Abstract
Background
Methamphetamine induces neuronal N-acetyl-aspartate synthesis in preclinical studies. In a preliminary human proton magnetic resonance spectroscopic imaging investigation, we also observed that N-acetyl-aspartate+N-acetyl-aspartyl-glutamate in right inferior frontal cortex correlated with years of heavy methamphetamine abuse. In the same brain region, glutamate+glutamine is lower in methamphetamine users than in controls and is negatively correlated with depression. N-acetyl and glutamatergic neurochemistries therefore merit further investigation in methamphetamine abuse and the associated mood symptoms.
Methods
Magnetic resonance spectroscopic imaging was used to measure N-acetyl-aspartate+N-acetyl-aspartyl-glutamate and glutamate+glutamine in bilateral inferior frontal cortex and insula, a neighboring perisylvian region affected by methamphetamine, of 45 abstinent methamphetamine-dependent and 45 healthy control participants. Regional neurometabolite levels were tested for group differences and associations with duration of heavy methamphetamine use, depressive symptoms, and state anxiety.
Results
In right inferior frontal cortex, N-acetyl-aspartate+N-acetyl-aspartyl-glutamate correlated with years of heavy methamphetamine use (r = +0.45); glutamate+glutamine was lower in methamphetamine users than in controls (9.3%) and correlated negatively with depressive symptoms (r = -0.44). In left insula, N-acetyl-aspartate+N-acetyl-aspartyl-glutamate was 9.1% higher in methamphetamine users than controls. In right insula, glutamate+glutamine was 12.3% lower in methamphetamine users than controls and correlated negatively with depressive symptoms (r = -0.51) and state anxiety (r = -0.47).
Conclusions
The inferior frontal cortex and insula show methamphetamine-related abnormalities, consistent with prior observations of increased cortical N-acetyl-aspartate in methamphetamine-exposed animal models and associations between cortical glutamate and mood in human methamphetamine users.
Keywords: methamphetamine, N-acetyl-aspartate, glutamate, abstinence, depression
Significance Statement
Methamphetamine Use Disorder is a prevalent, treatment-resistant, public health problem. Treatment often fails during early abstinence from the drug, when many patients experience depression and anxiety. In the inferior frontal cortex and the insula, which influence negative emotions, we measured levels of N-acetyl-compounds and glutamate compounds using magnetic resonance spectroscopy in methamphetamine users who had been <2 weeks abstinent and in healthy controls. Methamphetamine users, especially those with more years of heavy use, had higher levels of N-acetyl-metabolites than controls. Those with more severe depression and anxiety had lower levels of glutamate metabolites. These findings extend observations from animals and in vitro human cell cultures that methamphetamine increases neuronal N-acetyl aspartate concentration in living humans. They also motivate the development of medications that operate on N-acetyl and glutamatergic systems to assist treatment during early abstinence from methamphetamine.
Introduction
Amphetamine-type stimulants, particularly methamphetamine, are a rising class of abused drugs worldwide (UNODC, 2017). With few treatment options and no approved medications for Stimulant Use Disorders (Courtney and Ray, 2014), continued investigation of the neurochemical correlates of methamphetamine abuse is warranted. The present study focused on the neurometabolites N-acetyl-aspartate (NAA) and glutamate (Glu) in the inferior frontal cortex and the insula, 2 perisylvian brain regions that show abnormalities in methamphetamine users.
NAA production, or at least expression or activity of the enzyme that catalyzes NAA biosynthesis, is enhanced by methamphetamine in the ex vivo mouse nucleus accumbens (Niwa et al., 2007), in rat pheochromocytoma-12 cells (Niwa et al., 2008), and in human SH-SY5Y neuroblastoma cells (Ariyannur et al., 2010). The NAA synthase enzyme is denoted in the literature by several names, including N-acetyltransferase, N-acetyl-transferase-8-like protein, and shati, among others. Increase in the activity of this enzyme may represent an adaptive response to methamphetamine-induced elevation of extracellular dopamine, as the enzyme appears to diminish methamphetamine-associated behavioral effects such as hyperlocomotion, sensitization, and conditioned place preference (Niwa et al., 2007; Ariyannur et al., 2013). These observations and additional rodent studies intimate a more direct role for N-acetyl-compounds in higher brain functions than had previously been envisioned (Ariyannur et al., 2013) and suggest novel pharmacological pathways to management of methamphetamine abuse.
In human brain, NAA is measured with 1H MRS. It is commonly assayed alongside N-acetyl-aspartyl-glutamate (NAAG), and the combined NAA+NAAG signal is abbreviated tNAA (total N-acetyl-compounds). Contrasting with the aforementioned preclinical findings, MRS has indicated below-normal tNAA in multiple brain regions of methamphetamine users (Ernst et al., 2000; Nordahl et al., 2002, 2005; Salo et al., 2007, 2011a; Sung et al., 2007; Sailasuta et al., 2010). Because NAA and NAAG are abundant in neurons but not in other cells (Simmons et al., 1991; Urenjak et al., 1992), impaired neuronal metabolic activity in methamphetamine users is often offered as an explanation of low tNAA. The relevant studies, however, overwhelmingly sampled methamphetamine users in mid- to long-term abstinence from the drug and not during active abuse or early abstinence. Longitudinal investigations, including studies of cerebral glucose metabolism (Berman et al., 2008) and MRS (Ernst and Chang, 2008; Salo et al., 2011a), have shown that cortical metabolism changes over the course of methamphetamine abstinence. Such changes could involve tNAA. In our pilot study of methamphetamine users abstinent ≤2 weeks (O’Neill et al., 2010), tNAA in right inferior frontal cortex correlated positively with duration of heavy methamphetamine abuse. Here we report on such effects in our full sample in inferior frontal cortex and in a neighboring perisylvian brain region, the insula.
