Abstract
Ovarian cancer is the most aggressive type of gynecological cancer. The cause of the poor survival rate is the development of chemotherapy resistance to platinum-based therapies, including cisplatin. The present study aimed to investigate the mechanism of cancerous inhibitor of protein phosphatase 2A (CIP2A)-induced chemoresistance in ovarian cancer. The present study initially investigated the expression of CIP2A in the ovarian tumor tissue, cisplatin-sensitive SKOV-3 cell line, and cisplatin-resistant ovarian carcinoma SKOV-3CDDP/R cell line. In addition, CIP2A was knocked down using small interference RNA in ovarian cancer cells and the chemosensitivity of these cells was analyzed. The results demonstrated that CIP2A expression was significantly higher in patients with ovarian cancer and in the cisplatin-resistant ovarian carcinoma SKOV-3CDDP/R cell line at the mRNA and protein levels. The proliferation and chemosensitivity were decreased and enhanced, respectively, when CIP2A was knocked down. CIP2A silencing significantly promoted the apoptosis induced by cisplatin in SKOV-3CDDP/R cells, suggesting that CIP2A participated in the cisplatin resistance of ovarian cancer cells and that CIP2A silencing enhanced the apoptosis induced by cisplatin. CIP2A may be considered as a potential candidate for modulating cisplatin therapy in ovarian cancer.
Keywords: ovarian cancer, cancerous inhibitor of protein phosphatase 2A, cisplatin, proliferation, apoptosis, chemoresistance
Introduction
Ovarian cancer is the leading cause of cancer-associated mortality among all gynecological malignancies in China. Due to the fact that there are few specific or sensitive biomarkers for ovarian cancer monitoring, the majority of patients with ovarian cancer are diagnosed at late stages (1,2). The therapeutic guidelines for patients with ovarian cancer included aggressive surgical resection followed by adjuvant chemotherapy (3). Platinum-based chemotherapy has been the first-line chemotherapeutic treatment for patients with ovarian cancer. Despite recent improvements in the survival rate of patients with ovarian cancer, >80% of patients eventually relapse due in part to the acquisition of chemoresistance (4). Therefore, it is urgent to identify novel targets for the development of therapeutics for patients with ovarian cancer.
Several studies have attempted to uncovered the mechanisms of cisplatin resistance in various human carcinoma cell lines. Cancerous inhibitor of protein phosphatase 2A (CIP2A), a novel oncoprotein, is also known as KIAA1524 or p90 tumor-associated antigen (5,6). Overexpression of CIP2A contributed to cell proliferation and progression of malignancies (7,8). Previous studies have revealed that CIP2A enhanced the proliferation and invasion of ovarian cancer cells. A recent study reported that CIP2A has also been implicated in resistance to chemotherapy (9). However, it remains unclear whether CIP2A serves a critical role in cisplatin resistance in ovarian cancer. Therefore, we hypothesized that CIP2A is associated with chemoresistance in ovarian cancer. To the best of our knowledge, the present study was the first to demonstrate that CIP2A leads to cisplatin resistance in ovarian cancer and that inhibition of CIP2A may be a promising treatment for patients with ovarian cancer.
Materials and methods
Patients and specimens
Paraffin-embedded tissue blocks were collected from 36 patients with a median age of 56 years, age range 38–72 years) with epithelial ovarian cancer at the Affiliated Hospital of Jining Medical University between January 2008 and December 2014. Paired adjacent non-cancerous tissues were also taken from the distal resection margins (>5 cm). None of the patients had undergone chemotherapy, immunotherapy or radiotherapy prior to specimen resection. The clinicopathological characteristics of patients were recorded, including age, clinical stage and tumor differentiation. Additionally, half the samples available from 36 patients were snap-frozen in liquid nitrogen immediately following resection and were stored at −80°C until subsequent analysis. The present study was approved by the Local Institutional Review Board of the Affiliated Hospital of Jining Medical University, and written informed consent was obtained from all patients.
Cell lines
The cisplatin-resistant ovarian carcinoma SKOV-3CDDP/R cell line and the cisplatin-sensitive SKOV-3 cell line were purchased from the Cell Bank of the Chinese Academy of Sciences Institute (Shanghai, China). The two cell lines were cultured in RPMI-1640 medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA), supplemented with 10% (v/v) fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml penicillin and 100 g/ml streptomycin, and cells were maintained at 37°C in a humidified atmosphere containing 5% CO2 and 95% air. Cell culture medium was changed every 4 days depending on cell density. For routine passage, when cells reached 85–90% confluency, they were split at a ratio of 1:4.
