Abstract
Matrix metalloproteinase-9 (MMP9) has been recognized to be an important factor in cancer invasion and metastasis. In contrast, decorin has been revealed to inhibit primary tumor development. The aim of the present study was to investigate the function of MMP9 and decorin in cervical cancer. Three experiments were performed to analyze the function of MMP9 and decorin in the invasion of cervical cancer by: i) Analyzing the expression of MMP9 and decorin by immunohistochemistry in 100 cervical specimens; ii) determining the concentration of decorin by an enzyme-linked immunosorbent assay (ELISA) using the human squamous cervical cancer cell line CaSki and human endometrial stromal cell line CRL4003 and iii) evaluating the invasion ability of CaSki cells in a cervical invasion model by an invasion assay. Immunohistochemistry revealed that MMP9 was overexpressed in microinvasive carcinoma (100.0%) but was less strongly expressed in normal or pre-malignant squamous epithelium (0–41.9%). In contrast, the activity of decorin in stroma adjacent to neoplastic cells was lower in microinvasive carcinoma (9.1%) compared with in normal or pre-malignant lesions (74.2–100.0%). An ELISA revealed that MMP9 released from CaSki cells resolved the decorin released from CRL4003 cells. An invasion assay demonstrated that the invasive ability of CaSki cells was suppressed by an MMP inhibitor, and decorin was released from CRL4003 cells. These data suggested that decorin prevented the invasion of malignant cells in uterine cervical cancer; however, MMP9 promotes cell invasion by destroying decorin.
Keywords: cervical cancer, matrix metalloproteinase-9, decorin, invasion, metastasis
Introduction
Matrix metalloproteinases (MMPs) are a family of zinc- and carcium-dependent proteolytic enzymes (1). These enzymes are normally involved in the breakdown of the extracellular matrix within the context of physiological tissue remodeling and angiogenesis (2). MMP9 is normally associated with bone remodeling (2) and dysregulated states, such as rheumatoid arthritis and osteosarcoma (3). It also has a strong influence on many phases of cancer progression, including angiogenesis, invasiveness and metastasis. An excessive production of MMP9 has been recognized to be an important factor in cancer invasion and metastasis (4).
Decorin is a member of the extracellular matrix small leucine-rich proteoglycan (SLRP) family of proteins that exists and functions mainly in the stroma and epithelial cells (5). It also has been found to play an important role in tumor development and progression, angiogenesis and metastasis (6). Several studies have shown that decorin inhibits primary tumor development and progression by antagonistically targeting multiple tyrosine kinase receptors, such as epidermal growth factor receptor (EGFR) (7).
The data in the pertinent literature show that members of the MMP family are able to cleave some SLRPs (8–11) including decorin (9). We hypothesize that cervical cancer cells display enhanced MMP9 expression, which results in infiltration to the extracellular matrix and uterine stroma destroying the basement membranes. The resolution of decorin by MMP9 from cancer cells promotes further infiltration, which results in further destruction of the extracellular matrix and stroma, where decorin is produced. The aim of the present study was to investigate the role of MMP9 and decorin in cervical cancer.
Materials and methods
Participants
The present study included 100 randomly selected patients who underwent cervical conization for squamous cell neoplasia at Osaka Medical College between 2010 and 2012. The Institutional Review Board approved this study (Ethics Committee of Osaka Medical College, 0277), and informed consent was obtained from all patients for the use of their tissue samples.
Expression of MMP9 and decorin by immunohistochemistry
The specimens were fixed in 10% formalin and embedded in paraffin. Serial sections cut out from paraffin-embedded blocks were used for routine histopathology. A 4-µm section was cut from a paraffin embedded block and immunohistochemically analyzed for the expression of MMP9 and decorin. Deparaffinized and rehydrated sections (4 µm) were autoclaved in 0.01 mol/l citrate buffer Ph 6.0 for 15 min at 121°C for antigen retrieval. Endogenous peroxidase activity was blocked with 0.3% solution hydrogen peroxide in methanol for 30 min. Tumor sections were incubated at 4°C for 12 h with anti-MMP9 rabbit polyclonal antibodies (AB13458; 1:50 dilutio; EMD Millipore, Billerica, MA, USA) and anti-decorin rabbit polyclonal antibodies (ab151988, 1:100 dilution; Abcam, Cambridge, MA, USA). The sections were washed with 1X phosphate-buffered saline (PBS) and incubated with Histofine simple stain MAX PO (multi; Nichirei, Tokyo, Japan) for 30 min at room temperature. Finally, the sections were washed with 1X PBS, signals and then visualized by incubation with H2O2/diaminobenzidine substrate solution for 5 min. The sections were counterstained with hematoxylin prior to dehydration and mounting.