Much preclinical evidence also links methamphetamine with glutamatergic systems of the brain (Kalivas, 2007, 2009). In vivo in humans, Glu is also measured with MRS, but since the Glu spectrum overlaps with that of its derivative glutamine, the two are often assayed as a combined entity, Glx. One study showed no differences between methamphetamine users and controls in Glu or Glx in middle frontal cortex and anterior middle cingulate cortex (Howells et al., 2014), but others found differences in pregenual anterior cingulate cortex, anterior middle cingulate cortex, posterior cingulate cortex, and precuneus (Ernst and Chang, 2008; Crocker et al., 2014; O’Neill et al., 2014). In right inferior frontal cortex, Glx was lower in methamphetamine users than controls in a sample that overlapped with that of the current study (O’Neill et al., 2014), and Glx correlated negatively with severity of depressive symptoms. Depression and other mood disorders can contribute to relapse in methamphetamine abuse (Glasner-Edwards et al., 2008, 2009, 2010). We found no previous MRS reports of Glu or Glx in the insula of methamphetamine users. Glu interacts with NAA in multiple ways. NAAG is biosynthesized from NAA and Glu (Becker et al., 2010; Collard et al., 2010) and is biodecomposed into NAA and Glu (Robinson et al., 1987). NAAG colocalizes with Glu in synaptic vesicles (Neale et al., 2000) and is an antagonist of the N-methyl-D-aspartate glutamate receptor (Sekiguchi et al., 1989). Therefore, it is of interest to investigate MRS tNAA and Glx together.
Previous studies suggested that the insula and the inferior frontal gyrus show metabolical and structural abnormalities in chronic methamphetamine users (London et al., 2004; Thompson et al., 2004; Tabibnia et al., 2011; Morales et al., 2012), and neuroimaging endpoints in these regions are associated with affective symptoms. The insula participates in emotion processing (Medford and Critchley, 2010; Goerlich-Dobre et al., 2014), and anxiety covaries negatively with glucose metabolism in left insula of methamphetamine users (London et al., 2004). The right inferior frontal gyrus has a special role in response inhibition (Aron et al., 2014), which is impaired in methamphetamine users (Monterosso et al., 2005), and in inhibitory control more generally (Tabibnia et al., 2011). Therefore, the insula and the inferior frontal cortex were chosen for this planned analysis of tNAA and Glx in relation to methamphetamine use and symptoms of depression and anxiety.
Methods
Research Participants
All procedures were approved by the University of California Los Angeles Office for the Protection of Research Subjects. Participants (18–55 years old), who were recruited using Internet and newspaper advertisements, provided written informed consent. Forty-five individuals who met criteria for methamphetamine dependence per DSM-IV (Table 1) and were not seeking treatment agreed to remain abstinent from methamphetamine (verified by random urine screening) while residing at a research unit for 2 weeks. They were compared with 45 healthy controls without history of substance abuse (apart from tobacco and/or light use of marijuana or alcohol, defined as ≤1 joint per week or ≤10 drinks of liquor or the equivalent of beer or wine per week) or current use, as indicated by urine toxicology. The sample included here shared 42 methamphetamine and 23 control subjects from a previous study (O’Neill et al., 2014) in which all the controls were smokers; the control group in the present report included almost equal numbers of smokers and nonsmokers.
Table 1.
Characteristics of Research Participants
| Methamphetamine (n = 45) | Control (n = 45) | P | |
|---|---|---|---|
| Sex | .99 | ||
| Female | 24 | 23 | |
| Male | 21 | 22 | |
| Age, y | 33.0±9.3 | 32.9±8.6 | .94 |
| Education, y | 11.8±2.3 | 13.5±1.8 | <.0005 |
| Depression, BDI Score | 15.6±13.0 | 2.3±2.8 | <.0005 |
| Anxiety, STAI Y1 Score | 33.1±9.8 | 27.0±7.4 | .002 |
| Cigarette smoking | |||
| Smokers, n | 41 | 23 | <.0005 |
| Pack-years tobacco (smokers only) | 11.7±11.4 | 9.7±7.9 | .42 |
| Fagerström Score (smokers only) | 3.4±2.1 | 3.6±2.1 | .78 |
| Methamphetamine use | |||
| Duration of use, y | 11.1±7.8 | 0±0 | — |
| Duration of heavy use, y | 6.7±6.4 | 0±0 | — |
| Current use, g/wk | 2.0±1.4 | 0±0 | — |
| Marijuana use | |||
| Marijuana users, n | 27 | 4 | <.0005 |
| Current use, d/month | 5.5±9.2 | 0.2±0.8 | <.0005 |
Shown are numbers of participants or group means±SDs. P values are for Χ2 (sex, number of smokers, number of marijuana users) or 2-way independent t tests (all other variables), unless otherwise indicated for comparisons of the full methamphetamine to the full control sample.
Methamphetamine dependence and absence of other psychiatric disorders were established using the Structured Clinical Interview for DSM-IV Axis I Disorders. Heavy methamphetamine use was defined as using methamphetamine 3 times/wk or having a 2-day binge each week. Smoker status was verified by ≥10 ppm carbon monoxide in expired air (MicroSmokerlyzer; Bedfont Scientific Ltd) and the presence of urinary cotinine (≥200 ng/mL by Accutest NicAlert strips; JANT Pharmacal Corporation). The methamphetamine users were inpatients at the UCLA General Clinical Research Center, where they maintained abstinence from all drugs of abuse other than nicotine (in cigarettes) and caffeine (in beverages), as verified by urine toxicology. Control participants visited the research site on different days for psychological testing and imaging. The Beck Depression Inventory (BDI) and the Spielberger State-Trait Anxiety Inventory (STAI Y1 score) were administered within 1 week of admission to assess depressive symptoms and state anxiety, respectively.
