Abstract
Understanding the regulatory mechanisms of intestinal morphology and function is essential for improving postweaning growth in pigs. The objective of this study was to identify the relationships of enterocyte proliferation with intestinal villus height, crypt depth, and nutrient digestibility in piglets. Sixty-four 21-d-old weaned piglets were used. Gastrointestinal cell proliferation was evaluated via Ki-67 immunohistochemistry. Villus height and crypt depth were measured using hematoxylin and eosin (H&E)-stained sections. The apparent total tract digestibility (ATTD) of CP and GE was determined by chemical analysis. The activities of lactase and sucrase were determined with commercial kits. Western blot was carried out to assess the expression of nutrient transporters. The number of Ki-67 positive cells was associated with villus height (r = 0.548, P < 0.001) and crypt depth (r = 0.759, P < 0.001) in the jejunum. The number of Ki-67 positive cells was also associated with the ATTD of CP (r = 0.715, P = 0.001). Furthermore, a positive relationship between Ki-67 positive cell populations and lactase activity (r = 0.559, P < 0.001) was observed. Additionally, the number of Ki-67 positive cells was associated with the protein expression levels of nutrient transporters PEPT1 (r = 0.511, P = 0.030) and SGLT1 (r = 0.601, P = 0.014). Weak relationships were found between Ki-67 positive cell numbers and the ATTD of GE (r = 0.401, P = 0.099) and the activity of sucrase (r = 0.313, P = 0.087). In conclusion, enterocyte proliferation was positively associated with intestinal villus height, crypt depth, and nutrient digestibility in weaning piglets. Our findings suggested that intestinal morphology and function can be improved by regulating epithelial cell proliferation in piglets.
Keywords: enterocytes, intestine, morphology, nutrient digestibility, proliferation
INTRODUCTION
In swine production, the early weaning (21 to 28 d) is extensively applied in an effort to shorten the breeding period and enhance production efficiency (Zhong et al., 2016). However, weaning stresses induce gut architectural and functional alterations, which is characterized by shortened villus length, disturbed absorptive capacity, and decreased enzymatic activities, and thus result in enteric diseases and subsequent morbidity and mortality (Campbell et al., 2013; Yang et al., 2016). Understanding the regulatory mechanisms behind intestinal morphology and function is therefore important for improving gut development and piglet growth after weaning.
The small intestine digests and absorbs nutrients. Its integrity depends on a tightly regulated balance between proliferating and differentiating enterocytes (Mah et al., 2014). A previous study showed that enterocyte proliferation rates occurred markedly modification with the change in morphology of the small intestine (Montagne et al., 2007; Wang et al., 2016). Recent researches have also reported that they were strongly affected by various nutrients in piglets feed (Jonecova et al., 2015; Wang et al., 2015; Bilićšobot et al., 2016). However, the relationship of epithelial cell proliferation with intestinal morphology and function (e.g., digestion and absorption) has not yet been clearly elucidated. To evaluate this relationship, we performed the correlation analysis of enterocyte proliferation with intestinal villus height, crypt depth, nutrient digestibility, activities of digestive enzymes, and the expression of nutrient transporters in weaning piglets.
MATERIALS AND METHODS
Animals
The experimental design and procedures were reviewed and approved by the Animal Care and Use Committee of Hunan Normal University, Changsha City, Hunan, China. The animals in this study were Duroc × Landrace × Yorkshire piglets and weaned at 21 d of age. Sixty-four piglets were used to measure intestinal cell proliferation, villus height, and crypt depth. Among them 42 were selected to evaluate the activities of digestive enzymes and 18 were used for the analysis of nutrient digestibility and the protein synthesis of nutrient transporters. All pigs were harvested as they were taken off the sow. After a transport period of 90 min, they were fed a solid diet that contained 37.66% corn, 20% extruded corn, 8% soybean meal (43% CP), 7% concentrated soy protein, 10% whey; 5% fish meal (63% CP), 4.5% plasma protein powder, 2% glucose, 2% soybean oil, and 4.34% premix. All of the animals had free access to feed and water throughout the experimental period.
