Abstract
Background
The role of orexin-A in regulating metabolic homeostasis has been recognized, but its association with antipsychotic-induced metabolic abnormalities remains unclear. We investigated the association between orexin-A levels and metabolic syndrome in patients with schizophrenia treated with clozapine or less obesogenic antipsychotics compared with nonpsychiatric controls.
Methods
Plasma orexin-A levels and metabolic parameters were determined in 159 patients with schizophrenia: 109 taking clozapine; 50 taking aripiprazole, amisulpride, ziprasidone, or haloperidol; and 60 nonpsychiatric controls.
Results
Orexin-A levels were significantly higher in the group taking less obesogenic antipsychotics, followed by the clozapine group and the controls (F=104.6, P<.01). Higher orexin-A levels were correlated with better metabolic profiles in the patient groups but not in the controls. Regression analyses revealed that the patients with higher orexin-A levels had significantly lower risk of metabolic syndrome (adjusted odds ratio [OR]=0.04, 95% CI: 0.01–0.38 for the 2nd tertile; OR=0.04, 95% CI: 0.01–0.36 for the 3rd tertile, compared with the first tertile), after adjustment for age, sex, smoking history, types of antipsychotics (clozapine vs less obesogenic antipsychotics), duration of antipsychotic treatment, and disease severity.
Conclusions
Our results revealed that the orexin-A level was upregulated in patients with schizophrenia treated with antipsychotics, especially for the group taking less obesogenic antipsychotics. Furthermore, higher orexin-A levels were independently associated with better metabolic profiles. These observations suggest that an upregulation of orexin-A has a protective effect against the development of metabolic abnormalities in patients with schizophrenia receiving antipsychotic treatment.
Keywords: orexin-A, schizophrenia, antipsychotics, metabolic syndrome, clozapine
Significance Statement
Patients with schizophrenia have a greater risk of metabolic syndrome (MS) than the general population. Orexins are neuropeptides that have been suggested to integrate central and peripheral signals to regulate metabolic homeostasis. We found orexin-A level was upregulated in antipsychotics-treated patients with schizophrenia, and those taking clozapine had lower orexin-A levels than less obesogenic antipsychotics. A higher orexin-A level was independently associated with better metabolic profiles. Orexin-A may have a protective effect against the development of MS in schizophrenia patients receiving antipsychotic treatment.
Introduction
Although some studies have suggested that schizophrenia is an independent risk factor for glycemic abnormalities and insulin resistance (Bushe and Holt, 2004; Greenhalgh et al., 2017), studies have consistently shown that antipsychotic treatment is associated with increased risks of metabolic syndrome (MS), which comprises a cluster of risk factors, including increased blood pressure (BP), dyslipidemia (raised triglycerides [TG] and reduced high-density lipoprotein cholesterol [HDL-C]), increased fasting plasma glucose [FPG], and central obesity, which occur jointly to increase the risk of type 2 diabetes mellitus (DM) and cardiovascular disease (Alberti et al., 2009). Patients with schizophrenia who receive antipsychotics have a 2- to 4-fold higher risk of MS than that of the general population (McEvoy et al., 2005; Huang et al., 2009). The risk of MS and related metabolic abnormalities in relation to antipsychotic treatment varies according to the type of administered medication, with clozapine and olanzapine being associated with the highest risk among antipsychotics (Vancampfort et al., 2015). Furthermore, the susceptibility to metabolic abnormalities varies among individuals (Lieberman et al., 2005). However, the mechanisms underlying the different liabilities remain unclear.
Orexins (i.e., hypocretins) are neuropeptides mainly produced by neurons located in the lateral hypothalamic area and have been suggested to integrate central and peripheral signals to regulate metabolic homeostasis (Tsuneki et al., 2010). The orexin system comprises 2 neuropeptides, orexin-A and orexin-B (products of the same prepro-orexin gene), and their receptors (orexin receptor types 1 and 2) (Sakurai et al., 1998). Orexin-A has enhanced feeding behavior in preclinical studies (Sakurai et al., 1998). Furthermore, it has been shown to be beneficial for metabolic functions. Exogenous orexin-A administration stimulates thermogenesis and energy expenditure (Messina et al., 2014) by increasing sympathetic nervous systemic activity (Shirasaka et al., 1999; Messina et al., 2018), particularly the signals to brown fat tissues (Messina et al., 2017). Exogenous orexin-A administration in mice also reduces blood glucose levels by increasing insulin secretion and prevents insulin resistance (Park et al., 2015; Tsuneki et al., 2015). Preclinical evidence suggests that the neuronal activity of orexin-A, measured using immediate-early gene c-Fos induction, can be altered by a single dose of antipsychotics, with the alteration varying among antipsychotics with different weight gain liabilities (Fadel et al., 2002). Additionally, antipsychotic administration potentially modifies the ability of orexin-A to regulate energy expenditure. Acute antipsychotics treatment in rats, namely treatment with haloperidol (Monda et al., 2003), clozapine (Monda et al., 2004), and olanzapine (Monda et al., 2008), reduces the sympathetic activation induced by exogenous orexin-A administration, whereas quetiapine delays the sympathetic activation (Monda et al., 2006). This suggests that sympathetic activation induced by orexin-A and ensuing hyperthermic effect, which promotes energy expenditure, could be blocked by antipsychotic treatment and thus contributes to weight gain (Monda et al., 2018). However, the role of orexin-A in metabolic outcomes following long-term antipsychotic administration in human subjects remains unclear.
Plasma orexin-A levels linearly correlate with its levels in cerebrospinal fluid (CSF) in humans, suggesting that plasma orexin-A levels are an easily accessible and reliable index of central orexin activity (Strawn et al., 2010). Clinical studies investigating changes in orexin-A levels following antipsychotic treatment in patients with schizophrenia have reported conflicting findings (Dalal et al., 2003; Basoglu et al., 2010; Chien et al., 2015). Chien et al. (2015) reported that patients receiving chronic antipsychotic treatment had higher orexin-A levels compared with controls. Only 1 study has examined the association between orexin-A levels and metabolic parameters in patients with schizophrenia (Basoglu et al., 2010). Because the benefit of antipsychotic treatment in patients with schizophrenia is limited by the propensity of these drugs leading to metabolic disturbances, clarifying the pathways underlying antipsychotic-associated metabolic dysregulation is critical for improving clinical outcomes in this group.