The evaluation of the immunohistochemical data was performed by two independent observers who were blinded to the clinicopathological data. The expression of MMP9 for each sample was defined as detectable immunoreactions in cancer cells, as described previously (12). The evaluation of MMP9 expression was performed for stroma under the basement membrane or epithelial cells with no invasive neoplasia. In cases of invasive carcinoma, malignant cells or the stroma adjacent to malignant cells were evaluated. Briefly, the MMP9 expression was considered to be negative (no more than 10% of cells positive), low (more than 10% and up to 50% of cells positive) or high (more than 50% of cells positive). Based on these data, two groups were established: Negative MMP9 (no/low expression) and positive MMP9 expression (high expression).
The evaluation of the stromal decorin expression was also performed under the basement membrane with no invasive neoplasia. In cases of invasive carcinoma, the stroma adjacent to the malignant cells was evaluated. The expression of decorin was assessed using a semiquantitative system. Briefly, the decorin expression was scored as 0 (no stain), 1+ (weak immunoreactivity in more than 10% of stromal cells), 2+ (moderate immunoreactivity in more than 10% of stromal cells), or 3+ (strong immunoreactivity in more than 10% of stromal cells). Based on these data, two groups were established: Including a low decorin expression group (0 and 1+) and a high decorin expression group (2+ and 3+).
Cell lines
We used the human cervical cancer CaSki and the CRL-4003 immortalized human endometrial stromal cells, both purchased from the American Type Cultured Collection (Manassas, VA, USA). We also performed an STR polymorphism profiling analysis (Wakennyaku Co., Ibaraki, Japan) to confirm the cell line identity. These cells were cultured in growth media [DMEM/F12 10% FBS, 1% BD Insulin, Transferrin, Selenous (ITS) +Premix Universal Culture Supplement (catalog no: 354352; BD Biosciences, Franklin Lakes, NJ, USA)], in a humidified atmosphere of 5% CO2 with 95% air at 37°C.
Measurement of the concentration of decorin with an enzyme-linked immunosorbent (ELISA)
We used the cervical cancer cell line CaSki and endometrial stromal cell line CRL4003. DMEM was used as cell culture medium. A total of 106 CaSki cells and CRL4003 cells each were cultured at 37°C in a humidified, 5% CO2 atmosphere in DMEM for 96 h. CaSki culture medium was then obtained as medium containing MMP9. CRL4003 culture medium was also obtained as medium containing decorin. The concentration of decorin in the CRL4003 culture medium with or without CaSki culture medium or MMP inhibitor (MMP-9 inhibitor, ab142180; Abcam) was investigated by an ELISA. The experiment was carried out five times, and the concentration of decorin was expressed as the mean ± standard deviation (SD).
Influence of MMP9 and decorin on the invasion ability using an invasion assay
The invasive potential was assessed using an invasion assay. We examined the effects of MMP9 and decorin on the invasive potential of CaSki cells with or without CRL4003 cells and MMP inhibitor using this assay. A total of 5×105 CaSki cells were cultured at 37°C in a humidified, 5% CO2 atmosphere in serum-free DMEM for 24 h. These cells with or without MMP inhibitor were then seeded into upper wells coated with a thin layer of Matrigel. The lower chamber contained 600 µl of DMEM with or without 5×105 CRL4003 cells; we established four groups: CaSki, CaSki with CRL4003, CaSki with MMP inhibitor, CaSki with CRL4003 and MMP inhibitor. Following 24-h incubation at 37°C, noninvading cells on the surface of the Matrigel-coated membrane were removed by scraping with a cotton swab. Cells that migrated through the Matrigel were stained with hematoxylin. Following several washes with PBS, the stained cells were manually counted for two independent experiments. Each point represents the mean ± SD of four replicates.