Sampling
After euthanizing piglets, the jejunum was removed and immediately irrigated with physiological saline to remove intestinal contents. Approximately 2-cm intestinal segment was fixed in 4% neutral-buffered formalin and stored at 4 °C before morphology and immunohistochemistry measurements. The jejunal mucosal layer was scraped using a glass slide, immediately frozen in liquid nitrogen, and stored at −80 °C for analyzing enzyme activity and protein synthesis. During the final 3 d of the experiment, fecal samples were collected for evaluating nutrient digestibility.
Immunohistochemistry for Ki-67
Slides were dewaxed and rehydrated, endogenous peroxidases were inhibited with 3% hydrogen peroxide (H2O2) in methanol for 10 min. Antigen retrieval was performed by boiling twice in a sodium citrate buffer (0.01 M, pH 6.0). A 5% bovine serum albumin (BSA; Boster Biological Technology Co. Ltd, Wuhan, China) was used in a 1:10 dilution during 30 min incubation at 37 °C to block nonspecific binding. After overnight incubation with Ki-67 antibody (Abcam, ab15580; 1:600 dilution), sections were treated with a goat anti-rabbit IgG secondary antibody (ZSGB-BIO, Beijing, China) for 45 min at 37 °C. Every step, except the blocking step, was followed by three 5-min washes in PBS. Positive cells were visualized with diaminobenzidine (DAB) Kit (ZSGB-BIO, Beijing, China). Fifteen microscopic fields per sample were captured using light microscope under 20× magnification (Leica DM3000, Leica Microsystems, Wetzlar, Germany). The number of Ki-67 positive cells at per field was counted using Image-Pro Plus 6.0 software (Zorn et al., 2011).
Morphological Analysis
The formalin-fixed intestinal segments were dehydrated in graded ethanol and embedded in paraffin wax. Sections were cut into 5-μm thickness on a microtome (RM2235; Leica, Germany). They were subsequently placed on adhesion glass slides (CITOGLAS, Jiangsu, China). Sections were stained with hematoxylin and eosin for examination under a light microscope (DM3000; Leica). Measurements were blindly made using Image-Pro Plus 6.0 software (Media Cybernetics, San Diego, CA, USA) that allowed us to measure villus height and crypt depth. Each value is the average from 20 well-oriented, complete villus-crypt structures.
Chemical Analyses and Calculation of Apparent Total Tract Digestibility
All chemical analyses were conducted in duplicate with DM which was obtained by oven-drying at 105 °C. The GE was measured using benzoic acid as the calibration standard in an isothermal auto-calorimeter (5E-AC8018, China). Nitrogen was determined with AA3 flow injection analyzer (Seal, Germany), with CP content calculated using the factor of 6.25 (Hendriks et al., 2013). The concentrations of Chromium (Cr) in feed and fecal samples were analyzed by using flame atomic absorption spectrometer (novAA350, Jena, Germany). The apparent total tract digestibility (ATTD) of components was calculated using the following equation (Jang et al., 2017): ATTD, % = 100 − [100 × (concentration of Cr2O3 in feed × concentration of component in feces/concentration of Cr2O3 in feces × concentration of component in feed)].
Analyses of Digestive Enzyme Activities
The jejunal mucosa samples were pulverized in liquid nitrogen and mixed with a 0.9% NaCl solution, then centrifuged at 3,000 × g for 10 min at 4 °C. Using the resulting supernatant, the activities of lactase and sucrase were determined with commercial kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). All samples and standards were run in duplicate. The protein level of the supernatant fraction for each sample was measured using a bicinchoninic acid assay kit (BCA; Beyotime Biotechnology, Shanghai, China).