We investigated the relationship between orexin-A levels and MS in patients with schizophrenia who had been chronically treated with antipsychotics. Specifically, we compared orexin-A levels in nonpsychiatric controls and patients taking antipsychotics with different weight gain liabilities, namely clozapine (Baptista et al., 2008) and other less obesogenic drugs (namely aripiprazole, amisulpride [Vancampfort et al., 2015], ziprasidone, and haloperidol [Leucht et al., 2013]). Because evidence suggests a link between orexin-A and metabolic functions and that orexin-A plays an adaptive role to counteract antipsychotic-associated metabolic disturbances, we examined the hypothesis that orexin-A acts as an independent risk factor for the development of MS using age, sex, smoking, types of antipsychotics (i.e., clozapine vs less obesogenic drugs), duration of antipsychotic treatment, and disease severity as potential confounders.
Materials and Methods
Setting
This cross-sectional study was conducted at Taipei City Psychiatric Center, Taipei City Hospital from July 2011 to December 2014 after obtaining approval from the hospital’s Institutional Review Board (IRB nos: TCHIRB-991211, TCHIRB-1000407, and TCHIRB-1020812).
Study Participants
Patients with schizophrenia from either the outpatient department or inpatient wards were consecutively recruited. Their clinical diagnosis was verified by 2 psychiatrists with 10 or more years of clinical experience. The inclusion criteria were as follows: (1) a diagnosis of schizophrenia in accordance with the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (Text Revision) by the American Psychiatric Association, (2) 20 to 65 years of age, and (3) continuous use of either clozapine (Baptista et al., 2008) or a less obesogenic antipsychotic (i.e., aripiprazole, amisulpride, ziprasidone, or haloperidol) (Leucht et al., 2013; Vancampfort et al., 2015) for more than 6 months. Because of difficulty in enrolling drug-naïve patients with schizophrenia, nonpsychiatric controls were enrolled from the physical check-up service in the hospital by applying the following inclusion criteria: (1) no history of a major psychiatric disorder (including schizophrenia, schizoaffective disorder, bipolar disorder, major depressive disorder, organic mental disorders, and substance abuse disorders) screened using the Mini-International Neuropsychiatric Interview (Sheehan et al., 1998) and (2) 20 to 65 years of age. Written informed consent was obtained from each participant. Data, including demographic and clinical information (such as diagnoses), and the duration and type of antipsychotics were collected from clinical interviews and medical records. The severity of psychotic symptoms of patients with schizophrenia was assessed using the Brief Psychiatric Rating Scale (BPRS-18) (Lee et al., 1990).
Metabolic Parameter Measurements
Anthropometrical measurements, namely height, body weight (BW), waist circumference (WC), and BP, were taken by trained nurses. Body mass index (BMI) was calculated as the patient’s BW (kg) divided by height (m2). Venous blood samples were collected in tubes containing ethylenediaminetetraacetic acid (1 mg/mL of blood) anticoagulant at 8:00 to 9:00 am after an overnight fast to analyze FPG, glycated hemoglobin (HbA1c), insulin, TG, and HDL-C levels by using an automatic biochemistry analyzer. Plasma was separated by centrifugation, and aliquots of plasma were immediately frozen and stored at −80°C until analysis, with a storage time of 10 to 15 months. Plasma orexin-A levels were analyzed using an extraction-free enzyme immunosorbent assay (EIA) (catalog no. EKE-003-30, Phoenix Pharmaceuticals, Belmont, CA). The intra-assay CV was <5%, and the assay kit had no cross-reactivity with any other substance, namely orexin-B, agouti-related protein, neuropeptide, alpha-MSH, or leptin. The minimal detectable amount of orexin-A assay was 0.17 ng/mL (per the manufacturer).
MS was defined using the modified criteria of the National Cholesterol Education Program Adult Treatment Panel III (2005) (Grundy et al., 2005), which refers to at least 3 of the following component risk factors: (1) WC>90 cm for men or >80 cm for women; (2) TG≥150 mg/dL; (3) HDL-C<40 mg/dL for men and <50 mg/dL for women; (4) systolic BP≥130 mm Hg or diastolic BP≥85 mmHg or current use of antihypertensive drugs; and (5) FPG≥110 mg/dL or current use of antihyperglycemic drugs. Obesity and overweight were defined as a BMI≥27 kg/m2 and ≥24 kg/m2, respectively, according to the criteria of the Department of Health in Taiwan (Lin et al., 2003). DM was defined when FPG levels were ≥126 mg/dL or antidiabetic medications were prescribed. Hypertension was defined when systolic BP≥140 mmHg, diastolic BP≥90 mmHg, or antihypertensive medications were prescribed. The homeostasis model assessment for insulin resistance (HOMA-IR) value was calculated as FPG (mg/dL)×fasting insulin (mU/L)/405.
Statistical Analyses
Descriptive statistics were used to present the demographic, clinical, and metabolic characteristics of the 3 comparison groups (patients on clozapine, patients on less obesogenic antipsychotics, and nonpsychiatric controls). Logarithmic transformation was applied to data for which the assumption of normality had been violated. One-way ANOVA was then used to compare the continuous variables among the 3 groups with Games–Howell methods (Games and Howell, 1976), and posthoc tests were applied if significance was found. Chi-square tests were used to compare the categorical variables. Independent t tests were also performed for comparing the 2 groups, when appropriate. Pearson’s correlation coefficient (Pearson, 1909) was used for examining the correlations between orexin-A and the metabolic parameters, namely BW, BMI, systolic BP, diastolic BP, FPG, TG, HDL-C, HbA1c, insulin, and HOMA-IR. Using MS as a dichotomous outcome, we applied logistic regression to assess the association between the groups (i.e., patients taking clozapine, less obesogenic antipsychotics, and nonpsychiatric controls) (major exposure of interest) and the presence of MS (outcome) after adjusting for possible confounders, namely age, sex (Huang et al., 2009), and smoking history, without/with plasma orexin-A levels to examine its mediating effect (model 1/model 2). To maximize statistical power and avoid linear assumption of using orexin-A level as a continuous variable in the analyses, we categorized the orexin-A concentration into tertiles (33.3% percentiles) for further analysis. Two study participants with missing values of orexin-A concentration were not included to estimate the relative risk of orexin-A on MS development in further analysis by modeling. All P values were 2-sided. P<.05 was considered significant. All analyses were conducted using IBM SPSS Statistics version 22 (IBM Corporation, Armonk, NY).