Magnetic Resonance Acquisition
As described in O’Neill et al. (2014), magnetic resonance spectroscopic imaging (MRSI) using point-resolved spectroscopy (PRESS; repetition-time/echo-time = 1500/30 ms, voxels 11×11×9 mm3, 16×16 phase-encoding steps in-plane, 11:04-minute runtime including water-suppressed and non-water-suppressed acquisitions) of bilateral inferior frontal cortex and insula and whole-brain structural MRI (MPRAGE, 1×1×1 mm3 voxels) were acquired at 1.5 T (Siemens Sonata, standard quadrature head coil). MRSI was acquired from 2 sagittal-oblique 2D slices oriented parallel to the left, respectively, right temple, set ~2 cm deep into the brain, rotated parallel to the Sylvian fissure, and positioned to straddle this fissure dorsoventrally. Subject movement that could have occurred between the MPRAGE and MRSI scans was detected by comparing head position on the MPRAGE to that on a structural scan acquired after MRSI; scans were then repeated as needed. The MRSI prescription sampled inferior frontal cortex, insula, and superior temporal cortex. MRSI was also acquired from an axial-oblique (genu-splenium parallel) slice at the level of the basal ganglia and extending from the caudal edge of the corpus callosum anteriorly past the corpus callosum splenium posteriorly (Figure 1). These regions were sampled at a nominal voxel size of 1.1 cc. The effective voxel size, however, is larger, because the voxel is modestly smeared by spatial apodization and the point-spread function of MRSI. The effective voxel size is not trivial to calculate, but using factors obtained for other MRSI techniques (Théberge et al., 2005; Posse et al., 2007) we estimate it at 1.3 to 1.4 cc.
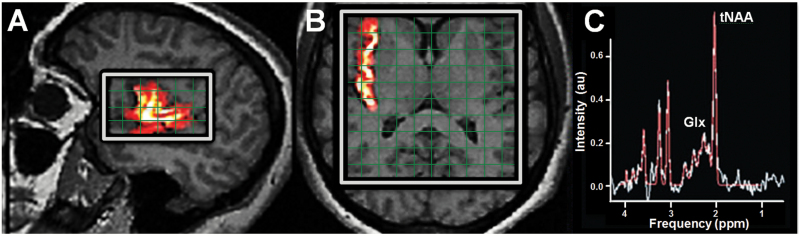
Figure 1.
(A) sagittal-oblique T1-weighted MRI of the brain showing sample coregistration of FreeSurfer right insula volume-of-interest (yellow-red) with superimposed right perisylvian 1H magnetic resonance spectroscopic imaging (MRSI) point-resolved spectroscopy (PRESS) excitation volume (“slice,” white rectangle). A similar sagittal-oblique PRESS slice sampled the left insula. (B) Overlap of the right insula volume with a third, axial-oblique PRESS slice. The opposite side of this slice cosampled the left insula. Each slice was 9 mm thick and consisted of a rectangular in-plane array of 11×11 mm2 voxels (green grids). (C) Sample right perisylvian MRSI spectrum showing high-amplitude, low-noise, well-separated peaks for the target neurometabolites N-acetyl-aspartate+N-acetyl-aspartyl-glutamate (tNAA) and glutamate+glutamine (Glx).
Magnetic Resonance Post-Processing
MRSI was post-processed as in O’Neill et al. (2014) with minor update in methods for the insula. Briefly, MR spectra were fit automatically with LCModel yielding levels of tNAA and Glx normed to the unsuppressed water resonance and expressed in Institutional Units (IU). Each MPRAGE was segregated in ICBM152 space into gray matter, white matter, and CSF subvolumes using SPM (http://www.fil.ion.ucl.ac.uk/spm/software/). The subvolumes were brought back into native space using the inverse of the MPRAGE-to-ICBM152 transform. Inferior frontal cortex MRSI voxels were selected manually as in O’Neill et al. (2014). For the insula, MRSI voxel selection was automated with the help of binary masks of left and right insula generated from the MPRAGE by FreeSurfer (https://surfer.nmr.mgh.harvard.edu/) in ICBM152 space. Again, masks were restored to native space with the inverse transform. Then, for both inferior frontal cortex and insula, using custom-written software, the MPRAGE, tissue subvolumes, and masks were aligned with each MRSI slice; the tissue-composition of each MRSI voxel was calculated; MRSI voxels were retained with >50% overlap with the target brain region (exclusive of CSF); and neurometabolite levels were corrected for voxel CSF content. Typically for all 4 brain regions and all 3 subject groups, 1 to 4 voxels per region passed all quality-control criteria and were included in the subject’s average for that region. Thus, differences in voxel sampling were unlikely to have affected results and—given the strict quality-control criteria, the voxel size relative to region volume, and the substantial local tissue content of voxels—the spectrum of even one voxel was deemed sufficient to represent a region.
Correction for Differences in MRSI Voxel Tissue-Composition
The group-mean volume fraction of gray matter, white matter, and CSF in MRSI voxels did not differ significantly between the 2 samples, except in the right insula, where the volume fraction of CSF was slightly (12% vs 14%) but significantly lower in methamphetamine users than in controls (P = .030, independent t test). MRSI neurometabolite levels were corrected for voxel CSF content to adjust for any such differences. Voxel white matter content was trendwise (P = .088) lower (3% vs 4%) while gray matter content was slightly higher (86% vs 84%; P = .052) in methamphetamine users; therefore, gray and white matter content were included as covariates in the relevant analyses to adjust for such differences.
Statistical Analyses
MRSI voxels within the PRESS excitation volume with ≥50 volume% of each target region were retained. Spectra with linewidth >0.10 ppm, signal-to-noise ratio <5, or not passing operator inspection were discarded, as were metabolite signals with Cramer-Rao Lower Bounds >20%. Metabolite data passing the foregoing quality-control criteria were averaged together for each subject to obtain a representative value for each metabolite region combination (e.g., tNAA in left insula). These quality control measures resulted in data from different numbers of participants contributing to the various analyses across regions and metabolites (Table 2).
Table 2.
