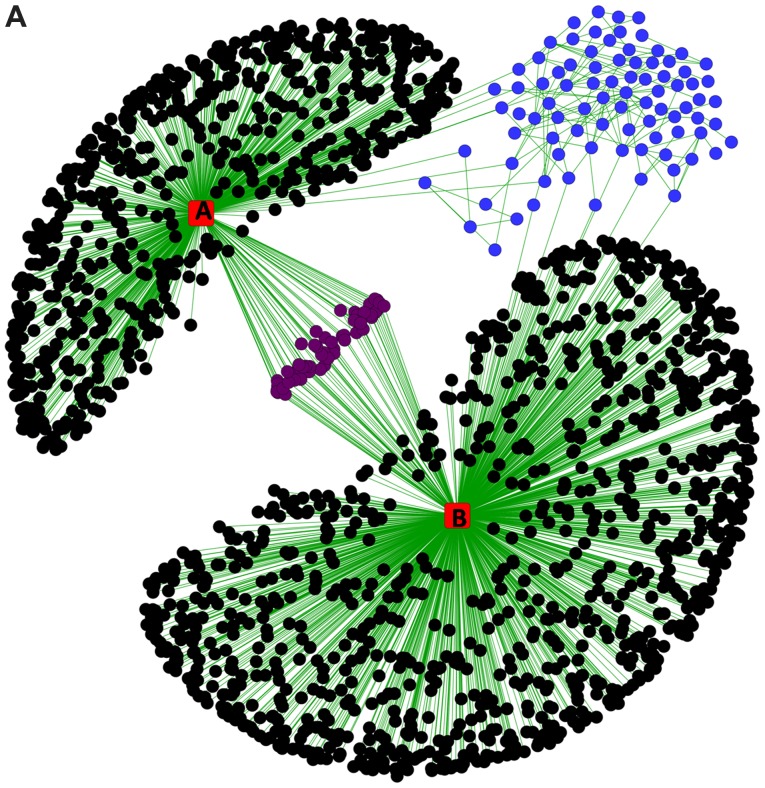
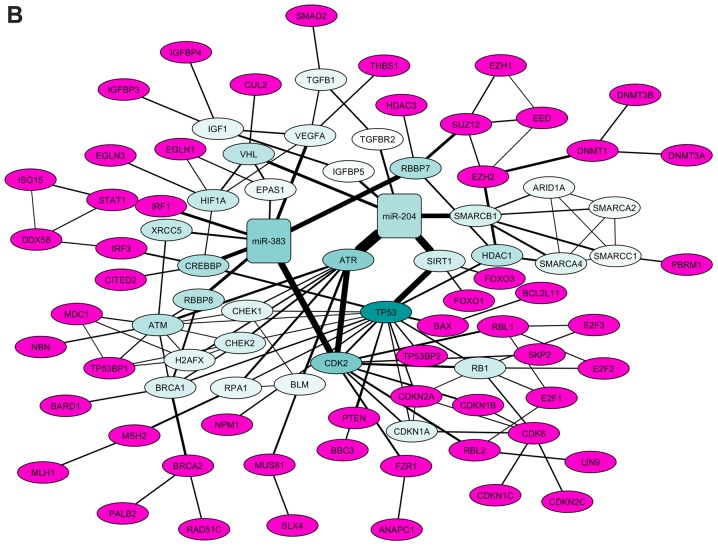
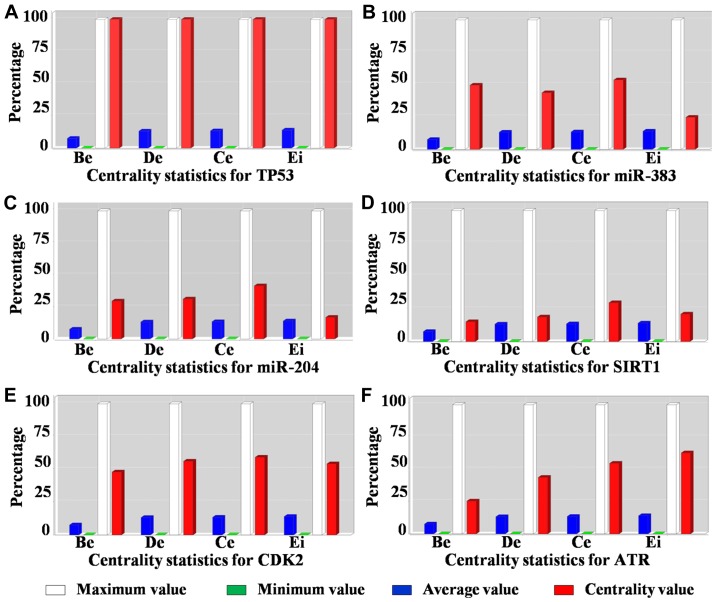
As indicated in Fig. 2A, the STRING protein interaction network revealed that miR-383 and miR-204 interacted with several common genes (proteins), as was reported previously (37,38). When we separated the connected network and calculated the basic parameters (degree, betweenness, centroid value and eigenvector) by Centiscape 2.2 through Cytoscape (Fig. 2B), we found all the basic parameters of TP53 (Fig. 3A) had higher value than any of the others. Further, the basic parameters of miR-383, miR-204, SIRT1, CDK2 and ATR (Fig. 3B-F) were higher than the average values, implying these miRNAs and proteins were the hub nodes of this biological network. In the separated miRNA-target interaction network we found that ATR and CDK2 were targets of miR-383 and miR-204 (Fig. 2B). Moreover, miR-204 could regulate the network through TP53 mediated by SIRT1. RBBP7, SMARCB1, and CREBBP were also connected with several nodes and may be related to the regulation of a small cluster network.
Figure 2.
miRNA-target regulatory interaction network. (A) miRNA-target regulatory network merged with the tumor suppressor genes protein interaction network. The red squares indicate miRNA nodes [(A) miR-383; (B) miR-204]. Black circles indicate targets (mRNAs) of single miRNAs, purple circles indicate targets shared by miRNAs and blue circles indicate tumor suppressor genes predicted to be targeted by one or both of the miRNA. The edges (lines) connecting two nodes are indicative of regulation (interaction). (B) Separated co-ordinate network showing the interactions between microRNAs and tumor suppressor genes. The node colors indicate the CV; pink gradient indicates CVs lower than average, blue gradient indicates CVs higher than average. Edge width indicates the betweenness measurement. miR/miRNA, microRNA; CV, centroid value.
Figure 3.