Results
Clinical Characteristics of Metabolic Profiles of the Participants
Overall, 219 participants were enrolled: 109 patients taking clozapine, 50 taking less obesogenic antipsychotics (aripiprazole, 19; haloperidol, 16; amisulpride, 12; and ziprasidone, 3), and 60 nonpsychiatric controls (Figure 1). The demographic data and metabolic profiles of the 3 groups are shown in Table 1. Notably, 36%, 20%, and 10% of the participants in the clozapine, less obesogenic antipsychotic, and nonpsychiatric control groups, respectively, fulfilled the criteria for MS, revealing a significant difference in the rate of MS among the groups (X2=14.58, P<.01). Continuous variables with a right-skewed distribution, namely BW, BMI, WC, systolic BP, diastolic BP, FPG, TG, HDL-C, HbA1c, insulin, plasma orexin-A levels, as well as BPRS scores, were logarithmically transformed before proceeding with ANOVA. The duration of antipsychotic treatment was significantly different between the less obesogenic antipsychotic (mean=14.94 years, SD=9.32) and clozapine (mean=19.03 years, SD=9.56) groups (t=-2.48, P=.01). The BPRS scores were higher in patents taking clozapine (mean=26.47, SD=5.95) than in those taking less obesogenic antipsychotics (mean=24.26, SD=5.34) (t=2.01, P=.04). Some metabolic parameters, namely WC, diastolic BP, and FPG, TG, and HDL-C levels, were significantly different among the 3 groups (Table 1).
Figure 1.
Consort diagram with flow chart for patient inclusion in the study.
Table 1.
Demographic and Metabolic Profiles of Patients Taking Clozapine, Less Obesogenic Antipsychotics, and Nonpsychiatric Controls (n=219)
| N (%) / Mean±SD | P valuea | Posthoc testc | |||
|---|---|---|---|---|---|
| Variables | Clozapine (n=109) |
Less obesogenic APs (n=50) |
Nonpsychiatric controls (n=60) |
||
| Age (years) | 41.97±9.26 | 39.28±9.15 | 41.10±9.65 | .24 | |
| Gender (male) | 61 (56.0) | 16 (32.0) | 29 (48.3) | .02 | |
| Antipsychotics treatment duration (years) | 19.03±9.56 | 14.94±9.32 | – | .01 | |
| BPRS scores | 26.47±5.95 | 24.26±5.34 | – | .04 | |
| Cigarette smoking | 25 (23.8) | 8 (16.7) | 7 (11.7) | .14 | |
| Metabolic profile | |||||
| MS | 39 (35.8) | 10 (20.0) | 6 (10.0) | <.01 | |
| DM | 17 (15.6) | 2 (4.0) | 4 (6.7) | .05b | |
| IR | 48 (44.0) | 22 (44.0) | 15 (25.0) | <.05 | |
| Hypertension | 33 (30.8) | 12 (24.0) | 5 (8.3) | <.01b | |
| Body weight (kg) | 67.68±13.04 | 67.07±13.94 | 65.66±18.44 | .71d | |
| BMI (kg/m2) | 25.05±4.29 | 25.14±4.97 | 24.00±6.11 | .37d | |
| Waist (cm) | 87.29±11.41 | 85.25±12.72 | 79.31±14.28 | <.01d | 1 > 3*, 2 > 3*** |
| Systolic BP (mmHg) | 120.28±14.94 | 118.50±18.08 | 114.71±16.27 | .10d | |
| Diastolic BP (mmHg) | 77.53±11.66 | 73.20±13.12 | 72.34±10.00 | .01d | 1 > 3** |
| Fasting glucose (mg/dL) | 104.78±16.36 | 92.48±10.50 | 94.70±23.23 | <.01d | 1 > 2***, 1 > 3*** |
| HbA1c (%) | 5.68±0.46 | 5.61±0.42 | – | .39d | – |
| Triglyceride (mg/dL) | 146.86±95.60 | 130.20±79.29 | 92.88±56.78 | <.01d | 1 > 3*, 2 > 3*** |
| HDL-C (mg/dL) | 48.33±14.22 | 53.63±15.82 | 59.07±16.61 | <.01d | 3 > 1*** |
| Total cholesterol (mg/dL) | 180.22±38.00 | 183.08±43.27 | 187.45±35.63 | .51d | |
| Insulin (uIU/mL) | 10.17±6.58 | 10.54±6.84 | 9.88±9.97 | .90d | |
| HOMA-IR (mmol/L) | 2.64±1.80 | 2.45±1.64 | 2.63±3.69 | .89d | |
| Orexin-A (ng/mL) | 1.54±0.60 | 1.89±0.50 | 0.60±0.17 | <.01d | 2 > 1***, 2 > 3***, 1 > 3*** |
Abbreviations: BMI, body mass index; BP, blood pressure; BPRS, Brief Psychiatric Rating Scale-18; DM, debates mellitus type 2; HbA1c,glycated hemoglobin; HDL-C, high density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment for insulin resistance; IR, insulin resistance; MS, metabolic syndrome.
a P values of 1-way ANOVA tests / independent t tests for continuous variables or chi-square tests for categorical variables.
b P value for Fisher’s exact test.
c 1=patients taking clozapine; 2=patients taking less obesogenic antipsychotics; 3=nonpsychiatric controls. *P<.05, **P<.01, *** P<01 for P value for posthoc tests.
d The data have been logarithmic transformed before proceeding with the statistical analyses.