Statistical analyses
All statistical analyses were performed using the JMP software package (v11.1.1; SAS Institute Inc, Tokyo, Japan). Continuous variables are expressed as the mean ± SD. The Mann-Whitney U-test was used to compare continuous variables between the two groups. When making multiple comparisons in datasets with continuous variables containing more than two groups, the Tukey honestly significant difference (HSD) was used. Fisher's exact test with Bonferroni's correction was used to compare frequencies between the three groups. Pearson's correlation coefficient was calculated to determine the correlation between two variables. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of MMP9 and decorin in surgical specimens
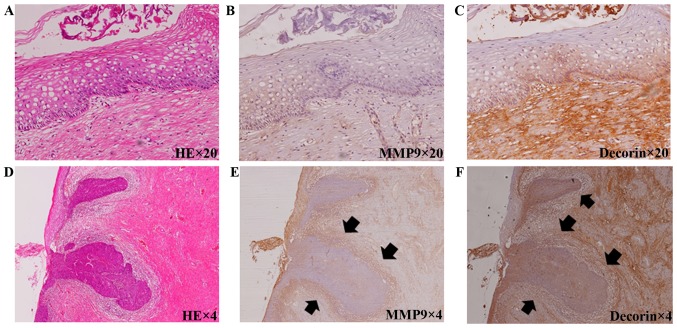
The immunochemical staining of MMP9 and decorin is shown in Fig. 1. In patients with cervical intraepithelial neoplasia (CIN) 1 (Fig. 1A), the expression of MMP9 was not seen under the basement membrane (Fig. 1B); however, decorin was expressed abundantly in the stroma (Fig. 1C). In contrast, in patients with microinvasive carcinoma (Fig. 1D), the expression of MMP9 was abundant (Fig. 1E); however, decorin was not seen in the stroma adjacent to malignant cells (Fig. 1F). The results of the immunohistochemical analyses are shown in Table I. The patients were categorized into three groups: those with normal to CIN2 were categorized as having low-grade neoplasia, those with CIN3 were categorized as being premalignant, and those with microinvasive carcinoma (FIGO stage IA1 squamous cervical cancer) were categorized as being malignant. Five patients had no neoplasia, 14 had CIN 1 and 14 had CIN 2; a total of 33 patients had normal to CIN2 (low-grade neoplasia group), 31 had CIN3 (premalignant group), and 36 had microinvasive carcinoma (malignant group). The mean ± SD age in the low-grade neoplasia, premalignant and malignant groups was 37.2±10.0, 40.9±11.2 and 38.6±10.4 years old, respectively. No significant difference was found between the groups. The rate of MMP9 positivity was 0% in the low-grade neoplasia group, 41.9% in the premalignant group and 100% in the malignant group. There were significant differences between each group (P<0.01). In contrast, the rate of high decorin expression was 100% in the low-grade neoplasia group, 74.2% in the premalignant group and 9.1% in the malignant group. There were significant differences between each group (P<0.01).
Figure 1.
Immunohistochemical staining of MMP9 and decorin in specimens from normal cervical epithelium (A-C) and microinvasive carcinoma (D-F). (A) Hematoxylin and eosin staining of normal epithelium. (B) MMP9 was not stained in the normal epithelium or stroma under the basement membrane. (C) Decorin was overexpressed in the stroma under the basement membrane. (D) Hematoxylin and eosin staining of microinvasive squamous cell carcinoma. (E) MMP9 was overexpressed in the stroma adjacent to cancer cells (arrows). (F) Decorin expression was weaker in the stroma adjacent to cancer cells (arrows). MMP9, matrix metalloproteinase-9.
Table I.
Expression of MMP9 and decorin in uterine cervical neoplasm.
| Variables | Low-grade neoplasia (n=33) | Premalignant (n=31) | Malignant (n=36) | P-value |
|---|---|---|---|---|
| Agea, years | 37.2±10.0 | 40.9±11.2 | 38.6±10.4 | N.S. |
| Positive for MMP9 (%) | 0/33 (0) | 13/31 (41.9) | 36/36 (100) | P<0.01 |
| High decorin expression (%) | 33/33 (100) | 23/31 (74.2) | 3/36 (9.1) | P<0.01 |
MMP9, Matrix metalloproteinase-9; N.S., not significant.
Based on an analysis of variance (mean ± standard deviation).
Relationship between MMP9 and decorin
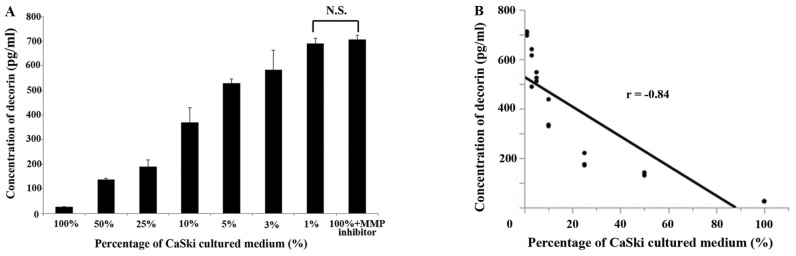
The concentration of decorin in CRL4003 culture medium with varying amounts of CaSki culture medium by an ELISA (Fig. 2A). When the ratio of CaSki culture medium to CRL4003 culture medium was 100, 50, 25, 10, 5, 3 and 1%, the concentration of decorin was 26.4±1.2, 138±6.4, 189±28, 368±61, 529±18, 582±81, 690±21 and 705±19 pg/ml, respectively. However, the concentration of decorin in CRL4003 culture medium with the same volume of CaSki culture medium and MMP inhibitor was 705±19 pg/ml. This value was not significantly different in comparison to the concentration of decorin in the CRL4003 cultured medium with 1% CaSki cultured medium (P=0.82). Pearson's correlation coefficient showed a strong correlation between the concentration of decorin and the amount of CaSki cultured medium (r=−0.84, P<0.05) (Fig. 2B). These findings indicate that the decorin released from CRL4003 cells was resolved by MMP9 released from CaSki cells.