Levels of tNAA and Glx in Perisylvian Cortices
| Left Inferior Frontal Cortex | Right Inferior Frontal Cortex | |||
|---|---|---|---|---|
| Methamphetamine | Control | Methamphetamine | Control | |
| tNAA | 8.2±1.2 | 8.1±1.0 | 8.2±1.0 | 8.2±0.9 |
| Participants | 41 | 44 | 37 | 41 |
| Glx | 12.4 ± 2.2 | 12.8±2.2 | 12.7±2.5* | 14.0±2.4 |
| Left insula | Right insula | |||
| Methamphetamine | Control | Methamphetamine | Control | |
| tNAA | 8.4±1.3** | 7.7±0.9 | 7.8±1.0 | 8.1±2.1 |
| Participants | 31 | 31 | 37 | 37 |
| Glx | 9.8±2.7 | 9.3±2.0 | 9.3±2.0* | 10.6±3.5 |
Indicated are numbers of participants with usable MRSI data for each metabolite in each target brain region.
tNAA and Glx levels are group means±SD in IU, corrected for voxel CSF content.
Significant between-group comparisons in bold (*P<.05, **P<.01 Bonferroni-corrected for multiple comparisons, ANCOVA covarying for age, sex, tobacco-smoking status, marijuana use status, and white and gray matter content).
Group differences in mean clinical endpoints were examined with independent t tests. Between-group differences in regional metabolite levels were tested using ANCOVA, Bonferroni-corrected for multiple comparisons. Covariates included age, sex, tobacco-smoking status, marijuana use status, and white mater and gray matter content. Given potential effects of tobacco use on neuroimaging endpoints, particularly in the insula (Morales et al., 2014; Naqvi et al., 2014), significant between-group differences in metabolite levels were retested comparing methamphetamine users with control smokers and control nonsmokers separately using independent t tests. Within-group relationships between regional metabolites and years of heavy methamphetamine abuse, BDI Score, and STAI Y1 Score were examined with Pearson correlation, Bonferroni-corrected for multiple comparisons. Significant findings were retested as Spearman correlations, partialling out demographic, clinical, or tissue content variables that may have significantly affected the metabolite level in question. Spearman rather than Pearson partial correlation was chosen, since the former is more conservative, less biased by outliers, and does not a priori presume linearly related and normally distributed variables. Based on our pilot study (O’Neill et al., 2010), we hypothesized that tNAA would be higher in the methamphetamine-dependent vs control subjects and/or would be correlated positively with years of heavy methamphetamine abuse in one or more perisylvian regions. The criterion for statistical significance was P<.05, whereby in cases with Bonferroni-correction the raw P value was multiplied by the number of multiple comparisons.
Results
Sample Characteristics
The methamphetamine users and controls were well matched for sex and age (Table 1), although the methamphetamine users had approximately 2 fewer years of education than controls on average (P<.0005) and most smoked cigarettes, whereas about one-half of the controls were smokers. Lifetime tobacco exposure (pack-years) and current tobacco dependence (Fagerström Score) did not differ significantly between smokers in the two groups. Although both groups reported light marijuana use, the methamphetamine users reported more (P<.0005); therefore, marijuana use was included as a covariate in between-group comparisons. The groups differed on self-reports of mood. The mean BDI score was ~6× higher (P<.0005, independent t test), and state anxiety was 23% higher (P = .002) in the methamphetamine users than controls.
Regional Neurometabolite Levels
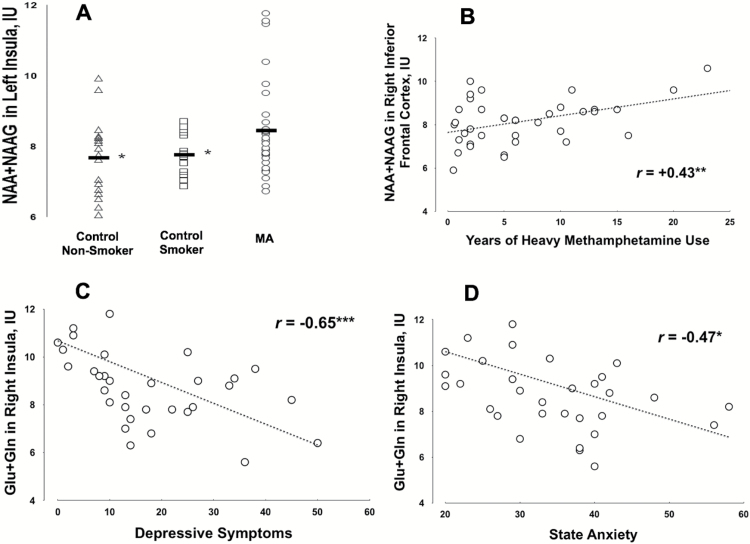
Across the various comparisons, the number of subjects per group with data passing quality control varied from 31 to 44 (Table 2). In left insula, tNAA was 9.1% higher in the methamphetamine than the control sample (Bonferroni-corrected P = .0064, ANCOVA covarying sex, age, smoking status, marijuana status, and gray and white matter). Of the 31 subjects with passing data quality in this region, 23 (74.2%) had tNAA values greater than the control mean. This difference remained significant when the methamphetamine users were compared separately with the smoking (8.8% higher; P = .019, independent t test) and nonsmoking (10.0% higher; P = .038) controls (Figure 2). The difference in tNAA, comparing the methamphetamine and total control samples, also remained significant when BDI score was added as a covariate (Bonferroni-corrected P = .004, ANCOVA). Although they were in no identifiable way atypical of the sample and none had a tNAA level ≥4 SDs above the mean of the remaining sample (a commonly used criterion for outliers), group-mean tNAA was still higher for methamphetamine than for control subjects at trend level (P = .086) when the 3 methamphetamine subjects with highest tNAA in this region were removed on an exploratory basis. When the full samples were compared using nonparametric statistics (less sensitive to outliers), tNAA was significantly higher for methamphetamine than for control subjects (P = .023). There were no significant group differences in tNAA in other regions.
Figure 2.