Immunohistochemistry (IHC)
For IHC, 4 µm, 10% formalin-fixed (4°C for ~24 h), paraffin-embedded tissue sections were deparaffinized with xylene, and rehydrated through a series of decreasing concentrations of ethanol (100, 95, 90, 80 and 70%). A high-temperature antigen retrieval method was performed using a citrate buffer solution (Maixin Bio, Fujian, China), and the slides were immersed in 100 µl 3% hydrogen peroxide for 10 min at 37°C to block endogenous peroxidase activity. Subsequent to washing with PBS, the sections were incubated with 5% bovine serum albumin (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at room temperature for 30 min, followed by incubation with anti-CIP2A monoclonal antibody (dilution, 1:400; cat. no. NB110-59722; Novus Biologicals, LLC, Littleton, CO, USA) at 4°C overnight. Following washing with PBS, the sections were incubated with a horseradish peroxidase-conjugated rabbit anti-mouse IgG secondary antibody (1:5,000; cat. no. ab6728; Abcam) for 30 min at 37°C.
The slides were stained with diaminobenzidine (as the color reagent) for 5 min at 37°C and hematoxylin (as a counterstain for the nuclei) for 2 min at 37°C. PBS was used as a negative control for the staining reactions. All slides were dehydrated in 70% ethanol (5 min), 75% ethanol (5 min), 80% ethanol (5 min), 90% ethanol (5 min), 95% ethanol (5 min) and 100% ethanol (10 min). Finally, all sections were mounted with neutral gum. IHC scores (between 0 and 8) were calculated by adding the intensity score (0–4) and the percentage score (0–4), with a maximum score of 8. A score of ≥4 was classified as high expression of CIP2A, while a score of ≤3 was classified as low expression of CIP2A. The Olympus CKX41SF (Olympus Corporation, Tokyo, Japan) light microscope was used at a magnification of ×200.
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from frozen tissues and cell lines using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) for 30 min at room temperature. Complementary DNA (cDNA) was synthesized using a SuperScript VILO cDNA Synthesis kit (Invitrogen; Thermo Fisher Scientific, Inc.). The reaction was incubated in a MyCycler Thermocycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) for 10 min at 25°C, 60 min at 42°C and 5 min at 85°C. cDNA samples were stored at −20°C prior to RT-qPCR amplification. RT-qPCR was performed using the SYBRGreen PCR master mix (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in a total volume of 20 µl on a 7900HT fast real-time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) as follows: 95°C for 30 sec, 40 cycles at 95°C for 5 sec, 60°C for 30 sec. A dissociation step was performed to generate a melting curve to confirm the specificity of the amplification. β-actin was used as the reference gene. The relative levels of gene expression were represented as ΔCq=Cq gene-Cq reference, and the fold change of gene expression was calculated using the 2−ΔΔCq method (10). Experiments were repeated in triplicate. The primer sequences used were as follows: CIP2A forward, 5′-ATACTTCAGGACCCACGTTTGAT-3′, CIP2A reverse, 5′-TCTCCAAGTACTAAAGCAGGAAAATCT-3′; β-actin forward, 5′-ATAGCACAGCCTGGATAGCAACGTAC-3′, β-actin reverse, 5′-CACCTTCTACAATGAGCTGCGTGTG-3′.
