Abstract
Omega-3 PUFA may benefit sow reproductive performance, but effects on weaned gilts are unknown. This study evaluated the effects of supplementing omega-3 PUFA to gilts after weaning on growth, metabolic markers, and gene expression of steroidogenic enzymes and hormone receptors. For 52 d, gilts in the control group were fed 100 g/d of regular diets, whereas gilts in the omega-3 group were fed 75 g/d of such diets plus 25 g/d of the microalgae Schizochytium sp. (3.5 g/d of omega-3 PUFA; n = 8 gilts/group). Blood samples were collected at day 0, day 21, and day 52. Total serum cholesterol levels were lower for the omega-3 group than for the control group (P < 0.05), but high-density lipoprotein-cholesterol levels were reduced at day 52 for both groups (P < 0.05). Gilts in the omega-3 group presented lower feed intake, better feed conversion, and less-intense immunolabeling for leptin and its receptor in the cytoplasm of oocytes included in primordial/primary follicles than gilts in the control group (P < 0.05). The expression of genes coding for cholesterol side-chain cleavage and aromatase enzymes and the LH receptor in follicular cells was lower for supplemented gilts (P < 0.05). Compared with controls, supplemented gilts presented decreased serum cholesterol levels and better feed conversion, but leptin presence and gene expression for steroidogenic enzymes and for the LH receptor were lower at ovarian level.
Keywords: genes, gilts, feed conversion, leptin, PUFA, steroidogeneses
INTRODUCTION
Despite being essential for domestic animals, PUFA are not naturally synthesized and only become available through diet supplementation (Kurlak and Stephenson, 1999). The docosahexaenoic acid (DHA) is one of the most important PUFA from the omega-3 series (reviewed by Rossi et al., 2010). When supplied to sows in gestation and lactation diets, omega-3 PUFA are incorporated into oocytes, embryos, and fetuses, benefiting fetal development (Brazle et al., 2009) and subsequent litter size (Smits et al., 2011). On the other hand, some studies reported either detrimental effects or no effect on reproductive performance (Perez Rigau et al., 1995; Smit et al., 2013; Mateo et al., 2014). The variety of omega-3 PUFA sources, such as vegetable, marine, and fish oils (Rossi et al., 2010; Smit et al., 2013), may be related to such inconclusive findings. Fish oils are the most frequent sources of omega-3 PUFA (Rooke et al., 2001; Mitre et al., 2004), but their use may be restrained by sustainability issues related to fish scarcity (Lorenzen, 2005). Algae are viable sources of omega-3 PUFA because they can be produced in controlled environments with high-quality control, no pathogenicity, and greater lipid content than other sources (Ryckebosch et al., 2012; Martins et al., 2013). Nevertheless, the efficacy of supplying omega-3 PUFA from algae to swine still deserves further research.
Diet supplementation with omega-3 PUFA affects metabolic parameters. In humans and mice, triglycerides serum levels were reduced after supplementation with omega-3 PUFA (Harris and Bulchandani, 2006). In swine, reduced cholesterol levels were reported for prepubertal gilts supplemented with fish oil (Moreira et al., 2016), but increased cholesterol levels occurred for boars supplemented with microalgae (Andriola et al., 2017). As cholesterol is the precursor of steroid hormones, omega-3 PUFA may affect steroidogenesis, as the action of some enzymes with relevant role in steroidogenesis is controlled by the expression of genes that share common pathways with hormones involved in steroid production. Some of such enzymes are cholesterol side-chain cleavage (CYP11A1), which converts cholesterol to pregnenolone, and aromatase (CYP19A1), which converts androstenedione to estrogen at granulosa cells (Lavoie and King, 2009; Robic et al., 2016). After supplementation with omega-3 PUFA, increased steroid concentration was observed in the follicular fluid of cows and ewes (Zachut et al., 2008; Wonnacott et al., 2010, respectively), and there was greater expression of steroidogenic enzymes in granulosa cells of supplemented cows (Zachut et al., 2008). In peripubertal rams supplemented with omega-3 PUFA, the expression of steroidogenesis-related genes was increased, resulting in stimulus to estradiol secretion and subsequent gonadal development (Li et al., 2017). Thus, investigating hormone receptor genes after supplementation with omega-3 PUFA is pertinent, but there are limited data about such associations in swine.
Metabolic effects of omega-3 PUFA on reproductive function may also be mediated through leptin. Leptin and its long-chain receptor (LEPR) have been identified in both gilts and sows, in the brain (Lin et al., 2001), and in oocytes at distinct developmental stages (Moreira et al., 2013, 2016). In gilts approaching puberty, circulating leptin levels are increased (Qian et al., 1999) and immunolabeling for LEPR becomes more evident at the hypothalamus (Moreira et al., 2014). In cycling gilts, high leptin levels have been associated with higher circulating levels of LH and steroid hormones, as well as increased intrafollicular estrogen levels, which may improve oocyte developmental competence (Ferguson et al., 2003), as also described for mice (Wakefield et al., 2008). Following supplementation with fish oil, the presence of leptin increased in oocytes of prepubertal gilts (Moreira et al., 2016). As leptin is produced in adipocytes, that may occur concomitantly with an increase in gilts’ fat reserves and BW (Amaral Filha et al., 2010), potentially helping to accelerate puberty onset (Zhuo et al., 2014). It is known that gilts with either light birth weight or slow growth rate are more likely to have impaired reproductive performance and longevity (Magnabosco et al., 2016; Almeida et al., 2017). When omega-3 PUFA were supplied to breeding sows during gestation and lactation, metabolic effects were evident on their offspring right after weaning (Gabler et al., 2007), which suggests potential effects on their subsequent growth and performance. Thus, supplementation with omega-3 PUFA to gilts at early growth stages may have relevant impact on their future reproductive performance. However, studies evaluating such effects on newly weaned gilts are scarce. This study evaluated the effects of supplementation with omega-3 PUFA from microalgae to gilts weaned with nearly 35 d of age on their: growth performance; metabolic parameters; presence of leptin and LEPR in oocytes; and expression of reproduction-related enzymes and hormone receptor genes.
MATERIALS AND METHODS
Study Design
The experiment was conducted at the Instituto Federal Catarinense (IFC), in the southern region of Brazil (26°23′35.6″S latitude, 48°44′31.9″W longitude). After weaning (at 35.5 ± 1.6 d), 16 crossbred F1 gilts (Landrace × Large White) were split at random in 2 groups (n = 8 each), grouped according to their BW: control (9.3 ± 0.9 kg) and omega-3 (9.4 ± 0.8 kg). Both groups were fed diets recommended for the nursery and growing stages (NRC, 2012). All procedures were approved by the IFC Ethics in Animal Experimentation Committee (process # 0128/2016).