Centrality measures of the hub nodes in the miRNA-target regulatory network. Betweenness (Be), degree (De), centroid value (Ce) and eigenvector (Ei) plots for (A) TP53, (B) miR-383, (C) miR-204, (D) SIRT1, (E) CDK2 and (F) ATR respectively. miR/miRNA, microRNA; TP53, tumor protein p53; SIRT1, sirtuin 1; CDK2, cyclin dependent kinase 2; ATR, ATR serine/threonine kinase.
Discussion
Some of the dysregulated miRNAs identified in the CMM tissues by microarray analysis were validated by qRT-PCR. The upregulation of seven miRNAs in CMM, namely miR-16, miR-21, miR-29b, miR-122, miR-125b, miR-204, and miR-383 was demonstrated here for the first time. In particular, miR-204 and miR-383 showed extra ordinarily high expression levels in the microarray and qRT-PCR analyses.
Downregulation of miR-145, miR-205 and miR-203 was detected in the microarray analysis, which is consistent with previous studies on CMM (16,17). However, we did not detect dysregulation of other miRNAs that have been reported previously to be downregulated (17). These inconsistencies might be because different microarray platforms and/or samples were used in the two studies. Noguchi et al (17) used a CombiMatrix array, whereas we used a miRCURY™ LNA microRNA Array. Thus, there were differences in the miRNAs that were spotted on the arrays. We used CMM tissues from three different dogs and Noguchi et al (17) used CMM tissue from only one dog. Finally, in the previous study, miRNA expression was compared between CMM tissue and normal oral mucosal tissue (17), whereas we compared CMM tissues with reference miRNAs from several cancers and normal tissues. We used mixed miRNA reference samples to avoid biases from low signal intensities in the microarray data. Using miRNAs from several different origins means different miRNAs will be included because miRNA expression is highly dependent on the tissue origin and status. Our approach should cover a broad range of miRNAs, thus avoiding misleading FC ratios as a result of weak signals (39). However, because our reference tissues were mostly tumor samples (70.8%), using this kind of miRNA reference samples may have caused miRNAs that are commonly dysregulated in tumors to be overlooked but, importantly, may have revealed miRNAs that are specifically dysregulated in melanoma.