Figure 2.
(A) Concentration of decorin in CRL4003 cell culture medium by enzyme-linked immunosorbent assay (ELISA). The concentration of decorin decreased when CaSki culture medium was added but not when MMP inhibitor was added. (B) Pearson's correlation coefficient showed a strong correlation between the concentration of decorin and the amount of CaSki cultured medium (r=−0.84, P<0.05). This finding indicates that MMP9 released from CaSki cells destroys decorin released from CRL4003 cells. MMP, matrix metalloproteinase.
Migration and invasion in the cervical invasion model
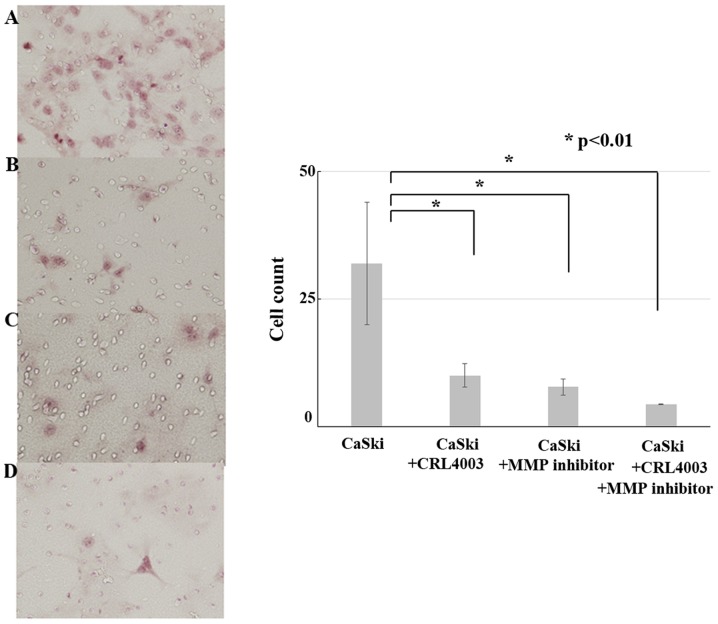
The results of migration and invasion assays is show in Fig. 3. The cell counts of CaSki, CaSki with CRL4003, CaSki with MMP inhibitor and CaSki with CRL4003 and MMP inhibitor were 32±12, 10±2.3, 7.8±1.6 and 4.4±1.1, respectively. CRL4003 culture medium and MMP inhibitor decreased the CaSki cell migration and invasion (P<0.01). CRL4003 culture medium with MMP inhibitor suppressed the CaSki cell migration and invasion the most effectively (P<0.01).
Figure 3.
Migration and invasion assays in a cervical cancer model. (A) CaSki cells without CRL4003 or matrix metalloproteinase (MMP) inhibitor. (B) CaSki cells with CRL4003. (C) CaSki cells with MMP inhibitor. (D) CaSki cells with CRL4003 and MMP inhibitor. CRL4003 and MMP inhibitor suppressed the invasive ability of CaSki cells.
Discussion
In the present study, immunohistochemical analyses in patients with cervical cancer showed an increased MMP9 expression and a decreased decorin expression in the stroma adjacent to malignant cells. In vitro, the endometrial stromal cell line CRL4003 produced decorin, which suppressed cell invasion. Furthermore, the cervical cancer cell line CaSki produced MMP9, which promoted cell invasion and decomposed decorin.
MMPs are a family of metalloendopeptidases that break down the protein components of the extracellular matrix; they play an important role in tissue remodeling and degradation (1). Angiogenesis is one of the most important process of tumor growth and requires the degeneration and remodeling of the extracellular matrix, cell migration and proliferation, and tube formation (13); the role of MMP in tumor growth and progression is associated with angiogenesis. MMP9, which is over-expressed in cancer cells, is a potent gelatinase that has been shown to be associated with the process of tumor cell invasion and metastasis (14,15). The overexpression of MMP9 has been observed in pre-cancer and cancer lesion of the uterine cervix. The published literature states that MMP9 is overexpressed in more than 90% of squamous cell carcinomas and 83–100% of high-grade squamous intraepithelial lesions (HSILs) but is less strongly expressed in low-grade squamous intraepithelial lesions (LSILs) and normal squamous epithelium (13%) (16–28). Most reports have shown that the activity of MMP9 tends to increase from normal cervix to HSIL and SCC, being more strongly expressed in more advanced stages (27,28). MMP9 is expressed in stromal cells and inflammatory cells around tumors (29). These findings suggest the importance of MMP9 in the pathogenesis of uterine cervical cancer; MMP9 affects various inflammatory cells infiltrating the tumor area, the extracellular matrix and stromal cells and proteins released from those cells.