Comparisons of Plasma Orexin-A Levels in Groups
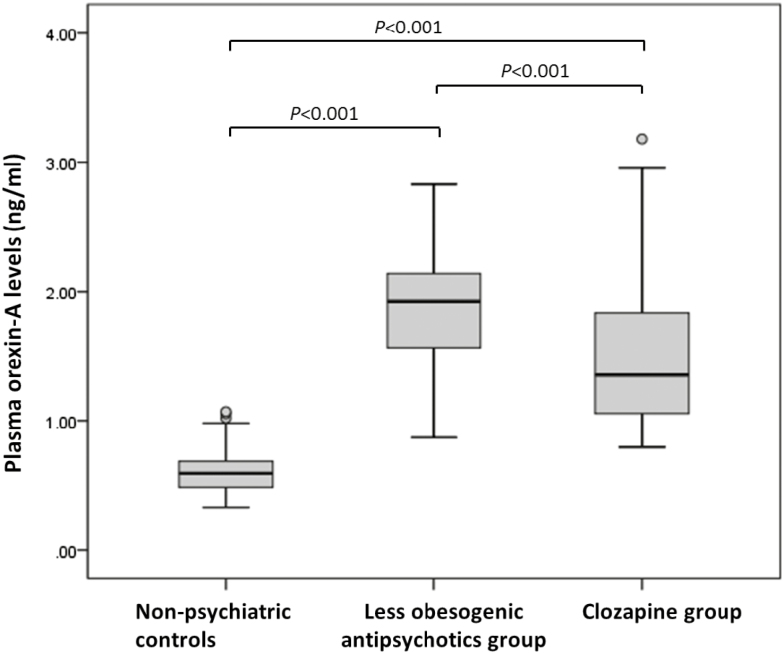
Figure 2 shows that the orexin-A levels differed across the 3 groups (P<.01). Posthoc comparisons using the Games–Howell test (Figure 2) also revealed significance for each pair of comparisons for orexin-A levels (all values P<.01), with the highest levels in the less obesogenic antipsychotic group (mean=1.89 ng/mL, SD=0.50), followed by the clozapine (mean=1.54 ng/mL, SD=0.60) and nonpsychiatric control (0.60 ng/mL, SD=0.17) groups. To estimate the effect of antipsychotic treatment on orexin-A levels, we conducted a multiple linear regression analysis and found that the antipsychotic treatment group was significantly associated with the orexin-A levels, after adjustment for age and sex (adjusted B-value=1.26, 95% CI: 1.07, adjusted B-value 1.45, P<.01 for the less obesogenic antipsychotic group vs the nonpsychiatric control group and adjusted B-value 0.94, 95% CI: 0.78, adjusted B-value 1.10, P<.01 for the clozapine group vs nonpsychiatric control group).
Figure 2.
Box plot of plasma orexin-A levels (ng/mL) in patients taking different antipsychotics and nonpsychiatric controls with comparisons by Welch’s 1-way ANOVA (posthoc multiple tests by the Games-Howell methods). The box plot whiskers indicate the highest, median, and lowest plasma orexin-A values that are no greater than 1.5 times the interquartile range, whereas the open circles are outlier values.
Correlation between Metabolic Parameters and Plasma Orexin-A Levels
In all the participants, plasma orexin-A levels were positively correlated with WC (r=0.17, P<.05) and TG levels (r=0.15, P<.05). Regarding the correlation between the orexin-A levels and metabolic parameters in each group, the orexin-A levels were positively correlated with HDL-C (r=0.26, P<.01) and HbA1C (r=0.28, P<.05) levels, but negatively correlated with systolic BP (r=−0.28, P<.01) in the clozapine group and with FPG (r=−0.31, P<.05) in the less obesogenic antipsychotic group. The orexin-A levels and metabolic parameters were not correlated in the nonpsychiatric control group (Supplementary Table 1).
Logistic Regression Analysis for MS Explanatory Variables
First, we conducted univariate analyses by using logistic regression to investigate the potential explanatory variables of MS in the sample by using age, sex, smoking history, type of antipsychotics (i.e., nonpsychiatric controls, patients taking less obesogenic antipsychotics, and clozapine) and orexin-A in tertiles as covariates (Table 2). The univariate analysis showed that older age, male sex, smoking, and treatment of clozapine were significantly associated with MS. Second, in the multivariate analysis in model 1, the clozapine group was significantly associated with MS risk (adjusted OR=5.10, 95% CI: 1.96–13.25, P<.01), with the nonpsychiatric controls as the reference group. Finally, by adding plasma orexin-A levels to the multivariate analysis in model 2, we observed that less obesogenic antipsychotics (adjusted OR=56.08, 95% CI: 5.22–602.67, P<.01), clozapine (adjusted OR=82.15, 95% CI: 8.82–765.07, P<.01), and orexin-A levels (adjusted OR=0.05, 95% CI: 0.01–0.39 for the 2nd tertile and adjusted OR=0.04, 95% CI: 0.01–0.36 for the 3rd tertile, with both values P<.01) were associated with MS risk, after adjustment for age, sex, and smoking history. Higher orexin-A levels were significantly associated with a lower risk of MS, even when the type of antipsychotics was considered a potential confounder.
Table 2.
Univariate and Multivariate Analyses of Risk Factors on Metabolic Syndrome in All Participants (Including Patients with Schizophrenia and Nonpsychiatric Participants) by Logistic Regression (n=217)
| Explanatory Variables | N (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|
| MS (n=55) | Non-MS (n=162) | Model 1a | Model 2b | |||||
| Age in years | 1.04 | (1.01–1.07)* | 1.03 | (0.99–1.07) | 1.03 | (0.99–1.07) | ||
| Gender | ||||||||
| Female | 19 (34.5) | 92 (56.8) | Ref | – | Ref | – | Ref | – |
| Male | 36 (65.5) | 70 (43.2) | 2.49 | (1.32–4.71)** | 2.08 | (1.01–4.32)* | 1.77 | (0.81–3.87) |
| Cigarette smoking | ||||||||
| No | 39 (70.9) | 133 (82.1) | Ref | – | Ref | – | Ref | – |
| Yes | 15 (27.3) | 24 (14.8) | 2.13 | (1.02–4.46)* | 1.22 | (0.52–2.84) | 1.57 | (0.65–3.79) |
| Missing | 1 (1.82) | 5 (3.1) | 0.68 | (0.08–6.01) | 0.36 | (0.04–3.55) | 0.06 | (0.01–1.41) |
| Type of antipsychotics | ||||||||
| Nonpsychiatric controls | 6 (10.9) | 54 (32.9) | Ref | – | Ref | – | Ref | – |
| Less obesogenic APs | 10 (18.2) | 39 (24.1) | 2.31 | (0.77–6.88) | 2.87 | (0.93–8.88) | 56.08 | (5.22–602.67)** |
| Clozapine | 39 (70.9) | 69 (42.6) | 5.09 | (2.01–12.90)** | 5.10 | (1.96–13.25)** | 82.15 | (8.82–765.07)** |
| Orexin-Ac | ||||||||
| 1st tertile (0.33–0.93) | 16 (29.09) | 52 (32.10) | Ref | – | Ref | – | ||
| 2nd tertile (0.94–1.59) | 21 (38.18) | 55 (33.95) | 1.24 | (0.58, 2.63) | 0.05 | (0.01, 0.39)** | ||
| 3rd tertile (1.60–3.18) | 18 (32.73) | 55 (33.95) | 1.06 | (0.49, 2.30) | 0.04 | (0.01, 0.36)** | ||
Abbreviations: Aps, antipsychotics; MS, metabolic syndrome; OR, odds ratio.
aModel 1: covariates including age, gender, cigarrete smoking, and participant groups (nonpsychiatric controls, patients taking less obesogenic antipsychotics or patients taking clozapine group).
bModel 2: covariates including age, gender, cigarrete smoking, participant groups (nonpsychiatric controls, patients taking less obesogenic antipsychotics or patients taking clozapine), and orexin-A in tertiles.
c The data have been logarithmic transformed before proceeding with the statistical analyses.