In this study, the microarray and qRT-PCR results were consistent for the relative expressions of miR-204, miR-383 and miR-205. However, the discrepant expressions of miR-122 and miR-143 between the microarray and qRT-PCR results may be explained by differences in the control samples that were used in the two experiments; that is, a mixed sample reference in the microarray analysis and normal oral tissues in the qRT-PCRs. For the same reason, differential expression of miR-16, miR-21, miR-29b and miR-125b was not detected in the microarray analysis but was detected by qRT-PCR. miR-21 and miR-29b are known to be upregulated in several tumors; for example, miR-21 in mouse BL/6 melanoma cells (40), miR-29b in human breast cancer (20) and both miRNAs in canine mammary cancer (14). These findings indicate that miR-21 and miR-29b are common oncomiRs in several species. Thus, the microarray screening method that we used may have masked the differential expression of these miRNAs because they are not specific to melanoma but commonly shared among several kinds of tumors.
While the significant downregulation of miR-205 can be explained, upregulation of miR-204 and miR-383 expression has not been reported in CMM until now. Indeed, miR-204 was reported to be upregulated in old HMM patients compared with young HMM patients (41); however, no comparison between melanoma and normal tissue was performed and the target mRNA was not defined. In another study, miR-204 was found to be downregulated in malignant melanoma compared with benign nevi (42), but the age of the patients was not considered and the comparisons were between malignant melanoma and benign nevi tissues. In prostate cancer and breast cancer studies, miR-204 was reported to be both up- and downregulated (43–47), maybe because of different experimental designs and individual identity.
TP53 is a well-known tumor suppressor gene located in the center of the network with a high centroid value (Fig. 3A). SIRT1, an indirect regulator of TP53, is a direct target of miR-204 in the network and has been reported to be downregulated in canine melanoma (48). SIRT1 acts as a tumor suppressor via β-catenin and has reminiscent effects on TP53 in colon cancer (49). Abnormal expression of β-catenin was reported in melanoma (50,51), so the miR-204-mediated downregulation of SIRT1 revealed in the network may cause β-catenin-mediated cell survival by evading TP53 in melanoma.
Up-regulation of miR-383 expression has been observed in primary HMM tumor cell lines compared with normal human epidermal melanocytes (52). In their study, Mueller et al (52) found that miR-383 was downregulated in snail stable knockdown melanoma cells by transfection of an antisense snail plasmid construct, named as-snail, compared with the parental melanoma cell line. Snail belongs to the snail superfamily of zinc finger transcription factors and is involved in the development of malignant melanoma through direct repression of E-cadherin expression (53). Indeed, the transcriptional profile of the as-snail cells was reported to be more similar to normal melanocytes than malignant melanoma cells (52). However, the detailed biological functions of miR-383 have not been reported so far. In our study, miR-383 was upregulated in CMM tissues. Liao et al (54) showed that ATR was the direct target of miR-383 and ATR was found to play a central role in the ATM/ATR pathway involved in DNA damage recognition and initial phosphorylation (55). Liao et al (54) also showed that GADD45γ, MDC1, and H2AX were all negatively correlated with miR-383 expression. Moreover, a recent study showed that loss of function or mutations of ATR lead to the development of melanoma (56). In testicular embryonal carcinoma miR-383 overexpression was found to reduce CDK2 expression at the protein level, which was also found to be necessary for proper DNA repair (57). Furthermore, CREB binding protein, a known co-activator of TP53, was found to be a direct target of miR-383 (58). There is also a possibility that miR-383 has indirect control over apoptosis via TP53 inhibition through CDK2. So, our network analysis and the above discussion suggest that miR-383 may be involved in DNA damage repair and apoptosis phenomena in melanoma. In this study, we demonstrated the dysregulation of 17 miRNAs in CMM and investigated the probable biological functions of these miRNAs based on their target genes. Our study is valid not only for dog but also for human because dog has been considered as a good preclinical model for human melanoma (10). Further studies are required to clarify the functions of the dysregulated miRNAs by for example, detecting the actual target genes and their pathways and analyzing their differential expression patterns in established canine melanoma cell lines (59,60) to determine the roles of the miRNA-target interactions in CMM tumor genesis and therapy.