Western Blot Analysis
Frozen samples of jejunal mucosa were powdered under liquid nitrogen and lysed in RIPA buffer with the protease inhibitor PMSF (Beyotime Biotechnology, Shanghai, China). This was followed by centrifugation at 12,000 × g for 10 min at 4 °C. The supernatant fluids were used for western blot analysis as previously described (Yang et al., 2016). Briefly, the denatured proteins were separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), and then transferred to polyvinylidene fluoride (PVDF) membranes at 200 mA for 30 min. The membranes were blocked with 5% nonfat milk in tris-buffered saline (TBS) mixed with 0.5% Tween-20 (TBST) at room temperature for 2 h and then incubated with antibody β-actin (Santa Cruz Biotechnology, SC-47778), sodium-glucose linked transporter 1 (SGLT1; Santa Cruz Biotechnology, SC-98974) or peptide transporter 1 (PEPT1; Santa Cruz Biotechnology, SC-19917) at 4 °C overnight. After being washed 3 times with TBST, the membranes were incubated with a secondary antibody (1:2,000 dilution) at room temperature for 2 h. Finally, the membranes were washed with TBST, and chemiluminescence was used to visualize the protein bands. Densitometric analyses of band intensities were performed with Gel-pro Analyzer software (Media Cybernetics, Inc., San Diego, CA, USA). The density of each band, which represented the abundance of protein expression, was expressed relative to the density of the corresponding β-actin band.
Statistical Analysis
All statistical analyses were performed using SPSS software (version 22.0; IBM Corp., Chicago, IL, USA). The relationship between proliferating cell numbers and parameters related to gut morphology and nutrient digestibility was tested with Pearson correlation coefficient. P-value of less than 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
The Relationship Between Enterocyte Proliferation and Intestinal Morphology
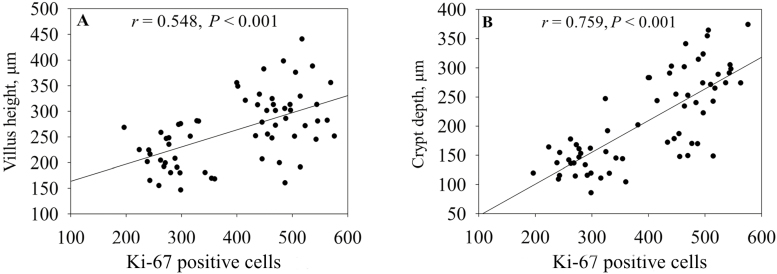
Protein Ki-67 is exclusively expressed in the nuclei of all proliferating cells from phase G1 to phase M, thereby served as a reliable marker of cell proliferation (Conto et al., 2010). The proliferation of intestinal cells was evaluated by counting Ki-67 positive cells. The same samples were used to measure villus height and crypt depth of small intestine. We found that there was a positive relationship between Ki-67 positive cell numbers and villus height (r = 0.548, P < 0.001; Fig. 1A). This provides an evidence for the finding of Jung et al., which suggested that the damaged villus in piglets infected with porcine epidemic diarrhea virus was recovered via stimulating proliferation of intestinal epithelial cells (Jung et al., 2008). In addition, the number of Ki-67 positive cells was associated with crypt depth (r = 0.759, P < 0.001; Fig. 1B) in this study. Our results confirmed the supposition that epithelial cell proliferation may positively influence the microscopic architecture of the intestine (Cheung et al., 2009).
Figure 1.
The relationship of Ki-67 positive cells with villus height (A) and crypt depth (B) in the small intestine of weaning piglets. The r is the correlation coefficient.