Decorin, which is a member of the extracellular matrix SLPR, has been thought to act exclusively as a structural component (5). It is expressed in a wide range of connective tissues, including skin, bone, cartilaginous tissue and stroma and is secreted into the extracellular matrix (30,31). Fibrillar collagen, which is the main structural constituent of tendons, ligaments, skin and other connective tissues, is bound and cross-linked by decorin (32–36). Decorin knockout mice show skin fragility and abnormal collagen fibril morphology (37), and a lack of decorin in these mice causes a significant delay in the healing of the excisional and incisional dermal wound compared to decorin wild-type mice (38). Furthermore, crossing decorin-null mice with P53-null mice caused early lethality of double-mutant animals because of massive organ infiltration by T-cell lymphoma (39). These findings indicate that decorin thus plays a role in tissue development.
As mentioned below, decorin has biological functions as a degenerator of endocytosis and inhibitor of tumor cell growth, migration, angiogenesis, endothelial cell proliferation and motility. Decorin affects signal transduction via erbB family receptors characterized by the stimulation of mitogen-activated protein kinases, mobilization of intracellular calcium and up-regulation of P21WAF1/CIP, a potent inhibitor of cyclin-dependent kinases, ultimately leading to growth suppression (31,40–43). These signal transductions are associated with modulating cell proliferation, cell cycle progression and apoptosis. Decorin is known to be a promoter of matrix organization (44), which constitutes a physical barrier against the migration/motility of cancerous cells; decorin-collagen interactions prevent metastasis (45). Decorin is also known to be a negative regulator of signal transduction by interfering with growth factor binding including TGF-β (46,47); the synthesis of matrix molecules and the growth factor-dependent modulation of cell proliferation and migration is inhibited by decorin. For these reasons, decorin has recently emerged as a potential natural anticancer agent produced by normal host cells against cancer cells. There have been a number of reports about the anti-tumor effects of decorin on various malignant tumors, including uterine cervical carcinoma cells (48), ovarian cancer cells (49), breast cancer cells (50), colon carcinoma cells (51) and pancreatic cancer cells (52).
The published literature shows that MMPs digest decorin into fragments by cleaving within the leucine-rich region at multiple site (9). Those previous authors examined the susceptibility of decorin to five different MMPs. MMP2, MMP3 and MMP7 exhibited digestive activity for decorin, with MMP1 and MMP9 showing lower activity. In the present study, concentration of decorin in CRL4003 cultured medium was increased by MMP9 inhibitor. These data suggest that MMP9 not only directly digests decorin but also suppresses the production of decorin by impairing the tissue.
In conclusion, MMP9 was found to be overexpressed in uterine cervical cancer, but it was less strongly expressed in normal or pre-malignant squamous epithelium. In contrast, the activity of decorin in stroma adjacent to neoplastic cells was lower in microinvasive carcinoma than in normal or pre-malignant lesions. Decorin prevents the invasion of malignant cells, but MMP9 promotes cell invasion by destroying decorin, the extracellular matrix and stroma.
Acknowledgements
The authors would like to thank Dr. Yoshihiro Joshua Ono (Department of Obstetrics and Gynecology, Osaka Medical College, Osaka, Japan) for advice on the experimental design, and Ms. Junko Hayashi and Ms. Kumiko Satoh (Department of Obstetrics and Gynecology, Osaka Medical College) for their secretarial assistance.
Glossary
Abbreviations
- MMP9
matrix metalloproteinase-9
- ELISA
enzyme-linked immunosorbent assay
- MMPs
matrix metalloproteinases
- SLRP
small leucine-rich proteoglycan
- EGFR
epidermal growth factor receptor
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.
Authors' contribution
TT and MO designed the study and performed the experimental data collection and analysis. TT and YT provided the diagnosis based on immunohistochemical analyses, and TT and MO wrote the paper.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Osaka Medical College, and written informed consent to participate in the study was obtained from all patients involved.