We have demonstrated the upregulation of potential oncomiRs, miR-16, miR-21, miR-29b, miR-122, miR-125b, miR-204 and miR-383 in CMM tissues. In particular, the strong upregulation of miR-383 in CMM tissues compared with normal oral tissues identified by microarray screening was confirmed by qRT-PCR. We conclude that miR-383 and miR-204 may promote melanoma development by regulating both the DNA repair/checkpoint and apoptosis. To identify therapeutic targets in melanoma, further studies are required to verify the biological significance of the miRNA target genes.
Acknowledgements
The authors would like to thank Dr Margaret Biswas for editing a draft of this manuscript.
Funding
The present study was supported by the Japan Society for the Promotion of Science KAKENHI (grant nos. 17H03926, 15H14872, 25292180 and 22780283).
Availability of data and materials
The datasets used and/or analyzed in the present study are available from the corresponding author on reasonable request.
Authors' contributions
NU, MR, TM and NaM were involved in designing the study. NU, MR, TM, TI, NoM and HK collaborated in the data analysis. NaM acquired the funding. NU, MR, YCL, TM, TI, HK, YM and NaM performed the experiments. NaM was involved in project administration. NU, TI, NoM and HK acquired the resources. NoM, YM and NaM supervised the study. NaM was involved in data validation. TM wrote the original draft of the manuscript. NU, MR, YM and NaM revised and edited the original draft. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Informed consent to use the specimens in this study was obtained from the dog patient's owners. The animal experiments were approved by the Kagoshima University's Laboratory Animal Committee (approval no. A10031).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 2.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 3.Cho WC. OncomiRs: The discovery and progress of microRNAs in cancers. Mol Cancer. 2007;6:60. doi: 10.1186/1476-4598-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manikandan J, Aarthi JJ, Kumar SD, Pushparaj PN. OncomiRs: The potential role of non-coding microRNAs in understanding cancer. Bioinformation. 2008;2:330–334. doi: 10.6026/97320630002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svoronos AA, Engelman DM, Slack FJ. OncomiR or tumor suppressor? The duplicity of MicroRNAs in cancer. Cancer Res. 2016;76:3666–3670. doi: 10.1158/0008-5472.CAN-16-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huynh C, Segura MF, Gaziel-Sovran A, Menendez S, Darvishian F, Chiriboga L, Levin B, Meruelo D, Osman I, Zavadil J, et al. Efficient in vivo microRNA targeting of liver metastasis. Oncogene. 2011;30:1481–1488. doi: 10.1038/onc.2010.523. [DOI] [PubMed] [Google Scholar]
- 7.Sun V, Zhou WB, Majid S, Kashani-Sabet M, Dar AA. MicroRNA-mediated regulation of melanoma. Br J Dermatol. 2014;171:234–241. doi: 10.1111/bjd.12989. [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Dou Y, Sheng M. Inhibition of microRNA-383 has tumor suppressive effect in human epithelial ovarian cancer through the action on caspase-2 gene. Biomed Pharmacother. 2016;83:1286–1294. doi: 10.1016/j.biopha.2016.07.038. [DOI] [PubMed] [Google Scholar]
- 9.Chang X, Zhu W, Zhang H, Lian S. Sensitization of melanoma cells to temozolomide by overexpression of microRNA 203 through direct targeting of glutaminase-mediated glutamine metabolism. Clin Exp Dermatol. 2017;42:614–621. doi: 10.1111/ced.13119. [DOI] [PubMed] [Google Scholar]
- 10.Simpson RM, Bastian BC, Michael HT, Webster JD, Prasad ML, Conway CM, Prieto VM, Gary JM, Goldschmidt MH, Esplin DG, Smedley RC, et al. Sporadic naturally occurring melanoma in dogs as a preclinical model for human melanoma. Pigment Cell Melanoma Res. 2014;27:37–47. doi: 10.1111/pcmr.12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uhl E, Krimer P, Schliekelman P, Tompkins SM, Suter S. Identification of altered MicroRNA expression in canine lymphoid cell lines and cases of B- and T-cell lymphomas. Genes Chromosomes Cancer. 2011;50:950–967. doi: 10.1002/gcc.20917. [DOI] [PubMed] [Google Scholar]