Western blot analysis
The SKOV-3 and SKOV-3CDDP/R ovarian carcinoma cells were quickly washed twice with pre-cooled PBS quickly and suspended in radioimmunoprecipitation assay buffer (Beyotime Institute of Biotechnology, Haimen, China). Proteins were quantified using a Bradford assay. Subsequently, 50 mg total protein extracts were fractionated by 10% SDS-PAGE, prior to being transferred onto polyvinylidene difluoride membranes (GE Healthcare Life Sciences, Little Chalfont, UK). The membranes were blocked with 5% milk at room temperature for 2 h. The membranes were subsequently incubated with the following primary antibodies: Mouse anti-CIP2A (dilution, 1:2,000; No: NB110-59722; Novus Biologicals, LLC, Littleton, CO, USA), mouse anti-β-actin (dilution, 1:2,000, cat no. ab8226; Abcam), rabbit anti-human p-Akt (Ser 473; dilution, 1:200; cat no. sc-7985-R, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and rabbit anti-human Akt antibody (dilution, 1:1,000; cat no: 4691, Cell Signaling Technology, Inc.) for 12 h at 4°C. Membranes were then washed three times with TBS-T and were incubated with horseradish peroxidase-conjugated secondary antibodies (goat anti-rabbit IgG, 1:2,000; cat. no. sc-2030, Santa Cruz Biotechnology, Inc., Dallas, TX, USA; goat anti-mouse IgG-B, 1:2,000; cat. no. sc-2039, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature. Membranes were then washed again three times for 10 min each time with TBS-T. Target protein bands were visualized using the enhanced chemiluminescence method (Pierce; Thermo Fisher Scientific, Inc.). The intensity of the bands was quantified using the Tanon GIS system (Tanon 2500R; Quantity One software; Tanon Science and Technology Co., Ltd., Shanghai, China) and the data were normalized to the β-actin loading controls. All western immunoblot analyses were performed three times.
Small interfering RNA (siRNA) and plasmid transfection and co-transfection
siRNA duplexes were prepared by Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The sequences of the siRNAs were as follows: CIP2A siRNA (siCIP2A)1, 5′-CUGUGGUUGUGUUUGCACUTT-3′; siCIP2A2, 5′-ACCAUUGAUAUCCUUAGAATT-3′; and negative control (NC) siRNA, 5′-UAACAAUGAGAGCACGGCTT-3′. Transfection or co-transfection with siRNA and plasmids were performed using Lipofectamine reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol.
Cell viability assay
Cell viability was measured using a Cell Counting kit-8 assay (CCK8; Dojindo Molecular Technologies, Inc., Shanghai, China). In brief, cells were plated at a density of 5×103 cells/well onto 96-well plates and were transfected with the control and siCIP2A for 48 h. Following 48 h incubation at 37°C in a humidified atmosphere containing 5% CO2, the samples were incubated for another 2 h with CCK8 reagent. The absorbance was measured at 450 nm using an FLx800 Fluorescence Microplate Reader (Biotek Instruments, Inc., Winooski, VT, USA).
Annexin V assay of apoptosis
At 48 h after siRNA transfection, the cells were exposed to cisplatin (1.5 mg/ml) for 24 h. Next, cells were harvested and washed twice with ice-cold PBS, suspended in Annexin V-binding buffer (BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA), and propidium iodide and Annexin V-FITC (BD Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA) was added. Following incubation for 20 min at room temperature in the dark, fluorescence was measured on a flow cytometer and analyzed with FlowJo 7.6 (BD Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Data analyses were performed using SPSS statistical package 15.0 (SPSS Inc., Chicago, IL, USA). Patient characteristics are expressed as the mean ± standard deviation for continuous variables, and as the count and percentage for discrete variables. All experiments were performed in triplicate. Comparisons between two groups were performed using Student's t-test, while comparisons among ≥3 groups were performed using one-way analysis of variance and post-hoc Student-Newman-Keuls test. P<0.05 was considered to indicate a statistically significant difference.
Results
CIP2A is upregulated in ovarian cancer tissues
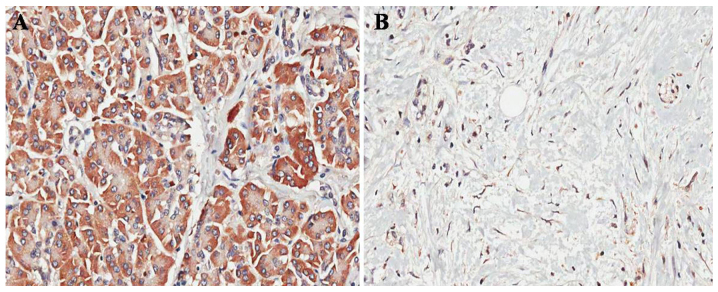
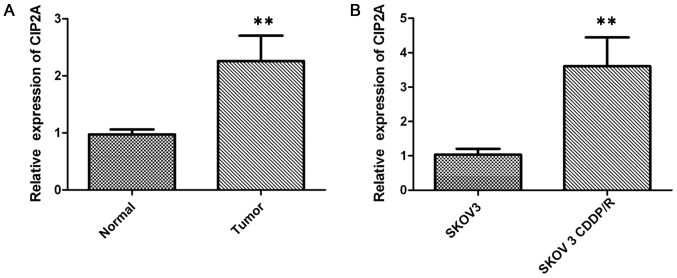
In order to investigate the role of CIP2A in ovarian cancer, the expression of CIP2A in 36 ovarian cancer tissue specimens was examined by IHC. Positive staining for CIP2A was observed in 83.33% (30/36) of the ovarian cancer tissues vs. 13.89% (5/36) of the paired adjacent non-cancerous tissues (P<0.001; Fig. 1A and B). In addition, CIP2A mRNA expression was also examined by RT-qPCR in cancer tissues. The CIP2A mRNA expression was significantly higher in ovarian cancer tissues than in paired adjacent non-cancerous tissues (P<0.01; Fig. 2A).