The Relationship Between Enterocyte Proliferation and Gut Digestive and Absorptive Function
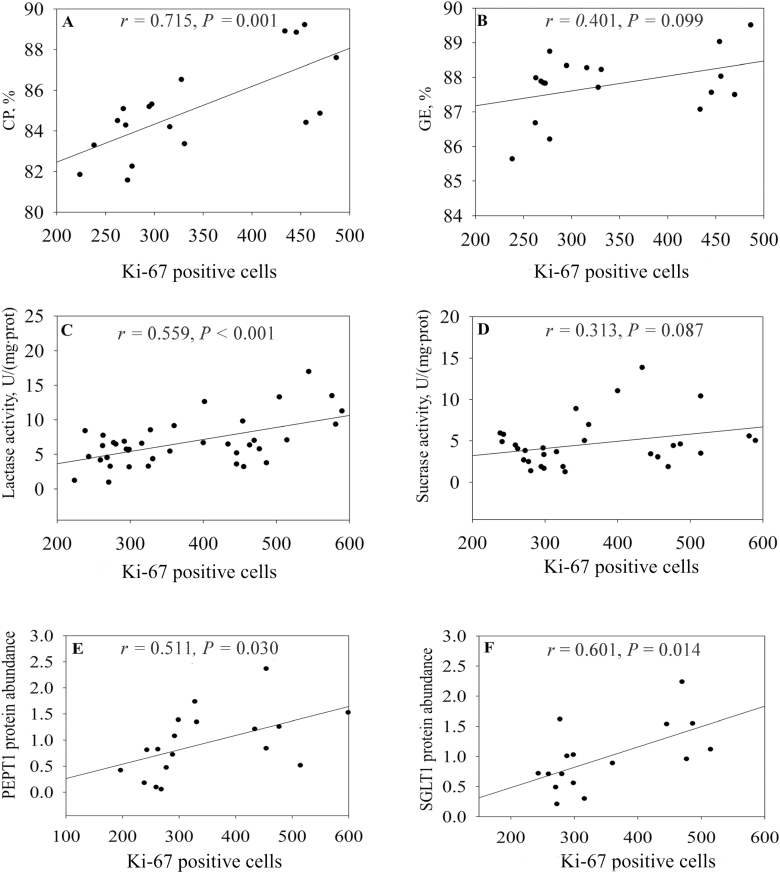
In this study, a positive relationship was observed between the number of Ki-67 positive cells and the ATTD of CP (r = 0.715, P = 0.001; Fig. 2A), although this number had a weak association with ATTD of GE (r = 0.401, P = 0.099; Fig. 2B). Enhanced digestibility involved in nutrient digestion and intake (Grenier and Applegate, 2013). Therefore, our finding indicated proliferation of epithelial cells may have a direct impact on digestive and absorptive function of the small intestine. Firstly, we examined the relationship between enterocytes proliferation and digestive enzymes activities and found that the number of Ki-67 positive cells was related with lactase activity (r = 0.559, P < 0.001; Fig. 2C). It is likely this reflected that with more proliferating enterocyte populations, digestive enzyme activity was greater and may thus contribute to enhanced digestive capacity of gastrointestinal tract. However, a weak relationship was observed between Ki-67 positive cell populations and sucrase activity (r = 0.313, P = 0.087; Fig. 2D).
Figure 2.
The relationship of Ki-67 positive cells with the apparent digestibility of CP (A) and GE (B), the activities of lactase (C) and sucrase (D), and the protein abundance of PEPT1 (E) and SGLT1 (F). The r is the correlation coefficient. SGLT1 = sodium-glucose linked transporter 1; PEPT1 = peptide transporter 1.
Sodium-glucose linked transporter 1-encoded sodium/glucose cotransporter 1 plays a critical role in glucose transport in the piglet small intestine, whereas PEPT1-encoded peptide transporter 1 mainly transports dipeptides and tripeptides from the digestive tract in the piglet small intestine (Xu et al., 2015). The current results revealed that correlation coefficients between Ki-67 positive cell numbers and the protein abundances of PEPT1 and SGLT1 were 0.511 (P = 0.030; Fig. 2E) and 0.601 (P = 0.014; Fig. 2F), respectively. This suggested that cell proliferation was well linked to the expression of nutrient transporters. It has been demonstrated that increasing intestinal absorption rates implicated in an overexpression of nutrient transporters (Choi et al., 2012). Therefore, our finding further suggested that enterocyte proliferation was closely and positively associated with the intestinal absorption, which supported the notion that cell production rate is proportional to absorptive function in animals (Goodlad et al., 1988). Notably, these relationships could be impacted by several factors (e.g., weaning age, mixed stresses, and feed restriction, etc.), which may cause the changes in gut-related parameters including villus height and enzyme activity (Moeser et al., 2007; Montagne., 2007; Pohl et al., 2017).