- 12.Gioia G, Mortarino M, Gelain ME, Albonico F, Ciusani E, Forno I, Marconato L, Martini V, Comazzi S. Immunophenotype-related microRNA expression in canine chronic lymphocytic leukemia. Vet Immunol Immunopathol. 2011;142:228–235. doi: 10.1016/j.vetimm.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 13.Vinall RL, Kent MS, deVere White RW. Expression of microRNAs in urinary bladder samples obtained from dogs with grossly normal bladders, inflammatory bladder disease, or transitional cell carcinoma. Am J Vet Res. 2012;73:1626–1633. doi: 10.2460/ajvr.73.10.1626. [DOI] [PubMed] [Google Scholar]
- 14.Boggs RM, Wright ZM, Stickney MJ, Porter WW, Murphy KE. MicroRNA expression in canine mammary cancer. Mamm Genome. 2008;19:561–569. doi: 10.1007/s00335-008-9128-7. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi M, Saito A, Tanaka Y, Michishita M, Kobayashi M, Irimajiri M, Kaneda T, Ochiai K, Bonkobara M, Takahashi K, et al. MicroRNA expression profiling in canine prostate cancer. J Vet Med Sci. 2017;79:719–725. doi: 10.1292/jvms.16-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noguchi S, Mori T, Hoshino Y, Yamada N, Nakagawa T, Sasaki N, Akao Y, Maruo K. Comparative study of anti-oncogenic microRNA-145 in canine and human malignant melanoma. J Vet Med Sci. 2012;74:1–8. doi: 10.1292/jvms.11-0264. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi S, Mori T, Hoshino Y, Yamada N, Maruo K, Akao Y. MicroRNAs as tumour suppressors in canine and human melanoma cells and as a prognostic factor in canine melanomas. Vet Comp Oncol. 2013;11:113–123. doi: 10.1111/j.1476-5829.2011.00306.x. [DOI] [PubMed] [Google Scholar]
- 18.Starkey MP, Compston-Garnett L, Malho P, Dunn K, Dubielzig R. Metastasis-associated microRNA expression in canine uveal melanoma. Vet Comp Oncol. 2018;16:81–89. doi: 10.1111/vco.12315. [DOI] [PubMed] [Google Scholar]
- 19.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Bian Z, Wei D, Zhang JG. miR-29b regulates migration of human breast cancer cells. Mol Cell Biochem. 2011;352:197–207. doi: 10.1007/s11010-011-0755-z. [DOI] [PubMed] [Google Scholar]
- 21.Latchana N, Ganju A, Howard JH, Carson WE III. MicroRNA dysregulation in melanoma. Surg Oncol. 2016;25:184–189. doi: 10.1016/j.suronc.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt MJ, Philippidou D, Reinsbach SE, Margue C, Wienecke-Baldacchino A, Nashan D, Behrmann I, Kreis S. Interferon-γ-induced activation of signal transducer and activator of transcription 1 (STAT1) up-regulates the tumor suppressing microRNA-29 family in melanoma cells. Cell Commun Signal. 2012;10:41. doi: 10.1186/1478-811X-10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Leva G, Garofalo M, Croce CM. MicroRNAs in cancer. Annu Rev Pathol. 2014;9:287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartwell LH, Hopfield JJ, Leibler S, Murray AW.From molecular to modular cell biology Nature 402(6761 Suppl)C47–C52.1999 10.1038/35011540 [DOI] [PubMed] [Google Scholar]
- 25.Zhu W, Yang L, Du Z. MicroRNA regulation and tissue-specific protein interaction network. PLoS One. 2011;6:e25394. doi: 10.1371/journal.pone.0025394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schroeder A, Mueller O, Stocker S, Salowsky R, Leiber M, Gassmann M, Lightfoot S, Menzel W, Granzow M, Ragg T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol Biol. 2006;7:3. doi: 10.1186/1471-2199-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao Y, Wang E, Liu H, Rotunno M, Koshiol J, Marincola FM, Landi MT, McShane LM. Evaluation of normalization methods for two-channel microRNA microarrays. J Transl Med. 2010;8:69. doi: 10.1186/1479-5876-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19:368–375. doi: 10.1093/bioinformatics/btf877. [DOI] [PubMed] [Google Scholar]