Patient consent for publication
No identifying patient information is included in the published manuscript.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Stamenkovic I. Extracellular matrix remodelling: The role of matrix metalloproteinases. J Pathol. 2003;200:448–464. doi: 10.1002/path.1400. [DOI] [PubMed] [Google Scholar]
- 2.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 3.Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit Rev Biochem Mol Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- 4.Moss Shuman LA, Jensen-Taubman S, Stetler-Stevenson WG. Matrix metalloproteinases: Changing roles in tumor progression and metastasis. Am J Pathol. 2012;181:1895–1899. doi: 10.1016/j.ajpath.2012.08.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaefer L, Iozzo RV. Biological functions of the small leucine-rich proteoglycans: From genetics to signal transduction. J Biol Chem. 2008;283:21305–21309. doi: 10.1074/jbc.R800020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bi XL, Yang W. Biological functions of decorin in cancer. Chin J Cancer. 2013;32:266–269. doi: 10.5732/cjc.012.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldoni S, Humphries A, Nyström A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monfort J, Tardif G, Reboul P, Mineau F, Roughley P, Pelletier JP, Martel-Pelletier J. Degradation of small leucine-rich repeat proteoglycans by matrix metalloprotease-13: Identification of a new biglycan cleavage site. Arthritis Res Ther. 2006;8:R26. doi: 10.1186/ar1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai K, Hiramatsu A, Fukushima D, Pierschbacher MD, Okada Y. Degradation of decorin by matrix metalloproteinases: Identification of the cleavage sites, kinetic analyses and transforming growth factor-beta1 release. Biochem J. 1997;322:809–814. doi: 10.1042/bj3220809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heathfield TF, Onnerfjord P, Dahlberg L, Heinegård D. Cleavage of fibromodulin in cartilage explants involves removal of the N-terminal tyrosine sulfate-rich region by proteolysis at a site that is sensitive to matrix metalloproteinase-13. J Biol Chem. 2004;279:6286–6295. doi: 10.1074/jbc.M307765200. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Aoki T, Mori Y, Ahmad M, Miyamori H, Takino T, Sato H. Cleavage of lumican by membrane-type matrix metalloproteinase-1 abrogates this proteoglycan-mediated suppression of tumor cell colony formation in soft agar. Cancer Res. 2004;64:7058–7064. doi: 10.1158/0008-5472.CAN-04-1038. [DOI] [PubMed] [Google Scholar]
- 12.Shirabe K, Shimada M, Kajiyama K, Hasegawa H, Gion T, Ikeda Y, Takenaka K, Sugimachi K. Expression of matrix metalloproteinase-9 in surgically resected intrahepatic cholangiocarcinoma. Surgery. 1999;126:842–846. doi: 10.1016/S0039-6060(99)70024-3. [DOI] [PubMed] [Google Scholar]
- 13.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 14.Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature. 1980;284:67–68. doi: 10.1038/284067a0. [DOI] [PubMed] [Google Scholar]
- 15.Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- 16.Sheu BC, Lien HC, Ho HN, Lin HH, Chow SN, Huang SC, Hsu SM. Increased expression and activation of gelatinolytic matrix metalloproteinases is associated with the progression and recurrence of human cervical cancer. Cancer Res. 2003;63:6537–6542. [PubMed] [Google Scholar]
- 17.Wang PH, Ko JL, Tsai HT, Yang SF, Han CP, Lin LY, Chen GD. Clinical significance of matrix metalloproteinase-2 in cancer of uterine cervix: A semiquantitative study of immunoreactivities using tissue array. Gynecol Oncol. 2008;108:533–542. doi: 10.1016/j.ygyno.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Gaiotto MA, Focchi J, Ribalta JL, Stavale JN, Baracat EC, Lima GR, da Silva Guerreiro ID. Comparative study of MMP-2 (matrix metalloproteinase 2) immune expression in normal uterine cervix, intraepithelial neoplasias and squamous cells cervical carcinoma. Am J Obstet Gynecol. 2004;190:1278–1282. doi: 10.1016/j.ajog.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Argüello-Ramírez J, Pérez-Cárdenas E, Delgado-Chávez R, Solorza-Luna G, Villa-Treviño S, Arenas-Huertero F. Matrix metalloproteinases-2, −a3 and −9 secreted by explants of benign and malignant lesions of the uterine cervix. Int J Gynecol Cancer. 2004;14:333–340. doi: 10.1111/j.1048-891x.2004.014218.x. [DOI] [PubMed] [Google Scholar]