In conclusion, the current study showed that enterocyte proliferation was positively associated with the villus height, crypt depth, nutrient digestion, and absorption of small intestine in weaning piglets. We provide a potential positive-feedback regulatory mechanism for intestinal morphology and function. It will, therefore, be of interest in future to modulate epithelial cell proliferation for improving intestinal morphology and function in weaning piglets.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (2016YFD0501201); Key Programs of frontier scientific research of the Chinese Academy of Sciences (QYZDY-SSW-SMC008), National Natural Science Foundation of China (31330075), Natural Science Foundation of Hunan Province (2017JJ1020), Huxiang Youth talent Support Program (2016RS3028), Young Elite Scientists Sponsorship Program by CAST (YESS20160086).
LITERATURE CITED
- Bilić-Šobot D., V. Kubale M. Škrlep M. Čandek-Potokar M. Prevolnik Povše G. Fazarinc, and Škorjanc D.. 2016. Effect of hydrolysable tannins on intestinal morphology, proliferation and apoptosis in entire male pigs. Arch. Anim. Nutr. 70:378–388. doi: 10.1080/1745039X.2016.1206735 [DOI] [PubMed] [Google Scholar]
- Campbell J. M., J. D. Crenshaw, and Polo J.. 2013. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 4:19. doi: 10.1186/2049-1891-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung Q. C., Z. Yuan P. W. Dyce D. Wu K. DeLange, and Li J.. 2009. Generation of epidermal growth factor-expressing Lactococcus lactis and its enhancement on intestinal development and growth of early-weaned mice. Am. J. Clin. Nutr. 89:871–879. doi: 10.3945/ajcn.2008.27073 [DOI] [PubMed] [Google Scholar]
- Choi H. J., J. H. Ahn S. H. Park K. H. Do J. Kim, and Moon Y.. 2012. Enhanced wound healing by recombinant Escherichia coli Nissle 1917 via human epidermal growth factor receptor in human intestinal epithelial cells: therapeutic implication using recombinant probiotics. Infect. Immun. 80:1079–1087. doi: 10.1128/IAI.05820-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Conto C., A. Oevermann I. A. Burgener M. G. Doherr, and Blum J. W.. 2010. Gastrointestinal tract mucosal histomorphometry and epithelial cell proliferation and apoptosis in neonatal and adult dogs. J. Anim. Sci. 88:2255–2264. doi: 10.2527/jas.2009-2511 [DOI] [PubMed] [Google Scholar]
- Goodlad R. A., J. A. Plumb, and Wright N. A.. 1988. Epithelial cell proliferation and intestinal absorptive function during starvation and refeeding in the rat. Clin. Sci. 74:301–306. [DOI] [PubMed] [Google Scholar]
- Grenier B. and Applegate T. J.. 2013. Modulation of intestinal functions following mycotoxin ingestion: meta-analysis of published experiments in animals. Toxins 5:396–430. doi: 10.3390/toxins5020396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks W. H., D. G. Thomas G. Bosch, and Fahey G. C. Jr. 2013. Comparison of ileal and total tract nutrient digestibility of dry dog foods. J. Anim. Sci. 91:3807–3814. doi: 10.2527/jas.2012-5864 [DOI] [PubMed] [Google Scholar]
- Jang Y. D., Wilcock P., Boyd R. D., and Lindemann M. D.. 2017. Effect of combined xylanase and phytase on growth performance, apparent total tract digestibility, and carcass characteristics in growing pigs fed corn-based diets containing high-fiber coproducts. J. Anim. Sci. 95:4005–4017. doi: 10.2527/jas2017.1781 [DOI] [PubMed] [Google Scholar]