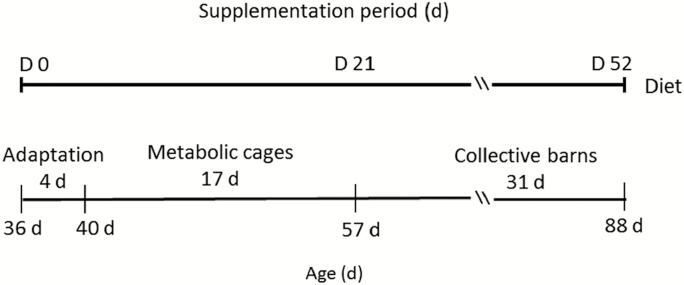
The experiment lasted 52 d (Fig. 1). During the first 4 d, both groups were housed in separate barns with slatted floor, to adapt to the nursery diet: 19.0% crude protein; 1.4% lysine; and 3,230 kcal/kg ME. After that, gilts were housed in individual size-adjustable metabolic cages equipped with a system for collection of feces and urine, to allow recording of their daily feed intake. Gilts remained in metabolic cages for 17 d (from 40 to 57 d of age). Then, both groups were moved to collective barns with slatted plastic floors, where they remained for 31 d. At 60 d of age, gilts started to receive the growing diet (18.0% crude protein, 1.15% lysine, and 3,350 kcal/kg ME).
Figure 1.
Timeline of the experimental design. Gilts were allocated to 2 groups after weaning (35.5 ± 1.6 d) during 52 d (from day 0 to day 52). Control = not supplemented (n = 8); omega-3 = supplemented with omega-3 PUFA in the diet after weaning during 52 d (n = 7).
In both nursery and growing stages, gilts had ad libitum access to diets. In addition, gilts from both groups were fed 100 g/d of the corresponding diets moistened in water, individually supplied using 10-mL syringes, once a day. Gilts in the control group were fed the same diets, but for gilts in the omega-3 group, that content corresponded to 75 g of each diet mixed to 25 g of a commercial seaweed meal of the heterotrophic microalgae Schizochytium sp. (All-G-Rich, Alltech Inc., Araucária, PR, Brazil) containing 140 g/kg DHA. The fatty acid profile of the microalgae has been described elsewhere (Andriola et al., 2017; Posser et al., 2018). Therefore, each gilt was supplemented with 3.5-g DHA daily, as reported in other studies (Smits et al., 2011; Moreira et al., 2016). Gilts from both groups had ad libitum access to water. All diets were isoenergetic and isoproteic across groups. One gilt from the omega-3 group died for a reason unrelated to the experiment.
All gilts were slaughtered at a commercial abattoir with 87.5 ± 1.6 d of age. Both ovaries were collected and the presence of structures was recorded. One ovary of each gilt was conditioned in formalin, for subsequent analysis through immunohistochemistry (IHQ). All available follicles of the other ovary were aspirated, and the follicular fluid was centrifuged at 1,000 × g for 1 min. The follicular pellet contained cumulus-oocyte complexes, which were not removed during aspiration. After discharging the supernatant, the pellet of follicular cells was stored in liquid nitrogen for subsequent evaluation of the gene expression of steroidogenic enzymes and hormone receptors.
Growth Performance
All gilts were weighted weekly (from day 0 to day 49). The average daily feed intake was obtained by dividing the total amount of the diets fed per gilt by the number of days spent in the metabolic cages (17 d). The average daily weight gain and feed conversion were also calculated the number of days spent in the metabolic cages.
Metabolic Markers
Blood samples were collected from all gilts at the beginning (day 0), 21 d after (day 21), and at the end of the period (day 52). Samples were collected through puncture of the jugular vein in 10-mL syringes attached to an 18 g × 1 ½” needle. Samples were placed into 10-mL vacutainer tubes (Vacuplast, Cral, São Paulo, Brazil) without clot activator and centrifuged at 2,500 × g for 10 min. Thereafter, the serum was stored in cryotubes at −20 °C.
Serum levels of triglycerides (#87-2/100: Labtest, Lagoa Santa, MG, Brazil; sensitivity 3.0 mg/dL), total cholesterol (#11539, BioSystems, Curitiba, PR, Brazil; sensitivity 0.3 mg/dL), and high-density lipoprotein (HDL)-cholesterol (#100-250/080: Vida Biotecnologia, Belo Horizonte, MG; sensitivity 0.905 mg/dL) were quantified through standard enzymatic procedures. Serum levels of low-density lipoprotein (LDL)-cholesterol were determined as described by Friedewald et al. (1972). Serum levels of aspartate aminotransferase (AST) were determined through UV kinetics (#109-2/100: Labtest; sensitivity 1.75 U/L), whereas serum levels of gamma glutamyl-transferase (GGT) were determined through colorimetric kinetics (#105-2/50: Labtest; sensitivity 2.48 U/L). Serum levels of estradiol were determined using eletrochemiluminescence (#06601811 Immulite Estradiol LKE21: Siemens Healthcare, São Paulo, SP, Brazil; sensitivity 11.8 pg/mL), but the reported results refer only to 2 collections (day 0 and day 52) because the values observed at day 21 were inferior to the limiting sensitivity of the test. All assays for metabolic indicators were conducted in a commercial laboratory, using kits developed for human serum, but with multispecies validation (CV inferior to 10%).
Immunohistochemistry
After conditioning in 10% buffer formalin solution for 24 h, dehydration, clarification, and inclusion in paraffin, 3-µm slices of ovarian tissues (2 per ovary) were obtained with an automatic microtome and fixed in ethanol on slides with 3% organosilane (Sigma Chemical Company, St. Louis, MO, EUA). Both slices were stained in the same slide: one received the antibody and the other was kept as a negative control.
The antileptin [Lep antibody; A-20, sc-842-rabbit (IgG)] diluted 1:2,000 and the anti-LEPR [pAb M-18-goat (IgG)] diluted 1:100 (Santa Cruz Biotechnology, Santa Cruz, CA) were the primary polyclonal antibodies, which were both diluted in 1.5% (wt/vol) BSA solution. The endogenous peroxidase activity was blocked with 3% (vol/vol) H2O2 (Spring Bioscience, Pleasanton, CA). The antigenic recovery was done through humid heat at 121 °C for 3 min after the boiling point, in citrate solution pH 6.0. Unspecific reactions were blocked using a protein blockage kit (Spring Bioscience, Pleasanton, CA). Slides were incubated overnight for 12 h with the primary antibody in humid chamber at 4 °C. Slides with anti-Lep and anti-LEPR were incubated with secondary antibodies: Reveal Polyvalente HRP Kit (Spring Bioscience, Pleasanton, CA) and Histofine Kit (Nichirei Bioscience, Tokyo, Japan), respectively. Subsequently, slides were incubated at room temperature for 1.15 min, with 3.3′ diaminobenzidine (DAB-K3468, DAKO Corporation, Carpinteria, CA), counterstained with aqueous hematoxylin (Merck, Darmstadt, Germany) during 45 s and mounted with coverslips and synthetic resin (Sigma).