*P<.05, **P<.01.
Furthermore, because the orexin-A levels and metabolic parameters were not correlated in the nonpsychiatric controls, we examined the explanatory variables (namely age, sex, smoking history, types of antipsychotics, duration of antipsychotic treatment, and BPRS scores) for MS only in the patient groups (Table 3). In the univariate model, only older age and male sex were significantly associated with MS, whereas the orexin-A levels were inversely associated with MS. Smoking history, duration of antipsychotic treatment, type of antipsychotics, or BPRS scores was not associated with MS risk. In the multivariate model, orexin-A level was a predictive variable of MS (adjusted OR=0.04, 95% CI: 0.01–0.38 for the 2nd tertile and adjusted OR=0.04, 95% CI 0.01–0.36 for the 3rd tertile, both values P<.01), after adjustment for age, sex, smoking history, BPRS, types and duration of antipsychotic treatment.
Table 3.
Univariate and Multivariate Analyses of Risk Factors on Metabolic Syndrome in Patients with Schizophrenia by Logistic Regression (n=157)
| Explanatory variables | N (%) | Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |||
|---|---|---|---|---|---|---|
| MS (n=49) | Non-MS (n=108) | |||||
| Age in years | 1.04 | (1.01–1.08)* | 1.03 | (0.98–1.09) | ||
| Gender | ||||||
| Female | 18 (36.7) | 62 (57.4) | Ref | – | Ref | – |
| Male | 31 (63.3) | 46 (42.6) | 2.32 | (1.16–4.65)* | 1.45 | (0.60–3.48) |
| Cigarette smoking | ||||||
| No | 34 (69.4) | 85 (78.7) | Ref | – | Ref | – |
| Yes | 14 (28.6) | 18 (16.7) | 1.94 | (0.87–4.34) | 1.82 | (0.69–4.80) |
| Missing | 1 (2.0) | 5 (4.6) | 0.50 | (0.06–4.44) | 0.06 | (0.01–1.51) |
| Type of antipsychotics | ||||||
| Less obesogenic APs | 10 (20.4) | 39 (36.1) | Ref | – | Ref | – |
| Clozapine | 39 (79.6) | 69 (63.9) | 2.20 | (0.99–4.90) | 1.55 | (0.62–3.87) |
| BPRS scores | 0.99 | (0.93–1.06) | 0.99 | (0.92–1.06) | ||
| APs treatment duration in years | 1.03 | (0.99–1.07) | 1.00 | (0.95–1.05) | ||
| Orexin-A | ||||||
| 1st tertile (0.33–0.93) | 10 (20.4) | 2 (1.9) | Ref | – | Ref | – |
| 2nd tertile (0.94–1.59) | 21 (42.9) | 51 (47.2) | 0.08 | (0.02, 0.41)** | 0.04 | (0.01, 0.38)** |
| 3rd tertile (1.60–3.18) | 18 (36.7) | 55 (50.9) | 0.07 | (0.01, 0.33)** | 0.04 | (0.01, 0.36)** |
Abbreviations: Aps, antipsychotics; BPRS, Brief Psychiatric Rating Scale-18; MS, metabolic syndrome; OR odds ratio.
*P<.05, **P<.01.
Discussion
By dividing patients with schizophrenia into 2 groups according to the weight gain liability of their administered antipsychotics (Lieberman et al., 2005; Leucht et al., 2013; Vancampfort et al., 2015) rather than by first- or second-generation antipsychotics, we found, consistent with the literature (Kang and Lee, 2015), that the patients taking clozapine had more unfavorable plasma lipid and glucose parameters than the patients taking less obesogenic antipsychotics. Furthermore, patients with schizophrenia had significantly higher levels of orexin-A compared with nonpsychiatric controls. Among the patients, individuals taking less obesogenic antipsychotics had higher orexin-A levels than those taking clozapine. This is the first study to show a significant correlation between orexin-A levels and various metabolic parameters in patients receiving long-term antipsychotic treatment. We propose that antipsychotics modify the effects of orexin-A on metabolic parameters and that higher orexin-A levels are associated with a lower risk of MS in patients with schizophrenia. These results highlight the potential protective role of orexin-A against the development of antipsychotic-related metabolic abnormalities.
Our observation of increased orexin-A levels in patients with schizophrenia receiving antipsychotic treatment is in agreement with a recent report by Chien et al. (Chien et al., 2015). They also showed that long-term antipsychotic treatment is associated with relatively high levels of orexin-A, although a specific antipsychotic-type difference was not found. Evidence of orexin-A changes (an increase or decrease) is inconclusive. For example, Dalal et al. reported that the CSF orexin-A levels in patients following treatment with haloperidol for 1 to 8 weeks (n=7) were lower than that in unmedicated patients (n=12), but the levels in patients treated with clozapine or olanzapine (n=8) did not differ from that in the unmedicated patients (Dalal et al., 2003). Basoglu et al. also demonstrated a decrease in plasma orexin-A levels following 6 weeks of olanzapine treatment in patients with first-episode schizophrenia (n=20) compared with controls (n=22) (Basoglu et al., 2010). These mixed results may be due to the sample characteristics, including sample size and duration of antipsychotic treatment, as well as different antipsychotic medications that were used across studies. Patients in our study had a longer treatment period (17.8±9.6 years, ranging from 6 months to 40 years) compared with relatively shorter periods (1–6 weeks) for those enrolled in previous studies. Moreover, our sample size was larger, especially the clozapine-administered group, which enabled us to divide the patients by the weight gain liability of the antipsychotics they were taking, and, thus, to present more homogenous groups (Chien et al., 2015). Therefore, our findings may reflect the adaptive responses of the orexin system to long-term antipsychotic exposure. Nevertheless, how the treatment affects orexin-A expression should be further examined in longitudinal follow-up studies.