Figure 1.
CIP2A is upregulated in ovarian cancer tissues compared with the corresponding adjacent non-cancerous ovarian tissues. (A) Representative CIP2A immunostained section of the ovarian cancer tissue (magnification, ×400). (B) Representative CIP2A immunostained section of the adjacent non-cancerous ovarian tissue. CIP2A, cancerous inhibitor of protein phosphatase 2A (magnification, ×400).
Figure 2.
CIP2A is upregulated in ovarian cancer tissues compared with the corresponding adjacent non-cancerous ovarian tissues. (A) The relative CIP2A expression levels were determined by RT-qPCR in ovarian cancer tissues and adjacent non-cancerous ovarian tissues. (B) The relative CIP2A expression levels were determined by RT-qPCR in chemosensitive SKOV-3 cells and chemoresistant SKOV-3CDDP/R cells. **P<0.01. CIP2A, cancerous inhibitor of protein phosphatase 2A; RT-qPCR, reverse transcription-quantitative polymerase chain reaction.
Cisplatin-resistant ovarian cancer cells exhibited higher CIP2A expression
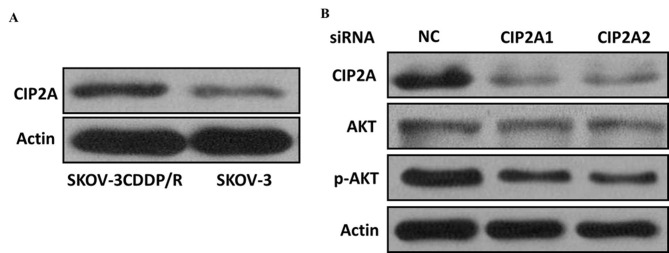
The expression level of CIP2A was examined by RT-qPCR to study its regulatory role in chemotherapy resistance in SKOV-3 (cisplatin-sensitive) and SKOV-3CDDP/R (cisplatin-resistant) ovarian carcinoma cells, with the IC50 value to cisplatin of the latter cell line being significantly higher than that of the SKOV-3 cells. The results demonstrated that the expression of CIP2A in the SKOV-3CDDP/R cells was nearly 3-fold higher than that in the SKOV-3 cells (P<0.001; Fig. 2B). Western blot analysis further confirmed that the CIP2A protein expression was elevated in cisplatin-resistant cells but decreased in cisplatin-sensitive cells (Fig. 3A), suggesting that CIP2A may serve an important role in inducing the cisplatin resistance of ovarian cancer cells.
Figure 3.
Cisplatin-resistant ovarian cancer cells exhibit increased CIP2A expression. (A) Relative expression levels of CIP2A were determined by western blot analysis in chemosensitive SKOV-3 and chemoresistant SKOV-3CDDP/R cells. (B) The knockdown effect of CIP2A siRNA was also manifested at the protein level, as determined by western blot analysis in chemoresistant SKOV-3CDDP/R cells. CIP2A, cancerous inhibitor of protein phosphatase 2A; siRNA, small interfering RNA.