Images were captured with a digital camera attached to a microscope (Eclipse E200, Nikon, Otawara, Japan) using the 40× objective with the Motic Images Plus 2.0 software. Protein staining was quantified by the Image J software, registering the mode value of each area in a 32-bit histogram, through a scale in which 0 represents the greatest staining intensity and 255 represents no staining (Moreira et al., 2013; Schneider et al., 2014). The means of the mode values were used for comparisons between groups.
Oocytes included in primordial/primary follicles were those surrounded by 1 layer of flat to cuboidal granulosa cells. Oocytes included in secondary follicles were those surrounded by 2 or more layers of cuboidal granulosa cells (Silva et al., 2011). The IHQ analyses considered 110 oocytes included in primordial and primary follicles and 90 oocytes included in secondary follicles for leptin, and 96 oocytes included in primordial and primary follicles and 75 oocytes included in secondary follicles for LEPR. No oocytes included in tertiary follicles were considered because their cytoplasm and nucleus were not clearly visible.
RNA Extraction, Reverse Transcription, and Real-Time PCR
After thawing at room temperature, a solution of phenol and guanidine isothiocyanate (Quick-Zol, Ludwig Biotec, Alvorada, RS, Brazil) was used to extract the total RNA from samples of follicular cells according to manufacturer’s instructions with slight modifications. The extracted RNA was quantified by NanoDrop (Thermo Scientific, Waltham, MA). The purity of the RNA was evaluated through the absorption rate of the OD260/OD280 ratio, considering values equal to or greater than 1.8. Any contaminant genomic DNA was digested by treating the total RNA with 0.1 U DNase I, Amplification Grade (Invitrogen, Life Technologies) at 37 °C for 5 min. The DNAse was inactivated at 65 °C for 10 min. The reverse transcriptase reaction was performed using iScript cDNA Synthesis kit (Bio-Rad, São Paulo, SP, Brazil). Gene expression was determined by real-time PCR (CFX384 real-time PCR, Bio-Rad) using GoTaq qPCR Master Mix (Promega, São Paulo, SP, Brazil) and swine specific primers (Table 1).
Table 1.
Sequence of primers used in the real-time PCR1
| Genes | Primers | Nomenclature |
|---|---|---|
| CYP11A1 F | TGCAATTGGTCCCACTCCTC | Cholesterol side-chain cleavage |
| CYP11A1 R | TTTGAGAAGAAGGCGGGGTC | |
| CYP19A1 F | TGCCAAGAATGTTCCTTACAGGTA | Aromatase |
| CYP19A1 R | CAGAGTGACCTTCATCATGACCAT | |
| FSHR F | CCAAGCTTCGAGTCATCCCA | FSH receptor |
| FSHR R | GAAGGCATCAGGGTCGATGT | |
| LHCGR F | CAGCCACTGCTGTGCTTTTA | LH receptor |
| LHCGR R | GAGTGTCTTGGGTGAGCAGA | |
| AR F | GGCCTTGCTTTCTAGCCTCA | Androgen receptor |
| AR R | AGCCCATGGCAAACACCATA | |
| PGR F | GCAGGTGTACCAGCCCTATC | Progesterone receptor |
| PGR R | GCTCCCACAGGTAAGGACAC | |
| RPL19 F | ATGAAATCGCCAACGCCAAC | Reference gene |
| RPL19 R | GGTGTTTTTCCGGCATCGAG | |
| GAPDH F | CAAGGGCATCCTGGGCTACA | Reference gene |
| GAPDH R | GCTTGACGAAGTGGTCGTTGA |
1F = forward primers; R = reverse primers.
To optimize gene expression, RNA from all samples suffered serial dilutions to generate a standard curve. Reactions having coefficient of determination greater than 0.98 and efficiency within 95% to 105% were considered optimized. All samples were analyzed in duplicate. The constitutive genes GAPDH and RPL19 selected through Bio-Rad CFX Manager Software Version 3.0 were used as internal controls.
Statistical Analyses
The Shapiro–Wilk test detected lack of normality for the serum levels of GGT, AST, estradiol, total, HDL-cholesterol, and LDL-cholesterol, which were transformed to the logarithmic scale. Serum levels of all metabolic markers were compared between treatments by ANOVA with repeated measures, with the individual effect of gilt nested within the period of sample collection. Interactions between treatment and collection period were tested. Comparisons of means were done using the LSD test. For transformed response variables, results were reported in their original scales.
The means for the mode values of immunolabeling intensity for leptin and LEPR on the cytoplasm and on the nucleus of oocytes included in primordial, primary and secondary follicles, BW, daily feed intake, daily weight gain, feed conversion, and gene expression were compared between treatments using 2-sample t tests. All statistical analyses were done using Statistix (2013).
RESULTS
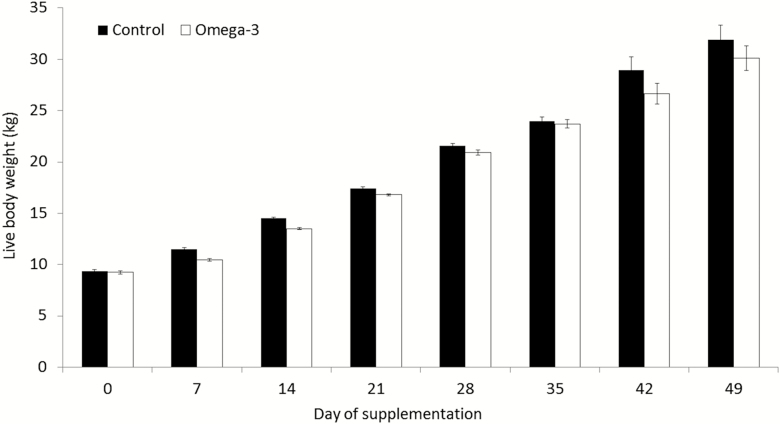
Body weight increased over time for gilts in both groups (Fig. 2). A treatment per period interaction indicated that BW was lower for supplemented gilts than for control gilts at day 7 and day 14 (P < 0.05), although gilts from both groups presented similar BW at the end of the period (P > 0.05). Daily weight gain did not differ between groups (P > 0.05), but supplemented gilts presented lower feed intake and better feed conversion (both P < 0.05) than control gilts (Table 2).
Figure 2.
Body weight for gilts at distinct periods. Means ± SEM indicate differences of at least P < 0.05 between groups at distinct periods. Control = not supplemented (n = 8); omega-3 = supplemented with omega-3 PUFA in the diet after weaning during 52 d (n = 7).
Table 2.