(A) CSF-corrected levels of N-acetyl-aspartate+N-acetyl-aspartyl-glutamate (tNAA) (NAA+NAAG) in Institutional Units (IU) in left insula of methamphetamine users (MA), control smokers, and control nonsmokers. Horizontal bars denote group means. *P<.05 for difference between the methamphetamine users and controls by independent t test. (B) Associations of tNAA in right inferior frontal cortex vs years of heavy methamphetamine use. P = .032, Pearson with Bonferroni correction. P = .047, (r = +0.34, Spearman) when effects of age, BDI Score, and pack-years of smoking were partialled out. (C–D) Negative associations of right insula glutamatergic compounds with symptoms of depression and anxiety in methamphetamine users. Y axes indicate CSF-corrected levels of Glx (Glu+ glutamine) in Institutional Units (IU). Depressive symptoms were self-reported using the Beck Depression Inventory (BDI), and state anxiety was self-reported using the Spielberger State-Trait Anxiety Inventory. P<*.05, ***.0005, Spearman with Bonferroni correction, partialling sex, age, smoking status, marijuana status, and gray and white matter.
Glx was significantly lower in the right inferior cortex (9.3% lower; Bonferroni-corrected P = .004, ANCOVA covarying sex, age, smoking status, marijuana status, and gray and white matter) and in the right insula (12.3% lower; Bonferroni-corrected P = .0004) in methamphetamine users than in controls (Table 2). There were no significant between-group differences in Glx in other regions.
There was a significant main effect of sex on Glx in left insula (P = .048), whereby Glx was 13.4% higher in males than in females. As indicated, groups were well balanced for sex (Table 1) and sex was included as a covariate in statistical analyses. There were no significant main effects of marijuana use status on regional metabolites.
Correlations of tNAA with Duration of Methamphetamine Abuse
In right inferior frontal cortex, tNAA correlated positively with years of heavy methamphetamine abuse (r = +0.43, Bonferroni-corrected P = .032, Pearson; Figure 2). This association remained significant when partialling age, BDI Score, and pack-years of smoking (r = +0.39, P = .047, Spearman). tNAA also correlated positively with years of any methamphetamine abuse, but this result did not survive Bonferroni correction. There were no significant correlations of tNAA with duration of heavy methamphetamine abuse in other regions or correlations of Glx with duration of heavy methamphetamine abuse in any region.
Correlations of Neurometabolite Levels with Symptoms of Depression and Anxiety
There were no significant associations of tNAA with BDI Score or STAI Y1 Score. Glx was negatively correlated with BDI Score in right inferior frontal gyrus (r = -0.51, Bonferroni-corrected P = .0052, Spearman, partialling sex, age, smoking status, marijuana status, and gray and white matter) and right insula (r = -0.44, Bonferroni-corrected P = .0004, partialling sex, age, smoking status, marijuana status, and gray and white matter). In right insula, Glx was additionally significantly negatively correlated with STAI Y1 Score (r = -0.47, Bonferroni-corrected P = .024, Spearman, partialling sex, age, smoking status, marijuana status, and gray and white matter).
Discussion
This study yielded 3 major findings: (1) tNAA in methamphetamine users was moderately elevated in the left insula and in the right inferior frontal cortex tNAA was positively correlated with years of heavy methamphetamine exposure; (2) in the right inferior frontal cortex and right insula, Glx was lower in methamphetamine users; and (3) in those regions, Glx was negatively correlated with severity of depression and anxiety symptoms in methamphetamine users. Thus, chronic methamphetamine users exhibit modest increments in regional cortical tNAA levels, similar to those observed in ex vivo and in vitro systems. Results also support previous findings associating the mood symptoms of early methamphetamine abstinence with glutamatergic metabolism.
Mean tNAA was greater than the control mean in one brain region of methamphetamine abusers, and tNAA correlated with duration of heavy methamphetamine exposure in another region. These effects remained significant after accounting for age, tobacco smoking, MRSI voxel tissue-composition, and BDI Score, and for multiple comparisons. Possible explanations include stimulation of NAA synthase by methamphetamine as in preclinical models (Niwa et al., 2007, 2008; Ariyannur et al., 2010), deceleration of NAA degradation, and (possibly untoward) neuroplastic increase in neuronal mass or metabolic activity, and others. Noninvasive in vivo human proton MRS is not capable of distinguishing among these various mechanisms, which are also not mutually exclusive. The tNAA effect was small, comprising an approximately 10% group-mean difference (left insula) and a 0.07 IU/y increment (right inferior frontal cortex) and was not statistically significant in all regions tested. Although significant effects on the order of 5% to 15% are common in MRS studies of brain N-acetyl- (and glutamatergic) compounds in methamphetamine abuse (Ernst et al., 2000; Nordahl et al. 2002; Salo et al., 2007, 2011a, 2011b; Sailasuta et al., 2010; Cloak et al., 2011; Crocker et al., 2014; Howells et al., 2014), the subtlety of the present tNAA effects may reflect superimposition of tNAA elevation from methamphetamine on tNAA declines due to aging (Aoki et al., 2012), chronic cigarette smoking (Durazzo et al., 2016), or other factors. That tNAA correlated with duration of heavy (as opposed to any) methamphetamine abuse is consistent with in vitro observations that methamphetamine significantly increased NAA only at higher methamphetamine doses (Ariyannur et al., 2010). Overall, these results are consistent with a gradual rise in tNAA with continuing methamphetamine exposure, perhaps as an adaptation to excess dopamine signaling (Niwa et al., 2007). It is recommended that tNAA in early abstinence from methamphetamine be reinvestigated for verification in further samples and at high-field strength.
Prior studies of methamphetamine users documented below-normal tNAA in some brain regions (Ernst et al., 2000; Nordahl et al., 2002, 2005; Salo et al., 2007, 2011a; Sung et al., 2007; Sailasuta et al., 2010). Rather than inferior frontal cortex and insula, as sampled here, these studies interrogated other brain regions (pregenual anterior and anterior middle cingulate cortices, prefrontal white matter, caudate-putamen, occipital cortex, and dorsal posterior cingulate cortex) than those examined here. Moreover, participants in those studies had been abstinent from methamphetamine for more than the 2-week maximum in this study. Methamphetamine effects on tNAA may vary with brain region or duration of abstinence. In early abstinence, tNAA elevations in compensation to dopaminergic hyperactivation due to methamphetamine may predominate in certain regions over tNAA decreases from neuronal loss or impairment. In mid-term abstinence, the former effect may fade leading the latter effects to predominate. Finally, in late abstinence rise of tNAA may be observed with recovery of function by some neurons (Salo et al., 2011a). Although, if methamphetamine enhances NAA production, one might anticipate a decrease in NAA production during abstinence due to removal of the enhancing factor; it may take time, especially after years of methamphetamine abuse as in our sample, for such decrease to become detectable. Hence, present tNAA observations may, in some aspects, reflect “overhang” from the chronic state of active abuse rather than acute changes precipitated by abstinence.