CIP2A induces cisplatin resistance and proliferation in vitro
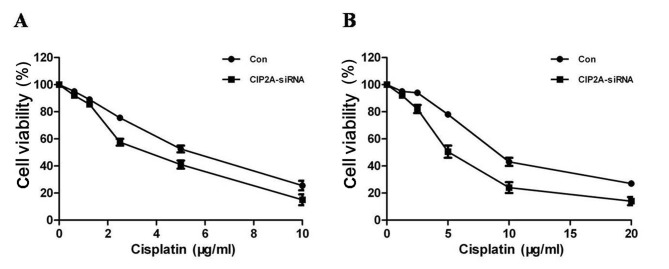
In order to further investigate the role of CIP2A in the cisplatin resistance of ovarian cancer cells, two siRNA constructs, siCIP2A1 and siCIP2A2, were utilized to interfere with CIP2A expression in ovarian cancer cells. SKOV-3 and SKOV-3 CDDP/R cells were transiently transfected with control and siCIP2A for 48 h. The two different CIP2A siRNAs acquired a knockdown efficiency of ~50%, which was confirmed by RT-qPCR. Furthermore, the CIP2A protein was inhibited using siCIP2A in SKOV-3 CDDP/R cells (Fig. 3B). The CCK8 assay revealed that CIP2A silencing decreased SKOV-3 cell proliferation (Fig. 4A), suggesting that CIP2A may participate in the development of ovarian cancer. In addition, SKOV-3 CDDP/R cells were more sensitive to cisplatin and exhibited a decreased proliferation rate following CIP2A suppression (Fig. 4B).
Figure 4.
CIP2A induces cisplatin resistance and proliferation (A) Knockdown of CIP2A in chemosensitive SKOV-3 cells decreased cell viability. (B) Knockdown of CIP2A in chemoresistant SKOV-3CDDP/R cells sensitizes the response of these cells to cisplatin and also depresses cell viability. CIP2A, cancerous inhibitor of protein phosphatase 2A.
Depletion of CIP2A induces apoptosis
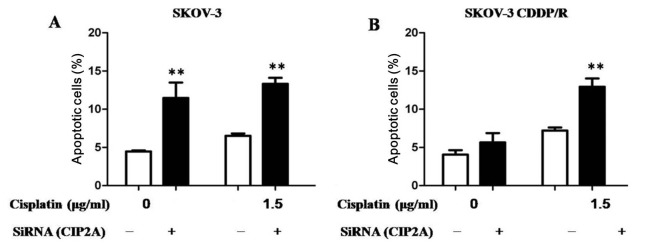
The primary antitumor mechanism for cisplatin results from the ability of cisplatin to induce apoptosis. Subsequently, the CIP2A-mediated chemosensitivity of ovarian cancer cells was determined using an apoptosis assay. Regardless of whether the cells were transfected with CIP2A or the NC siRNA, cisplatin was able to significantly induce the apoptosis of ovarian cancer SKOV-3 cells, indicating that depletion of CIP2A has no additive effects to cisplatin in SKOV-3 cells (Fig. 5A). Notably, it was revealed that cisplatin induced a higher proportion of apoptotic SKOV-3CDDP/R cells following CIP2A knockdown compared with transfection with NC siRNA, suggesting that CIP2A silencing may enhance cisplatin-mediated cell apoptosis (Fig. 5B).
Figure 5.
Depletion of CIP2A induces apoptosis. (A) Cisplatin significantly potentiated apoptosis in chemosensitive SKOV-3 cells transfected with either CIP2A or control siRNA. (B) CIP2A silencing significantly potentiated the apoptosis induced by cisplatin in chemoresistant SKOV-3CDDP/R cells with CIP2A knockdown compared with chemoresistant SKOV-3CDDP/R cells transfected with control siRNA. **P<0.01 CIP2A, cancerous inhibitor of protein phosphatase 2A; siRNA, small interfering RNA.
Discussion
Ovarian cancer is one of the leading causes of mortality of all gynecological tumors worldwide (11). The development of chemoresistance is a major challenge for patients with ovarian cancer (12). Although certain patients with ovarian cancer exhibit favorable responses to the initial chemotherapy treatment, the majority of them eventually succumb to chemoresistance. In those patients exhibiting chemoresistance, the 5-year overall survival rate is only 31% (13). Therefore, novel approaches to restoring the chemotherapy sensitivity of ovarian cancer are urgently required.