Growth performance for gilts from 40 to 57 d of age in distinct groups1
| Treatment | Feed intake (kg/d) | Weight gain (kg) | Feed conversion |
|---|---|---|---|
| Control | 0.76 ± 0.05a | 0.43 ± 0.05a | 1.78 ± 0.17a |
| Omega-3 | 0.66 ± 0.05b | 0.41 ± 0.03a | 1.60 ± 0.12b |
a,bMeans ± SEM having distinct superscript differ between treatments by at least P < 0.05.
1Control = not supplemented (n = 8); omega-3 = supplemented with omega-3 PUFA in the diet after weaning during 52 d (n = 7).
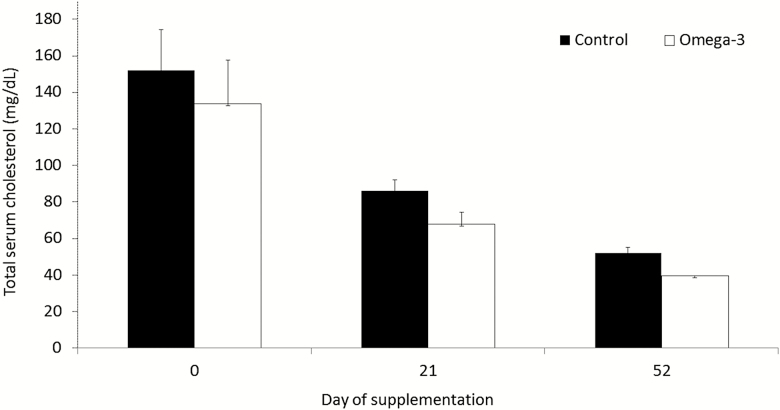
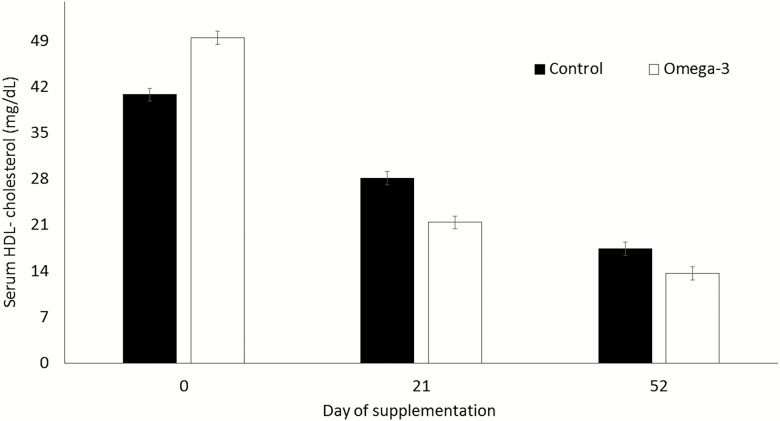
Cholesterol levels were lower for supplemented gilts (P < 0.01) than for control gilts at day 52 (Fig. 3). Compared with the omega-3 group, serum levels of HDL-cholesterol for control gilts were lower at day 0 (P < 0.05), but greater (P < 0.05) at both day 21 and day 52 (Fig. 4). Serum estrogen levels did not differ (P > 0.05) for control and for supplemented gilts (31.0 ± 3.3 and 33.3 ± 3.5 pg/mL, respectively). No differences (P > 0.05) were observed for the other evaluated metabolic indicators between groups (Table 3).
Figure 3.
Total serum cholesterol levels for gilts at distinct periods. Means ± SEM indicate differences of at least P < 0.05 between groups at distinct periods. Control = not supplemented (n = 8); omega-3 = supplemented with omega-3 PUFA in the diet after weaning during 52 d (n = 7).
Figure 4.
Serum high-density lipoprotein (HDL)-cholesterol levels for gilts at distinct periods. Means ± SEM indicate differences of at least P < 0.05 between groups at distinct periods. Control = not supplemented (n = 8); omega-3 = supplemented with omega-3 PUFA in the diet after weaning during 52 d (n = 7).
Table 3.
Serum levels of metabolic parameters for gilts in distinct groups1
| Parameters2 | Control | Omega-3 |
|---|---|---|
| Triglycerides (mg/dL) | 62.1 ± 4.8 | 57.3 ± 4.9 |
| Total cholesterol (mg/dL) | 96.7 ± 12.1 | 80.3 ± 10.5 |
| LDL-cholesterol3 (mg/dL) | 54.9 ± 9.2 | 40.6 ± 6.5 |
| HDL-cholesterol4 (mg/dL) | 28.6 ± 2.8 | 28.1 ± 3.9 |
| Gamma glutamyl transpeptidase (U/L) | 21.8 ± 2.1 | 24.9 ± 2.8 |
| Aspartate aminotransferase (U/L) | 72.3 ± 9.9 | 92.4 ± 17.1 |
Means ± SEM did not differ between treatments (P > 0.05).
1Control = not supplemented (n = 8); omega-3 = supplemented with omega-3 PUFA in the diet after weaning during 52 d (n = 7).
2Samples collected at day 0, day 21, and day 52.
3LDL-cholesterol = low-density lipoprotein-cholesterol.
4HDL-cholesterol = high-density lipoprotein-cholesterol.
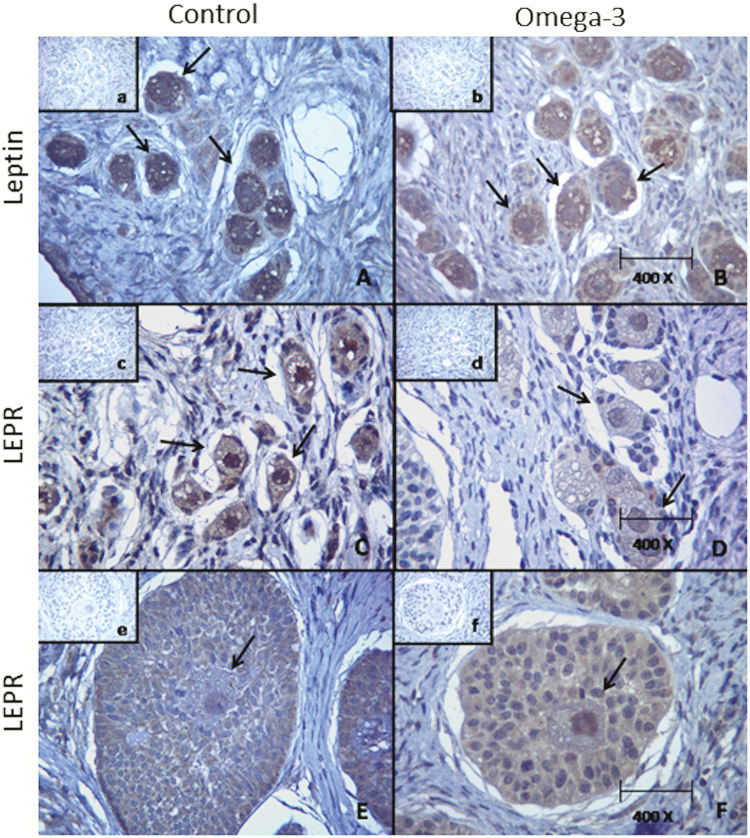
Twelve out of 15 gilts examined postmortem presented antral follicles in their ovaries (6 from each group). No corpora lutea were observed. Both leptin and LEPR were identified in the cytoplasm and in the nucleus of oocytes included in primordial, primary, and secondary follicles of gilts from both groups (Fig. 5). Immunolabeling for leptin and LEPR in the cytoplasm was less intense in oocytes included in primordial and primary follicles of supplemented gilts (P < 0.05) than in similar oocytes of control gilts (Table 4). Immunolabeling intensity for leptin and LEPR in the nucleus of oocytes included in primordial, primary, and secondary follicles and in the cytoplasm of oocytes included in secondary follicles did not differ between groups (P > 0.05).