The literature on the correlation between orexin-A levels and metabolic parameters in nonpsychiatric controls is also contradictory. Although some studies have reported reduced levels of orexin-A in individuals with morbid obesity compared with individuals with normal weight (Baranowska et al., 2005) and a negative correlation with BMI (Adam et al., 2002), other studies have found that the orexin-A levels in individuals with MS (Tabak et al., 2012) or morbid obesity were increased, with a potentially positive correlation between orexin-A levels and BMI (Heinonen et al., 2005; Cigdem Arica et al., 2013). Researchers have suggested that these conflicting results are due to complex compensatory changes of orexin during the progression of metabolic abnormalities (Adam et al., 2002; Baranowska et al., 2005; Heinonen et al., 2005; Tabak et al., 2012; Cigdem Arica et al., 2013). In our nonpsychiatric controls, no correlation was found between orexin-A levels and BMI or other metabolic parameters. We speculate that the relatively few participants with morbid obesity (n=7) and MS (n=6) in our control group might have contributed to the lack of correlation.
Accumulating evidence suggests that the orexin neuron system plays a governing role in regulating eating behavior, metabolic function, and energy expenditure by integrating central and peripheral signals in a complex manner (Tsuneki et al., 2010). Preclinical studies have reported that orexin-A promotes feeding behavior (Sakurai et al., 1998), whereas ablation of the orexin neurons suppresses this behavior (Inutsuka et al., 2014). The orexin neuron system can also prevent the development of obesity, reduce adiposity by stimulating thermogenesis and energy expenditure through increasing sympathetic nerve activity (Messina et al., 2018), and reduce peripheral insulin resistance (Tsuneki et al., 2015). Peripheral orexin-A may exert its own direct metabolic effects while simultaneously interacting with the central orexin system and acting synergistically to affect metabolism. Furthermore, exogenous orexin-A administration has been shown to be beneficial in certain metabolic functions, including reducing blood glucose levels after a glucose load by enhancing the insulin secretion and reducing plasma glucagon in both normal and diabetic mice (Park et al., 2015). In line with the notion that orexin-A might oppose metabolic disturbances, our clinical observation that orexin-A is associated with a relatively low risk of MS also suggests that an increase in orexin-A counteracts the adverse metabolic outcomes related to chronic antipsychotic treatment. The protective effect of orexin-A may be attributable to an increase in the sympathetic tone and thermogenesis (Tsuneki et al., 2015; Messina et al., 2017). Studies have shown that a single dose of various antipsychotic treatments suppressed the sympathetic activation and thermogenesis effect that was induced by a central orexin-A injection (Monda et al., 2003, 2004, 2008, 2018). An extended exposure of antipsychotics may cause a compensatory upregulation of orexin-A to restore the sympathetic balance.
The lower levels of orexin-A in the clozapine group compared with the less obesogenic group might reflect a reduced ability to upregulate orexin-A, thereby causing a higher susceptibility to weight gain. Moreover, studies have shown that the suppression effect of antipsychotics on orexin-A-induced sympathetic activation varies across different drugs: haloperidol exhibited a partial suppression effect (Monda et al., 2003), whereas clozapine (Monda et al., 2004) and olanzapine (Monda et al., 2008) completely blocked sympathetic activation. Orexin neurons are regulated by serotoninergic, dopaminergic, and adrenergic neurons (Yamanaka et al., 2006). Therefore, it is likely that antipsychotics with diverse neurotransmitter receptor affinity profiles affect the action of orexin neurons differently. The differential influence on the orexin system between different antipsychotics was observed by Fadel et al., who reported that acute treatment of antipsychotics with higher weight gain liabilities induced immediate-early gene c-Fos expression in orexin neurons, but such induction was not seen in antipsychotics with a lower weight gain liability (Fadel et al., 2002). Although this observation may appear to be in contrast with our findings, which instead reported decreased orexin levels in patients receiving clozapine, it is probable that the compensatory response of the orexin system following long-term antipsychotic treatment is distinct from an acute effect. Nevertheless, additional studies are required to investigate whether antipsychotic-induced suppression on the sympathetic effect of orexin-A directly contributes to the metabolic adversities associated with long-term antipsychotic treatment.
Our study has several limitations. Because of the small sample size, we pooled patients under aripiprazole, haloperidol, amisulpride, and ziprasidone treatment into 1 group, less obesogenic antipsychotics, even though each antipsychotic has a distinct neurotransmitter profile and metabolic adversity liability. Although no difference was detected in the metabolic profiles of the participants, in MS prevalence, or in the plasma orexin-A levels in patients taking different types of antipsychotics with less obesogenic liability (data not shown), the number of participants for each antipsychotic agent in this group was small, which did not allow us to detect differences among them. Second, we included a nonpsychiatric control rather than drug-naïve patients. Although a previous study reported no difference in CSF orexin-A levels between patients with schizophrenic disorder and normal controls (Nishino et al., 2002), we cannot exclude the effect of schizophrenia per se on orexin-A levels. Third, our patients displayed heterogeneity in their clinical profiles (i.e., those taking clozapine showed higher psychotic severity, and a greater proportion of those taking less obesogenic antipsychotics were women). However, by using the multivariate model, we found that the effect of the severity of psychotic symptoms or the sex distribution on the association of orexin-A levels with risk of MS was not significant. Fourth, we did not have information regarding the type of antipsychotic treatment administered 6 months prior to enrollment as well as diet and level of physical activity; thus, we cannot eliminate a long-lasting metabolic influence from previous antipsychotic treatments or from diet or exercise variance. Fifth, the causality in the association between orexin-A and metabolic measurements could not be examined in this cross-sectional study. Further prospective longitudinal studies measuring orexin levels during antipsychotic treatment may help to clarify this. Finally, orexin-A concentrations in previous studies using radioimmunoassay or EIA methods distributed in a considerably wide range from a mean of 2430 ng/mL to a median of 0.00065 ng/mL (Cintron et al., 2017). Although the orexin-A levels in our study (mean=1.36 ng/mL, SD=0.70, ranging from 0.33 ng/mL to 3.18 ng/mL) were of a similar range to previous studies using EIA methods (Sanchez-de-la-Torre et al., 2011; Chen et al., 2016; Cintron et al., 2017), both radioimmunoassay and EIA methods are subjective to different antibodies, radioligands, reagents, and other experimental conditions and thus can only produce “relative” instead of absolute measurements, precluding us from a direct comparison of the values across studies (Nishino, 2005).