Recent studies have indicated that elevated CIP2A expression in numerous types of cancer is associated with a poor prognosis (14–18); however, the clinical significance of CIP2A in ovarian cancer, including chemoresistance, has yet to be reported. To begin with, the expression levels of CIP2A in ovarian cancer tissues and the cisplatin-resistant ovarian cancer cell line were analyzed, in order to determine the influence of CIP2A in the drug resistance of patients with ovarian cancer. The results of the present study revealed that the mRNA and protein expression of CIP2A was significantly upregulated in ovarian cancer tissues compared with that in the paired adjacent non-cancerous tissues. In addition, it was revealed that CIP2A expression was significantly higher in the cisplatin-resistant ovarian cancer SKOV-3CDDP/R cell line, compared with that in the cisplatin-sensitive ovarian cancer SKOV-3 cell line. Using siCIP2A, the effect of CIP2A knockdown in SKOV-3CDDP/R and SKOV-3 cells was analyzed. Transient CIP2A depletion was able to suppress ovarian cancer cell proliferation while enhancing the sensitivity of these cells to cisplatin suggesting that CIP2A is involved in ovarian carcinogenesis and progression. In cisplatin resistant cells, CIP2A inhibition significantly sensitized cancer cells to cisplatin compared with control cells, suggesting that directly targeting CIP2A may be a promising approach to increase the sensitivity of ovarian cancer cells to cisplatin.
Cisplatin-induced cancer cell apoptosis has been previously studied in detail (19). The decrease in CIP2A expression, as well as the inactivation of the Akt pathway, may attenuate the proliferation and induce the apoptosis of numerous types of cancer cells (20). However, whether or not CIP2A is a key protein that affects cisplatin-induced apoptosis remains unknown. The results of the present study demonstrated that, compared with NC siRNA, suppression of CIP2A contributed to a significant increase in the apoptosis of SKOV-3 cells when treated with cisplatin (Fig. 3). Notably, knockdown of CIP2A in the cisplatin resistant SKOV-3 CDDP/R cells promoted the apoptotic cell death induced by cisplatin and decreased the expression of phosphorylated Akt. Therefore, appropriate combination of cisplatin and CIP2A depletion would be a potential strategy for the treatment of ovarian cancer.
In conclusion, cisplatin resistance is a complicated molecular process. The results of the present study revealed that CIP2A is highly expressed in ovarian cancer tissues and cisplatin-resistant cells. CIP2A knockdown may increase the chemotherapeutic sensitivity of ovarian cancer cells to cisplatin. Therefore, CIP2A may be a potential therapeutic target for improving cisplatin sensitivity in ovarian cancer. Future clinical trials should be designed to evaluate the usefulness of CIP2A inhibitors in cisplatin-based chemotherapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data that support the findings of the present study are available from JMU but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of JMU.
Authors' contributions
HZ conceived and designed the experiments. WL and HZ performed the experiments. LY and YW performed the proliferative ability analysis. WL and HZ performed the cell migration assay. WL and HZ performed the cell cycle analysis and statistical analysis. HZ provided oversight for all aspects of the study. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Local Institutional Review Board of the Affiliated Hospital of Jining Medical University, and written informed consent was obtained from all patients.
Patient consent for publication
The patients provided written informed consent for the publication of the present study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Vaughan S, Coward JI, Bast RC, Jr, Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, et al. Rethinking ovarian cancer: Recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capoluongo E, Ellison G, López-Guerrero JA, Penault-Llorca F, Ligtenberg MJL, Banerjee S, Singer C, Friedman E, Markiefka B, Schirmacher P, et al. Guidance statement On BRCA1/2 tumor testing in ovarian cancer patients. Semin Oncol. 2017;44:187–197. doi: 10.1053/j.seminoncol.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Vargas-Hernández VM, Moreno-Eutimio MA, Acosta-Altamirano G, Vargas-Aguilar VM. Management of recurrent epithelial ovarian cancer. Gland Surg. 2014;3:198–202. doi: 10.3978/j.issn.2227-684X.2013.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W, Ge Z, Liu C, Liu Z, Björkholm M, Jia J, Xu D. CIP2A is overexpressed in gastric cancer and its depletion leads to impaired clonogenicity, senescence, or differentiation of tumor cells. Clin Cancer Res. 2008;14:3722–3728. doi: 10.1158/1078-0432.CCR-07-4137. [DOI] [PubMed] [Google Scholar]