- 20.Sato T, Sakai T, Noguchi Y, Takita M, Hirakawa S, Ito A. Tumor-stromal cell contact promotes invasion of human uterine cervical carcinoma cells by augmenting the expression and activation of stromal matrix metalloproteinases. Gynecol Oncol. 2004;92:47–56. doi: 10.1016/j.ygyno.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed N, Riley C, Oliva K, Barker G, Quinn MA, Rice GE. Expression and localization of alphavbeta6 integrin in extraplacental fetal membranes: Possible role in human parturition. Mol Hum Reprod. 2004;10:173–179. doi: 10.1093/molehr/gah025. [DOI] [PubMed] [Google Scholar]
- 22.Yang SF, Wang PH, Lin LY, Ko JL, Chen GD, Yang JS, Lee HS, Hsieh YS. A significant elevation of plasma level of matrix metalloproteinase-9 in patients with high-grade intraepithelial neoplasia and early squamous cell carcinoma of the uterine cervix. Reprod Sci. 2007;14:710–718. doi: 10.1177/1933719107307916. [DOI] [PubMed] [Google Scholar]
- 23.Baltazar-Rodriguez LM, Anaya-Ventura A, Andrade-Soto M, Monrroy-Guizar EA, Bautista-Lam JR, Jonguitud-Olguin G, Cepeda-Lopez FR, Centeno-Aguilar VA, Gonzalez-Hernandez NA, Soriano-Hernández AD, et al. Polymorphism in the matrix metalloproteinase-2 gene promoter is associated with cervical neoplasm risk in Mexican women. Biochem Genet. 2008;46:137–144. doi: 10.1007/s10528-007-9136-4. [DOI] [PubMed] [Google Scholar]
- 24.Rauvala M, Aglund K, Puistola U, Turpeenniemi-Hujanen T, Horvath G, Willén R, Stendahl U. Matrix metalloproteinases-2 and −9 in cervical cancer: Different roles in tumor progression. Int J Gynecol Cancer. 2006;16:1297–1302. doi: 10.1111/j.1525-1438.2006.00448.x. [DOI] [PubMed] [Google Scholar]
- 25.Nasr M, Ayyad SB, El-Lamie IK, Mikhail MY. Expression of matrix metalloproteinase-2 in preinvasive and invasive carcinoma of the uterine cervix. Eur J Gynaecol Oncol. 2005;26:199–202. [PubMed] [Google Scholar]
- 26.Yoshida H, Sumi T, Hyun Y, Nakagawa E, Hattori K, Yasui T, Morimura M, Honda K, Nakatani T, Ishiko O. Expression of survivin and matrix metalloproteinases in adenocarcinoma and squamous cell carcinoma of the uterine cervix. Oncol Rep. 2003;10:45–49. [PubMed] [Google Scholar]
- 27.Talvensaari-Mattila A, Turpeenniemi-Hujanen T, Puistola U. Matrix metalloproteinase 9 and relapse in patients with early stage squamous cervical carcinoma. Int J Gynaecol Obstet. 2005;91:75–76. doi: 10.1016/j.ijgo.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 28.Talvensaari-Mattila A, Turpeenniemi-Hujanen T. Matrix metalloproteinase 9 in the uterine cervix during tumor progression. Int J Gynaecol Obstet. 2006;92:83–84. doi: 10.1016/j.ijgo.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 29.Tellier E, Nègre-Salvayre A, Bocquet B, Itohara S, Hannun YA, Salvayre R, Augé N. Role for furin in tumor necrosis factor alpha-induced activation of the matrix metalloproteinase/sphingolipid mitogenic pathway. Mol Cell Biol. 2007;27:2997–3007. doi: 10.1128/MCB.01485-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corsi A, Xu T, Chen XD, Boyde A, Liang J, Mankani M, Sommer B, Iozzo RV, Eichstetter I, Robey PG, et al. Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res. 2002;17:1180–1189. doi: 10.1359/jbmr.2002.17.7.1180. [DOI] [PubMed] [Google Scholar]
- 31.Iozzo RV. The biology of the small leucine-rich proteoglycans. Functional network of interactive proteins. J Biol Chem. 1999;274:18843–18846. doi: 10.1074/jbc.274.27.18843. [DOI] [PubMed] [Google Scholar]
- 32.Keene DR, Antonio San JD, Mayne R, McQuillan DJ, Sarris G, Santoro SA, Iozzo RV. Decorin binds near the C terminus of type I collagen. J Biol Chem. 2000;275:21801–21804. doi: 10.1074/jbc.C000278200. [DOI] [PubMed] [Google Scholar]
- 33.Bidanset DJ, Guidry C, Rosenberg LC, Choi HU, Timpl R, Hook M. Binding of the proteoglycan decorin to collagen type VI. J Biol Chem. 1992;267:5250–5256. [PubMed] [Google Scholar]
- 34.Schonherr E, Hausser H, Beavan L, Kresse H. Decorin-type I collagen interaction. Presence of separate core protein-binding domains. J Biol Chem. 1995;270:8877–8883. doi: 10.1074/jbc.270.15.8877. [DOI] [PubMed] [Google Scholar]
- 35.Kinsella MG, Bressler SL, Wight TN. The regulated synthesis of versican, decorin, and biglycan: Extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr. 2004;14:203–234. doi: 10.1615/CritRevEukaryotGeneExpr.v14.i3.40. [DOI] [PubMed] [Google Scholar]
- 36.Nareyeck G, Seidler DG, Troyer D, Rauterberg J, Kresse H, Schönherr E. Differential interactions of decorin and decorin mutants with type I and type VI collagens. Eur J Biochem. 2004;271:3389–3398. doi: 10.1111/j.1432-1033.2004.04273.x. [DOI] [PubMed] [Google Scholar]