Inflammatory processes may influence MRS neurometabolite levels (Chang et al., 2013) and the evolution of brain metabolism during recovery from methamphetamine abuse (Volkow et al., 2001; Berman et al., 2008). We previously suggested that higher cortical tNAA in early abstinence from methamphetamine could be due to local invasion of microglia as part of the inflammatory response (O’Neill et al., 2010), since activated microglia contain high concentrations of NAAG (Passani et al., 1998). NAA itself, moreover, acts as an antiinflammatory agent for reactive astroglia (Rael et al., 2004). Hence, an inflammatory response in early abstinence could initiate a transient rise in tNAA levels. Longitudinal cortical MRS measurements may help answer such questions.
Findings also included Glx approximately 10% lower in right inferior frontal cortex and insula in methamphetamine users and that Glx in these regions was negatively correlated with depression and anxiety. The findings in right inferior frontal cortex largely replicated those in O’Neill et al. (2014), which had essentially the same methamphetamine sample. However, the control sample in the present analysis was now as large as the methamphetamine sample, rather than being about one-half the size. The present study extends findings to the neighboring right insula, where a negative relation of Glx with anxiety was also found. As discussed in O’Neill et al. (2014), depression appears to be associated with glutamatergic systems and with the right forebrain. Glutamatergic agents might offer promise in combatting the often severe depressive symptoms of early abstinence from methamphetamine (Zorick et al., 2011). Present findings also motivate exploration of MRS Glx as a potential biomarker of relapse in methamphetamine abuse in systematic clinical trials.
Overall, the current findings add to evidence pointing to (especially right) inferior frontal cortex and insula as sites of sequelae of methamphetamine abuse (London et al., 2004; Thompson et al., 2004; Tabibnia et al., 2011; Morales et al., 2012; O’Neill et al., 2014; Okita et al., 2016). That the major findings were hemispherically lateralized is not surprising, as functional lateralization within perisylvian cortices is well known. Prominent examples include lateralization of language (Ojemann, 1991), mood symptoms following cerebrovascular insult (Starkstein et al., 1989; Morris et al., 1996), and motor response inhibition (Aron et al., 2014). In fact, a possible alternative explanation of our Glx findings in right inferior frontal cortex and insula (Table 2) is that controls have a higher Glx on the right side, perhaps reflecting functional lateralization that does not pertain in the methamphetamine subjects. We have previously observed effects on glucose metabolism lateralized to the left insula in methamphetamine abuse (London et al., 2004). It remains a challenge of neuroimaging research to delineate more fully the course, character, and extent of methamphetamine-associated neurophysiological effects on these regions and their possible role in therapeutic response.
This investigation had limitations including MRS acquisition at 1.5 T. Studies at 3 T would yield larger and better separated metabolite signals. Acquisition at ultrahigh-field (e.g., 7 T) would further enable separation of the NAA and NAAG components of the tNAA signal. In addition, cross-sectional, rather than longitudinal, data were used. As is common in studies of stimulant abuse, marijuana use was much more prevalent in the methamphetamine than in the control sample, representing a potential confound. Marijuana use status, however, did not affect regional metabolite endpoints and was included as a statistical covariate. These weaknesses are balanced by certain strengths, including: high spatial resolution (nominally approximately 1.1 cc, effectively approximately 1.3–1.4 cc) of MRSI; a rarely used sagittal-oblique MRSI prescription to access inferior frontal cortex and insula; water-referenced neurometabolite levels rather than ratios to creatine+phosphocreatine; determination of MRSI voxel tissue composition and its use in quantitation; a well-characterized methamphetamine user sample in recent abstinence from drug; samples fairly well matched for sex; and consideration of tobacco smoking. The tNAA findings represent a possible human in vivo expression of methamphetamine effects previously observed only preclinically, and the Glx findings reinforce notions of a possible glutamatergic basis for the mood symptoms of early abstinence from methamphetamine.
Acknowledgments
This work was supported by the National Institute on Drug Abuse (P20DA022539 to E.D.L., R01DA020726 to E.D.L., R03DA20512 to J.O.N., and R21DA023192 to J.O.N.); the National Center for Research Resources (MOIRR00865 from the UCLA General Clinical Research Center); and endowments from the Thomas P. and Katherine K. Pike Chair in Addiction Studies (E.D.L.) and the Marjorie M. Greene Trust.
Statement of Interest
None.