- 30.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/S0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 31.Lopes CT, Franz M, Kazi F, Donaldson SL, Morris Q, Bader GD. Cytoscape Web: An interactive web-based network browser. Bioinformatics. 2010;26:2347–2348. doi: 10.1093/bioinformatics/btq430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. (Database Issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scardoni G, Petterlini M, Laudanna C. Analyzing biological network parameters with CentiScaPe. Bioinformatics. 2009;25:2857–2859. doi: 10.1093/bioinformatics/btp517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Felicetti F, Errico MC, Bottero L, Segnalini P, Stoppacciaro A, Biffoni M, Felli N, Mattia G, Petrini M, Colombo MP, et al. The promyelocytic leukemia zinc finger-microRNA-221/-222 pathway controls melanoma progression through multiple oncogenic mechanisms. Cancer Res. 2008;68:2745–2754. doi: 10.1158/0008-5472.CAN-07-2538. [DOI] [PubMed] [Google Scholar]
- 35.Kappelmann M, Kuphal S, Meister G, Vardimon L, Bosserhoff AK. MicroRNA miR-125b controls melanoma progression by direct regulation of c-Jun protein expression. Oncogene. 2013;32:2984–2991. doi: 10.1038/onc.2012.307. [DOI] [PubMed] [Google Scholar]
- 36.Si H, Sun X, Chen Y, Cao Y, Chen S, Wang H, Hu C. Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J Cancer Res Clin Oncol. 2013;139:223–229. doi: 10.1007/s00432-012-1315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creighton CJ, Hernandez-Herrera A, Jacobsen A, Levine DA, Mankoo P, Schultz N, Du Y, Zhang Y, Larsson E, Sheridan R, et al. Integrated analyses of microRNAs demonstrate their widespread influence on gene expression in high-grade serous ovarian carcinoma. PLoS One. 2012;7:e34546. doi: 10.1371/journal.pone.0034546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim YK, Yu J, Han TS, Park SY, Namkoong B, Kim DH, Hur K, Yoo MW, Lee HJ, Yang HK, Kim VN. Functional links between clustered microRNAs: Suppression of cell-cycle inhibitors by microRNA clusters in gastric cancer. Nucleic Acids Res. 2009;37:1672–1681. doi: 10.1093/nar/gkp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parker BJ, Günter S, Bedo J. Stratification bias in low signal microarray studies. BMC Bioinformatics. 2007;8:326. doi: 10.1186/1471-2105-8-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang CH, Yue J, Pfeffer SR, Handorf CR, Pfeffer LM. MicroRNA miR-21 regulates the metastatic behavior of B16 melanoma cells. J Biol Chem. 2011;286:39172–39178. doi: 10.1074/jbc.M111.285098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jukic DM, Rao UN, Kelly L, Skaf JS, Drogowski LM, Kirkwood JM, Panelli MC. Microrna profiling analysis of differences between the melanoma of young adults and older adults. J Transl Med. 2010;8:27. doi: 10.1186/1479-5876-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luan W, Qian Y, Ni X, Bu X, Xia Y, Wang J, Ruan H, Ma S, Xu B. miR-204-5p acts as a tumor suppressor by targeting matrix metalloproteinases-9 and B-cell lymphoma-2 in malignant melanoma. Onco Targets Ther. 2017;10:1237–1246. doi: 10.2147/OTT.S128819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ding M, Lin B, Li T, Liu Y, Li Y, Zhou X, Miao M, Gu J, Pan H, Yang F, et al. A dual yet opposite growth-regulating function of miR-204 and its target XRN1 in prostate adenocarcinoma cells and neuroendocrine-like prostate cancer cells. Oncotarget. 2015;6:7686–7700. doi: 10.18632/oncotarget.3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Findlay VJ, Turner DP, Moussa O, Watson DK. MicroRNA-mediated inhibition of prostate-derived Ets factor messenger RNA translation affects prostate-derived Ets factor regulatory networks in human breast cancer. Cancer Res. 2008;68:8499–8506. doi: 10.1158/0008-5472.CAN-08-0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imam JS, Plyler JR, Bansal H, Prajapati S, Bansal S, Rebeles J, Chen HI, Chang YF, Panneerdoss S, Zoghi B, et al. Genomic loss of tumor suppressor miRNA-204 promotes cancer cell migration and invasion by activating AKT/mTOR/Rac1 signaling and actin reorganization. PLoS One. 2012;7:e52397. doi: 10.1371/journal.pone.0052397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, Jin X, Zhang Q, Zhang G, Deng X, Ma L. Decreased expression of miR-204 is associated with poor prognosis in patients with breast cancer. Int J Clin Exp Pathol. 2014;7:3287–3292. [PMC free article] [PubMed] [Google Scholar]