Figure 5.
Immunolabeling for leptin and its receptor (LEPR) in oocytes from gilts in distinct groups. Control = not supplemented (on the left side); omega-3 = supplemented with omega-3 PUFA in the diet after weaning during 52 d (on the right side). (A) Oocytes in primordial/primary follicles: immunolabeling for leptin in the cytoplasm is more intense (P < 0.05) for gilts in the control group than in the omega-3 group (B). (C) Oocytes in primordial/primary follicles: immunolabeling for LEPR in the cytoplasm is more intense (P < 0.05) for gilts in the control group than in the omega-3 group (D). (E) Oocytes in secondary follicles: immunolabeling for LEPR in the nucleus and in the cytoplasm did not differ (P > 0.05) for gilts in both groups (F). a, b, c, d, e, and f: negative controls for A, B, C, D, E, and F, respectively.
Table 4.
Intensity of immunolabeling (means of the mode observed values) for leptin and its receptor (LEPR) in the cytoplasm and in the nucleus of oocytes from gilts in distinct groups (87.5 ± 1.6 d of age)1
| Immunolabeling | Treatment2 | Primordial/primary follicles | ||
|---|---|---|---|---|
| n | Cytoplasm | Nucleus | ||
| Leptin | Control | 59 | 115.1 ± 2.1a | 125.4 ± 1.9 |
| Omega-3 | 51 | 123.4 ± 2.8b | 127.2 ± 3.2 | |
| LEPR | Control | 50 | 146.9 ± 3.6x | 125.3 ± 4.4 |
| Omega-3 | 46 | 157.8 ± 3.6y | 129.4 ± 3.2 | |
| Secondary follicles | ||||
| n | Cytoplasm | Nucleus | ||
| Leptin | Control | 46 | 135.4 ± 2.5 | 132.5 ± 2.5 |
| Omega-3 | 44 | 139.6 ± 2.7 | 136.8 ± 2.6 | |
| LEPR | Control | 42 | 164.1 ± 3.6 | 128.1 ± 3.2 |
| Omega-3 | 33 | 168.4 ± 4.7 | 130.1 ± 4.4 | |
a,bMeans ± SEM having distinct superscript differ between treatments by at least P < 0.05.
x,yMeans ± SEM having distinct superscript differ between treatments by at least P < 0.05.
10 = greatest intensity; 255 = no staining.
2Control = not supplemented; omega-3 = supplemented with omega-3 PUFA in the diet after weaning during 52 d.
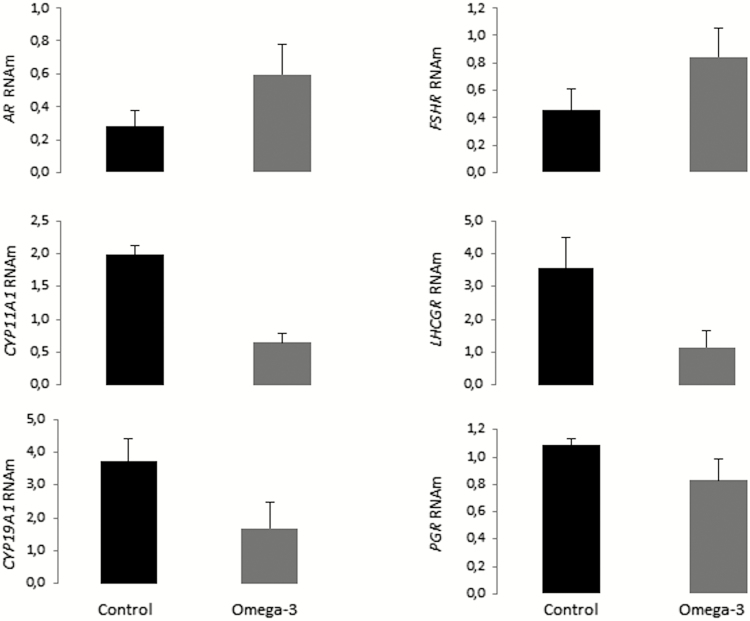
Expression of CYP11A1, CYP19A1, and LHCGR in follicular cells was lower (P < 0.05) for supplemented gilts than for control gilts (Fig. 6). No difference between groups was observed for the expression of AR, FSHR, and PGR (P > 0.05). Transcripts of the genes CYP17A1 e HSD3B mRNA were absent or marginally detected in most samples indicating low cross-contamination with theca cells and low granulosa cells luteinization, respectively (data not shown).
Figure 6.
mRNA expression of steroidogenic enzymes and hormone receptors in follicular cells from gilts in distinct groups. Means ± SEM indicate differences of at least P < 0.05 between groups. Control = not supplemented; omega-3 = supplemented with omega-3 PUFA in the diet after weaning during 52 d.
DISCUSSION
Compared with control gilts, supplemented gilts presented decreased serum levels of total and HDL-cholesterol at day 21 and day 52. As gilts were randomly allocated across groups and no other differences in metabolic parameters were observed between groups at day 0, the greater level of HDL-cholesterol observed for supplemented gilts at day 0 was probably due to random effects. Because the levels of GGT and AST were unaffected, remaining within the reference values for gilts at the age group of interest (Kaneko et al., 1989), the increased lipid metabolization probably did not compromise the liver function of supplemented gilts. Decrease in serum cholesterol levels after supplementation with omega-3 PUFA was also reported for older prepubertal gilts (Moreira et al., 2016) and for rats (Sengupta and Ghosh, 2015). Hence, supplementation with omega-3 PUFA from microalgae was efficient in reducing serum levels of both total and HDL-cholesterol, but such levels appear to decline naturally as gilts age. No effects on serum triglyceride levels were evident in the present study, despite the reports of declined levels of triglycerides after supplementation with omega-3 PUFA from fish sources (Harris and Bulchandani, 2006). On the other hand, reduced serum levels of triglycerides were detected for sows supplemented with omega-3 PUFA from microalgae during gestation, in other study conducted by our research group (Posser et al., 2018).