References
- Aoki Y, et al. (2012)Absence of age-related prefrontal NAA change in adults with autism spectrum disorders. Transl Psychiatry 2:e178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyannur PS, Moffett JR, Manickam P, Pattabiraman N, Arun P, Nitta A, Nabeshima T, Madhavarao CN, Namboodiri AM(2010)Methamphetamine-induced neuronal protein NAT8L is the NAA biosynthetic enzyme: implications for specialized acetyl coenzyme A metabolism in the CNS. Brain Res 1335:1–13. [DOI] [PubMed] [Google Scholar]
- Ariyannur PS, Arun P, Barry ES, Andrews-Shigaki B, Bosomtwi A, Tang H, Selwyn R, Grunberg NE, Moffett JR, Namboodiri AM(2013)Do reductions in brain N-acetylaspartate levels contribute to the etiology of some neuropsychiatric disorders?J Neurosci Res 91:934–942. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA(2014)Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18:177–185. [DOI] [PubMed] [Google Scholar]
- Arun P, Madhavarao CN, Moffett JR, Namboodiri AM(2008)Antipsychotic drugs increase N-acetylaspartate and N-acetylaspartylglutamate in SH-SY5Y human neuroblastoma cells. J Neurochem 106:1669–1680. [DOI] [PubMed] [Google Scholar]
- Becker I, Lodder J, Gieselmann V, Eckhardt M(2010)Molecular characterization of N-acetylaspartylglutamate synthetase. J Biol Chem 285:29156–29164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SM, Voytek B, Mandelkern MA, Hassid D, Isaacson A, Monterosso J, Miotto K, Ling W, London ED(2008)Changes in regional cerebral metabolism during early abstinence from chronic methamphetamine abuse. Molec Psychiatry 13:897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Munsaka SM, Kraft-Terry S, Ernst T(2013)Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol 8:576–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloak CC, Alicata D, Chang L, Andrews-Shigaki B, Ernst T(2011)Age and sex effects levels of choline compounds in the anterior cingulate cortex of adolescent methamphetamine users. Drug Alcohol Depend 119:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collard F, Stroobant V, Lamosa P, Kapanda CN, Lambert DM, Muccioli GG, Poupaert JH, Opperdoes F, Van Schaftingen E(2010)Molecular identification of N-acetylaspartylglutamate synthase and beta-citrylglutamate synthase. J Biol Chem 285:29826–29833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ray LA(2014)Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend 143:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker CE, Bernier DC, Hanstock CC, Lakusta B, Purdon SE, Seres P, Tibbo PG(2014)Prefrontal glutamate levels differentiate early phase schizophrenia and methamphetamine addiction: a (1)H MRS study at 3tesla. Schizophr Res 157:231–237. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Mon A, Abé C, Gazdzinski S, Murray DE(2016)Chronic cigarette smoking in healthy middle-aged individuals is associated with decreased regional brain N-acetylaspartate and glutamate levels. Biol Psychiatry 79:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L(2008)Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. J Neuroimmune Pharmacol 3:165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L, Leonido-Yee M, Speck O(2000)Evidence for long-term neurotoxicity associated with methamphetamine abuse: A 1H MRS study. Neurology 54:1344–1349. [DOI] [PubMed] [Google Scholar]
- Glasner-Edwards S, Marinelli-Casey P, Hillhouse M, Ang A, Mooney LJ, Rawson R, the Methamphetamine Treatment Project Corporate Authors (2009)Depression among methamphetamine users association with outcomes from the Methamphetamine Treatment Project at 3-year follow-up. Am J Addictions 17:99–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson R, the Methamphetamine Treatment Project Corporate Authors (2008)Identifying methamphetamine users at risk for major depressive disorder: findings from the methamphetamine treatment project at three-year follow-up. Drug Alcohol Rev 29:12–20. [DOI] [PubMed] [Google Scholar]
- Glasner-Edwards S, Mooney LJ, Marinelli-Casey P, Hillhouse M, Ang A, Rawson RA, Methamphetamine Treatment Project Corporate Authors (2010)Psychopathology in methamphetamine-dependent adults 3 years after treatment. Drug Alcohol Rev 29:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerlich-Dobre KS, Bruce L, Martens S, Aleman A, Hooker CI(2014)Distinct associations of insula and cingulate volume with the cognitive and affective dimensions of alexithymia. Neuropsychologia 53:284–292. [DOI] [PubMed] [Google Scholar]
- Howells FM, Uhlmann A, Temmingh H, Sinclair H, Meintjes E, Wilson D, Stein DJ(2014)(1)H-magnetic resonance spectroscopy ((1)H-MRS) in methamphetamine dependence and methamphetamine induced psychosis. Schizophr Res 153:122–128. [DOI] [PubMed] [Google Scholar]
- Kalivas PW.(2007)Cocaine and amphetamine-like psychostimulants: neurocircuitry and glutamate neuroplasticity. Dialogues Clin Neurosci 9:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW.(2009)The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572. [DOI] [PubMed] [Google Scholar]
- London ED, Simon S, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, Shinn AK, Miotto K, Learn J, Dong Y, Matochik JA, Kurian V, Newton T, Woods R, Rawson R, Ling W(2004)Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry 61:73–84. [DOI] [PubMed] [Google Scholar]
- Medford N, Critchley HD(2010)Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct 214:535–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monterosso JR, Aron AR, Cordova X, Xu J, London ED(2005)Deficits in response inhibition associated with chronic methamphetamine abuse. Drug Alcohol Depend 79:273–277. [DOI] [PubMed] [Google Scholar]