- 47.Turner DP, Findlay VJ, Moussa O, Semenchenko VI, Watson PM, LaRue AC, Desouki MM, Fraig M, Watson DK. Mechanisms and functional consequences of PDEF protein expression loss during prostate cancer progression. Prostate. 2011;71:1723–1735. doi: 10.1002/pros.21389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marfe G, De Martino L, Tafani M, Irno-Consalvo M, Pasolini MP, Navas L, Papparella S, Gambacurta A, Paciello O. A multicancer-like syndrome in a dog characterized by p53 and cell cycle-checkpoint kinase 2 (CHK2) mutations and sirtuin gene (SIRT1) down-regulation. Res Vet Sci. 2012;93:240–245. doi: 10.1016/j.rvsc.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 49.Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pećina-Slaus N, Zigmund M, Kusec V, Martić TN, Cacić M, Slaus M. E-cadherin and beta-catenin expression patterns in malignant melanoma assessed by image analysis. J Cutan Pathol. 2007;34:239–246. doi: 10.1111/j.1600-0560.2006.00601.x. [DOI] [PubMed] [Google Scholar]
- 51.Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of beta-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- 52.Mueller DW, Rehli M, Bosserhoff AK. miRNA expression profiling in melanocytes and melanoma cell lines reveals miRNAs associated with formation and progression of malignant melanoma. J Invest Dermatol. 2009;129:1740–1751. doi: 10.1038/jid.2008.452. [DOI] [PubMed] [Google Scholar]
- 53.Kuphal S, Palm HG, Poser I, Bosserhoff AK. Snail-regulated genes in malignant melanoma. Melanoma Res. 2005;15:305–313. doi: 10.1097/00008390-200508000-00012. [DOI] [PubMed] [Google Scholar]
- 54.Liao XH, Zheng L, He HP, Zheng DL, Wei ZQ, Wang N, Dong J, Ma WJ, Zhang TC. STAT3 regulated ATR via microRNA-383 to control DNA damage to affect apoptosis in A431 cells. Cell Signal. 2015;27:2285–2295. doi: 10.1016/j.cellsig.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Rouse J, Jackson SP. Interfaces between the detection, signaling, and repair of DNA damage. Science. 2002;297:547–551. doi: 10.1126/science.1074740. [DOI] [PubMed] [Google Scholar]
- 56.Chen CF, Ruiz-Vega R, Vasudeva P, Espitia F, Krasieva TB, de Feraudy S, Tromberg BJ, Huang S, Garner CP, Wu J, et al. ATR mutations promote the growth of melanoma tumors by modulating the immune microenvironment. Cell Rep. 2017;18:2331–2342. doi: 10.1016/j.celrep.2017.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Satyanarayana A, Kaldis P. A dual role of Cdk2 in DNA damage response. Cell Div. 2009;4:9. doi: 10.1186/1747-1028-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grossman SR. p300/CBP/p53 interaction and regulation of the p53 response. Eur J Biochem. 2001;268:2773–2778. doi: 10.1046/j.1432-1327.2001.02226.x. [DOI] [PubMed] [Google Scholar]
- 59.Ohashi E, Hong SH, Takahashi T, Nakagawa T, Mochizuki M, Nishimura R, Sasak N. Effect of retinoids on growth inhibition of two canine melanoma cell lines. J Vet Med Sci. 2001;63:83–86. doi: 10.1292/jvms.63.83. [DOI] [PubMed] [Google Scholar]
- 60.Inoue K, Ohashi E, Kadosawa T, Hong SH, Matsunaga S, Mochizuki M, Nishimura R, Sasaki N. Establishment and characterization of four canine melanoma cell lines. J Vet Med Sci. 2004;66:1437–1440. doi: 10.1292/jvms.66.1437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in the present study are available from the corresponding author on reasonable request.