Decrease in serum cholesterol levels may reduce the availability of substrates for steroidogenesis, as suggested by the decreased expression of CYP11A1 and CYP19A1 in the ovarian tissue of supplemented gilts. In accordance with our results, treatment of cultured granulosa cells from women undergoing in vitro fertilization with the eicosapentaenoic acid resulted in increased expression of PPARγ (an aromatase inhibitor), whereas expression of CYP19A1 was decreased (Zaree et al., 2015). The expression of CYP19A1 in follicular cells would be related to mechanisms of follicular dominance and may be a marker for oocyte quality and competence, as suggested in studies with women (Hamel et al., 2008). The lower expression of genes related to steroidogenesis and of LHCGR observed in our study suggests that antral follicles of the supplemented gilts may have compromised development and differentiation. Despite those findings, no effect on serum estradiol levels was observed in the present study. During most of the prepubertal period, estradiol circulatory levels are commonly low (reviewed by Evans and Doherty, 2001), which justifies the difficulties in determining serum estradiol levels throughout the experimental period. Estradiol concentration may be as low as 14 pg/mL prior to puberty, increased up to 26 pg/mL after puberty, and greater than 50 pg/mL during the last 2 d prior to estrous (Li et al., 2016).
In addition, supplemented gilts presented less-intense immunolabeling for leptin and LEPR in oocytes included in primordial and primary follicles, but not in oocytes included in secondary follicles. Generally, as females grow older and follicle development accelerates, leptin concentration in the ovaries increases (Gregoraszczuk et al., 2007) and becomes more evident in the cytoplasm of follicles (Moreira et al., 2013). That was also observed after supplementation with fish oil to gilts near to puberty (Moreira et al., 2016), suggesting that increased leptin levels may help to stimulate puberty onset (Zhuo et al., 2014). Considering the role of leptin on regulating the action of GnRH, FSH, and LH (Barash et al., 1996) and the action of LEPR on regulating circulatory levels of steroid hormones (Farooqi et al., 2007), such findings might suggest that supplementation with omega-3 PUFA from microalgae to gilts at early growth stages (e.g., after weaning) would impair their ovarian function. However, as the gilts evaluated in the present study were less than 3 mo old at the time of their slaughter, it is possible that truly relevant effects could be more evident at older ages. A recent study with breeding sows reported that omega-3 PUFA modulates the endometrial expression of genes with relevant roles in pathways of prostaglandin synthesis during early gestation, probably influencing subsequent reproductive events (Gokuldas et al., 2018). Thus, although supplementation with omega-3 PUFA may be helpful to improve female reproductive performance, long-term effects on ovarian function still need to be investigated in future studies, preferably evaluating gilts until puberty.
Feed conversion was improved in the omega-3 group as a function of the reduced feed intake, which may be due to the greater lipid content in the tested supplement compared with other sources of omega-3 PUFA (Ryckebosch et al., 2012; Martins et al., 2013). Similar benefits have been reported for piglets at the same age group of the gilts evaluated in the present study that were fed diets supplemented with fat sources, such as vegetable oils (Cera et al., 1990; Myer et al., 1992). Reduced feed intake may result from a feeling of satiety, which commonly occurs when increased leptin circulating levels signal the hypothalamic–pituitary axis about the animal’s nutritional status (Barash et al., 1996). Improved feed conversion has been reported following supplementation of growing piglets with omega-3 PUFA from microalgae (Abril et al., 2003). Nonetheless, in the present study, supplementation of gilts after weaning had no effect on their BW.
CONCLUSIONS
Potential effects of supplementing gilts with omega-3 PUFA from microalgae during 52 d after their weaning appeared to be driven toward growth performance and metabolic status, as feed conversion was improved and serum cholesterol levels were decreased. No benefits were evident at ovarian level because leptin immunostaining was less intense in oocytes included in primordial and primary follicles and gene expression for the LH receptor and for steroidogenic enzymes in follicular cells was reduced.
Footnotes
This research was funded with a scholarship given to the first author by CAPES and with research grants given to I.B. and T.L. by CNPq.
LITERATURE CITED
- Abril R., Garrett J., Zeller S. G., Sander W. J., and Mast R. W.. 2003. Safety assessment of DHA-rich microalgae from Schizochytrium sp. Part V: Target animal safety/toxicity study in growing swine. Regul. Toxicol. Pharmacol. 37:73–82. doi: 10.1016/S0273-2300(02)00030-2 [DOI] [PubMed] [Google Scholar]
- Almeida F., Alvarenga Dias A., Moreira L. P., Fiúza A., and Chiarini-Garcia H.. 2017. Ovarian follicle development and genital tract characteristics in different birthweight gilts at 150 days of age. Reprod. Domest. Anim. 52:756–762. doi: 10.1111/rda.12976 [DOI] [PubMed] [Google Scholar]
- Amaral Filha W. S., Bernardi M. L., Wentz I., and Bortolozzo F. P.. 2010. Reproductive performance of gilts according to growth rate and backfat thickness at mating. Anim. Reprod. Sci. 121:139–144. doi: 10.1016/j.anireprosci.2010.05.013 [DOI] [PubMed] [Google Scholar]
- Andriola Y. T., Moreira F., Anastácio E., Camelo F. A. Jr, Silva A. C., Varela A. S. Jr, Gheller S. M. M., Goularte K. L., Corcini C. D., and Lucia T. Jr. 2017. Boar sperm quality after supplementation of diets with omega-3 polyunsaturated fatty acids extracted from microalgae. Andrologia 50:e12825. doi: 10.1111/and.12825 [DOI] [PubMed] [Google Scholar]
- Barash I. A., Cheung C. C., Weigle D. S., Ren H., Kabigting E. B., Kuijper J. L., Clifton D. K., and Steiner R. A.. 1996. Leptin is a metabolic signal to the reproductive system. Endocrinology 137:3144–3147. doi: 10.1210/en.137.7.3144 [DOI] [PubMed] [Google Scholar]