- Morales A, Lee B, Hellemann G, O’Neill J, London ED(2012)Gray-matter volume in methamphetamine dependence: cigarette smoking and changes with abstinence from methamphetamine. Drug Alcohol Depend 125:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales AM, Ghahremani DG, Kohno M, London ED(2014)Cigarette exposure, dependence and craving are related to insula thickness in young adult smokers. Neuropsychopharmacology 39:1816–1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris PL, Robinson RG, Raphael B, Hopwood MJ(1996)Lesion location and poststroke depression. J Neuropsychiatry Clin Neurosci 8:399–403. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, Bechara A(2014)The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci 1316:53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale JH, Bzdega T, Wroblewska B(2000)N-acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem 75:443–452. [DOI] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Mizoguchi H, Ito Y, Noda Y, Nagai T, Nabeshima T(2007)A novel molecule “shati” is involved in methamphetamine-induced hyperlocomotion, sensitization, and conditioned place preference. J Neurosci 27:7604–7615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa M, Nitta A, Cen X, Kitaichi K, Ozaki N, Yamada K, Nabeshima T(2008)A novel molecule ‘shati’ increases dopamine uptake via the induction of tumor necrosis factor-alpha in pheochromocytoma-12 cells. J Neurochem 107:1697–1708. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Possin K, Gibson DR, Flynn N, Leamon M, Galloway GP, Pfefferbaum A, Spielman DM, Adalsteinsson E, Sullivan EV(2002)Low N-acetyl-aspartate and high choline in the anterior cingulum of recently abstinent methamphetamine-dependent subjects: a preliminary proton MRS study. Magnetic resonance spectroscopy. Psychiatry Res 116:43–52. [DOI] [PubMed] [Google Scholar]
- Nordahl TE, Salo R, Natsuaki Y, Galloway GP, Waters C, Moore CD, Kile S, Buonocore MH(2005)Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry 62:444–452. [DOI] [PubMed] [Google Scholar]
- Ojemann GA.(1991)Cortical organization of language. J Neurosci 11:2281–2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K, Ghahremani DG, Payer DE, Robertson CL, Mandelkern MA, London ED(2016)Relationship of alexithymia ratings to dopamine D2-type receptors in anterior cingulate and insula of healthy control subjects but not methamphetamine-dependent individuals. Int J Neuropsychopharm 19:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Thomas S, Hudkins M, Dean A, Tobias MC, London ED(2010)MRSI and a model of metabolic dysfunction in human methamphetamine dependence. Paper presented at: NIDA Translational Research in Methamphetamine Addiction Conference;July 21, 2010; Pray, MT. [Google Scholar]
- O’Neill J, Tobias MC, Hudkins M, London ED(2014)Glutamatergic neurometabolites during early abstinence from chronic methamphetamine abuse. Int J Neuropsychopharmacol 18:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passani L, Elkabes S, Coyle JT(1998)Evidence for the presence of N-acetylaspartylglutamate in cultured oligodendrocytes and LPS activated microglia. Brain Res 794:143–145. [DOI] [PubMed] [Google Scholar]
- Posse S, Otazo R, Caprihan A, Bustillo J, Chen H, Henry PG, Marjanska M, Gasparovic C, Zuo C, Magnotta V, Mueller B, Mullins P, Renshaw P, Ugurbil K, Lim KO, Alger JR(2007)Proton echo-planar spectroscopic imaging of J-coupled resonances in human brain at 3 and 4 tesla. Magn Reson Med 58:236–244. [DOI] [PubMed] [Google Scholar]
- Rael LT, Thomas GW, Bar-Or R, Craun ML, Bar-Or D(2004)An anti-inflammatory role for N-acetyl aspartate in stimulated human astroglial cells. Biochem Biophys Res Commun 319:847–853. [DOI] [PubMed] [Google Scholar]
- Robinson MB, Blakely RD, Couto R, Coyle JT(1987)Hydrolysis of the brain dipeptide N-acetyl-L-aspartyl-L-glutamate. Identification and characterization of a novel N-acetylated alpha-linked acidic dipeptidase activity from rat brain. J Biol Chem 262:14498–14506. [PubMed] [Google Scholar]
- Sailasuta N, Abulseoud O, Hernandez M, Haghani P, Ross BD(2010)Metabolic abnormalities in abstinent methamphetamine dependent subjects. Subst Abuse 2010:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Natsuaki Y, Leamon MH, Galloway GP, Waters C, Moore CD, Buonocore MH(2007)Attentional control and brain metabolite levels in methamphetamine abusers. Biol Psychiatry 61:1272–1280. [DOI] [PubMed] [Google Scholar]
- Salo R, Buonocore MH, Leamon M, Natsuaki Y, Waters C, Moore CD, Galloway GP, Nordahl TE(2011a) Extended findings of brain metabolite normalization in MA-dependent subjects across sustained abstinence: a proton MRS study. Drug Alcohol Depend 113:133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo R, Nordahl TE, Buonocore MH, Natsuaki YT, Moore CD, Waters C, Leamon MH (2011b) Spatial inhibition and the visual cortex: a magnetic resonance spectroscopy imaging study. Neuropsychologia 49:830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi M, Okamoto K, Sakai Y(1989)Low-concentration N-acetylaspartylglutamate suppresses the climbing fiber response of purkinje cells in guinea pig cerebellar slices and the responses to excitatory amino acids of Xenopus laevis oocytes injected with cerebellar mRNA. Brain Res 482:87–96. [DOI] [PubMed] [Google Scholar]
- Simmons ML, Frondoza CG, Coyle JT(1991)Immunocytochemical localization of N-acetyl-aspartate with monoclonal antibodies. Neuroscience 45:37–45. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Robinson RG, Honig MA, Parikh RM, Joselyn J, Price TR(1989)Mood changes after right-hemisphere lesions. Br J Psychiatry 155:79–85. [DOI] [PubMed] [Google Scholar]
- Sung YH, Cho SC, Hwang J, Kim SJ, Kim H, Bae S, Kim N, Chang KH, Daniels M, Renshaw PF, Lyoo IK(2007)Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: a proton magnetic resonance spectroscopy study. Drug Alcohol Depend 88:28–35. [DOI] [PubMed] [Google Scholar]
- Tabibnia G, Monterosso JR, Baicy K, Aron AR, Poldrack RA, Chakrapani S, Lee B, London ED(2011)Different forms of self-control share a neurocognitive substrate. J Neurosci 31:4805–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Théberge J, Menon RS, Williamson PC, Drost DJ(2005)Implementation issues of multivoxel STEAM-localized 1H spectroscopy. Magn Reson Med 53:713–718. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi K, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED(2004)Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci 24:6028–6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (NODC) (2017)World Drug Report 2017, United Nations Publication, Vienna: Available at https://www.unodc.org/wdr2017/index.html. [Google Scholar]
- Urenjak J, Williams SR, Gadian DG, Noble M(1992)Specific expression of N-acetylaspartate in neurons, oligodendrocyte-type-2 astrocyte progenitors, and immature oligodendrocytes in vitro. J Neurochem 59:55–61. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Wong C, Logan J(2001)Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am J Psychiatry 158:383–389. [DOI] [PubMed] [Google Scholar]
- Zorick T, Sugar C, Hellemann G, Shoptaw S, London ED(2011)Poor response to sertraline in methamphetamine dependence is associated with sustained craving for methamphetamine. Drug Alcohol Depend 118:500–503. [DOI] [PMC free article] [PubMed] [Google Scholar]