- 6.Junttila MR, Puustinen P, Niemelä M, Ahola R, Arnold H, Böttzauw T, Ala-aho R, Nielsen C, Ivaska J, Taya Y, et al. CIP2A inhibits PP2A in human malignancies. Cell. 2007;130:51–62. doi: 10.1016/j.cell.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 7.Khanna A, Böckelman C, Hemmes A, Junttila MR, Wiksten JP, Lundin M, Junnila S, Murphy DJ, Evan GI, Haglund C, et al. MYC-dependent regulation and prognostic role of CIP2A in gastric cancer. J Natl Cancer Inst. 2009;101:793–805. doi: 10.1093/jnci/djp103. [DOI] [PubMed] [Google Scholar]
- 8.Côme C, Laine A, Chanrion M, Edgren H, Mattila E, Liu X, Jonkers J, Ivaska J, Isola J, Darbon JM, et al. CIP2A is associated with human breast cancer aggressivity. Clin Cancer Res. 2009;15:5092–5100. doi: 10.1158/1078-0432.CCR-08-3283. [DOI] [PubMed] [Google Scholar]
- 9.Choi YA, Park JS, Park MY, Oh KS, Lee MS, Lim JS, Kim KI, Kim KY, Kwon J, Yoon DY, et al. Increase in CIP2A expression is associated with doxorubicin resistance. FEBS Lett. 2011;585:755–760. doi: 10.1016/j.febslet.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Yin X, Zhang N, Di W. Regulation of LC3-dependent protective autophagy in ovarian cancer cells by protein phosphatase 2A. Int J Gynecol Cancer. 2013;23:630–641. doi: 10.1097/IGC.0b013e3182892cee. [DOI] [PubMed] [Google Scholar]
- 12.Miller DS, Blessing JA, Krasner CN, Mannel RS, Hanjani P, Pearl ML, Waggoner SE, Boardman CH. Phase II evaluation of pemetrexed in the treatment of recurrent or persistent platinum-resistant ovarian or primary peritoneal carcinoma: A study of the gynecologic oncology group. J Clin Oncol. 2009;27:2686–2691. doi: 10.1200/JCO.2008.19.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burger RA. A new model of ovarian carcinogenesis may influence early detection strategies. Am J Obstet Gynecol. 2008;198:349–350. doi: 10.1016/j.ajog.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Wang M, Zhang X, Wang Q, Qi M, Hu J, Zhou Z, Zhang C, Zhang W, Zhao W, Wang X. CIP2A is associated with multidrug resistance in cervical adenocarcinoma by a P-glycoprotein pathway. Tumour Biol. 2016;37:2673–2682. doi: 10.1007/s13277-015-4032-8. [DOI] [PubMed] [Google Scholar]
- 15.Chen KF, Liu CY, Lin YC, Yu HC, Liu TH, Hou DR, Chen PJ, Cheng AL. CIP2A mediates effects of bortezomib on phospho-Akt and apoptosis in hepatocellular carcinoma cells. Oncogene. 2010;29:6257–6266. doi: 10.1038/onc.2010.357. [DOI] [PubMed] [Google Scholar]
- 16.Chen KF, Pao KC, Su JC, Chou YC, Liu CY, Chen HJ, Huang JW, Kim I, Shiau CW. Development of erlotinib derivatives as CIP2A-ablating agents independent of EGFR activity. Bioorg Med Chem. 2012;20:6144–6153. doi: 10.1016/j.bmc.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 17.Tseng LM, Liu CY, Chang KC, Chu PY, Shiau CW, Chen KF. CIP2A is a target of bortezomib in human triple negative breast cancer cells. Breast Cancer Res. 2012;14:R68. doi: 10.1186/bcr3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu HC, Hou DR, Liu CY, Lin CS, Shiau CW, Cheng AL, Chen KF. Cancerous inhibitor of protein phosphatase 2A mediates bortezomib-induced autophagy in hepatocellular carcinoma independent of proteasome. PLoS One. 2012;8:e55705. doi: 10.1371/journal.pone.0055705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chattopadhyay S, Machado-Pinilla R, Manguan-Garcı'a C, Belda-Iniesta C, Moratilla C, Cejas P, Fresno-Vara JA, de Castro-Carpeño J, Casado E, Nistal M, et al. MKP1/CL100 controls tumor growth and sensitivity to cisplatin in non-small-cell lung cancer. Oncogene. 2006;25:3335–3345. doi: 10.1038/sj.onc.1209364. [DOI] [PubMed] [Google Scholar]
- 20.Ma L, Wen ZS, Liu Z, Hu Z, Ma J, Chen XQ, Liu YQ, Pu JX, Xiao WL, Sun HD, Zhou GB. Overexpression and small molecule-triggered downregulation of CIP2A in lung cancer. PLoS One. 2011;6:e20159. doi: 10.1371/journal.pone.0020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of the present study are available from JMU but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of JMU.