- Brazle A. E., Johnson B. J., Webel S. K., Rathbun T. J., and Davis D. L.. 2009. Omega-3 fatty acids in the gravid pig uterus as affected by maternal supplementation with omega-3 fatty acids. J. Anim. Sci. 87:994–1002. doi: 10.2527/jas.2007-0626 [DOI] [PubMed] [Google Scholar]
- Cera K. R., Mahan D. C., and Reinhart G. A.. 1990. Evaluation of various extracted vegetable oils, roasted soybeans, medium-chain triglyceride and an animal-vegetable fat blend for postweaning swine. J. Anim. Sci. 68:2756–2765. doi: 10.2527/1990.6892756x [DOI] [PubMed] [Google Scholar]
- Evans A. C. O., and Doherty J. V.. 2001. Endocrine changes and management factors affecting puberty in gilts. Livest. Prod. Sci. 68:1–12. doi:10.1016/S0301-6226(00)00202-5 [Google Scholar]
- Farooqi I. S., Wangensteen T., Collins S., Kimber W., Matarese G., Keogh J. M., Lank E., Bottomley B., Lopez-Fernandez J., Ferraz-Amaro I.,. et al. 2007. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. N. Engl. J. Med. 356:237–247. doi: 10.1056/NEJMoa063988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson E. M., Ashworth C. J., Edwards S. A., Hawkins N., Hepburn N., and Hunter M. G.. 2003. Effect of different nutritional regimens before ovulation on plasma concentrations of metabolic and reproductive hormones and oocyte maturation in gilts. Reproduction 126:61–71. [DOI] [PubMed] [Google Scholar]
- Friedewald W. F., Levy R. Y., and Frederickson D. S.. 1972. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18:499–502. [PubMed] [Google Scholar]
- Gabler N. K., Spencer J. D., Webel D. M., and Spurlock M. E.. 2007. In utero and postnatal exposure to long chain (n−3) PUFA enhances intestinal glucose absorption and energy stores in weanling pigs. J. Nutr. 137:2351–2358. doi: 10.1093/jn/137.11.2351 [DOI] [PubMed] [Google Scholar]
- Gokuldas P. P., Singh S. K., Tamuli M. K., Naskar S., Vashi Y., Thomas R., Barman K., Pegu S. R., Chethan S. G., and Agarwal S. K.. 2018. Dietary supplementation of n−3 polyunsaturated fatty acid alters endometrial expression of genes involved in prostaglandin biosynthetic pathway in breeding sows (Sus scrofa). Theriogenology 110:201–208. doi: 10.1016/j.theriogenology.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Gregoraszczuk E. Ł., Ptak A., Wojciechowicz T., and Nowak K.. 2007. Action of IGF-I on expression of the long form of the leptin receptor (ObRb) in the prepubertal period and throughout the estrous cycle in the mature pig ovary. J. Reprod. Dev. 53:289–295. doi:10.1262/jrd.18071 [DOI] [PubMed] [Google Scholar]
- Hamel M., Dufort I., Robert C., Gravel C., Leveille M. C., Leader A., and Sirard M. A.. 2008. Identification of differentially expressed markers in human follicular cells associated with competent oocytes. Hum. Reprod. 23:1118–1127. doi: 10.1093/humrep/den048 [DOI] [PubMed] [Google Scholar]
- Harris W. S., and Bulchandani D.. 2006. Why do omega-3 fatty acids lower serum triglycerides?Curr. Opin. Lipidol. 17:387–393. doi: 10.1097/01.mol.0000236363.63840.16 [DOI] [PubMed] [Google Scholar]
- Kaneko J. J., Harvey J. W., and Bruss M. L.. 1989. Clinical biochemistry of domestic animals. 4th ed. Academic Press, London, UK. [Google Scholar]
- Kurlak L. O., and Stephenson T. J.. 1999. Plausible explanations for effects of long chain polyunsaturated fatty acids (LCPUFA) on neonates. Arch. Dis. Child. Fetal Neonat. Ed. 80:148–154. doi: 10.1136/fn.80.2.F148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie H. A., and King S. R.. 2009. Transcriptional regulation of steroidogenic genes: STARD1, CYP11A1 and HSD3B. Exp. Biol. Med. (Maywood). 234:880–907. doi: 10.3181/0903-MR-97 [DOI] [PubMed] [Google Scholar]
- Li W., Tang D., Li F., Tian H., Yue X., Li F., Weng X., Sun W., Wang W., and Mo F.. 2017. Supplementation with dietary linseed oil during peri-puberty stimulates steroidogenesis and testis development in rams. Theriogenology 102:10–15. doi: 10.1016/j.theriogenology.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Li F., Zhu Y., Ding L., and Zhang Y.. 2016. Effects of dietary glucose on serum estrogen levels and onset of puberty in gilts. Asian-Australas. J. Anim. Sci. 29:1309–1313. doi: 10.5713/ajas.15.0444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Richard Barb C., Kraeling R. R., and Rampacek G. B.. 2001. Developmental changes in the long form leptin receptor and related neuropeptide gene expression in the pig brain. Biol. Reprod. 64:1614–1618. doi:10.1095/biolreprod64.6.1614 [DOI] [PubMed] [Google Scholar]
- Lorenzen K. 2005. Population dynamics and potential of fisheries stock enhancement: Practical theory for assessment and policy analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360:171–189. doi: 10.1098/rstb.2004.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnabosco D., Bernardi M. L., Wentz I., Cunha E. C. P., and Bortolozzo F. P.. 2016. Low birth weight affects lifetime productive performance and longevity of female swine. Livest. Sci. 184:119–125. doi: 10.1016/j.livsci.2015.12.008 [DOI] [Google Scholar]
- Martins D. A., Custódio L., Barreira L., Pereira H., Ben-Hamadou R., Varela J., and Abu-Salah K. M.. 2013. Alternative sources of n−3 long-chain polyunsaturated fatty acids in marine microalgae. Mar. Drugs 11:2259–2281. doi: 10.3390/md11072259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo R. D., Carroll J. A., Hyun Y., Smith S., and Kim W. S.. 2014. Effect of dietary supplementation of n−3 fatty acids and elevated concentrations of dietary protein on the performance of sows. J. Anim. Sci. 87:948–959. doi: 10.2527/jas.2008-0964 [DOI] [PubMed] [Google Scholar]
- Mitre R., Cheminade C., Allaume P., Legrand P., and Legrand A. B.. 2004. Oral intake of shark liver oil modifies lipid composition and improves motility and velocity of boar sperm. Theriogenology 62:1557–1566. doi: 10.1016/j.theriogenology.2004.02.004 [DOI] [PubMed] [Google Scholar]
- Moreira F., Cheuiche Z. M., Rizzoto G., Santos M. Q., Schuch M. S., Flach M. J., Gasperin B. G., Bianchi I., and Lucia T. Jr. 2016. Metabolic and reproductive parameters in prepubertal gilts after omega-3 supplementation in the diet. Anim. Reprod. Sci. 170:178–183. doi: 10.1016/j.anireprosci.2016.05.008 [DOI] [PubMed] [Google Scholar]