- Jonecova Z., S. Toth R. Ciccocioppo L. Rodrigo P. Kruzliak, and Nemcova R.. 2015. Influence of dietary supplementation with flaxseed and lactobacilli on the mucosal morphology and proliferative cell rate in the jejunal mucosa of piglets after weaning. Int. J. Exp. Pathol. 96:163–171. doi: 10.1111/iep.12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung K., B. K. Kang J. Y. Kim K. S. Shin C. S. Lee, and Song D. S.. 2008. Effects of epidermal growth factor on atrophic enteritis in piglets induced by experimental porcine epidemic diarrhoea virus. Vet. J. 177:231–235. doi: 10.1016/j.tvjl.2007.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah A. T., L. Van Landeghem H. E. Gavin S. T. Magness, and Lund P. K.. 2014. Impact of diet-induced obesity on intestinal stem cells: hyperproliferation but impaired intrinsic function that requires insulin/IGF1. Endocrinology 155:3302–3314. doi: 10.1210/en.2014-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeser A. J., K. A. Ryan P. K. Nighot, and Blikslager A. T.. 2007. Gastrointestinal dysfunction induced by early weaning is attenuated by delayed weaning and mast cell blockade in pigs. Am. J. Physiol. Gastrointest. Liver Physiol. 293:G413–G421. doi: 10.1152/ajpgi.00304.2006 [DOI] [PubMed] [Google Scholar]
- Montagne L., G. Boudry C. Favier I. Le Huërou-Luron J. P. Lallès, and Sève B.. 2007. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 97:45–57. doi: 10.1017/S000711450720580X [DOI] [PubMed] [Google Scholar]
- Pohl C. S., Medland J. E., Mackey E., Edwards L. L., Bagley K. D., Dewilde M. P., Williams K. J., and Moeser A. J.. 2017. Early weaning stress induces chronic functional diarrhea, intestinal barrier defects, and increased mast cell activity in a porcine model of early life adversity. Neurogastroenterol. Motil. 29:11. doi: 10.1111/nmo.13118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., G. R., Li B. E., Tan X., Xiong X. F., Kong D. F., Xiao L. W., Xu M. M., Wu B., Huang S. W., Kim, et al. 2015. Oral administration of putrescine and proline during the suckling period improves epithelial restitution after early weaning in piglets. J. Anim. Sci. 93:1679–1688. doi: 10.2527/jas.2014-8230 [DOI] [PubMed] [Google Scholar]
- Wang J., L. Zeng B. Tan G. Li B. Huang X. Xiong F. Li X. Kong G. Liu, and Yin Y.. 2016. Developmental changes in intercellular junctions and Kv channels in the intestine of piglets during the suckling and post-weaning periods. J. Anim. Sci. Biotechnol. 7:4. doi: 10.1186/s40104-016-0063-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S., D. Wang P. Zhang Y. Lin Z. Fang L. Che, and Wu D.. 2015. Oral administration of Lactococcus lactis-expressed recombinant porcine epidermal growth factor stimulates the development and promotes the health of small intestines in early-weaned piglets. J. Appl. Microbiol. 119:225–235. doi: 10.1111/jam.12833 [DOI] [PubMed] [Google Scholar]
- Yang H., X. Xiong X. Wang T. Li, and Yin Y.. 2016. Effects of weaning on intestinal crypt epithelial cells in piglets. Sci. Rep. 6:36939. doi: 10.1038/srep36939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong J. F., W. G. Wu X. Q. Zhang W. Tu Z. X. Liu, and Fang R. J.. 2016. Effects of dietary addition of heat-killed Mycobacterium phlei on growth performance, immune status and anti-oxidative capacity in early weaned piglets. Arch. Anim. Nutr. 70:249–262. doi: 10.1080/1745039X.2016.1183365 [DOI] [PubMed] [Google Scholar]
- Zorn T. M., M. Zúñiga E. Madrid R. Tostes Z. Fortes F. Giachini, and San Martín S.. 2011. Maternal diabetes affects cell proliferation in developing rat placenta. Histol. Histopathol. 26:1049–1056. doi: 10.14670/HH-26.1049 [DOI] [PubMed] [Google Scholar]