- 37.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Järveläinen H, Puolakkainen P, Pakkanen S, Brown EL, Höök M, Iozzo RV, Sage EH, Wight TN. A role for decorin in cutaneous wound healing and angiogenesis. Wound Repair Regen. 2006;14:443–452. doi: 10.1111/j.1743-6109.2006.00150.x. [DOI] [PubMed] [Google Scholar]
- 39.Iozzo RV, Chakrani F, Perrotti D, McQuillan DJ, Skorski T, Calabretta B, Eichstetter I. Cooperative action of germ-line mutations in decorin and p53 accelerates lymphoma tumorigenesis. Proc Natl Acad Sci USA. 1999;96:3092–3097. doi: 10.1073/pnas.96.6.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csordás G, Santra M, Reed CC, Eichstetter I, McQuillan DJ, Gross D, Nugent MA, Hajnoczky G, Iozzo RV. Sustained down-regulation of the epidermal growth factor receptor by decorin. A mechanism for controlling tumor growth in vivo. J Biol Chem. 2000;275:32879–32887. doi: 10.1074/jbc.M005609200. [DOI] [PubMed] [Google Scholar]
- 41.Zhu JX, Goldoni S, Bix G, Owens RT, McQuillan DJ, Reed CC, Iozzo RV. Decorin evokes protracted internalization and degradation of the epidermal growth factor receptor via caveolar endocytosis. J Biol Chem. 2005;280:32468–32479. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]
- 42.Seidler DG, Faiyaz-Ul-Haque M, Hansen U, Yip GW, Zaidi SH, Teebi AS, Kiesel L, Götte M. Defective glycosylation of decorin and biglycan, altered collagen structure, and abnormal phenotype of the skin fibroblasts of an Ehlers-Danlos syndrome patient carrying the novel Arg270Cys substitution in galactosyltransferase I (beta4GalT-7) J Mol Med (Berl) 2006;84:583–594. doi: 10.1007/s00109-006-0046-4. [DOI] [PubMed] [Google Scholar]
- 43.Seidler DG, Goldoni S, Agnew C, Cardi C, Thakur ML, Owens RT, McQuillan DJ, Iozzo RV. Decorin protein core inhibits in vivo cancer growth and metabolism by hindering epidermal growth factor receptor function and triggering apoptosis via caspase-3 activation. J Biol Chem. 2006;281:26408–26418. doi: 10.1074/jbc.M602853200. [DOI] [PubMed] [Google Scholar]
- 44.Koninger J, Giese T, di Mola FF, Wente MN, Esposito I, Bachem MG, Giese NA, Büchler MW, Friess H. Pancreatic tumor cells influence the composition of the extracellular matrix. Biochem Biophys Res Commun. 2004;322:943–949. doi: 10.1016/j.bbrc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Skandalis SS, Kletsas D, Kyriakopoulou D, Stavropoulos M, Theocharis DA. The greatly increased amounts of accumulated versican and decorin with specific post-translational modifications may be closely associated with the malignant phenotype of pancreatic cancer. Biochim Biophys Acta. 2006;1760:1217–1225. doi: 10.1016/j.bbagen.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Border WA, Noble NA, Yamamoto T, Harper JR, Yamaguchi YU, Pierschbacher MD, Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 47.Hildebrand A, Romaris M, Rasmussen LM, Heinegård D, Twardzik DR, Border WA, Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302:527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant DS, Yenisey C, Rose RW, Tootell M, Santra M, Iozzo RV. Decorin suppresses tumor cell-mediated angiogenesis. Oncogene. 2002;21:4765–4777. doi: 10.1038/sj.onc.1205595. [DOI] [PubMed] [Google Scholar]
- 49.Nash MA, Loercher AE, Freedman RS. In vitro growth inhibition of ovarian cancer cells by decorin: Synergism of action between decorin and carboplatin. Cancer Res. 1999;59:6192–6196. [PubMed] [Google Scholar]
- 50.Reed CC, Waterhouse A, Kirby S, Kay P, Owens RT, McQuillan DJ, Iozzo RV. Decorin prevents metastatic spreading of breast cancer. Oncogene. 2005;24:1104–1110. doi: 10.1038/sj.onc.1208329. [DOI] [PubMed] [Google Scholar]
- 51.Santra M, Skorski T, Calabretta B, Lattime EC, Iozzo RV. De novo decorin gene expression suppresses the malignant phenotype in human colon cancer cells. Proc Natl Acad Sci USA. 1995;92:7016–7020. doi: 10.1073/pnas.92.15.7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Köninger J, Giese NA, di Mola FF, Berberat P, Giese T, Esposito I, Bachem MG, Buchler MW, Friess H. Overexpressed decorin in pancreatic cancer: Potential tumor growth inhibition and attenuation of chemotherapeutic action. Clin Cancer Res. 2004;10:4776–4783. doi: 10.1158/1078-0432.CCR-1190-03. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed in the current study are available from the corresponding author on reasonable request.