In conclusion, our study suggests that long-term antipsychotic treatment is associated with increased levels of orexin-A. Patients treated with clozapine had lower orexin-A levels than those treated with less obesogenic agents. Higher plasma orexin-A levels were associated with favorable lipid and BP profiles in the antipsychotic-treated patients. Additionally, higher orexin-A levels were associated with a lower risk of MS development. In summary, our study supports the role of the orexin system in the regulation of antipsychotic-related metabolic abnormalities. Moreover, upregulation of orexin-A potentially exerts a protective effect. Additional studies are required to examine the causal relationship between adaptive responses of orexin-A levels and metabolic dysregulation as well as to investigate whether enhancing the orexin-A system would be advantageous in patients with schizophrenia who display or carry a risk of developing MS.
Supplementary Material
Acknowledgments
We thank Ren-Hui Zheng, Jia-Ru Chen, Su-Chen Chang, Shu-Pin Lo, and Hsiao-Tien Wu for their assistance with patient recruitment, data management, and administrative affairs.
This work was supported by the Ministry of Science and Technology, Taiwan (100-2314-B-532-001, 101-2314-B-532-003, 102-2314-B-532-001, 104-2314-B-532-003, 105-2314-B-532-005, 106-2314-B-532-005-MY3); Taipei City Government, Taiwan (10101-62-067, 10201-62-046, 10301-62-006, 10701-62-029); and Taipei Institute of Pathology, Taiwan.
Statement of Interest
None.
References
- Adam JA, Menheere PP, van Dielen FM, Soeters PB, Buurman WA, Greve JW(2002)Decreased plasma orexin-A levels in obese individuals. Int J Obes Relat Metab Disord 26:274–276. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC Jr, International Diabetes Federation Task Force on Epidemiology and Prevention, Hational Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, International Association for the Study of Obesity (2009)Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120:1640–1645. [DOI] [PubMed] [Google Scholar]
- Baptista T, ElFakih Y, Uzcátegui E, Sandia I, Tálamo E, Araujo de Baptista E, Beaulieu S(2008)Pharmacological management of atypical antipsychotic-induced weight gain. CNS Drugs 22:477–495. [DOI] [PubMed] [Google Scholar]
- Baranowska B, Wolińska-Witort E, Martyńska L, Martyńska M, Chmielowska M, Baranowska-Bik A(2005)Plasma orexin A, orexin B, leptin, neuropeptide Y (NPY) and insulin in obese women. Neuro Endocrinol Lett 26:293–296. [PubMed] [Google Scholar]
- Basoglu C, Oner O, Gunes C, Semiz UB, Ates AM, Algul A, Ebrinc S, Cetin M, Ozcan O, Ipcioglu O(2010)Plasma orexin A, ghrelin, cholecystokinin, visfatin, leptin and agouti-related protein levels during 6-week olanzapine treatment in first-episode male patients with psychosis. Int Clin Psychopharmacol 25:165–171. [DOI] [PubMed] [Google Scholar]
- Bushe C, Holt R(2004)Prevalence of diabetes and impaired glucose tolerance in patients with schizophrenia. Br J Psychiatry Suppl 47:S67–S71. [DOI] [PubMed] [Google Scholar]
- Chen WY, Kao CF, Chen PY, Lin SK, Huang MC(2016)Orexin-A level elevation in recently abstinent male methamphetamine abusers. Psychiatry Res 239:9–11. [DOI] [PubMed] [Google Scholar]
- Chien YL, Liu CM, Shan JC, Lee HJ, Hsieh MH, Hwu HG, Chiou LC(2015)Elevated plasma orexin A levels in a subgroup of patients with schizophrenia associated with fewer negative and disorganized symptoms. Psychoneuroendocrinology 53:1–9. [DOI] [PubMed] [Google Scholar]
- Cigdem Arica P, Kocael A, Tabak O, Taskin M, Zengin K, Uzun H(2013)Plasma ghrelin, leptin, and orexin-A levels and insulin resistance after laparoscopic gastric band applications in morbidly obese patients. Minerva Med 104:309–316. [PubMed] [Google Scholar]
- Cintron D, Beckman JP, Bailey KR, Lahr BD, Jayachandran M, Miller VM(2017)Plasma orexin A levels in recently menopausal women during and 3 years following use of hormone therapy. Maturitas 99:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal MA, Schuld A, Pollmächer T(2003)Lower CSF orexin A (hypocretin-1) levels in patients with schizophrenia treated with haloperidol compared to unmedicated subjects. Mol Psychiatry 8:836–837. [DOI] [PubMed] [Google Scholar]
- Fadel J, Bubser M, Deutch AY(2002)Differential activation of orexin neurons by antipsychotic drugs associated with weight gain. J Neurosci 22:6742–6746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games PA, Howell JF(1976)Pairwise multiple comparison procedures with unequal N’s and/or variances: A Monte Carlo Study. J Educ Stat 1:113–125. [Google Scholar]
- Greenhalgh AM, Gonzalez-Blanco L, Garcia-Rizo C, Fernandez-Egea E, Miller B, Arroyo MB, Kirkpatrick B(2017)Meta-analysis of glucose tolerance, insulin, and insulin resistance in antipsychotic-naïve patients with nonaffective psychosis. Schizophr Res 179:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F, American Heart Association, National Heart, Lung, and Blood Institute (2005)Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- Heinonen MV, Purhonen AK, Miettinen P, Pääkkönen M, Pirinen E, Alhava E, Akerman K, Herzig KH(2005)Apelin, orexin-A and leptin plasma levels in morbid obesity and effect of gastric banding. Regul Pept 130:7–13. [DOI] [PubMed] [Google Scholar]
- Huang MC, Lu ML, Tsai CJ, Chen PY, Chiu CC, Jian DL, Lin KM, Chen CH(2009)Prevalence of metabolic syndrome among patients with schizophrenia or schizoaffective disorder in taiwan. Acta Psychiatr Scand 120:274–280. [DOI] [PubMed] [Google Scholar]
- Inutsuka A, Inui A, Tabuchi S, Tsunematsu T, Lazarus M, Yamanaka A(2014)Concurrent and robust regulation of feeding behaviors and metabolism by orexin neurons. Neuropharmacology 85:451–460. [DOI] [PubMed] [Google Scholar]
- Kang SH, Lee JI(2015)Metabolic disturbances independent of body mass in patients with schizophrenia taking atypical antipsychotics. Psychiatry Investig 12:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MB, Lee YJ, Yen LL, Lin MH, Lue BH(1990)Reliability and validity of using a brief psychiatric symptom rating scale in clinical practice. J Formos Med Assoc 89:1081–1087. [PubMed] [Google Scholar]
- Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, Samara M, Barbui C, Engel RR, Geddes JR, Kissling W, Stapf MP, Lässig B, Salanti G, Davis JM(2013)Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 382:951–962. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK, Clinical Antipsychotic Trials of Intervention Effectiveness I (2005)Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 353:1209–1223. [DOI] [PubMed] [Google Scholar]
- Lin YC, Yen LL, Chen SY, Kao MD, Tzeng MS, Huang PC, Pan WH(2003)Prevalence of overweight and obesity and its associated factors: findings from National Nutrition and Health Survey in Taiwan, 1993–1996. Prev Med 37:233–241. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Meyer JM, Goff DC, Nasrallah HA, Davis SM, Sullivan L, Meltzer HY, Hsiao J, Scott Stroup T, Lieberman JA(2005)Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the clinical antipsychotic trials of intervention effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 80:19–32. [DOI] [PubMed] [Google Scholar]
- Messina A, Monda M, Valenzano A, Messina G, Villano I, Moscatelli F, Cibelli G, Marsala G, Polito R, Ruberto M, Carotenuto M, Monda V, Viggiano A, Daniele A, Nigro E(2018)Functional changes induced by orexin A and adiponectin on the sympathetic/parasympathetic balance. Front Physiol 9:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina G, Dalia C, Tafuri D, Monda V, Palmieri F, Dato A, Russo A, De Blasio S, Messina A, De Luca V, Chieffi S, Monda M(2014)Orexin-A controls sympathetic activity and eating behavior. Front Psychol 5:997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina G, Valenzano A, Moscatelli F, Salerno M, Lonigro A, Esposito T, Monda V, Corso G, Messina A, Viggiano A, Triggiani AI, Chieffi S, Guglielmi G, Monda M, Cibelli G(2017)Role of autonomic nervous system and orexinergic system on adipose tissue. Front Physiol 8:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monda M, Viggiano A, De Luca V(2003)Haloperidol reduces the sympathetic and thermogenic activation induced by orexin A. Neurosci Res 45:17–23. [DOI] [PubMed] [Google Scholar]
- Monda M, Viggiano A, Viggiano A, Fuccio F, De Luca V(2004)Clozapine blocks sympathetic and thermogenic reactions induced by orexin A in rat. Physiol Res 53:507–513. [PubMed] [Google Scholar]
- Monda M, Viggiano A, Viggiano A, Viggiano E, Messina G, Tafuri D, De Luca V(2006)Quetiapine lowers sympathetic and hyperthermic reactions due to cerebral injection of orexin A. Neuropeptides 40:357–363. [DOI] [PubMed] [Google Scholar]
- Monda M, Viggiano A, Viggiano A, Mondola R, Viggiano E, Messina G, Tafuri D, De Luca V(2008)Olanzapine blocks the sympathetic and hyperthermic reactions due to cerebral injection of orexin A. Peptides 29:120–126. [DOI] [PubMed] [Google Scholar]
- Monda V, Salerno M, Sessa F, Bernardini R, Valenzano A, Marsala G, Zammit C, Avola R, Carotenuto M, Messina G, Messina A(2018)Functional changes of orexinergic reaction to psychoactive substances. Mol Neurobiol 55:6362–6368. [DOI] [PubMed] [Google Scholar]
- Nishino S.(2005)Hypocretin measurements in the CSF, and blood and brain tissue. In: The Orexin/Hypocretin System: Physiology and Pathophysiology (Nishino S, Sakurai T, eds), pp73–82. Totowa, NJ: Humana Press. [Google Scholar]
- Nishino S, Ripley B, Mignot E, Benson KL, Zarcone VP(2002)CSF hypocretin-1 levels in schizophrenics and controls: relationship to sleep architecture. Psychiatry Res 110:1–7. [DOI] [PubMed] [Google Scholar]
- Park JH, Shim HM, Na AY, Bae JH, Im SS, Song DK(2015)Orexin A regulates plasma insulin and leptin levels in a time-dependent manner following a glucose load in mice. Diabetologia 58:1542–1550. [DOI] [PubMed] [Google Scholar]
- Pearson K.(1909)Determination of the coefficient of correlation. Science 30:23–25. [DOI] [PubMed] [Google Scholar]
- Sakurai T, et al. (1998)Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585. [DOI] [PubMed] [Google Scholar]
- Sanchez-de-la-Torre M, Barcelo A, Pierola J, Esquinas C, de la Pena M, Duran-Cantolla J, Capote F, Masa JF, Marin JM, Vila M, Cao G, Martinez M, de Lecea L, Gozal D, Montserrat JM, Barbe F(2011)Plasma levels of neuropeptides and metabolic hormones, and sleepiness in obstructive sleep apnea. Respir Med 105:1954–1960. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC(1998)The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59:22–33. [PubMed] [Google Scholar]
- Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H(1999)Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol 277:R1780–R1785. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Pyne-Geithman GJ, Ekhator NN, Horn PS, Uhde TW, Shutter LA, Baker DG, Geracioti TD Jr(2010)Low cerebrospinal fluid and plasma orexin-A (hypocretin-1) concentrations in combat-related posttraumatic stress disorder. Psychoneuroendocrinology 35:1001–1007. [DOI] [PubMed] [Google Scholar]
- Tabak O, Gelişgen R, Cicekçi H, Senateş E, Erdenen F, Müderrisoğlu C, Aral H, Uzun H(2012)Circulating levels of adiponectin, orexin-A, ghrelin and the antioxidant paraoxonase-1 in metabolic syndrome. Minerva Med 103:323–329. [PubMed] [Google Scholar]
- Tsuneki H, Wada T, Sasaoka T(2010)Role of orexin in the regulation of glucose homeostasis. Acta Physiol (Oxf) 198:335–348. [DOI] [PubMed] [Google Scholar]
- Tsuneki H, Tokai E, Nakamura Y, Takahashi K, Fujita M, Asaoka T, Kon K, Anzawa Y, Wada T, Takasaki I, Kimura K, Inoue H, Yanagisawa M, Sakurai T, Sasaoka T(2015)Hypothalamic orexin prevents hepatic insulin resistance via daily bidirectional regulation of autonomic nervous system in mice. Diabetes 64:459–470. [DOI] [PubMed] [Google Scholar]
- Vancampfort D, Stubbs B, Mitchell AJ, De Hert M, Wampers M, Ward PB, Rosenbaum S, Correll CU(2015)Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry 14:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Muraki Y, Ichiki K, Tsujino N, Kilduff TS, Goto K, Sakurai T(2006)Orexin neurons are directly and indirectly regulated by catecholamines in a complex manner. J Neurophysiol 96:284–298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.