- Moreira F., Corcini C. D., Mondadori R. G., Gevehr-Fernandes C., Mendes F. F., Araújo E. G., and Lucia T. Jr. 2013. Leptin and mitogen-activated protein kinase (MAPK) in oocytes of sows and gilts. Anim. Reprod. Sci. 139:89–94. doi: 10.1016/j.anireprosci.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Moreira F., Gheller S. M., Mondadori R. G., Varela Júnior A. S., Corcini C. D., and Lucia T. Jr. 2014. Presence of leptin and its receptor in the hypothalamus, uterus and ovaries of swine females culled with distinct ovarian statuses and parities. Reprod. Domest. Anim. 49:1074–1078. doi: 10.1111/rda.12438 [DOI] [PubMed] [Google Scholar]
- Myer R. O., Lamkey J. W., Walker W. R., Brendemuhl J. H., and Combs G. E.. 1992. Performance and carcass characteristics of swine when fed diets containing canola oil and added copper to alter the unsaturated: Saturated ratio of pork fat. J. Anim. Sci. 70:1414–1423. doi: 10.2527/1992.7051417x [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th rev. ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Perez Rigau A., Lindemann M. D., Kornegay E. T., Harper A. F., and Watkins B. A.. 1995. Role of dietary lipids on fetal tissue fatty acid composition and fetal survival in swine at 42 days of gestation. J. Anim. Sci. 73:1372–1380. doi: 10.2527/1995.7351372x [DOI] [PubMed] [Google Scholar]
- Posser C. J. M., Almeida L. M., Moreira F., Bianchi I., Gasperin B. G., and Lucia T. Jr. 2018. Supplementation of diets with omega-3 fatty acids from microalgae: Effects on sow reproductive performance and metabolic parameters. Livest. Sci. 207:59–62. doi: 10.1016/j.livsci.2017.11.006 [DOI] [Google Scholar]
- Qian H., Barb C. R., Compton M. M., Hausman G. J., Azain M. J., Kraeling R. R., and Baile C. A.. 1999. Leptin MRNA expression and serum leptin concentrations as influenced by age, weight, and estradiol in pigs. Domest. Anim. Endocrinol. 16:135–143. doi: 10.1016/S0739-7240(99)00004-1 [DOI] [PubMed] [Google Scholar]
- Robic A., Feve K., Louveau I., Riquet J., and Prunier A.. 2016. Exploration of steroidogenesis-related genes in testes, ovaries, adrenals, liver and adipose tissue in pigs. Anim. Sci. J. 87:1041–1047. doi: 10.1111/asj.12532 [DOI] [PubMed] [Google Scholar]
- Rooke J. A., Sinclair A. G., Edwards S. A., Cordoba R., Pkiyach S., Penny P. C., Penny P., Finch A. M., and Horgan G. W.. 2001. The effect of feeding salmon oil to sows throughout pregnancy on pre-weaning mortality of piglets. Anim. Sci. 73:489–500. doi: 10.1017/S135772980005846X [DOI] [Google Scholar]
- Rossi R., Pastorelli G., Cannata S., and Corino C.. 2010. Recent advances in the use of fatty acids as supplements in pig diets: A review. Anim. Feed Sci. Technol. 162:1–11. doi: 10.1016/j.anifeedsci.2010.08.013 [DOI] [Google Scholar]
- Ryckebosch E., Bruneel C., Muylaert K., and Foubert I.. 2012. Microalgae as an alternative source of omega-3 long chain polyunsaturated fatty acids. Lipid Technol. 24:128–130. doi: 10.1002/lite.201200197 [DOI] [PubMed] [Google Scholar]
- Schneider A., Zhi X., Moreira F., Lucia T. Jr, Mondadori R. G., and Masternak M. M.. 2014. Primordial follicle activation in the ovary of Ames dwarf mice. J. Ovarian Res. 7:120. doi: 10.1186/s13048-014-0120-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta A., and Ghosh M.. 2015. Reduction of cardiac and aortic cholesterol in hypercholesterolemic rats fed esters of phytosterol and omega-3 fatty acids. J. Food Sci. Technol. 52:2741–2750. doi: 10.1007/s13197-014-1346-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva R. C., Báo S. N., Jivago J. L., and Lucci C. M.. 2011. Ultrastructural characterization of porcine oocytes and adjacent follicular cells during follicle development: Lipid component evolution. Theriogenology 76:1647–1657. doi: 10.1016/j.theriogenology.2011.06.029 [DOI] [PubMed] [Google Scholar]
- Smit M. N., Patterson J. L., Webel S. K., Spencer J. D., Cameron A. C., Dyck M. K., Dixon W. T., and Foxcroft G. R.. 2013. Responses to n−3 fatty acid (LCPUFA) supplementation of gestating gilts, and lactating and weaned sows. Animal 7:784–792. doi: 10.1017/S1751731112002236 [DOI] [PubMed] [Google Scholar]
- Smits R. J., Luxford B. G., Mitchell M., and Nottle M. B.. 2011. Sow litter size is increased in the subsequent parity when lactating sows are fed diets containing n−3 fatty acids from fish oil. J. Anim. Sci. 89:2731–2738. doi: 10.2527/jas.2010-3593 [DOI] [PubMed] [Google Scholar]
- Statistix 2013. Statistix® 10 analytical software. Statistix: Tallahassee, FL. [Google Scholar]
- Wakefield S. L., Lane M., Schulz S. J., Hebart M. L., Thompson J. G., and Mitchell M.. 2008. Maternal supply of omega-3 polyunsaturated fatty acids alter mechanisms involved in oocyte and early embryo development in the mouse. Am. J. Physiol. Endocrinol. Metab. 294:E425–E434. doi: 10.1152/ajpendo.00409.2007 [DOI] [PubMed] [Google Scholar]
- Wonnacott K. E., Kwong W. Y., Hughes J., Salter A. M., Lea R. G., Garnsworthy P. C., and Sinclair K. D.. 2010. Dietary omega-3 and -6 polyunsaturated fatty acids affect the composition and development of sheep granulosa cells, oocytes and embryos. Reproduction 139:57–69. doi: 10.1530/REP-09-0219 [DOI] [PubMed] [Google Scholar]
- Zachut M., Arieli A., Lehrer H., Argov N., and Moallem U.. 2008. Dietary unsaturated fatty acids influence preovulatory follicle characteristics in dairy cows. Reproduction 135:683–692. doi: 10.1530/REP-07-0556 [DOI] [PubMed] [Google Scholar]
- Zaree M., Shahnazi V., Fayezi S., Darabi M., Mehrzad-Sadaghiani M., Darabi M., Khani S., and Nouri M.. 2015. Expression levels of PPARγ and CYP-19 in polycystic ovarian syndrome primary granulosa cells: Influence of ω-3 fatty acid. Int. J. Fertil. Steril. 9:197–204. doi: 10.22074/ijfs.2015.4240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo Y., Zhou D., Che L., Fang Z., Lin Y., and Wu D.. 2014. Feeding prepubescent gilts a high-fat diet induces molecular changes in the hypothalamus-pituitary-gonadal axis and predicts early timing of puberty. Nutrition 30:890–896. doi: 10.1016/j.nut.2013.12.019 [DOI] [PubMed] [Google Scholar]